Abstract
Background and Aim
Tricuspid regurgitation (TR) is a frequent complication in various cardiovascular diseases. However, few studies have reported the prevalence of TR especially the moderate to severe or significant TR (ms-TR) maintenance dialysis patients. Thus, we aimed to identify the prevalence of ms-TR and its associated factors.
Methods
A total of 491 maintenance dialysis patients underwent echocardiographic examinations, while a subgroup (n = 283) also received routine blood tests, renal function examinations, and electrolyte analysis. We first compared the differences in abovementioned parameters among groups with various TR areas (TRAs). Finally, univariate and adjusted regression were also used to identify factors that were independently associated with ms-TR.
Results
The incidence of TR jets was 62.6%, which included a mildly increased TRA (47.8%), moderately increased TRA (10.4%), and severely increased TRA (3.5%). Most of the cardiac structures and functional parameters, such as the end-diastolic internal diameters of the left atrium (LA), left ventricle (LVDD), right atrium (RA), right ventricle (RV), left ventricular ejection fraction (LVEF), and fractional shortening (FS), were significantly associated with ms-TR. Among serum ions, only total CO2 (TCO2; r = −0.141, p = 0.047) was negatively correlated with TRA. After adjusted, only Na+ [odds ratio (OR): 0.871 0.888, p = 0.048], RA (OR: 1.370, p < 0.001), and FS (OR: 0.887, p < 0.001) were independently associated with ms-TR.
Conclusion
Tricuspid regurgitation occurs in maintenance hemodialysis patients with ESRD. Na+ FS and RA were independently associated with ms-TR, and these parameters may be potential risk factors/predictors for ms-TR.
Keywords: prevalence, tricuspid regurgitation, maintenance dialysis, end-stage renal disease, retrospective study
Introduction
End-stage renal disease (ESRD) has been demonstrated to be accompanied by various cardiovascular complications, which usually have poor outcomes (Kim and Parekh, 2015; Ramesh et al., 2016; Samanta et al., 2019). It has been reported that cardiovascular events, including heart failure, are the leading causes of death in ESRD patients (Kooman and van der Sande, 2019; Sugiura et al., 2019; Yogasundaram et al., 2019). The progressively deteriorating structure and function of the heart may be the consequence of renal failure (Cutshall et al., 2018; Unlu et al., 2018). On other hand, the insufficient perfusion of the kidney from the heart may aggravate renal dysfunction. Previous studies mainly focused on structural and functional cardiac changes in the left heart in ESRD patients and identified several biochemical markers, clinical factors, and hemodynamic predictors (Mahfouz et al., 2013; Minami et al., 2016; Lemor et al., 2018; Khan et al., 2019; Yogasundaram et al., 2019).
However, the structure and function of the right heart in ESRD patients, especially those who underwent dialysis treatment, have not been extensively studied. Tricuspid regurgitation (TR), which includes functional or mild TR, moderate TR and significant severe TR, is one of the most important functions of the right heart and is highly prevalent in various populations (Agricola et al., 2017; Al-Hijji et al., 2017; Sun and O’Gara, 2017). However, the prevalence and associated factors have not been identified in ESRD patients who received maintenance dialysis. TR may contribute to renal dysfunction in heart failure patients (Maeder et al., 2008).
Many previous studies have been studied the roles of tricuspid regurgitation in right heart failure in hemodialysis patients (Gumus and Saricaoglu, 2020). Part of the associated factors have also been found. However, interaction and relationship between TR especially ms-TR or significant TR and renal dysfunction attached less attentions. Furthermore, the prevalence of ms-TR in the special subgroup populations of dialysis patients has not been studied deeply (Cakici et al., 2020). Thus, we performed this retrospective study to identify the prevalence of ms-TR and potentially associated factors in a large sample size of a special population: ESRD patients who underwent maintenance dialysis.
Materials and Methods
Study Design and Populations
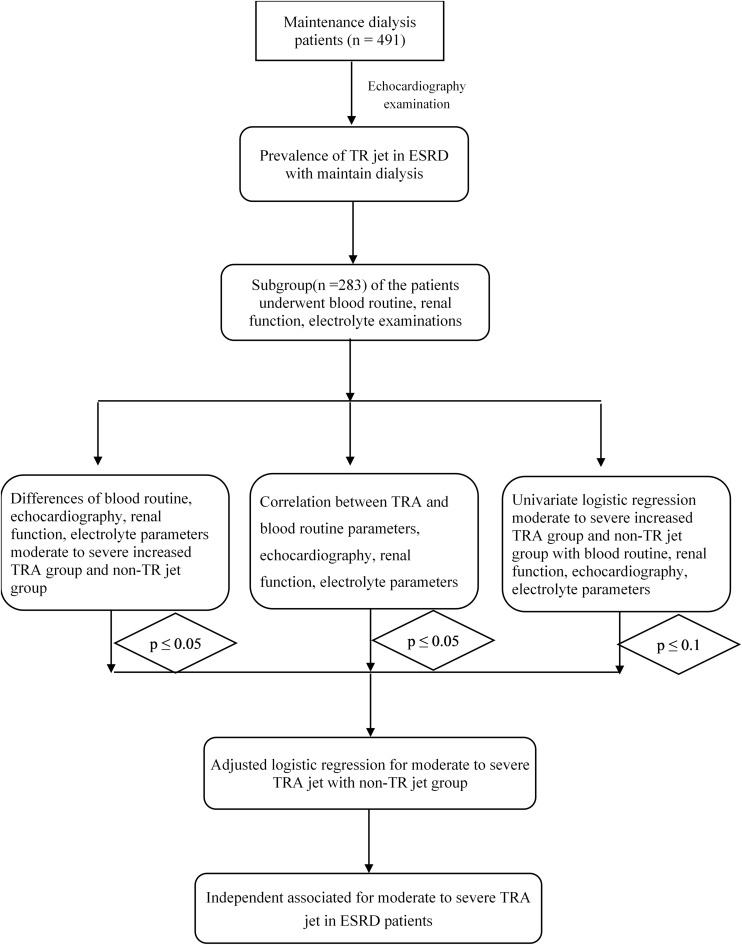
From March 2011 to June 2019, ESRD patients who received maintenance dialysis (n = 491) at the Nephrology of Chongqing and Kidney Center of PLA, Xinqiao Hospital, and Army Medical University (Third Military Medical University) were included in this retrospective study. These hemodialysis patients underwent echocardiographic examinations, while a subgroup (n = 283) of this population also received routine blood tests, renal function examinations, and electrolyte analysis, which are shown in Figure 1.
FIGURE 1.
