Abstract
The tumor development and metastasis are closely related to the structure and function of the tumor microenvironment (TME). Recently, TME modulation strategies have attracted much attention in cancer immunotherapy. Despite the preliminary success of immunotherapeutic agents, their therapeutic effects have been restricted by the limited retention time of drugs in TME. Compared with traditional delivery systems, nanoparticles with unique physical properties and elaborate design can efficiently penetrate TME and specifically deliver to the major components in TME. In this review, we briefly introduce the substitutes of TME including dendritic cells, macrophages, fibroblasts, tumor vasculature, tumor-draining lymph nodes and hypoxic state, then review various nanoparticles targeting these components and their applications in tumor therapy. In addition, nanoparticles could be combined with other therapies, including chemotherapy, radiotherapy, and photodynamic therapy, however, the nanoplatform delivery system may not be effective in all types of tumors due to the heterogeneity of different tumors and individuals. The changes of TME at various stages during tumor development are required to be further elucidated so that more individualized nanoplatforms could be designed.
Keywords: Nanoparticles, Tumor microenvironment, Cancer immunotherapy, Hypoxia
Abbreviations: Ab, antibodies; AC-NPs, antigen-capturing nanoparticles; Ag, antigen; ANG2, angiopoietin-2; APCs, antigen-presenting cells; AuNCs, gold nanocages; AuNPs, gold nanoparticles; α-SMA, alpha-smooth muscle actin; BBB, blood-brain barrier; Bcl-2, B-cell lymphoma 2; BTK, Bruton's tyrosine kinase; CaCO3, calcium carbonate; CAR-T, Chimeric antigen receptor-modified T-cell therapy; CAFs, cancer associated fibroblasts; CAP, cleavable amphiphilic peptide; CCL, chemoattractant chemokines ligand; cDCs, conventional dendritic cells; CTL, cytotoxic T lymphocytes; CTLA4, cytotoxic lymphocyte antigen 4; DCs, dendritic cells; DMMA, 2,3-dimethylmaleic anhydrid; DMXAA, 5,6-dimethylxanthenone-4-acetic acid; DSF/Cu, disulfiram/copper; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; EPG, egg phosphatidylglycerol; EPR, enhanced permeability and retention; FDA, the Food and Drug Administration; FAP, fibroblast activation protein; HA, hyaluronic acid; HB-GFs, heparin-binding growth factors; HIF, hypoxia-inducible factor; HPMA, N-(2-hydroxypropyl) methacrylamide; HSA, human serum albumin; IBR, Ibrutinib; IFP, interstitial fluid pressure; IL, interleukin; LMWH, low molecular weight heparin; LPS, lipopolysaccharide; IFN-γ, interferon-γ; M2NP, M2-like TAM dual-targeting nanoparticle; MCMC, mannosylated carboxymethyl chitosan; MDSCs, myeloid-derived suppressor cells; melittin-NP, melittin-lipid nanoparticle; MnO2, manganese dioxide; MPs, microparticles; NF-κB, nuclear factor κB; NK, nature killer; nMOFs, nanoscale metal-organic frameworks; NO, nitric oxide; NPs, nanoparticles; ODN, oligodeoxynucleotides; PD-1, programmed cell death protein 1; PDT, photodynamic therapy; PFC, perfluorocarbon; PHDs, prolyl hydroxylases; PLGA, poly(lactic-co-glycolic acid); PS, photosensitizer; PSCs, pancreatic stellate cells; PTX, paclitaxel; RBC, red-blood-cell; RLX, relaxin-2; ROS, reactive oxygen species; SA, sialic acid; scFv, single-chain variable fragment; siRNA, small interfering RNA; SPARC, secreted protein acidic and rich in cysteine; TAAs, tumor-associated antigens; TAMs, tumor-associated macrophages; tdLNs, tumor-draining lymph nodes; TDPA, tumor-derived protein antigens; TfR, transferrin receptor; TGF-β, transforming growth factor β; TIE2, tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2; TIM-3, T cell immunoglobulin domain and mucin domain-3; TLR, Toll-like receptor; TME, tumor microenvironment; TNF-α, tumor necrosis factor alpha; Tregs, regulatory T cells; UPS-NP, ultra-pH-sensitive nanoparticle; VEGF, vascular endothelial growth factor; VDA, vasculature disrupting agent
Graphical abstract
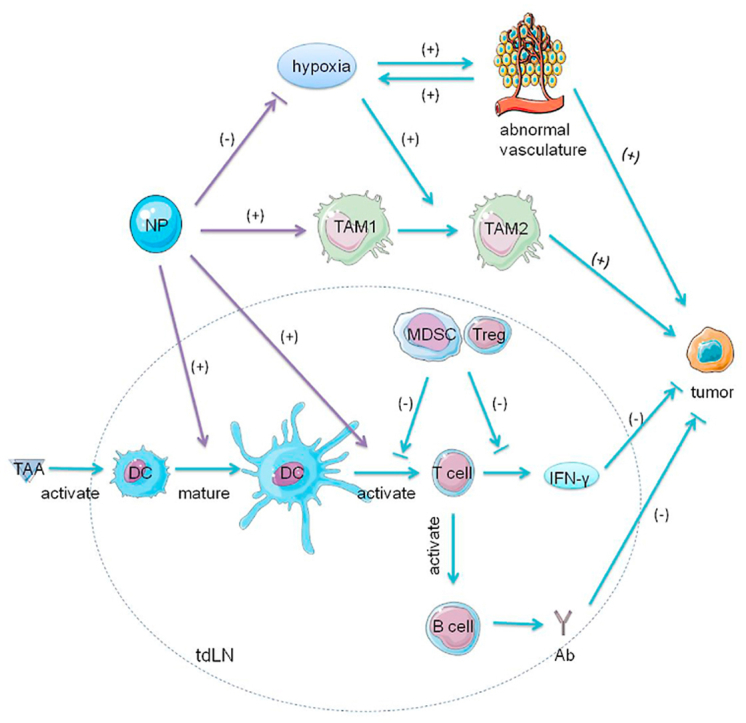
Highlights
-
•
In responsive to the changes in TME, nanoparticles target tumor microenvironment and enhance the therapeutic effect.
-
•
Nanoparticles modulate the activation and maturation of DC.
-
•
Nanoparticles could reprogram polarization of TAM and relieve hypoxia.
-
•
Nanoparticles could transfer the immunosuppressive TME to immunosupportive.
1. Introduction
Tumor microenvironment (TME) consists of various cells (including immune cells,fibroblasts, endothelial cells, inflammatory cells and lymphocytes), extracellular matrix (ECM), vasculature and chemokines [[1], [2], [3]]. The immune cells mediate innate and adaptive immune responses. Innate immune cells including macrophages and dendritic cells (DCs) are both pro- and anti-tumorigenic depending on complex cross-talk and different chemokines in TME. The adaptive immune system activated by innate immune cells can specifically attack tumor cells and is regarded as most effective for tumor eradication. The activated fibroblasts in TME, known as cancer-associated fibroblasts (CAFs), construct the structure of microenvironment by synthesizing a large proportion of ECM in TME and have a significant impact on tumor progression and tumor therapy [4]. The TME is characterized by the aberrant tumor vasculature with abnormal appearance and function, which results in a hypoxic state. Moreover, hypoxia in TME can alter the function of the normal microenvironment, promote tumor progression, and restrict therapeutic effects. Therefore, the interactions between tumor cells and the components in TME affect tumor progression and metastasis as well as the therapeutic effect. It has been demonstrated that TME has a profound influence on drug penetration and function and is associated with drug resistance and low response rates. To this end, modulation of TME has attracted much attention among researchers interested in cancer immunotherapy [5,6].
The TME modulation strategies targeting extracellular ligand-receptor interactions and downstream pathways can improve therapeutic efficacy and achieve durable responses [7,8]. The TME-modulated agents including a cytotoxic lymphocyte antigen 4 (CTLA4) antagonist plerixafor [9], an anti-interleukin-6 monoclonal antibody siltuximab [10], an antibody to chemoattractant chemokines ligand 2 (CCL 2) carlumab, and a novel selective antagonist of α4β1 and α4β7 integrins natalizumab [11] have shown preliminary success in tumor therapy. Moreover, the Food and Drug Administration (FDA) has granted accelerated approval to pembrolizumab, an inhibitory antibody that targets the immune-checkpoint receptor programmed cell death protein 1 (PD-1) in May 2017 [12], however, some patients do not benefit from these drugs. In addition, many patients have experienced severe adverse events, including gastrointestinal, hematologic and endocrine disorders, arthritis, rash, neuropathy, and acute kidney injury [[13], [14], [15]]. Chimeric antigen receptor-modified T-cell therapy (CAR-T) against CD19 has been proved to be effective in treating acute lymphoblastic leukemia, however, it has so far shown limited therapeutic effects in melanoma [16] and non-small-cell lung cancer [17] and adverse events have been reported.
In comparison with these drugs, nanoparticles can prolong retention time and achieve targeted delivery, thus reducing toxicity. Moreover, nanoparticles could change the immunosuppressive environment in TME by targeting the major components of TME. The distorted blood vessels in TME and rapid growth of tumor cells directly result in hypoxia, which leads to immunosuppression in TME by accumulating immunosuppressive cells including regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) and secreting immunosuppressive factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β). These substitutes inhibit the functions of DCs, transfer macrophages to the pro-tumorigenic M2 phenotype and lead to abnormal fibrosis. Nanoparticles with specific design can target these components in TME and transform the immunosuppressive TME to an immunosupportive state, thus promoting the efficacy of cancer immunotherapy. Nanoparticles have been extensively designed as drug delivery systems [18]. They can be passively delivered to tumor tissue and prolong the retention time of carried drugs. Owing to the enhanced permeability and retention (EPR) effect they tend to accumulate in tumor much more than in normal tissue because of the leaky tumor vasculature and damaged lymphatic drainage [19,20]. On the other hand, modification of their structures can further enhance their efficacy and compatibility. Conjugating with specific ligands, nanoparticles achieve targeted delivery to TME as well as modulate the TME, thus enhancing therapeutic efficacy [[21], [22], [23]]. Nanoplatforms could combine with other therapies by carrying various drugs. Numerous nanostructures with different compositions, sizes, shapes, and functions have been developed [24]. Several nanodrugs, such as Doxil [25] and Abraxane [26], have been approved by the FDA for clinical use. In this review, we summarized the current targeted delivery of nanoparticles to the components in TME. We briefly introduce the major components of TME including DCs, macrophages, fibroblasts, tumor vasculature, tumor-draining lymph nodes (tdLNs) and the hypoxic state, then review various nanoparticles targeting these components and their applications in tumor therapy.
2. Targeting dendritic cells
DCs derived from bone marrow are the most powerful antigen-presenting cells (APCs) [27]. They could be found in blood, but once activated, they migrate to lymph nodes where they interact with T cells. They are the center of immune responses, linking innate and adaptive immunity. They recognize endogenous and exogenous proteins, and degrade them into small pieces, and then present them on the cell surface to naive T cells, thus initiating and modulating adaptive immunity (Fig. 1). Conventional DCs (cDCs), one subset of the DC lineage, are thought to be closely associated with tumor development, which internalize tumor-associated antigens and deliver them to draining lymph nodes [28], activating immunity against tumor. However, a number of molecules found in TME as well as hypoxic environment and lactic acid inhibit the functions of cDCs [29,30]. Nanoparticles conjugated with specific ligands could target DCs and modulate their activation and maturation (Table 1).
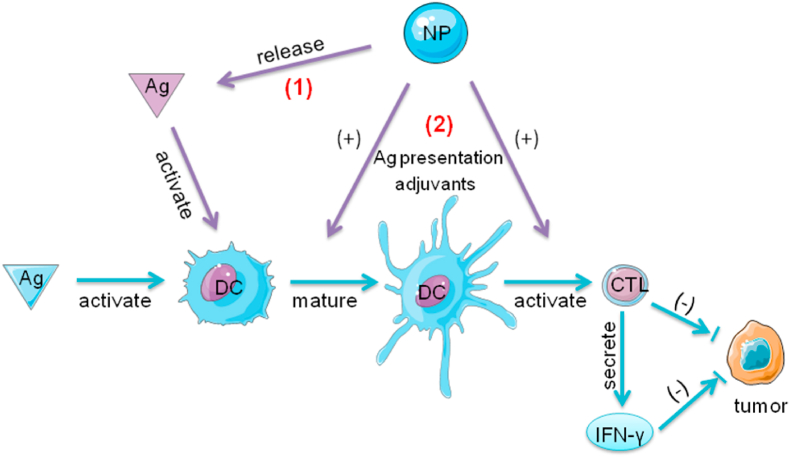
Fig. 1.
The role of DCs and the function of NPs in the tumor immunity. In response to endogenous and exogenous antigens DCs activate and maturate. They recognize these antigens and degrade them, then present them on the cell surface to naive T cells. The T cells become activated and transform to CTLs. The CTLs can attack tumor cells via direct killing or IFN-γ dependent pathways. (1) NPs modified with antigens and adjuvants specifically deliver antigens to DCs, (2) and promote DCs maturation and CTL activation by antigen presentation or the assistance of adjuvants. DCs present antigen fragments to naïve T cells. The CD4+ and CD8+ T cells become activated, undergo clonal expansion, and acquire cytotoxic abilities or helper functions (such as IFN-γ secretion). The addition of TLR ligands in NPs induces strong immune responses. Apart from extra addition of adjuvants in nanocomplex, Fe3O4 NPs as nano-immunopotentiators could promote the maturation of dendritic cells and immune responses; Abbreviations: Ag, antigen; DCs, dendritic cells; CTL, cytotoxic T lymphocyte; IFN-γ, interferon-γ; NPs, nanoparticles.
