Abstract
The COVID-19, caused by a novel coronavirus, was declared as a global pandemic by WHO more than five months ago, and we are still experiencing a state of global emergency. More than 74.30 million confirmed cases of the COVID-19 have been reported globally so far, with an average fatality rate of almost 3.0%. Seven different types of coronaviruses had been detected from humans; three of them have resulted in severe outbreaks, i.e., MERS-CoV, SARS-CoV, and SARS-CoV-2. Phylogenetic analysis of the genomes suggests that the possible occurrence of recombination between SARS-like-CoVs from pangolin and bat might have led to the origin of SARS-CoV-2 and the COVID-19 outbreak.
Coronaviruses are positive-sense, single-stranded RNA viruses and harbour a genome (30 kb) consisting of two terminal untranslated regions and twelve putative functional open reading frames (ORFs), encoding for non-structural and structural proteins. There are sixteen putative non-structural proteins, including proteases, RNA-dependent RNA polymerase, helicase, other proteins involved in the transcription and replication of SARS-CoV-2, and four structural proteins, including spike protein (S), envelope (E), membrane (M), and nucleocapsid (N). SARS-CoV-2 infection, with a heavy viral load in the body, destroys the human lungs through cytokine storm, especially in elderly persons and people with immunosuppressed disorders. A number of drugs have been repurposed and employed, but still, no specific antiviral medicine has been approved by the FDA to treat this disease. This review provides a current status of the COVID-19, epidemiology, an overview of phylogeny, mode of action, diagnosis, and possible treatment methods and vaccines.
1. Introduction
Coronaviruses (CoVs) belong to a large group of enveloped, single-stranded, positive-sense RNA viruses having the capability of infecting a wide variety of animals, including humans, birds, rodents, carnivores, chiropters and other mammals [1], [2]. Though they have been known for many years and have been considered as one of the viral sources responsible for respiratory diseases, they caught the attention of the whole world in December 2019, when an epidemic episode of cases with respiratory tract infections was reported in Wuhan, the largest metropolitan area in the province of Hubei, China. The outbreak was first treated as a complication of pneumonia with unknown etiology, but then the Centre for Disease Control in China declared that the respiratory infection was caused by a novel CoV named as 2019-nCoV, at that time [3], [4], [5], [6]. Later, the virus spread so enormously and rapidly that the WHO (World Health Organization) declared a global emergency amid this pandemic and called it coronavirus disease-2019 (COVID-19) while this novel 2019-nCoV was renamed as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). When the clinical spectrum of COVID-2019 was observed, it was noticed that few patients were asymptomatic, and some patients have mild to severe symptoms like severe respiratory discomfortness, fever, cough and flu [7], [8], [9]. During the past twelve months, COVID-19 has spread worldwide hitting some countries with extreme cruelty including the USA, India, Brazil, Russian Federation, France, the United Kingdom, Italy, Spain, Argentina, Colombia, Germany, Mexico, Poland, Iran, Turkey, each with more than 1 million confirmed COVID-19 cases (https://covid19.who.int/ accessed on December 19, 2020). Death toll has been extremely high in some countries including the USA, Brazil, India, Mexico, Italy, the UK, France, Iran, Russian Federation and Spain, each reporting greater than 50,000 COVID-19 related deaths as of December 19, 2020. According to WHO, 216 countries and territories around the world have reported more than 74.30 million confirmed COVID-19 cases with a death toll of above 1.67 million (https://covid19.who.int/ accessed on December 19, 2020).
Without any proper treatment and vaccine for COVID-19, we are currently experiencing a worldwide emergency affecting all societies, and it has sent billions of people into lockdown. Around the world, desperate efforts are underway to curtail this pandemic while it has resulted in the collapsing of health systems and has triggered lasting geopolitical and economic changes. To date, no approved medical treatment is available, that makes social distancing only best possible solution to stop the spread of the virus [10]. It is thought that future outbreaks of CoVs are unavoidable because of changes in the climate and ecology and increased interaction of humans with animals. Therefore, there is a need to develop effective therapeutics and vaccines against CoVs [11].
In this review, we briefly highlight the history, phylogeny, genomics, epidemiology, mode of action, disease symptoms, diagnosis, and possible treatment methods of COVID-19 and the research progress in the development of vaccines against SARS-Cov-2.
2. History of CoV-related diseases in humans
Human coronaviruses (HCoVs) were first reported in the mid-1960s when two species were isolated from persons with the common cold: HCoV-229E [12] and HCoV-OC43 [13]. Since then, seven different types of CoVs had been detected from humans, three of them happened to be highly pathogenic, and all suggested to be originated from bats: the Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), and SARS-CoV-2 [14].
First time, CoV wreaked global havoc in 2002 when SARS-CoV caused a severe acute respiratory syndrome and emerged as highly pandemic disease. SARS-CoV was thought to be an animal virus with the genetic ability to cross the species barrier that spreads to humans through an unknown intermediate host(s) [4]. It first appeared as a human pathogen in the Guangdong province of southern China in 2002. Later, it spread to 26 countries and resulted in more than 8000 cases and 774 deaths in 2003 (http://www.who.int/csr/sars/country/table2004_04_21/en/). World Health Organization declared the end of this outbreak in July 2003.
Another respiratory syndrome outbreak similar to that of SARS-CoV emerged in June 2012 in Saudi Arabia and was named as MERS-CoV [15]. MERS-CoV outbreak infected 2494 individuals exclusively travelling through the Middle East and caused 858 deaths [16]. This virus originated from bats and possibly camels as its intermediate host, got passed genetic recombinations across different species to infect human beings [14].
A few months ago, a novel CoV emerged and caused a serious disaster across the whole world. During the last two months of 2019, several cases of ‘viral pneumonia’ in Wuhan, People’s Republic of China, were reported [17], [18]. The cause of this infectious disease was identified as a natural virus of an animal origin with spillover infection potential [19]. It was traced that the geographical source of this virus was Huanan South China Seafood Market, but the actual animal source of this CoV was not known. It is now thought that this virus came from bats as their primary hosts, then it passed through one or multiple intermediate hosts, possibly including pangolins, to infect human beings [20]. International Committee on Taxonomy of Viruses (ICTV) announced SARS-CoV-2 as the name of the new virus on February 11, 2020, because of the genetic resemblance of the virus to the CoV responsible for the outbreak of 2003. Following guiding principles previously developed with the World Organization for Animal Health (OIE) and the Food and Agriculture Organization (FAO) of the United Nations, WHO named the disease “COVID-19” and announced it as a global pandemic on March 11, 2020.
3. Epidemiology
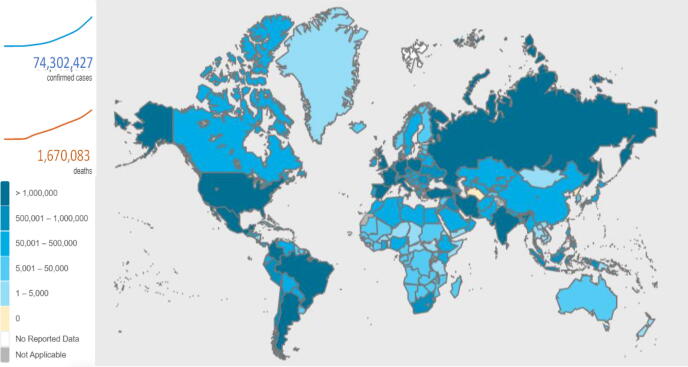
Since the first confirmed diagnosis of SARS-CoV-2 in China, more than 74.30 million people have been affected, from which more than 1.67 million lives have been claimed (https://covid19.who.int/, assessed on December 19, 2020). Although more than 52 million people have defeated COVID-19 and recovered from the disease, yet the battle between SARS-CoV-2 and humans is continued, and still, no specific therapeutics are available. The United States of America (USA) shares 22.7% of total infection cases, followed by India and Brazil, sharing 13.5% and 9.6% of cases, respectively (https://covid19.who.int/, assessed on December 19, 2020) (Fig. 1). Although a decrease in death rate is observed (September 10, 3.22; July 20, 6.65%; April 10, 22.36% and Feb 2, 41.80%), there is no substantial reduction in active COVID-19 cases (>700,000daily cases on December 19, 2020). The cumulative incidences for COVID-19 vary by a multitude of factors, including comorbidities, age, gender, health and living conditions [21], [22]. The disease severity was found to increase in diabetes, cardiovascular, lung, kidney, and renal diseases [23]. Upon infection, one in five persons, with developed comorbidities, is at increased risk of severe COVID-19 infection [24]. Case studies from China show that COVID-19 is more severe in older adults aged 50–60 years [25], while it became more fatal in people above 70 years old regardless of any chronic disease complications. In a gender-based meta-analysis study of European countries, it is observed that COVID-19 was significantly fatal in men compared to women [26].