The flow chart of this study.
All of the ESRD patients were informed about the purpose and procedures of our study and provided written informed consent. Our present study complied with the Declaration of Helsinki.
In addition, the ethics committee of the Second Affiliated Clinic Hospital (Xinqiao Hospital) and Army Medical University (Third Military Medical University) approved the study.
Procedures of Echocardiographic Examinations
The echocardiographic examinations of each patient were carried out by a skilled echocardiographer, Dr. Rongsheng Rao, according to the American Society of Echocardiography recommendations (Mansour et al., 2010). Echocardiographic examinations were performed in a supine position after resting for 10 min after hemodialysis. Two-dimensional echocardiographically guided motion mode (M-mode) images were obtained from standardized views by using an ultrasonography system (CX50, Philips, United States) with an S5 probe.
Among the echocardiographic parameters, our trained physicians recorded the left atrial end-diastolic internal diameter (LA), left ventricular end-diastolic internal diameter (LVDD), right atrial end-diastolic internal diameter (RA), right ventricular end-diastolic internal diameter (RV), and pulmonary arterial end-diastolic internal diameter (PA).
Additionally, the mobility and size of the left ventricular posterior wall (LVPW), the mobility and the size of the interventricular septum (IVS), fractional shortening (FS) and left ventricular ejection fraction (LVEF) and stroke volume (SV) were also recorded. Finally, TR-related parameters, such as TR area (TRA), TR velocity (TRV), and TR pressure, were also measured and recorded.
Biomarker Determination
After admission to the hospital, we collected venous blood samples early in the morning after patients had fasted for more than 12 h before dialysis. The blood samples have also been obtained after dialysis in order to assess the effect of dialysis.
First, renal function, such as eGFR, plasma creatinine (Cr) and the blood urea nitrogen (BUN) concentration, was examined. Cr was also tested with Roche Diagnostics GmbH products (enzyme assay, Abbott, i2000, United States). BUN was also measured with ferene methods (Beckman AU5821, GA, United States).
Then, we performed routine blood examinations. An automated hematology chemistry analyzer was used to examine the hemoglobin concentration (Hb), red blood cell (RBC) count (RBC), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW) (type: AU400; Olympus Optical, Co., Tokyo, Japan). Furthermore, we also examined the white blood cell (WBC) and platelet (PLT) counts. The lymphocyte ratio (LYM), basophil ratio (BAS), neutrophil ratio (NEU), eosinophil ratio (EO), and monocyte ratio (MONO) were also tested and recorded.
Finally, we also performed an electrolyte examination, which included the sodium concentration (Na+, indirect ion selective electrode assay with EX-Z, JOKOH, Japan), serum calcium concentration (Ca2+, tri-azo methods), and phosphate concentration (P, phosphomolybdate ultraviolet assay, Roche Diagnostics GmbH, United States). In addition, we also measured the serum potassium concentration (K+, ferene methods, Beckman AU5821).
Variable Definitions
First, patients were divided into the non-TR group (without a TR jet), the mildly increased TRA group (0 < TRA < 5 cm2) and the moderately to severely increased TRA group (ms-TR, TRA ≥ 5 cm2) which is also called significant TR (ms-TR in this study).
We calculated the estimated glomerular filtration rate (eGFR) by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula: eGFR (ml/min/1.73 m2) = 1.41 × min(Cr/k,1)α × max(Cr/,1)–1.209 × 0.993age × 1.018 [k = 0.7 (female) and 0.9 (male); α = −0.329 (female) and −0.411 (male)].
Statistical Analysis
If a continuous variable was normally distributed, it is presented as the mean ± standard deviation (SD). We employed one-way ANOVA to compare differences among the non-TR, mildly increased TRA and moderately to severely increased TRA groups. Further analysis comparing two groups was performed using least significant difference (LSD) methods after one-way ANOVA.
If one variable was non-normally distributed, it is presented as the median (25–75%). Comparisons among the non-TR, mildly increased TRA and moderately to severely increased TRA groups of variables with non-normal distributions were performed by one-way ANOVA after being transformed to achieve normality.
We performed univariate logistic regression analyses with each variable to screen for factors associated with moderate to severe TR. Variables with a p value less than 0.1 in the univariate logistic regression were included in multivariate (adjusted) logistic regression analyses to identify factors that were independently associated with moderate to severe TR. Our statistical analyses were performed by using the statistical software SPSS 22.0 (CA, United States) for Mac.
Results
Basic Information and the Prevalence of TR in the Study Populations
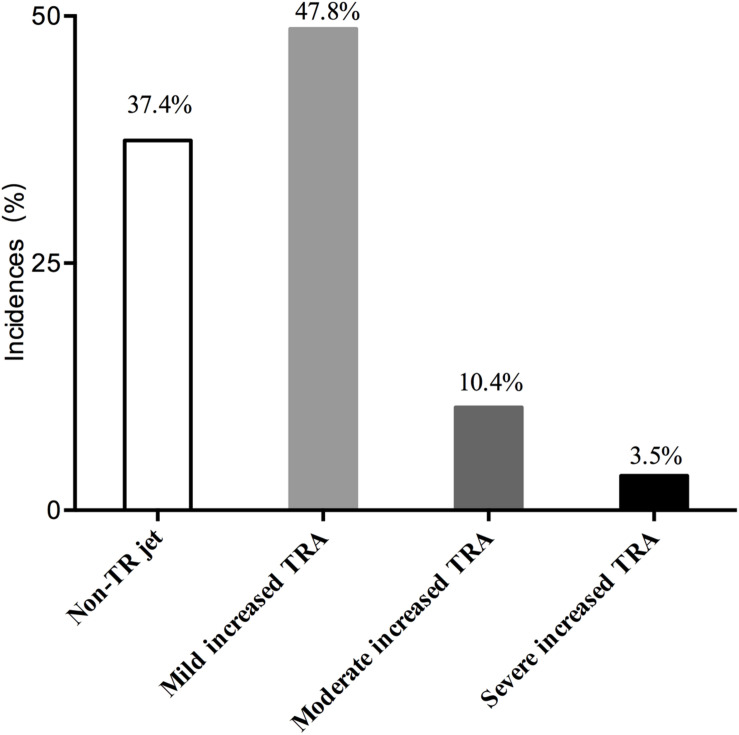
In our study, 491 ESRD patients were included. The mean age was 52.95 ± 14.21 years, and the mean body-mass index (BMI) was 21.89 ± 4.89 kg/m2. The incidence of a TR jet was 62.6%, which included a mildly increased TRA (47.8%), a moderately increased TRA (10.4%), and a severely increased TRA (3.5%). Thus, the incidence of ms-TR was 13.9%. These results are shown in Figure 2.