Table 1.
Summary of the application of nanoparticles.
| Target | NP | Diameter | Mechanism | Animal model | Cell lines | Ref |
|---|---|---|---|---|---|---|
| DC | PLGA NPs loaded with ovalbumin and TLR 3 and 7 ligands | 186.6 ± 9.0–242.3 ± 9.0 nm | target CD40, DEC-205, CD11c on DCs | WT C57BL/6 mice, Albino B6, Ly5.1/CD45.1, transgenic OT-I/Thy1.1/CD45.2, transgenic OT-II/Ly5.1/CD45.1 mice | WT C57BL/6 or CD40 KO BMDCs | [34] |
| DC | lipid-coated calcium phosphate NPs delivered specific tumor antigen BRAFV600E peptide | 30 nm | antigen presentation | female C57BL/6 mice (6–8 weeks old) | murine BRAF-mutant melanoma cell line BPD6 | [35] |
| DC | erythrocyte membrane-enveloped PLGA NPs | 131.3 ± 0.6 nm - 149.2 ± 0.6 nm | antigen presentation | adult C57BL/6 mice | murine melanoma cell line B16F10 (syngeneic with C57BL/6) and DC2.4 cells | [36] |
| DC | ultra-small Fe3O4 NPs loaded with ovalbumin | 20–40 nm | Fe3O4 NPs as adjuvants promote DC activation | female C57BL/6 mice (4–6 weeks old) | BMDCs from C57BL/6 mice and B16–F10 melanoma cell line | [37] |
| DC | antigen-capturing NPs | / | antigen presentation | female C57BL/6 mice (6–8 weeks old) | B16–F10 and 4T1 cell lines | [38] |
| DC | liposomes-coated gold nanocages modified with DCs specific antibody aCD11c for targeted delivery of adjuvant and melanoma antigen peptide TRP2 | 59 ± 5 nm | target CD11c on DCs | female C57BL/6 mice (6–8 weeks old) | C57BL/6 (C57) mice bone marrow | [39] |
| TAM | ferumoxytol NPs | 30 nm | iron oxides attract macrophages and repolarize M2 to M1 | female FVB/N mice (8–10 weeks old) | MMTV-PyMT mammary cancer cells, MDA-MB-468 human breast carcinoma cells, HT1080 human fibrosarcoma cells, RAW264.7 macrophages, human dermal fibroblasts and human umbilical vein endothelial cells | [52] |
| TAM | nano-Fe3O4 CpG-loaded liposomes with a cancer cell membrane-derived antigenic microparticles | / | iron oxides attract macrophages and repolarize M2 to M1 | female C57BL/6 mice (6 weeks old) | B16F10 or CT26 cells | [53] |
| TAM | albumin NPs modified with dual ligands, a transferrin receptor-binding peptide T12 and mannose | less than 135 nm | target SPARC and mannose receptors on TAM2 | male Balb/c nude mice (4–5 weeks old), male C57BL/6 mice (4–5 weeks old), and male Sprague Dawley rats (220 g) | U87 glioma cells, murine glioma cell line GL261 | [54] |
| TAM | a dual-targeting system composed of MCMC/HA for macrophage targeting and protamine sulfate for ODN complexation | 145 ± 9–210 ± 5 nm | target mannose receptors and CD44 receptors | / | J774A.1 cells and MCF-7 cells | [55] |
| TAM | NK cell-membrane-decorated NPs | 85 ± 1.2 nm | NK cell membranes could induce or enhance pro-inflammatory M1-macrophages polarization | female BALB/c mice (6 weeks old, 18–22 g) | MCF-10A, HepG2, A549, MCF-7, 4T1, and NK-92 cells line | [56] |
| TAM | M2-like TAM dual-targeting NP loaded with anti-colony stimulating factor-1 receptor siRNA | 18 nm | The anti-colony stimulating factor-1 receptor siRNA specifically block M2 survival signals | female C57BL/6 mice (8–12 weeks old) | ldlA7 and ldlA(mSR-B1) cell lines and B16F10 cells | [57] |
| TAM | NPs consisting of amphiphilic egg phosphatidylglycerol, sialic acid and ibrutinib | 30.3 ± 3.1 nm | target Siglec-1 on TAMs | male Kunming mice (6–7 weeks; 18–22 g) and Wistar rats (7–8 weeks; 180–220 g) | RAW264.7 macrophage and S180 murine sarcoma cell lines | [59] |
| TAM | photosensitizer-loaded upconversion nanocrystals nanoconjugate by integrating MnO2 nanosheets and HA biopolymer | 38 ± 3 nm | HA could reprogram the polarization of pro-tumor M2-type TAMs to anti-tumor M1-type | / | RAW264.7 cells and B16F10 cells | [60] |
| CAF | redox-active polymer-coated cerium oxide NPs | 5 nm | inhibit myofibroblast formation | CD-1 mice (8 weeks old) | human dermal fibroblasts and squamous cancer cells (SCL-1) | [73] |
| CAF | Fe3O4 NPs | 65 nm | inhibit myofibroblast formation | / | human dermal fibroblasts and squamous cancer cells (SCL-1) | [74] |
| CAF | a superparamagnetic iron oxide NP modified with relaxin-2 | 60 nm | relaxin-2 inhibits PSC differentiation by inhibiting pSmad2 signaling pathway | male CB17 SCID mice (6 weeks old) | primary human pancreatic stellate cells, Panc-1 cancer cells | [75] |
| CAF | cleavable amphiphilic peptide | 58.5 ± 4.2–75.8 ± 5.2 nm | could be specifically cleaved by fibroblast activation protein-α expressed on fibroblasts | male BALB/c nude mice (6 weeks age, 16–18 g) | PF179T-CAF cells | [76] |
| CAF | navitoclax-loaded nanoliposomes modified with peptide FH | 90.76 ± 2.36 nm | navitoclax specifically induces apoptosis of CAFs | female BALB/c nude mice | human hepatic stellate cell line LX-2, mouse fibroblast cell line NIH/3T3 | [77] |
| CAF | a ferritin-NP modified with a fibroblast-activation protein-specific single chain variable fragment | 12 nm | NP modified with a fibroblast-activation protein-specific single chain variable fragment | female C57BL/6 mice | 4T1 murine breast cancer cell line | [79] |
| tumor vasculature | a polymeric NP system encapsulating erlotinib and doxorubicin | 70–80 nm | codelivery of EGFR inhibitor erlotinib and doxorubicin | syngeneic FVB female mice | R7 murine breast cancer cell line | [93] |
| tumor vasculature | a lipid derivative NP modified with anti-angiogenic agents (LMWH and gemcitabine) and cytotoxic drugs paclitaxel | 152.6 ± 3.1 nm | LMWH inhibits the binding of VEGF to their receptors on endothelial cells and blocks the VEGF signaling pathway | Institute of Cancer Research (ICR) mice | human hepatoma cells and primary human umbilical vein endothelial cells | [95] |
| tumor vasculature | PLGA NPs loaded with VDA-DMXAA and TLR 7/8 agonist-gardiquimod | 194 ± 50 nm | disrupt the tumor vasculature | female C57BL/6 (H-2b) mice (5–6 weeks old) | B16–F10 murine melanoma cell line | [97] |
| tumor vasculature | copper chelator polymer NP loaded with resiquimod | 200 nm | copper can stimulate endothelial cell proliferation and migration | female BALB/c mice (5–6 weeks old) | normal lung epithelial BEAS-2B cells and breast cancer MCF-7, MDA-MB-231 and 4T1 cells | [100] |
| tdLN | carboxylated polystyrene NP loaded with antigens | 0.02–2 μm | lymphatic drainage of antigens | H-2Kb C57BL/6 mice (6–8 weeks old) | OVA-expressing EG7 cells | [106] |
| tdLN | immunologically inert NP with a synthetic long peptide antigen within hydrogel of cholesteryl pullulan | 60 nm | lymphatic drainage of antigens | female BALB/c mice (6–12 weeks old) | CT26 cells | [107] |
| tdLN | NP loaded with TAA and TLR9 ligand CpG DNA | 30 nm | lymphatic drainage of antigens | E.G7-OVA or B16–F10 tumor-bearing mice | E.G7-OVA (CRL-2113), EL-4 (TIB-39), and B16–F10 (CRL-6475) cells | [110] |
| tdLN | PLGA NPs encapsulating TLR 7/8 agonists | 210 ± 12 nm | migrate to draining lymph node | female C57BL/6 mice (6–8 weeks old) and female Balb/c mice (7–8 weeks old) | ovalbumin expressing murine melanoma cell line B16F10-OVA, Murine bladder cancer cell line MB49 | [111] |
| tdLN | biomimetic “artificial necroptotic cancer cell” system consisting of cancer membrane proteins, HSP70 peptide and the phosphate calcium core | 31 nm | lymphatic drainage of TAA | C57BL/6 mice (8–10 weeks old) | BMDCs from C57BL/6 mice | [114] |
| tdLN | a melittin-lipid NP | 10–20 nm | lymphatic drainage of the whole-tumor antigens released from tumor cells which are destroyed by melittin | female C57BL/6 mice (6–8 weeks old) | B16F10 cell line and E0771 cell line | [115] |
| hypoxia | albumin-MnO2 NPs | 50 nm | H2O2 released oxygen | nude mice bearing subcutaneous 4T1 tumors | 4T1 murine breast cancer cell line | [125] |
| hypoxia | MnO2 particles loaded with chlorine e6 and doxorubicin | 3.94 nm | H2O2 released oxygen | female Balb/c mice (6–8 weeks old) | 4T1 murine breast cancer cell line | [126] |
| hypoxia | hypoxia-responsive mesoporous silica nanostructure | 90 nm | transform in hypoxic conditions | B16.F1-bearing mouse allograft model | ovalbumin-expressing melanoma cell line, B16.Mo5 | [127] |
| hypoxia | a nanoscale red-blood-cell system by encapsulating PFC within PLGA | 20 nm | PFC can load large amounts of oxygen and release oxygen in situ | female nude mice | 4T1 murine breast cancer cell line and CT26 murine colon cancer cell line | [130] |
| hypoxia | PFC-loaded hollow Bi2Se3 NP | 35 nm | PFC can load large amounts of oxygen and release oxygen in situ | Balb/c mice | murine breast cancer 4T1 cells and human epithelial carcinoma HeLa cells | [131] |
| hypoxia | polyethylene glycol stabilized PFC nano-droplets with TaOx NPs | 150 nm | PFC gradually released oxygen | Balb/c mice | murine breast cancer 4T1 cells | [133] |
| hypoxia | gold-manganese oxide NPs | / | the levels of HIF-1α decreased | male Swiss albino mice (6–8 weeks old; 20 ± 2 g) | / | [141] |
| hypoxia | an amino-functionalized nanogel with DMMA | 122 nm | pH-responsive charge conversion | Balb/c-nu mice | MDA-MB-435s cells | [148] |
| hypoxia | Mn2+-doped upconversion NPs | 20 nm | pH-responsive charge conversion | female Balb/c mice | 4T1 murine breast cancer cells and HeLa human epithelial carcinoma cells | [151] |
| hypoxia | N-(2-hydroxypropyl) methacrylamide polymer-based nanovehicle | 55 nm | pH-responsive size reduction | HeLa tumor-bearing mice | HeLa cells | [153] |
AbbreviationsDC, dendritic cells; TAM, tumor-associated macrophages; CAF, cancer associated fibroblast; tdLN, tumor-draining lymph nodes; PLGA, poly(lactic-co-glycolic acid); TLR, Toll-like receptor; ODN, oligodeoxynucleotides; BBB,blood-brain barrier; MCMC, mannosylated carboxymethyl chitosan; HA, hyaluronan; HSA, human serum albumin; PTX, paclitaxel; VDA, vasculature disrupting agent; SPARC, secreted protein acidic and rich in cysteine; DMXXA, 5,6-dimethylxanthenone-4-acetic acid; TAA, tumor-associated antigens; HIF, hypoxia-inducible factor, NP, nanoparticle; DMMA, 2,3-dimethylmaleic anhydrid; LMWH, low molecular weight heparin; PFC, perfluorocarbon; siRNA, small interfering RNA.
Nanoparticles specific to CD40, CD11c, mannose receptor, Fc receptor or CD205 on DCs have been reported and all resulted in enhanced immune responses compared with non-specific delivery [[31], [32], [33]]. To determine which cell surface marker is the optimal target, Cruz et al. designed pegylated poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with ovalbumin and Toll-like receptor (TLR) 3 and 7 ligands [34]. Decorated with specific antibodies these particles can target different cell surface molecules on DCs, respectively, including CD40, DEC-205, and CD11c. Among them, nanoparticle targeted to CD40 achieved the highest binding and uptake capacity and the maximum production of interleukin-12 (IL-12) in vitro. However, no significant difference was observed in T cell proliferation in the in vivo assays. It seems that the targeted molecules only exerted a slight influence, whereas the presence of TLR ligands had a significant effect on T cell responses.
