Abstract
Mechanical ventilation (MV) is often a life-saving intervention for patients in respiratory failure. Unfortunately, a common and undesired consequence of prolonged MV is the development of diaphragmatic atrophy and contractile dysfunction. This MV-induced diaphragmatic weakness is commonly labeled “ventilator-induced diaphragm dysfunction” (VIDD). VIDD is an important clinical problem because diaphragmatic weakness is a major risk factor for the failure to wean patients from MV; this inability to remove patients from ventilator support results in prolonged hospitalization and increased morbidity and mortality. Although several processes contribute to the development of VIDD, it is clear that oxidative stress leading to the rapid activation of proteases is a primary contributor. While all major proteolytic systems likely contribute to VIDD, emerging evidence reveals that activation of the calcium-activated protease calpain plays a required role. This review highlights the signaling pathways leading to VIDD with a focus on the cellular events that promote increased cytosolic calcium levels and the subsequent activation of calpain within diaphragm muscle fibers. In particular, we discuss the emerging evidence that increased mitochondrial production of reactive oxygen species promotes oxidation of the ryanodine receptor/calcium release channel, resulting in calcium release from the sarcoplasmic reticulum, accelerated proteolysis, and VIDD. We conclude with a discussion of important and unanswered questions associated with disturbances in calcium homeostasis in diaphragm muscle fibers during prolonged MV.
Keywords: oxidative stress, reactive oxygen species, muscle atrophy, ryanodine receptors, calpain, proteolysis
Introduction
Mechanical ventilation (MV) is often a life-saving intervention for both critically ill patients and patients undergoing surgery. An unwanted side-effect of prolonged MV is the rapid development of inspiratory muscle weakness that occurs due to both diaphragmatic atrophy and contractile dysfunction. Collectively, this syndrome has been labeled ventilator-induced diaphragm dysfunction (VIDD) (Vassilakopoulos and Petrof, 2004). VIDD is a serious clinical problem because diaphragmatic weakness is a major risk factor contributing to the failure to wean patients from the ventilator (Petrof et al., 2010).
Abundant evidence confirms that MV-induced diaphragmatic atrophy occurs due to both a decrease in muscle protein synthesis and increased proteolysis with proteolysis playing a dominant role (Whidden et al., 1985b; Shanely et al., 2002, 2004; Agten et al., 2011; Powers et al., 2013; Smuder et al., 2014, 2018; Hudson et al., 2015). The MV-induced increase in proteolysis within diaphragm fibers is triggered by increases in mitochondrial production of reactive oxygen species (ROS); this redox imbalance contributes to the activation of the four major proteolytic systems in skeletal muscle (i.e., ubiquitin-proteasome, autophagy, calpain, and caspase-3) (Powers et al., 2011). Although all of these proteolytic systems contribute to MV-induced diaphragmatic atrophy, activation of the calcium (Ca2+)-activated protease, calpain, plays a central role in MV-induced diaphragmatic atrophy. Indeed, blockade of calpain activation in the diaphragm can markedly reduce both MV-induced diaphragmatic atrophy and contractile dysfunction (Maes et al., 2007; Nelson et al., 2012).
This review provides a summary of the cell signaling events leading to VIDD with a focus on the cellular processes that result in disturbed Ca2+ homeostasis and the subsequent activation of calpain within diaphragm muscle fibers. More specifically, we discuss the evidence that increased mitochondrial production of ROS results in modification of the ryanodine receptor/Ca2+ release channel in the sarcoplasmic reticulum (SR), resulting in the release of Ca2+ from the SR, calpain activation, and VIDD. In an effort to stimulate future research, we also highlight unanswered questions associated with signaling events.
The Problem: Ventilator-Induced Diaphragmatic Weakness
The observation that prolonged MV results in diaphragmatic wasting was first reported in a retrospective study revealing that diaphragmatic atrophy was present in infants and neonates exposed to prolonged MV (Knisely et al., 1988). Direct evidence to support this conjecture was later provided by a preclinical study revealing that 48 h of MV results in marked diaphragmatic atrophy and contractile dysfunction (Le Bourdelles et al., 1996). Since these initial reports, numerous studies have confirmed that as few as 12–24 h of MV results in VIDD in both animals and humans [reviewed in Powers et al. (2013)].
Two primary modes of MV exist: (1) partial support and (2) full support MV. During partial support MV, the ventilator assists during inspiration, but the patients’ inspiratory muscles remain engaged in breathing. During full support MV, the ventilator performs all of the work of breathing, resulting in inactivity of the diaphragm and other inspiratory muscles; compared to partial support, full support MV results in a more rapid rate of VIDD. Indeed, when contrasted with inactivity-induced limb muscle atrophy (e.g., prolonged bedrest), full support MV-induced diaphragmatic atrophy is a unique form of skeletal muscle atrophy that occurs extremely rapidly after the onset of MV. For instance, the cross-sectional area (CSA) of diaphragm muscle fibers is reduced by >15% within the first 12–18 h of MV in both rats and humans (Whidden et al., 1985a; Shanely et al., 2002; Levine et al., 2008; Nelson et al., 2012). In comparison with inactivity-induced atrophy in limb muscles, 5–7 days of inactivity would be required to achieve this magnitude of fiber atrophy in locomotor skeletal muscles (Powers et al., 2013). In this regard, the diaphragm muscle differs from limb muscle in several aspects. First, the diaphragm is chronically active, contracting several times per minute even during sleep (Lessa et al., 2016; Fogarty et al., 2018). Moreover, the diaphragm also contributes to several non-respiratory activities including swallowing and vocalization (Fogarty et al., 2018). Further, limb skeletal muscles exert force along the longitudinal axis of the fiber because diaphragm fibers are exposed to a pressure load both longitudinally and perpendicularly to the axis of the muscle (Lessa et al., 2016).