FIGURE 2.
Distributions of TRA in the ESRD patients.
Differences in Demographic, Echocardiographic, Routine Blood, Electrolyte and Renal Function Parameters Among Various TRA Groups
First, we did not find any differences in the demographic data (age and BMI, p > 0.05) among the non-TR, mildly increased TRA and ms-TR groups.
Second, in the echocardiographic parameters, both left heart and right heart structures and functions showed significant differences among these three groups. LA (43.93 ± 6.58 vs. 36.74 ± 4.75) and LVDD (54.11 ± 7.18 vs. 48.80 ± 5.25) were significantly higher in the ms-TR group than in the non-TR group (all p values < 0.001). These parameters were also significantly higher in the ms-TR group than in the mildly increased TRA group (all p values < 0.001). However, there were no differences in LA and LVDD between the mildly increased TRA and non-TR groups (all p values > 0.05). Though there were no differences in IVS and LVPW thickness among the three subpopulations, and their mobility was significantly different among the various TRA groups. ms-TR patients had worse IVS mobility [3.00 (6.00–6.00)] and a more dispersive LVPW mobility [10.00 (7.00–10.00)] than the other two groups (all p values < 0.001, Table 1). FS was also significantly lower in the ms-TR population [29.00 (18.75–33.25)] than in the non-TR [35.00 (32.00–37.00), p < 0.001] and mildly increased TRA [34.00 (32.00–37.00), p < 0.001] populations. The left heart functions, including LVEF, were significantly different among the three groups. The ms-TR group was characterized by a lower LVEF (50.28 ± 13.93%) than the other two subgroups (62.55 ± 8.01 and 62.28 ± 7.78, p < 0.001). However, SV was similar in the three groups (all p values > 0.05). In addition, PA was also significantly higher in the ms-TR group than in the other groups (25.14 ± 2.58 vs. 23.65 ± 2.44 and 23.68 ± 2.49 mm, p = 0.001, Table 1).
TABLE 1.
Differences between of the hemodynamics, demographic data, renal function, electrolyte and routine blood examination parameters among various TRA groups.
| Overall | Non –TR patients | TRA increased groups | |||
| Parameters | (n = 491 Subgroup n1 = 283) | (n = 84) | Mild increased TR (n = 153) | ms-TR group (n = 46) | p value |
| Demographic data | |||||
| Age (years) | 52.54 ± 14.32 | 52.23 ± 12.50 | 53.22 ± 15.25 | 51.41 ± 14.19 | 0.719 |
| BMI (kg/m2) | 22.45 ± 3.56 | 22.54 ± 3.17 | 22.56 ± 3.79 | 21.87 ± 3.92 | 0.503 |
| Echocardiographic parameters | |||||
| LA (mm) | 38.41 ± 5.77 | 36.74 ± 4.75 | 38.02 ± 4.86 | 43.93 ± 6.58**## | <0.001 |
| LVDD (mm) | 49.30 ± 6.29 | 48.80 ± 5.25 | 48.92 ± 5.93 | 54.11 ± 7.18**## | <0.001 |
| RA (mm) | 37.15 ± 5.11 | 35.52 ± 3.78 | 36.43 ± 4.03 | 44.41 ± 5.36**## | <0.001 |
| RV (mm) | 35.46 ± 5.56 | 34.40 ± 3.40 | 34.90 ± 3.51 | 40.98 ± 5.03**## | <0.001 |
| PA (mm) | 23.82 ± 2.70 | 23.65 ± 2.44 | 23.68 ± 2.49 | 25.14 ± 2.58**## | 0.001 |
| IVS (mm) | 13.04 ± 8.57 | 12.65 ± 1.64 | 12.47 ± 1.57 | 12.22 ± 1.73 | 0.345 |
| IVS mobility | 6.00 (6.00–6.00) | 6.00 (6.00–6.00) | 6.00 (6.00–6.00) | 3.00 (6.00–6.00) **## | <0.001 |
| LVPW (mm) | 11.00 (10.00–12.00) | 11.00 (10.00–12.00) | 11.80 (10.00–12.00)* | 11.20 (10.00–12.00) | 0.144 |
| LVPW mobility | 10.00 (10.00–10.00) | 10.00 (10.00–10.00) | 10.00 (10.00–10.00) | 10.00 (7.00–10.00)**## | <0.001 |
| FS (%) | 34.00 (31.00–37.00) | 35.00 (32.00–37.00) | 34.00 (32.00–37.00) | 29.00 (18.75–33.25)**## | <0.001 |
| LVEF (%) | 60.83 ± 9.44 | 62.55 ± 8.01 | 62.28 ± 7.78 | 50.28 ± 13.93**## | <0.001 |
| SV (ml) | 75.10 ± 20.22 | 74.57 ± 15.53 | 78.41 ± 21.86 | 75.20 ± 18.43**## | 0.301 |
| TRV (cm/s) | 281.00 (243.00–336.00) | – | 265.00 (240.00–311.50) | 354.00 (322.00–384.25) | <0.001 |
| ΔP (mmHg) | 33.00 (22.00–46.00) | – | 28.00 (23.00–38.25) | 50.00 (40.50–59.00) | <0.001 |
| Renal function (n = 283) | |||||
| eGFR [mL/(min⋅1.73 m2)] | 10.47 (5.62–13.00) | 10.47 (5.88–12.04) | 10.03 (5.88–13.00) | 10.34 (5.28–13.50) | 0.981 |
| Cr (μmol/L) | 815.50 (618.40–1016.90) | 821.80 (618.92–1017.45) | 814.40 (598.00–998.25) | 817.35 (643.95–1126.22) | 0.448 |
| BUN (μmoI/L) | 21.75 ± 7.82 | 21.26 ± 7.64 | 21.77 ± 7.58 | 22.57 ± 8.98 | 0.659 |
| Electrolyte parameters | |||||
| Mg2+ (mmol/L) | 0.982 ± 0.158 | 0.983 ± 0.175 | 0.978 ± 0.153 | 0.991 ± 0.143 | 0.877 |
| K+ (mmol/L) | 4.79 ± 0.787 | 4.78 ± 0.72 | 4.78 ± 0.79 | 4.83 ± 0.89 | 0.944 |
| Ca2+ (mmol/L) | 2.15 (2.03–2.28) | 2.15 (2.05–2.280) | 2.15 (2.02–2.28) | 2.14 (1.97–2.36) | 0.957 |
| Na+(mmol/L) | 137.85 (136.40–139.80) | 138.40 (136.50–140.00) | 137.84 (136.35–140.0) | 137.35 (136.07–139.20) | 0.149 |
| Cl– (mmol/L) | 102.45 (99.60–105.30) | 102.45 (99.10–105.20) | 102.45 (99.60–105.50) | 102.45 (99.98–105.80) | 0.882 |
| TCO2 (mmol/L) | 20.66 (18.00–23.00) | 21.05 (18.78–23.95) | 20.50 (18.10–23.00) | 19.90 (16.75–22.02) | 0.075 |