Several studies focused on the potency of nanoparticles encapsulating DC-activating molecules to activate and maturate DCs. Liu et al. used a lipid-coated calcium phosphate nanoparticle to deliver specific tumor antigen BRAFV600E peptide to DCs via antigen presentation. These lipid bilayers can efficiently penetrate physical barriers and prevent the degradation and aggregation of cargo-drugs. The results showed that nanoparticles significantly increased antigen-specific T cell responses and interferon-γ (IFN-γ) production. After treatment 20% of mice achieved tumor-free survival [35]. In another study Guo et al. designed an erythrocyte membrane-enveloped nanoplatform modified with antigenic peptide (hgp10025-33), TLR4 agonist, and mannose [36]. The erythrocyte membrane might be a desirable option for its convenient isolation and biocompatibility. Remarkably, the erythrocyte membrane also acts as an adjuvant and forms a “depot effect” at the administration site, thereby prolonging the exposure time of antigens. With mannose binding to its receptor on DCs, this nanoparticle efficiently activates DCs and significantly inhibits tumor growth. Apart from extra addition of adjuvants in the nanocomplex, studies have discovered that some nanoparticles themselves exhibit immunomodulatory activity. A recent study has reported that synthesized ultra-small Fe3O4 nanoparticles used as nano-immunopotentiators in combination with ovalbumin could promote the maturation of DCs and the following immune responses [37]. Notably, it was found for the first time that this Fe3O4 nanoparticle not only served as a delivery system but also participated in immunotherapy. This inherent immunomodulatory property of Fe3O4 might be attractive in cancer immunotherapy. However, the immune responses initiated by one antigen are limited. A broader spectrum of tumor antigens is required. Min et al. utilized antigen-capturing nanoparticles (AC-NPs) which could capture tumor-derived protein antigens (TDPA) by a variety of mechanisms, including ionic interactions, covalent interactions and noncovalent hydrophobic-hydrophobic interactions. These TDPA-bound AC-NPs efficiently targeted DCs and then induced more robust CD8+ cell responses, achieving 20% cure rate in melanoma models compared with 0% in controls [38]. Different from traditional immunotherapy of delivering one or several specific antigens, AC-NPs capture a variety of tumor antigens, therefore magnifying therapeutic effects and reducing the effect of tumor heterogeneity on cancer immunotherapy.
In addition to their influence on DC function, nanoparticles have more value. As a natural tracer, nanoparticles could reflect their location and migration in real-time, indicating the association between the more robust anti-tumor responses and the accumulation of nanoparticles. Gold nanocages (AuNCs) with unique optical properties have been applied in photoacoustic bioimaging and luminescence imaging. Liang et al. encapsulated AuNCs and CD11c, a common biomarker on DCs, within liposomes. These nanoparticles can be directly delivered to DCs and document this process in real-time by in vivo noninvasive fluorescence and photoacoustic bioimaging. The results have shown a significant accumulation in lymph nodes within 1 h and a maximum value at 12 h [39]. They then evaluated the levels of CD86, one of the differentiation proteins expressed on mature DCs, and CD107a expressed on activated cytotoxic CD8+ T cells. After injection of AuNCs, elevated levels of DC maturation and T cell activation were observed. This study revealed the value of AuNCs in immunotherapy and in vivo tracking.
Given the crucial role of DCs as the most powerful APC in immunity, great efforts have been devoted to the development of DC-targeted therapy. The cancer vaccines which deliver antigens to enhance anti-tumor immunity have attracted increasing attention in recent years. The major type of cancer vaccines is a cell-based vaccine which is time-consuming, expensive, and bears potential risks due to autoimmunity. The development of nanomedicine seems to provide a better option. With their tailored properties, nanoparticles can specifically deliver antigens or adjuvants to DCs, preventing their degradation and promoting the efficient activation of DCs. However, the therapeutic efficacy of nanomedicine largely depends on proper carriers and antigens. Additionally, the variation of DC subsets (e.g., plasmacytoid or myeloid) has not been fully elucidated. So more extensive studies are needed to illuminate the most appropriate design of nanoparticles and the functions of different DC subsets before they can be translated to clinical practice.
3. Targeting tumor-associated macrophages
Macrophages are differentiated cells of the mononuclear phagocyte system and play an important role in homeostasis, wound healing, tissue regeneration, and immunity. Their main function is phagocytosis such that they could engulf and digest foreign substances, cell debris, and other substances that could not be recognized as normal cells [[40], [41], [42]]. After exposure to different signals, macrophages undergo polarized activation. In response to IFN-γ and lipopolysaccharide (LPS), macrophages transform to classically activated (M1) macrophages and secrete high levels of IL-12, inhibiting tumor development. Alternative (M2) activated macrophages following exposure to IL-4 or IL-13 produce IL-10, promoting tissue repair, wound healing, and tumor growth [[43], [44], [45], [46]]. Given these two activated states, macrophages are regarded as “a double-edged sword” with both pro-tumor and anti-tumor ability [47,48]. Tumor-associated macrophages (TAMs), a major component in TME, activate anti-tumor immunity at the tumor initiation stage, however, once the tumor is established, they contribute to tumor angiogenesis and immune suppression as well as tumor invasion and metastasis [49]. This discrepancy might be due to the plasticity nature of macrophages that, in response to the changes in microenvironment during tumor development (e.g., hypoxic state), the TAMs undergo a transition of polarized states from M1 to M2 (Fig. 2) [50,51]. Reprogramming the polarization of TAMs could therefore influence the functions of TAMs and has attracted great attention. Nanoparticles which can specifically deliver drugs to TAMs and modulate their polarized states might be an effective method in cancer immunotherapy (Table 1).
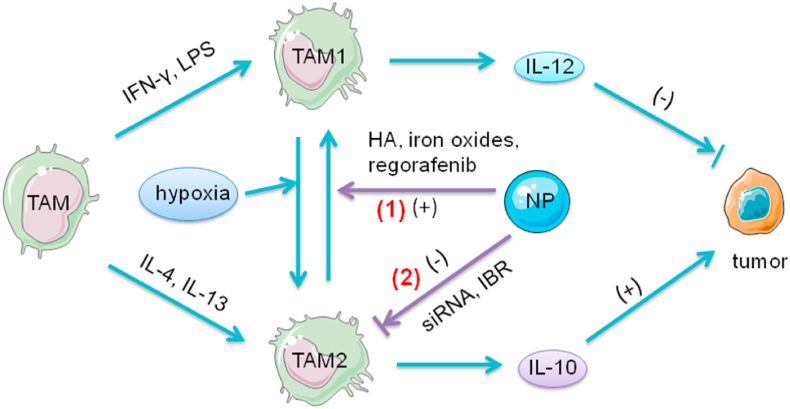
Fig. 2.
The role of TAMs and the function of NPs in the tumor immunity. In response to IFN-γ and LPS, TAMs transform to TAM1 phenotype and secrete high levels of IL-12, inhibiting tumor development. After exposure to IL-4 or IL-13 TAMs undergo the transition to TAM2 and produce IL-10, promoting tumor growth. In the hypoxic state in TME, TAM1 can repolarize to TAM2, which contributes to the immunosuppressive environment in TME. (1) NPs modified with HA, iron oxides or regorafenib can reprogram TAMs activities from TAM2 to TAM1 polarization. HA modulates the activation states of TAM by binding to CD44 on TAMs and activation of TLR4 pathways. Iron oxides attract macrophages and promote macrophage recruitment. After exposure to NPs, TAMs upregulate M1-related CD86 and TNF-α markers, and reduce the levels of M2-related IL-10 and CD206 markers. Iron oxides repolarize M2 to M1 and induce the Fenton reaction which can generate ROS and promote the apoptosis of tumor cells. The apoptotic tumor cells induce M1 polarization, which forms a feedback loop. The expression of TIE2, the receptor of ANG2, has been detected on TAM2. Regorafenib, an oral multi-kinase inhibitor, reduces TAM accumulation by ANG2/TIE2 blockade and modulates TAM polarization. (2) In addition, NPs can directly inhibit the survival and function of TAM2 by delivering siRNA or IBR. The anti-colony stimulating factor-1 receptor siRNA could specifically block M2 survival signals. IBR, an irreversible BTK inhibitor, can diminish the contribution of TAMs to tumorigenesis, thus reversing the immunosuppression established by TAMs.; Abbreviations: ANG2, angiopoietin-2; HA, hyaluronic acid; IFN-γ, interferon-γ; IBR, ibrutinib; IL, interleukin; LPS, lipopolysaccharide; NPs, nanoparticles; TAMs, tumor-associated macrophages; TIE2, tyrosine kinase with immunoglobulin and epidermal growth factor homology domain 2; TLR, toll-like receptor; TME, tumor microenvironment.
Several studies have investigated the applications of nanoparticles in modulating TAM polarization. Iron oxide nanoparticles have been approved for treating iron deficiency by the FDA. Zanganeh et al. discovered their off-labeled uses that they can induce a phenotypic transition from M2 towards M1 and inhibit tumor growth [52]. Iron oxides repolarize M2 to M1 and induce the Fenton reaction which can generate ROS and promote the apoptosis of tumor cells. The apoptotic tumor cells induce M1 polarization, which forms a feedback loop producing a large amount of nitric oxide (NO) and tumor necrosis factor alpha (TNF-α). After injection of ferumoxytol nanoparticles, an 11-fold increase in hydrogen peroxide and a 16-fold increase in hydroxyl radical production were observed, and the levels of M1-related biomarkers TNF-α and CD86 significantly increased, whereas the levels of M2-related CD206 and IL-10 decreased. It suggested that ferumoxytol had a cytotoxic effect which depends on M1 polarization. The anti-tumor effect was also confirmed in animal studies. This study demonstrated that iron oxide could inhibit tumor growth and metastasis in a TAM-dependent manner. In another study nano-Fe3O4 CpG-loaded liposomes (Lipo) with a cancer cell membrane-derived antigenic microparticles (MPs) which share surface markers with tumor cells have been developed [53]. This Fe3O4/MPs-CpG/Lipo nanoplatform presents an immunomodulator, tumor antigens, and an immunostimulatory adjuvant in one vehicle and achieves maximal anti-tumor immunity. The MPs with a variety of tumor antigens promote APC maturation and activate more T cells. Consistent with previous studies, Fe3O4 can reprogram M2 TAMs to the M1 phenotype based on the Fenton reaction, thus this nanostructure has partially changed the immuno-suppressive TME to immuno-supportive and transformed a “cold” tumor into a “hot” tumor. The Fe3O4/MPs-CpG/Lipo-treated group exhibited a 2.55-fold and a 3.84-fold increase in CD4+ and CD8+ T cell proliferation in the spleen compared with the control group, respectively. The median survival time of C57BL/6 mice has shown a 4-fold increase after treatment. The preliminary success in mammary and lung cancer stimulated the application of nanoparticles in other tumors, however, their functions have been restricted in brain tumors because of the blood-brain barrier (BBB). To overcome this difficulty, Zhao et al. developed a “three-bird-one-stone” delivery system which simultaneously targeted the transferrin receptors (TfRs) and the albumin-binding receptor (e.g., secreted protein acidic and rich in cysteine (SPARC)) expressed on the BBB, the SPARC, and TfRs on the glioma cells, and the SPARC and mannose receptors on macrophages [54]. This nanoparticle can pass through the BBB via TfRs and SPARC, then it targets M2 by binding to the SPARC and mannose receptors. This complex contains disulfiram/copper (DSF/Cu) complex and regorafenib, an oral multi-kinase inhibitor. After penetration through BBB and targeted delivery to M2, regorafenib modulates TAM polarization and DSF/Cu as a chemotherapy agent kills cancer cells and releases tumor-associated antigens, in this way magnifying the effect of immunotherapy. The in vivo studies have shown prolonged survival time compared with control groups. After treatment the transition from M2 to M1, as well as Treg suppression and enhanced cytotoxic T lymphocyte (CTL) activation, was observed, suggesting its underlying mechanisms. This dual-targeting delivery system has shown preliminary success. In another study, He et al. constructed dual-targeting nanoparticles modified with mannosylated carboxymethyl chitosan and hyaluronan and the results have shown better delivery efficacy and higher immune responses [55]. To further enhance their delivery efficacy and reduce the toxicity of nanoparticles, Deng et al. prepared nature killer (NK) membrane cloaked photosensitizer-loaded nanoparticles. The NK membrane enables this nanoparticle to induce or enhance M1 polarization and has unique properties such as prolonged retention time and low toxicity [56].
Apart from modulation of TAM polarization, another strategy is to inhibit the survival and function of TAMs. Qian et al. designed a novel M2-like TAM dual-targeting nanoparticle (M2NP) containing a scavenger receptor B type 1 and M2pep (an M2 macrophage binding peptide) [57]. Loaded with anti-colony stimulating factor-1 receptor small interfering RNA (siRNA) on the M2NP, this nanocomplex has shown high affinity for TAMs than for other macrophages and specifically blocks M2 survival signals. After administration of the M2NP, 52% of M2 macrophages have been eliminated, accompanied by decreased tumor sizes and increased survival rates. Moreover, increased levels of immunostimulatory cytokines (IFN-γ and IL-12) and reduced immunosuppressive cytokines (TGF-β and IL-10) have been observed. The results have suggested the potential value of molecular-targeted cancer immunotherapy. In addition, nanoparticles can deliver small-molecule drugs to inhibit TAM function. Bruton's tyrosine kinase (BTK) is overexpressed on TAMs and promotes tumor progression, angiogenesis, and immunosuppression [58]. Ibrutinib (IBR), an irreversible BTK inhibitor, can diminish the contribution of TAMs to tumorigenesis, thus reversing the immunosuppression established by TAMs. Therefore, IBR has shown great potentials in cancer immunotherapy. However, as a small molecule, IBR is rapidly excreted by the kidneys, which restricts its efficacy. An efficient drug delivery system is required. Qiu et al. developed a nanoplatform consisting of amphiphilic egg phosphatidylglycerol (EPG), sialic acid (SA) and IBR (SA/IBR/EPG) [59]. With its amphiphilic EPG structure, this nanoparticle has high IBR binding capacity and prolonged circulation time. The SA has a high affinity for Siglec-1 which is overexpressed on TAMs. After injection, the SA/IBR/EPG nanoparticle was preferentially delivered IBR to TAMs through ligand-receptor binding and regulated anti-tumor immunity. The results have shown an increase in immunosupportive cytokines and a decline in tumor volume.
