Abstract
Primary ovarian insufficiency (POI) is an ovarian dysfunction that affects more than 1 % of women and is characterized by hormone imbalances that afflict women before the age of 40. The typical perimenopausal symptoms result from abnormal levels of sex hormones, especially estrogen. The most prevalent treatment is hormone replacement therapy (HRT), which can relieve symptoms and improve quality of life. However, HRT cannot restore ovarian functions, including secretion, ovulation, and fertility. Recently, as part of a developing field of regenerative medicine, stem cell therapy has been proposed for the treatment of POI. Thus, we recapitulate the literature focusing on the use of stem cells and biomaterials for POI treatment, and sum up the underlying mechanisms of action. A thorough understanding of the work already done can aid in the development of guidelines for future translational applications and clinical trials that aim to cure POI by using regenerative medicine and biomedical engineering strategies.
Keywords: Stem cells, Primary ovarian insufficiency, Cell therapy, Biomaterials
Graphical abstract
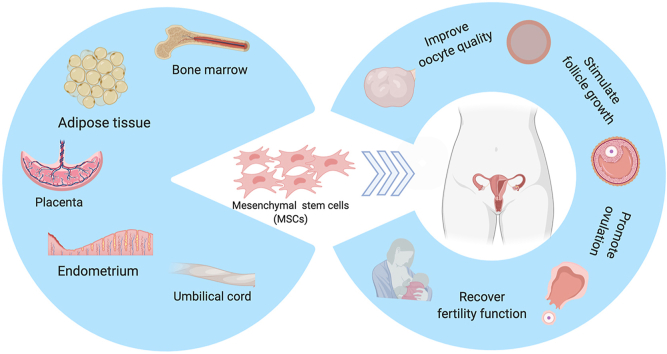
Graphical abstract: Multiple sources of MSCs and their roles in improving ovarian functions. MSCs isolated from various kinds of tissues and organs, such as bone marrow, adipose tissue, placenta, endometrium, and umbilical cord have been used as treatments for POI to stimulate follicle growth and improve oocyte quality, ovulation, and fertility.
Highlights
-
•
This paper illustrates the in-vivo, in-vitro, and cell-free treatments for POI using stem cells and biomaterials.
-
•
We provide basic theories and suggestions for future research and clinical therapy translation.
-
•
This review can help researcher to develop guidelines on stem cells treating POI.
1. Introduction
Primary ovarian insufficiency (POI) or premature ovarian failure (POF), is a clinical syndrome that leads to the loss of ovarian activity before the age of 40 [1]. It is characterized by amenorrhea lasting more than four months, which results from increased follicle-stimulating hormone (FSH) and decreased estradiol (E2) levels, a condition defined as hypergonadotropic hypogonadism (HH) [[2], [3], [4]]. The prevalence of POI is 1% according to Coluam et al. [5], who first described the syndrome in 1986. However, the most recent research conducted by Golezar et al. [6] reported a higher POI prevalence of 3.7% after an analysis of 31 epidemiological studies between 1987 and 2017. In addition, it was reported that the prevalence of early menopause was 12.2%, which means that more than 10% of women will experience menopause between the ages of 40 and 45, as opposed to the mean age of naturally occurring menopause, which is 51.4 [7].
Most POI symptoms result from estrogen deficiency, including hot flashes, sweating, sleep disturbance, vaginal dryness and irritation, loss of skin elasticity, decreased size of the uterus and breasts, infertility, and compromised mental health [4,8]. The management of POI involves a healthy lifestyle, quitting smoking, hormone replacement therapy (HRT) [9], and the induction of puberty [[10], [11], [12], [13], [14]]. However, these therapies are palliative [12,14,15], and while they can improve women's quality of life, they cannot restore ovarian functions such as secretion, follicle growth, or ovulation. Moreover, POI-derived infertility cannot be improved with assisted reproductive technologies.
Nowadays, the use of biomaterials, coupled with stem cell-based regenerative medicine, holds great promise for the restoration of injured, and non-functional tissues or organs. Mesenchymal stem cells (MSCs), a cell type commonly used in regenerative medicine, have been reported as an effective treatment for POI [[16], [17], [18], [19], [20]]. The efficacy of MSCs in restoring ovarian function has been confirmed in a number of molecular biology studies, which explored the signaling pathways, genetic modulations, and paracrine messaging proteins that drive the cell's mechanisms of action. MSCs have also been used to treat cellular and animal models of POI and changes in protein expression patterns, the estrus cycle, sexual hormone levels, and animal fertility have been elucidated. In addition, acellular therapies, such as MSC-derived secretomes, cytokines, and exosomes, have been studied for their ability to circumventing the potential immunogenic and carcinogenic risks of using cells. Researchers have integrated these regenerative therapies with biocompatible materials to create ovarian patches that provide efficient mechanical support and sustained cellular delivery to help restore ovarian function and improve follicle development [21]. This comprehensive review of current advances in biomaterials and regenerative medicine for POI treatment will help to provide support and guidance for future scientific research and clinical applications.
2. Characterization of MSCs
MSCs are multipotent stem cells that were firstly reported by Friedenstein [22] as fibroblast-like cells expanded from bone marrow via attachment to plastic culture flasks. Then, MSCs were defined by Caplan [23] as adherent, fibroblast-like cells with the potentials to differentiate into osteoblasts, adipocytes, and chondrocytes in vitro. In 2006, the International Society for Cellular Therapy (ISCT) officially defined MSCs as multipotent mesenchymal stromal cells, which are isolated from stromal tissues and have the properities of plastic-adherent, self-renew and multiple-lineage differentiateion [24].
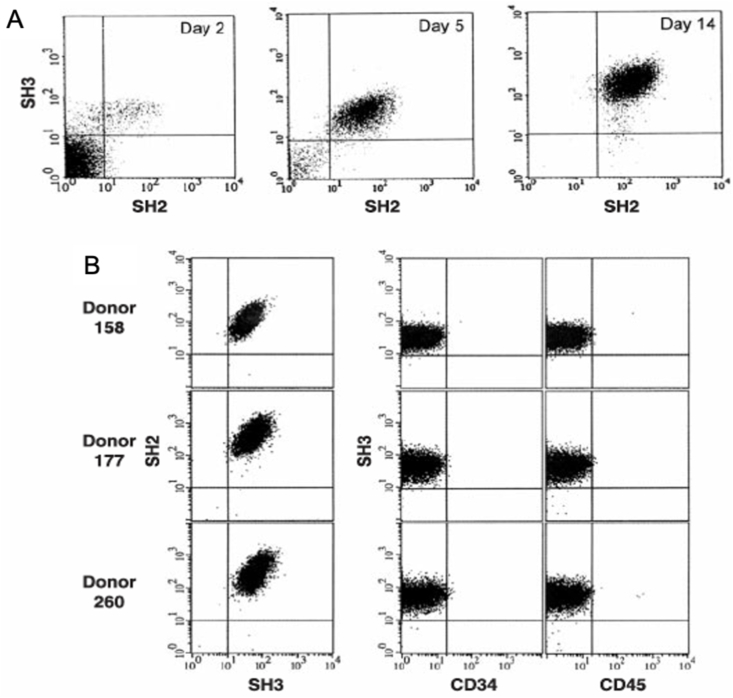
MSCs can be acquired from various organs and tissues of the body, such as the lungs, adipose tissue, umbilical cord blood, umbilical cord (Wharton's jelly), placenta and the endometrium. These cells display a stable phenotype and create a monolayer in vitro [25]. MSCs can be induced to differentiate exclusively into the adipocyte, chondrocyte, or osteocyte lineages. MSCs commonly express a number of surface receptors, including SH2, SH3, CD29, CD44, CD49a-f, CD51, CD71, CD73, CD90, CD105, CD106, CD120a, CD124, and CD166, while not express the hematopoietic lineage markers CD11b, CD14, CD34, and the leukocyte common antigen CD45 (Fig. 1) [23,[26], [27], [28], [29]]. In addition, Nestin-expressing MSCs have been shown to co-localize and support hematopoietic stem cells (HSCs). These nestin+ MSCs contain all the bone-marrow colony-forming-unit fibroblastic activity and can be propagated as non-adherent ‘mesenspheres’ that can self-renew and expand in serial transplantations. Nestin+ MSC-HSC pairings are part of a unique niche in the bone-marrow, which is tightly regulated by homeostatic neuronal and hormonal mechanisms that guiding HSC maintenance, MSCs proliferation and differentiation [30].
Fig. 1.
Characterization of isolated bone marrow stromal cells. (A) Flow cytometry shows the enrichment of these cells as they were cultured at days 2, 5, and 14 with antibodies of SH2 and SH3, which were raised against surface markers. At 14 days, the cells were 95–99% homogeneous and were negative for reactivity to antigens CD14, CD34 and CD45, which are makers of the hematopoietic lineages. (B) Homogeneity and reproducibility of the isolation procedure were demonstrated by flow cytometry [29].
In 2008, Katarina Le Blanc et al. [31] reported the therapeutic potential of MSCs for the treatment of acute steroid-refractory graft-versus-host disease (GVHD), which with high mortality rate, is a serious complication that occurs after allogeneic stem-cell transplantation. The multi-center, non-randomized trial showed that the transfer of 1.4 × 10⁶ MSCs (median) per kg of patient bodyweight induced complete responses in 55 % of patients, and partial or complete responses in 71 % of patients with acute GVHD (grade 2–4). The study was done on 55 patients who had not responded to steroid treatment. This result established innovative cell-based therapies for the treatment of diseases characterized by overreactive immune responses [32].
3. Cellular and animal models for POI research
To explore the potentials of MSCs in POI therapies, in vitro cell models and animal models are used to explore the therapeutic efficacy and mechanism. Ovarian granulosa cells (OGCs) play a critical role in ovarian function, including the secretion of estrogen, progestin, and the sexual hormones that maintain female secondary sex characteristics, regulation of the menstrual cycle and the stimulation of follicle growth and ovulation. For this reason OGCs can be considered as models for in vitro studies [33]. Moreover, animal models are established to proceed the therapies.
3.1. OGCs as a proliferative and easy to isolate cell model
OGCs are used as the in vitro cellular model for POI because of its roles in exerting ovary functions. Moreover, OGCs are able to proliferate and can be acquired easily. Human OGCs are obtained from human follicular fluid, commonly donated by women who undergo in vitro fertilization [34]. These cells tend to proliferate in culture after red blood cells are separated and removed. Primary OGCs are dendritic- or spindle-shaped cells connected by elongated pseudopodia. They have a large and round nucleus with a conspicuous nucleolus and abundant cytoplasmic particles. After OGCs are passaged for the first time, they show a fibroblastic morphology with attachment, which helps to distinguish them from oocytes [[35], [36], [37], [38]]. Rat and mouse OGCs share similar characteristics with human OGCs. Therefore, either rat or mouse OGCs can be induced into the POI model by chemotherapeutic medications like cisplatin and epirubicin [39,40]. The specific marker of OGCs is the follicle stimulating hormone receptor (FSHR), present in both the cell membrane and the cytoplasm. It can be detected by immunocytochemistry (ICC), Western blot, and flow cytometry.
MSCs are able to influence the apoptotic behavior and functions of OGCs. When OGCs are co-cultured with MSCs, the total cell count, hormone secretion (E2, progesterone, anti-müllerian hormone (AMH), inhibin A, and inhibin B), and gene expression (Gadd45b and MT-1) were increased compared to OGCs not co-cultured with MSCs [41,42].
In 2020, Yang et al. [43] studied the effects of MSC-derived exosomes on the oocytes in primordial follicles acquired from the ovaries of newborn mice. After dyeing and co-culturing with MSCs-derived exosomes, they found that the exosomes were successfully internalized by and accumulated in the oocytes. Then, they cultured the ovaries of new born mice with or without MSCs-derived exosomes for 24 h and transplanted them into the kidney capsules of adult female mice. The size and weight of the ovaries cultured with MSC-derived exosomes was significantly greater than those cultured without MSC-derived exosomes. In addition, the percentage of follicles was higher in ovaries co-cultured with MSCs exosomes.
In vitro studies are the precursors to animal experiments. OGCs and oocytes can be used to represent ovaries in researches, especially because they express specific ovarian protein markers of functional importance. Ovaries, however, are hard to experiment with in vitro because they are often transplanted into animals after the experimental therapies are administered. Thus, alternative in vivo studies are necessary [44].
3.2. Establishment and evaluation of animal models of POI
3.2.1. Commonly used methods for POI induction in animals
POI models using animals such as mice, rats, or rabbits give researchers the opportunity to explore the mechanisms of action, study therapeutic effects, and optimize the routes of administration of proposed therapies. These studies can mimic procedures used in a clinical setting and provide information and evidence for clinical trials. There are three major methods used to create a non-congenital POI animal model for MSCs studies: chemotherapy, radiotherapy, and d-galactose administration.
