Introduction
In 1975 Giulio D’Angio stated that “cure is not enough” and late effects research was declared an essential priority.1 As detailed in the preceding chapters, there is a growing, global population of childhood cancer survivors who face unique health and psychosocial challenges in adulthood as a result of their prior cancer treatment. Decades of studies have described the long-term and late health outcomes experienced by survivors including risks conferred by specific therapy exposures, demographic and lifestyle factors.2–6 However, the future of cancer survivorship research must focus on novel approaches to identify, prevent, and mitigate adverse effects of cancer therapy. Attention should also be paid to disseminate and implement uniform and cost-effective patient-centered survivorship care within diverse clinical settings. In order to do this, future research priorities must include multi-disciplinary and internationally collaborative approaches to better understand the toxicity of novel agents, contribution of genetic factors and aging to late effects, targets for intervention, and the role of health services research and implementation science to assure universal access to evidence-based and comprehensive survivorship care.
Large Cohort Collaboration: Pooling of Data to Answer the Unanswered Questions
Despite growing numbers of survivors, childhood cancer remains a rare disease, and although most survivors experience early and late complications of disease and treatment, identification of low prevalence, yet clinically important late effects, such as rare subsequent neoplasms, are difficult to ascertain due to the small number of events. Multiple institutions and cooperative groups have developed and followed childhood cancer survivors in large cohorts.5,7–9 Still, the numbers of childhood cancer survivors with a given combination of treatment exposures or specific late effects are often not large enough to answer key research questions regarding risk for toxicity. Optimally, the pooling of data across groups and projects could address this issue.
Combining data from various cohorts can facilitate early identification of late complications, both known and novel, and updating of surveillance recommendations in long-term follow-up clinical practice guidelines. Two examples of recently completed studies highlight the power of using pooled cohort data to more accurately define late effect risks of specific therapeutic exposures as well as model an individual’s risk for a specific late effect. In the first, demographic, treatment and late-outcomes data from the Childhood Cancer Survivor Study (CCSS), the Dutch Children’s Oncology Group’s (DCOG) LATER study, and the St Jude Lifetime (SJLIFE) study were used to evaluate the assumption that the long-term cardiotoxicity of anthracycline and anthraquinone agents was equivalent to their established hematological toxicity profile.10,11 This analysis including data from nearly 30,000 childhood cancer survivors demonstrated that daunorubicin, previously thought to be roughly isoequivalent to doxorubin, was only half as cardiotoxic when cardiomyopathy was used as an endpoint, while mitoxantrone was at least 10-times as cardiotoxic as doxorubicin.10,11 These findings prompted a change in the previous doxorubicin equivalent conversion of 1:1 for daunorubicin and reconsideration of the prior conversion of 4:1 for mitoxantrone. These findings will certainly impact the screening recommendations for individuals who received mitoxantrone or daunorubicin by modifying their cumulative anthracycline exposure and will also inform future frontline and relapse protocol development. In the second example, data from the CCSS, SJLIFE, and Emma Children’s Hospital/Academic Medical Center were used to develop and validate a risk-prediction model for heart failure, ischemic heart disease and stroke among five-year childhood cancer survivors through age 50.12–14 These models allow for individualized risk prediction based on demographic factors, chemotherapy and radiation doses, and traditional cardiovascular risk factors (hypertension, diabetes, dyslipidemia) that may assist in patient education as well as clinical decision making regarding potentially risk-reducing interventions.
Finally, if a late complication of cancer treatment has occurred, care should focus on ameliorating the effects and providing the necessary support to the survivor. By pooling cohort data, we are more likely to understand the magnitude and severity of late complications that should inform better understanding of the interventions and support services that may benefit the affected survivor. Optimizing care for childhood cancer survivors requires new collaborations across cooperative groups, institutional studies and national and continental borders.
Defining Genetic Contributions to Late Effects Risk
Research in the general population has demonstrated that genetic factors contribute to many of the conditions for which childhood cancer survivors have elevated risks, such as subsequent malignancies, cardiovascular disease, obesity, and hearing loss.15 Advances in technology and concurrent reductions in genotyping and sequencing costs have facilitated research in large study populations, which has enabled the identification of both common and rare genetic risk variants in novel biological pathways that underlie these conditions in the general population.16,17
The contribution of inherited factors to the development of selected conditions in childhood cancer survivors is currently unknown.18,19 However, as the technological advances used in the general population are leveraged to study cancer survivors, the future holds tremendous promise for defining the genetic contributions to a range of disease risks after childhood cancer. Preliminary evidence suggests that certain rare and common genetic factors act similarly in both the general population and among survivors,20–22 though further research is needed to understand whether the joint effect of genetic susceptibility and treatment-related risks is additive or multiplicative. Other studies have identified novel genetic risk factors among survivors,23 suggesting that the pathophysiology underlying treatment-related conditions may differ from that seen in the general population.