Nanoparticles can be combined with other therapies to magnify therapeutic effects and reduce toxicity. The studies of Zhao et al. mentioned above used nanoparticles to specifically deliver a cytotoxic drug to macrophages and the results have shown a successful combination of immunotherapy with chemotherapy [54]. In another study, nanoparticles have shown their value in photodynamic therapy (PDT). The anti-tumor effect of PDT which relies on photosensitizer (PS) agents and tissue oxygen to produce reactive oxygen species (ROS) has been greatly restricted by the hypoxic microenvironment. Ai et al. constructed a nanoplatform modified with PS, integrating manganese dioxide (MnO2) nanosheets and hyaluronic acid (HA) [60]. MnO2 could react with the overproduced H2O2 in TME to produce large amounts of oxygen, thus enhancing therapeutic effect of PDT. Moreover, thanks to the HA this nanoparticle achieves TAM-targeted delivery and reprograms TAMs from an M2 to an M1 phenotype, inhibiting tumor relapse after PDT therapy. These studies suggested that with delicate design nanoparticles, when used as a carrier, could combine immunotherapy with other therapeutic methods to achieve better outcomes.
Nanomedicine overcomes the obstacles of traditional TAM-targeted therapies (e.g., nucleic acids and peptides), including short circulation time, non-specific delivery and poor drug solubility [61,62]. Nonetheless, the origin and nature of TAMs remain unclear. Different macrophages, such as M1 or M2 phenotypes, locally proliferating or systemically recruited TAMs, coexist in TME. The complex origin of TAMs restricts the therapeutic efficacy, especially when using a strategy aimed at inhibition of TAM survival. Moreover, despite some surface markers on TAMs having been employed for the development of nanoparticles, they are also present on other cells. For example, mannose receptor is also expressed on DCs [59,63]. However, with deeper understanding of TAMs, nanomedicine will bring new insights to cancer immunotherapy.
4. Targeting cancer-associated fibroblasts
Fibroblasts regulate the structure and function of normal tissue by synthesizing ECM and accelerate tissue repair [64]. Fibroblasts remain constantly activated in cancer tissue where “wounds do not heal” and these “activated” fibroblasts have been termed CAFs. They are the main components of tumor stroma and play a crucial role in tumor progression and invasion with overexpressed levels of VEGF, alpha-smooth muscle actin (α-SMA) and other pro-angiogenic factors that could promote tumor angiogenesis [[65], [66], [67], [68], [69], [70]]. The increased expression of these molecules was observed in almost all solid tumors but not in normal tissue. CAFs produce the main components of ECM, including type I, type III, and type V collagen and fibronectin. As the efficacy of traditional anti-tumor drugs, such as chemotherapeutic or immunomodulatory agents, has been restricted by the ECM barriers which limit the diffusion of drugs [71], CAF-targeted nanoparticles used in remodeling TME have attracted great attention (Table 1).
Extensive efforts have been devoted to the investigation of CAFs as a potential therapeutic target. The first strategy is to regulate the formation of CAFs. Myofibroblasts, modulated fibroblasts that are characterized by their function of collagen contraction, could produce pro-invasive molecules, promoting tumor invasion. Their formation relies on TGF-β1 which initiates the ROS-dependent expression of α-SMA and could be inhibited by antioxidants [72]. Alili et al. developed cerium oxide nanoparticles (nanoceria) to injure tumor cells directly as well as inhibit myofibroblast formation [73]. With Ce4+, nanoceria exerts pro-oxidant effects. It can increase intracellular ROS and attack tumor cells. Remarkably, the concentrations of nanoceria are non-toxic for normal stromal cells, whereas they are toxic for tumor cells. Nanoceria has shown unique properties such that, at non-toxic concentration, it prevents the TGF-β1-initiated and ROS-triggered formation of myofibroblasts, thereby achieving CAF-targeted delivery. Reduced levels of α-SMA expression were observed after injection of nanoceria, evincing the inhibition of fibroblast-to-myofibroblast transition. Then they evaluated the effect of nanoceria on tumor invasive ability and the nanoceria-treated group showed a 70% decrease in invasive capacity of tumor cells. The results suggested nanoceria had dual functions in cancer immunotherapy. Furthermore, they wondered whether the Fe3O4 nanoparticle, which has been reported to have cytotoxic effect on mammalian cells, could be another therapeutic agent [74], so they evaluated its influence on tumor and normal squamous cells. According to in vitro studies, although Fe3O4 nanoparticles could decrease myofibroblast formation by reducing the expression of α-SMA and generating ROS, they had a pro-invasive effect on squamous cells and may not be an appropriate approach to tumor therapy. Pancreatic stellate cells (PSCs) are the precursors of CAFs in pancreatic ductal adenocarcinoma. Mardhian et al. designed a superparamagnetic iron oxide nanoparticle modified with relaxin-2 [75]. Relaxin-2 inhibits TGF-β-induced PSC differentiation by inhibiting pSmad2 signaling pathway. With relaxin-2, this nanoparticle achieves targeted delivery and significant anti-tumor effects in pancreatic tumor. Another strategy is using cytotoxic drugs to destroy CAFs. Ji et al. developed nanoparticles with a cleavable amphiphilic peptide (CAP) which could be specifically cleaved by fibroblast activation protein (FAP) expressed on fibroblasts. In aqueous solutions CAP monomers easily self-assembled due to their amphiphilic properties, whereas in TME cleaved by FAP on CAFs they quickly dissembled and released chemotherapeutic drugs, specifically killing CAFs. This nanostructure with rapid enzymatic reaction could enhance penetration distance and provide an efficient drug delivery system [76]. In another study a CAF-targeted nanoliposome encapsulating navitoclax was developed [77]. It further linked with peptide FH, which binds to tenascin C, a protein mainly expressed on CAFs. Navitoclax specifically induces apoptosis of CAFs. After treatment, the percentages of CAFs were 18% compared to 77% in controls. However, as certain subsets of CAFs at certain stages of tumor progression are anti-tumorigenic [68], complete eradication of CAFs may not be an ideal option.
Furthermore, several studies combine CAF-targeted therapy with PDT. FAP which is overexpressed on CAFs has been thought to be a promising target. Therefore, anti-FAP antibody and its humanized version sibrotuzumab were investigated in clinical setting. However, after preliminary studies, researchers found that the expression of FAP in normal tissue could not be neglected [78]. The treatment of sibrotuzumab has severe side effects. A more specific CAF-targeted delivery approach is required. Zhen et al. developed a ferritin-nanoparticle modified with a fibroblast-activation protein-specific single-chain variable fragment (scFv) and a PS [79]. With the scFv and PS this nanoparticle can selectively home to CAFs and eradicate CAFs by photo-irradiation. This nanoparticle-based photo-immunotherapy navigates photodynamic injury to CAFs and enhances T cell infiltration. Further studies demonstrated that this CAF-eradication therapy promoted penetration of nanoparticles and other drugs [80]. Compared with conventional PDT which often uses unspecific PS, nanomedicine with unique properties can not only achieve simultaneous targeted delivery of two or more therapeutic drugs, but also magnify the effects of immunotherapy and PDT.
Despite the evidence of CAFs acting as a barrier hindering anti-tumor therapy, recent studies have revealed that, under certain circumstances, certain subsets of CAFs have anti-tumor effects. Similar to TAMs, recent studies have suggested quiescent fibroblasts might differentiate into cancer-restraining (F1 subtype) and cancer-promoting (F2 subtype) CAFs [81], which depends on the stage of tumorigenesis. Notably, it has been found that the same pathway could have opposite functions during tumor development, therefore precise modulation instead of complete depletion of CAFs might be a more appropriate strategy.
5. Modulating tumor vasculature
Tumor vasculature has an abnormal appearance and impaired functions [82]. The hypoxia in TME induces continued production of pro-angiogenic molecules, such as VEGF and TGF-β [[83], [84], [85], [86]]. The imbalance between the pro-angiogenic and anti-angiogenic factors results in rapid but aberrant tumor vessel formation. These vessels are tortuous, unevenly distributed, and have increased permeability due to the detachment of pericytes which normally surround the endothelial cells [87,88]. The increased permeability results in the leakiness of proteins from vessels and thus increases interstitial fluid pressure (IFP) in TME [89]. The elevated IFP compresses blood vessels and exacerbates the hypoxic state thus creating a vicious cycle. Therefore aberrant vasculature contributes greatly to the abnormality of TME. In addition, the dysfunctional vessels restrict the infiltration of immune cells as well as the efficient delivery of nanoparticles and therapeutic drugs [90].
The abnormal vasculature in TME obstructs the efficacy of tumor therapy, so vascular normalization strategies might be a promising solution. However, the therapeutic effects of anti-angiogenic therapy have been obstructed by acquired resistance of the endothelium towards anti-angiogenic drugs, for example, anti-VEGF therapy. After treatment tumor cells increase the expression of other pro-angiogenic cytokines, compensating for the inhibitory effects. Based on the hypothesis of Rakesh K. Jain, anti-angiogenic therapy only transiently reverses abnormal vessels, but does not simply destroy them. This transient vasculature normalization was termed the “normalization window” [91,92], which differs between various tumor types and tumor states. As the main purpose of these vascular normalization strategies is to promote other anti-tumor therapies, the “normalization window” is vital for anti-tumor therapies. However, it is difficult in clinical practice to seize the therapeutic window with precision. Nanoparticles, as an effective drug delivery platform, can simultaneously deliver anti-angiogenic drugs and other anti-tumor drugs to achieve optimal anti-tumor therapeutic effects, obviating the need to identify the therapeutic window. Zhou et al. developed a polymeric nanoparticle system encapsulating epidermal growth factor receptor (EGFR) inhibitor erlotinib and doxorubicin. This nanoplatform targets tumor tissue via the EPR effect and achieves a slow but sustained release of doxorubicin with the assistance of erlotinib [93]. Treatment of anti-angiogenic drugs significantly magnifies the effects of nanomedicine and chemotherapy [93,94]. Nanoparticles have significant advantages over traditional delivery systems with improved drug stability, reduced non-specific toxicity and high drug loading capacity. Du et al. developed a lipid derivative nanoparticle modified with anti-angiogenic agents (low molecular weight heparin (LMWH) and gemcitabine (Gem)) and cytotoxic drugs paclitaxel [95]. LMWH inhibits the binding of VEGF to its receptors on endothelial cells and blocks the VEGF signaling pathway. Gem can promote the normalization of tumor vessels in a metronomic manner, i.e., administration of a low dose at a high frequency. After injection this nanoplatform degraded in acidic TME and efficiently released Gem and paclitaxel. Increased flow perfusion could be observed. The fluorescence microscopic evaluation of tumor sections showed that in nanoparticle-treated group the abnormal tumor blood vessels have become regular during the normalization window. These vessels then degraded and the density of blood vessels significantly decreased. A recent study implied that, compared with anti-angiogenesis alone, normalizing tumor vasculature and ECM simultaneously further enhanced the efficacy of nanomedicine [96].
Apart from VEGF inhibitor, vasculature disrupting agents (VDAs) are another class of drugs, which can disrupt existing tumor blood vessels, leading to necrosis in the center of solid tumors because of reduced blood supply. However, tumor cells in the periphery of solid tumors where the influence of VDAs is limited could survive and migrate. This drawback would be overcome when VDAs were combined with immunotherapy which can modulate the TME in the periphery of tumor. Seth et al. designed PLGA nanoparticles loaded with VDA 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and TLR7/8 agonist-gardiquimod [97]. DMXAA can induce apoptosis of endothelial cells by TNF-α mediated pathways [98]. Although both DMXAA and gardiquimod are non-toxic to tumor cells, they have synergistic effects on tumor suppression with gardiquimod activating immune responses through MyD88 and nuclear factor κB (NF-κB) pathways and DMXAA releasing necrotic particles, which as tumor antigens, magnify immune reactions. The in vivo studies in a melanoma model have shown vasculature disruption and increased APC, achieving 63.6% of the survival rate compared with monotherapy of PLGA (18.1%) and DMXAA (9%). The results revealed the value of this combinational therapy in treating solid tumors. In addition, previous studies have demonstrated that copper contributes to angiogenesis by promoting migration and invasion of endothelial cells [99]. Zhou et al. used a copper chelator polymer to construct nanoparticles loaded with resiquimod, a TLR7 and TLR8 agonist [100]. This system combined anti-angiogenesis with immunotherapy and had a synergistic anti-tumor effect in breast cancer mice with smaller tumor size and slower tumor growth.
However, several issues must be addressed in anti-angiogenic therapy. On the one hand, the development of anti-angiogenic drugs requires a deep understanding of the mechanisms of tumor angiogenesis. Therefore, more studies investigating the detailed mechanisms in different tumor types at different periods during tumor progression are needed. On the other hand, to achieve uniform distribution of nanoparticles in solid tumors remains an obstacle in nanomedicine. Notably, nanomedicine itself benefits from anti-angiogenesis therapy, so the combination of nanoparticles, anti-angiogenic drugs, and cytotoxic drugs might be an ideal option.