The most common induction method of the POI model is chemotherapy. The animals used in this model are stable and rarely sacrificed prematurely. Chemotherapeutic drugs are easily acquired, stored, and administered. The drugs used include cyclophosphamide (CTX), cisplatin (CDDP), busulfan, and tripterygium wilfordii polyglycoside. They are administered, respectively, using the following protocols: 1) a single intraperitoneal injection of 150 mg per kg of body weight; 2) consecutive intraperitoneal injections of 2.5 mg per kg of body weight per day, for 7 days; 3) a single intraperitoneal injection of 36 mg per kg of body weight during the first estrus cycle; or 4) consecutive oral administrations of 50 mg per kg of body weight per day, for 14 days [[45], [46], [47], [48]]. The mechanisms of action for these drugs is believed to be the induction of chromosomal aberrations, sister chromatid exchange, DNA adducts, single-strand breaks, and the formation of double DNA crosslinks [49,50]. In mice, primary follicles, antral follicles, and granulosa cells are the most sensitive to chemotherapy‐induced ovarian damage.
Tan et al. [51] and Gao et al. [52] established a POI mouse model by exposing the whole body under a single dose (4Gy) of X-ray irradiation. After one week, the estrus cycle, hormone levels, and size of the ovaries changed significantly compared to non-irradiated mice, which confirmed the success of POI induction..
The final common method is the administration of d-galactose, which is fed to the animals. This strategy is popular because it protects researchers from toxicity and radiation exposure [53]. Oral administration in prenatal and postnatal mice for 2–6 weeks can induce neonatal POI mice and adult POI mice, respectively. Three enzymes are involved in the creation of the POI model: galactokinase (GALK), galactose 1-phosphate uridylyltransferase (GALT), and UDP-galactose 4 epimerase (GALE). Galactose accumulation happens in the absence of GALT in galactosemic females, which leads to the accelerated depletion of ovarian follicle reserves and the onset of POI.
Autoimmune pathways and gene knock-outs can also induce POI. The most well-known gene, detected by Rajkovic et al. [54] is NOBOX. This is an oocyte-specific homeobox gene expressed in germ cell cysts and in primordial and growing oocytes. They firstly disrupted the NOBOX locus in embryonic stem cells, which accelerated postnatal oocyte loss and impedded the transition from primordial to growing follicles in mice. Furthermore, Zhou et al. [55] reported that the Wdr62 (WD40-repeat protein 62) gene was involved in meiotic initiation and knocking out this gene in mouse resulted in germ cell loss and defects in the initiation of meiosis, which induced the reduction of ovaries size, absent growth follicles, and completely infertile. Franca et al. [56] revealed that a mutation of the POLR3H gene can cause POI, based on the whole-exome sequencing of 11 independent families with POI. Then, they generated POLR3H knock-out mouse model and found that those mice had early embryonic lethality, delayed puberty, decreased fertility, and decreased quantities of primary follicles.
3.2.2. Indicators for successful POI model establishment
To assess ovarian function, the animal's body and ovaries are weighted, their estrous cycles are tracked with vaginal smears, serum sexual hormone levels are evaluated, ovarian histopathological analysis is performed, and fertility is tested.
Body and organ weights usually decrease in POI animals, and can be improved significantly after MSCs treatment in most studies [47,[57], [58], [59]]. Vaginal smears are used to track the estrous cycle through the diestrus, proestrus, estrus, and metestrus stages. POI animals treated with MSCs remained at diestrus for less time than those that did not receive MSCs treatment [57,60,61]. Serum sexual hormone level testing of FSH, E2, and AMH is not consistently applied and varies widely [46,47,57,60,61]. Moreover, there is a significant increase in the number of primordial, primary, secondary follicles, and sinus follicles in POI animals treated with MSCs compared to untreated POI animals. However, more atretic follicles appeared in POI animals that were not treated with MSCs. The ovary morphologies and number of follicles observed in different stages can be compared to the gold standard for ovarian function [50,58,[62], [63], [64]]. Finally, mating, pregnancy, and pup delivery represent the fertility function. Su et al. [65] confirmed that the rates of vaginal plug visualization (a marker of mating success) and pregnancy in the MSCs-treated POI animals increased significantly compared to the untreated groups.
The timelines of the animal experiments depend on the criteria. For instance, one whole estrous cycle takes 5–10 days, which means that it is necessary to conduct vaginal smears every day for at least one week. It takes 21 days from pregnancy to pup delivery. Thus, the age of the animal used to establish a POI model should not be higher than 12 weeks to avoid age-related infertility issues, such as those seen in older humans undergoing menopause (Fig. 2).
Fig. 2.
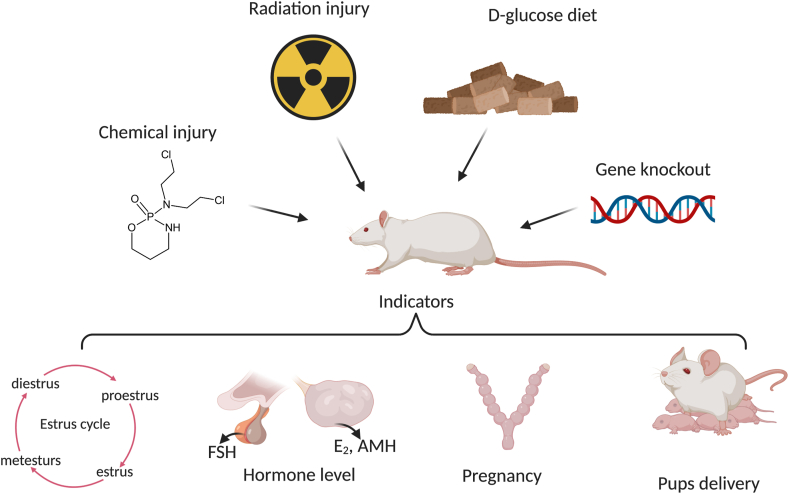
POI induction and indicators for successful model establishment in rodents. POI models can be established via injection of cyclophosphamide (chemical injury), radiation, a diet high in d-glucose, and genetic manipulation (gene knockout animals). The successful establishment of POI models can be verified by observing the estrus cycle, hormone levels, pregnancy success rates, and the rate of pup delivery after mating.
3.3. Mechanisms underlying MSCs therapies for POI
MSCs exert their therapeutic benefits through direct differentiation and paracrine effects, of which the paracrine effects are the major therapeutic mechanism of action. MSCs secrete many kinds of cytokines and growth factors, such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and hepatocyte growth factor (HGF) [19], which modulate downstream signaling pathways to stimulate pro-reparative actions. Moreover, through exosome-mediated cellular communications, MSCs can also regulate gene expression in target cells.
3.4. Signaling pathways involved in MSC therapeutics for POI
MSCs from different origins affect ovarian function through several signaling pathways, which are associated with cellular migration, homing, anti-apoptosis, and anti-inflammation. Multiple studies confirm that the phosphatidylinositol 3-kinase (PI3K) signaling pathway plays a critical role in the MSCs-mediated treatment of POI in vitro and in vivo [43]. PI3K is involved in ovarian dysfunction by reducing phosphorylation of Akt. It is also a regulator of key biological activities in human and other mammals, such as cell differentiation, proliferation, apoptosis, and transportation. Another key player is the mTOR protein, which plays roles in fragile X-associated POI (Fig. 3A) [66]. Human umbilical cord MSC (HucMSC)-derived exosomes failed to activate oocytes growth in newborn mice by decreasing phosphorylation of mTOR, rpS6, and Akt. Also, human placenta MSC (hpMSC) transplantation can restore ovarian function by changing the ratios of Th17 (T-helper 17 cells) to Tc17 (cytotoxic T cells) cells, and that of Th17 to Treg cells (regulatory T cells), which might regulate the PI3K/Akt signaling pathway and curb inflammation and autoimmunity (Fig. 3B) [67]. When these ratios significantly increase, proliferation of OGCs decreases and apoptosis of OGCs increases. In addition, an in vitro study demonstrated that microRNA-21(miR-21), regulated in part by the PI3K/Akt pathway, promoted bone marrow MSC (BMMSCs) migration by upregulating the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9 [68].
Fig. 3.
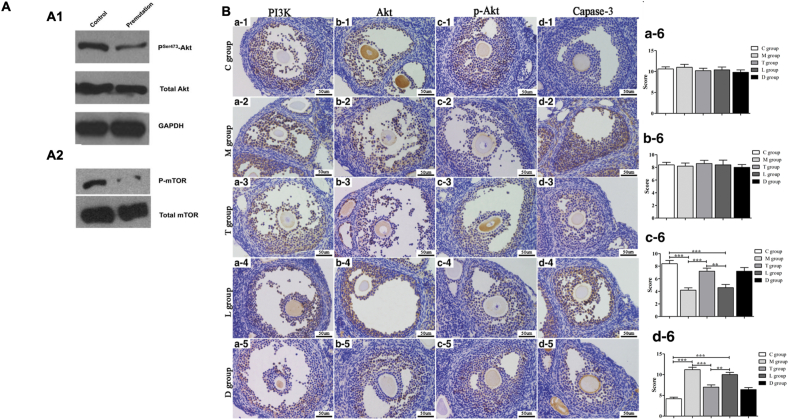
PI3K/Akt signaling pathway plays a critical role in ovarian dysfunction. (A) Western blot analysis of Akt and mTOR phosphorylation in ovaries isolated from normal and adult fragile X premutation mice. (A1) A significant reduction of phosphorylated Akt, but not total Akt, in premutation ovaries. (A2) A dramatic reduction of phosphorylated mTOR protein in premutation ovaries. GAPDH was used as a loading control. (B) IHC analysis of PI3K, Akt, p-Akt, and capase-3 in the ovarian tissues of mice. Photomicrographs show hematoxylin and DAB-stained ovaries. (a1-d1) Control group (C group). (a2-d2) POI group (M group). (a3-d3) POI + hPMSCs group (T group). (a4-d4) POI + hPMSCs + LY294002 group (L group). (a5-d5) POF + hPMSCs + DMSO group (D group). (a6-d6) Bar graphs summarizing the four kinds of cytokines expressed in the five groups. Brown in the cytoplasm indicates positive expression of the protein of interest. Blue represents cell nuclear staining. **p < 0.01, ***p < 0.001 vs POI group. Scale bar = 50 μm. POI, primary ovarian insufficiency; hPMSC, human placenta-derived mesenchymal stem cell; DMSO, dimethylsulfoxide; and PI3K, phosphatidylinositol 3-kinase [67].
Other studies involving the pathways JNK/Bcl2, IRE1α, NGF/TrkA, and SMAD have been reported. Yin et al. [69] suggested that the activation of the JNK/Bcl-2 signaling pathway regulated autophagy and circulation of CD8+/CD28− T cells, which led to hemeoxygenase-1 (HO-1) expression in umbilical cord MSCs. HO-1 has a crucial role in restoring the ovarian function of POI mice with HucMSC transplantation. Another study using hpMSCs to treat POI, indicated that the IRE1α pathway was activated both in POI mice and in OGCs from POI mice [70], which was mediated by increased X‐box binding protein 1 (XBP1) and upregulated 78 kDa glucose‐regulated protein (GRP78) and caspase‐12. In addition, the importance of the SMAD pathway was confirmed by a study involving human adipose MSCs-derived exosomes (hADMSC-Exos) [71]. The study showed that the ovarian granulosa cells of POI patients had decreased protein levels of SMAD2, SMAD3, and SMAD5. When POI mice were treated with hADMSC-Exos, the gene expression and protein levels of SMAD2, 3, and 5 were significantly upregulated. In addition, Cui et al. [72]revealed that HucMSC transplantation can restore ovarian function in rats with cisplatin-induced POI. The recovery was associated with the inhibition of ovarian fibrosis through the regulation of stromal cell differentiation by transforming growth factor-β1 (TGF-β1)/SMAD3 signaling pathway. According to these studies, hADMSC-Exos improved ovarian function by regulating the SMAD pathway.
Then, Lu et al. [73] presented the role of theca-interstitial cells (TICs), instead of OGCs, as a model cell line in POI therapy analysis. TICs are the ovary's primary androgen-producing cells. They explored the mechanisms underneath the observed protection of ovarian function by HucMSCs. The main driver of therapeutic efficacy seems to be the regulation of TIC autophagy. Results indicate that HucMSCs can reduce the autophagy levels of TICs by reducing oxidative stress and regulating the amp-activated protein kinase (AMPK)/mTOR signaling pathway, thereby alleviating the apoptosis of TICs.
Future studies will require the identification of specific targets involved in clinical therapeutic applications. When they have been confirmed, the proteins, the nuclei acids, or other biological factors can be more thoroughly investigated and exploited as therapeutic mediators.
3.5. MSCs restore ovarian function by modulating multiple signaling pathways
POI can be regulated through the modification of gene and protein expressions. A research study from Gao et al. [74] indicated that radiation-induced ovarian damage caused an imbalance in sex hormones and the distribution of mineral elements, decreasing fertility in mice. They also found 223 upregulated genes and 65 downregulated genes of known function. They found that Cyp17a1 expression can increase during estradiol biosynthesis and that AKR1c18 can accelerate progesterone metabolism. Another case control study from South Korea showed that tumor necrosis factor (TNF) -α-1031C and TNF-α −238A, alleles of TNF-α, had a strong association with POI [75]. The TNF-α-308A allele, however, did not significantly increase POI risk in the presence of the TNF-α-1031T allele, which suggests that TNF-α promoter polymorphisms may increase POI risk.