To comprehensively define the genetic contributions to disease risk after childhood cancer, international, interdisciplinary collaborations will be required to create studies with sufficient sample sizes and diversity of patient characteristics (e.g., specific treatment exposures, race/ethnicity, and ages). As demonstrated in the general population, large sample sizes that enable replication of results in independent populations are especially important in genomics research to protect against false positive reports. However, the challenges of developing such collaborations are dwarfed by the importance of discovering the biological pathways that underlie these conditions – thereby providing clues to prevention approaches and informing clinical decisionmaking for frontline treatment recommendations and long-term surveillance. The coming years should see a dramatic expansion in the consideration of genetic susceptibility in survivorship after childhood cancer.
Identifying Late Effects of Novel Therapies
In recent years, improved understanding of the biology of many pediatric cancers has led to the development of multiple new agents that offer the promise of more effective and less toxic treatment (Table 1). For example, an initial success came with Philadelphia-positive acute lymphoblastic leukemia, where the tyrosine kinase inhibitor, imatinib, plus chemotherapy, transformed 3-year event-free survival from <50% to ~80%.24 The addition of other molecularly-targeted agents to conventional chemotherapy is now routine in subsets of acute myeloid leukemia, lymphoma, and sarcoma patients where specific tumor mutations appear amenable to such inhibitors (e.g., FLT3-internal tandem duplications, anaplastic large cell kinase mutations, NTRK fusions, respectively).25–27 The addition of antibody-based therapies to conventional chemotherapy has also improved outcomes for many pediatric malignancies. For example, dinutuximab, rituximab, brentuximab, and gemtuzumab are already considered standard of care for certain newly diagnosed or relapsed neuroblastomas, lymphomas and leukemias, respectively.28–31 Ongoing trials are testing the efficacy of other promising antibodies such as blinatumomab and inotuzumab,32,33 and the optimal role of immune checkpoint inhibitors and genetically engineered chimeric antigen receptor (CAR) T cells.34,35 For local control, surgery and radiotherapy have also evolved to become less invasive, or feature new techniques and particles (e.g., protons) that more precisely target the tumor and limit dose to normal tissues.36
Table 1.
Molecularly targeted agents being used or under consideration for pediatric cancers (Adapted from Chow EJ, Antal Z, Constine LS, et al. New agents, emerging late effects, and the development of precision survivorship. J Clin Oncol. 2018;36(21):2231-2240; with permission)
| Target(s) | Drug(s) | Notable toxicities* |
|---|---|---|
| ALK/ROS | Ceritinib Crizotinib Ensartinib Lorlatinib |
Arrhythmia Dyslipidemia (lorlatinib) Hyperglycemia (ceritinib) Hallucinations / psychiatric (lorlatinib) Neuropathy/neuromuscular Pulmonary embolism (crizotinib) Respiratory Vision changes |
| BCR/ABL, KIT, PDGFR | Dasatinib Imatinib Nilotinib Ponatinib |
Cardiac dysfunction Edema, effusions Growth & stature* Pulmonary hypertension (dasatinib) Thyroid dysfunction Vascular events, including myocardial ischemia, peripheral arterial occlusion, and stroke (ponatinib) |
| BRAF | Dabrafenib Vemurafenib |
Hyperglycemia (dabrafenib) New acute development of skin cancers QT-prolongation (vemurafenib) Radiation sensitivity |
| CD3 | Blinatumomab | B cell aplasia (CAR T cells)* Cytokine release syndrome Neurotoxicity |
| CD19 | Blinatumomab Chimeric antigen receptor (CAR) T cells |
Same as with CD3-targeted agents above |
| CD20 | Rituximab | B cell aplasia |
| CD30 | Brentuximab vedotin | Neuropathy Progressive multifocal leukoencephalopathy |
| CD33 | Gemtuzumab ozogamicin | Hepatotoxicity, sinusoidal obstruction syndrome |
| CDK/cyclin (cell cycle) | Palbociclib Ribociclib |
QT-prolongation (ribociclib) |
| EZH2 | Tazemetostat | Limited experience to date |
| GD2 | Dinutuximab Hu3F8 Hu14.18K322A |
Capillary leak syndrome Neuropathic pain Reversible posterior leukoencephalopathy |
| HDAC (histone deacetylase) | Entinostat Fimepinostat Panobinostat Romidepsin Vorinostat |
Arrhythmia / myocardial infarction Pulmonary embolus (vorinostat) Limited experience for entinostat, fimepinostat |
| MEK/MAPK | Binimetinib Cobimetinib Selumetinib Trametinib |
Cardiac dysfunction Skin toxicity Vision changes, retinopathy |
| mTOR | Everolimus Sirolimus Temsirolimus ABI-009 (Nab-Rapamycin) LY3023414 |
Dyslipidemia Hyperglycemia |
| PD-1, PDL-1, CTL4 (immune checkpoint) | Atezolizumab Avelimumab Cemiplimab Durvalumab Ipilimumab Nivolumab Pembrolizumab |
Auto-immune/inflammatory, including: Endocrinopathies Myocarditis Neurotoxicity Pneumonitis |
| PI3K | Fimepinostat LY3023414 |
Hyperglycemia |
| TRK | Entrectinib Larotrectinib |
Limited experience to date |
| VEGF, VEGFR, PDGFR, RET | Axitinib Bevacizumab Cabozantinib Lenvantinib Pazopanib Sorafenib Vandetanib |
Cardiac dysfunction Hemorrhage, impaired wound healing Hepatotoxicity (pazopanib) Hypertension, proteinuria Intestinal perforation/fistula (bevacizumab) Thromboembolism Thyroid dysfunction (axitinib, pazopanib, sorafenib) |
Toxicities that may persist well after cessation of therapy or develop later.
