Abstract
In around the world, mosquito control is considered a most important because of the incapable of synthetic insecticides and the ecological pollution about by them. In this manner, need the eco-friendly insecticides to efficient control the mosquito disease is the need of the hour. We synthesized the eco-friendly of zinc oxide nanoparticles (ZnO-NPs) using the Knoxia sumatrensis aqueous leaf extract (Ks-ALE) as a reducing and stabilizing agent. The synthesis of ZnO-NPs was confirmed by UV with an absorption peak at 354 nm. ZnO-NPs crystal structure was analyzed by X-ray diffraction (XRD). Fourier transform infrared spectroscopy (FT-IR) spectra revealed the chloride, cyclic alcohols, sulfonamies, carboxylic acids, oximes, phosphines, alkenes and alcohol & phenol. Field emission-scanning electron microscopy (FE-SEM) showed that the NP’s are rod shaped with 50–80 nm size and also energy dispersive spectra (EDaX) spectra showed presence of zinc. Antioxidant assay showed superior activity and evidenced by DPPH, ABTS and H2O2 radical assays. Furthermore, the ZnO-NPs exhibited strong activity in MCF-7 cell line with IC50 value is 58.87 μg/mL. Mosquito larvicidal activity of ZnO-NPs produced significant activity and excellent larvicidal activity was noticed in Cx. quinquefasciatus with LC50 0.08, mg/mL and LC9019.46 mg/mL. This study suggests that synthesized ZnO-NPs using Knoxia sumatrensis leaf extract have good biological activities and it makes them an ideal candidate for pharmacological studies.
Keywords: K. sumatrensis, FE-SEM, Antioxidant, AO/EtBr, Cx. quinquefasciatus
1. Introduction
Nanotechnology has extensive use in science and medicine. Nanoparticles (1−100 nm) are used in various biomedical applications and also create a major impact in modern technology [1]. In recent times, zinc, iron, copper and cerium which have been used due to unique properties [2]. Metal-oxide and metal-sulfide nanoparticles (NPs), including cadmium and zinc sulfides (CdS and ZnS), are regularly utilized for oxidation of natural issue during sewage water treatment attributable to their photocatalytic properties. In addition, CdS and ZnS nanocrystals are known for their effective applications in toxicity evacuation as well as for the decrease of carbon dioxide, aldehydes, water parting, and reductive dehalogenation of benzene subsidiaries. For example, toxic impact of cadmium selenide and zinc selenide NPs was shown in tests with Daphnia magna. The various types of CdS NPs toxicity was reported in Vibrio fischeri, Raphidoxelis subcapitata and Chlorella vulgaris and also toxic for aquatic plants Spirodela polyrrhiza. While Ruditapes decussates were tested using ZnS NPs [3]. Cadmium (Cd) is a heavy metal that found in the earth’s crust as well as primary industrial, ecological poison, and a critical anthropogenic poison. It is additionally present in different food things and its toxicity has been generally archived in both people and animal models [4]. A widespread of NPs comprise tested for their toxicity towards various life forms however the mode of toxic action of metal sulfide NPs isn't completely seen at this point.
Among these nanoparticles, particularly zinc oxide nanoparticles (ZnO-NPs) are frequently favoured because of their low toxicity and varied therapeutic applications [5]. The green synthesis method are more advantageous when compare to physical and chemical methods because of their eco-friendly, low cost, increase economic and large-scale synthesis. ZnO nanoparticles have been effectively orchestrated utilizing plants such as, Musa ornate and Zea mays [6], Drosophila melanogaster [7], Lycopersicon esculentum [8] and Murraya koenigii [9]. Medicinal plants have a rich source of secondary metabolites and also folkloric sacred texts reports a large number of medicinal plants for different therapeutic applications.
Knoxia sumatrensis is a traditional medicinal plant and it contain high amount of bioactive compounds. It belongs to Rubiaceae family. It is an erect perennial herb, with height ranging from 40−90 cm. K. sumatrensis has worldwide distribution with Indo-Malaya and Australia regions. The entire plant is utilized for the preparation of alcoholic and non-alcoholic beverages [10]. K. sumatensis leaf paste is applied to wound for healing [11].
Breast cancer is an emerging problem in the world and so, there is a new need to create promising cancer treatment modalities [12]. Nanotechnology is innovative method of treatment of different cancer studies. The combination of macromolecules and NPs is a powerful tool for cancer treatment [13]. At present, cancer treatments such as chemotherapy, surgery and radiotherapy are high cost, relatively ineffective and not widely available. Current chemotherapies are limited because of the low selectivity of cancer cells, which causes unpleasant side effects and harm the healthy cells. Irinotecan hydrochloride (IRI) is a potential chemotherapeutic agent and also good activity against malignancies. IRI-loaded liposome has declared sizable therapeutic application in opposition to colorectal cancer [14]. Magnetite nanoparticles contain a good targeted drug delivery of last three years and also this process allows treatment of tumor diseases via hyperthermia and development of antibody sensors in immunoassay [15]. The amphiphilic compounds (Liposomes and Micelles) are generally used as nanoparticle drug transporters. In this manner, poly (ethylene oxide), poly (L-lactide), poly (epsiloncarprolactone), poly (lactic-co-glycolic acid) and poly-N-vinyl-2-pyrrolidone (PVP) contain gained wide acknowledgment of disease treatment [16].
Mosquitoes belong to family Culicidae and are exclusively responsible for dispersal of several diseases. Ae. aegypti is a vector of dengue, and last 30 years more than 50–100 million infections have been estimated in worldwide [17]. An. stephensi Liston is a malarial vector. Globally, 219 million cases have been reported with 4,350,000 death rates [18]. Cx. quinquefasciatus Say transmit lymphatic filariasis. Worldwide, 40 million people are affected [19]. The chemical synthetic insecticides such as, organophosphates, diflubenzuron, methoprene and bacterial larvicides are effective against mosquitoes [20]. Although, the regular use of chemical insecticides has affected the environmental related problems [21]. To overcome the issue need alternative way to control mosquito. Therefore, the green-synthesized metal and metal oxide nanoparticles are good in controlling the mosquito problems because of the reduce eco-pollution.
Mosquito borne and Cancer infections are interrelated because of mosquito related disease attack the human metabolic pathways and it prompting cancer. In, malaria infection few cofactors exist due to require for tumor improvement in Burkitt’s lymphoma. It was accounted for that an indistinct virus is moved by Anopheles vectors that basically causes gentle sickness and later transforms into brain cancer. Furthermore, Ae. aegypti can be spread hamster reticulum cell sarcoma cancer through tumor’s cells [22]. Plant based nanotechnology is seeing a rising trend in applied sciences and medical application due to relatively high biocompatibility [23]. The objective of the study is synthesis, characterization and biological potential of zinc oxide nanoparticles using K. sumatrensis (Retz.) DC. aqueous leaf extract (ALE).