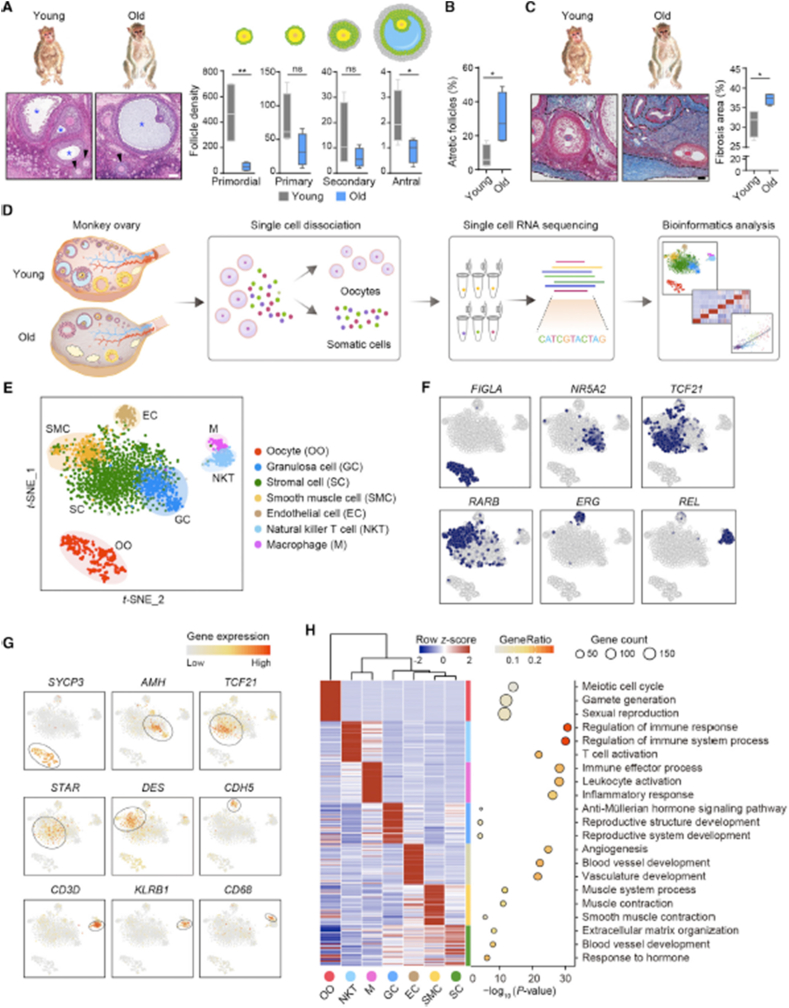
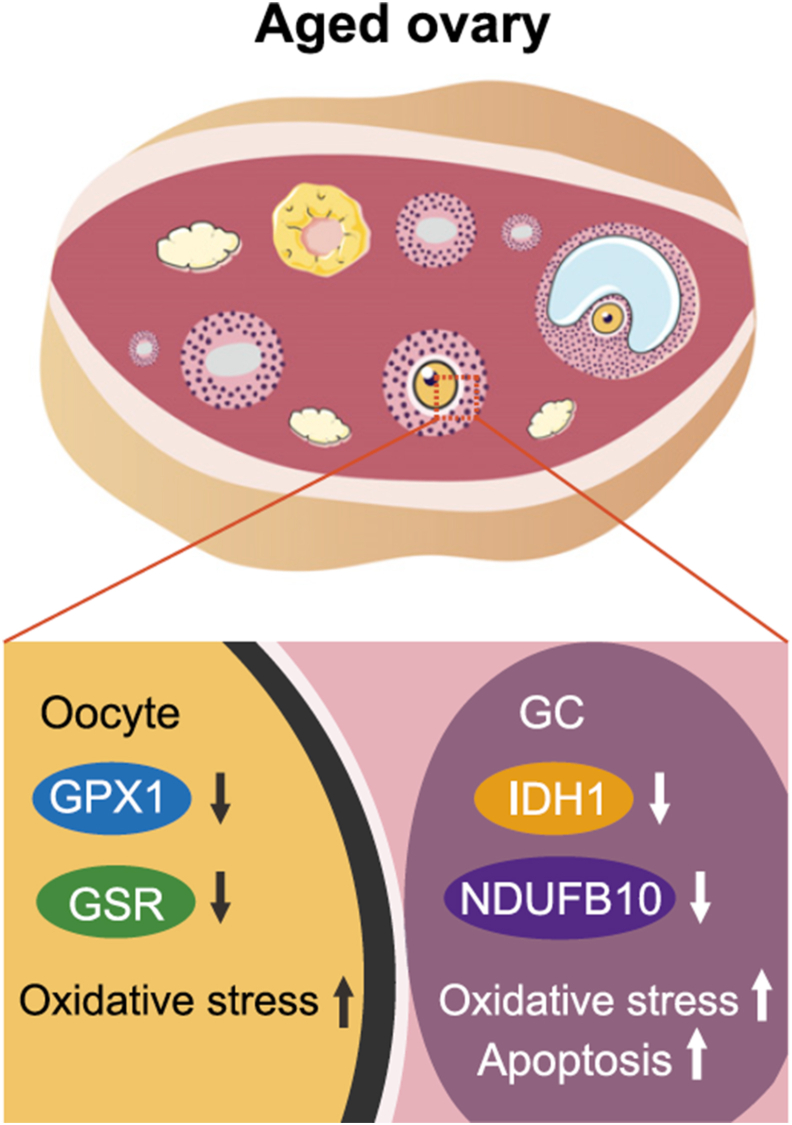
A study conducted by Wang et al. [76] revealed the mechanisms responsible for ovarian aging. They indicated that the onset of POI in young patients was also mediated by these same mechanisms. Their analyses involved the single-cell RNA sequencing (scRNA-seq) of ovaries, obtained from monkeys, which closer represents human physiology than rodents (Fig. 4). They explored the gene-expression dynamics of oocytes during folliculogenesis, and they identified four subtypes of oocytes. Their findings suggest that the downregulation of antioxidant genes, including GPX1 and GSR, was a unique aging feature of early-stage oocytes, likely contributing to the increased oxidative damage during ovarian aging. Lastly, they isolated OGCs from both aging and young female patients in an effort to discover and isolate the genes regulating ovarian aging. Their results indicate that IDH1 and NDUFB10 protect OGCs from aging-related oxidative stress in both humans and monkeys (Fig. 5). Altogether, this study provided the first comprehensive single-cell transcriptomic atlas of the ovaries of young and aged non-human primates (NHPs), and broadened the understanding of cell identities and cell-type-specific gene signatures in the primate ovary. The mechanistic insights attained this study can establish new avenues for developing targeted antioxidant interventions to protect against physiological ovarian aging and related diseases, and to develop new tools for the rejuvenation of oocytes in POI patients.
Fig. 4.
Distinct ovarian cell subpopulations with transcriptional signatures determined by single-cell RNA-Seq analysis. (A) Left: H&E stained sections of young and old monkey ovaries. Arrowheads and asterisks denote secondary and antral follicles, respectively. Right: density quantification of different stages of follicles indicated by the morphology shown by the cartoons. Scale bar, 100 mm n = 4 monkeys. ns, not significant, *p < 0.05, and **p < 0.01 (one-tailed t-test). (B) Percentage of atretic follicles with respect to total follicles based on H&E stained sections. n = 4 monkeys. *p < 0.05 (two-tailed t-test). (C) Masson's trichrome staining of young and old monkey ovaries. Dashed lines denote fibrotic areas. Scale bar, 100 mm n = 4 monkeys. *p < 0.05 (two-tailed t-test). (D)Flowchart overview of monkey ovarian scRNA-seq. (E) t-SNE plot showing seven ovarian cell types. (F) t-SNE plots characterizing representative transcriptional regulators for different cell types. Blue color denotes the cells with the activation of indicated transcriptional regulators. (G) t-SNE plots showing expression levels of oocyte and somatic cell marker genes. (H) Left: heatmap showing expression signatures of top 50 specifically expressed genes in each cell type; the value for each gene is a row-scaled Z score. Right: representative gene terms [76].
Fig. 5.
A schematic illustration of gene regulation. The figure shows the cell-type-specific downregulation of genes involved in redox homeostasis maintenance in aged primate oocytes and GCs, which could contribute to increased oxidative damage and cell apoptosis [76].
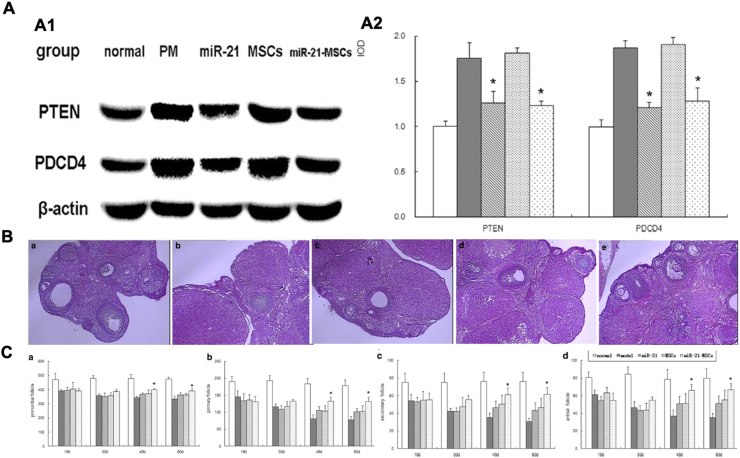
MSCs may upregulate or downregulate gene expression in ovarian cells by participating in cell proliferation, apoptosis, death, and cytotoxicity. Fu et al. [77] revealed that BMMSCs transfected with miR-21 led to the decrease in apoptosis of OGCs in vitro. While in vivo, POI rats injected with miR-21-BMMSCs had increased ovary weight and follicle counts, increased E2 levels, as well as decreased FSH levels (Fig. 6). On the other hand, Yan et al. [41] found that menstrual blood mesenchymal stem cells (MB-MSCs) repaired epirubicin-induced damage in OGCs associated with Gadd45b, CyclinB1, and CDC2, the differentially expressed genes of OGCs. In detail, epirubicin upregulated Gadd45b gene expression and downregulated CyclinB1 and CDC2 gene expression. MB-MSC treatment resulted in the downregulation of Gadd45b gene expression and upregulation of CyclinB1 and CDC2 gene expression. Thus, it was concluded that these genes were an important part of the mechanism of action of MB-MSC-mediated POI treatment. In addition, the Sry gene has been identified in the ovarian tissue of POI animals that received BMMSCs, but not in POI animals that did not receive BMMSCs treatment [78]. One study used fetal liver MSCs (fMSCs) to treat POI animals and found that they upregulated melatonin receptor 1 (MT1), JNK1, PCNA, and AMPK at both the mRNA and protein levels. When OGCs from POI patients were subjected to MT1 knockdown or antagonist treatment, their expression of JNK1, PCNA, and AMPK was reduced, and the percentage of proliferative OGCs was impaired [42].
Fig. 6.
MiR-21 affects OGCs in vitro and follicle growth in vivo. (A) Expression of miR-21 and protein expression of target genes (PTEN and PDCD4) in ovarian granulosa cells; PM, phosphamide mustard. (A1) Western blot detection of PTEN and PDCD protein expression. (A2) Quantification, *P < 0.05, compared to the MSC group. (B) Ovarian structure in H&E staining. (a) normal group, with the observation of follicles at different developmental stages and corpora lutea. (b) model group (PM injection), with a dramatic reduction in follicle counts. (c) miR-21 group, with an increase in follicle counts as compared with the model group. (d) mesenchymal stem cell (MSC) group, with an increase in follicle counts as compared with the model group but comparable to that in the miR-21 group. (e) miR-21-MSC group, with an increase in follicle counts as compared with the miR-21 group and MSC group. (C) Comparison of follicle counts among the groups at 15, 30, 45, and 60 days after injection. (a) Count of primordial follicles. (b) Count of primary follicles. (c) Count of secondary follicles. (d) Count of antral follicles. *P < 0.05, compared to the miR-21 group and the mesenchymal stem cell (MSC) group [77].
Consequently, gene mutation has been confirmed to play a critical role in POI pathogenesis, regardless of whether or not the POI is congenital in nature. MSCs improved POI by regulating gene and protein expressions, suggesting that the genetic mechanisms involved in POI may be viable therapeutic targets. There are multiple studies that explore the efficacy of gene regulation as a treatment for POI. This avenue of research has broadened our etiological understanding of POI. However, these genetic approaches have not yet been translated into clinical treatments.
3.6. MSC paracrine factors mediate ovarian functional improvement
One therapeutic characteristic of MSCs is their ability to secrete cytokines that reduce apoptosis, fibrosis, and inflammation, and promote angiogenesis, which results in POI recovery. The most commonly secreted cytokine is vascular endothelial growth factor (VEGF), which has been reported to be anti-apoptotic (Table 1) and anti-fibrotic. It has also been shown to participate in arteriogenesis, improving in vitro and in vivo growth of granular cells by increasing the length, area, and branch point number of the vessels [[79], [80], [81], [82], [83]]. Another secreted cytokine, hepatocyte growth factor (HGF), with the anti-apoptotic effect on OGCs and oocytes, promoted vascular outgrowth and improved ovarian functions. It is possible that HGF and VEGF function synergistically to increase the vascular diameter [80,83]. In addition, basic fibroblast growth factor (bFGF) plays an inhibitory role on the apoptotic pathway. Huang et al. [84] demonstrated that granulocyte colony-stimulating factor (G–CSF)–mobilized peripheral blood mononuclear cells (PBMCs), combined with platelet-rich plasma (PRP), can restore ovarian function in POI rats by increasing ovarian neovascularization, reducing granulosa cell apoptosis, and promoting follicle growth. Also, anti-inflammation may be mediated by tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interferon type II (IFN-γ) [85,86]. In addition, IFN-γ may regulate immune function in POI animals.