As mentioned previously, the diaphragmatic weakness associated with VIDD is a primary risk factor for the failure to wean patients from the ventilator. In this context, weaning is defined as the ability to remove patients from ventilator support and restore spontaneous breathing. The incidence of difficult weaning of patients from MV is variable but can reach >30% or higher in those ventilated for more than 3 days (Funk et al., 2010). The inability to wean patients from MV is a serious clinical problem that results in prolonged stays in the intensive care unit and significant increases in both morbidity and mortality (Funk et al., 2010). Developing a therapy to prevent VIDD and reduce the risk of weaning problems requires a thorough understanding of the signaling events that promote VIDD. The next segments provide a summary of our current knowledge of the cellular processes that promote MV-induced diaphragmatic weakness.
MV-Induced Diaphragmatic Atrophy: Proteolysis vs Depressed Protein Synthesis
The size of skeletal muscle fibers is controlled by the relative balance between rates of protein synthesis and protein breakdown. It is well established that prolonged MV results in a rapid increase in proteolysis and a decline in protein synthesis in the rodent diaphragm (Shanely et al., 2004). In regard to the rates of diaphragmatic protein synthesis, both mixed and myofibrillar protein synthesis rates decline within the first 6 h of MV (Shanely et al., 2004). While this MV-induced depression in protein synthesis rates clearly contributes to diaphragmatic atrophy during prolonged MV (i.e., days to weeks), the rapid atrophy that occurs in the human or rat diaphragm (i.e., >15% reduction in diaphragm CSA) within the first 12–24 h of MV is largely driven by a swift increase in proteolysis (Powers et al., 2013). In reference to the proteolytic systems that contribute to MV-induced diaphragm atrophy, abundant evidence indicates that all four of the major proteolytic systems in skeletal muscle (i.e., calpains, caspase-3, ubiquitin-proteasome system, and autophagy) are active during prolonged MV (DeRuisseau et al., 1985; Kwon et al., 1985; Maes et al., 2007; McClung et al., 2007, 2008; Agten et al., 2011; Nelson et al., 2012; Smuder et al., 2018). Although each of these proteolytic systems contributes to MV-induced diaphragmatic wasting, evidence reveals that the calcium-activated protease calpain plays a key role. Because increases in cytosolic free Ca2+ are required to activate calpains, the next segment provides a brief overview of the regulation of cellular Ca2+ levels.
Regulation of Ca2+ Homeostasis in Skleletal Muscle
Calcium ions play important roles in several signaling events in skeletal muscle fibers including excitation-contraction (E-C) coupling and a variety of cell signaling pathways. Importantly, high levels of free Ca2+ in the cytosol can be toxic to cells by promoting increased mitochondrial ROS production, protease activation, and myonuclear apoptosis (Bogeski et al., 2011; Vallejo-Illarramendi et al., 2014; Rossi et al., 2019). Therefore, it is not surprising that skeletal muscle fibers contain an intricate regulatory system that tightly manages the levels of free Ca2+ in the fiber. Indeed, Ca2+ concentration is controlled in muscle fibers via a network of voltage sensors, Ca2+ transporters, Ca2+ channels, Na+/Ca2+ exchangers, Ca2+-binding proteins, and ion pumps/exchangers. A detailed discussion of the regulation of Ca2+ homeostasis is beyond the scope of this review, and the reader is directed to comprehensive reviews on this topic for more details (Berridge et al., 2003; Bogeski et al., 2011; Vallejo-Illarramendi et al., 2014; Belosludtsev et al., 2019; Rossi et al., 2019). Nevertheless, prior to a discussion of the mechanisms responsible for MV-induced disturbances in Ca2+ homeostasis in diaphragm fibers, a brief introduction to some of the key checkpoints in cellular Ca2+ homeostasis is provided in the next segments.
Sarcolemma Regulation of Ca2+ Influx/Efflux
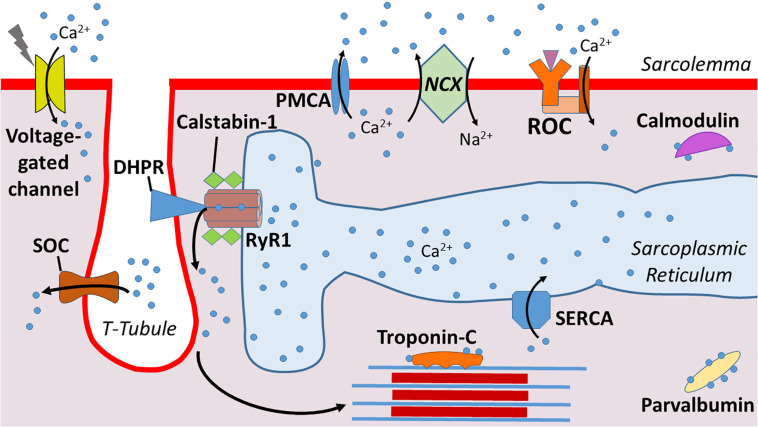
By design, the sarcolemma has a low permeability for Ca2+ as evidenced by the large difference (∼10,000-fold) between the extracellular and cytosolic Ca2+ concentration (Vallejo-Illarramendi et al., 2014). Because of this significant concentration gradient, Ca2+ movement from the extracellular fluid into the cytosol of muscle fibers is driven by the large electrochemical gradient across the sarcolemma. The major influx pathways for Ca2+ entry into the cytosol include voltage-gated channels, receptor operated channels (ROC), and store operated entry channels (SOC) (Vallejo-Illarramendi et al., 2014; Figure 1).
FIGURE 1.
Illustration of calcium (Ca2+) regulation at the sarcolemma. Dihydropyridine receptors (DHPR) mediate Ca2+ release from the sarcoplasmic reticulum into the cytosol. Calstabin-1 is bound to RyR1 and stabilizes this complex to aid in maintaining a closed channel. Ca2+ is pumped back into the sarcoplasmic reticulum via sarcoplasmic reticulum Ca2+-ATPase (SERCA). Ca2+ enters from the extracellular space via voltage-gated channels, store-operated channels (SOC), and receptor operated channels (ROC). Ca2+ is excreted from the cytosol via plasma membrane Ca2+ ATPases (PMCA) and Na+/Ca2+ exchanger (NCX). Levels of cytosolic free Ca2+ are also regulated by Ca2+ binding proteins such as Troponin-C, parvalbumin, and calmodulin.