2. Materials and methods
2.1. Materials
Zinc acetate dehydrade [Zn (CH3COO)2]. 2 H2O, Sodium hydroxide (NaOH), 2,2-Diphenyl-1-picrylhydrazyl assay (DPPH), 80% Methanol, Butylated hydroxyl anisole, 2, 2΄ Azinobis (3-ethyl benzo-thizoline-6-sulfonic acid (ABTS), Potassium persulphate, Ascorbic acid, Ferrous sulphate (FeSO4), Hydrogen peroxide (H2O2), Sodium salicylate, Fetal bovine serum (FBS), Dimethyl sulfoxide (DMSO), Dulbecco in modified eagle medium (DMEM), 3-[4,5-Dimethylthiazol-2-yl] 2,5-diphenyltetrazolium bromide (MTT), Phosphate buffered saline (PBS), Penicillin/Streptomycin antibiotic solution, Trypsin-Ethylene diamine tetra acetic acid (EDTA) was purchased from Gibco (USA), Ethidium bromide (ETBr) and Acridine Orange (AO) was purchased from Sigma Aldrich (USA). All other chemicals and reagents used in this study were of analytical grade. The experiments in this study were done using sterile distilled water.
2.2. Collection and extraction
Knoxia sumatrensis (Retz). DC. Plant was collected in September 2018 from vathalmalai hills (12.0464 N, 78.2220 E), located in Dharmapuri district, Tamil Nadu, India. The collected plant was authenticated by Botanical survey of India (BSI), Coimbatore, Tamil nadu, India and the specimen voucher number (BSI/SRC/4/23/2018/Tech/620). Plant leaves were shade dried for 2 weeks at room temperature. After shade dried, the leaves were powdered by steel blender. Leaf powder (5 g) was taken in 300 mL beaker having 100 mL of distilled water. The mixture was boiled for 20–25 min s after the extract was filtered using Whatman no.1 filter paper and the solutions store at 4 °C.
2.3. Phytosynthesis of zinc oxide nanoparticle
The method was followed by Vijayakumar et al. [8]. The solution was centrifuged at 10,000 rpm (10,956 g) for 12 min. and the pellet was obtained and dried. After, the dried Zn powder was calcinated under a muffle furnace at 350 °C for 3 h.
2.4. Characterization study
The synthesized zinc oxide nanoparticle using K. sumatrensis leaf extract were characterized by following the instrument analysis. Ultraviolet-visible spectroscopy (UV–vis-Shimadzu UV-1800) operated at the subsequent 400–700 nm wavelength. X-Ray Diffraction (XRD-Rigaku MiniFlex, operating at 40 kV with 30 mA using CuKα(λ = 0.154 Å) radiation) was analysis the crystalline structure and it’s sizes of NP’s were calculated via the Debye–Scherrer. The diffraction data were recorded for 2θ range between 20° and 80°. The functional groups were analyzed through Fourier transform infrared spectroscopy (FTIR-Perkin Elmer-Tensor 27, Bruker, Germany) and wavelength for 400–4000 cm−1. The NP’s shape and size were analyzed by scanning electron microscopy (FE-SEM-EVO 18, ZEISS, UK) with element and mapped analyzed (EDAX-Quorum Technologies, U.K).
2.5. Antioxidant assays
2.5.1. 2,2-Diphenyl-1-Picrylhydrazyl assay (DPPH)
DPPH study was performed by ZnO-NPs was described by earlier, Shimada et al. [24]. In briefly, 1.0 mL of DPPH solution (0.2 mM) was taken having ZnO-NPs various dose (20, 40, 60, 80, and 100 μg/mL) are used and stand for 30 min under dark conditions. After 30 min the absorbance was read at 517 nm.
A0 - Control, A1- ZnO-NPs.
2.5.2. Hydroxyl scavenging
In this assay was performed by Rajeshwar et al. [25]. As regards 1 mL of various concentrations in ZnO-NPs was mixed into 3 mL of hydrogen peroxide solution (1.0 mL of 1.5 mM FeSO4, 0.7 mL of 6 mM hydrogen peroxide and 0.3 mL of 20 mM sodium salicylate). The reaction mixture is incubated for 37 °C as well as the absorbance was measured on 562 nm.
A0 - Control, A1 - ZnO-NPs and A2 - absorbance without sodium salicylate.
2.5.3. 2, 2′- Azino-bis-3-Ethylbenzothiazoline-6-Sulfonic acid (ABTS)
This assay was followed by Giao et al. [26]. The reaction was began via the adding of 1.0 mL of diluted ABTS towards 10 μL of different concentrations (20, 40, 60, 80 and 100 μg/mL) of sample and also 10 μL of ethanol as a control. The absorbance was read at 734 nm after 6 min.
Ao - Control reaction, A1- ZnO-NPs.
2.6. Anti-proliferative study
2.6.1. Culture
Breast cancer cell line (MCF-7) was get into National Centre for Cell Science. It was growing in Dulbecco in modified eagle medium (DMEM) elevated glucose medium (Sigma Aldrich, USA), as increase with 10 % fetal bovine serum and antibiotics (20 mL of penicillin), (Hi-Media, India). This cell line was stored on 37 °C and 5% humidified CO2 atmosphere.
2.6.2. Viable assay
This assay was followed by earlier, Mosmann, [27]. Breast cancer cell line cells were seeded in a 96 - well plate (cells/well) and the plates were incubated at 37 °C for 24 h in a humidified CO2. A various dosages of ZnO-NPs (6.5, 12.5, 25, 50, and 100 μg/mL) were consistently treated in cancer cell at incubation time. The cell viability was calculated (MTT 10 μL for 4 h at 37 °C) later than 24 h exposure. The treated cells were dissolved with Dimethyl sulfoxide (DMSO) for precipitate of MTT. The formation of crystals were measure optical density (OD) at 540 nm (reference: 630 nm) using ELISA plate reader (Epoch 2.0, USA). For cell morphology images were captured in confocal microscope (Olympus, Japan).
2.6.3. Dual staining
The apotoptic morphological features were done by dual staining (AO/EtBr) in ZnO-NPs against MCF-7 cell line. Briefly, cancer cells (5 × 105) cells/wells were seeded into the 24 well tissue culture plates treated with IC50 concentration of ZnO-NPs for 24 h then washed with phosphate-buffered saline (PBS). Then 50 μL of 1 mg/mL AO/EtBr were additional to the every well. The change of morphological was observed through visualized and fluorescence microscope (Nikon Eclipse, Inc, Japan).
2.7. Larvicidal activity
2.7.1. Collection and rearing
Ae. aegypti, An. stephensi and Cx. quinquefasciatus species were acquire from Indian Council of Medical Research (ICMR)-Vector Control Research Centre, Madurai. It’s larvae maintained at 14: 10 h light and dark photoperiod with temperatures 25 ± 2 °C, RH-70 ± 5%. Larvae were fed on (3:1 ratio-dog biscuit and yeast powder).