Nevertheless, “targeted” agents, like conventional chemotherapy, radiotherapy, and surgery, may have off-target effects.36 For example, growth issues have been reported in children treated long-term with imatinib.37,38 Although not yet reported in children, rare but serious immune-related toxicities (e.g., autoimmune myocarditis) have been observed in adults, including young adults, treated with immune checkpoint inhibitors.39 As experience with new agents and modalities grows, the established paradigm of requiring large phase 2 or 3 trials to establish efficacy prior to an agent becoming standard of care may no longer be feasible in all situations.40 Given that pediatric cancer is relatively rare and, hopefully, that delayed but serious off-target toxicities are even less frequent, longitudinal systematic follow-up of children treated with novel emerging therapies is critical to determining whether these therapies are truly associated with improved long-term outcomes compared to historical treatments. Decades of follow-up are required to demonstrate improvements in long-term pediatric cancer outcomes.41,42 To facilitate this, a joint effort by the pharmaceutical industry, government, and non-governmental professional societies to organize infrastructure that enables such long-term follow-up is recommended. Such infrastructure should include, at minimum, the creation of a registry that allows for later linkage and ability to recontact patients or families for follow-up information, or if possible, a more resourceintensive prospective cohort.
Identifying Frailty and Accelerated Aging
Studies conducted in past decades identified increased risk for chronic health conditions, multiple health conditions and mortality compared to the general population and have suggested a process of accelerated aging among adult childhood cancer survivors.2,3 More recently, measures generally utilized in geriatric populations, such as frailty, characterized by decreased physiologic reserve, have been identified as a prevalent condition among young adult childhood cancer survivors. At a median age of 33 years, survivors were observed to have rates of frailty higher than those observed in the general population in their seventh decade of life (Figure 1).43,44 Frailty, in the general population, identifies individuals who are at risk for poor health outcomes including early mortality.43 Aging may be accelerated among childhood cancer survivors due to chemotherapy- and radiation-induced damage to normal, non-malignant cells. Some evidence suggests that molecular mechanisms associated with physiologic aging in the general population may potentiate premature aging among survivors including cellular senescence, telomere attrition, changes in DNA methylation patterns, accumulation of somatic DNA mutations and loss of mitochondrial fidelity (Figure 2).45,46 However, few studies have evaluated specific associations of these mechanisms with accelerated aging in childhood cancer survivors.
Figure 1.
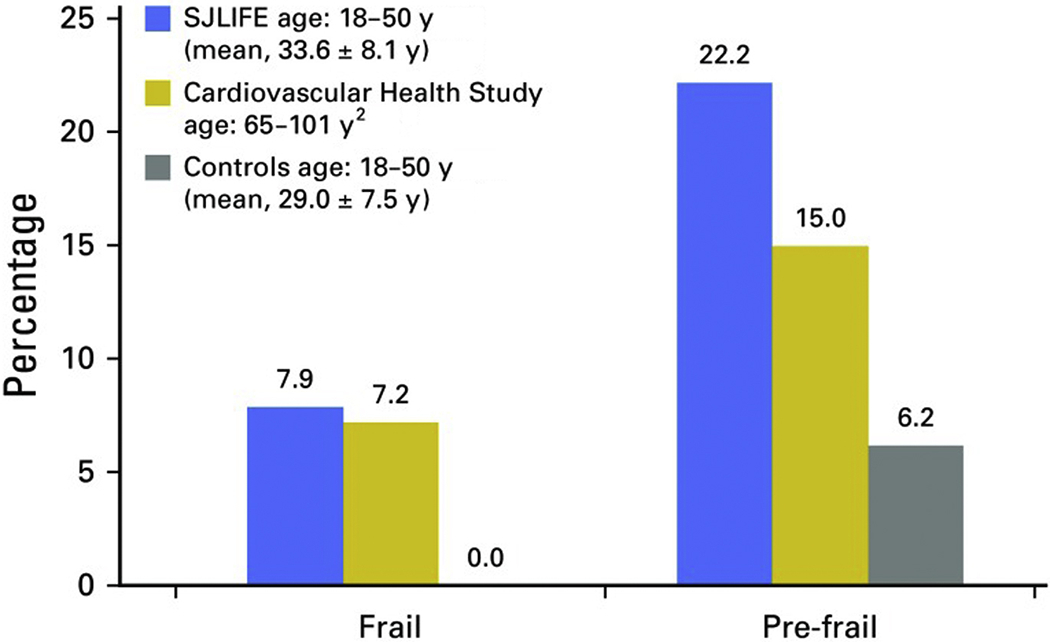
Percentage of survivors in the St. Jude Lifetime Cohort Study (SJLIFE) who meet the criteria for frailty compared with participants in the Cardiovascular Health Study and normal controls. N = 1,922 (50.3% male); mean time since diagnosis, 25.5 ± 7.7 years; mean age at diagnosis, 8.2 ± 5.6 years; 43% leukemia; 33% with cranial radiation exposure.
From Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. JClin Oncol. 2018;36(21):2206-2215; with permission.
Figure 2.
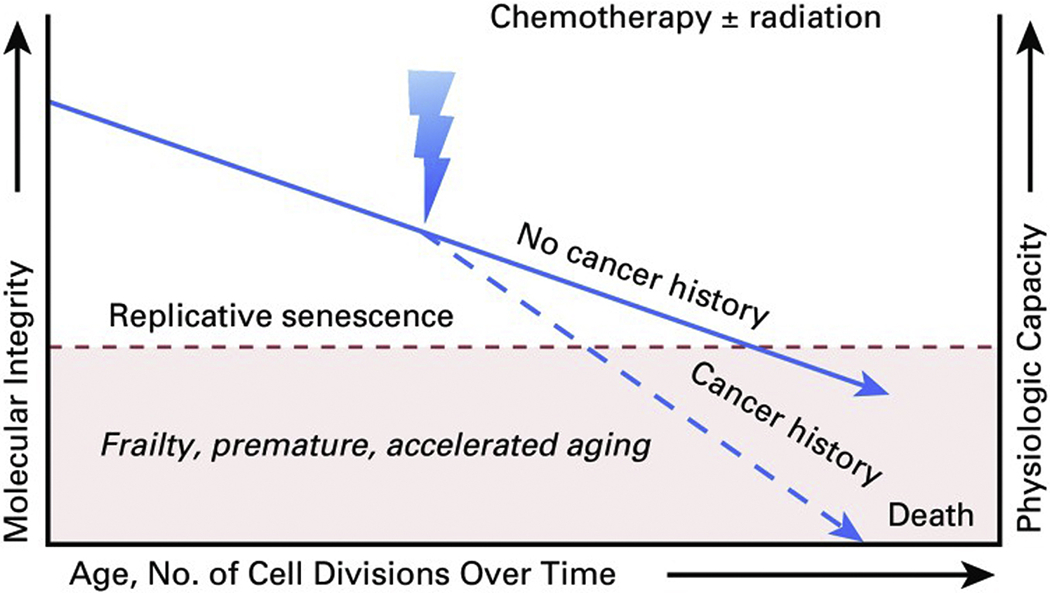
Extrapolated figure hypothesizing that molecular integrity and associated physiologic capacity provoked by exposure to chemotherapy and/or radiation may be associated with excess risk and advanced onset of age-related diseases and frailty.
From Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol. 2018;36(21):2206-2215; with permission.
Although better understanding of the underlying mechanisms is needed, existing data may also be used to guide effective clinical trial development. Markers of aging have been shown to be responsive to exercise in the general population.46 Among childhood cancer survivors, exercise intervention led to increased lean mass, strength and walking speed;47 however, future research is needed to specifically measure the impact of exercise on biomarkers of aging. Senolytic agents selectively eliminate senescent cells which are no longer dividing and have demonstrated efficacy in the treatment of multiple chronic diseases in animal models including frailty and radiation-induced muscle wasting.48,49 Clinical trials are needed to assess the impact of these and other pharmacologic agents on delaying or reversing markers of aging.
However, as premature aging among survivors differs from physiologic aging in the general population, improving not only the lifespan but also the health span of an aging population of adult childhood cancer survivors necessitates a better understanding of the molecular mechanisms most critical in potentiating early aging. With this knowledge, identification of specific subgroups of survivors at highest risk and subsequent development of novel pharmacologic and lifestyle interventions that target biologic pathways to prevent, or delay, aging associated with cancer and cancer treatment will be possible in the future.