Fig. 1.
Total number of confirmed cases and deaths due to Coronavirus disease-2019 (COVID-19). Adapted from COVID-19 dashboard by WHO (https://covid19.who.int/) accessed on December 19, 2020.
In the USA, the situation is still aggravating, where COVID-19 death toll is over 300,000 and the rate is still rising as 95 deaths per 100,000 since January 2020, across the country (https://www.cdc.gov/coronavirus/2019-ncov, accessed December 20, 2020). Amongst the countries reporting at least 50,000 COVID-19 cases, Singapore has the lowest COVID-19 fatality count with just 27 deaths with more than 57,000 persons who tested positive for COVID-19, with a death rate of below 0.05% compared to the global average of 3%. Singapore’s COVID-19 pandemic response that includes, mass testing, contact tracing of COVID-19 positive patient, rapid response public health preparedness clinics across the country, public awareness and countrywide lockdown [27] can be adapted as a successful model framework for other countries. In case no viable vaccine is available for low income countries, Africa, South Asia and South America can become unfortunate regions severely affected by SARS-CoV-2. A recent estimate put 23 million African population at the risk of severe COVID-19, whereas the current infection rate is exponentially increasing by 0.22 per day [28], [29]. Overloading poorly established health systems in underdeveloped countries may lead to numerous causalities.
4. Taxonomy and phylogeny of SARS-CoV-2
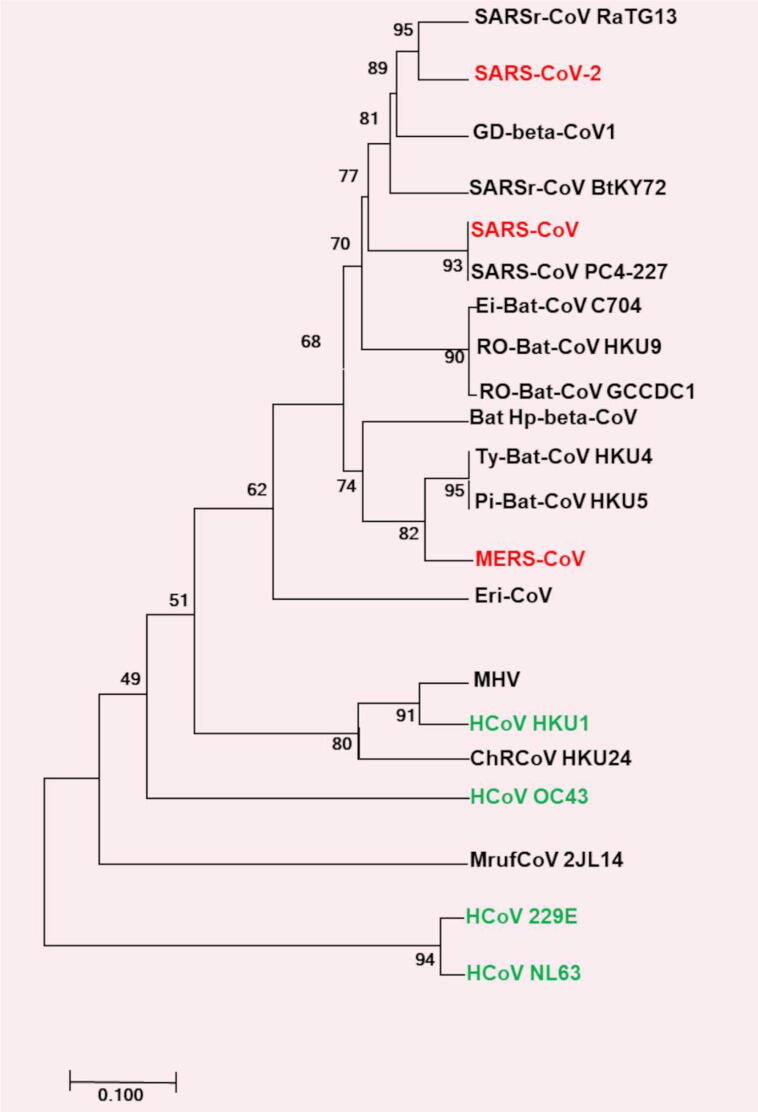
CoVs are positive-sense, single-stranded RNA viruses belonging to the order Nidovirales, suborder Cornidovirineae, family Coronaviridae and subfamily Orthocoronavirinae [16], [30]. The subfamily Orthocoronavirinae is further divided into Alpha-, Beta-, Gamma- and Delta- CoVs [31]. Alpha- and Beta- CoVs are pathogenic to mammals, including human beings, bats, pigs, mice, and cats. Gamma- and Delta- CoVs are usually pathogenic to birds but rarely infectious to mammals [32]. Phylogenetic analysis of SARS-CoV-2, SARS-CoV, and MERS-CoV suggests that it is more closely related to bat-CoVs of the Sarbecovirus subgenus isolated from bats (Fig. 2). A SARS related (SARSr) bat-CoV strain named SARSr-CoV-RaTG13 detected in an Intermediate Horseshoe bat (Rhinolophus affinis) [33], [34], was found very similar to SARS-CoV-2. The comparison of genome sequences revealed that SARSr-CoV-RaTG13 and SARS-CoV-2 sequences shared a similarity of more than 96% over a large part of the genome. However, the genomic region spanning the 3′-end of ORF1a, ORF1b and almost half of the spike (S) protein region of SARS-CoV-2 is divergent to SARSr-CoV-RaTG13 [35] but more closely related to pangolin CoV [36]. Considering that bats were in hibernation when the outbreak occurred [37], and the phylogenetic resemblance of Pangolin CoV strain to SARS-CoV-2, suggest that the virus is more likely to have been transmitted via other species. This also suggests that the possible occurrence of recombination between SARS-like-CoVs from pangolin and bats might have led to the origin of SARS-CoV-2 [36] and the COVID-19 outbreak. Dorp et al., (2020) analyzed the emergence of genomic diversity over time and reported that all CoV sequences share a common ancestor towards the end of 2019, supporting this as the period when SARS-CoV-2 jumped into its human host. They further identified several recurrent mutations producing non-synonymous changes in the virus at the protein level, suggesting possible ongoing adaptation of SARS-CoV-2 to the human host [38]. Various sequencing projects and phylogenetic studies involving SARS-CoV-2 genomes from COVID-19 patients during this pandemic have revealed that how fast the virus is mutating and adapting to its novel human host, providing information to direct drug and vaccine design [39], [40], [41].
Fig. 2.