2.7.2. Bioassay
The study was followed by World Health Organization (WHO), [28] standard procedure with some modification as per the method of Thandapani et al. [29]. The IVth ‘instar’ 20 numbers of larvae was added into at every plastic cups having 200 mL of double distilled water. K. sumatrensis-ALE and ZnO-NPs extract various concentrations (5, 10, 15, 20 and 25 mg/L) were added. The control treatment was also maintained (Distilled water). Larva mortality was counted (12, 24 and 48 h) and the percentages of mortality were described from the average of three replicates. The larva mortality with number of death was calculated by the method of Abbott, [30].
2.8. Statistical investigation
The results were described as Mean ± SD assays which were subjected to One - Way and Two - Way analysis of variance, which Dunnett’s multiple comparison tests using PRISM software version 5.2 (Graph Pad Software Inc, USA). The larval death was subjected to log Probit analysis after 12, 24 and 48 h for calculation LC50 and LC90 values. The chi square values were also calculated by SPSS software version 20.0 (SPSS., USA).
3. Results and discussion
3.1. UV–vis spectrophotometer
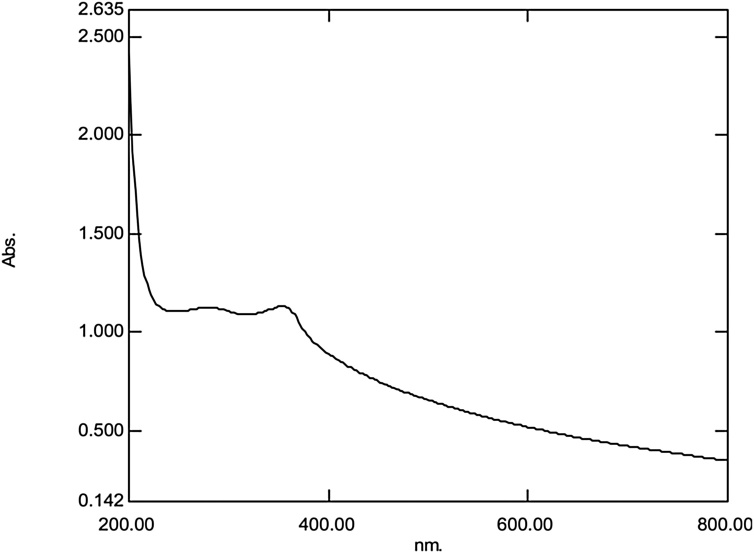
The confirmation of K. sumatrensis leaf extract of ZnO-NPs were observed by colour change from greenish yellow to pale white precipitate. The UV spectrum of synthesized ZnO-NPs was shown in (Fig. 1). It’s absorbance peak at 354 nm due to Surface Plasmon Resonance (SPR). Similar, results were observed by ZnO-NPs using Calotropis gigantea leaf extract [31]. In previous report, the ZnO-NPs using Drosophila melanogaster leaf extract showed UV absorption peak at 357 nm [7].
Fig. 1.
UV–Vis absorption spectra of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE). The UV–Vis absorption spectrum of synthesized ZnO-NPs absorption peak at 354 nm.
3.2. XRD study
The structural properties as well as crystalline size of ZnO-NPs were analyzed by XRD. The 2 theta value at 31.77°, 34.43°, 36.26°, 47.55°, 56.61°, 62.87°, 66.39°, 67.96°, 69.10°, 72.59°, and 76.98°. These peaks index to (100), (002), (004), (101), (102), (103), (110), (200), (112), (201), and (202) are represented in Fig. 2. The same diffraction value was noticed in ZnO-NPs using Lycopersicon esculentum and Cynara scolymus leaf extract [8,32]. In this study, the lattice planes clearly indicate that ZnO-NPs formation and its hexagonal phase and wurtzite structure of ZnO-NPs. The lattice planes which are matched to JCPDS file no. (89− 0511). The average crystal size of NP’s measure by Scherrer's formula and it’s shows range from 64.74 nm.
Fig. 2.
XRD analysis of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE).
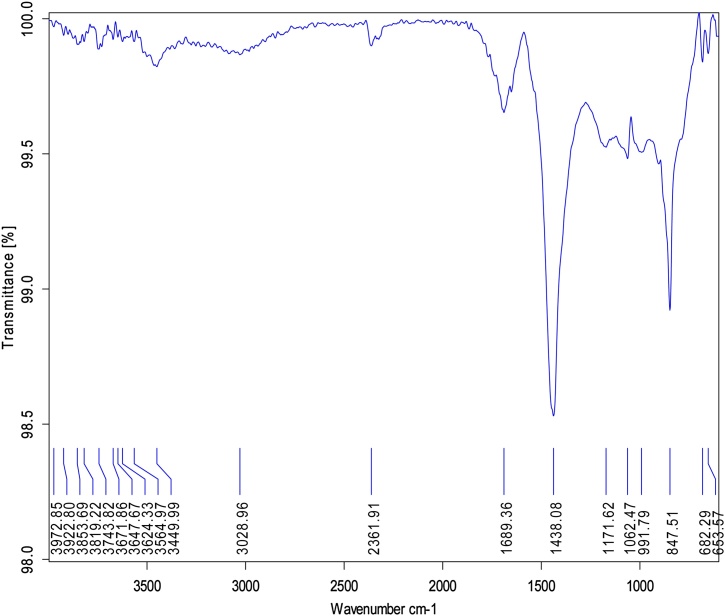
3.3. FT - IR study
Fig. 3 shows that the FT-IR spectra of ZnO-NPs and also it’s characterize peaks at 653.57 cm−1 for C–X bending (Chloride), 1062.47 cm−1 for CH—O—H stretch (Cyclic alcohols), 1171.62 cm−1 for S O stretch (Sulfonamies), 1438.08 cm–1 for OH bending (Carboxylic acids), 1689.36 cm−1 for C N Stretch (Oximes), 2361.91 cm−1 for P—H stretch (Phosphines) and 3028.96 cm−1 for C—C C Stretch (Alkenes) and 3449.99 cm−1 for O—H stretch (Alcohols and Phenols) are represented in (Table 1). In earlier report, were observed by Ulva lactuca- fabricated of ZnO-NPs [33]. In other results were finding in ZnO-NPs using Cynara scolymus leaf extract [32]. The spectra band at 434 cm−1 which is indicates that the formation of ZnO-NPs [34]. K. Sumatrensis−ALE few molecules which are responsible for reduction and capping agent of NP’s.
Fig. 3.
FT-IR analysis of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE).
Table 1.