| P (mmol/L) | 1.91 ± 0.64 | 1.96 ± 0.63 | 1.89 ± 0.62 | 1.91 ± 0.69 | 0.722 |
| Dialysis time (days) | 1230.00 (541.00–2549.00) | 1214.00 (497.00–2101.25 | 1312.00 (539.00–2619.00) | 1368.00 (555.750–2632.75) | 0.183 |
| Routine blood examination parameters | |||||
| RBC (1012/L) | 3.39 ± 0.72 | 3.37 ± 0.62 | 3.40 ± 0.73 | 3.39 ± 0.86 | 0.950 |
| Hb (g/dl) | 100.52 ± 19.42 | 99.98 ± 19.46 | 100.45 ± 19.07 | 101.74 ± 20.83 | 0.884 |
| Hct (L/L) | 31.85 ± 6.12 | 31.85 ± 6.07 | 31.70 ± 5.98 | 32.37 ± 6.78 | 0.809 |
| MCV (fl) | 95.14 ± 8.02 | 95.11 ± 8.11 | 94.75 ± 8.14 | 96.50 ± 7.46 | 0.431 |
| MCH (pg) | 30.03 ± 2.70 | 29.84 ± 2.57 | 30.03 ± 2.78 | 30.39 ± 2.65 | 0.547 |
| MCHC (g/dl) | 315.72 ± 12.98 | 313.92 ± 11.22 | 316.99 ± 13.97 | 314.79 ± 12.38 | 0.191 |
| RDW-CV (%) | 14.57 ± 1.65 | 14.65 ± 1.61 | 14.48 ± 1.71 | 14.69 ± 1.56 | 0.649 |
| RDW-SD (%) | 51.30 (47.00–55.40) | 51.84 (47.00–55.78) | 51.20 (46.75–54.15) | 51.47 (48.70–56.60) | 0.342 |
| PLT (109/L) | 167.00 (124.00–211.00) | 171.756 (127.50–212.50) | 165.00 (123.00–221.00) | 150.00 (126.75–197.75) | 0.258 |
| WBC (109/L) | 6.61 (5.22–8.18) | 6.44 (5.24–8.09) | 6.87 (5.22–8.40) | 6.07 (5.10–6.98) | 0.445 |
| EO (%) | 2.80 (1.60–4.70) | 2.65 (1.50–4.35) | 2.70 (1.50–4.85) | 3.35 (1.88–5.00) | 0.277 |
| BASO (%) | 0.500 (0.300–0.700) | 0.500 (0.200–0.700) | 0.500 (0.300–0.700) | 0.500 (0.200–0.800) | 0.763 |
| MONO (%) | 6.00 (4.60–7.50) | 6.05 (4.48–6.98) | 5.90 (4.55–7.60) | 6.38 (4.80–7.62) | 0.771 |
| NEU (%) | 71.43 ± 9.54 | 70.77 ± 9.82 | 72.16 ± 9.36 | 70.19 ± 9.59 | 0.352 |
| LYMP (%) | 17.78 ± 7.23 | 18.20 ± 6.87 | 17.16 ± 7.05 | 19.03 ± 8.37 | 0.249 |
p presents differences among various groups.
*: p < 0.05 compared with non-TR group; **: p < 0.01 compared with non-TR group; ##: p < 0.01 compared with mild increased TR group.
In regard to renal function, none of the parameters (eGFR, Cr, and BUN) were significantly different among the three groups of patients (all p values were greater than 0.05, Table 1).
We further compared the differences in serum ions [Mg2+, K+, Ca2+, Na+, Cl–, total CO2 (TCO2), and P]. However, none of these ions were significantly different among the non-TR, mildly increased TRA and ms-TR groups (Table 1).
Finally, we also investigated routine blood examinations. However, we did not find any differences among the three groups (Table 1).
Correlation Between TRA and Demographic, Echocardiographic, Routine Blood, Electrolyte and Renal Function Parameters
To identify associations between TRA and other parameters, we performed Spearman’s correlation analysis. We found that LA (r = 0.510, p < 0.001), LVDD (r = 0.389, p < 0.001), RA (r = 0.615, p < 0.001), RV (r = 0.586, p < 0.001), and PA (r = 0.322, p < 0.001) were closely positively related to TRA. Meanwhile, the IVS mobility (r = −0.338, p < 0.001), LVPW mobility (r = −0.344, p < 0.001), LVEF (r = −0.401, p < 0.001), and FS (r = −0.403, p < 0.001) as well as TCO2 (r = −0.141, p = 0.047) were negatively correlated with TRA. We did not find other associations between TRA and the rest of the parameters (Table 2).
TABLE 2.
Relationship between TRA and other parameters.
| Relationship with TRA | ||
| Parameters | R | p value |
| Demographic data | ||
| Age (years) | –0.064 | 0.371 |
| BMI (kg/m2) | ||
| Echocardiographic parameters | ||
| LA (mm) | 0.510 | <0.001 |
| LVDD (mm) | 0.389 | <0.001 |
| RA (mm) | 0.615 | <0.001 |
| RV (mm) | 0.586 | <0.001 |
| PA (mm) | 0.322 | <0.001 |
| IVS (mm) | 0.086 | 0.228 |
| IVS mobility | –0.338 | <0.001 |
| LVPW (mm) | 0.084 | 0.237 |
| LVPW mobility | –0.344 | <0.001 |
| FS (%) | –0.403 | <0.001 |
| LVEF (%) | –0.401 | <0.001 |
| SV (ml) | 0.095 | 0.184 |
| Renal function | ||
| eGFR [mL/(min⋅1.73 m2)] | 0.047 | 0.510 |
| Cr (umol/L) | 0.013 | 0.858 |
| BUN (μmoI/L) | 0.056 | 0.433 |
| Electrolyte parameters | ||
| Mg2+ (mmol/L) | 0.080 | 0.263 |
| K+ (mmol/L) | 0.088 | 0.215 |
| Ca2+ (mmol/L) | –0.039 | 0.583 |
| Na+(mmol/L) | –0.012 | 0.867 |
| Cl– (mmol/L) | 0.109 | 0.127 |
| TCO2 (mmol/L) | –0.141 | 0.047 |
| P (mmol/L) | 0.067 | 0.345 |
| Dialysis time (days) | –0.046 | 0.518 |
| Blood routine examination parameters | ||
| RBC (1012/L) | 0.033 | 0.641 |
| Hb (g/dl) | 0.062 | 0.381 |
| Hct (L/L) | 0.075 | 0.292 |
| MCV (fl) | 0.063 | 0.376 |
| MCH (pg) | 0.043 | 0.546 |
| MCHC (g/dl) | –0.027 | 0.709 |
| RDW-CV (%) | 0.021 | 0.765 |
| RDW-SD (%) | 0.060 | 0.402 |
| PLT (109/L) | –0.080 | 0.260 |
| WBC (109/L) | –0.114 | 0.109 |
| EO (%) | –0.054 | 0.449 |
| BASO (%) | 0.041 | 0.563 |
| MONO (%) | 0.058 | 0.418 |
| NEU (%) | –0.023 | 0.750 |
Logistic Regressions for ms-TR With Demographic, Echocardiographic, Routine Blood, Electrolyte and Renal Function Parameters
In univariate regression analysis, demographic data (age and BMI) were not associated with ms-TR (all p values > 0.05) (Table 3).