Because high cytosolic Ca2+ levels are toxic to muscle fibers, the sarcolemma is equipped with ATPases – e.g., plasma membrane Ca2+ ATPases (PMCA) – to pump Ca2+ from the cytosol across the sarcolemma (DeSantiago et al., 2007). Further, the Na+/Ca2+ exchanger (NCX) also contributes to Ca2+ exchange across the sarcolemma (Burr et al., 2014). Compared to the PMCA, the NCX has a greater Ca2+ transport rate and therefore, can protect against Ca2+ overload in the cytosol over a wider range of Ca2+ transients (Brini and Carafoli, 2011). In healthy muscle fibers, the combined properties of these Ca2+ transport proteins are effective in maintaining cytosolic Ca2+ homeostasis.
Cytosolic Ca2+ Buffers
Cytosolic Ca2+ buffers are Ca2+ binding proteins that provide another means of regulating cytosolic levels of free Ca2+. Indeed, the rapid binding of Ca2+ to cytosolic buffers make Ca2+ binding molecules an ideal strategy to regulate free Ca2+ levels in the cytosol (Schwaller, 2010). Important Ca2+ binding proteins in skeletal muscle fibers include troponin C, parvalbumin, and calmodulin (Kretsinger, 1976). Collectively, these Ca2+ binding proteins compose a network of molecules that modulate signaling (Yanez et al., 2012).
Ca2+-Handling Within the Sarcoplasmic Reticulum
The SR, a highly specialized type of endoplasmic reticulum, is the primary reservoir for Ca2+ storage in muscle fibers (Pietrobon et al., 1990; Vallejo-Illarramendi et al., 2014). The SR is an organized tubular network that surrounds myofibrils with a dilated end sac labeled as the terminal cisternae (Figure 1). An extension of the sarcolemma forms the central T-tubule responsible for penetrating into the muscle cell and facilitating Ca2+ release. This structure is flanked on each side by terminal cisternae that form a region within the fiber called the triad; the triad serves as the anatomical structure responsible for triggering excitation-contraction coupling in skeletal muscle fibers.
The SR plays an essential role in the management of intracellular Ca2+ within skeletal muscle fibers. In particular, three major categories of Ca2+ handling proteins exist within the SR: (1) SR Ca2+ release channels; (2) SR Ca2+ binding proteins; and (3) Ca2+ ATPase pumps. A brief summary of these classes of SR handling proteins follows.
The primary Ca2+ release channel in the SR is the ryanodine receptor (RyR). Three isoforms of the RyR (i.e., RyR1, RyR2, and RyR3) exist in mammals, but RyR1 is the dominant isoform expressed in adult skeletal muscle and is crucial for muscular contraction (Capes et al., 2011; Vallejo-Illarramendi et al., 2014). RyR1 is the largest ion channel known (∼2.3 MDa) and is composed of four identical subunits; this organization forms a pore within the SR membrane that serves as the Ca2+ release channel (Hernandez-Ochoa et al., 2015). Importantly, the subunit structure of RyR1 is associated with several accessory proteins including four calstabin-1 subunits (also known as FK506 binding protein 1A) (Wehrens et al., 2005). Calstabin is a key molecule regulating RyR1 function because, in a resting (non-contracting muscle), the binding of calstabin to RyR1 maintains the channel in a closed state to prevent leakage of Ca2+ from the SR into the cytoplasm (Brillantes et al., 1994).
Upon depolarization of the sarcolemma, the opening of the RyR1 is regulated by dihydropyridine receptors (DHPRs) (Capes et al., 2011; Vallejo-Illarramendi et al., 2014). DHPRs are mechanically linked to RyR1, and the voltage change induced by depolarization of the sarcolemma results in a conformational change in DHPR that opens the RyR1 (Protasi et al., 2002). This activation and opening of the RyR1 releases Ca2+ from the SR into the cytosol of muscle fibers, triggering E-C coupling and increased muscle force production. Notably, the magnitude of Ca2+ release from the SR into the cytosol is a primary determinant of the amount of force generated during muscular contraction (Wehrens et al., 2005).
In addition to the modulatory effects of DHPRs and calstabin-1, Ca2+ release through the RyR1 is also regulated by several post-translational events along with numerous proteins and small molecules. Cytosolic regulators of RyR1 include ATP, calmodulin II, and protein kinase A (PKA) (Vallejo-Illarramendi et al., 2014). RyR1 activity can also be regulated by oxidative stress, as oxidative modification of cysteine thiol residues such as S-nitrosylation, S-glutathionylation, and disulfide oxidation have been shown to modulate RyR1 function. In particular, oxidation, coupled with PKA-induced phosphorylation of RyR1, decreases the binding association between calstabin-1 and the RyR1 complex; this results in a leak of Ca2+ from the SR into the cytosol (Reiken et al., 2003; Ward et al., 2003).
Mitochondrial Ca2+ Handling
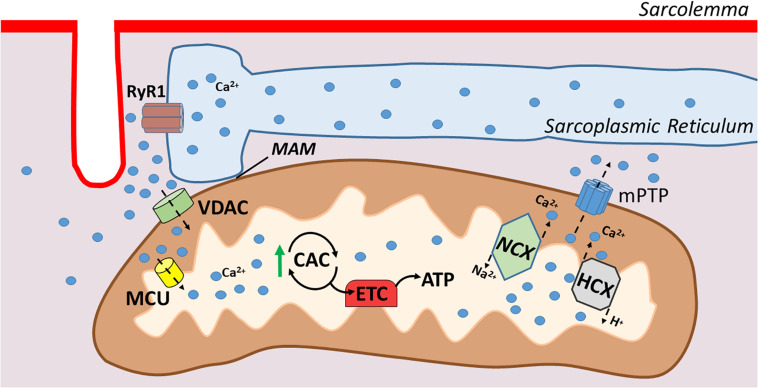
Along with their key role in muscle bioenergetics, mitochondria can also take up and store Ca2+ transiently (Rossi et al., 2019). Two subpopulations of mitochondria exist in skeletal muscle: (1) subsarcolemmal and (2) intermyofibrillar. As the names imply, subsarcolemmal mitochondria are located directly beneath the sarcolemma whereas intermyofibrillar mitochondria are found surrounding the myofibrillar contractile proteins. The adjacent locations of the subsarcolemmal mitochondria and the SR allow for significant interaction between these organelles; indeed, areas of adjacent SR and mitochondria form microdomains of contact that are described as mitochondria associated membranes (MAMs). MAMs describe the areas where SR proteins directly associate with components of the outer mitochondrial membrane (Csordas et al., 2006). This linkage facilitates efficient Ca2+ transfer between these two organelles and therefore, mitochondria actively contribute to Ca2+ homeostasis in skeletal muscles.