FT-IR analysis of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE).
| Wave number (cm−1) |
Intensity | Group compound |
Functional group |
|---|---|---|---|
| 653.57/Bending | Strong | C–X | Chloride |
| 1062.47/Stretch | VS | CH-O-H | Cyclic alcohols |
| 1171.62/Stretch | VS | S = O | Sulfonamies |
| 1438.08/Bending | Medium | OH | Carboxylic acids |
| 1689.36/Stretch | Srong | C = N | Oximes |
| 2361.91/Stretch | Medium | P-H | Phosphines |
| 3028.96/Stretch | Strong | C-C = C | Alkenes |
| 3449.99/Stretch | Medium | O-H | Alcohols and Phenols |
3.4. SEM, EdaX and mapping analysis
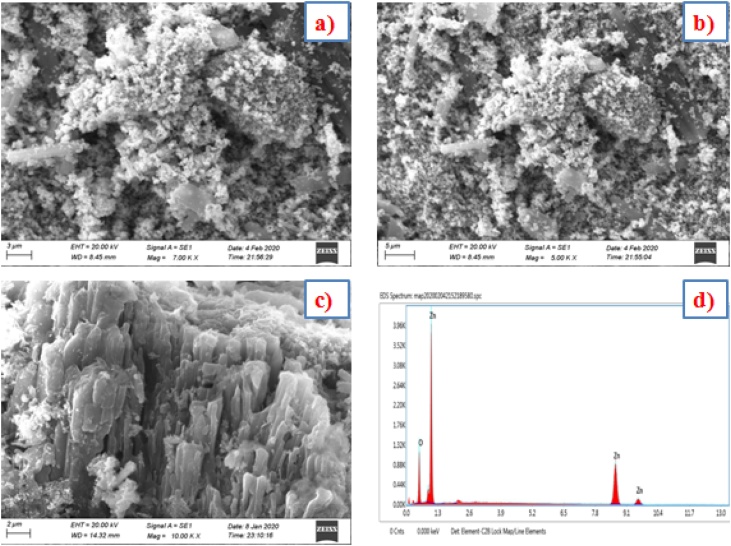
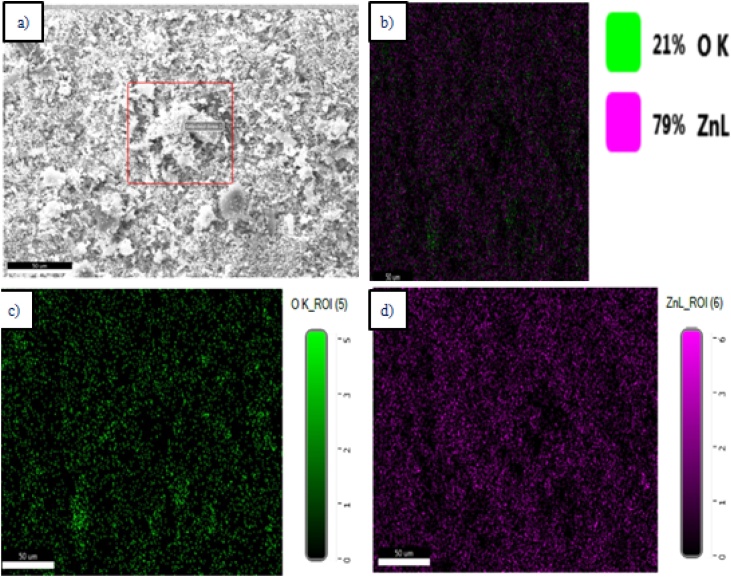
Fig. 4 a–c was showing the NPs were Rod shaped and 64−80 nm size. The EDX profile showed a strong Zn signal and other signal value Oxygen which obviously display the emergence of ZnO-NPs lacking any rubbish (Fig. 4d). Fig. 5 illustrates the corresponding Elemental mapping showed that the (Zinc 79 % and Oxygen 21 %) cleanliness of the Zn nanoparticles. In Similar, SEM and Edax results were searched by ZnO-NPs using biofavonoid rutin [5]. In another reported on Zinc oxide nanorods (ZnO) showed NP’s were rod shaped [35].
Fig. 4.
(a−c) Scanning electron microscope images of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE) at different magnification (a) 700x-3 μm, (b) 500x-5 μm, (c)1000x-2 μm) (d) Edax Spectrum showed the presence of Zn signals.
Fig. 5.
Elemental mapping of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE) (a) Selected area (b) Overview of Elemental map (c) Percentage of Oxyxen (21 %) (d) Percentage of Zinc (79 %).
3.5. Antioxidant assays
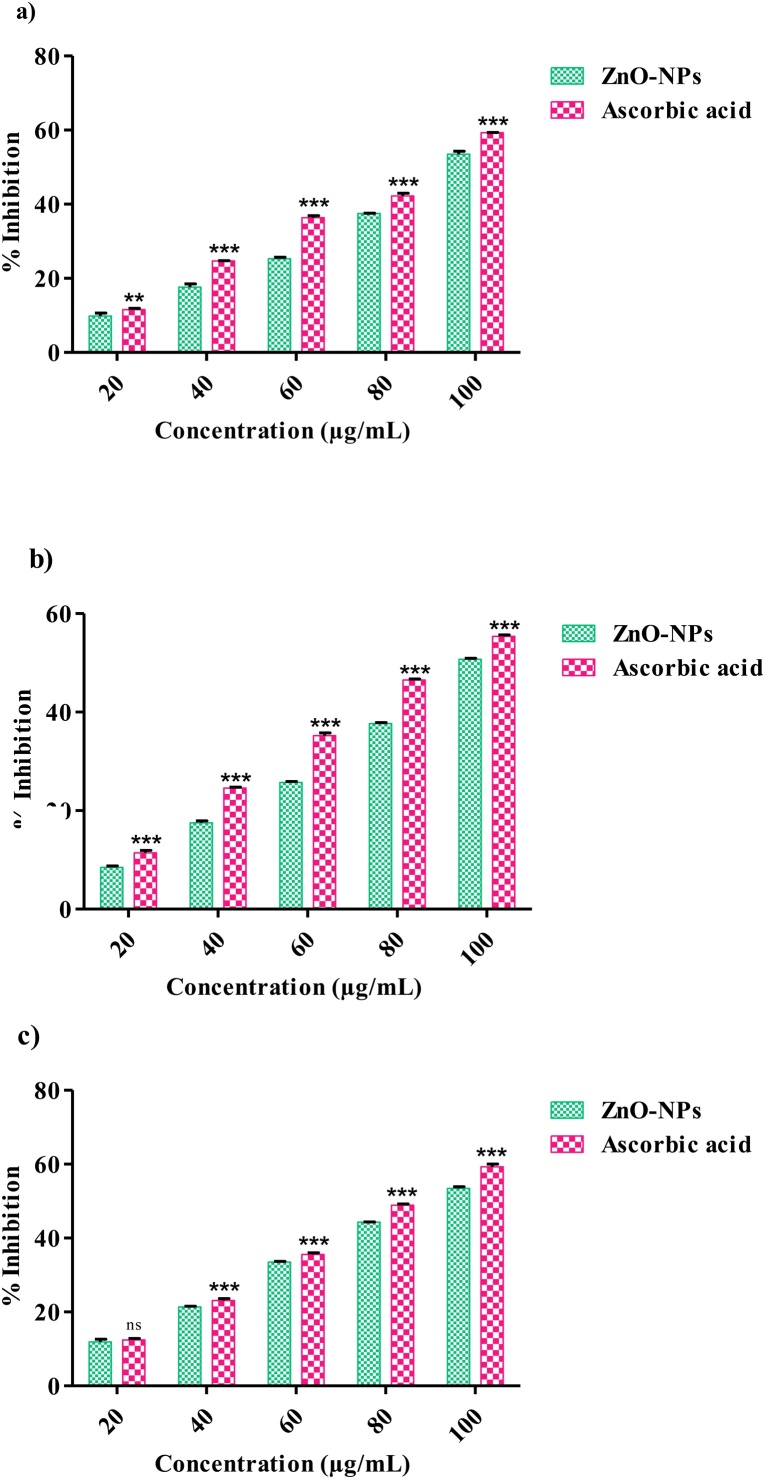
The antioxidant potential of K. sumatrensis leaf extract of Zno-NPs was determined by DPPH, ABTS and H2O2 assays. The percentage of inhibition of DPPH radical scavenging activity is shown in Fig. 6a. In this study, DPPH activity was dose depended manner. In similar, dose depended activity were find out in ZnO-NPs using Barberis aristata leaf extract [36]. DPPH IC50 value for sample is 95.80 μg/mL and standard (Vitamin−C) IC50 is 87.62 μg/mL. The free radical scavenging activity was tested using ABTS assay. When ZnO-NPs and Standard (Vitamin−C) showed 50.70 % as well as 55.39 % scavenging activity respectively at 100 μg/mL (Fig. 6b). This study showed dose dependent radical scavenging activity of ZnO-NPs. Similar dose depended scavenging activity were highlighted in Aspergillus niger