Table 1.
Determination of granular cell apoptosis by Annexin-V/propidium iodide (PI) staining A: before freezing; B: immediately after thawing; C: after thawing and culturing for 24 h. In the VEGF pretreated group, increases in the proportions of late apoptotic cells [Annexin-V (+)/PI (+)] were significantly lower in the un-pretreated control immediately after thawing (***p < 0.05), and reductions in the proportions of viable cells [Annexin-V (−)/PI (−)] (*p < 0.05) and increases in the proportions of early apoptotic cells [Annexin-V (+)/PI (−)] were significantly lower after culturing for 24 h (**p < 0.05) [81].
| Viable cellsAnnexin-V(−)PI(−) | Early apoptotic cellsAnnexin-V(+)PI(−) | Late apoptotic cells Annexin-V(+)PI(+) | Necrotic cellsAnnexin-V(−)PI(+) | |
|---|---|---|---|---|
| VEGF(+) | ||||
| A | 35.66 ± 0.48 | 55.66 ± 0.30 | 7.74 ± 0.16 | 0.94 ± 0.02 |
| B | 24.75 ± 0.45 | 64.54 ± 1.01 | 9.89 ± 0.34*** | 0.83 ± 0.23 |
| C | 32.69 ± 2.07* | 60.75 ± 1.78** | 6.30 ± 0.38 | 0.27 ± 0.17 |
| VEGF(−) | ||||
| A | 40.75 ± 0.04 | 51.84 ± 0.47 | 6.37 ± 0.26 | 1.04 ± 0.25 |
| B | 26.62 ± 1.49 | 58.64 ± 0.28 | 13.79 ± 1.41*** | 1.0 ± 0.11 |
| C | 23.09 ± 0.89 | 66.91 ± 0.19** | 9.66 ± 1.04 | 0.34 ± 0.02 |
In summary, the paracrine effects of MSCs make them a promising candidate for cell-free POI therapies. Cell-free therapies that use allogeneic MSC secretions are even less immuno-stimulating than transplanted autologous MSCs. This makes them a safer alternative for clinic translation, with a number of advantages including preventing immune rejection and obviating the invasive procedures required to isolate autologous MSCs.
4. Acellular treatment strategies for POI
4.1. MSCs derived secretomes or exosomes for POI therapy
Even though stem cells are a promising therapy option for POI, they are also hindered by a number of limitations that cannot be ignored, including the risks of creating carcinomas, inducing autoimmune diseases, and the lack of knowledge about their mechanisms of action. Therefore, an increasing number of studies are focusing on stem cell-derived secretomes, cytokines, and exosomes for regenerative applications. Exosomes are a type of extracellular vesicles that mediate cellular communications by enveloping and transferring proteins, nucleic acids as well as the other active components [87].
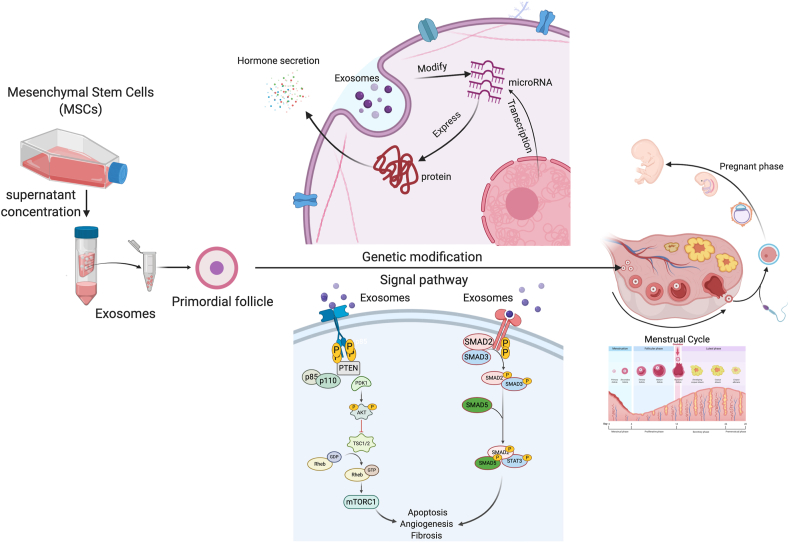
Wang et al. [58] indicated that menstrual stem cells (MenSCs) might not differentiate into oocytes directly. After injecting MenSC-conditioned media into POI rodents, serum FSH levels decreased, and E2 levels increased. Their analyses revealed improved ovarian function, reduced fibrosis, and lower levels of apoptosis in OGCs. Meanwhile, Khanmohammadi et al. [88] concluded that the ovarian structure and serum hormone levels were similar in BMMSC-treated and BMMSC secretome-treated POI rats. Some researchers explored the therapeutic effects of stem cell exosomes, and explored their mechanisms of action for treating POI. HucMSCs promoted ovarian expression of HGF, VEGF, and IGF-1 through their secretion of cytokines, resulting in restored ovarian function and resistance to ovarian senescence [89]. Sun et al. [90] showed that HucMSC-derived exosomes regulate apoptosis in OGCs through the modification of gene and protein expression. In addition, Yang et al. [43] confirmed that HucMSC-derived exosomes stimulated primordial follicles to activate oocyte development. The injection of HucMSC-derived exosomes into elder mice (>10 months) restored fertility and increased oocyte quantity and quality. Huang et al. [91] showed that hADMSC-Exos can inhibit apoptosis in the ovarian granulosa cells of POI patients by down-regulating certain genes in the SMAD pathway (Fig. 7). Altogether, these cell-derived agents make up a host of possible cell-free therapies that may be used to treat POI.
Fig. 7.
Acellular therapy strategies for POI. Stem cell exosomes can be acquired from cell culture supernatant and used for POI treatment. Following the uptake by primordial follicles, exosome treatment improved ovarian hormone secretory functions, ovulation, and fertility through genetic modification and modulation of multiple signaling pathways.
In recapitulation, MSCs improved ovary outcomes in POI animal models by secreting various paracrine molecules and vesicles. Instead, paracrine signaling has become the more accepted mechanism of action thought to account for the therapeutic benefits of MSCs. Therefore, MSC-derived secretomes and exosomes are broadly employed in acellular treatment strategies. In addition to exosomes, secretomes derived from stem cells are expected to overcome the restrictions of stem cell transplantations and may establish the basis of a cell-free therapy for POI in clinical trials.
4.2. Biomaterials for the treatment of POI
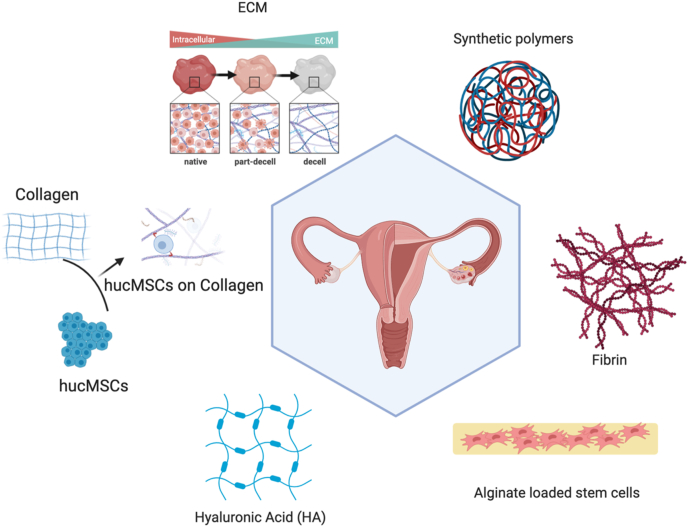
One of the vital components in regenerative medicine is biomaterials [92] that are designed based on multiple factors, such as the fabrication [93], biocompatibility, and promoting favorable cellular interaction [94,95] (Fig. 8). There are two types of biomaterials used in the formula of hydrogels include natural materials such as extracellular matrix (ECM), collagen, alginate, and hyaluronic acid, and synthetic polymers such as polytetrafluoroethylene (PTFE). Ovarian patch is also used for POI treatment, which providing efficient mechanical support, restored ovarian function and improved the development of follicles. The ideal biomaterial should be non-toxic, biocompatible, biodegradable, and bioresorbable. It should be able to support the regeneration of new cells and tissue without producting an inflammatory reaction [96]. The use of these biomaterials and their success in reversing ovarian dysfunction will be discussed in detail in the following sections [97] (Table 2).
Fig. 8.
Biomaterial carriers for the delivery of stem cells and stem cell derivatives for the treatment of POI. To regenerative tissues damaged in POI models, biomaterials ranging from natural components to synthetic products have been employed. They serve as carriers for stem cells or stem cell derivatives. The natural biomaterials studied include extracellular matrix (ECM), collagen, hyaluronic acid (HA), alginate, and fibrin.
Table 2.
List of biomaterials used for ovarian function restoration.
| Categories | Author | Year | Materials | Major finding |
|---|---|---|---|---|
| Extracellular matrix (ECM) | J. Smitz [102] | 2010 | ECM is composed of a variety of molecules, which can include collagens, laminin, fibronectin, proteoglycans, and polysaccharides. | ECM improved the secretion of E2 and AMH from follicles. It also improved follicle development |
| Wen-Yue Liu [103] | 2016 | Xenogeneic decellularized ovary (D-ovary) scaffold, as a platform derived from pigs, and used in rats. | The D-ovary tissues successfully supported rat granulosa cell penetration in vivo and showed an improvement in estradiol (E2) hormone secretion. | |
| Ebru Ersoy [104] | 2017 | The mechanical exploration of ECM to support ovarian function. | The disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) enzymes in ECM contribute to ovulation and differentiation of the follicular cells into the luteal cells. | |
| Collagen | Xiaoming He [107] | 2017 | A 3D microfluidic encapsulation of ovarian follicles in biomimetic ovarian microtissues. | Due to the mechanical heterogeneity of the resulting culture capsule, this 3D microfluidic technology and biomimetic ovarian microtissue system could facilitate follicular culture and preservation. This might be a feasible option of assisted reproduction and may help restore women's fertility in the clinic. |
| Collagen | Yanjun Yang [109] | 2019 | Gelatinous scaffolds carried type I collagen and were loaded with HucMSCs in animal experiments. | Collagen/HucMSC transplantation may be a potential and promising treatment for POI |
| Lijun Ding [110] | 2018 | Gelatinous scaffolds carried type I collagen and were loaded with HucMSCs for clinical use. | Collagen/HucMSC transplanted into the ovaries improved the fertility of women with POI. Therefore, collagen/HucMSC transplantation may bet a promising approach for POI treatment. | |
| Hyaluronic Acid (HA) | Guangfeng Zhao [113] | 2014 | Hyaluronic acid was injected into ovaries of POI rats. | MiR-139–5p might be a candidate miRNA in the up-regulation of HA on PGRMC1 expression. |
| Guangfeng Zhao [114] | 2014 | Hyaluronic acid was injected into ovaries of POI rats. | HA has a preventative effect on POI rats, which was associated with promotion of GC proliferation. These findings support the applicability and efficacy of HA in preventing POI. | |
| Alginate | Lisa J. Green [125] | 2019 | Adipose-derived stem cells (ADSC) were suspended in alginate solution and encapsulated in crosslinked alginate beads. | Co-encapsulation of early-stage murine follicles with ADSCs supports the in vitro maturation of murine follicles. |
| Alginate | Sivanandane Sittadjody1 [118] | 2017 | 3D bioengineered ovarian constructs that recapitulate native cell–cell interactions between ovarian granulosa and theca cells. | A 3D bioengineered ovarian construct for the treatment and study of conditions associated with functional loss of the ovaries, which may be suitable for young patients suffering from malignant tumors or congenital diseases. |
| Fibrin | Ariella Shikanov [120] | 2011 | Cryopreserved and thawed ovarian tissue was encapsulated in fibrin-(heparin-binding peptide)HBP-VEGF hydrogels. | Potential of engineering biomaterials to enhance angiogenesis during engraftment of ovarian tissue and biomaterials for ovarian transplantation. |
| E. Kniazeva [117] | 2015 | Primordial and primary ovarian follicles from young female mice were extracted and encapsulated into biomaterials for subsequent transplantation into adult mice with ovariectomy. | Obtain live births by transplanting isolated primordial and primary follicles, and reducing the risk of re-seeding disease relative to ovarian tissue transplantation. | |
| Synthetic polymers | Anu David [122] | 2017 | Ovarian grafts were picked up in a pipette and inserted into the TheraCyte® capsule, which is an FDA approved device made of polytetrafluoroethylene (PTFE). | The TheraCyte® devices can successfully support the function of ovaries and avoid immune sensitization of the host. |
4.2.1. Extracellular matrix
Extracellular matrix (ECM) is a three-dimensional network of extracellular macromolecules that provides structural and biochemical support for cells and helps to maintain homeostasis [98]. ECM is widely used as a supporting biomaterial in regenerative medicine. When using ECM to create scaffolds, it's important to ensure a porous 3-D microenvironment with an interconnected structure of well-distributed pores large enough for cell seeding [99]. In addition, the technique used to manufacture ECM must produce scaffolds with the correct physical attributes without affecting the material's biocompatibility [100]. ECM is commonly used in therapeutic research involving myocardial infarction, lung fibrosis, and skin and hair regeneration [101]. It has also been studied for the treatment of POI, and has been shown to be effective at helping to restore ovarian function. In 2010, Smith [102] produced an ECM based material composed of a variety of molecules that were particularly useful for ovarian research. It included collagens, laminin, fibronectin, proteoglycans, and polysaccharides. Collagen IV is abundant around theca cells in the follicles. It is also found at lower-levels around the stroma and granulosa cells of the ovaries. Fibronectin is presented around the stroma and theca cells. As follicles develop, its abundance increases, except around the granulosa cells, where it actually decreases. Laminin is localized primarily around the theca cells, with a defined ring at the exterior of the follicular granulosa cells marking the basement membrane. When laminin is co-cultured with growing follicles, at various developmental stages, derived from human and non-human ovarian tissues, it can improve their secretion of E2 and AMH secretion.