The cytosolic Ca2+ fluxes that occur during muscular contraction result in a transient uptake of Ca2+ into the mitochondria (Rossi et al., 2019). This Ca2+ uptake at the outer mitochondrial membrane is facilitated by the voltage-dependent anion channel (VDAC), which allows for the exchange of molecules and ions between the cytosol and mitochondria. However, the mitochondrial inner membrane is impermeable to ions, and therefore, highly specialized channels are required for Ca2+ ions to enter the mitochondrial matrix (Vallejo-Illarramendi et al., 2014; Rossi et al., 2019). Ca2+ transport into the mitochondrial matrix is primarily facilitated by the mitochondrial uniporter (MCU) (Vallejo-Illarramendi et al., 2014; Belosludtsev et al., 2019; Rossi et al., 2019; Figure 2). This ruthenium-red-sensitive uniporter utilizes the negative membrane potential across the inner mitochondrial membrane to facilitate Ca2+ entry into the mitochondrial matrix. In particular, MAMs form junctures that facilitate microdomains of high Ca2+ levels due to their positioning near the SR, and these high Ca2+ levels induce the opening of the MCU pore, permitting Ca2+ entry into the matrix (Vallejo-Illarramendi et al., 2014; Rossi et al., 2019).
FIGURE 2.
Illustration of mitochondrial calcium transport. Mitochondrial associated membranes (MAMs) result in microdomains of high Ca2+ levels that facilitate Ca2+ entry through the outer mitochondrial membrane via voltage dependent anion channel (VDAC). Ca2+ crosses the inner mitochondrial membrane via the mitochondrial uniporter (MCU), which then stimulates respiration via increases in citric acid cycle enzymes. Ca2+ is extruded from the mitochondria via Na+/Ca2+ exchanger (NCX) and H+/Ca2+ exchanger (HCX) in the inner membrane and the outer membrane via the mitochondrial permeability transition pore (mPTP).
The discovery that mitochondria are capable of Ca2+ uptake led to studies revealing that Ca2+ is an important regulator of oxidative phosphorylation. For example, Ca2+ is a positive allosteric activator for several key enzymes that influence flux through the citric acid cycle (e.g., pyruvate dehydrogenase, isocitrate dehydrogenase, and 2-oxoglutarate dehydrogenase) (Denton et al., 1980; McCormack and Denton, 1980). Moreover, recent evidence shows that Ca2+ activates the entire oxidative phosphorylation cascade in skeletal muscle mitochondria (Glancy et al., 2013). In contrast to these metabolically beneficial effects of moderate Ca2+ uptake in mitochondria, excessive Ca2+ uptake resulting in a Ca2+ overload elevates mitochondrial ROS production and increases mitochondrial sensitivity to apoptotic stimuli (Pinton et al., 2008).
The primary means for extrusion of Ca2+ from the mitochondria in skeletal muscle is the Na+/Ca2+ exchanger and the H+/Ca2+ exchanger located within the inner mitochondrial membrane (Pizzo et al., 2012). Further, during Ca2+ overload in the mitochondria, a large conductance channel known as the mitochondrial permeability transition pore (mPTP) can be formed and operates as a conduit for release (Vallejo-Illarramendi et al., 2014). Nonetheless, at present, details about the role that the mPTP plays in regulating mitochondrial Ca2+ levels remains poorly understood (Giorgio et al., 2018).
Disturbances in Diaphragmatic Ca2+ Homeostasis Is Required for VIDD
Shanely et al. (2002) provided the earliest evidence that prolonged MV results in disturbances of Ca2+ homeostasis in rat diaphragm fibers. Although this study did not directly measure cytosolic Ca2+ levels, the results revealed that prolonged MV activates calpain in diaphragm fibers and increased cytosolic levels of free Ca2+ is a requirement for calpain activation (Goll et al., 2003). MV-induced activation of calpains in diaphragm fibers has since been confirmed in many studies involving both humans and animals (Whidden et al., 1985b; Maes et al., 2007; Levine et al., 2008; McClung et al., 2008; Nelson et al., 2012; Dridi et al., 2020a). Moreover, two independent studies using calpain inhibitors have demonstrated that calpain is a major contributor to VIDD (Maes et al., 2007; Nelson et al., 2012). A brief overview of calpains and their activation in skeletal muscle follows.
Calpains are a family of Ca2+ activated proteases that cleave target substrates at specific sites (Goll et al., 2003). Three primary calpain isoforms exist in skeletal muscle (i.e., calpain1, calpain2, and calpain3); although evidence reveals that calpains 1 and 2 play an important role in promoting disuse muscle atrophy, it remains unknown as to whether calpain1 or calpain2 plays the dominant role in promoting fiber atrophy (Hyatt and Powers, 2020).
While activation of calpain involves several post-translational modifiers, it is established that calpain activation in muscle fibers requires increased cytosolic levels of free Ca2+. Though the intracellular concentrations of free Ca2+ required to activate calpains in skeletal muscle in vivo remain unknown, it is clear that the proteolytic removal of the N-terminal domain of the calpain molecule allows for both calpain1 and calpain2 to be activated at significantly lower Ca2+ concentrations (Goll et al., 2003). For a detailed discussion of the factors regulating the activity of calpains see the classic review of Goll et al. (2003) and the recent review by Hyatt and Powers (2020).