derived ZnO-NPs [37]. ABTS IC50 value for sample is 92.29 μg/mL and standard (Vitamin−C) IC50 is 81.85 μg/mL. The radical scavenging activity was tested using H2O2 assay. The percentage of inhibition was shown in Fig. 6c. With increasing concentrations of ZnO-NPs increased percentage of inhibition is observed. H2O2 − IC50 value for sample is 98.92 μg/mL and standard (Vitamin−C) IC50 is 87.24 μg/mL. In recent study, were find ZnO-NPs has good radical scavenging activity against H2O2 with IC50 value is 417.22 μg/mL [38]. Hydroxyl assay mainly depends on the presence of phenolic compounds in the ZnO-NPs [39]. K. sumatrensis is a better antioxidant activity due to presence of some secondary metabolites.
Fig. 6.
Antioxidant activity of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE). (a) DPPH radical scavenging activity. The values are expressed as mean ± SD values and analyzed by Two−Way analysis of variance (ANOVA). Asterisk (**, ***) indicates significant different among treatments with respect to control (P < 0.01 and P < 0.001). (b) ABTS radical scavenging activity. The values are expressed as mean ± SD and analyzed by Two−Way analysis of variance (ANOVA). Asterisk (***) indicates significant different among treatments with respect to control (P < 0.001). (c) Hydroxyl scavenging activity. The values are expressed as mean ± SD and analyzed by Two−Way analysis of variance (ANOVA). Not Significant (ns). Asterisk (***) indicates significant different among treatments with respect to control (P < 0.001).
3.6. Anti-proliferative activity
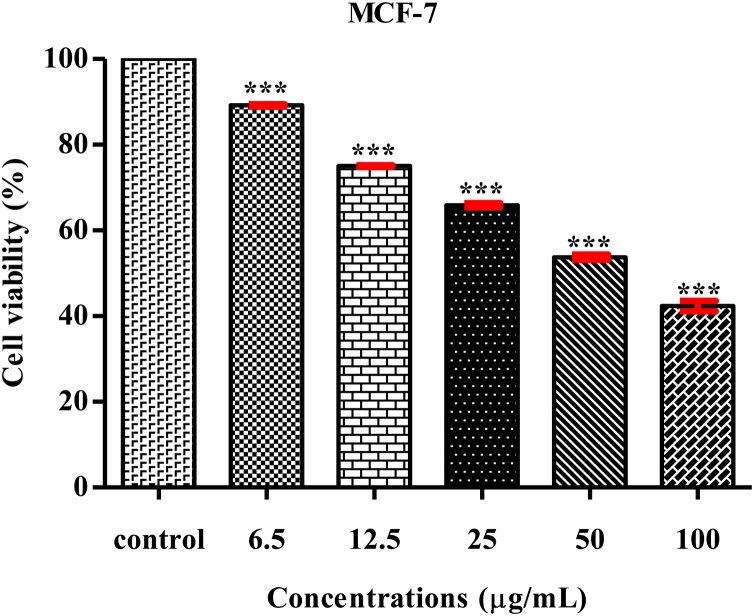
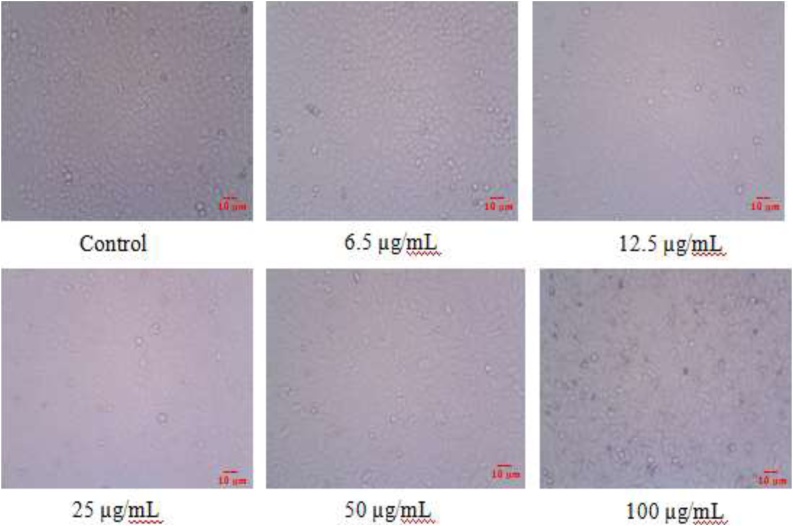
In this study, ZnO-NPs using K. sumatrensis−ALE against human breast cancer cell line (MCF-7) by MTT assay at 24 h. The percentage of cell viability was gradually decreased in increasing of ZnO-NPs concentrations (6.5–100 μg/mL) (Fig. 7). The MCF-7 cell treated image is shown in Fig. 8. In this study showed dose depended anti-proliferative activity. In similar dose depended anti-proliferative activity were noticed in ZnO-NPs against MCF-7 cell line [40,35]. Further, the IC50 value was calculated from ZnO-NPs against MCF-7 cell line and it’s found to be 58.87 μg/mL. In similar study, were observed by Solanum trilobatum derived ZnO-NPs showed IC50 value is 85.05 μg/mL [41]. Our present result showed that, the biosynthesis ZnO-NPs exhibit higher rate of breast cancer activity of 6.5 μg/mL at their lower concentration of 100 μg/mL.