Phylogenetic tree of representative species of SARS-CoV-2, SARS-CoV, and MERS-CoV. Red text highlights zoonotic viruses with pathogenicity in humans and green text highlights common respiratory viruses that circulate in humans. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) was shown next to the branches. Figure adapted from Gorbalenya et al., [30]. The tree was drawn based on the sequence information of following species: Severe acute respiratory syndrome related Bat Hp-betacoronavirus Zhejiang2013 (SARSr-CoV Ratg13), Rousettus bat coronavirus GCCDC1 (RO-Bat-CoV GCCDC1), Rousettus bat coronavirus HKU9 (RO-Bat-CoV HKU9), Eidolon bat coronavirus C704 (Ei-Bat-CoV C704), Pipistrellus bat coronavirus HKU5 (Pi-Bat-CoV HKU5), Tylonycteris bat coronavirus HKU4 (Ty-Bat-CoV HKU4), Middle East respiratory syndrome-related coronavirus (MERS-CoV), Hedgehog coronavirus OC43 (HCoV OC43), Murine coronavirus (MHV), Human coronavirus HKU1 (HCoV HKU1), China Rattus coronavirus HKU24 (ChRCoV HKU24), Pangolin Beta-coronavirus (GD-beta-CoV1), Bat Betacoronavirus 1 (Bat Hp-beta-CoV1), Myodes coronavirus 2JL14 (MrufCoV 2JL14), Human coronavirus NL63 (HCoV NL63), Human coronavirus 229E (HCoV 229E). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Genomic features and life cycle of SARS-CoV-2
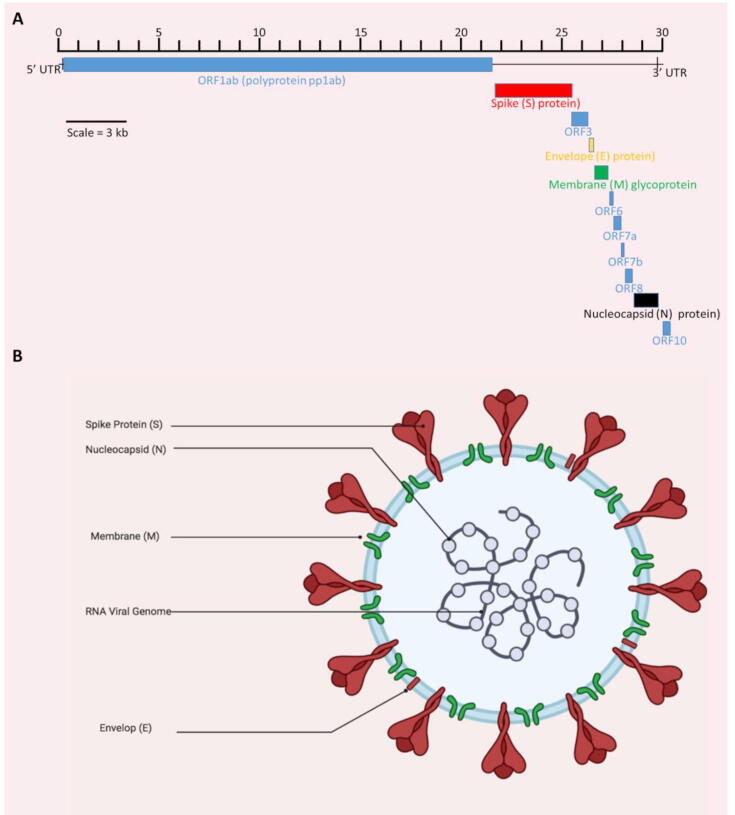
Several genome sequences of SARS-CoV-2 retrieved from the COVID-19 patients have been reported by researchers from various countries. According to the NCBI database, there are more than 28,000 (full length) SARS-CoV-2 genome sequences from human hosts and more than 113,000 Sequence Read Archive (SRA) high throughput sequence submissions through multiple cloud providers and NCBI servers (December 20, 2020). The single-stranded positive-sense RNA genome of SARS-CoV-2 is around ~ 30 kb that starts with a 5′‐cap structure and ends with a 3′‐poly‐A tail. Chan et al. (2020) [42] reported detailed genomic characterization of SARS-CoV-2, which consists of two terminal untranslated regions (5′- and 3′- UTRs) and twelve putative functional open reading frames (ORFs) (Fig. 3A). ORFs 1a and 1b, spanning over two-thirds of the genome, encode the large replicase polyproteins (pp1a and pp1ab), which are post-translationally cleaved into 16 putative non-structural proteins (nsps) involving proteases, RNA polymerase, helicase, and other proteins involved in the transcription and replication of SARS-CoV-2 [4], [11], [42]. There are four structural proteins, including S protein, envelope (E), membrane (M), and nucleocapsid (N) (Fig. 3B) [43]. Most of the nonstructural proteins are known to have a role in the replication of the viral genome, whereas these four structural proteins are essential for the assembly and release of SARS-CoV-2 [43]. S protein is responsible for the binding of virion on the cell surface [44]. M protein has three transmembrane domains, whereas E protein plays its role in the assembly and release of viral particles from the cell. It is also involved in viral pathogenesis [45]. N protein has two domains, both of which can attach to the viral RNA in order to assist replication, and it also acts as a repressor of the RNAi system of the host cell, hence supporting the viral replication [46]
Fig. 3.
Genomic features and structure of SARS-CoV-2. (A) Genomic organization of SARS-CoV-2 reference genome (isolate Wuhan-Hu-1) from NCBI (accession number NC_045512.2). All genomic regions or open-reading frames (ORFs) are presented i.e. untranslated regions at both 5′ and 3′ ends (5′-UTR, 3′-UTR), polyproteins (pp1ab), structural proteins including spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins. (B) Structure (created with BioRender.com) of SARS-CoV-2 showing its major structural proteins.
The initial attachment of the virus to the host cell is mediated by S protein, which has two subunits, S1 (specific receptor binding domain known as RBD) and S2 (CoV S2 glycoprotein). S protein, through its specific RBD, binds to its receptor on the host cell [47]. Wan et al. [48] reported that SARS-CoV-2 is optimized to bind on angiotensin-converting enzyme II (ACE2) human receptors. RBD in the S protein is the most variable part, and it differs for each type of CoV. After binding to the receptor, the host protease cleaves the S protein, which causes the release of the spike fusion peptide, facilitating the entry of the virus into the host cell [49]. S protein cleavage takes place at two sites. The first cleavage causes the separation of RBD and fusion peptide, whereas the second cleavage exposes the fusion peptide that inserts into the cell membrane [50], which ultimately causes the formation of a six-helix bundle. The formation of this bundle allows the fusion of virus cell membrane and host cell membrane, causing the release of the viral genome into the cytoplasm [51].
As the genome of CoV consists of positive-sense single-stranded RNA, it is used as a template directly to translate pp1a and pp1b, which are processed further to proteins essential for the formation of replication transcription complex (RTC) present in double-membrane vesicles [52]. Subsequently, RTC synthesizes a set of sub-genomic RNAs (sgRNAs) in a discontinuous manner [53]. The positive sgRNA serves as an mRNA for all structural and accessory genes, whereas the negative-sense strand of sgRNA serves as a template for the production of sub genomic and genomic positive-sense mRNAs [54]. Following the replication and synthesis of mRNAs, structural proteins get transcribed [55]. These structural proteins are inserted into the endoplasmic reticulum and transferred to endoplasmic reticulum-Golgi intermediate compartments [56]. Here, the genomes are encapsulated by N proteins and budded into membranes of ERGIC containing viral structural proteins, ultimately causing the release of the mature virion. This causes an increase in the viral load in the body.