Intervention and Precision Prevention of Late Effects
The National Institutes of Health has defined precision medicine as “an emerging approach for disease prevention and treatment that takes into account people’s individual variation in genes, environment, and lifestyle.” The use of cancer therapies targeted at specific molecular abnormalities in tumors has led to a revolution in the precision medicine approach to treating cancer. However, development of precision approaches to preventing or treating the late effects that can arise from cancer therapies has lagged. Precision survivorship36 integrates a cancer patient’s genomic features with traditional risk factors such as demographics (e.g. age, sex, race/ethnicity) and treatment exposures (e.g. cumulative chemotherapy doses, radiation) to more precisely predict late effects risk. Better risk prediction can be applied at the time of cancer therapy50 to improve counseling or inform the use of protective agents or modification of chemotherapy dose or type, or in follow-up to guide the efficient use of surveillance, prophylaxis or early therapy of sub-clinical late effects. This can be enhanced by integrating individual data about lifestyle, comorbid health conditions and other chronic disease risk factors.13 As other elements of a survivor’s -omics profile become available (e.g. proteomics, metabolomics),51 risk-prediction models could be further enriched.
A majority of the intervention studies aimed at improving survivor outcomes have focused on the completion of recommended late effects surveillance.52–54 Despite this, adherence to published late effects guidelines is generally poor.55 Similarly, trials of secondary chemoprevention of late effects (e.g. use of low-dose tamoxifen to reduce breast cancer risk in irradiated survivors) have generally struggled to accrue patients, in part because of the lack of precision in determining at-risk survivors and survivors’ reluctance to take medications with adverse side effect profiles to prevent a late effect that they may or may not develop. More precise classification of “at-risk” status can improve the focus of interventions on those survivors at particularly elevated risk, potentially improving survivors’ willingness to participate in such trials. In addition, true precision prevention acknowledges that interventions may need to be tailored to specific survivor phenotypes. For example, a recent study identified survivors are at increased risk for poor exercise intolerance for many reasons (cardiac, pulmonary, neuropathic and muscular impairments, among others).56 Based on these findings, survivors will need interventions tailored to their specific deficits to improve their exercise tolerance.
Mobile health (mHealth) interventions offer an opportunity for better targeting of risk-based survivor care. In the survivorship context, mHealth platforms will allow for easier, real-time communication between survivors and their care providers, and remote collection of biometric data (e.g. blood pressure), medical data (e.g. teledermatology),57 or patient-reported outcome measures (e.g. pain). “Real time” access to such individualized patient data will guide the provision of appropriate survivor care, even remotely. Thus, the combination of more precise predictors of long-term risk and new tools to facilitate the collection of data and provision of survivor care will herald a new era of precision survivorship.
The Future of Health Services Research for Childhood Cancer Survivors
Previous health service research in childhood cancer survivors has largely focused on determining how social factors and health behaviors among survivors affect their access and use of health services.58–60 This knowledge has been helpful in characterizing survivors at high risk for morbidity and guiding the development of interventions for populations vulnerable to specific adverse health outcomes. Surprisingly, few studies have addressed the quality and cost-effectiveness of survivorship care delivered within health care systems or its impact on important outcomes such as survivor satisfaction, health-related quality of life, chronic symptoms and disease burden, health care utilization, and survival.61,62 Correspondingly, despite a plethora of publications describing models of survivorship care, evaluating the use of cancer treatment summaries and survivorship care plans, and promoting risk-based health surveillance recommendations,63,64 studies are lacking that demonstrate how these resources can be feasibly and effectively implemented in oncology and primary care settings to facilitate care coordination, reduce duplication of services, and improve survivorship outcomes.
Progress in survivorship care and enhancement of the health span of the growing population of childhood cancer survivors requires methodologically rigorous research guided by an analytic framework of established health care quality measures, particularly timeliness, patient-centeredness, efficiency and equity of care. Table 2 summarizes the common domains of health care quality measures and potential survivorship topics relevant to these measures advocated by the National Academy of Medicine (formerly the Institute of Medicine).65 To address knowledge deficits related to survivorship care, careful conceptualization of research aims within the context of survivor, health care provider, community and health care system factors influencing access to services and survivorship outcomes is essential. In the absence of an integrated health services system, robust infrastructure will need to be developed to support longitudinal investigations of factors influencing access and utilization of health services across the survivorship spectrum from diagnosis, to after completion of therapy, and into long-term follow-up. Improving care coordination should focus on investigation of scalable methods of communication and education at transition milestones, particularly during the transition from oncology to primary care. Historically, survivorship care plans aimed to serve this purpose but, considering the lack of uniform evidence to demonstrate benefit,66 evaluation of stakeholder perspective (both survivors and providers) is critical to advance understanding about barriers to coordination and implementation of quality, patient-centered survivorship care.
Table 2.