TABLE 3.
Univariate logistic analysis for ms-TR jet.
| 95CI% | |||||
| Parameters | β | p value | OR | Lower borderline | Upper borderline |
| Demographic data | |||||
| Age (years) | –0.005 | 0.734 | 0.995 | 0.968 | 1.023 |
| BMI (kg/m2) | –0.057 | 0.291 | 0.945 | 0.850 | 1.050 |
| Echocardiographic parameters | |||||
| LA (mm) | 0.248 | <0.001 | 1.281 | 1.166 | 1.408 |
| LVDD (mm) | 0.139 | <0.001 | 1.149 | 1.076 | 1.227 |
| RA (mm) | 0.362 | <0.001 | 1.437 | 1.281 | 1.612 |
| RV (mm) | 0.343 | <0.001 | 1.410 | 1.251 | 1.589 |
| PA (mm) | 0.233 | 0.003 | 1.262 | 1.083 | 1.471 |
| IVS (mm) | –0.162 | 0.163 | 0.850 | 0.677 | 1.068 |
| IVS mobility | –0.821 | <0.001 | 0.440 | 0.295 | 0.655 |
| LVPW (mm) | 0.058 | 0.559 | 1.060 | 0.872 | 1.288 |
| LVPW mobility | –0.531 | <0.001 | 0.588 | 0.449 | 0.770 |
| FS (%) | –0.159 | <0.001 | 0.853 | 0.801 | 0.909 |
| LVEF (%) | –0.100 | <0.001 | 0.905 | 0.869 | 0.943 |
| SV (ml) | 0.002 | 0.835 | 1.002 | 0.981 | 1.024 |
| Dialysis time (days) | 0.001 | 0.203 | 1.000 | 1.000 | 1.001 |
| Renal function | |||||
| eGFR [mL/(min⋅1.73 m2)] | 0.023 | 0.374 | 1.023 | 0.973 | 1.075 |
| Cr (umol/L) | 0.001 | 0.456 | 1.000 | 0.999 | 1.001 |
| BUN (μmoI/L) | 0.020 | 0.380 | 1.020 | 0.976 | 1.066 |
| Electrolyte parameters | |||||
| Mg2+ (mmol/L) | 0.029 | 0.796 | 1.337 | 0.149 | 12.006 |
| K+ (mmol/L) | 0.077 | 0.744 | 1.080 | 0.682 | 1.709 |
| Ca2+ (mmol/L) | 0.118 | 0.861 | 1.125 | 0.300 | 4.218 |
| Na+(mmol/L) | –0.146 | 0.044 | 0.864 | 0.749 | 0.996 |
| Cl– (mmol/L) | 0.022 | 0.608 | 1.022 | 0.939 | 1.113 |
| TCO2 (mmol/L) | –0.116 | 0.017 | 0.890 | 0.809 | 0.980 |
| P (mmol/L) | –0.105 | 0.711 | 0.901 | 0.517 | 1.568 |
| Routine blood examination parameters | |||||
| RBC (1012/L) | 0.057 | 0.827 | 1.058 | 0.637 | 1.759 |
| Hb (g/dl) | 0.005 | 0.628 | 1.005 | 0.986 | 1.023 |
| Hct (L/L) | 0.013 | 0.655 | 1.013 | 0.957 | 1.073 |
| MCV (fl) | 0.023 | 0.339 | 1.023 | 0.976 | 1.072 |
| MCH (pg) | 0.084 | 0.255 | 1.088 | 0.941 | 1.258 |
| MCHC (g/dl) | 0.007 | 0.681 | 1.007 | 0.976 | 1.039 |
| RDW-CV (%) | 0.014 | 0.903 | 1.014 | 0.808 | 1.273 |
| RDW-SD (%) | 0.026 | 0.336 | 1.026 | 0.974 | 1.081 |
| PLT (109/L) | –0.003 | 0.242 | 0.997 | 0.991 | 1.002 |
| WBC (109/L) | –0.032 | 0.710 | 0.969 | 0.820 | 1.145 |
| EO (%) | –0.006 | 0.742 | 0.994 | 0.958 | 1.031 |
| BASO (%) | –0.025 | 0.544 | 0.976 | 0.901 | 1.057 |
| MONO (%) | 0.030 | 0.951 | 1.030 | 0.404 | 2.625 |
| NEU (%) | 0.015 | 0.542 | 1.015 | 0.967 | 1.066 |
| RBC (1012/L) | 0.063 | 0.441 | 1.065 | 0.907 | 1.252 |
Primary screen for the independently associated factors.
Among the echocardiographic parameters, we identified several factors that were potentially associated with ms-TR. First, the diameter of the left and right heart as well as the PA, LA [odds ratio (OR): 1.281, 95% confidence interval (CI): 1.166–1.408, p < 0.001], LVDD (OR: 1.149, 95% CI: 1.076–1.227, p < 0.001), RA (OR: 1.437, 95% CI: 1.281–1.612, p < 0.001), RV (OR: 1.410, 95% CI: 1.251–1.589, p < 0.001), and PA (OR: 1.262, 95% CI: 1.083–1.471, p = 0.003) were associated with ms-TR (Table 3).