To determine the source of increased free Ca2+ in the diaphragm during prolonged MV, Talbert et al. (2016) tested the hypothesis that leakage of Ca2+ from the RyR1 is required for MV-induced calpain activation in the diaphragm. Cause and effect were determined by treating ventilated animals with the RyR1 blocker, azumolene. Their results revealed that while azumolene did block stimulation-induced Ca2+ release from the RyR1, treatment with this RyR1 channel blocker was not sufficient to prevent MV-induced calpain activation or VIDD (Talbert et al., 2016). However, azumolene may not prevent all forms of Ca2+ release from RyR1s. For example, while it is established that azumolene prevents neural stimulation-induced Ca2+ release from the RyR1, it remains unknown if this compound blocks oxidation-induced release of Ca2+ from the RyR1. Hence, experiments using a different investigative approach are required to delineate the role that leaky RyR1s play in MV-induced disturbances in Ca2+ homeostasis in diaphragm fibers.
Matecki et al. (2016) performed the first study to directly evaluate the impact of prolonged MV on Ca2+ homeostasis in the diaphragm. This elegant study provided robust evidence that prolonged MV increases the opening of RyR1 in diaphragm muscle fibers (Matecki et al., 2016); this increased spontaneous opening of RyR1 results in increased Ca2+ release (i.e., Ca2+ sparks) and disrupts Ca2+ homeostasis (Matecki et al., 2016). Analysis of both human and mouse diaphragm fibers has established that prolonged MV results in oxidation, S-nitrosylation, and Ser-2844 phosphorylation of the RyR1. Importantly, these post-translational modifications of the RyR1 accompany the dissociation of calstabin1 (Matecki et al., 2016). The importance of calstabin-1 association with RyR1 for preventing RyR1 leak was confirmed by findings that treatment of animals with S107, a small molecule that stabilizes the RyR1-calstabin1 interaction, prevents MV-induced Ca2+ sparks in diaphragm fibers, and protects against VIDD (Matecki et al., 2016). Furthermore, animals treated with the antioxidant trolox do not experience MV-induced oxidation of RyR1 and RyR1-mediated Ca2+ leak in diaphragm fibers. Therefore, this landmark study demonstrates that RyR1 dysfunction is an early pathological event leading to VIDD in both mice and humans, and confirms that oxidative stress is a prerequisite for MV-induced RyR1 dysfunction (Matecki et al., 2016).
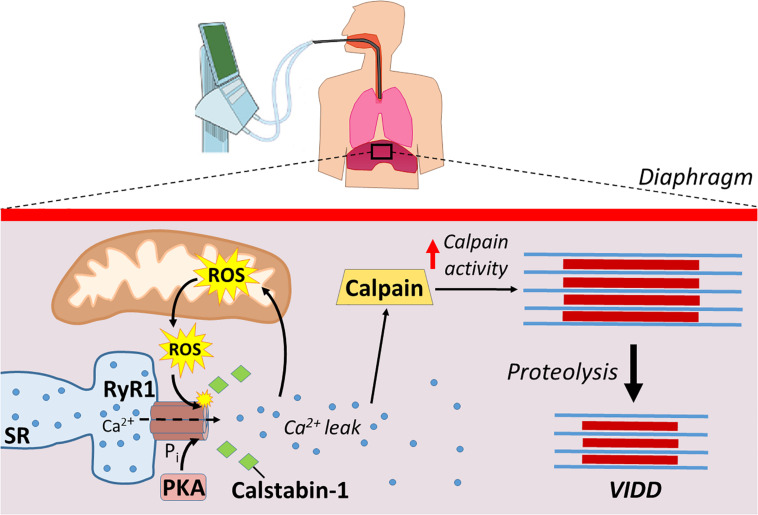
While the MV-induced increases in ROS production in the diaphragm is derived from several sources, mitochondrial ROS production is the dominant site of ROS emission in diaphragm fibers during prolonged MV (Falk et al., 1985, 2011; Whidden et al., 1985a; McClung et al., 2009; Powers et al., 2011). Recently, this supposition has gained additional support from a study concluding that MV-induced mitochondrial ROS production in the diaphragm is responsible for the oxidative remodeling of the RyR1 (Dridi et al., 2020b). Specifically, using the mitochondrial-targeted antioxidant SS-31 to prevent MV-induced increases in mitochondrial ROS production, Dridi et al. (2020a) provide further evidence that mitochondrial oxidative stress is responsible for oxidation of RyR1s resulting in SR Ca2+ leak, calpain activation, and VIDD (Figure 3). Moreover, prolonged MV is also associated with increased PKA activity, which is posited to play a role in the hyperphosphorylation of the RyR1 within diaphragm fibers (Dridi et al., 2020b). This MV-induced increase in PKA activity and hyperphosphorylation of RyR1 was abolished by treatment of animals with the mitochondrial-targeted antioxidant (SS-31). These findings are consistent with reports that oxidative stress increases PKA activity and decreases the activity of several phosphatases (Brennan et al., 2006; Steinberg, 2013). Collectively, these studies demonstrate that mitochondrial ROS emissions are key in the disruptions of Ca2+ homeostasis.
FIGURE 3.
Mechanical ventilation causes Ca2+ disruptions that lead to increased proteolysis. ROS production from mitochondria, along with increased protein kinase A (PKA) activity, modify ryanodine receptors (RyR1) leading to the disassociation of calstabin-1. Disassociation of calstabin-1 from RyR1 leads to Ca2+ leak, activation of calpains, and increased proteolysis that contributes to VIDD.
Investigations into the signaling pathways responsible for the MV-induced increases in mitochondrial ROS emission in the diaphragm have provided increased insight into the pathologic events underlying VIDD. Specifically, a growing number of studies suggest that MV-induced mitochondrial dysfunction is controlled by several interacting events including mechanical stimulation of angiotensin II type 1 receptors, along with activation of several signaling molecules including FoxO, STAT3, and Smad3 (Kwon et al., 1985; Smith et al., 2014; Smuder et al., 2015; Tang et al., 2015, 2017). Further, emerging evidence also suggests that accelerated autophagy and increased cytosolic levels of free Ca2+ are potential modulators of mitochondrial ROS production, acting as a positive feedback loop (Smuder et al., 2018; Tang and Shrager, 2018). Despite these recent advances in our knowledge of the signaling network responsible for MV-induced increases in mitochondrial ROS production, numerous questions remain unanswered about the pathways responsible for MV-induced mitochondrial dysfunction.