Institute of Medicine Domains of Health Care Quality
| Domain | Aim for health care quality | Relevance to childhood cancer |
|---|---|---|
| Safe | Avoiding harm to patients from the care that is intended to help them. | Are there efforts and systems in place to monitor late effects of childhood cancer and its treatment? |
| Effective | Providing services based on scientific knowledge to all who could benefit and refraining from providing services to those not likely to benefit (avoiding underuse and misuse, respectively) | Do survivors have access to surveillance, treatment and interventions known to impact late effects? |
| Patient-centered | Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions. | Is survivorship care designed around the preferences and needs of and resources available to survivors? Is financial toxicity and survivor engagement considered in care planning? |
| Timely | Reducing waits and sometimes harmful delays for both those who receive and those who give care. | Are diagnoses of post-treatment health problems made quickly and without delay? |
| Efficient | Avoiding waste, including waste of equipment, supplies, ideas, and energy. | Are resources used efficiently? Is there communication between survivors and providers over time and across settings of care? |
| Equitable | Providing care that does not vary in quality because of personal characteristics such as gender, ethnicity, geographic location, and socioeconomic status. | Are there disparities in care and outcomes based on social risk? Are survivors able to receive care in a nearby and convenient location? |
Dissemination and Implementation Science to Improve Survivorship Care
In 2001, the National Academy of Medicine brought to light the major gap between the quality and efficiency of care that could be delivered when health care was informed by scientific evidence and the care that was delivered in routine practice – termed the quality chasm.65 Dissemination and implementation (D&I) science is designed to bridge that chasm and translate research findings into clinical practice. D&I research in survivors is needed to fill the identified gap between what survivorship care could be with improved care coordination, seamless transition from oncology to primary care, and uniform access to evidence based screening or intervention strategies outlined above, and what is the reality of often fractured and inconsistent, if any, survivorship care today. Given the emphasis on guideline development and intervention, collaboration with experts in dissemination and implementation will be essential to translate these findings to the almost half million survivors in the U.S. and many more around the world.67
The Future of Evidence Based Guideline Development
In Chapter 4 the international collaboration for guideline development (IGHG) has been described. International collaboration is needed to keep up-to-date with the increasing number of new papers on (interventions for) health problems in childhood cancer survivors, to avoid duplication of work, and to make the process of translating evidence to recommendation transparent. As new knowledge is always emerging, it is essential that a process is in place to update guidelines regularly or perform continuous review of new evidence. Unfortunately, development of guidelines and updating them is a time consuming process. New methods for development and timely updating of these guidelines is essential to bridge the gap between knowledge and practice. The IGHG is developing a living guidelines ICT (information and communication technology) tool in collaboration with a European project (PanCareFollowUp). This ICT tool “the living guideline” will be able to regularly search the literature and to provide guideline groups with new evidence. It will also support the guideline groups to grade new evidence and, if needed, adjust current recommendations. This will help to translate new knowledge into guidelines in an efficient way.
Keeping guidelines up-to-date is important, but simply having guidelines in place does not mean that clinicians are utilizing them for survivorship care in their clinical practice. The complexity of survivorship care may contribute to difficulties with implementation. Many health problems can occur, and the recommendations are based on many different types of cancer treatment. Future solutions could include implementation of a digital decisions support tool, based on previous treatment, that could to generate a treatment summary and an individual care plan for survivors. Such a tool can help to implement all recommendations in survivorship care.68 Moreover risk prediction tools, such as those discussed for cardiac risk above, may help to refine future recommendations for an individual survivor.
Guidelines are also important to inform survivors and encourage survivorship care. Every survivor of childhood cancer should be aware of their specific treatment-related risks for health problems and suggested risk-based care recommendations from current guidelines. Every survivor of childhood cancer should be aware of their specific treatment-related risks for health problems and suggested risk-based care recommendations from current guidelines. A nice example is the Survivorship Passport initiative of the European Network for Cancer research in Children and Adolescents (ENCCA) in collaboration with other key stakeholders across Europe to develop individualized, web-based, survivorship care plans utilizing follow-up and screening recommendations from the International Guidelines Harmonization Group (IGHG) and PanCare Childhood and Adolescent Cancer Survivor Care and Follow-Up Studies (www.PanCareFollowUp.eu and www.survivorshippassport.org).68
In addition to having an individualized survivorship care plan, ensuring availability of cancer survivorship guidelines in lay language and translation of cancer survivorship guidelines into multiple languages, utilizing non-medical terminology, will enable survivors to manage their own care. Nice examples are the lay language summaries for guideline recommendations described in the Survivorship Passport (www.survivorshippassport.org) and at the Together website from St. Jude Children’s Research Hospital (together.stjude.org/en-us).
Conclusion
As survival continues to improve for children diagnosed with cancer and the growing population of childhood cancer survivors in the United States alone now approaches 500,000,67 we must focus on how to improve not just the duration but also the quality of life after childhood cancer. The future of survivorship research must prioritize expansion of knowledge of late health and psychosocial outcomes, including toxicity of novel therapies and genetic contribution to risk, by leveraging large-scale, collaborative research, and efforts toward prediction and prevention of late effects at the individual survivor level. Further, for these findings to translate into improved outcomes among survivors, they must be adopted by providers and health systems outside of pediatric oncology, which will require coordinated, national and international efforts to overcome barriers to successful implementation of high-quality, patient-centered survivorship care.