Furthermore, it was also found that IVS mobility (OR: 0.440, 95% CI: 0.295–0.655, p < 0.001), LVPW mobility (OR: 0.588, 95% CI: 0.449–0.770, p < 0.001), FS (OR: 0.853, 95% CI: 0.801–0.909, p < 0.001), and LVEF (OR: 0.905, 95% CI: 0.869–0.943, p < 0.001) may also have been associated with ms-TR (Table 3).
However, none of the renal function parameters showed significant associations with ms-TR (Cr, BUN, and eGFR, Table 3).
Among the serum ions, we found that only Na+ (OR: 0.864, 95% CI: 0.749–0.996, p = 0.044) and TCO2 (OR: 0.890, 95% CI: 0.809–0.980, p = 0.017) were potentially related to ms-TR (Table 3).
Finally, in the routine blood examination, RBC- and WBC-related parameters showed no potential associations with ms-TR. In addition, the other parameters, such as the ratios of monocytes, lymphocytes, eosinophils, and basophilic granulocytes, were not associated with ms-TR (Table 3).
To identify factors that were independently associated with ms-TR, we performed an adjusted (multivariate) logistic regression. We found that only Na+ (OR: 0.871 95% CI: 0.760–0.999, p = 0.048), RA (OR: 1.370, 95% CI: 1.234–1.520, p < 0.001), and FS (OR: 0.887, 95% CI: 0.822–0.957, p < 0.001) were independently associated with ms-TR (Table 4).
TABLE 4.
Adjusted logistic regressions for ms-TR.
| 95%CI | |||||
| Parameters | β | p value | OR | Lower borderline | Upper borderline |
| RA (mm) | 0.3150 | <0.001 | 1.370 | 1.234 | 1.520 |
| FS (%) | −0.120 | <0.001 | 0.887 | 0.822 | 0.957 |
| Na+ (mmol/L) | −0.137 | 0.048 | 0.871 | 0.760 | 0.999 |
Na+, RA, and FS have been identified to be independently associated with ms-TR.
Discussion
In our present study, we found that TR was prevalent (62.6%) in 491 ESRD patients who received maintenance dialysis treatment. Though TCO2 and other renal functions were potentially associated with TR, adjusted regression showed that only Na+, FS, and RA were independently associated with ms-TR; thus, further cohort studies are warranted to confirm their causality.
TR Was Prevalent in ESRD Patients Who Received Maintenance Dialysis Treatment
As discussed above, though TR is common in normal populations or populations with other cardiovascular diseases (Arsalan et al., 2017), TR was more prevalent and had a relatively higher incidence in a special subgroup, maintenance dialysis patients with ESRD (Walsh et al., 1998; Maeder et al., 2008; Al-Hijji et al., 2017; Del Forno et al., 2018; Dietz et al., 2019). Furthermore, we also found that ms-TR showed a higher incidence than other degrees of TRA. In particular, a few individuals had a TRA that was larger than 15 cm2; these patients needed further examination or treatment by the cardiovascular disease department. In the past, few studies had investigated the roles of TR, as well as the function and structure of the left heart, in renal function in heart failure patients (Minami et al., 2016; Cutshall et al., 2018; Konigsbrugge and Ay, 2019). However, no papers have reported the prevalence of TR in a large population of maintenance dialysis patients with ESRD. Our study was the first to describe the prevalence and TRA degree in patients who received dialysis at our center. Our data revealed that a TR jet occurred in maintenance dialysis patients with ESRD. Some patients had a TR jet with an extremely large area and may have needed cardiac surgery interventions. Thus, echocardiographic examinations are a highly valuable non-invasive method to evaluate the cardiovascular complications of ESRD, providing guidelines for the treatment of patients.
TR Was Not Closely Associated With Renal Function in ESRD Patients Undergoing Hemodialysis
It has been indicated that kidney dysfunction may contribute to cardiovascular complications (Yogasundaram et al., 2019). We investigated renal function in ESRD patients, but the eGFR, Cr and BUN were not significantly different among the various TRA groups and were not associated with TRA. Though few previous studies have indicated that renal dysfunction affects cardiac structure and function (Minami et al., 2016; Cutshall et al., 2018; Lemor et al., 2018), we did not find a potential association of eGFR, BUN and Cr with ms-TR. Thus, these parameters were not included in the final adjusted logistic regression. Though our present study did not find an association of renal functions with ms-TR, the causal relationship between renal function and TR jets may need further cohort studies or basic studies to be confirmed.
TR Was Significantly Associated With Serum Ions
Ions are known to play numerous key roles in signal transduction, protein synthesis, cell proliferation and differentiation. In ESRD patients, serum ion disturbances are the most common clinical manifestations. Thus, we also investigated the associations between serum ions (Mg2+, Ca2+, K+, Cl–, TCO2, Na+, and P) and TR jets. First, there were no significant differences in the abovementioned ions in the various TRA groups. Then, we found that TCO2 was closely negatively correlated with TRA. In the past several decades, researchers have found that TCO2 levels changed in uremia, indicating that renal dysfunction may alter TCO2 levels, resulting in changes in respiration and oxygen usage. If this situation is persistent, it may cause hypoxia-induced cardiac injury or changes in cardiac structure and function. Through further univariate regression analysis, we found that TCO2 and Na+ were potentially associated with an ms-TR jet. However, in the adjusted regression analysis, these factors were excluded by other variables and did not show independent associations with ms-TR.
TR Showed No Close Relationship With Routine Blood Parameters
Anemia, which is the consequence of the insufficient production of EPO and a higher demand for EPO, is another critical complication of ESRD and leads to insufficient Hb and RBC production (Santoro and Canova, 2005; van der Meer et al., 2008; Yokoro et al., 2017). It proposed that the anemia may contribute to TR jet. Thus, we investigated the association between RBC-related parameters and WBC-related parameters. However, we did not find any associations between RBC/WBC-related parameters and TR. This result may have been caused by the effect of anemia on cardiovascular diseases; this may involve a long-term process that requires cohort studies to identify their associations.
Na+, FS, and RA May Contribute to the Development of ms-TR
Our present study found that Na+ FS and RA were independently associated with ms-TR. The structure and function of the left heart showed an association with ms-TR, which may have been caused by the interactions between the left and right heart. Furthermore, changes in the right heart may result in relative or absolute tricuspid insufficiency, which may aggravate changes in structure and function (enlargement and right heart failure) (Al-Hijji et al., 2017; Sun and O’Gara, 2017; Hahn, 2019).
We have identified that Na+ was independently associated with TR which was in concordant with the previous studies that Na+ was correlated to the cardiovascular diseases in ERSD dialysis patients. This may be caused by the roles of Na+ in the vascular systems. In according to the previous studies, cardiovascular diseases are the common complications in ESRD patients especially in the maintenance dialysis subset populations (Cakici et al., 2020; Gumus and Saricaoglu, 2020). The precise mechanisms of Na+ in TR have not been widely investigated.