Fig. 7.
MTT assay confirming the anti-proliferative activity of ZnO-NPs against MCF-7 cell line. The values are expressed as mean ± SD values and analyzed by One -Way analysis of variance (ANOVA). Asterisk (***) indicates significant different among treatments with respect to control (P < 0.001).
Fig. 8.
Anti−proliferative observed from confocal microscope (340 pixel); Control and various concentrations (6.5, 12.5, 25, 50 and 100 μg/mL) of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE).
3.7. AO/EtBr staining
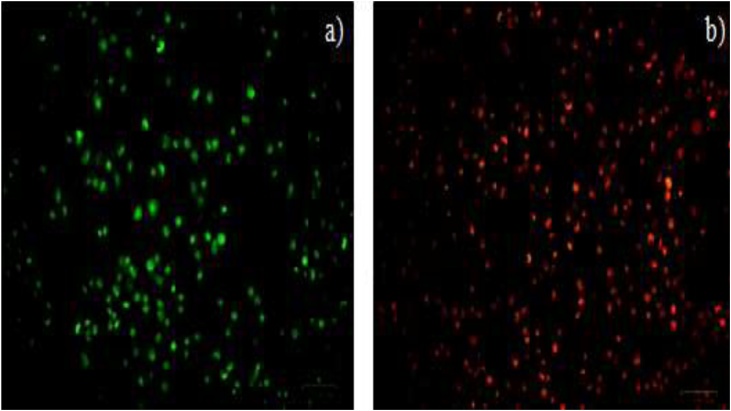
The apoptotic morphology changes of MCF-7 cell line were stained through AO/Etbr using ZnO-NPs (Fig. 9). Ordinary tumor cells, early and late apoptotic cells, and necrotic cells were inspected by means of fluorescent microscopy. In this study, the staining normal cells were showed the green in colour and treated cells showed orange colour which are apoptotic bodies characterized by chromatin. Furthermore, same stain was noted that the Aspergillus niger against human hepatocellular carcinoma cells (HepG2) cell line [37]. When Similar, AO/EtBr staining was finding in santalum album against MCF-7 [42].
Fig. 9.
AO/EB staining of MCF-7 cells of ZnO-NPs using K. sumatrensis aqueous leaf extract (ALE) (a) Control-Live green cells (b) Treated cells Orange are apoptotic bodies.
3.8. Larvicidal activity
ZnO-NPs and K. sumatrensis−ALE were tested against IVth instar larvae of Ae. aegypti, An. stephensi and Cx. quinquefasciatus vectors was assessed. Both extract (ZnO-NPs and K. sumatrensis – ALE) at different concentration like as 5, 10, 15, 20, and 25 mg/L were tested (Table 2, Table 3, Table 4). The maximum larval die was observed by ZnO-NPs when compared to K. sumatrensis – ALE. The both extract concentration when increased result in increased mosquito mortality rate. Similar dose-depended larvicidal activity was noticed in AgNPs in Elytraria acaulis leaf extract [43]. The synthesized ZnO-NPs shows LC50 and LC90 value for Cx. quinquefasciatus, 0.08 and 19.46 mg/mL followed by An. stephensi and Ae. aegypti is 04.67, 22.95, 0.98, 22.68 at 48 h respectively. In previous study, Yaziniprabha et al. [9] reported that ZnO-NPs using Murraya koenigii were showed significant larvicidal activity against Cx. quinquefasciatus larvae with LC50 and LC90 value is 2.1 and 12.1 μg/mL. In this investigation, ZnO-NPs indicated potential larvicidal action and results can be useful to create nanobased mosquito larvicides, due to less hazardous to the environment.
Table 2.
Larvicidal activity of K. sumatrensis aqueous leaf extract (ALE) and synthesized ZnO-NPs against IVth instar larvae of Aedes aegypti.
| Time (Hour) |
Samples | LC50 (mg/mL) (LCL-UCL) |
LC90 (mg/mL) (LCL-UCL) |
χ2 | df |
|---|---|---|---|---|---|
| 12 | Plant extract | 37.36 (29.08−66.62) | 67.29 (48.42−137.29) | 1.79 | 13 |
| ZnO-NPs | 23.19 (19.42−31.37) | 51.82 (39.85−84.76) | 1.74 | 13 | |
| 24 | Plant extract | 26.01 (21.88−35.34) | 52.35 (40.70−82.84) | 1.32 | 13 |
| ZnO-NPs | 10.35 (07.08−12.66) | 29.56 (25.30−37.51) | 1.49 | 13 | |
| 48 | Plant extract | 16.06(13.05−19.47) | 41.56 (33.42−60.71) | 0.76 | 13 |
| ZnO-NPs | 0.98 (3.55−5.27) | 22.68 (19.09−30.21) | 1.67 | 13 |
LC50-Lethal concentration kills 50 % of the exposed larvae, LC90-Lethal concentration kills 90 % of the exposed larvae. LCL-Lower confidence limit, UCL-Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
Table 3.
Larvicidal activity of K. sumatrensis aqueous leaf extract (ALE) and synthesized ZnO-NPs against IVth instar larvae of Anopheles stephensi.
| Time (Hour) |
Samples | LC50 (mg/mL) (LCL-UCL) |
LC90 (mg/mL) (LCL-UCL) |
χ2 | df |
|---|---|---|---|---|---|
| 12 | Plant extract |
28.13 (23.92−37.23) | 50.87 (40.47−75.78) | 1.38 | 13 |
| ZnO-NPs | 17.87 (14.95−21.90) | 43.56 (34.84−64.41) | 1.72 | 13 | |
| 24 | Plant extract | 24.24 (20.34−32.92) | 30.75 (26.78−37.61) | 0.867 | 13 |
| ZnO-NPs | 06.31 (01.12−09.25) | 26.71 (22.67−34.65) | 2.32 | 13 | |
| 48 | Plant extract | 15.54 (12.32−18.99) | 42.05 (33.54−62.83) | 0.812 | 13 |
| ZnO-NPs | 04.67 (0.8−7.65) | 22.95 (19.74−28.81) | 1.85 | 13 |
LC50-Lethal concentration kills 50 % of the exposed larvae, LC90-Lethal concentration kills 90 % of the exposed larvae. LCL-Lower confidence limit, UCL-Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
Table 4.