6. Targeting tumor-draining lymph nodes
TdLNs are the first site of metastasis and abound with immunosuppressive factors, including Tregs and MDSCs [[101], [102], [103]]. TdLNs are immuno-privileged where APCs and T cells are tolerized, contributed to the immune escape of tumor [104]. Although they are immunosuppressive, they experience antigen priming during lymphatic drainage of tumor-associated antigens (TAAs). Infiltrated with both tumor immune suppressor and effector cells, tdLNs have a crucial role in generating and regulating tumor-related immune responses (Fig. 3) [105]. Apart from conventional surgical removal of tdLNs (sentinel lymph nodes), modulation of their functions in tumor progression has aroused increasing attention. As for nanomedicine, nanoparticles have been developed for specific delivery to tdLNs and promote anti-tumor immune responses (Table 1).
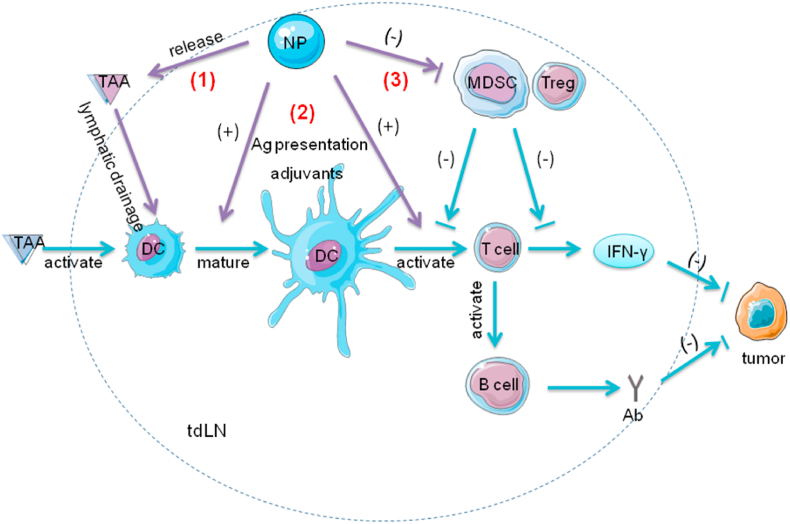
Fig. 3.
Graphic illustration of tdLNs and the function of NPs in the tumor immunity. TdLNs abound with both immunosupportive factors (e.g., DCs, T cells and B cells) and immunosuppressive factors (e.g., Tregs, MDSCs). TAAs are delivered to tdLNs via lymphatic drainage. DCs recognize these antigens and present them to T cells. The T cells produce IFN-γ and activate B cells to generate antibodies. These factors collectively contribute to anti-tumor immunity. MDSCs and Tregs inhibit the activation and function of T cells. (1) NPs encapsulating TAAs efficiently target tdLNs via lymphatic drainage. NPs with a medium size (10–100 nm) achieve maximum efficacy. (2) These TAAs then activate DCs and significantly promote the following T cell responses by antigen presentation or adjuvants. The addition of TLR ligands in NPs significantly promotes immune responses. (3) In addition, NPs with CpG, the TLR9 ligand, can reduce the number of MDSCs and Tregs. After exposure of CpG, DCs which express TLR9 produce proinflammatory cytokines and promote differentiation of MDSCs, blocking the inhibition of MDSCs on T cell proliferation.; Abbreviations: Ab, antibodies; IFN-γ, interferon-γ; tdLNs, tumor-draining lymph nodes; TAAs, tumor-associated antigens; DCs, dendritic cells; MDSCs, myeloid-derived suppressor cells; TLR, Toll-like receptor; Tregs, regulatory T cells; NPs, nanoparticles.
Particle size is closely associated with the lymphatic drainage of nanoparticles and therapeutic efficacy of nanomedicine. Large particles could be trapped by ECM, prohibiting their further penetration. Small particles, however, rapidly diffusing out of lymph nodes, have limited effect on immune responses. Fifis et al. compared the effect of different nanoparticles with sizes ranging from 0.02 to 2 μm on T cell activation. Among them, solid core nano-beads conjugated with antigens with a diameter of 40–50 nm have achieved 2 to 10-fold stronger responses than other particles, producing high levels of IFN-γ and antibodies [106]. Muraoka et al. used an immunologically inert nanoparticle with a synthetic long peptide antigen within hydrogel of cholesteryl pullulan, with a diameter of 60 nm. It could be delivered to tdLNs via lymphatic flow and engulfed by medullary macrophages without detection of other immune cells. These macrophages then efficiently activated CD8+ T cells in the presence of TLRs. With high specificity, this immunologically stealth nanoparticle provides a new strategy for development of a tumor vaccine [107]. Similarly, the study of Kang et al. showed that the optimal size of gold nanoparticles was 10–22 nm to induce maximum immune responses [108]. Based on these studies, we conclude that nanoparticles of medium size (10–100 nm) might be desirable. Small particles (<10 nm) are subject to kidney clearance, whereas large particles (>100 nm) stimulate the reticuloendothelial system, thus rapidly cleared by the immune system [109].
The applications of nanoparticles as anticancer vaccines in tumor treatment have been extensively studied. These nanoparticles modified with TAAs can target tdLNs by lymphatic drainage. TdLNs are immunosuppressive but primed of TAAs. Jeanbart et al. wondered whether vaccines targeting tdLNs would be more effective than targeting naive non-tumor-draining lymph nodes [110]. They compared these two approaches in lymphoma and melanoma models. The results showed that, although tdLNs are immunosuppressive, targeting tdLN vaccine could induce a stronger immune reaction in response to TAAs in both models. More CD8+ T cells and a lower tumor volume were observed in groups with TAAs delivered to tdLNs. These nanoparticles could promote CD8+ T cell responses both locally and systemically and modulate the immunosuppressive state in tdLNs by reducing the MDSCs and Tregs. Therefore the antigen priming effect in tdLNs outweighs the immunosuppression of tdLNs, suggesting the value of tdLNs as a target in cancer immunotherapy. In addition, Kim et al. discovered that PLGA nanoparticle loaded with TLR7/8 agonists can efficiently migrate to tdLNs and trigger DC expansion and activation. After treatment the total number of DCs and CD8+ T cells increased 3-fold and 4.5-fold, respectively. This robust and specific immune activation results in significant prophylactic and therapeutic efficacy. The nanoparticle inhibits primary and secondary tumor growth in melanoma and renal cell carcinoma models. Remarkably, this nanoparticle vaccine could reduce systemic metastasis by inducing protective immunity [111]. Decorated with TAAs, the cancer vaccine strategies achieve targeted delivery to tdLNs and have shown promising results. However, the available TAAs, the key components of vaccine, are limited for most cancers [112,113]. A specific TAA only induces limited immune responses and adjuvants are needed to realize desirable outcomes, so a broad spectrum of TAAs is required. Kang et al. designed a biomimetic “artificial necroptotic cancer cell” system consisting of cancer membrane proteins, HSP70 peptide, and a phosphate calcium core [114]. The cancer membrane proteins present a wide range of TAAs, reducing immune escape of tumor cells. Simultaneous delivery of TAAs and adjuvants to lymph nodes promotes DC maturation and antigen presentation, activates NK cells, and induces stronger cell immunity. This nanoplatform has shown prolonged retention time in tdLNs, enabling gradual and sustained release of TAAs. After administration to mice B16OVA melanoma models, the number of metastasis nodules was reduced by 77%. Significantly, when combined with checkpoint inhibitors anti-PD-1 it was declined by 89%, exhibited significant tumor regression. Therefore this biomimetic nano-vaccine has unique advantages and has shown a synergistic effect in combination with checkpoint inhibitors. In a recent study vaccines based on whole-cell tumor antigens have been proposed [115]. Yu et al. developed a melittin-lipid nanoparticle (melittin-NP) without extra TAAs. Melittin can destroy cell membrane and release the whole-tumor antigens. With an optimal size of 20 nm, the melittin-NP was easily drained to tdLNs, where it can exert its maximum immunomodulatory effect. The results showed a 3.6-fold increase in CD8+ T cell responses, along with an inhibition rate of 95% and 92% in primary and distant tumor growth, respectively. The whole-tumor antigens provide more antigenic epitopes to generate a wide range of anti-tumor immunity.
7. Modulating hypoxic condition
The rapid growth of tumor cells and the distorted blood vessels result in insufficient oxygen supply. The insufficient oxygenation in TME leads to a hypoxic state which triggers a cascade of events that contribute to tumor angiogenesis, tumor growth, and metastasis. Hypoxia induces immunosuppression in TME by upregulating expression of CCL22 and CCL28 and accumulating MDSCs and Tregs [[116], [117], [118]]. Hypoxia also facilitates the conversion of macrophages to pro-tumorigenic phenotype M2 [119]. In addition, hypoxia promotes the secretion of immunosuppressive factors, such as VEGF and TGF-β, and the expression of immune checkpoint molecules such as PDL1 on tumor cells as well as T cell immunoglobulin domain and mucin domain-3 (TIM-3) and CTLA4 on MDSCs, TAMs, and Tregs [[120], [121], [122]]. Furthermore, the elevated levels of anaerobic metabolites, such as adenosine and lactate, impair the functions of CTL by affecting the production of IFN-γ (Fig. 4) [123]. Apart from immunosuppression, hypoxia is also responsible for therapeutic resistance, especially to photodynamic therapy and radiotherapy, in which the oxygen molecule is essential for tumor cell eradication [124].
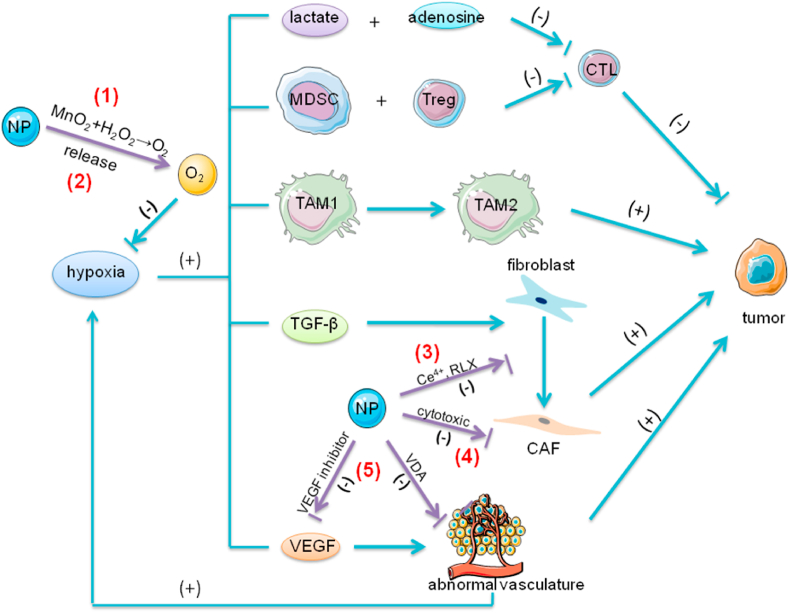
Fig. 4.
The effect of hypoxia and the role of NPs in tumor immunity. The hypoxic state in TME contributes to the immunosuppression in TME in multiple ways. First, the elevated levels of anaerobic metabolites, such as adenosine and lactate, impair the functions of CTL by affecting the production of IFN-γ. Second, hypoxia promotes the accumulation of immunosuppressive cells, such as MDSCs and Tregs, and also facilitates the conversion of macrophages from anti-tumorigenic phenotype TAM1 to pro-tumorigenic TAM2. Finally, hypoxia promotes the secretion of immunosuppressive factors, such as TGF-β and VEGF. TGF-β is a crucial factor in the transition of fibroblasts to CAF. VEGF promotes tumor angiogenesis. The excessive production of VEGF leads to the imbalance between the pro-angiogenic and anti-angiogenic factors, which results in rapid but aberrant tumor vessel formation, further exacerbating the hypoxic state in TME. (1) NPs modified with MnO2 react with H2O2 in TME and produce sufficient oxygen. (2) Another strategy is that NPs loaded with oxygen directly release oxygen in TME. PFC with extremely high oxygen solubility has the ability to load large amounts of oxygen. NPs encapsulating PFC exhibit high oxygen binding capacity and release oxygen in situ. (3) In addition, NPs with Ce4+ or RLX can inhibit the formation of CAF. Ce4+ prevents the TGF-β1-initiated and ROS-triggered formation of myofibroblasts which produce pro-invasive molecules promoting tumor invasion. RLX inhibits the differentiation of pancreatic stellate cells, the precursors of CAFs, by inhibiting pSmad2 signaling pathway. (4) NPs also can deliver cytotoxic drugs (such as chemotherapeutic drugs or navitoclax) to directly attack CAFs. Navitoclax, an inhibitor of Bcl-2, induces apoptosis of CAFs. (5) NPs regulate abnormal tumor vasculature by delivering VEGF inhibitor or VDA. The VEGF inhibitor can suppress the binding of VEGF to their receptors on endothelial cells and block the VEGF signaling pathway, inhibiting tumor angiogenesis. VDAs disrupt existing tumor blood vessels by the induction of endothelial cells apoptosis mediated by TNFα.; Abbreviations: Bcl-2, B-cell lymphoma 2; CAFs, cancer-associated fibroblasts; TAMs, tumor-associated macrophages; CTL, cytotoxic T lymphocytes; VEGF, vascular endothelial growth factor; MDSCs, myeloid-derived suppressor cells; TGF-β1, transforming growth factor β1; TME, tumor microenvironment; TNF-α, tumor necrosis factor alpha; Tregs, regulatory T cells; NPs, nanoparticles; VDA, vasculature disrupting agent; RLX, relaxin-2.