6. Pathogenesis of SARS-CoV-2
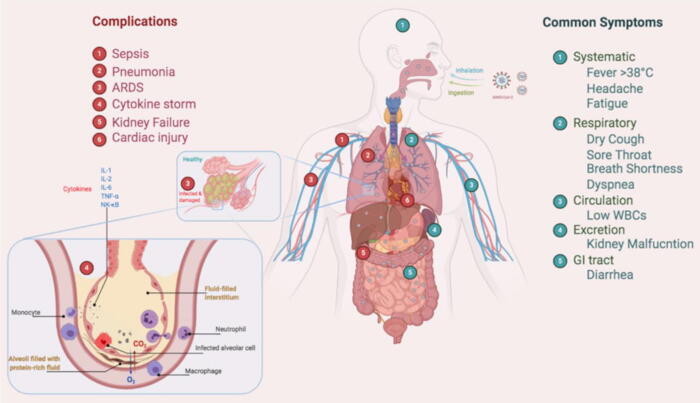
SARS-CoV-2 manipulates host’s receptor ACE-2 and a serine protease TMPRSS2, to activate viral S protein and entry inside the host cell [57], [58], [59]. SARS-CoV-2 infection, with heavy viral load in the body, destroys the human lungs through cytokine storm that refers to the overreaction of the body’s immune system [60]. Cytokines released by different types of cells in the body, are signals to attract immune cells to the site of infection, which allows the immune cells to coordinate their response against the virus. During a viral infection, the body produces large amounts of cytokines, causing a significant burden on the immune system, referred to as cytokine storm syndrome [61]. This burden forces the immune system to send more and more immune cells to the site of infection, leading to hyper-inflammation (Fig. 4). CoVs, after entering into the lungs, reaches the lower respiratory tract where alveoli are present and start to replicate there [62]. As a result, the cytokine storm causes the destruction of alveoli by the immune system. More and more immune cells are recruited to the site of infection that leads to the thickening of the lung lining and ultimately causes pneumonia with shortness of breath, the main symptom of COVID-19 [62]. Moreover, this cytokine storm forces the immune cells to destroy the healthy cell lining of the lungs that may leads to secondary bacterial pneumonia, causing the lungs to become less functional. Owing to the malfunctioning of lungs, other organs such as the brain, kidney, and liver become deprived of oxygen. Eventually, patients require ventilators to receive enough oxygen [63].
Fig. 4.
Common symptoms and complication related to the patients of coronavirus disease-2019 (COVID-19). (Figure created with BioRender.com).
7. Symptoms
The effect of COVID-19 may vary from person to person, and it may be from mild to moderate with an incubation period of 6 to 41 days (median of 14 days) [64]. The manifestation of multiple COVID-19 symptoms, as well as the duration of incubation time, depends on age groups, health conditions, and exposure times [65]. Old age people and patients with immunosuppressed disorders are the most susceptible to the infection. On average, symptoms appear in 5 days after exposure [66]. These symptoms may range from headache, fatigue with pain and aches, cough, sore throat to high fever, GI distress, diarrhea, nausea, myalgia, dyspnea, lymphopenia, difficulty in breathing and pneumonia [67], [68], [69], [70]. Unfortunately, COVID-19 symptoms, at the initial stage cannot make the basis for diagnosis as they mimic many respiratory and common infections (Table 1). Moreover, SARS-CoV-2 infected persons might also be asymptomatic carriers.
Table 1.
Characteristics, symptoms and epidemiology of respiratory viruses.
 |
COVID-19 may progress with cytokine storm leading to acute pneumonia, acute respiratory distress syndrome (ARDS) (Fig. 4), respiratory failure and even death [71]. Acute lung and kidney injuries, shock and heart failure have also been observed as complications of the disease [69], [70], [71]. Some individuals fail to respire in the fulminant disease that causes septic shock, multiple organ dysfunction (MOD), multiple organs failure (MOF), and its frequency is 5% patients [69], [70], [71]. Chest radiographs from COVID-19 patients indicate pneumonia with peripheral and subpleural ground-glass lung opacities [72], [73]. The proinflammation state with cytokine storm and an imbalance expression of ACE receptors are associated with the progression of COVID-19 [74]. SARS-CoV-2 interacts with sialic acids present at the surface of ACE2 receptors [74], reduces their expression, and triggers proinflammatory mediators, including IL-6, IL2, NF-κB, and TNF-alpha [75], [76].
8. Diagnostics
Unbiased next-generation sequencing tools were used for the confirmed diagnosis of earlier cases after initial screening for common causes of respiratory infections of patients with atypical pneumonia due to an unidentified microbial agent gave negative results [77], [78]. Next-generation sequencing of bronchoalveolar lavage was performed, and a novel CoV later named SARS-CoV-2 was subsequently identified as the causative pathogen [78], [79]. Few weeks following the preliminary characterization of COVID-19, molecular assays for detection of the virus in clinical samples were rapidly developed using the sequenced genomic information. Corman et al., developed a diagnostic qRT-PCR-based protocol for COVID-19 using swabbed samples from a patient’s nose and throat that has since been selected by the World Health Organization (https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn = a9ef618c_2). The Chinese and American Centers for Disease Control and Prevention and other research groups also described the development of real-time PCR methods to diagnose COVID-19, mainly targeting various combinations of the ORF1ab, E, N, and RNA-dependent RNA polymerase (RdRp) genes [80], [81], [82], [83]. Few cases of the COVID-19 reinfection have also been reported based on re-positive PCR test [84]. Though few studies have suggested short lived immunity to be a reason of reinfection [85], while some reports suggests the involvement of false positives, wrong sampling and medical diagnosis [86].
Although qRT-PCR has been a gold standard for the diagnostic of COVID-19 and the detection of SARS-CoV-2, several other methods have also been developed. A new molecular approach for the diagnosis of COVID-19 is Loop-mediated isothermal amplification (LAMP) being emerged as a great alternative to the qRT-PCR method. Amplification at a constant temperature (60 to 65 ͦC), exclusion of fancy lab instruments, rapid test results, naked-eye visible results, and potentially a numerically large diagnostic capacity, while maintaining similar sensitivity and specificity [87] are the advantages LAMP assay possesses thus making it more suitable than the RT-PCR during a pandemic period. LAMP assay is relatively a newer technique and therefore, there is less evidence on its use, but several studies have reported the development of LAMP assays for the detection of SARS-CoV-2 in clinical [88], [89], [90], [91] as well as environmental samples (Farhan Ul Haque et al., unpublished). Triggering the neutralizing antibody response to CoV infections [68] also allowed the development of serological testing. Several serodiagnostic methods have been developed for the rapid detection of COVID-19 [92], [93], [94], [95], [96], [97], but some of these methods have been reported inadequate in clinical settings due to very low sensitivity [98].
9. Treatments and vaccines
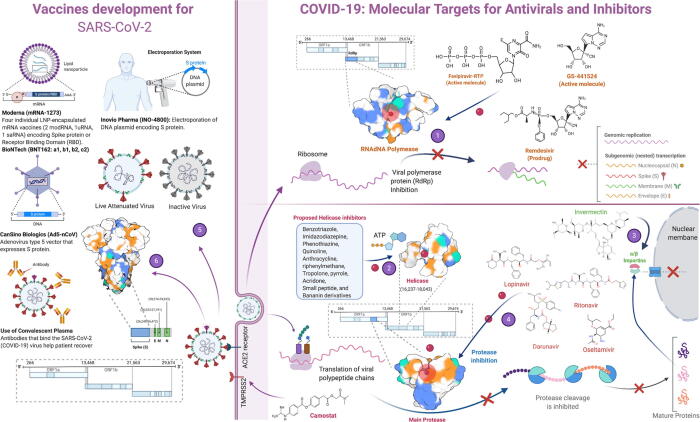
A number of drugs have been repurposed and employed (Fig. 5) for COVID-19 treatment [99] but still, no specific and effective drug has been approved to treat this disease. By 3rd September 2020, 321 vaccine candidates had been proposed and 33 of them were in clinical trials [100]. Along with traditional therapeutics, monoclonal antibodies, [101], [102], [103] convalescent blood plasma, [104], [105] peptide-based [106] and oligonucleotide medicines, [107] and interferon therapies (Inhaled interferon beta) [108], [109] have been used to treat COVID-19. As 80% of the COVID-19 victims suffer from mild symptoms, they do not need any special medical care. The best treatment for those people is to self-isolate themselves along with a healthy diet. Old-age and patients with comorbidities are required to be admitted to the hospital and sometimes may need oxygen and ventilator support [9].