Discussion
Mechanical ventilation is a life-saving clinical intervention used to provide adequate alveolar ventilation in patients that are incapable of doing so on their own. Common applications of MV include patients suffering from chronic obstructive pulmonary disease, heart failure, acute myocardial infarction, and other critical illnesses. While MV is a life-saving intervention for many patients, prolonged MV promotes the rapid development of VIDD. VIDD is clinically significant because diaphragmatic weakness is a key risk factor contributing to problems in weaning patients from the ventilator. The inability to wean patients from the ventilator results in prolonged hospitalization and increased morbidity and mortality.
Mechanical ventilation-induced diaphragmatic atrophy results from both decreased protein synthesis and accelerated proteolysis. However, MV-induced activation of proteases in the diaphragm play a dominant role in the development of VIDD. Although all major proteolytic systems contribute to this MV-induced increase in proteolysis, evidence suggests that activation of the Ca2+-activated protease, calpain, plays a required role in this process. The steps leading to MV-induced calpain activation begin with an increase in mitochondrial ROS emission resulting in redox disturbances in diaphragm fibers. This MV-induced oxidative stress in diaphragm fibers results in post-translation modifications of the RyR1 (e.g., phosphorylation and oxidation) leading to the disassociation of calstabin1 from RyR1 and Ca2+ leak from the SR. This disturbance in Ca2+ homeostasis provides a required stimulus for calpain activation in the diaphragm leading to accelerated proteolysis and VIDD (Figure 3).
Although significant progress has been made toward understanding the mechanisms responsible for the MV-induced disturbance in Ca2+ homeostasis in the diaphragm, several important unanswered questions remain. For example, although it is established that an increase in mitochondrial ROS production is a requirement for MV-induced RyR1 leak leading to increased cytosolic levels of free Ca2+, the precise mechanisms responsible for this increased oxidant production remain undefined.
Evidence also exists that accelerated autophagy also promotes an increase in mitochondrial ROS emission in the diaphragm during prolonged MV (Smuder et al., 2018). However, the signaling pathways that connect these two events remain unclear.
Finally, it is generally agreed that an uptake of cytosolic Ca2+ into mitochondria can produce a mitochondrial Ca2+ overload, resulting in significant increases in mitochondria ROS production (Brookes et al., 2004; Peng and Jou, 2010). Nonetheless, it is currently unknown as to whether the MV-induced increases in cytosolic levels of free Ca2+ in the diaphragm are sufficient to produce mitochondrial Ca2+ overload and stimulate additional mitochondrial ROS production. This is an important issue that warrants future study.
Author Contributions
HH and SP formulated the outline of the review and contributed to writing and editing of the manuscript. Both authors approved the final submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer WN declared a past co-authorship with one of the author SP to the handling editor.
Abbreviations
- MV
Mechanical ventilation
- VIDD
Ventilator-induced diaphragm dysfunction
- ROS
Reactive oxygen species
- SR
sarcoplasmic reticulum
- CSA
cross-sectional area
- EC
excitation-contraction
- SOC
store-operated calcium channel
- ROC
receptor-operated calcium channel
- PMCA
plasma membrane Ca2+ ATPases
- NCX
Na+/Ca2+ exchanger
- RyR
ryanodine receptor
- DHPRs
dihydropyridine receptors
- PKA
Protein Kinase A
- MAMs
mitochondria associated membranes
- VDAC
voltage dependent anion channel
- MCU
mitochondrial uniporter.
Footnotes
Funding. This research was supported by the National Institutes of Health R21 AR073956.
References
- Agten A., Maes K., Smuder A., Powers S. K., Decramer M., Gayan-Ramirez G. (2011). N-Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit. Care Med. 39 777–782. 10.1097/ccm.0b013e318206cca9 [DOI] [PubMed] [Google Scholar]
- Belosludtsev K. N., Dubinin M. V., Belosludtseva N. V., Mironova G. D. (2019). Mitochondrial Ca2+ transport: mechanisms, molecular structures, and role in cells. Biochemistry 84 593–607. 10.1134/s0006297919060026 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Bootman M. D., Roderick H. L. (2003). Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell. Biol. 4 517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Bogeski I., Kappl R., Kummerow C., Gulaboski R., Hoth M., Niemeyer B. A. (2011). Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium 50 407–423. 10.1016/j.ceca.2011.07.006 [DOI] [PubMed] [Google Scholar]
- Brennan J. P., Bardswell S. C., Burgoyne J. R., Fuller W., Schroder E., Wait R., et al. (2006). Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J. Biol. Chem. 281 21827–21836. 10.1074/jbc.m603952200 [DOI] [PubMed] [Google Scholar]
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasova E., Moschella M. C., et al. (1994). Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77 513–523. 10.1016/0092-8674(94)90214-3 [DOI] [PubMed] [Google Scholar]
- Brini M., Carafoli E. (2011). The plasma membrane Ca(2)+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect. Biol. 3:a004168. 10.1101/cshperspect.a004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P. S., Yoon Y., Robotham J. L., Anders M. W., Sheu S. S. (2004). Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 287 C817–C833. [DOI] [PubMed] [Google Scholar]
- Burr A. R., Millay D. P., Goonasekera S. A., Park K. H., Sargent M. A., Collins J., et al. (2014). Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice. Mol. Cell Biol. 34 1991–2002. 10.1128/mcb.00339-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes E. M., Loaiza R., Valdivia H. H. (2011). Ryanodine receptors. Skelet. Muscle 1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K. F., et al. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell. Biol. 174 915–921. 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. (1980). Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J. 190 107–117. 10.1042/bj1900107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuisseau K. C., Kavazis A. N., Deering M. A., Falk D. J., Van Gammeren D., Yimlamai T., et al. (1985). Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J. Appl. Physiol. 98 1314–1321. 10.1152/japplphysiol.00993.2004 [DOI] [PubMed] [Google Scholar]