Larvicidal activity of K. sumatrensis aqueous leaf extract (ALE) and synthesized ZnO-NPs against IVth instar larvae of Culex quinquefasciatus.
| Time (Hours) |
Samples | LC50(mg/mL) (LCL-UCL) |
LC90(mg/mL) (LCL-UCL) | χ2 | df |
|---|---|---|---|---|---|
| 12 | Plant extract | 25.06 (21.45−32.37) | 48.85 (38.97−72.17) | 1.19 | 13 |
| ZnO-NPs | 12.72 (10.22−14.83) | 30.85 (26.59−38.54) | 1.67 | 13 | |
| 24 | Plant extract | 20.68 (17.33−26.92) | 49.06 (38.03−78.35) | 0.93 | 13 |
| ZnO-NPs | 3.24 (3.18−6.69) | 22.81 (19.44−29.17) | 1.80 | 13 | |
| 48 | Plant extract | 10.99 (6.99−13.72) | 34.60 (28.52−47.88) | 0.85 | 13 |
| ZnO-NPs | 0.08 (08.70−4.29) | 19.46 (16.46−24.89) | 2.53 | 13 |
LC50-Lethal concentration kills 50 % of the exposed larvae, LC90-Lethal concentration kills 90 % of the exposed larvae. LCL-Lower confidence limit, UCL-Upper confidence limit, χ2 Chi-square value, df = degrees of freedom.
4. Conclusion
Over all this study, the green synthesized of ZnO-NPs using K. sumatrensis Aqueous leaf extract (ALE) is eco-friendly approached and to effectively control of tested mosquito vectors and also potentially decrease the cell viability against MCF-7 cell line. The UV visible spectral result shows the absorbance peak at 354 nm. The Characterized in FT-IR functional groups and the compounds names are C—X, CH—O—H, S O, OH, C N, P—H, C—C C and O—H. The NP’s were crystalline structure were analyzed by XRD. SEM characterizations shows rod shaped. Besides, the ZnO-NPs have fine antioxidant activity. In addition, the anti-proliferative study showing excellent activity and also IC50 value is 58.87 μg/mL against MCF-7. On other hand, fine larvicidal activity was observed in Cx. quinquefasciatus larvae. Later on, making a new path for mosquito control and various biomedical applications using biologically synthesized ZnO-NPs.
CRediT authorship contribution statement
Settu Loganathan: Lab related work to bioassay tests, preparation of the plant extract and data analysis. Settu Loganathan and Sengodan Karthi: larvicidal activity was analyzed. Sengodan Karthi: the experimental work designed. Kuppusamy Selvam: Manuscript corrected. Kuppusamy Selvam, Sengottayan Senthil Nathan and Muthugounder Subaramanian Shivakumar: All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors Acknowledge to Periyar University, Salem to providing University Research Fellowship (Ref. No. PU/AD-3/URF/2016). The authors also thank to Department of Botany, School of Life Sciences, Periyar University, Salem, Tamil Nadu-636 011, India, for providing infrastructural facility.
Edited by Dr. A.M Tsatsaka
References
- 1.Suganya P., Vaseeharan B., Vijayakumar S., Balan B., Govindarajan M., Alharbi N.S., Benelli G. Biopolymer zinc-coated gold nanoparticles: synthesis, antibacterial potential, toxicity and histopathological effects against the Zika virus vector Aedes aegypti. J. Photochem. Photobiol. B, Biol. 2017;173:404–411. doi: 10.1016/j.jphotobiol.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran S.P., Sengodan K. Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. Int. J. Nanosci. 2017;2017:1–7. [Google Scholar]
- 3.Pikula K., Mintcheva N., Kulinich S.A., Zakharenko A., Markina Z., Chaika V., Golokhvast K. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ. Res. 2020 doi: 10.1016/j.envres.2020.109513. [DOI] [PubMed] [Google Scholar]
- 4.Varmazyari A., Taghizadehghalehjoughi A., Sevim C., Baris O., Eser G., Yildirim S., Aschner M. Cadmium sulfide-induced toxicity in the cortex and cerebellum: in vitro and in vivo studies. Toxicol. Rep. 2020;7:637–648. doi: 10.1016/j.toxrep.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharathi D., Bhuvaneshwari V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: antibacterial, antioxidant and cytotoxic activities. Res. Chem. Intermed. 2019;45:2065–2078. [Google Scholar]
- 6.Saranya S., Vijayaranai K., Pavithra S., Raihana N., Kumanan K. In vitro cytotoxicity of zinc oxide, iron oxide and copper nanopowders prepared by green synthesis. Toxicol. Rep. 2017;4:427–430. doi: 10.1016/j.toxrep.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sood K., Kaur J., Singh H., Arya S.K., Khatri M. Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol. Rep. 2019;6:768–781. doi: 10.1016/j.toxrep.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayakumar S., Vaseeharan B., Sudhakaran R., Jeyakandan J., Ramasamy P., Sonawane A., Faggio C. Bioinspired zinc oxide nanoparticles using Lycopersicon esculentum for antimicrobial and anticancer applications. J. Clust. Sci. 2019;30:1465–1479. [Google Scholar]
- 9.Yazhiniprabha M., Vaseeharan B., Sonawane A., Behera A. In vitro and in vivo toxicity assessment of phytofabricated ZnO nanoparticles showing bacteriostatic effect and larvicidal efficacy against Culex quinquefasciatus. J. Photochem. Photobiol. B, Biol. 2019;192:158–169. doi: 10.1016/j.jphotobiol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Vivek K., Rao R. Some interesting indigenous beverages among the tribals of Central India. Ind. J. Trad. Knowl. 2007;6:141–143. [Google Scholar]
- 11.Ayyanar M., Ignacimuthu S. Herbal medicines for wound healing among tribal people in Southern India: ethnobotanical and Scientific evidences. Int. J. Appl. Res. Nat. Prod. 2009;2:29–42. [Google Scholar]
- 12.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro. Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patra S., Mukherjee S., Barui A.K., Ganguly A., Sreedhar B., Patra C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. 2015;53:298–309. doi: 10.1016/j.msec.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 14.Taghizadehghalehjoughi A., Hacimuftuoglu A., Cetin M., Ugur A.B., Galateanu B., Mezhuev Y., Gundogdu B. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: in vitro and in vivo studies. Nanomedicine. 2018;13:1595–1606. doi: 10.2217/nnm-2017-0386. [DOI] [PubMed] [Google Scholar]
- 15.Strakhov I.S., Mezhuev Y.O., Korshak Y.V., Kovarskii A.L., Shtil’man M.I. Preparation of magnetite nanoparticles modified with poly (о-phenylenediamine) and their use as drug carriers. Russ. J. Appl. Chem. 2016;89:447–450. [Google Scholar]
- 16.Luss A.L., Kulikov P.P., Romme S.B., Andersen C.L., Pennisi C.P., Docea A.O., Tsatsakis A.M. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomedicine. 2018;13:703–715. doi: 10.2217/nnm-2017-0311. [DOI] [PubMed] [Google Scholar]
- 17.World health organization (WHO), Dengue (2018a). https://www.who.int/denguecontrol/disease/en/.