Fig. 5.
Development of repurposed drugs and vaccines against COVID-19. (1) Inhibition of RNA-dependent RNA polymerase by Favipiravir, Remdesivir and GS-441524. Inhibition of the enzyme halts genomic replication and stops viral dissemination (2) Several drugs have been proposed against helicase but none of them is approved yet (3) Ivermectin dissociates IMP α/β1 (importins) heterodimer, which is responsible for nuclear transport of viral protein cargos, so viral proteins cannot enter into the nucleus to continue vital processes like replication (4) Lopinavir, Ritonavir, Darunavir and Oseltamivir inhibit Main Protease (MPro) enzyme which is involved in maturation of viral proteins (5) Inactive and attenuated SARS-CoV-2 can be used for vaccine production (6) Use of spike protein for development of vaccine candidates by different labs and pharma companies. (Figure created with BioRender.com).
During the early days of the COVID-19 pandemic, synthetic forms of quinine, chloroquine (CQ), and hydroxychloroquine (HCQ) were proposed as medicine of choice against COVID-19. In contrast, HCQ was previously found potent against several viral diseases including avian influenza A H5N1, HIV-1/AIDS, hepatitis C virus, Dengue virus, Zika virus, Ebola virus and SARS [110], [111], [112], [113], [114], [115], [116], [117]. These drugs have been proposed to inhibit posttranslational modifications in ACE2 receptors in humans and interfere with SARS-CoV-2 interactions with cells. HCQ, in combination with a macrolide antibiotic, azithromycin, was widely proposed as COVID-19 treatment, but the recent metanalyses show the combination of two medicines increased the mortality rate in the treated patients [118], [119]. Later, a debate was started for CQ and HCQ use against SARS-CoV-2 as some clinical trials reported adverse events during the treatment, including arrhythmia, heart failure and increased death rate [120], [121]. World Health Organization (WHO) and the National Institute of Health (NIH) stopped many trials using HCQ treatments afterwards and FDA revoked the use of HCQ and CQ for COVID-19 treatment [122]. Moreover, the “Randomised Evaluation of COVid-19 thERapY (RECOVERY)” trial conclusively showed that hydroxychloroquine is not an effective treatment in patients hospitalized with COVID-19. The RECOVERY trial was established during March 2020 as a randomized clinical trial in the UK to test a range of potential drugs for COVID-19 cure (https://www.recoverytrial.net/). The trial is still going on and several drugs or treatments including Colchicine, Dexamethasone, Lopinavir-Ritonavir, Azithromycin, Tocilizumab, Convalescent plasma (collected from donors who have recovered from COVID-19 and contain antibodies against SARS-CoV-2) and Aspirin, have been repurposed in an attempt to find an effective cure for COVID-19 patients. Montelukast, a cysteinyl leukotriene receptor antagonist used to treat asthmatic attacks, has been found better than HCQ in a randomized observational study, where it not only tames the cytokine storm in severe COVID-19 patients but also decreases the duration of the disease (Rehman et al., unpublished).
Antiviral drugs, including Galidesivir, Favipiravir, Remdesivir, Lopinavir/Ritonavir, Umifenovir (Arbidol), Ostalmovir have also been found active against SARS-CoV-2 [123], [124]. Galidesivir, Favipiravir and Remdesivir are nucleoside analogues and inhibit viral RNA-dependent RNA polymerase [123], [124]. Galidesivir has been previously used against the Ebola virus, HCV and Marburg virus [125]. Favipiravir is an antiviral effective against the influenza virus and in recent clinical trials, Favipiravir was found useful for COVID-19 [126]. Remdesivir, a non-FDA-approved antiviral was previously found active against SARS-CoV and MERS-CoV. It has also shown promising effects against SARS-CoV-2, and the FDA has allowed the emergency use of the drug to treat severe COVID-19 patients on 1 May 2020. Lopinavir/Ritonavir is a protease enzyme inhibitor, Ostalmovir is a Neuraminidase inhibitor, and Umifenovir interferes with virus interactions with host cells [123]. All of these antivirals have been employed in many clinical trials and found effective against COVID-19 to some extent [127], [128], [129].
Nutraceuticals, food supplements and phytochemicals have shown great potential to fight against many deadly viral diseases including SARS-CoV, HIV, HBV, HCV and Dengue fever [130], [131], [132]. Several plant-derived constituents including the polyphenols, alkaloids, terpenoids have been studied as potential inhibitors of SARS-CoV-2 surface protein (S protein) [133], [134] and key enzymes i.e., proteases, helicase, and polymerase [135], [136], [137]. In silico approaches have found more than 100 phytoconstituents including hesperidin, rhodiolin, baicalin, glycyrrhizin, and 18, ß-Glycyrrhetinic acid to interact with SARS-CoV-2 enzymes/proteins with high binding affinity [137], [138] (Rehman et al., unpublished) where as these phytochemical have already been experimentally found active against many viral enzymes [139], [140], [141]. Some traditional Chinese medicines (TCM) and phytoextracts are also being used in clinical trials against SARS-CoV-2 [142], [143].
A number of monoclonal antibodies act against inflammatory cytokines. Tocilizumab, a recombinant humanised monoclonal antibody (Anti IL-6), is conventionally used to treat rheumatoid arthritis and has shown promising effects in taming the cytokine storm in severe COVID-19 patients [79], [144]. Sarilumab is another antagonist to the IL-6 receptor that is undergoing phase 2/3 trials for the treatment of COVID-19 [145]. Camostat mesylate is usually used for the treatment of pancreatitis, is approved to be effective against SARS-CoV-2 by preventing its entry into the host cell [146]. For the treatment of COVID-19, α, and β- interferon therapy was found very useful, especially when used in combination with other drugs like lopinavir or ribavirin. But like the number of other factors, delay in treatment reduces the effectiveness of interferon [147]. The use of interferons is not recommended for the treatment of COVID-19. Treatment of severe COVID-19 patients using convalescent plasma or immunoglobulins from the recovered patients has also been found successful against SARS-CoV-2 [148], [149]. Corticosteroids can help in alleviating lung inflammation, but their uses may lead to other complications like hyperglycemia, avascular necrosis, and psychosis [150]. The use of dexamethasone has been found safer to lower down the mortality rate in COVID-19 patients [151].
According to WHO, till the end of November 2020, almost a year into this on-going pandemic, there was no FDA-approved vaccine available against COVID-19. Considering the severity of the current public health emergency worldwide, FDA has issued an emergency use authorization for two vaccines: mRNA-1273 vaccine from Moderna and BNT162b2 from Pfizer – BioNtech. Apart from these two vaccines, currently 172 countries are working on the development of an efficient and safe vaccine [152]. S protein of SARS-CoV-2 has been targeted for the development of the majority of vaccines [153], [154]. Microneedle array delivered recombinant CoV vaccine, PittCoVacc, has been proposed to develop immunity in mice against the CoV within just two weeks of microneedle pricks [155]. Lab mice produced specific antibodies in amounts sufficient to neutralize the virus. The vaccine was delivered via a fingertip-sized patch of 400 tiny needles, which are designed to provide the S protein pieces directly into the skin, where the immune system is strongest. Bacillus Calmette-Guerin (BCG) is also another potential candidate to fight against COVID-19 that offers a boarder protection against various respiratory infections [156]. Countries that have a late start of universal BCG vaccine policy also had a high mortality rate, supporting the idea that BCG protects the vaccinated population from SARS-CoV-2 [156], [157].