Many studies have applied nanoparticles to relieve the hypoxic state in combination with other therapies, including chemotherapy, photodynamic therapy, and radiotherapy. Chen et al. designed pH/H2O2 dual-responsive nanoparticles using albumin-coated MnO2. When penetrating the tumor, MnO2 reacted with H2O2 and H+ to produce oxygen. By relieving the hypoxic state, they enhanced the therapeutic effects of chemotherapy and photodynamic therapy [125]. Yang et al. fabricated hollow MnO2 particles loaded with a PS chlorine e6 and a chemotherapy drug doxorubicin [126]. Reacting with H2O2, this nanoshell degraded in an acidic environment and released oxygen and drugs. This nanoplatform can serve as an effective PDT agent even in the hypoxic state and modulate the immunosuppressive TME by increasing CTL infiltration and TNF-α excretion. This combinational therapy has shown to be most effective compared with monotherapy in both in vivo and in vitro studies. Similarly, Im et al. developed a hypoxia-responsive mesoporous silica nanostructure that could transform under hypoxic conditions releasing photodynamic agents, thereby promoting its penetration and magnifying the efficacy of photodynamic therapy [127]. In addition, nanoparticles can also enhance the efficacy of radiotherapy which is restricted by limited energy accumulation within tumor sites and hypoxia-associated resistance [128,129]. Apart from in-situ oxygen generation, several studies used nanoparticles to deliver oxygen to TME to relieve hypoxia [130,131]. Gao et al. designed a nanoscale red-blood-cell (RBC) system by encapsulating perfluorocarbon (PFC) within PLGA, which is further coated with an RBC membrane [130]. PFC, with its extremely high oxygen solubility, can be loaded with large amounts of oxygen. This nanoparticle exhibits a high oxygen binding capacity and prolonged retention time. Due to its much smaller size, it could easily leak from blood vessels and penetrate solid tumors where it releases oxygen and reduces hypoxia in TME. This RBC-mimic system has utilized the inherent ability of PFC, however, the release of oxygen by diffusion alone may not be enough [132]. Song et al. developed a PFC-loaded hollow Bi2Se3 nanoparticle. Bi2Se3, an effective photothermal agent, generates heat upon exposure to near-infrared laser irradiation and the heat then increases the release of oxygen. This nanoplatform realized efficient oxygen supply when exposed to near-infrared irradiation [131]. In another study they developed polyethylene glycol stabilized PFC nano-droplets with TaOx nanoparticles [133]. PFC could gradually release oxygen destroying the hypoxic environment, whereas TaOx nanoparticles, as a radiotherapy sensitizer could absorb X-rays and concentrate energy within the tumors to attack tumor cells. The tumor oxygenation increased 3-fold after injection of nanoparticles and smaller hypoxic areas were found. Using this nanoparticle radiotherapy with lower radiation energy can achieve desirable therapeutic effects with lower toxicity. Nanoparticles are also applied in combination with PDT to enhance therapeutic effects, for example, nanoscale metal-organic frameworks (nMOFs). The porous and ordered structure of nMOFs with high-Z elements averts self-quenching of PS and allows a higher probability for the secondary photons to interact with other metal clusters, significantly enhancing the therapeutic effect of PDT [[134], [135], [136], [137]]. Nanoparticles used for TME modulation are non-toxic by themselves, so their application in modulating TME to enhance the therapeutic effects of other existing cancer therapies might be a better option.
The change of the hypoxic state also affects other components in TME. Hypoxia-inducible factor (HIF) which is secreted by TAMs is a key molecule in signaling pathways and plays a critical role in maintaining the hypoxic state and stimulating pro-tumorigenic events [138]. HIF is a heterodimer with an O2-regulated α subunit and a constitutively expressed β subunit. In normoxic conditions, the HIF-1α subunit is widely expressed and degraded by O2 and iron-dependent enzymes prolyl hydroxylases (PHDs) through the proteasomal pathway. However in hypoxic conditions, as the iron compound bound to PHDs is chelated and oxidized by ROS and NO, PHDs become inactive and thus HIF-1α accumulates in TME [139,140]. After injection of a gold-manganese oxide nanoparticle, the level of HIF-1α was shown to be significantly decreased, indicating its potential in relieving hypoxia. The decline in the levels of TNF-α and IL-10 along with the increase in IL-12 suggested the improved TME might repolarize macrophages to M1 [141]. This implied that nanoparticles might be able to transform the immunosuppressive TME to an immunosupportive state by relieving hypoxia.
The hypoxic environment promotes glycolysis which produces a significant amount of lactate and results in an acidic microenvironment (pH 6.5–6.8). This small pH drop is enough to change the properties of the functional groups, among which the most liable groups including tertiary amine-, histidine- and sulfonamide-containing groups. In addition, the changes in pH might destroy the pH-sensitive linkages and change their structures [142]. These nanoparticles are inactivated in blood circulation but once triggered by the acidic environment, they promptly convert to an activated state and release drugs. These pH-responsive drug delivery systems have been widely investigated and have shown preliminary success in enhancing therapeutic effects and reducing drug resistance [[143], [144], [145], [146]]. Notably, in a recent study an ultra-pH-sensitive nanoparticle (UPS-NP) has been developed. The UPS-NP responds to a minor pH change (<0.3 pH units) and exhibits an “all-or-nothing” protonation cooperativity during micelle assembly or disassembly. This unique property enables instant and pH-triggered drug release in acidic sites, such as TME [147]. Therefore nanoparticles with delicate design respond to the pH change in TME and achieve specific tumor therapy and tumor detection.
It has been found that positively charged nanoparticles are more easily internalized due to the negatively charged cell membrane, whereas they often experience more rapid clearance owing to stronger interactions with serum. Du et al. designed an amino-functionalized nanogel with negative-to-positive charge conversion property [148]. This nanogel remains stable under neutral and alkali conditions, but at acidic pH the amide bond is immediately cleaved and the nanogel exposes positively charged amino groups. This charge conversion promotes the internalization of nanoparticles and drug delivery. The following studies also indicated this charge conversion system enhanced the cellular uptake of nanoparticles in tumor cells [149,150]. Similarly, Wang et al. designed upconversion nanoparticles with charge conversion in response to change in pH. At pH 6.8 they obtained a positive surface charge, which could promote cellular uptake of the nanoparticles and enhance the PDT efficacy [151]. In addition, pH-responsive size-switchable or dissociable nanoparticles were developed [152,153]. The diameter of nanoparticles was found to be closely associated with their blood circulation time and the depth of penetration in tumor tissue. Small nanocargos could efficiently diffuse into tumor, whereas they tend to be rapidly excreted by the kidneys, so the size-switchable nanoparticles might be an ideal option. Li et al. developed N-(2-hydroxypropyl) methacrylamide (HPMA) polymer-based nanovehicles which realized stepwise size reduction in response to the change in pH to deliver chemotherapeutic drugs to the nucleus [153]. Apart from organic nanoparticles, the inorganic nanostructures also seem promising. Recent studies have shown that calcium carbonate (CaCO3) nanoparticles are sensitive to slight pH change and are capable of drug loading [[154], [155], [156]]. In addition, amorphous iron nanoparticles can induce the Fenton reaction with H2O2 at weakly acidic pH and produce hydroxyl groups to attack tumor cells [157,158]. One of the major advantages of nanoparticles is that they can be delicately designed to optimize their size, charge or material to meet different requirements during drug delivery.
8. Conclusions and perspectives
In recent years, the rapid development of nanomedicine has offered new insights to cancer immunotherapy. Nanoparticles have yielded advantages over traditional drug delivery systems. First, the most obvious advantage of nanoparticles is their tunability such that they can be designed to various sizes, shapes and functions. They can be modified with targeting molecules or loaded with various drugs, thus achieving targeted delivery and simultaneous delivery of therapeutic agents. Second, they tend to accumulate in tumor much more than in normal tissue because of the leaky tumor vasculature and damaged lymphatic drainage, i.e., the EPR effect, which significantly increases the accumulation of drugs in tumor and reduces off-target effects. Finally, the release of drugs at a precise place during a precise period is required considering the complexity of TME during tumor development. With unique properties, nanoparticles can protect the degradation or aggregation of drugs and in response to specific stimuli (e.g., pH, hypoxia, or H2O2) they release drugs in a spatio-temporal manner. In this review we summarized how nanoparticles act as an efficient drug delivery system when modified with various ligands that could specifically target components in TME, including DCs, macrophages, fibroblasts, tumor vasculature, tdLNs and the hypoxic state. Moreover, nanoparticles could influence the aberrant structures and functions of TME, thus reducing the development of drug resistance and magnifying the therapeutic effects of chemotherapy, radiotherapy, and photodynamic therapy.
There are, however, several challenges to be faced en route to the translation of nanomedicine to clinical practice. First, the limitations of nanoparticles in cancer immunotherapy are largely attributed to the limited knowledge of immune network during tumorigenesis. The innate and adaptive immunity consists a complex network, so the influence of depletion or inhibition of one component on the whole network remains unclear. Especially for those targeted therapies, the inhibition of one or several components might be compensated by upregulation of other pathways. Second, there are individual variations in the therapeutic effects of nanomedicine. Considering the heterogeneity of the structure in different tumors, the responses of different tumors to the same therapy are quite different, mainly due to the different tumor vasculature. In addition, there are several concerns about the potential risks of nanoparticles. First, the major concern is the potential immunogenicity. Nanoparticles themselves can be antigenic. The immune responses towards nanoparticles would accelerate their clearance, thus limiting their efficacy. Moreover, robust activation of immunity can result in serious complications, such as hemolysis and thrombogenesis [159]. Second, the TME-modulation strategies which partially change the structure of TME and reverse the immunosuppressive microenvironment to an immunosupportive one incur potential risks related to promotion of tumor metastasis, so the long-term effects of TME-modulation strategy should be further investigated. Finally, the current toxicity assays for nanoparticles are immature. Given the fact that the physico-chemical properties of nanoparticles could change upon reaction with other biological substances in body, their final forms should be carefully evaluated. Therefore, great efforts are still needed to optimize the size, shape, ligands, and other properties and to assess the potential risks of nanoparticles before they can be translated to clinical practice. With deeper knowledge of cancer immunology and nanomedicine, nanoparticles will revolutionize cancer immunotherapy in the near future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Key Research and Development program of China (Grant number 2018YFC1106100, 2018YFC1106101), the project was also funded by National Natural Science Foundation of China (Grant number 81930024, 81770974), the Science and Technology Commission of Shanghai (Grant Number 17DZ2260100).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Ping Gu, Email: guping2009@126.com.
Xianqun Fan, Email: fanxq@sjtu.edu.cn.