- 18.World health organization (WHO), 2018 Malaria. https://www.who.int/malaria/media/worldmalaria-report-2018/en/.
- 19.World health organization (WHO), Filariasis (2018b). https://www.who.int/lymphaticfilariasis/managing-morbidity/en/f.
- 20.González J.O.W., Jesser E.N., Yeguerman C.A., Ferrero A.A., Band B.F. Polymer nanoparticles containing essential oils: new options for mosquito control. Environ. Sci. Pollut Res. 2017;24:17006–17015. doi: 10.1007/s11356-017-9327-4. [DOI] [PubMed] [Google Scholar]
- 21.Ga’al H., Fouad H., Mao G., Tian J., Jianchu M. Larvicidal and pupicidal evaluation of silver nanoparticles synthesized using Aquilaria sinensis and Pogostemon cablin essential oils against dengue and zika viruses vector Aedes albopictus mosquito and its histopathological analysis. Artif. Cells Nanomed. Biotechnol. 2018;46:1171–1179. doi: 10.1080/21691401.2017.1365723. [DOI] [PubMed] [Google Scholar]
- 22.Saini H., Yadav R., Kumar D., Kumar G., Agrawal V. Cullen corylifolium (L.) Medik. Seed extract, an excellent system for fabrication of silver nanoparticles and their multipotency validation against different mosquito vectors and human cervical cancer cell line. J. Clust. Sci. 2020;31:161–175. [Google Scholar]
- 23.Rajkumar R., Shivakumar M.S., Nathan S.S., Selvam K. Pharmacological and larvicidal potential of green synthesized silver nanoparticles using Carmona retusa (Vahl) Masam leaf extract. J. Clust. Sci. 2018;29:1243–1253. [Google Scholar]
- 24.Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agr. Food. Chem. 1992;40:945–948. [Google Scholar]
- 25.Rajeshwar Y., Senthilkumar G.P., Malay A.G., Mazumder U.K. Studies on in vitro antioxidant activities of methanol extract of Mucuma pruriens (Fabaceae) seeds. Europ. Bull. Drug Res. 2005;13:131–138. [Google Scholar]
- 26.Giao M.S., Gonzalez S.M.L., Rivero P.M.D., Pereira C.I., Pintado M.E., Malcata F.X. Infusions of Portuguese medicinal plants: dependence of final antioxidant capacity and phenolic content on extraction features. J. Sci. Food Agric. 2007;87:2638–2647. doi: 10.1002/jsfa.3023. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunologic. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO) World Health Organization; Sudan: 2005. Communicable Disease Tool Kit. WHO/CDS/2005.26. [Google Scholar]
- 29.Thandapani K., Kathiravan M., Namasivayam E., Padiksan I.A., Natesan I.A., Tiwari M., Perumal V. Environ. Sci. Pollut. R. 2018;25:10328–10339. doi: 10.1007/s11356-017-9177-0. [DOI] [PubMed] [Google Scholar]
- 30.Abbott W.S. A method of computing the effectiveness of an insecticide, J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- 31.Chaudhuri S.K., Malodia L. Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosc. 2017;7:501–512. [Google Scholar]
- 32.Rajapriya M., Sharmili S.A., Baskar R., Balaji R., Alharbi N.S., Kadaikunnan S., Vaseeharan B. Synthesis and characterization of zinc oxide nanoparticles using Cynara scolymus leaves: enhanced hemolytic, antimicrobial, antiproliferative, and photocatalytic activity. J. Clust. Sci. 2019;31:791–801. [Google Scholar]
- 33.Ishwarya R., Vaseeharan B., Kalyani S., Banumathi B., Govindarajan M., Alharbi N.S., Benelli G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B, Biol. 2018;178:249–258. doi: 10.1016/j.jphotobiol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Hussain A., Oves M., Alajmi M.F., Hussain I., Amir S., Ahmed J., Ali I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv. 2019;9:15357–15369. doi: 10.1039/c9ra01659g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadhukhan P., Kundu M., Rana S., Kumar R., Das J., Sil P.C. Microwave induced synthesis of ZnO nanorods and their efficacy as a drug carrier with profound anticancer and antibacterial properties. Toxicol. Rep. 2019;6:176–185. doi: 10.1016/j.toxrep.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra H., Patel D., Kumari P., Jangwan J.S., Yadav S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. 2019;102:212–220. doi: 10.1016/j.msec.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y., Anand M.A.V., Ramachandran V., Karthikkumar V., Shalini V., Vijayalakshmi S., Ernest D. Biofabrication of zinc oxide nanoparticles from Aspergillus niger, their antioxidant, antimicrobial and anticancer activity. J. Clust. Sci. 2019;30:937–946. [Google Scholar]
- 38.Manimaran K., Balasubramani G., Ragavendran C., Natarajan D., Murugesan S. Biological applications of synthesized zno nanoparticles using Pleurotus djamor against mosquito larvicidal, histopathology, antibacterial, antioxidant and anticancer effect. J. Clust. Sci. 2020 doi: 10.1007/s10876-020-01927-z. [DOI] [Google Scholar]
- 39.Anbukkarasi M., Thomas P.A., Sheu J.R., Geraldine P. In vitro antioxidant and anticataractogenic potential of silver nanoparticles biosynthesized using an ethanolic extract of Tabernaemontana divaricata leaves. Biomed. Pharmacother. 2017;91:467–475. doi: 10.1016/j.biopha.2017.04.079. [DOI] [PubMed] [Google Scholar]
- 40.Shwetha U.R., Latha M.S., Kumar C.R., Kiran M.S., Betageri V.S. Facile synthesis of zinc oxide nanoparticles using novel Areca catechu leaves extract and their in vitro antidiabetic and anticancer studies. J. Inorg. Organomet. Polym. Mater. 2020 doi: 10.1007/s10904-020-01575-w. [DOI] [Google Scholar]
- 41.Guruviah K., Annamalai S.K., Ramaswamy A., Sivasankaran C., Ramasamy S., Barabadi H., Saravanan M. Comparative antimicrobial and anticancer activity of biologically and chemically synthesized zinc oxide nanoparticles toward breast cancer cells. Nanomed. J. 2020;7:272–283. [Google Scholar]
- 42.Kavithaa K., Paulpandi M., Ponraj T., Murugan K., Sumathi S. Induction of intrinsic apoptotic pathway in human breast cancer (MCF-7) cells through facile biosynthesized zinc oxide nanorods. Karb. Inter. J. Moder. Sci. 2016;2:46–55. [Google Scholar]
- 43.Rangayasami A., Kannan K., Joshi S., Subban M. Bioengineered silver nanoparticles using Elytraria acaulis (Lf) Lindau leaf extract and its biological applications. Biocatal. Agric. Biotechnol. 2020;27 [Google Scholar]