Inovio, a Pennsylvania-based biotech company, is using DNA instead of RNA for making the candidate vaccine (INO-4800) against the COVID-19 [158]. Zydus Cadlia, an India-based pharmaceutical company, is using two approaches for the development of the COVID-19 vaccine [18]. First, is the use of DNA to produce CoV protein in the human body and second deals with the genetically manipulating an attenuated measles virus to boost the immune response against COVID-19. Novavax, a Maryland-based company, announced that they had generated a candidate vaccine using recombinant proteins nanoparticles derived from the S proteins of SARS-CoV-2 in February [159]. Altimmune is developing a candidate vaccine that gets sprayed into patient’s noses instead of injecting them into their arms [160]. Vaxart is developing an oral vaccine against COVID-19, whereas another company, Expression, is using insect cells from fruit fly to produce viral antigens [161]. Many other pharmaceutical companies are using different approaches to develop candidate vaccines, but Codagenix is the only company to attenuate a live SARS-CoV-2 virus to develop a vaccine [162]. They are using the deoptimization approach to manipulate the virus in such a way that it may replicate inside both without causing any disease. COVAX (COVID-19 Vaccines Global Access) COVAX has the largest vaccine portfolio for the COVID-19 [163]. They have nine candidate vaccines and nine more vaccines under consideration. COVAX is co-led by CEPI (The Coalition for Epidemic Preparedness Innovations), ACT (Access to COVID-19 Tools), WHO, the vaccine Alliance and Gavi. One vaccine is approved by the Ministry of Health of the Russian Federation on 11 August. The name of the vaccine is Sputnik-V, previously known as COVID-Vac [164]. The Gamaleya Research institute developed this vaccine in Moscow. Medicago is going to develop a vaccine that is a SARS-CoV-2 like particle. They proposed that this virus-like particle would force the immune system of humans to produce antibodies against SARS-CoV-2. Overall, without approved and specific anti-SARS-CoV-2 drugs and vaccines, it is very difficult to treat the patients with severe COVID-19, so currently, the main focus during the treatment of COVID-19 patients is to maintain the functions of patients’ organs.
Nano-formulations of the proposed therapeutics and vaccines can target the effected cells through inhalation or intravenous injection in a way with better efficiency and efficacy [165]. Nano-antibodies (nanobodies) have been developed to treat COVID-19 patients. Nanodrugs based on Silver (Ag), gold (Au) and zinc (Zn) nanoparticles and nanoparticle bases drug delivery systems have been found effective against many viral infections like HIV, HSV, HCV, monkey pox virus and zika virus [166]. Currently under clinical trial nanobodies includes BGB-DXP592 (US clinical trial # NCT04551898), LY3819252 (US clinical trial #NCT04497987), REGN10933/REGN10987 monoclonal antibodies (US clinical trial # NCT04426695) and antibody fragments (INOSARS) (US clinical trial # NCT04514302). Dexamethasone, which has been recommended to treat COVID-19 patients (https://www.recoverytrial.net/results), nano-formulations have been proposed to be more effective [167]. In addition to the two approved vaccines i.e. mRNA-1273 from Moderna and BNT162b2 from Pfizer – BioNtech, many other vaccines under clinical trials have been lipid nanoparticle–formulated (Fig. 5) [168].
While scientists around the world are working on the development of effective therapeutics and vaccines against COVID-19. Further research studies are needed to understand the SARS-CoV-2 infections in humans and the zoonotic transmission of CoVs through clinical manifestations and study these viruses in detail. On the other hand, the pandemic’s catastrophic economic impact is pushing governments to reopen their economies, and this creates a public health quandary. Currently, the option is to minimize viral transmission through social distancing and efficient public health policy.
Funding
We thank Higher Education Commission (HEC), Pakistan and School of Biological Sciences, University of the Punjab, Lahore, Pakistan for providing funding and research facilities for this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Salma Mukhtar, Email: salmamukhtar85@gmail.com.
Muhammad Farhan Ul Haque, Email: mfarhan.sbs@pu.edu.pk.
References
- 1.Fan Y., Zhao K., Shi Z.-L., Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3):210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G. SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2003;2020 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y.-Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020 doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan X., Chen D., Xia Y., Wu X., Li T., Ou X. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20(4):410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;105924 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatrics. 2020;1–6 doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramphul K., Mejias S.G. Coronavirus disease: A review of a new threat to public health. Cureus. 2020;12(3) doi: 10.7759/cureus.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020 doi: 10.1002/jmv.26234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamre D., Procknow J.J. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121(1):190–193. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh K., Becker W.B., Chanock R.M. Growth in suckling-mouse brain of“ IBV-like” viruses from patients with upper respiratory tract disease. Proc Natl Acad Sci USA. 1967;58(6):2268. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B., Ge X., Wang L.-F., Shi Z. Bat origin of human coronaviruses. Virol J. 2015;12(1):1–10. doi: 10.1186/s12985-015-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang P., Wang H., Cao Z., Jin H., Chi H., Zhao J. A rapid and specific assay for the detection of MERS-CoV. Front Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:1–9. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagliusi S., Jarrett S., Hayman B., Kreysa U., Prasad S.D., Reers M. Emerging Manufacturers engagements in the COVID-19 vaccine research, development and supply. Vaccine. 2020 doi: 10.1016/j.vaccine.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meo S., Alhowikan A., Al-Khlaiwi T., Meo I., Halepoto D., Iqbal M. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24(4):2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A., Beatrice G., Dalbeni A. Coronavirus disease (COVID-19) and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2019;2020:40. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 22.Khalili M., Karamouzian M., Nasiri N., Javadi S., Mirzazadeh A., Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2020;148 doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. The Lancet. Global Health. 2020 doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-López F.R., Tajada M., Savirón-Cornudella R., Sánchez-Prieto M., Chedraui P., Terán E. Coronavirus disease 2019 and gender-related mortality in European countries: A meta-analysis. Maturitas. 2020;141:59–62. doi: 10.1016/j.maturitas.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuguyo O., Kengne A.P., Dandara C. Singapore COVID-19 pandemic response as a successful model framework for low-resource health care settings in Africa? OMICS: A Journal of. Integr Biol. 2020;24(8):470–478. doi: 10.1089/omi.2020.0077. [DOI] [PubMed] [Google Scholar]
- 28.Africa UNECf. COVID-19 in Africa: protecting lives and economies. 2020.