References
- 1.Wu T., Dai Y. Tumor microenvironment and therapeutic response. Canc. Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Denton A.E., Roberts E.W., Fearon D.T. Stromal cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2018;1060:99–114. doi: 10.1007/978-3-319-78127-3_6. [DOI] [PubMed] [Google Scholar]
- 3.Guo S., Deng C.X. Effect of stromal cells in tumor microenvironment on metastasis initiation. Int. J. Biol. Sci. 2018;14:2083–2093. doi: 10.7150/ijbs.25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirata E., Sahai E. Tumor microenvironment and differential responses to therapy. Cold Spring Harb Perspect. Med. 2017;7:a026781. doi: 10.1101/cshperspect.a026781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musetti S., Huang L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy. ACS Nano. 2018;12:11740–11755. doi: 10.1021/acsnano.8b05893. [DOI] [PubMed] [Google Scholar]
- 6.Rajendrakumar S.K., Uthaman S., Cho C.S., Park I.K. Nanoparticle-based phototriggered cancer immunotherapy and its domino effect in the tumor microenvironment. Biomacromolecules. 2018;19:1869–1887. doi: 10.1021/acs.biomac.8b00460. [DOI] [PubMed] [Google Scholar]
- 7.Casey S.C., Amedei A., Aquilano K., Azmi A.S., Benencia F., Bhakta D., Bilsland A.E., Boosani C.S., Chen S., Ciriolo M.R. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Canc. Biol. 2015;35:S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougan M., Dougan S.K. Targeting immunotherapy to the tumor microenvironment. J. Cell. Biochem. 2017;118:3049–3054. doi: 10.1002/jcb.26005. [DOI] [PubMed] [Google Scholar]
- 9.Uy G.L., Rettig M.P., Motabi I.H., McFarland K., Trinkaus K.M., Hladnik L.M., Kulkarni S., Abboud C.N., Cashen A.F., Stockerl-Goldstein K.E. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L., Smith M.A., Doshi P., Sasser K., Fulp W., Altiok S., Haura E.B. Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer. J. Thorac. Oncol. 2014;9:974. doi: 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podar K., Zimmerhackl A., Fulciniti M., Tonon G., Hainz U., Tai Y.T., Vallet S., Halama N., Jäger D., Olson D.L., Sattler M., Chauhan D., Anderson K.C. The selective adhesion molecule inhibitor Natalizumab decreases multiple myeloma cell growth in the bone marrow microenvironment: therapeutic implications. Br. J. Haematol. 2011;155:438–448. doi: 10.1111/j.1365-2141.2011.08864.x. [DOI] [PubMed] [Google Scholar]
- 12.Liao X., Zhao L., Wu S., Zheng H., Chen H., Zhang H., Wang Z., Lin Q. Microsatellite stability and mismatch repair proficiency in nasopharyngeal carcinoma may not predict programmed death-1 blockade resistance. Oncotarget. 2017;8:113287–113293. doi: 10.18632/oncotarget.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A. KEYNOTE-177: randomized phase III study of pembrolizumab versus investigator-choice chemotherapy for mismatch repair-deficient or microsatellite instability-high metastatic colorectal carcinoma. J. Clin. Oncol. 2017;35:815. [Google Scholar]
- 16.Lebbé C., Weber J.S., Maio M., Neyns B., Harmankaya K., Hamid O., O'Day S.J., Konto C., Cykowski L., McHenry M.B. Survival follow-up and ipilimumab retreatment of patients with advanced melanoma who received ipilimumab in prior phase II studies. Ann. Oncol. 2014;25:2277–2284. doi: 10.1093/annonc/mdu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gettinger S.N., Horn L., Gandhi L., Spigel D.R., Antonia S.J., Rizvi N.A., Powderly J.D., Heist R.S., Carvajal R.D., Jackman D.M. Overall survival and long-term safety of Nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overchuk M., Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–237. doi: 10.1016/j.biomaterials.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Canc. Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 20.Prabhakar U., Maeda H., Jain R.K., Sevick-Muraca E.M., Zamboni W., Farokhzad O.C., Barry S.T., Gabizon A., Grodzinski P., Blakey D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Canc. Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q., Liu G., Liu S., Su H., Wang Y., Li J., Luo C. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol. Sci. 2018;39:59–74. doi: 10.1016/j.tips.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Li M., Zhang F., Su Y., Zhou J., Wang W. Nanoparticles designed to regulate tumor microenvironment for cancer therapy. Life Sci. 2018;201:37–44. doi: 10.1016/j.lfs.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 24.Muntimadugu E., Kommineni N., Khan W. Exploring the potential of nanotherapeutics in targeting tumor microenvironment for cancer therapy. Pharmacol. Res. 2017;126:109–122. doi: 10.1016/j.phrs.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J. Contr. Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Miele E., Spinelli G.P., Miele E., Tomao F., Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantino J., Gomes C., Falcão A., Neves B.M., Cruz M.T. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol. Res. 2017;65:798–810. doi: 10.1007/s12026-017-8931-1. [DOI] [PubMed] [Google Scholar]
- 28.Gardner A., Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doedens A.L., Stockmann C., Rubinstein M.P., Liao D., Zhang N., DeNardo D.G., Coussens L.M., Karin M., Goldrath A.W., Johnson R.S. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Canc. Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colegio O.R., Chu N.Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M., Cline G.W., Phillips A.J., Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi M.D., Unger W.J., Storm G., Kooyk Y., Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J. Contr. Release. 2012;161:25–37. doi: 10.1016/j.jconrel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Dominguez A.L., Lustgarten J. Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine. 2010;28:1383–1390. doi: 10.1016/j.vaccine.2009.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosalia R.A., Cruz L.J., Duikeren S., Tromp A.T., Silva A.L., Jiskoot W., Gruijl T., Löwik C., Oostendorp J., Burg S.H., Ossendorp F. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 34.Cruz L.J., Rosalia R.A., Kleinovink J.W., Rueda F., Löwik C.W., Ossendorp F. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study. J. Contr. Release. 2014;192:209–218. doi: 10.1016/j.jconrel.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 35.Liu Q., Zhu H., Liu Y., Musetti S., Huang L. BRAF peptide vaccine facilitates therapy of murine BRAF-mutant melanoma. Cancer Immunol. Immunother. 2018;67:299–310. doi: 10.1007/s00262-017-2079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y., Wang D., Song Q., Wu T., Zhuang X., Bao Y., Kong M., Qi Y., Tan S., Zhang Z. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 37.Luo L., Iqbal M.Z., Liu C., Xing J., Akakuru O.U., Fang Q., Li Z., Dai Y., Li A., Guan Y., Wu A. Engineered nano-immunopotentiators efficiently promote cancer immunotherapy for inhibiting and preventing lung metastasis of melanoma. Biomaterials. 2019;223:119464. doi: 10.1016/j.biomaterials.2019.119464. [DOI] [PubMed] [Google Scholar]
- 38.Min Y., Roche K.C., Tian S., Eblan M.J., McKinnon K.P., Caster J.M., Chai S., Herring L.E., Zhang L., Zhang T., DeSimone J.M., Tepper J.E., Vincent B.G., Serody J.S., Wang A.Z. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang R., Xie J., Li J., Wang K., Liu L., Gao Y., Hussain M., Shen G., Zhu J., Tao J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. 2017;149:41–50. doi: 10.1016/j.biomaterials.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 40.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNardo D.G., Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 43.Mantovani A., Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Kim J., Bae J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat. Inflamm. 2016;(2016):6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuccarese M.F., Dubach J.M., Pfirschke C., Engblom C., Garris C., Miller M.A., Pittet M.J., Weissleder R. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 2017;8:14293. doi: 10.1038/ncomms14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ostuni R., Kratochvill F., Murray P.J., Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–239. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Palma M., Lewis C.E. Macrophage regulation of tumor responses to anticancer therapies. Canc. Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Sica A., Larghi P., Mancino A., Rubino L., Porta C., Totaro M.G., Rimoldi M., Biswas S.K., Allavena P., Mantovani A. Macrophage polarization in tumour progression. Semin. Canc. Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sica A., Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ngambenjawong C., Gustafson H.H., Pun S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017;114:206–221. doi: 10.1016/j.addr.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., Pajarinen J.S., Nejadnik H., Goodman S., Moseley M., Coussens L.M., Daldrup-Link H.E. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H., Zhao B., Wu L., Xiao H., Ding K., Zheng C., Song Q., Sun L., Wang L., Zhang Z. Amplified cancer immunotherapy of a surface-engineered antigenic microparticle vaccine by synergistically modulating tumor microenvironment. ACS Nano. 2019;13:12553–12566. doi: 10.1021/acsnano.9b03288. [DOI] [PubMed] [Google Scholar]
- 54.Zhao P., Wang Y., Kang X., Wu A., Yin W., Tang Y., Wang J., Zhang M., Duan Y., Huang Y. Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophage-mediated immunotherapy. Chem. Sci. 2018;9:2674–2689. doi: 10.1039/c7sc04853j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He X.Y., Liu B.Y., Wu J.L., Ai S.L., Zhuo R.X., Cheng S.X. A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression. ACS Appl. Mater. Interfaces. 2017;9:42566–42576. doi: 10.1021/acsami.7b13594. [DOI] [PubMed] [Google Scholar]
- 56.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., Ma Y., Gong P., Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 57.Qian Y., Qiao S., Dai Y., Xu G., Dai B., Lu L., Yu X., Luo Q., Zhang Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 58.Weber A.N.R., Bittner Z., Liu X., Dang T.M., Radsak M.P., Brunner C. Bruton's tyrosine kinase: an emerging key player in innate immunity. Front. Immunol. 2017;8:1454. doi: 10.3389/fimmu.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu Q., Li C., Song Y., Shi T., Luo X., Zhang H., Hu L., Yan X., Zheng H., Liu M.Y., Liu M.Q., Liu M., Yang S., Liu X., Chen G., Deng Y. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy. Acta Biomater. 2019;92:184–195. doi: 10.1016/j.actbio.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 60.Ai X., Hu M., Wang Z., Lyu L., Zhang W., Li J., Yang H., Lin J., Xing B. Enhanced cellular ablation by attenuating hypoxia status and reprogramming tumor-associated macrophages via NIR light-responsive upconversion nanocrystals. Bioconjugate Chem. 2018;29:928–938. doi: 10.1021/acs.bioconjchem.8b00068. [DOI] [PubMed] [Google Scholar]
- 61.Pathria P., Louis T.L., Varner J.A. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–327. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song M., Liu T., Shi C., Zhang X., Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10:633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Canc. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 65.Ireland L.V., Mielgo A. Macrophages and fibroblasts, key players in cancer chemoresistance. Front. Cell Dev. Biol. 2018;6:131. doi: 10.3389/fcell.2018.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Canc. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 67.Räsänen K., Vaheri A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 68.Ishii G., Ochiai A., Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 69.Kubo N., Araki K., Kuwano H., Shirabe K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J. Gastroenterol. 2016;22:6841–6850. doi: 10.3748/wjg.v22.i30.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bussard K.M., Mutkus L., Stumpf K., Gomez-Manzano C., Marini F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem. Int. Ed. Engl. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 72.Kunz-Schughart L.A., Knuechel R. Tumor-associated fibroblasts (part II): functional impact on tumor tissue. Histol. Histopathol. 2002;17:623–637. doi: 10.14670/HH-17.623. [DOI] [PubMed] [Google Scholar]
- 73.Alili L., Sack M., Karakoti A.S., Teuber S., Puschmann K., Hirst S.M., Reilly C.M., Zanger K., Stahl W., Das S., Seal S., Brenneisen P. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 74.Alili L., Chapiro S., Marten G.U., Schmidt A.M., Zanger K., Brenneisen P. Effect of Fe3O4 nanoparticles on skin tumor cells and dermal fibroblasts. BioMed Res. Int. 2015;2015:530957. doi: 10.1155/2015/530957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mardhian D.F., Storm G., Bansal R., Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Contr. Release. 2018;290:1–10. doi: 10.1016/j.jconrel.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 76.Ji T., Zhao Y., Ding Y., Wang J., Zhao R., Lang J., Qin H., Liu X., Shi J., Tao N., Qin Z., Nie G., Zhao Y. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem. Int. Ed. Engl. 2016;55:1050–1055. doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen B., Wang Z., Sun J., Song Q., He B., Zhang H., Wang X., Dai W., Zhang Q. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine. 2016;12:131–141. doi: 10.1016/j.nano.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Truffi M., Mazzucchelli S., Bonizzi A., Sorrentino L., Allevi R., Vanna R., Morasso C., Corsi F. Nano-strategies to target breast cancer-associated fibroblasts: rearranging the tumor microenvironment to achieve antitumor efficacy. Int. J. Mol. Sci. 2019;20:1263. doi: 10.3390/ijms20061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhen Z., Tang W., Wang M., Zhou S., Wang H., Wu Z., Hao Z., Li Z., Liu L., Xie J. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 80.Li L., Zhou S., Lv N., Zhen Z., Liu T., Gao S., Xie J., Ma Q. Photosensitizer-encapsulated ferritins mediate photodynamic therapy against cancer-associated fibroblasts and improve tumor accumulation of nanoparticles. Mol. Pharm. 2018;15:3595–3599. doi: 10.1021/acs.molpharmaceut.8b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Canc. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 82.Krishna Priya S., Nagare R.P., Sneha V.S., Sidhanth C., Bindhya S., Manasa P., Ganesan T.S. Tumour angiogenesis-origin of blood vessels. Int. J. Canc. 2016;139:729–735. doi: 10.1002/ijc.30067. [DOI] [PubMed] [Google Scholar]
- 83.Jain R.K. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 84.De Palma M., Biziato D., Petrova T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Canc. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 85.Maity A., Pore N., Lee J., Solomon D., O'Rourke D.M. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3'-kinase and distinct from that induced by hypoxia. Canc. Res. 2000;60:5879–5886. [PubMed] [Google Scholar]