Our present results were consistent with previous studies on various cardiovascular diseases that are induced by a TR jet (Lee et al., 2010; Al-Hijji et al., 2017; Kopic et al., 2017; Mangieri et al., 2017). However, the roles of FS in TR jets have not been reported before. We found that FS was significantly associated with ms-TR, which was a novel finding that revealed that left heart functions may affect the right heart (due to preloading or other hemodynamic changes), which is partly in according with the previous studies (Badano et al., 2019; Dietz et al., 2019; Prihadi et al., 2019). However, the precise cause and mechanisms were not confirmed; thus, further cohort studies and research to uncover the basic mechanisms are warranted.
Limitations
This was a retrospective study focusing on the prevalence of TR and its associated factors. However, there are several limitations that need to be improved in our future studies. First, our present study was a retrospective study that identified only the factors associated with ms-TR. The causality between clinical factors and ms-TR needs to be analyzed by further cohort studies. Another limitation is that we investigated only a few of the frequently used clinical parameters; however, there may be other valuable factors that should be examined. Finally, the parameters after dialysis were not included in this study, but these factors should be included in future research.
Conclusion
Tricuspid regurgitation is also prevalent in maintenance hemodialysis patients with ESRD. Na+, FS, and RA were independently associated with ms-TR; these may be potential risk factors/predictors for ms-TR and warrant further cohort studies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Second Affiliated Clinic Hospital (Xinqiao Hospital) and Army Medical University (Third Military Medical University). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
S-ZB and YZ designed the study, drafted the manuscript, and performed the statistical analysis. S-ZB and X-HD critically reviewed and revised the manuscript for important intellectual content. RR performed the echocardiographic examinations and LZ recorded the measurements of TR-related parameters. YZ and X-HD collected the demographic data and performed routine blood examinations. YW, FP, and LZ performed the laboratory measurements. YW and WW obtained the dialysis-related data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all the individuals who participated in this study for their support. Finally, S-ZB thank his wife Meng Zhou for her help and care during the preparation of this manuscript.
Abbreviations
- BAS
basophil ratio
- BUN
blood urea nitrogen concentration
- Ca2+
serum calcium concentration
- Cr
plasma creatinine
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- EO
eosinophil ratio
- FS
fractional shortening
- Hb
hemoglobin concentration
- HCT
hematocrit
- IVS
interventricular septum
- K+
serum potassium concentration
- LA
left atrial end-diastolic internal diameter
- LVDD
left ventricular end-diastolic internal diameter
- LVEF
left ventricular ejection fraction
- LVPW
left ventricular posterior wall
- LYM
lymphocyte ratio
- MCH
mean corpuscular hemoglobin
- MCHC
mean corpuscular hemoglobin concentration
- MCV
mean corpuscular volume
- MONO
monocyte ratio
- Na+
sodium concentration
- NEU
neutrophil ratio
- ms-TR
moderate to severe TRA
- P
phosphate concentration
- PA
pulmonary arterial end-diastolic internal diameter
- PLT
platelet
- RA
right atrial end-diastolic internal diameter
- RBC
red blood cell count
- RDW
red blood cell distribution width
- RV
right ventricular end-diastolic internal diameter
- SV
stroke volume
- TR
tricuspid regurgitation
- TRA
TR area
- TRV
TR velocity
- WBC
white blood cell count
- Δ P
mitral transmitral pressure gradient.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (grant no. 81901916), the Excellent Scientist Pool Program of Army Medical University (“Miaopu Program 2019R046”), and The Major Clinical Program of Xinqiao Hospital (2018JSLC0024).
References
- Agricola E., Marini C., Stella S., Monello A., Fisicaro A., Tufaro V., et al. (2017). Effects of functional tricuspid regurgitation on renal function and long-term prognosis in patients with heart failure. J. Cardiovasc. Med. 18 60–68. 10.2459/jcm.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Al-Hijji M., Fender E. A., El Sabbagh A., Holmes D. R. (2017). Current treatment strategies for tricuspid regurgitation. Curr. Cardiol. Rep. 19:106. [DOI] [PubMed] [Google Scholar]
- Arsalan M., Walther T., Smith R. L., II, Grayburn P. A. (2017). Tricuspid regurgitation diagnosis and treatment. Eur. Heart J. 38 634–638. [DOI] [PubMed] [Google Scholar]
- Badano L. P., Hahn R., Rodriguez-Zanella H., Araiza Garaygordobil D., Ochoa-Jimenez R. C., Muraru D. (2019). Morphological assessment of the tricuspid apparatus and grading regurgitation severity in patients with functional tricuspid regurgitation: thinking outside the box. JACC Cardiovasc. Imaging 12 652–664. 10.1016/j.jcmg.2018.09.029 [DOI] [PubMed] [Google Scholar]
- Cakici E. K., Cakici M., Gumus F., Tan Kurklu T. S., Yazilitas F., Orun U. A., et al. (2020). Effects of hemodialysis access type on right heart geometry in adolescents. J. Vasc. Access. 21 658–664. 10.1177/1129729819897454 [DOI] [PubMed] [Google Scholar]
- Cutshall B. T., Duhart B. T., Jr., Saikumar J., Samarin M., Hutchison L., Hudson J. Q. (2018). Assessing guideline-directed medication therapy for heart failure in end-stage renal disease. Am. J. Med. Sci. 355 247–251. 10.1016/j.amjms.2017.11.008 [DOI] [PubMed] [Google Scholar]
- Del Forno B., Lapenna E., Dalrymple-Hay M., Taramasso M., Castiglioni A., Alfieri O., et al. (2018). Recent advances in managing tricuspid regurgitation. F1000Res 7:355. 10.12688/f1000research.13328.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz M. F., Prihadi E. A., van der Bijl P., Goedemans L., Mertens B. J. A., Gursoy E., et al. (2019). Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation 140 836–845. 10.1161/circulationaha.119.039630 [DOI] [PubMed] [Google Scholar]