- 29.Musa S.S., Zhao S., Wang M.H., Habib A.G., Mustapha U.T., He D. Estimation of exponential growth rate and basic reproduction number of the coronavirus disease 2019 (COVID-19) in Africa. Infect Diseases Poverty. 2020;9(1):96. doi: 10.1186/s40249-020-00718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorbalenya A., Baker S., Baric R., de Groot R., Drosten C., Gulyaeva A. The species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet Microbiol. 2020;108693 doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Morales A.J., Bonilla-Aldana D.K., Balbin-Ramon G.J., Rabaan A.A., Sah R., Paniz-Mondolfi A. History is repeating itself: Probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infez Med. 2020;28(1):3–5. [PubMed] [Google Scholar]
- 33.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau S.K.P., Luk H.K.H., Wong A.C.P., Li K.S.M., Zhu L., He Z. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1542–1547. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paraskevis D., Kostaki E.G., Magiorkinis G., Panayiotakopoulos G., Sourvinos G., Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect Genet Evol. 2020;79 doi: 10.1016/j.meegid.2020.104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes L.R., de Mattos Cardillo G, Paiva P.B. Molecular evolution and phylogenetic analysis of SARS-CoV-2 and hosts ACE2 protein suggest Malayan pangolin as intermediary host. Braz J Microbiol. 2020;1–7 doi: 10.1007/s42770-020-00321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereson M.J., Mojsiejczuk L., Martínez A.P., Flichman D.M., Garcia G.H., Di Lello F.A. Phylogenetic analysis of SARS-CoV-2 in the first few months since its emergence. J Med Virol. 2020 doi: 10.1002/jmv.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020;371 doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- 40.Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H., Dingens A.S. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182(5) doi: 10.1016/j.cell.2020.08.012. pp. 1295–310. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4) doi: 10.1016/j.cell.2020.06.043. pp. 812–27. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beniac D.R., Andonov A., Grudeski E., Booth T.F. Architecture of the SARS coronavirus prefusion spike. Nat Struct Mol Biol. 2006;13(8):751–752. doi: 10.1038/nsmb1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeDiego M.L., Álvarez E., Almazán F., Rejas M.T., Lamirande E., Roberts A. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J Virol. 2007;81(4):1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui L., Wang H., Ji Y., Yang J., Xu S., Huang X. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J Virol. 2015;89(17):9029–9043. doi: 10.1128/JVI.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J Virol. 1994;68(9):5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci. 2009;106(14):5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snijder E.J., Van Der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussain S., Chen Y., Yang Y., Xu J., Peng Y., Wu Y. Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J Virol. 2005;79(9):5288–5295. doi: 10.1128/JVI.79.9.5288-5295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J Virol. 2007;81(1):20–29. doi: 10.1128/JVI.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331(5):991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krijnse-Locker J., Ericsson M., Rottier P., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994;124(1):55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kupferschmidt K., Cohen J. American Association for the Advancement of Science; 2020. Race to find COVID-19 treatments accelerates. [DOI] [PubMed] [Google Scholar]
- 58.Mollica V., Rizzo A., Massari F. The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol. 2020;16(27):2029–2033. doi: 10.2217/fon-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). StatPearls [Internet] StatPearls Publishing. 2020 [PubMed] [Google Scholar]
- 61.Channappanavar R, Perlman S, editors. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in immunopathology; 2017: Springer. [DOI] [PMC free article] [PubMed]
- 62.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020,:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020 doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 67.Ren L.-L., Wang Y.-M., Wu Z.-Q., Xiang Z.-C., Guo L., Xu T. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Diseases. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao R., Qiu Y., He J.-S., Tan J.-Y., Li X.-H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):18-. [DOI] [PMC free article] [PubMed]
- 73.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y., Liu D.X., Tam J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369(2):344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xing Z., Gauldie J., Cox G., Baumann H., Jordana M., Lei X.-F. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Investig. 1998;101(2):311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Binnicker M.J. Emergence of a novel coronavirus disease (COVID-19) and the importance of diagnostic testing: why partnership between clinical laboratories, public health agencies, and industry is essential to control the outbreak. Clin Chem. 2020;66(5):664–666. doi: 10.1093/clinchem/hvaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li B., Si H.-R., Zhu Y., Yang X.-L., Anderson D.E., Shi Z.-L. Discovery of bat coronaviruses through surveillance and probe capture-based next-generation sequencing. Msphere. 2020;5(1) doi: 10.1128/mSphere.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alagarasu K., Choudhary M., Lole K., Abraham P., Potdar V. Evaluation of RdRp & ORF-1b-nsp14-based real-time RT-PCR assays for confirmation of SARS-CoV-2 infection: An observational study. Indian J Med Res. 2020;151(5):483. doi: 10.4103/ijmr.IJMR_1256_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dharavath B., Yadav N., Desai S., Sunder R., Mishra R., Ketkar M. A one-step, one-tube real-time RT-PCR based assay with an automated analysis for detection of SARS-CoV-2. Heliyon. 2020;6(7) doi: 10.1016/j.heliyon.2020.e04405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu W, Chen CZ, Gorshkov K, Xu M, Lo DC, Zheng W. RNA-Dependent RNA Polymerase as a Target for COVID-19 Drug Discovery. SLAS DISCOVERY: Advancing the Science of Drug Discovery. 2020:2472555220942123. [DOI] [PMC free article] [PubMed]
- 83.Wang X., Yao H., Xu X., Zhang P., Zhang M., Shao J. Limits of detection of 6 approved RT–PCR kits for the novel SARS-Coronavirus-2 (SARS-CoV-2) Clin Chem. 2020;66(7):977–979. doi: 10.1093/clinchem/hvaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang B., Liu S., Dong Y., Zhang L., Zhong Q., Zou Y. Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID-19) J Infect. 2020 doi: 10.1016/j.jinf.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parry J. Covid-19: Hong Kong scientists report first confirmed case of reinfection. British Medical Journal Publishing Group; 2020. [DOI] [PubMed]
- 86.Alizargar J. Risk of reactivation or reinfection of novel coronavirus (COVID-19) J Formos Med Assoc. 2020;119(6):1123-. doi: 10.1016/j.jfma.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thompson D., Lei Y. Mini Review: Recent progress in RT-LAMP enabled COVID-19 detection. Sensors and Actuators Reports. 2020;100017 doi: 10.1016/j.snr.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang M., Pan W., Arasthfer A., Fang W., Ling L., Fang H. Development and validation of a rapid, single-step reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system potentially to be used for reliable and high-throughput screening of COVID-19. Front Cell Infect Microbiol. 2020;10(331) doi: 10.3389/fcimb.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kashir J., Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med Hypotheses. 2020;109786 doi: 10.1016/j.mehy.2020.109786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buck M.D., Poirier E.Z., Cardoso A., Frederico B., Canton J., Barrell S. Standard operating procedures for SARS-CoV-2 detection by a clinical diagnostic RT-LAMP assay. medRxiv. 2020 doi: 10.12688/wellcomeopenres.16517.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thi V.L.D., Herbst K., Boerner K., Meurer M., Kremer L.P., Kirrmaier D. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci Transl Med. 2020;12(556) doi: 10.1126/scitranslmed.abc7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng M.P., Yansouni C.P., Basta N.E., Desjardins M., Kanjilal S., Paquette K. Serodiagnostics for severe acute respiratory syndrome-related coronavirus-2: A narrative review. Ann Intern Med. 2020 doi: 10.7326/M20-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vandergaast R., Carey T., Reiter S., Lech P., Gnanadurai C., Tesfay M. Development and validation of IMMUNO-COV: a high-throughput clinical assay for detecting antibodies that neutralize SARS-CoV-2. bioRxiv. 2020 [Google Scholar]
- 95.Bonelli F., Sarasini A., Zierold C., Calleri M., Bonetti A., Vismara C.S. Clinical And Analytical Performance Of An Automated Serological Test That Identifies S1/S2 Neutralizing IgG In Covid-19 Patients Semiquantitatively. bioRxiv. 2020 doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perera R.A., Mok C.K., Tsang O.T., Lv H., Ko R.L., Wu N.C. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance. 2020;25(16):2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muruato A.E., Fontes-Garfias C.R., Ren P., Garcia-Blanco M.A., Menachery V.D., Xie X. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. bioRxiv. 2020 doi: 10.1038/s41467-020-17892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cassaniti I., Novazzi F., Giardina F., Salinaro F., Sachs M., Perlini S. Performance of VivaDiag COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. J Med Virol. 2020 doi: 10.1002/jmv.25800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li G., De Clercq E. Nature Publishing Group; 2020. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) [DOI] [PubMed] [Google Scholar]
- 100.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020 doi: 10.1038/d41573-020-00151-8. Advance online publication:10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 101.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac J Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 103.Marovich M., Mascola J.R., Cohen M.S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 2020;324(2):131–132. doi: 10.1001/jama.2020.10245. [DOI] [PubMed] [Google Scholar]
- 104.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Investig. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pant S., Singh M., Ravichandiran V., Murty U., Srivastava H.K. Peptide-like and small-molecule inhibitors against Covid-19. J Biomol Struct Dyn. 2020;1–10 doi: 10.1080/07391102.2020.1757510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rossi J.J., Rossi D. Oligonucleotides and the COVID-19 Pandemic: A Perspective. Nucleic Acid Ther. 2020 doi: 10.1089/nat.2020.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park A., Iwasaki A. Type I and Type III Interferons-Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan Y., Zou Z., Sun Y., Li X., Xu K.-F., Wei Y. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mizui T., Yamashina S., Tanida I., Takei Y., Ueno T., Sakamoto N. Inhibition of hepatitis C virus replication by chloroquine targeting virus-associated autophagy. J Gastroenterol. 2010;45(2):195–203. doi: 10.1007/s00535-009-0132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farias K.J.S., Machado P.R.L., de Almeida Junior R.F., de Aquino A.A., da Fonseca B.A.L. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol Immunol. 2014;58(6):318–326. doi: 10.1111/1348-0421.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Delvecchio R., Higa L.M., Pezzuto P., Valadão A.L., Garcez P.P., Monteiro F.L. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses. 2016;8(12):322. doi: 10.3390/v8120322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dowall S.D., Bosworth A., Watson R., Bewley K., Taylor I., Rayner E. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gener Virol. 2015;96(Pt 12):3484. doi: 10.1099/jgv.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology journal. 2005;2(1):1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fiolet T., Guihur A., Rebeaud M., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roustit M., Guilhaumou R., Molimard M., Drici M.D., Laporte S., Montastruc J.L. Chloroquine and hydroxychloroquine in the management of COVID-19: Much kerfuffle but little evidence. Therapies. 2020;75(4):363–370. doi: 10.1016/j.therap.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. medRxiv. 2020:2020.04.10.20060558.