- DeSantiago J., Batlle D., Khilnani M., Dedhia S., Kulczyk J., Duque R., et al. (2007). Ca2+ /H+ exchange via the plasma membrane Ca2+ ATPase in skeletal muscle. Front. Biosci. 12 4641–4660. 10.2741/2414 [DOI] [PubMed] [Google Scholar]
- Dridi H., Liu X., Yuan Q., Reiken S., Mohamad Y., Sittenfeld L. R., et al. (2020a). Role of defective calcium regulation in cardiorespiratory dysfunction in Huntington’s disease. JCI Insight 5:e140614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dridi H., Yehya M., Barsotti R., Reiken S., Angebault C., Jung B., et al. (2020b). Mitochondrial oxidative stress induces leaky ryanodine receptor during mechanical ventilation. Free Radic. Biol. Med. 146 383–391. 10.1016/j.freeradbiomed.2019.11.019 [DOI] [PubMed] [Google Scholar]
- Falk D. J., Deruisseau K. C., Van Gammeren D. L., Deering M. A., Kavazis A. N., Powers S. K. (1985). Mechanical ventilation promotes redox status alterations in the diaphragm. J. Appl. Physiol. 101 1017–1024. 10.1152/japplphysiol.00104.2006 [DOI] [PubMed] [Google Scholar]
- Falk D. J., Kavazis A. N., Whidden M. A., Smuder A. J., McClung J. M., Hudson M. B., et al. (2011). Mechanical ventilation-induced oxidative stress in the diaphragm: role of heme oxygenase-1. Chest 139 816–824. 10.1378/chest.09-2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M. J., Mantilla C. B., Sieck G. C. (2018). Breathing: motor control of diaphragm muscle. Physiology 33 113–126. 10.1152/physiol.00002.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk G. C., Anders S., Breyer M. K., Burghuber O. C., Edelmann G., Heindl W., et al. (2010). Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur. Respir. J. 35 88–94. 10.1183/09031936.00056909 [DOI] [PubMed] [Google Scholar]
- Giorgio V., Guo L., Bassot C., Petronilli V., Bernardi P. (2018). Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 70 56–63. 10.1016/j.ceca.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Glancy B., Willis W. T., Chess D. J., Balaban R. S. (2013). Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 52 2793–2809. 10.1021/bi3015983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003). The calpain system. Physiol. Rev. 83 731–801. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ochoa E. O., Pratt S. J. P., Lovering R. M., Schneider M. F. (2015). Critical role of intracellular RyR1 calcium release channels in skeletal muscle function and disease. Front. Physiol. 6:420. 10.3389/fphys.2015.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. B., Smuder A. J., Nelson W. B., Wiggs M. P., Shimkus K. L., Fluckey J. D., et al. (2015). Partial support ventilation and mitochondrial-targeted antioxidants protect against ventilator-induced decreases in diaphragm muscle protein synthesis. PLoS One 10:e0137693. 10.1371/journal.pone.0137693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt H. W., Powers S. K. (2020). The role of calpains in skeletal muscle remodeling with exercise and inactivity-induced atrophy. Int. J. Sports Med. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Knisely A. S., Leal S. M., Singer D. B. (1988). Abnormalities of diaphragmatic muscle in neonates with ventilated lungs. J. Pediatr. 113 1074–1077. 10.1016/s0022-3476(88)80585-7 [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. (1976). Calcium-binding proteins. Annu. Rev. Biochem. 45 239–266. [DOI] [PubMed] [Google Scholar]
- Kwon O. S., Smuder A. J., Wiggs M. P., Hall S. E., Sollanek K. J., Morton A. B., et al. (1985). AT1 receptor blocker losartan protects against mechanical ventilation-induced diaphragmatic dysfunction. J. Appl. Physiol. 119 1033–1041. 10.1152/japplphysiol.00237.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourdelles G., Mier L., Fiquet B., Djedaini K., Saumon G., Coste F., et al. (1996). Comparison of the effects of heat and moisture exchangers and heated humidifiers on ventilation and gas exchange during weaning trials from mechanical ventilation. Chest 110 1294–1298. 10.1378/chest.110.5.1294 [DOI] [PubMed] [Google Scholar]
- Lessa T. B., de Abreu D. K., Bertassoli B. M., Ambrosio C. E. (2016). Diaphragm: a vital respiratory muscle in mammals. Ann. Anat. 205 122–127. 10.1016/j.aanat.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Levine S., Nguyen T., Taylor N., Friscia M. E., Budak M. T., Rothenberg P., et al. (2008). Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 358 1327–1335. 10.1056/nejmoa070447 [DOI] [PubMed] [Google Scholar]
- Maes K., Testelmans D., Powers S., Decramer M., Gayan-Ramirez G. (2007). Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am. J. Respir. Crit. Care Med. 175 1134–1138. 10.1164/rccm.200609-1342oc [DOI] [PubMed] [Google Scholar]
- Matecki S., Dridi H., Jung B., Saint N., Reiken S. R., Scheuermann V., et al. (2016). Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc. Natl. Acad. Sci. U.S.A. 113 9069–9074. 10.1073/pnas.1609707113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J. M., Kavazis A. N., DeRuisseau K. C., Falk D. J., Deering M. A., Lee Y., et al. (2007). Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am. J. Respir. Crit. Care Med. 175 150–159. 10.1164/rccm.200601-142oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J. M., Van Gammeren D., Whidden M. A., Falk D. J., Kavazis A. N., Hudson M. B., et al. (2009). Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit. Care Med. 37 1373–1379. 10.1097/ccm.0b013e31819cef63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J. M., Whidden M. A., Kavazis A. N., Falk D. J., Deruisseau K. C., Powers S. K. (2008). Redox regulation of diaphragm proteolysis during mechanical ventilation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294 R1608–R1617. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. (1980). Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem. J. 190 95–105. 10.1042/bj1900095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. B., Smuder A. J., Hudson M. B., Talbert E. E., Powers S. K. (2012). Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit. Care Med. 40 1857–1863. 10.1097/ccm.0b013e318246bb5d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T. I., Jou M. J. (2010). Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 1201 183–188. 10.1111/j.1749-6632.2010.05634.x [DOI] [PubMed] [Google Scholar]