- Gumus F., Saricaoglu M. C. (2020). Assessment of right heart functions in the patients with arteriovenous fistula for hemodialysis access: right ventricular free wall strain and tricuspid regurgitation jet velocity as the predictors of right heart failure. Vascular 28 96–103. 10.1177/1708538119866616 [DOI] [PubMed] [Google Scholar]
- Hahn R. T. (2019). Assessment and procedural guidance with echocardiography for transcatheter tricuspid regurgitation devices. Prog. Cardiovasc. Dis. 62 452–458. 10.1016/j.pcad.2019.09.003 [DOI] [PubMed] [Google Scholar]
- Khan R. A., Khan N. A., Bauer S. R., Li M., Duggal A., Wang X., et al. (2019). Association between volume of fluid resuscitation and intubation in high-risk patients with sepsis, heart failure, end-stage renal disease, and cirrhosis. Chest 3692:7. [DOI] [PubMed] [Google Scholar]
- Kim E. D., Parekh R. S. (2015). Calcium and sudden cardiac death in end-stage renal disease. Semin. Dial. 28 624–635. 10.1111/sdi.12419 [DOI] [PubMed] [Google Scholar]
- Konigsbrugge O., Ay C. (2019). Atrial fibrillation in patients with end-stage renal disease on hemodialysis: magnitude of the problem and new approach to oral anticoagulation. Res. Pract. Thromb. Haemost. 3 578–588. 10.1002/rth2.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooman J. P., van der Sande F. M. (2019). Body fluids in end-stage renal disease: statics and dynamics. Blood Purif. 47 223–229. 10.1159/000494583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopic S., Stephensen S. S., Heiberg E., Arheden H., Bonhoeffer P., Ersboll M., et al. (2017). Isolated pulmonary regurgitation causes decreased right ventricular longitudinal function and compensatory increased septal pumping in a porcine model. Acta Physiol. 221 163–173. 10.1111/apha.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Song J. M., Park J. P., Lee J. W., Kang D. H., Song J. K. (2010). Long-term prognosis of isolated significant tricuspid regurgitation. Circ. J. 74 375–380. 10.1253/circj.cj-09-0679 [DOI] [PubMed] [Google Scholar]
- Lemor A., Hernandez G. A., Lee S., Patel N., Blumer V., Badiye A., et al. (2018). Impact of end stage renal disease on in-hospital outcomes of patients with systolic and diastolic heart failure (insights from the Nationwide Inpatient Sample 2010 to 2014). Int. J. Cardiol. 266 174–179. 10.1016/j.ijcard.2018.02.117 [DOI] [PubMed] [Google Scholar]
- Maeder M. T., Holst D. P., Kaye D. M. (2008). Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J. Card. Fail. 14 824–830. 10.1016/j.cardfail.2008.07.236 [DOI] [PubMed] [Google Scholar]
- Mahfouz R. A., Elawady W., Hossein E., Yosri A. (2013). Impact of atrioventricular compliance on clinical outcome of patients undergoing successful percutaneous balloon mitral valvuloplasty. Echocardiography 30 1187–1193. 10.1111/echo.12256 [DOI] [PubMed] [Google Scholar]
- Mangieri A., Montalto C., Pagnesi M., Jabbour R. J., Rodes-Cabau J., Moat N., et al. (2017). Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ. Cardiovasc. Interv. 10:12. [DOI] [PubMed] [Google Scholar]
- Mansour I. N., Lang R. M., Aburuwaida W. M., Bhave N. M., Ward R. P. (2010). Evaluation of the clinical application of the ACCF/ASE appropriateness criteria for stress echocardiography. J. Am. Soc. Echocardiogr. 23 1199–1204. 10.1016/j.echo.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Minami Y., Kajimoto K., Sato N., Hagiwara N., Takano T. Attend study investigators. (2016). End-stage renal disease patients on chronic maintenance hemodialysis in a hospitalized acute heart failure cohort: prevalence, clinical characteristics, therapeutic options, and mortality. Int. J. Cardiol. 224 267–270. 10.1016/j.ijcard.2016.09.038 [DOI] [PubMed] [Google Scholar]
- Prihadi E. A., Delgado V., Leon M. B., Enriquez-Sarano M., Topilsky Y., Bax J. J. (2019). Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. JACC Cardiovasc. Imaging 12 491–499. 10.1016/j.jcmg.2018.09.027 [DOI] [PubMed] [Google Scholar]
- Ramesh S., Zalucky A., Hemmelgarn B. R., Roberts D. J., Ahmed S. B., Wilton S. B., et al. (2016). Incidence of sudden cardiac death in adults with end-stage renal disease: a systematic review and meta-analysis. BMC Nephrol. 17:78. 10.1186/s12882-016-0293-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta R., Chan C., Chauhan V. S. (2019). Arrhythmias and sudden cardiac death in end stage renal disease: epidemiology, risk factors, and management. Can. J. Cardiol. 35 1228–1240. 10.1016/j.cjca.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Santoro A., Canova C. (2005). Anemia and erythropoietin treatment in chronic kidney diseases. Minerva Urol. Nefrol. 57 23–31. [PubMed] [Google Scholar]
- Sugiura E., Dohi K., Tanimura M., Kumagai N., Ishikawa E., Ito M. (2019). Successful peritoneal dialysis for the treatment of inotrope-dependent end-stage heart failure. Int. Heart J. 60 1211–1218. 10.1536/ihj.18-550 [DOI] [PubMed] [Google Scholar]
- Sun Y. P., O’Gara P. T. (2017). Epidemiology, anatomy, pathophysiology and clinical evaluation of functional tricuspid regurgitation. Minerva Cardioangiol. 65 469–479. [DOI] [PubMed] [Google Scholar]
- Unlu S., Sahinarslan A., Gokalp G., Seckin O., Arinsoy S. T., Boyaci N. B., et al. (2018). The impact of volume overload on right heart function in end-stage renal disease patients on hemodialysis. Echocardiography 35 314–321. 10.1111/echo.13768 [DOI] [PubMed] [Google Scholar]
- van der Meer P., Lipsic E., van Gilst W. H., van Veldhuisen D. J. (2008). Anemia and erythropoietin in heart failure. Heart Fail. Monit. 6 28–33. [PubMed] [Google Scholar]
- Walsh J. T., Glennon P., Schofield P. M. (1998). Tricuspid regurgitation following central line insertion in a patient undergoing haemodialysis. Postgrad. Med. J. 74 631–632. 10.1136/pgmj.74.876.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogasundaram H., Chappell M. C., Braam B., Oudit G. Y. (2019). Cardiorenal syndrome and heart failure-challenges and opportunities. Can. J. Cardiol. 35 1208–1219. 10.1016/j.cjca.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoro M., Nakayama Y., Yamagishi S. I., Ando R., Sugiyama M., Ito S., et al. (2017). Asymmetric dimethylarginine contributes to the impaired response to erythropoietin in CKD-Anemia. J. Am. Soc. Nephrol. 28 2670–2680. 10.1681/asn.2016111184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.