In another study involving ECM, Liu et al. [103] investigated xenogeneic decellularized ovary (D-ovary) scaffolds, which were isolated from pigs and was used in rats. They confirmed that D-ovary tissues were noncytotoxic to rat ovarian cells in vitro and caused only a minimal immunogenic response in vivo. D-ovary tissues successfully supported rat granulosa cell penetration in vitro and improved its secretion of estradiol (E2). Also, the safety and efficacy of D-ovary tissues have been demonstrated with rat ovarian cells in vitro and caused only a minimal immunogenic response in vivo. However, the mechanisms of action behind ECM-mediated ovarian therapies are still unknown. In 2019, Ersoy et al. [104] discovered that several enzymes, like matrix metalloproteinases, plasminogen activators, and disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) in ECM contribute to ovulation and differentiation of follicular cells into luteal cells. The ADAMTS enzymes are a zinc metalloproteinase gene family with roles in vascular biology, inflammation, and especially in the control of the function and structure of the ECM.
These studies demonstrated that ECM can improve ovarian functions of secretion and ovulation. ECM is safe and viable for clinical applications. However, these studies did not evaluate the effect of ECM on fertility, and the proposed clinical applications of ECM have been focusing on procedures requiring ovary cryopreservation for patients suffering from gynecological malignant tumors and undergoing ovariectomies.
4.2.2. Collagen
Collagen as the main structural protein, is found in the ECM. On average, it makes up 30% of the total protein mass of the body [105]. Its ubiquity is indicative of an important role in maintaining biological viability. There are three signature features of collagen. First, it is composed of repeating sequences of amino acids, both with and without interruptions. Next, within those repeating sequences, proline and hydroxyproline occupy the X and Y positions, respectively. Finally, its right-handed triple helix structure is formed from three left-handed polyproline α-chains of identical length, which gives collagen a unique quaternary structure [106]. More than twenty types of collagen have been identified, and numerous functional characteristics, including high mechanical strength, good biocompatibility, low antigenicity, and ability to crosslink with other molecules have been reported. These traits enable the tailoring of collagen's mechanical, degradation, and swelling properties [96].
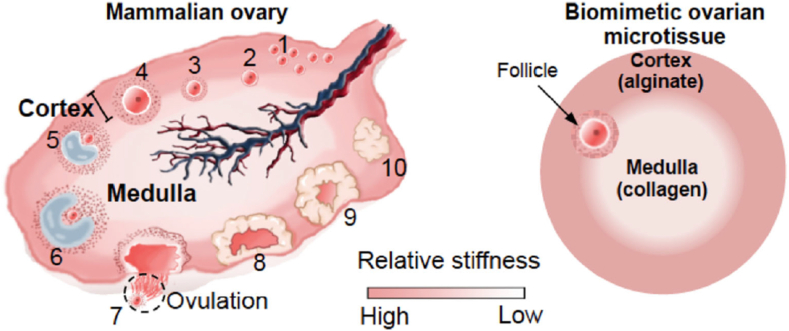
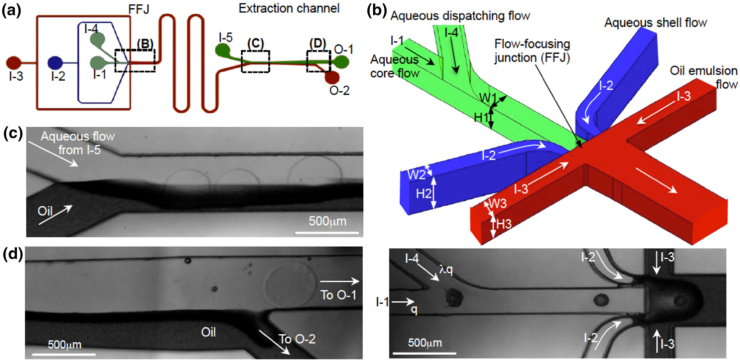
He et al. [107] used microfluidic encapsulation to create collagen-rich, biomimetic 3D shells to culture rodent ovarian follicles. This culture technique recapitulated the mechanical heterogeneity of the ovaries (Fig. 9) by creating capsules with a relatively soft collagen core (ovarian medulla) surrounded by a relatively harder alginate shell (ovarian cortex). The microfluidic device, which is used to create the capsules, integrated the core and shell ingredients using an oil emulsion flow that pinched the ingredients into droplets (Fig. 10). This device was achieved using a computational fluid dynamics model and capable of predicting the complex multiphase microfluidic flow in the non-planar flow-focusing junction [108]. The biomimetic capsules resulted in follicle development into the antral stage, accompanied by estradiol production. This study showed the power of collagen microenvironments in advancing follicular culture in vitro. In addition, because of the exceptional of alginate shells at inhibiting ice crystal formations, this culture paradigm improved the outcome of follicular cryopreservation, which is a key feature of assisted reproduction. However, while this study provides an example of collagen application in vitro, supporting data from in vivo experiments will be necessary for its clinical applicability.
Fig. 9.
A schematic illustration showing the development of follicles at various stages and the difference in mechanical properties between the ovarian cortex and medulla of the mammalian ovary, together with the biomimetic ovarian microtissue that recapitulates the mechanical heterogeneity experienced by follicles in the ovary. (1) primordial follicles; (2) primary follicles; (3–4) pre-antral follicles; (5–6) antral follicles; (7) cumulus–oocyte complex (COC); and (8-10) corpus luteum [107].
Fig. 10.
Microfluidic generation of biomimetic ovarian microtissue and on-chip extraction of the microtissue from oil emulsion into isotonic aqueous solution: (a) A schematic overview of the microchannel system. (b) A 3D zoomed-in view and real image of the dispatching channel and flow-focusing junction (FFJ) showing the non-planar design of the FFJ. (c) An image of the extraction channel at its entrance. (d) An image of the extraction channel at its end. The symbol q represents the flow rate of the aqueous core flow of sodium alginate; I-1, I-2, I-3, I-4, and I-5 represent the five inlets; O-1 and O-2 represent the two outlets; and λ≤1 [107].
Indeed, the use of collagen as a scaffold for stem cell-based POI therapy is well documented. Yang et al. [109] investigated the use of gelatinous scaffolds made from type I collagen and loaded with HucMSCs as a treatment for POI mice. Their results showed that transplantation of the HucMSC-loaded collagen scaffolds improved ovary size, follicles growth, and follicles development. Moreover, the potential mechanism of action may involve the proliferation of granulosa cells and the increase of vascularization in the ovaries. While it is still not clear what the mechanistic details are, with further research development, HucMSC-loaded collagen scaffolds may represent an ideal and promising treatment for POI patients.
Ding et al. [110] reported that transplantation of HucMSC-loaded collagen scaffolds to the POI patients can restore ovarian function. Their treatment caused hormone levels of FSH to decrease and E2 to increase. It also resulted in restored blood flow in the ovaries. Follicles, 12 mm in size or greater, were observed via ultrasounds conducted after the transplantation. Furthermore, 2 patients became pregnant and one baby was delivered at full-term. They also mentioned that no severe side effects, such as hemorrhage, inflammation, or abdominal pain were observed in any of the cases. Therefore, HucMSCs/collagen may represent a promising approach for POI treatment in the clinic. In the future, and as a necessary stepping stone to achieve clinical trials, the safety and biocompability of Huc-MSCs/collagen needs to be more thoroughly assessed.
In summary, collagen can be used in combination with biologic therapies to restore ovarian function. It is easy to produce, biocompatible, and abundant. In order to advance as a viable therapeutic option, however, the mechanism of action needs further elucidation.
4.2.3. Hyaluronic acid
Hyaluronic acid (HA), or hyaluronan, is a linear polysaccharide that consists of alternating units of repeating disaccharides made up of β-1,4-d-glucuronic acid and β-1,3-N-acetyl-d-glucosamine. Currently, HA is widely used in tissue bioengineering studies due to its superior biocompatibility and hyaluronidase-mediated biodegradability [111]. An advantage of HA is that it can achieve targeted therapy delivery due to the presence of HA receptors on the cells of specific tissues such as the liver, uterus, and ovaries [112].
Zhao et al. [113] tested serum HA levels in POI patients, and found that they were significantly lower than in healthy women. Then, in vitro experiments showed that HA reversed the damage that immunosuppressive agents caused to OGCs. Subsequently, they injected HA into POI rats and found that it prevented chemically induced ovarian damage and improved ovarian function. Mechanistic research showed that HA promoted the proliferation and function of OGCs via the P4 receptor membrane component 1 (PGRMC1), which was regulated by miR-139–5p [114].
Above research indicated that HA is efficacious in preventing POI. However, before clinical studies commence, the safety and biocompatibility of HA as well the appropriate therapeutic concentrations, volumes, and delivery routes should be further detailed.
4.2.4. Alginates
Alginates are polysaccharides isolated from brown algae, such as Laminaria hyperborea and Lessonia, found in coastal waters. They are linear, unbranched copolymers of (1,4)-linked beta-d-mannuronic acid (M), and its C-5 epimer, alpha-l-guluronic acid (G). The mannuronic acid and guluronic acid are covalently linked together in different sequences, or blocks [115]. Alginates can be used as biomaterials for clinical drug delivery, wound healing, tissue engineering, and cell barriers due to their advantageous material properties, including biocompatibility, non-immunogenicity, and hydrophilicity [116].
Kniazeva et al. [117] developed alginate grafts to encapsulate primordial and primary follicles into POI rats. After treatment, the rats showed higher levels of Luteinizing Hormone(LH), E2, and progestin, less body fat, and reduced body weight. Bone metabolism and architecture were also improved, as did genitourinary tract health. Moreover, the rats did not show signs of hyperplasia or hypertrophy. In another study, Green et al. [118] produced alginate beads to encapsulate adipose-derived stem cells, which were then co-cultured with primary and secondary mouse follicles. The data showed that follicle survival and growth increased, as did the rates of antrum formation and oocyte maturation.
These researches provided insights into new alginate-based approaches for treatments of ovarian dysfunction. However, the efficacy and safety information obtained in these studies are limited to small animal data. To move forward, translational large animal study and preclinical trial data will be essential in determining the actual utility of alginate as a biomaterial for POI treatment in clinics.
4.2.5. Fibrin
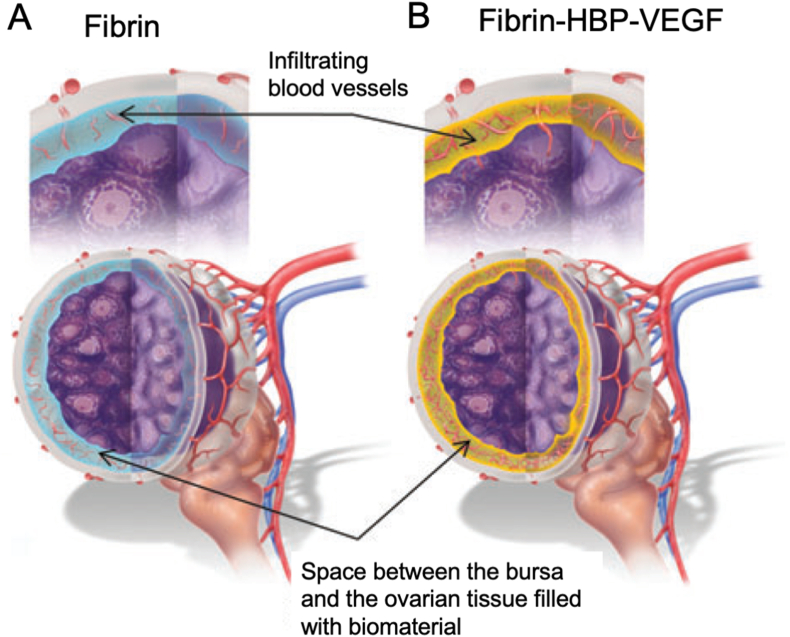
Fibrin is formed via the polymerization of fibrinogen and thrombin in blood plasma, which plays a critical role in the blood clotting process. Fibrin has been isolated and approved as a therapeutic to stop patients from hemorrhaging in the clinic. It has also been used in bioengineering and regenerative medicine because of its biological and structural properties [119]. Shikanov et al. [120] designed a hydrogel made up of fibrin that was modified with heparin binding protein and heparin, and loaded with VEGF. They used this hydrogel to encapsulate ovarian grafts that were transplanted into mice. They found that fibrin encapsulation preserved ovarian tissue morphology, leading to successful integration of the transplanted tissue with the host (Fig. 11). The encapsulating fibrin was fully degraded after 3 weeks, eliminating all barriers between the grafted tissue and the host bursa. The transplanted ovaries had similar morphologies to those of a healthy fertile adult mouse female.