- 121.Borba MGS, Val FdA, Sampaio VS, Alexandre MAA, amp, amp, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). medRxiv. 2020:2020.04.07.20056424.
- 122.Infante M., Ricordi C., Alejandro R., Caprio M., Fabbri A. Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Review of Anti-infective. Therapy. 2020:1–12. doi: 10.1080/14787210.2020.1799785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jomah S., Asdaq S.M.B., Al-Yamani M.J. Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. J Infect Public Health. 2020;13(9):1187–1195. doi: 10.1016/j.jiph.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Coomes E.A., Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75(7):2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lou Y., Liu L., Qiu Y. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID-19 patients: an exploratory randomized. Controlled Trial medRxiv. 2020 doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 129.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naithani R., Mehta R.G., Shukla D., Chandersekera S.N., Moriarty R.M. Springer; 2010. Antiviral activity of phytochemicals: a current perspective. Dietary components and immune function; pp. 421–468. [Google Scholar]
- 131.Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother Res. 2018;32(12):2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ashok A., Ravivarman J., Kayalvizhi K. Nutraceutical value of salad vegetables to combat COVID 19. J Pharmacog Phytochem. 2020;9(3):2144–2148. [Google Scholar]
- 133.Basu A., Sarkar A., Maulik U. Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci Rep. 2020;10(1):17699. doi: 10.1038/s41598-020-74715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pandey P., Rane J.S., Chatterjee A., Kumar A., Khan R., Prakash A. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development. J Biomol Struct Dyn. 2020;1–11 doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Swargiary A., Mahmud S., Saleh M.A. Screening of phytochemicals as potent inhibitor of 3-chymotrypsin and papain-like proteases of SARS-CoV2: an in silico approach to combat COVID-19. J Biomol Struct Dyn. 2020;1–15 doi: 10.1080/07391102.2020.1835729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar S., Kashyap P., Chowdhury S., Kumar S., Panwar A., Kumar A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine. 2020;153317 doi: 10.1016/j.phymed.2020.153317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Islam R., Parves M.R., Paul A.S., Uddin N., Rahman M.S., Mamun A.A. A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2. J Biomol Struct Dyn. 2020;1–12 doi: 10.1080/07391102.2020.1761883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chandel V, Raj S, Rathi B, Kumar D. In Silico Identification of Potent COVID-19 Main Protease Inhibitors from FDA Approved Antiviral Compounds and Active Phytochemicals through Molecular Docking: A Drug Repurposing Approach. 2020
- 139.Miyake K., Tango T., Ota Y., Mitamura K., Yoshiba M., Kako M. Efficacy of Stronger Neo-Minophagen C compared between two doses administered three times a week on patients with chronic viral hepatitis. J Gastroenterol Hepatol. 2002;17(11):1198–1204. doi: 10.1046/j.1440-1746.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- 140.Rehman M.F., Batool A.I., Qadir R., Aslam M. Hesperidin and naringenin. In: Mushtaq M., editor. A centum of valuable plant bioactives. Elsevier; In Press: 2021. [Google Scholar]
- 141.Sasaki H., Takei M., Kobayashi M., Pollard R.B., Suzuki F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology. 2002;70(4):229–236. doi: 10.1159/000069334. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williamson G., Kerimi A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem Pharmacol. 2020;114123 doi: 10.1016/j.bcp.2020.114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cortegiani A., Ippolito M., Greco M., Granone V., Protti A., Gregoretti C. Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Della-Torre E, Campochiaro C, Cavalli G, De Luca G, Napolitano A, La Marca S, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Annals of the Rheumatic Diseases. 2020:annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed]
- 146.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother. 2020;64(6):e00754–e820. doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shalhoub S. Interferon beta-1b for COVID-19. The Lancet. 2020;395(10238):1670–1671. doi: 10.1016/S0140-6736(20)31101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Malani AN, Sherbeck JP, Malani PN. Convalescent Plasma and COVID-19. JAMA. 2020;324(5):524-. [DOI] [PubMed]
- 149.Zeng F, Chen X, Deng G. Convalescent plasma for patients with COVID-19. Proceedings of the National Academy of Sciences. 2020;117(23):12528. [DOI] [PMC free article] [PubMed]
- 150.Ellsworth G.B., Glesby M.J., Gulick R.M. The Uncertain Role of Corticosteroids in the Treatment of COVID-19. JAMA. Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2444. [DOI] [PubMed] [Google Scholar]
- 151.Group TRC Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020 [Google Scholar]
- 152.Callaway E. Coronavirus vaccines: five key questions as trials begin. Nature. 2020;579(7800):481. doi: 10.1038/d41586-020-00798-8. [DOI] [PubMed] [Google Scholar]
- 153.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;102743 doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020 [Google Scholar]
- 157.Marciano B.E., Huang C.-Y., Joshi G., Rezaei N., Carvalho B.C., Allwood Z. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. 2014;133(4):1134–1141. doi: 10.1016/j.jaci.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mahase E. Covid-19: What do we know so far about a vaccine? : British Medical Journal Publishing Group; 2020.
- 159.Jaklevic M.C. Researchers strive to recruit hard-hit minorities into COVID-19 vaccine trials. JAMA. 2020 doi: 10.1001/jama.2020.11244. [DOI] [PubMed] [Google Scholar]
- 160.Wu S.C. Progress and concept for COVID-19 vaccine development. Biotechnol J. 2020 doi: 10.1002/biot.202000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moreno-Fierros L., García-Silva I., Rosales-Mendoza S. Taylor & Francis; 2020. Development of SARS-CoV-2 vaccines: should we focus on mucosal immunity? [DOI] [PubMed] [Google Scholar]
- 162.Chen W.-H., Strych U., Hotez P.J., Bottazzi M.E. The SARS-CoV-2 vaccine pipeline: an overview. Current Trop Med Rep. 2020:1–4. doi: 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Torjesen I. Covid-19: Pre-purchasing vaccine—sensible or selfish? : British Medical Journal Publishing Group; 2020. [DOI] [PubMed]
- 164.Burki T.K. The Russian vaccine for COVID-19. The Lancet. Respir Med. 2020 doi: 10.1016/S2213-2600(20)30402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jones G.W., Monopoli M.P., Campagnolo L., Pietroiusti A., Tran L., Fadeel B. No small matter: a perspective on nanotechnology-enabled solutions to fight COVID-19. Nanomedicine. 2020;15(24):2411–2427. doi: 10.2217/nnm-2020-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F. Dexamethasone nanomedicines for COVID-19. Nat Nanotechnol. 2020;15(8):622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]