KEY POINTS.
Childhood cancer survivorship research has made substantial advances towards improving understanding of treatment-related toxicities.
- Future efforts should:
- Emphasize collaborative approaches, including pooling of data across studies, to address a range of research questions.
- Integrate individualized approaches to risk-prediction, including integration of genomic data, to provide opportunities to prevent late effects in survivors.
- Maximize knowledge of late effects of novel therapies, intervention strategies, aging and accelerated aging, health services utilization, and dissemination and implementation science to better promote adoption of survivorship care recommendations by aging adult survivors and their providers.
SYNOPSIS.
As the population who survive childhood cancer ages and increases in number, and as treatment continues to evolve, cancer survivorship researchers must utilize novel approaches to prevent, identify and mitigate adverse effects of cancer treatment. Priorities for the future include collaborative efforts to pool data from large cohorts to improve power for detection of late effects, identify the late effects of novel therapies as well as determine the contribution of genetic factors and both physiologic and accelerated aging among survivors. Beyond adding to our knowledge of late effects risks and mechanisms, this knowledge should translate to individual risk prediction and prevention strategies. Finally, we must employ approaches from health services research and implementation science to improve adoption of survivorship care recommendations outside of specialized pediatric oncology centers. Without dissemination of these findings through coordinated, national and international efforts, the decades of research may not translate into improved health and psychosocial outcomes for the growing community of survivors of childhood cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
Contributor Information
Stephanie B. Dixon, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN.
Eric J. Chow, Fred Hutchinson Cancer Research Center, University of Washington, Seattle, WA.
Lars Hjorth, Department of Paediatrics, Skåne University Hospital, Lund, Sweden.
Melissa M. Hudson, Division of Cancer Survivorship, Department of Oncology, St. Jude. Children’s Research Hospital, Memphis, TN.
Leontien C. M. Kremer, Princess Maxima Center, Utrecht. Emma Children’s Hospital, Amsterdam UMC.
Lindsay M. Morton, Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
Paul C. Nathan, Division of Hematology/Oncology, The Hospital for Sick Children, Toronto, ON, Canada.
Kirsten K. Ness, Department of Epidemiology and Cancer Control, St. Jude. Children’s Research Hospital, Memphis, TN.
Kevin C. Oeffinger, Duke Center for Onco-Primary Care, Duke Cancer Institute, Durham, NC.
Gregory T. Armstrong, Department of Epidemiology and Cancer Control, St. Jude. Children’s Research Hospital, Memphis, TN.
References
- 1.D’Angio GJ. Pediatric cancer in perspective: cure is not enough. Cancer. 1975;35(3 suppl):866–870. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32(12):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teepen JC, van Leeuwen FE, Tissing WJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study Cohort: role of chemotherapy. J Clin Oncol. 2017;35(20):2288–2298. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon SB, Bjornard KL, Alberts NM, et al. Factors influencing risk-based care of the childhood cancer survivor in the 21st century. CA Cancer J Clin. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011. ;56(5):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winther JF, Kenborg L, Byrne J, et al. Childhood cancer survivor cohorts in Europe. Acta Oncol. 2015;54(5):655–668. [DOI] [PubMed] [Google Scholar]
- 10.Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer. J Clin Oncol. 2015;33(32):3774–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow EJ, Chen Y, Hudson MM, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. Journal of Clinical Oncology. 2018;36(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Chow EJ, Oeffinger KC, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price AL, Spencer CC, Donnelly P. Progress and promise in understanding the genetic basis of common diseases. Proc Biol Sci. 2015;282(1821):20151684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michailidou K, Beesley J, Lindstrom S, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline MS, Liao RG, Parsons MT, et al. BRCA Challenge: BRCA Exchange as a global resource for variants in BRCA1 and BRCA2. PLoS Genet. 2018;14(12):e1007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatia S Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer. 2015;121(5):648–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gramatges MM, Bhatia S. Evidence for genetic risk contributing to long-rerm adverse treatment effects in childhood cancer survivors. Annu Rev Med. 2018;69:247–262. [DOI] [PubMed] [Google Scholar]
- 20.Opstal-van Winden AWJ, de Haan HG, Hauptmann M, et al. Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood. 2019;133(10):1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong SL, Chaiyakunapruk N, Lee SW. Candidate gene association studies of anthracycline-induced cardiotoxicity: a systematic review and meta-analysis. Sci Rep. 2017;7(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Wilson CL, Easton J, et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36(20):2078–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton LM, Sampson JN, Armstrong GT, et al. Genome-wide association study to identify susceptibility loci that modify radiation-related risk for breast cancer after childhood cancer. J Natl Cancer Inst. 2017;109(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol. 2009;27(31):5175–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rollig C, Serve H, Huttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699. [DOI] [PubMed] [Google Scholar]