Fig. 11.
Schematic presentation of the ovarian tissue in fibrin-HBP hydrogels containing heparin-bound VEGF. (A) Ovarian tissue encapsulated in degradable fibrin allows physical connectivity that may result in improved communication between the host and the graft and create a continuous path for cell infiltration. (B) Controlled release of VEGF promoted revascularization [120].
Much like the other natural biomaterials that were previously described, fibrin holds great promise as a scaffold or encapsulating polymer for therapies targeting ovarian damage. However, the mechanisms of action still need to be determined, and the safety as well as efficacy profiles need further study.
4.2.6. Synthetic biomaterials
Synthetic biomaterials are usually polymers, or other organic chemical materials, which are approved by the Food and Drug Administration (FDA) as safe to use in humans. They can be synthesized with biological components, like protein or cells, to increase their therapeutic efficacy [121].
David et al. [122] used TheraCyte®, a porous polytetrafluoroethylene (PTFE) membrane that is impermeable to cells but allows for the diffusion of their soluble molecules. In this study, ovarian grafts were inserted into the TheraCyte® capsule and transplanted into mouse POI models to restore ovarian endocrine functions. After one week of incubation, they found that new blood vessels had formed around the TheraCyte® capsule, indicative of successful host integration. Primordial, primary, and antral follicles were observed in the retrieved grafts. Moreover, the TheraCyte® capsule restore ovarian endocrine function, which was confirmed by the marked decrease in FSH levels.
In another study, Chen et al. [123] designed triptolide-loaded nanoparticles that significantly increased serum AMH levels in POI mice. In addition, most mice appeared to be fertile after triptolide-loaded nanoparticle administration. Additionally, safety tests indicated that the therapy had low toxicity and high biocompatibility.
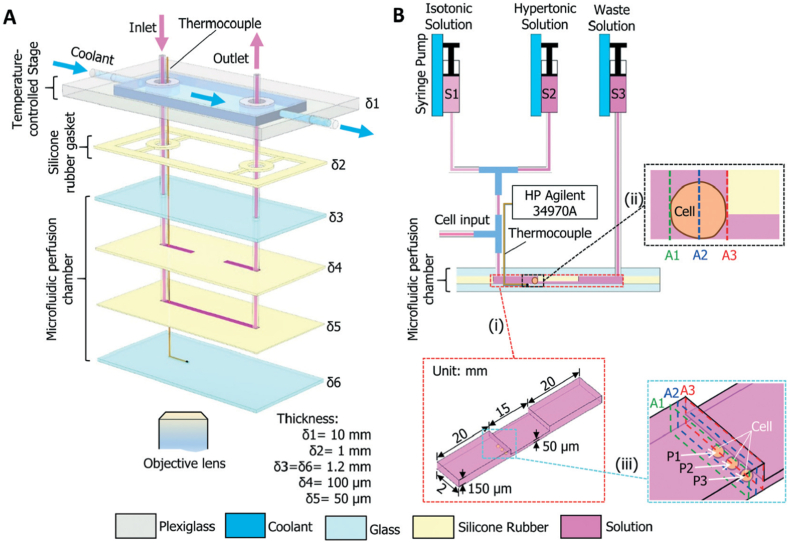
Zhao et al. [124] designed a novel sandwich structured microfluidic device that can characterize oocyte membrane transport properties and reduce measurement variability. The device consists of a temperature-controlled stage, and a microfluidic perfusion chamber (Fig. 12). The cost-effectiveness, ease of fabrication, long-term durability, and robustness of this approach makes the microfluidic perfusion system a powerful tool for studying oocyte membrane transport properties. The device has applications in fertility preservation and assisted reproduction.
Fig. 12.
A schematic illustration of the microfluidic perfusion system. (A) An expanded view showing the configuration of the microdevice with a microfluidic perfusion chamber and an integrated temperature-controlled stage. (B) A diagram showing the connections between the microdevice and three syringes and the microchannels. The arrows indicate the direction of fluid flow. The HP Agilent 34970A data acquisition system is used for monitoring and recording the temperature with the thermocouple. δ1–δ6, thickness; S1–S3, syringes; A1–A3, representative cross sections; P1– P3, typical cell locations. Note: drawing not to scale [124].
The utility of these synthetic biomaterials as carriers of ovarian follicles or oocytes is made by their ability to help improve sexual hormone secretion and fertility after transplantation in POI animal models. However, the number of studies involving synthetic biomaterials as carriers of MSC-derived secretomes or exosomes is limited. In addition, more detailed information about the toxicity, biosafety, and efficacy will be needed for the translational application of synthetic materials.
5. Conclusion and perspective
The therapeutic efficacy of MSCs has been demonstrated in multiple disease models. Although more insight is needed, the overarching mechanism of action seems to be the secretion of paracrine signaling molecules into the affected area. These signals can stimulate proliferation, angiogenesis, and tissue regeneration. These beneficial mechanisms have made MSCs a promising therapeutic candidate in the field of regenerative medicine. Ongoing preclinical and clinical trials employing MSCs and MSC-derivatives are studying their effects on diseases of the heart, lung, and other organs. Ultimately, the success of the MSC therapeutic model, as well as its applicability in POI, will be determined by its success in these ongoing clinical studies.
POI is an ovarian disfunction that leads to premature infertility. Because of the lack of intrinsic stem cells, endogenous reparation of the ovaries after the onset of POI is limited. Given the reported therapeutic potential of MSCs, the revitalization of ovarian functions via regenerative medicine is a sought-after treatment option in POI research. Stem cells are versatile players in regenerative medicine, and direct delivery of MSCs has been reported to cure POI in rodents. Despite long-standing issues with MSC retention and survival rates, the field's interest in MSCs continues, not just because the preclinical data shows promise, but also because of novel solutions that involve the use of biomaterials as cell carriers.
The field of biomaterials research is vast and ever-growing. A wide range of natural and synthetic biomaterials have been isolated and/or developed. Decellularized extracellular matrix (ECM), collagen, fibrin, and hyaluronic acid are natural biomaterials that are commonly used for MSC/exosome/secretome delivery for POI treatments. These natural materials are derived from animal tissues/organs, which make sure the biocompatiblity, biodegradation and safe-to-use of natural materials in vivo. However, there are also properties like stiffness, elasticity, and viscoelasticity which may not always be ideal for implantation. With improved material properties, therefore, synthetic polymers and aggregates, such as synthetic hydrogels, nanoparticles, and biofilms may optimize delivery efficacy and compatibility profiles. Despite of the advances, the potentials of cytotoxicity as a result of chemical leaching is causing massive concerns, and becoming the issue that limits the in practice use of synthetic biomaterials. Currently, only PLGA, PCL, etc. have been approved for biomedical use by the FDA. Nevertheless, the biosafety, biodegradation, and biocompatibility of novel materials are the most important regulatory considerations.
The other problem with stem cell therapy and regenerative medicine is the potential for tumorigenesis and immunogenicity. However, both of these risks can be overcome by using acellular treatments. These are cell-derived therapies that make use of the contents of the cells, without needing the cell itself. A promising acellular strategy is the use of exosomes and secretome, coupled with encapsulating or carrier biomaterials.
Taken together, the data hints at the possibility for a promising future for stem cell- and biomaterials-based POI therapy. Recent studies aimed at understanding their mechanisms of action are revealing novel targets for future treatments. As the field progresses, it should aim for clinical translations that bridge the gap between the promising scientific work being done, and the clinical applicability of these treatment modalities [126]. For POI patients, the main concern involves the invasiveness of the procedures to deploy the therapeutic product. Thanks to the recent development in the field of medical devices, many non- or minimally-invasive methods have been invented to delivery therapeutics to internal organs including the ovary. Those technologies can be applied to deliver stem cells and biomaterials to the ovary. Patients in other groups may also benefit from such therapies. For example, patients who receive pelvic surgeries can take advantage of the regenerative medicine strategies to aid the ovary tissue recovery process.
Author disclosure statement
The authors declare that no competing financial interests exist.
Acknowledgements
This work was supported by the Beijing Dongcheng Department of Science, Technology, and Information (BJ-2019-103 to Shaowei Wang).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Shaowei Wang, Email: w_sw999@163.com.
Ke Cheng, Email: ke_cheng@unc.edu.
References
- 1.European Society for Human R., Embryology Guideline Group on P.O.I., Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B., Cifkova R., de Muinck Keizer-Schrama S., Hogervorst E., Janse F., Liao L., Vlaisavljevic V., Zillikens C., Vermeulen N. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016;31(5):926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 2.Wu X., Cai H., Kallianpur A., Li H., Yang G., Gao J., Xiang Y.B., Ji B.T., Yu T., Zheng W., Shu X.O. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PloS One. 2014;9(3) doi: 10.1371/journal.pone.0089597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzen J.G., Forman J.L., Larsen E.C., Pinborg A., Johannsen T.H., Schmidt L., Friis-Hansen L., Nyboe Andersen A. Maternal menopause as a predictor of anti-Mullerian hormone level and antral follicle count in daughters during reproductive age. Hum. Reprod. 2013;28(1):247–255. doi: 10.1093/humrep/des356. [DOI] [PubMed] [Google Scholar]
- 4.Ossewaarde M.E., Bots M.L., Verbeek A.L., Peeters P.H., van der Graaf Y., Grobbee D.E., van der Schouw Y.T. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 5.Coulam C.B., Adamson S.C., Annegers J.F. Incidence of premature ovarian failure. Obstet. Gynecol. 1986;67(4):604–606. [PubMed] [Google Scholar]
- 6.Golezar S., Ramezani Tehrani F., Khazaei S., Ebadi A., Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric : the journal of the International Menopause Society. 2019:1–9. doi: 10.1080/13697137.2019.1574738. [DOI] [PubMed] [Google Scholar]
- 7.casper R.F. Up To Date; 2011. Clinical Manifestations and Diagnosis of Menopause. [Google Scholar]
- 8.Driscoll M.A., Davis M.C., Aiken L.S., Yeung E.W., Sterling E.W., Vanderhoof V., Calis K.A., Popat V., Covington S.N., Nelson L.M. Psychosocial vulnerability, resilience resources, and coping with infertility: a longitudinal model of adjustment to primary ovarian insufficiency. Ann. Behav. Med. 2016;50(2):272–284. doi: 10.1007/s12160-015-9750-z. [DOI] [PubMed] [Google Scholar]
- 9.Yen M. Samuel S.C., Sci D. W.B. Saunders, Harcourt Asia; 2001. Reproductive Endocrinology. [Google Scholar]
- 10.De Vos M., Devroey P., Fauser B.C. Primary ovarian insufficiency. Lancet. 2010;376(9744):911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 11.Nelson L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009;360(6):606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S., Sun M., Yu L., Wang Y., Yao Y., Wang D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell. Physiol. Biochem. 2018;50(6):2060–2070. doi: 10.1159/000495051. [DOI] [PubMed] [Google Scholar]
- 13.He L., Ling L., Wei T., Wang Y., Xiong Z. Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp. Biol. Med. 2017;242(7):683–691. doi: 10.1177/1535370217693323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikami Y., Kanemaru K., Okubo Y., Nakaune T., Suzuki J., Shibata K., Sugiyama H., Koyama R., Murayama T., Ito A., Yamazawa T., Ikegaya Y., Sakurai T., Saito N., Kakizawa S., Iino M. Nitric oxide-induced activation of the type 1 ryanodine receptor is critical for epileptic seizure-induced neuronal cell death. EBioMedicine. 2016;11:253–261. doi: 10.1016/j.ebiom.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang H., Na Y., Hong K., Lee S., Moon S., Cho M., Park M., Lee O.-H., Chang E.M., Lee D.R., Ko J.J., Lee W.S., Choi Y. Synergistic effect of melatonin and ghrelin in preventing cisplatin-induced ovarian damage via regulation of FOXO3a phosphorylation and binding to thep27Kip1promoter in primordial follicles. J. Pineal Res. 2017;63(3) doi: 10.1111/jpi.12432. [DOI] [PubMed] [Google Scholar]