- Petrof B. J., Jaber S., Matecki S. (2010). Ventilator-induced diaphragmatic dysfunction. Curr. Opin. Crit. Care 16 19–25. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Di Virgilio F., Pozzan T. (1990). Structural and functional aspects of calcium homeostasis in eukaryotic cells. Eur. J. Biochem. 193 599–622. 10.1111/j.1432-1033.1990.tb19378.x [DOI] [PubMed] [Google Scholar]
- Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. (2008). Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27 6407–6418. 10.1038/onc.2008.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P., Drago I., Filadi R., Pozzan T. (2012). Mitochondrial Ca(2)(+) homeostasis: mechanism, role, and tissue specificities. Pflugers Arch. 464 3–17. 10.1007/s00424-012-1122-y [DOI] [PubMed] [Google Scholar]
- Powers S. K., Hudson M. B., Nelson W. B., Talbert E. E., Min K., Szeto H. H., et al. (2011). Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 39 1749–1759. 10.1097/ccm.0b013e3182190b62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S. K., Wiggs M. P., Sollanek K. J., Smuder A. J. (2013). Ventilator-induced diaphragm dysfunction: cause and effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305 R464–R477. [DOI] [PubMed] [Google Scholar]
- Protasi F., Paolini C., Nakai J., Beam K. G., Franzini-Armstrong C., Allen P. D. (2002). Multiple regions of RyR1 mediate functional and structural interactions with alpha(1S)-dihydropyridine receptors in skeletal muscle. Biophys. J. 83 3230–3244. 10.1016/s0006-3495(02)75325-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiken S., Lacampagne A., Zhou H., Kherani A., Lehnart S. E., Ward C., et al. (2003). PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J. Cell. Biol. 160 919–928. 10.1083/jcb.200211012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Pizzo P., Filadi R. (2019). Calcium, mitochondria and cell metabolism: a functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell. Res. 1866 1068–1078. 10.1016/j.bbamcr.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Schwaller B. (2010). Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2:a004051. 10.1101/cshperspect.a004051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanely R. A., Van Gammeren D., Deruisseau K. C., Zergeroglu A. M., McKenzie M. J., Yarasheski K. E., et al. (2004). Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Respir. Crit. Care Med. 170 994–999. 10.1164/rccm.200304-575oc [DOI] [PubMed] [Google Scholar]
- Shanely R. A., Zergeroglu M. A., Lennon S. L., Sugiura T., Yimlamai T., Enns D., et al. (2002). Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am. J. Respir. Crit. Care Med. 166 1369–1374. 10.1164/rccm.200202-088oc [DOI] [PubMed] [Google Scholar]
- Smith I. J., Godinez G. L., Singh B. K., McCaughey K. M., Alcantara R. R., Gururaja T., et al. (2014). Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 28 2790–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder A. J., Nelson W. B., Hudson M. B., Kavazis A. N., Powers S. K. (2014). Inhibition of the ubiquitin-proteasome pathway does not protect against ventilator-induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology 121 115–126. 10.1097/aln.0000000000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder A. J., Sollanek K. J., Min K., Nelson W. B., Powers S. K. (2015). Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit. Care Med. 43 e133–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuder A. J., Sollanek K. J., Nelson W. B., Min K., Talbert E. E., Kavazis A. N., et al. (2018). Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic. Biol. Med. 115 179–190. 10.1016/j.freeradbiomed.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. F. (2013). Oxidative stress and sarcomeric proteins. Circ. Res. 112 393–405. 10.1161/circresaha.111.300496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert E. E., Smuder A. J., Kwon O. S., Sollanek K. J., Wiggs M. P., Powers S. K. (2016). Blockage of the ryanodine receptor via azumolene does not prevent mechanical ventilation-induced diaphragm atrophy. PLoS One 11:e0148161. 10.1371/journal.pone.0148161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Kennedy C. L., Lee M., Gao Y., Xia H., Olguin F., et al. (2017). Smad3 initiates oxidative stress and proteolysis that underlies diaphragm dysfunction during mechanical ventilation. Sci. Rep. 7:14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Shrager J. B. (2018). The signaling network resulting in ventilator-induced diaphragm dysfunction. Am. J. Respir. Cell. Mol. Biol. 59 417–427. 10.1165/rcmb.2018-0022tr [DOI] [PubMed] [Google Scholar]
- Tang H., Smith I. J., Hussain S. N., Goldberg P., Lee M., Sugiarto S., et al. (2015). The JAK-STAT pathway is critical in ventilator-induced diaphragm dysfunction. Mol. Med. 20 579–589. 10.2119/molmed.2014.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A., Toral-Ojeda I., Aldanondo G., de Munain A. Lopez. (2014). Dysregulation of calcium homeostasis in muscular dystrophies. Expert Rev. Mol. Med. 16:e16. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T., Petrof B. J. (2004). Ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 169 336–341. [DOI] [PubMed] [Google Scholar]
- Ward C. W., Reiken S., Marks A. R., Marty I., Vassort G., Lacampagne A. (2003). Defects in ryanodine receptor calcium release in skeletal muscle from post-myocardial infarct rats. FASEB J. 17 1517–1519. [DOI] [PubMed] [Google Scholar]
- Wehrens X. H., Lehnart S. E., Reiken S., van der Nagel R., Morales R., Sun J., et al. (2005). Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc. Natl. Acad. Sci. U.S.A. 102 9607–9612. 10.1073/pnas.0500353102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whidden M. A., McClung J. M., Falk D. J., Hudson M. B., Smuder A. J., Nelson W. B., et al. (1985a). Xanthine oxidase contributes to mechanical ventilation-induced diaphragmatic oxidative stress and contractile dysfunction. J. Appl. Physiol. 106 385–394. 10.1152/japplphysiol.91106.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whidden M. A., Smuder A. J., Wu M., Hudson M. B., Nelson W. B., Powers S. K. (1985b). Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J. Appl. Physiol. 108 1376–1382. 10.1152/japplphysiol.00098.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez M., Gil-Longo J., Campos-Toimil M. (2012). Calcium binding proteins. Adv. Exp. Med. Biol. 740 461–482. [DOI] [PubMed] [Google Scholar]