- 26.Mosse YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: a Children’s Oncology Group study. J Clin Oncol. 2017;35(28):3215–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laetsch TW, DuBois SG, Nagasubramanian R, et al. A pediatric phase 1 study of larotrectinib, a highly selective inhibitor of the tropomyosin receptor kinase (TRK) family. J Clin Oncol. 2017;35(suppl; abstr 10510). [Google Scholar]
- 28.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard JA, Loken M, Gerbing RB, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2016;34(7):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minard-Colin V, Auperin A, Pillon M, et al. Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): Evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. J Clin Oncol. 2016;34(15s):10507–10507. [Google Scholar]
- 32.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(36):4381–4389. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto N, Park JR, Murphy E, et al. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 35.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371 (16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow EJ, Antal Z, Constine LS, et al. New agents, emerging late effects, and the development of precision survivorship. J Clin Oncol. 2018;36(21):2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shima H, Tokuyama M, Tanizawa A, et al. Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr. 2011;159(4):676–681. [DOI] [PubMed] [Google Scholar]
- 38.Tauer JT, Hofbauer LC, Jung R, et al. Impact of long-term exposure to the tyrosine kinase inhibitor imatinib on the skeleton of growing rats. PLoS One. 2015;10(6):e0131192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DuBois SG, Corson LB, Stegmaier K, Janeway KA. Ushering in the next generation of precision trials for pediatric cancer. Science. 2019;363(6432):1175–1181. [DOI] [PubMed] [Google Scholar]
- 41.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017;317(8):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001. ;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 44.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31 (36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ness KK, Kirkland JL, Gramatges MM, et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol. 2018;36(21):2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016;3:CD008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aminkeng F, Ross CJ, Rassekh SR, et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016;82(3):683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biro K, Dombradi V, Jani A, Boruzs K, Gray M. Creating a common language: defining individualized, personalized and precision prevention in public health. J Public Health (Oxf). 2018;40(4):e552–e559. [DOI] [PubMed] [Google Scholar]
- 52.Oeffinger KC, Ford JS, Moskowitz CS, et al. Promoting breast cancer surveillance: the EMPOWER study, a randomized clinical trial in the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(24):2131–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson MM, Leisenring W, Stratton KK, et al. Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: a randomized controlled trial. J Clin Oncol. 2014; 32(35):3974–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zabih V, Kahane A, O’Neill NE, Ivers N, Nathan PC. Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: a systematic review. J Cancer Surviv. 2019;13(5):713–729. [DOI] [PubMed] [Google Scholar]
- 55.Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153(7):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ness KK, Plana JC, Joshi VM, et al. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J Clin Oncol. 2019:JCO1901661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniel CL, Armstrong GT, Keske RR, et al. Advancing Survivors’ Knowledge (ASK) about skin cancer study: study protocol for a randomized controlled trial. Trials. 2015; 16: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caplin DA, Smith KR, Ness KK, et al. Effect of population socioeconomic and health system factors on medical care of childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Adolesc Young Adult Oncol. 2017;6(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casillas J, Oeffinger KC, Hudson MM, et al. Identifying predictors of longitudinal decline in the level of medical care received by adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Health Serv Res. 2015;50(4):1021–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mueller EL, Park ER, Kirchhoff AC, et al. Insurance, chronic health conditions, and utilization of primary and specialty outpatient services: a Childhood Cancer Survivor Study report. J Cancer Surviv. 2018;12(5):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siembida EJ, Kadan-Lottick NS, Moss K, Bellizzi KM. Adolescent cancer patients’ perceived quality of cancer care: The roles of patient engagement and supporting independence. Patient Educ Couns. 2018;101(9):1683–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutradhar R, Agha M, Pole JD, et al. Specialized survivor clinic attendance is associated with decreased rates of emergency department visits in adult survivors of childhood cancer. Cancer. 2015;121(24):4389–4397. [DOI] [PubMed] [Google Scholar]
- 63.Kadan-Lottick NS, Ross WL, Mitchell HR, Rotatori J, Gross CP, Ma X. Randomized Trial of the Impact of Empowering Childhood Cancer Survivors With Survivorship Care Plans. J Natl Cancer Inst. 2018;110(12):1352–1359. [DOI] [PubMed] [Google Scholar]
- 64.Landier W, Chen Y, Namdar G, et al. Impact of tailored education on awareness of personal risk for therapy-related complications among childhood cancer survivors. J Clin Oncol. 2015;33(33):3887–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Institute of Medicine Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, D.C.: National Academy Press; 2001. [PubMed] [Google Scholar]
- 66.Jacobsen PB, DeRosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018;36(20):2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haupt R, Essiaf S, Dellacasa C, et al. The ‘Survivorship Passport’ for childhood cancer survivors. European Journal of Cancer. 2018; 102:69–81. [DOI] [PubMed] [Google Scholar]