- 86.Hinz B. The extracellular matrix and transforming growth factor-β1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 87.Barlow K.D., Sanders A.M., Soker S., Ergun S., Metheny-Barlow L.J. Pericytes on the tumor vasculature: jekyll or hyde? Canc. Microenviron. 2013;6:1–17. doi: 10.1007/s12307-012-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y., Kim B.Y.S., Chan C.K., Hahn S.M., Weissman I.L., Jiang W. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018;18:195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heldin C.H., Rubin K., Pietras K., Ostman A. High interstitial fluid pressure-an obstacle in cancer therapy. Nat. Rev. Canc. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 90.Azzi S., Hebda J.K., Gavard J. Vascular permeability and drug delivery in cancers. Front. Oncol. 2013;3:211. doi: 10.3389/fonc.2013.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mattheolabakis G., Mikelis C.M. Nanoparticle delivery and tumor vascular normalization: the chicken or the egg? Front. Oncol. 2019;9:1227. doi: 10.3389/fonc.2019.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arvizo R.R., Rana S., Miranda O.R., Bhattacharya R., Rotello V.M., Mukherjee P. Mechanism of anti-angiogenic property of gold nanoparticles: role of nanoparticle size and surface charge. Nanomedicine. 2011;7:580–587. doi: 10.1016/j.nano.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Z., Kennell C., Jafari M., Lee J.Y., Ruiz-Torres S.J., Waltz S.E., Lee J.H. Sequential delivery of erlotinib and doxorubicin for enhanced triple negative Breast cancer treatment using polymeric nanoparticle. Int. J. Pharm. 2017;530:300–307. doi: 10.1016/j.ijpharm.2017.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Q., Xu L., Chen J., Yang Z., Liang C., Yang Y., Liu Z. Tumor vasculature normalization by orally fed erlotinib to modulate the tumor microenvironment for enhanced cancer nanomedicine and immunotherapy. Biomaterials. 2017;148:69–80. doi: 10.1016/j.biomaterials.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 95.Du S., Xiong H., Xu C., Lu Y., Yao J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019;7:1147–1160. doi: 10.1039/c8bm01350k. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y., Liu X., Yuan H., Yang Z., von Roemeling C.A., Qie Y., Zhao H., Wang Y., Jiang W., Kim B. Therapeutic remodeling of the tumor microenvironment enhances nanoparticle delivery. Adv. Sci. 2019;6:1802070. doi: 10.1002/advs.201802070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seth A., Lee H., Cho M.Y., Park C., Korm S., Lee J.Y., Choi I., Lim Y.T., Hong K.S. Combining vasculature disrupting agent and Toll-like receptor 7/8 agonist for cancer therapy. Oncotarget. 2017;8:5371–5381. doi: 10.18632/oncotarget.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smolarczyk R., Czapla J., Jarosz-Biej M., Czerwinski K., Cichoń T. Vascular disrupting agents in cancer therapy. Eur. J. Pharmacol. 2020:173692. doi: 10.1016/j.ejphar.2020.173692. [DOI] [PubMed] [Google Scholar]
- 99.McAuslan B.R., Reilly W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res. 1980;130:147–157. doi: 10.1016/0014-4827(80)90051-8. [DOI] [PubMed] [Google Scholar]
- 100.Zhou P., Qin J., Zhou C., Wan G., Liu Y., Zhang M., Yang X., Zhang N., Wang Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials. 2019;195:86–99. doi: 10.1016/j.biomaterials.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat. Rev. Canc. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hatziioannou A., Alissafi T., Verginis P. Myeloid-derived suppressor cells and T regulatory cells in tumors: unraveling the dark side of the force. J. Leukoc. Biol. 2017;102:407–421. doi: 10.1189/jlb.5VMR1116-493R. [DOI] [PubMed] [Google Scholar]
- 103.Sarvaria A., Madrigal J.A., Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell. Mol. Immunol. 2017;14:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fransen M.F., Schoonderwoerd M., Knopf P., Camps M.G., Hawinkels L.J., Kneilling M., Hall T., Ossendorp F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rotman J., Koster B.D., Jordanova E.S., Heeren A.M., Gruijl T.D. Unlocking the therapeutic potential of primary tumor-draining lymph nodes. Cancer Immunol. Immunother. 2019;68:1681–1688. doi: 10.1007/s00262-019-02330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fifis T., Gamvrellis A., Crimeen-Irwin B., Pietersz G.A., Li J., Mottram P.L., McKenzie I.F., Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 107.Muraoka D., Harada N., Hayashi T., Tahara Y., Momose F., Sawada S., Mukai S.A., Akiyoshi K., Shiku H. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano. 2014;8:9209–9218. doi: 10.1021/nn502975r. [DOI] [PubMed] [Google Scholar]
- 108.Kang S., Ahn S., Lee J., Kim J.Y., Choi M., Gujrati V., Kim H., Kim J., Shin E.C., Jon S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Contr. Release. 2017;256:56–67. doi: 10.1016/j.jconrel.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 109.Guo J., Rahme K., He Y., Li L.L., Holmes J.D., O'Driscoll C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017;12:6131–6152. doi: 10.2147/IJN.S140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jeanbart L., Ballester M., Titta A., Corthésy P., Romero P., Hubbell J.A., Swartz M.A. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Canc. Immunol. Res. 2014;2:436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 111.Kim H., Niu L., Larson P., Kucaba T.A., Murphy K.A., James B.R., Ferguson D.M., Griffith T.S., Panyam J. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53. doi: 10.1016/j.biomaterials.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 112.Wang H., Mooney D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018;17:761–772. doi: 10.1038/s41563-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 113.Lewis J.D., Reilly B.D., Bright R.K. Tumor-associated antigens: from discovery to immunity. Int. Rev. Immunol. 2003;22:81–112. doi: 10.1080/08830180305221. [DOI] [PubMed] [Google Scholar]
- 114.Kang T., Huang Y., Zhu Q., Cheng H., Pei Y., Feng J., Xu M., Jiang G., Song Q., Jiang T., Chen H., Gao X., Chen J. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials. 2018;164:80–97. doi: 10.1016/j.biomaterials.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 115.Yu X., Dai Y., Zhao Y., Qi S., Liu L., Lu L., Luo Q., Zhang Z. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 2020;11:1110. doi: 10.1038/s41467-020-14906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taylor C.T., Colgan S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Facciabene A., Peng X., Hagemann I.S., Balint K., Barchetti A., Wang L.P., Gimotty P.A., Gilks C.B., Lal P., Zhang L., Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 118.Doedens A.L., Stockmann C., Rubinstein M.P., Liao D., Zhang N., DeNardo D.G., Coussens L.M., Karin M., Goldrath A.W., Johnson R.S. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Canc. Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corzo C.A., Condamine T., Lu L., Cotter M.J., Youn J.I., Cheng P., Cho H.I., Celis E., Quiceno D.G., Padhya T., McCaffrey T.V., McCaffrey J.C., Gabrilovich D.I. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barsoum I.B., Smallwood C.A., Siemens D.R., Graham C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Canc. Res. 2014;74:665–674. doi: 10.1158/0008-5472.CAN-13-0992. [DOI] [PubMed] [Google Scholar]
- 121.Zhou Y., Miao J., Wu H., Tang H., Kuang J., Zhou X., Peng Y., Hu D., Shi D., Deng W., Cao X., Zhao C., Xie C. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget. 2017;8:51210–51223. doi: 10.18632/oncotarget.17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ruf M., Moch H., Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int. J. Canc. 2016;139:396–403. doi: 10.1002/ijc.30077. [DOI] [PubMed] [Google Scholar]
- 123.Carmona-Fontaine C., Deforet M., Akkari L., Thompson C.B., Joyce J.A., Xavier J.B. Metabolic origins of spatial organization in the tumor microenvironment. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2934–2939. doi: 10.1073/pnas.1700600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Silva V.L., Al-Jamal W.T. Exploiting the cancer niche: tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J. Contr. Release. 2017;253:82–96. doi: 10.1016/j.jconrel.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 125.Chen Q., Feng L., Liu J., Zhu W., Dong Z., Wu Y., Liu Z. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy [published correction appears in Adv Mater. 2018 Feb;30(8):] Adv. Mater. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- 126.Yang G., Xu L., Chao Y., Xu J., Sun X., Wu Y., Peng R., Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Im S., Lee J., Park D., Park A., Kim Y.M., Kim W.J. Hypoxia-triggered transforming immunomodulator for cancer immunotherapy via photodynamically enhanced antigen presentation of dendritic cell. ACS Nano. 2019;13:476–488. doi: 10.1021/acsnano.8b07045. [DOI] [PubMed] [Google Scholar]
- 128.Kobayashi K., Usami N., Porcel E., Lacombe S., Le Sech C. Enhancement of radiation effect by heavy elements. Mutat. Res. 2010;704:123–131. doi: 10.1016/j.mrrev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 129.Yoshimura M., Itasaka S., Harada H., Hiraoka M. Microenvironment and radiation therapy. BioMed Res. Int. 2013;2013:685308. doi: 10.1155/2013/685308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gao M., Liang C., Song X., Chen Q., Jin Q., Wang C., Liu Z. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv. Mater. 2017;29:35. doi: 10.1002/adma.201701429. [DOI] [PubMed] [Google Scholar]
- 131.Song G., Liang C., Yi X., Zhao Q., Cheng L., Yang K., Liu Z. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv. Mater. 2016;28:2716–2723. doi: 10.1002/adma.201504617. [DOI] [PubMed] [Google Scholar]
- 132.Jägers J., Wrobeln A., Ferenz K.B. Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflügers Archiv. 2020;3:1–12. doi: 10.1007/s00424-020-02482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song G., Ji C., Liang C., Song X., Yi X., Dong Z., Yang K., Liu Z. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials. 2017;112:257–263. doi: 10.1016/j.biomaterials.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 134.Lan G., Ni K., Veroneau S.S., Song Y., Lin W. Nanoscale metal-organic layers for radiotherapy-radiodynamic therapy. J. Am. Chem. Soc. 2018;140:16971–16975. doi: 10.1021/jacs.8b11593. [DOI] [PubMed] [Google Scholar]
- 135.Ni K., Lan G., Veroneau S.S., Duan X., Song Y., Lin W. Nanoscale metal-organic frameworks for mitochondria-targeted radiotherapy-radiodynamic therapy. Nat. Commun. 2018;9:4321. doi: 10.1038/s41467-018-06655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ni K., Lan G., Chan C., Quigley B., Lu K., Aung T., Guo N., La Riviere P., Weichselbaum R.R., Lin W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018;9:2351. doi: 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu K., He C., Guo N., Chan C., Ni K., Lan G., Tang H., Pelizzari C., Fu Y.X., Spiotto M.T., Weichselbaum R.R., Lin W. Low-dose X-ray radiotherapy-radiodynamic therapy via nanoscale metal-organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2018;2:600–610. doi: 10.1038/s41551-018-0203-4. [DOI] [PubMed] [Google Scholar]
- 138.Schito L., Semenza G.L. Hypoxia-inducible factors: master regulators of cancer progression. Trends Canc. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 139.Wu G., Luo J., Rana J.S., Laham R., Sellke F.W., Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc. Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 140.Airley R.E., Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 141.Nath A., Pal R., Singh L.M., Saikia H., Rahaman H., Ghosh S.K., Mazumder R., Sengupta M. Gold-manganese oxide nanocomposite suppresses hypoxia and augments pro-inflammatory cytokines in tumor associated macrophages. Int. Immunopharm. 2018;57:157–164. doi: 10.1016/j.intimp.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 142.Du J., Lane L.A., Nie S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Contr. Release. 2015;219:205–214. doi: 10.1016/j.jconrel.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Du J.Z., Mao C.Q., Yuan Y.Y., Yang X.Z., Wang J. Tumor extracellular acidity-activated nanoparticles as drug delivery systems for enhanced cancer therapy. Biotechnol. Adv. 2014;32:789–803. doi: 10.1016/j.biotechadv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 144.Ganta S., Devalapally H., Shahiwala A., Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Contr. Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 145.Zheng H., Xing L., Cao Y., Che S. Coordination bonding based pH-responsive drug delivery systems. Coord. Chem. Rev. 2013;257:1933–1944. [Google Scholar]
- 146.Li Y., Xiao K., Zhu W., Deng W., Lam K.S. Stimuli-responsive cross-linked micelles for on-demand drug delivery against cancers. Adv. Drug Deliv. Rev. 2014;66:58–73. doi: 10.1016/j.addr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li S., Bennett Z.T., Sumer B.D., Gao J. Nano-immune-engineering approaches to advance cancer immunotherapy: lessons from ultra-pH-sensitive nanoparticles. Acc. Chem. Res. 2020;53:2546–2557. doi: 10.1021/acs.accounts.0c00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Du J.Z., Sun T.M., Song W.J., Wu J., Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew Chem. Int. Ed. Engl. 2010;49:3621–3626. doi: 10.1002/anie.200907210. [DOI] [PubMed] [Google Scholar]
- 149.Yuan Y.Y., Mao C.Q., Du X.J., Du J.Z., Wang F., Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012;24:5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 150.Feng L., Li K., Shi X., Gao M., Liu J., Liu Z. Smart pH-responsive nanocarriers based on nano-graphene oxide for combined chemo-and photothermal therapy overcoming drug resistance. Adv. Healthc. Mater. 2014;3:1261–1271. doi: 10.1002/adhm.201300549. [DOI] [PubMed] [Google Scholar]
- 151.Wang C., Cheng L., Liu Y., Wang X., Ma X., Deng Z., Li Y., Liu Z. Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light. Adv. Funct. Mater. 2013;23:3077–3086. [Google Scholar]
- 152.Li H.J., Du J.Z., Du X.J., Xu C.F., Sun C.Y., Wang H.X., Cao Z.T., Yang X.Z., Zhu Y.H., Nie S., Wang J. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4164–4169. doi: 10.1073/pnas.1522080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li L., Sun W., Zhong J., Yang Q., Zhu X., Zhou Z., Zhang Z., Huang Y. Multistage nanovehicle delivery system based on stepwise size reduction and charge reversal for programmed nuclear targeting of systemically administered anticancer drugs. Adv. Funct. Mater. 2015;25:4101–4113. [Google Scholar]
- 154.Dong Z.L., Feng L.Z., Zhu W., Sun X., Gao M., Zhao H., Chao Y., Liu Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials. 2016;110:60–70. doi: 10.1016/j.biomaterials.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 155.Ueno Y., Futagawa H., Takagi Y., Ueno A., Mizushima Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Contr. Release. 2005;103:93–98. doi: 10.1016/j.jconrel.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 156.Zhao Y., Luo Z., Li M., Qu Q., Ma X., Yu S.H., Zhao Y. A preloaded amorphous calcium carbonate/doxorubicin@ silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem. Int. Ed. 2015;54:919–922. doi: 10.1002/anie.201408510. [DOI] [PubMed] [Google Scholar]
- 157.Li W.P., Su C.H., Chang Y.C., Lin Y.J., Yeh C.S. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a fenton reaction activable polymersome. ACS Nano. 2016;10:2017–2027. doi: 10.1021/acsnano.5b06175. [DOI] [PubMed] [Google Scholar]
- 158.Zhang C., Bu W., Ni D., Zhang S., Li Q., Yao Z., Zhang J., Yao H., Wang Z., Shi J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew. Chem. Int. Ed. 2016;55:2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 159.Li Y., Ayala-Orozco C., Rauta P.R., Krishnan S. The application of nanotechnology in enhancing immunotherapy for cancer treatment: current effects and perspective. Nanoscale. 2019;11:17157–17178. doi: 10.1039/c9nr05371a. [DOI] [PMC free article] [PubMed] [Google Scholar]