- 16.Herraiz S., Pellicer N., Romeu M., Pellicer A. Treatment potential of bone marrow-derived stem cells in women with diminished ovarian reserves and premature ovarian failure. Curr. Opin. Obstet. Gynecol. 2019;31(3):156–162. doi: 10.1097/GCO.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 17.Sheikhansari G., Aghebati-Maleki L., Nouri M., Jadidi-Niaragh F., Yousefi M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed. Pharmacother. 2018;102:254–262. doi: 10.1016/j.biopha.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 18.He Y., Chen D., Yang L., Hou Q., Ma H., Xu X. The therapeutic potential of bone marrow mesenchymal stem cells in premature ovarian failure. Stem Cell Res. Ther. 2018;9(1):263. doi: 10.1186/s13287-018-1008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Liu Y., Li P., Zhu D. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J. Natl. Cancer Inst. 2018;110(3):304–315. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang J.N., Cores J., Huang K., Cui X.L., Luo L., Zhang J.Y., Li T.S., Qian L., Cheng K. Concise review: is cardiac cell therapy dead? Embarrassing trial outcomes and new directions for the future. Stem Cells Transl Med. 2018;7(4):354–359. doi: 10.1002/sctm.17-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huhtaniemi I., Hovatta O., La Marca A., Livera G., Monniaux D., Persani L., Heddar A., Jarzabek K., Laisk-Podar T., Salumets A., Tapanainen J.S., Veitia R.A., Visser J.A., Wieacker P., Wolczynski S., Misrahi M. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol. Metabol. 2018;29(6):400–419. doi: 10.1016/j.tem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Friedenstein A.J., Chailakhjan R.K., Lalykina K.S. The development of fibroblast colonies in monolayer cultures of Guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 23.Caplan A.I. Mesenchymal stem cells: the past, the present, the future. Cartilage. 2010;1(1):6–9. doi: 10.1177/1947603509354992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss M.L., Troyer D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang H., Huang K., Su T., Li Z., Hu S., Dinh P.U., Wrona E.A., Shao C., Qiao L., Vandergriff A.C., Hensley M.T., Cores J., Allen T., Zhang H., Zeng Q., Xing J., Freytes D.O., Shen D., Yu Z., Cheng K. Mesenchymal stem cell/red blood cell-inspired nanoparticle therapy in mice with carbon tetrachloride-induced acute liver failure. ACS Nano. 2018;12(7):6536–6544. doi: 10.1021/acsnano.8b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caplan A.I. Adult mesenchymal stem cells and women's health. Menopause. 2015;22(2):131–135. doi: 10.1097/GME.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caplan A.I. Adult mesenchymal stem cells: when, where, and how. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianco P., Robey P.G., Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Mendez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Dini G., Egeler R.M., Bacigalupo A., Fibbe W., Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 32.Wolf D., Wolf A.M. Mesenchymal stem cells as cellular immunosuppressants. Lancet. 2008;371(9624):1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 33.Stoklosowa S., Bahr J., Gregoraszczuk E. Some morphological and functional characteristics of cells of the porcine theca interna in tissue culture. Biol. Reprod. 1978;19(4):712–719. doi: 10.1095/biolreprod19.4.712. [DOI] [PubMed] [Google Scholar]
- 34.Yin N., Zhao W., Luo Q., Yuan W., Luan X., Zhang H. Restoring ovarian function with human placenta-derived mesenchymal stem cells in autoimmune-induced premature ovarian failure mice mediated by Treg cells and associated cytokines. Reprod. Sci. 2018;25(7):1073–1082. doi: 10.1177/1933719117732156. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Luo Q., Lu X., Yin N., Zhou D., Zhang L., Zhao W., Wang D., Du P., Hou Y., Zhang Y., Yuan W. Effects of hPMSCs on granulosa cell apoptosis and AMH expression and their role in the restoration of ovary function in premature ovarian failure mice. Stem Cell Res. Ther. 2018;9(1):20. doi: 10.1186/s13287-017-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Belli M., Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam. Horm. 2018;107:317–348. doi: 10.1016/bs.vh.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong Y., Liu T., Wang S., Chi H., Chen C., Zheng J. Cyclophosphamide promotes the proliferation inhibition of mouse ovarian granulosa cells and premature ovarian failure by activating the lncRNA-Meg3-p53-p66Shc pathway. Gene. 2017;596:1–8. doi: 10.1016/j.gene.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Conca Dioguardi C., Uslu B., Haynes M., Kurus M., Gul M., Miao D.Q., De Santis L., Ferrari M., Bellone S., Santin A., Giulivi C., Hoffman G., Usdin K., Johnson J. Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol. Hum. Reprod. 2016;22(6):384–396. doi: 10.1093/molehr/gaw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu X., He Y., Wang X., Peng D., Chen X., Li X., Wang Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017;8(1) doi: 10.1186/s13287-017-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S., Yu L., Sun M., Mu S., Wang C., Wang D., Yao Y. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. BioMed Res. Int. 2013;2013:690491. doi: 10.1155/2013/690491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan Z., Guo F., Yuan Q., Shao Y., Zhang Y., Wang H., Hao S., Du X. Endometrial mesenchymal stem cells isolated from menstrual blood repaired epirubicin-induced damage to human ovarian granulosa cells by inhibiting the expression of Gadd45b in cell cycle pathway. Stem Cell Res. Ther. 2019;10(1):4. doi: 10.1186/s13287-018-1101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang B., Qian C., Ding C., Meng Q., Zou Q., Li H. Fetal liver mesenchymal stem cells restore ovarian function in premature ovarian insufficiency by targeting MT1. Stem Cell Res. Ther. 2019;10(1) doi: 10.1186/s13287-019-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W., Zhang J., Xu B., He Y., Liu W., Li J., Zhang S., Lin X., Su D., Wu T., Li J. HucMSC-derived exosomes mitigate the age-related retardation of fertility in female mice. Mol. Ther. 2020;28(4):1200–1213. doi: 10.1016/j.ymthe.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu J., Warmflash A., Lutolf M.P. Stem-cell-based embryo models for fundamental research and translation. Nat. Mater. 2020 doi: 10.1038/s41563-020-00829-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed S., Shalaby S., Brakta S., Elam L., Elsharoud A., Al-Hendy A. Umbilical cord blood mesenchymal stem cells as an infertility treatment for chemotherapy induced premature ovarian insufficiency. Biomedicines. 2019;7(1):7. doi: 10.3390/biomedicines7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manshadi M.D., Navid S., Hoshino Y., Daneshi E., Noory P., Abbasi M. The effects of human menstrual blood stem cells-derived granulosa cells on ovarian follicle formation in a rat model of premature ovarian failure. Microsc. Res. Tech. 2019;82(6):635–642. doi: 10.1002/jemt.23120. [DOI] [PubMed] [Google Scholar]
- 47.Mohamed S.A., Shalaby S.M., Abdelaziz M., Brakta S., Hill W.D., Ismail N., Al-Hendy A. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod. Sci. 2018;25(1):51–63. doi: 10.1177/1933719117699705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li N., Liu L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 2018;44(8):1431–1438. doi: 10.1111/jog.13680. [DOI] [PubMed] [Google Scholar]
- 49.Delkhosh A., Delashoub M., Tehrani A.A., Bahrami A.M., Niazi V., Shoorei H., Banimohammad M., Kalarestaghi H., Shokoohi M., Agabalazadeh A., Mohaqiq M. Upregulation of FSHR and PCNA by administration of coenzyme Q10 on cyclophosphamide-induced premature ovarian failure in a mouse model. J. Biochem. Mol. Toxicol. 2019;33(11) doi: 10.1002/jbt.22398. [DOI] [PubMed] [Google Scholar]
- 50.Chen X., Wang Q., Li X., Wang Q., Xie J., Fu X. Heat shock pretreatment of mesenchymal stem cells for inhibiting the apoptosis of ovarian granulosa cells enhanced the repair effect on chemotherapy-induced premature ovarian failure. Stem Cell Res. Ther. 2018;9(1) doi: 10.1186/s13287-018-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan R., He Y., Zhang S., Pu D., Wu J. Effect of transcutaneous electrical acupoint stimulation on protecting against radiotherapy- induced ovarian damage in mice. J. Ovarian Res. 2019;12(1):65. doi: 10.1186/s13048-019-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao W., Liang J.X., Ma C., Dong J.Y., Yan Q. The protective effect of N-acetylcysteine on ionizing radiation induced ovarian failure and loss of ovarian reserve in female mouse. BioMed Res. Int. 2017;2017:4176170. doi: 10.1155/2017/4176170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rostami Dovom M., Noroozzadeh M., Mosaffa N., Zadeh-Vakili A., Piryaei A., Ramezani Tehrani F. Induced premature ovarian insufficiency by using D galactose and its effects on reproductive profiles in small laboratory animals: a systematic review. J. Ovarian Res. 2019;12(1):96. doi: 10.1186/s13048-019-0565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajkovic A., Pangas S.A., Ballow D., Suzumori N., Matzuk M.M. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305(5687):1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y., Qin Y., Qin Y., Xu B., Guo T., Ke H., Chen M., Zhang L., Han F., Li Y., Chen M., Behrens A., Wang Y., Xu Z., Chen Z.J., Gao F. Wdr62 is involved in female meiotic initiation via activating JNK signaling and associated with POI in humans. PLoS Genet. 2018;14(8) doi: 10.1371/journal.pgen.1007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franca M.M., Han X., Funari M.F.A., Lerario A.M., Nishi M.Y., Fontenele E.G.P., Domenice S., Jorge A.A.L., Garcia-Galiano D., Elias C.F., Mendonca B.B. Exome sequencing reveals the POLR3H gene as a novel cause of primary ovarian insufficiency. J. Clin. Endocrinol. Metab. 2019;104(7):2827–2841. doi: 10.1210/jc.2018-02485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herraiz S., Buigues A., Diaz-Garcia C., Romeu M., Martinez S., Gomez-Segui I., Simon C., Hsueh A.J., Pellicer A. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil. Steril. 2018;109(5):908–918. doi: 10.1016/j.fertnstert.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z., Wang Y., Yang T., Li J., Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017;8(1):11. doi: 10.1186/s13287-016-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu S.F., Hu H.B., Xu H.Y., Fu X.F., Peng D.X., Su W.Y., He Y.L. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J. Cell Mol. Med. 2015;19(9):2108–2117. doi: 10.1111/jcmm.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su J., Ding L., Cheng J., Yang J., Li X.a., Yan G., Sun H., Dai J., Hu Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum. Reprod. 2016;31(5):1075–1086. doi: 10.1093/humrep/dew041. [DOI] [PubMed] [Google Scholar]
- 61.Lai D., Wang F., Yao X., Zhang Q., Wu X., Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J. Transl. Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song D., Zhong Y., Qian C., Zou Q., Ou J., Shi Y., Gao L., Wang G., Liu Z., Li H., Ding H., Wu H., Wang F., Wang J., Li H. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. BioMed Res. Int. 2016;2016:2517514. doi: 10.1155/2016/2517514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu T., Huang Y., Zhang J., Qin W., Chi H., Chen J., Yu Z., Chen C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cell. Dev. 2014;23(13):1548–1557. doi: 10.1089/scd.2013.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun M., Wang S., Li Y., Yu L., Gu F., Wang C., Yao Y. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res. Ther. 2013;4(4):80. doi: 10.1186/scrt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su J., Ding L., Cheng J., Yang J., Li X., Yan G., Sun H., Dai J., Hu Y. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum. Reprod. 2016;31(5):1075–1086. doi: 10.1093/humrep/dew041. [DOI] [PubMed] [Google Scholar]
- 66.Lu C., Lin L., Tan H., Wu H., Sherman S.L., Gao F., Jin P., Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum. Mol. Genet. 2012;21(23):5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin N., Wang Y., Lu X., Liu R., Zhang L., Zhao W., Yuan W., Luo Q., Wu H., Luan X., Zhang H. hPMSC transplantation restoring ovarian function in premature ovarian failure mice is associated with change of Th17/Tc17 and Th17/Treg cell ratios through the PI3K/Akt signal pathway. Stem Cell Res. Ther. 2018;9(1):37. doi: 10.1186/s13287-018-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Lv C., Yang S., Chen X., Zhu X., Lin W., Wang L., Huang Z., Wang M., Tu G. MicroRNA-21 promotes bone mesenchymal stem cells migration in vitro by activating PI3K/Akt/MMPs pathway. J. Clin. Neurosci. : official journal of the Neurosurgical Society of Australasia. 2017;46:156–162. doi: 10.1016/j.jocn.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 69.Yin N., Wu C., Qiu J., Zhang Y., Bo L., Xu Y., Shi M., Zhu S., Yang G., Mao C. Protective properties of heme oxygenase-1 expressed in umbilical cord mesenchymal stem cells help restore the ovarian function of premature ovarian failure mice through activating the JNK/Bcl-2 signal pathway-regulated autophagy and upregulating the circulating of CD8+CD28− T cells. Stem Cell Res. Ther. 2020;11(1) doi: 10.1186/s13287-019-1537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H., Zhao W., Wang L., Luo Q., Yin N., Lu X., Hou Y., Cui J., Zhang H. Human placenta‐derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol. Int. 2019;43(8):899–909. doi: 10.1002/cbin.11165. [DOI] [PubMed] [Google Scholar]
- 71.Huang B., Lu J., Ding C., Zou Q., Wang W., Li H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018;9(1) doi: 10.1186/s13287-018-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui L., Bao H., Liu Z., Man X., Liu H., Hou Y., Luo Q., Wang S., Fu Q., Zhang H. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-beta1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res. Ther. 2020;11(1):386. doi: 10.1186/s13287-020-01904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu X., Bao H., Cui L., Zhu W., Zhang L., Xu Z., Man X., Chu Y., Fu Q., Zhang H. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res. Ther. 2020;11(1):268. doi: 10.1186/s13287-020-01784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao G., Ze Y., Li B., Zhao X., Zhang T., Sheng L., Hu R., Gui S., Sang X., Sun Q., Cheng J., Cheng Z., Wang L., Tang M., Hong F. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.H., Jeon Y.J., Rah H., Lee B.E., Choi D.H., Lee W.S., Kim N.K. Tumor necrosis factor-alpha promoter polymorphisms are associated with idiopathic primary ovarian insufficiency in Korean women. Fertil. Steril. 2012;98(5):1260. doi: 10.1016/j.fertnstert.2012.07.1111. 5 e1-2. [DOI] [PubMed] [Google Scholar]
- 76.Wang S., Zheng Y., Li J., Yu Y., Zhang W., Song M., Liu Z., Min Z., Hu H., Jing Y., He X., Sun L., Ma L., Esteban C.R., Chan P., Qiao J., Zhou Q., Izpisua Belmonte J.C., Qu J., Tang F., Liu G.H. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180(3):585–600. doi: 10.1016/j.cell.2020.01.009. e19. [DOI] [PubMed] [Google Scholar]
- 77.Fu X., He Y., Wang X., Peng D., Chen X., Li X., Wang Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017;8(1):187. doi: 10.1186/s13287-017-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abd-Allah S.H., Shalaby S.M., Pasha H.F., El-Shal A.S., Raafat N., Shabrawy S.M., Awad H.A., Amer M.G., Gharib M.A., El Gendy E.A., Raslan A.A., El-Kelawy H.M. Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy. 2013;15(1):64–75. doi: 10.1016/j.jcyt.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Xia X., Yin T., Yan J., Yan L., Jin C., Lu C., Wang T., Zhu X., Zhi X., Wang J., Tian L., Liu J., Li R., Qiao J. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant. 2015;24(10):1999–2010. doi: 10.3727/096368914X685267. [DOI] [PubMed] [Google Scholar]
- 80.Fu X., He Y., Xie C., Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4):353–363. doi: 10.1080/14653240802035926. [DOI] [PubMed] [Google Scholar]
- 81.Shin S.Y., Lee J.Y., Lee E., Choi J., Yoon B.K., Bae D., Choi D. Protective effect of vascular endothelial growth factor (VEGF) in frozen-thawed granulosa cells is mediated by inhibition of apoptosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;125(2):233–238. doi: 10.1016/j.ejogrb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 82.Kinnaird T., Stabile E., Burnett M.S., Lee C.W., Barr S., Fuchs S., Epstein S.E. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ. Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 83.Beilmann M., Birk G., Lenter M.C. Human primary co-culture angiogenesis assay reveals additive stimulation and different angiogenic properties of VEGF and HGF. Cytokine. 2004;26(4):178–185. doi: 10.1016/j.cyto.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Huang Q., Liu B., Jiang R., Liao S., Wei Z., Bi Y., Liu X., Deng R., Jin Y., Tan Y., Yang Y., Qin A. G-CSF-mobilized peripheral blood mononuclear cells combined with platelet-rich plasma accelerate restoration of ovarian function in cyclophosphamide-induced POI ratsdagger. Biol. Reprod. 2019;101(1):91–101. doi: 10.1093/biolre/ioz077. [DOI] [PubMed] [Google Scholar]
- 85.Liang C., Jiang E., Yao J., Wang M., Chen S., Zhou Z., Zhai W., Ma Q., Feng S., Han M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology. 2018;23(1):44–49. doi: 10.1080/10245332.2017.1333245. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Q., Xu M., Yao X., Li T., Wang Q., Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res. Ther. 2015;6(1):152. doi: 10.1186/s13287-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiao L., Hu S., Liu S., Zhang H., Ma H., Huang K., Li Z., Su T., Vandergriff A., Tang J., Allen T., Dinh P.U., Cores J., Yin Q., Li Y., Cheng K. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Invest. 2019;129(6):2237–2250. doi: 10.1172/JCI123135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khanmohammadi N., Sameni H.R., Mohammadi M., Pakdel A., Mirmohammadkhani M., Parsaie H., Zarbakhsh S. Effect of transplantation of bone marrow stromal cell- conditioned medium on ovarian function, morphology and cell death in cyclophosphamide-treated rats. Cell J. 2018;20(1):10–18. doi: 10.22074/cellj.2018.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J., Mao Q., He J., She H., Zhang Z., Yin C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res. Ther. 2017;8(1):55. doi: 10.1186/s13287-017-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun L., Li D., Song K., Wei J., Yao S., Li Z., Su X., Ju X., Chao L., Deng X., Kong B., Li L. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci. Rep. 2017;7(1):2552. doi: 10.1038/s41598-017-02786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao G.-Y., Cheng C.-C., Chiang Y.-S., Cheng W.T.-K., Liu I.H., Wu S.-C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016;6(1) doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen D., Cheng K., Marban E. Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction. J. Cell Mol. Med. 2012;16(9):2112–2116. doi: 10.1111/j.1582-4934.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Azhar Z., Haque N., Ali S., Mozafari M., Sefat F. 2019. Bioengineered cardiac patch scaffolds; pp. 705–728. (Handbook of Tissue Engineering Scaffolds). [Google Scholar]
- 94.Nikolova M.P., Chavali M.S. Recent advances in biomaterials for 3D scaffolds: a review. Bioact Mater. 2019;4:271–292. doi: 10.1016/j.bioactmat.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu F., Hu S., Wang S., Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen Biomater. 2019;6(3):141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mano J.F., Silva G.A., Azevedo H.S., Malafaya P.B., Sousa R.A., Silva S.S., Boesel L.F., Oliveira J.M., Santos T.C., Marques A.P., Neves N.M., Reis R.L. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface. 2007;4(17):999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma D., Ferguson M., Kamp T.J., Zhao F. Constructing biomimetic cardiac tissues: a review of scaffold materials for engineering cardiac patches. Emergent Materials. 2019;2(2):181–191. doi: 10.1007/s42247-019-00046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghahremani-Nasab M., Ghanbari E., Jahanbani Y., Mehdizadeh A., Yousefi M. Premature ovarian failure and tissue engineering. J. Cell. Physiol. 2020;235(5):4217–4226. doi: 10.1002/jcp.29376. [DOI] [PubMed] [Google Scholar]
- 99.Ermis M., Antmen E., Hasirci V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: a review from the tissue engineering perspective. Bioact Mater. 2018;3(3):355–369. doi: 10.1016/j.bioactmat.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corrigendum to: cardiovascular disease in Europe: epidemiological update 2016. Eur. Heart J. 2019;40(2):189. doi: 10.1093/eurheartj/ehy342. [DOI] [PubMed] [Google Scholar]
- 101.Huang K., Ozpinar E.W., Su T., Tang J., Shen D., Qiao L., Hu S., Li Z., Liang H., Mathews K., Scharf V., Freytes D.O., Cheng K. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020;12(538) doi: 10.1126/scitranslmed.aat9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smitz J., Dolmans M.M., Donnez J., Fortune J.E., Hovatta O., Jewgenow K., Picton H.M., Plancha C., Shea L.D., Stouffer R.L., Telfer E.E., Woodruff T.K., Zelinski M.B. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum. Reprod. Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu W.Y., Lin S.G., Zhuo R.Y., Xie Y.Y., Pan W., Lin X.F., Shen F.X. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng. C Methods. 2017;23(2):61–71. doi: 10.1089/ten.tec.2016.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ersoy E., Ozler S., Oztas E., Ersoy A.O., Ergin M., Tokmak A., Yilmaz N. Comparison of serum A disintegrin and metalloproteinase with thrombospondin motifs-19 levels in different fertility situations: could it Be a serum marker of ovarian function and oocyte pool? Gynecol. Obstet. Invest. 2019;84(1):6–11. doi: 10.1159/000490665. [DOI] [PubMed] [Google Scholar]
- 105.Shekhter A.B., Fayzullin A.L., Vukolova M.N., Rudenko T.G., Osipycheva V.D., Litvitsky P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019;26(3):506–516. doi: 10.2174/0929867325666171205170339. [DOI] [PubMed] [Google Scholar]
- 106.Sorushanova A., Delgado L.M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A.M., Bayon Y., Pandit A., Raghunath M., Zeugolis D.I. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater. 2019;31(1) doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 107.He X. Microfluidic encapsulation of ovarian follicles for 3D culture. Ann. Biomed. Eng. 2017;45(7):1676–1684. doi: 10.1007/s10439-017-1823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He X. Microscale biomaterials with bioinspired complexity of early embryo development and in the ovary for tissue engineering and regenerative medicine. ACS Biomater. Sci. Eng. 2017;3(11):2692–2701. doi: 10.1021/acsbiomaterials.6b00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y., Lei L., Wang S., Sheng X., Yan G., Xu L., Liu J., Liu M., Zhen X., Ding L., Sun H. Transplantation of umbilical cord-derived mesenchymal stem cells on a collagen scaffold improves ovarian function in a premature ovarian failure model of mice. In Vitro Cell. Dev. Biol. Anim. 2019;55(4):302–311. doi: 10.1007/s11626-019-00337-4. [DOI] [PubMed] [Google Scholar]
- 110.Ding L., Yan G., Wang B., Xu L., Gu Y., Ru T., Cui X., Lei L., Liu J., Sheng X., Wang B., Zhang C., Yang Y., Jiang R., Zhou J., Kong N., Lu F., Zhou H., Zhao Y., Chen B., Hu Y., Dai J., Sun H. Transplantation of UC-MSCs on collagen scaffold activates follicles in dormant ovaries of POF patients with long history of infertility. Sci. China Life Sci. 2018;61(12):1554–1565. doi: 10.1007/s11427-017-9272-2. [DOI] [PubMed] [Google Scholar]
- 111.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23(12):H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim H., Shin M., Han S., Kwon W., Hahn S.K. Hyaluronic acid derivatives for translational medicines. Biomacromolecules. 2019;20(8):2889–2903. doi: 10.1021/acs.biomac.9b00564. [DOI] [PubMed] [Google Scholar]
- 113.Zhao G., Zhou X., Fang T., Hou Y., Hu Y. Hyaluronic acid promotes the expression of progesterone receptor membrane component 1 via epigenetic silencing of miR-139-5p in human and rat granulosa cells. Biol. Reprod. 2014;91(5):116. doi: 10.1095/biolreprod.114.120295. [DOI] [PubMed] [Google Scholar]
- 114.Zhao G., Yan G., Cheng J., Zhou X., Fang T., Sun H., Hou Y., Hu Y. Hyaluronic acid prevents immunosuppressive drug-induced ovarian damage via up-regulating PGRMC1 expression. Sci. Rep. 2015;5:7647. doi: 10.1038/srep07647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Augst A.D., Kong H.J., Mooney D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006;6(8):623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 116.Tonnesen H.H., Karlsen J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002;28(6):621–630. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 117.Kniazeva E., Hardy A.N., Boukaidi S.A., Woodruff T.K., Jeruss J.S., Shea L.D. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep. 2015;5:17709. doi: 10.1038/srep17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sittadjody S., Saul J.M., McQuilling J.P., Joo S., Register T.C., Yoo J.J., Atala A., Opara E.C. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat. Commun. 2017;8(1):1858. doi: 10.1038/s41467-017-01851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park C.H., Woo K.M. Fibrin-based biomaterial applications in tissue engineering and regenerative medicine. Adv. Exp. Med. Biol. 2018;1064:253–261. doi: 10.1007/978-981-13-0445-3_16. [DOI] [PubMed] [Google Scholar]
- 120.Shikanov A., Zhang Z., Xu M., Smith R.M., Rajan A., Woodruff T.K., Shea L.D. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. 2011;17(23–24):3095–3104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng K., Kisaalita W.S. Exploring cellular adhesion and differentiation in a micro-/nano-hybrid polymer scaffold. Biotechnol. Prog. 2010;26(3):838–846. doi: 10.1002/btpr.391. [DOI] [PubMed] [Google Scholar]
- 122.David A., Day J.R., Cichon A.L., Lefferts A., Cascalho M., Shikanov A. Restoring ovarian endocrine function with encapsulated ovarian allograft in immune competent mice. Ann. Biomed. Eng. 2017;45(7):1685–1696. doi: 10.1007/s10439-016-1780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen X.Y., Chen W.L., Ma M., Gu C., Xiao X.R., Li B. The potential of follicle-stimulating hormone peptide-modified triptolide-loaded nanoparticles to induce a mouse model of premature ovarian insufficiency. Int. J. Nanomed. 2015;10:2765–2774. doi: 10.2147/IJN.S72593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao G., Zhang Z., Zhang Y., Chen Z., Niu D., Cao Y., He X. A microfluidic perfusion approach for on-chip characterization of the transport properties of human oocytes. Lab Chip. 2017;17(7):1297–1305. doi: 10.1039/c6lc01532h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Green L.J., Zhou H., Padmanabhan V., Shikanov A. Adipose-derived stem cells promote survival, growth, and maturation of early-stage murine follicles. Stem Cell Res. Ther. 2019;10(1):102. doi: 10.1186/s13287-019-1199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Junlang, Hu Shiqi, Cheng Ke. Engineering better stem cell therapies for treating heart diseases. Ann. Transl. Med. 2020;8(8):569–580. doi: 10.21037/atm.2020.03.44. [DOI] [PMC free article] [PubMed] [Google Scholar]