Abstract
Background
Wounds are important health problems that cause significant financial burden and loss of time to work, more so in low and lower middle income countries. Negative pressure wound therapy (NPWT) is widely established in managing acute and chronic extremity wounds. We studied the effects of addition of normal saline instillation to NPWT in terms of changes in granulation tissue, bacterial-burden and overall wound healing using readily available means and materials including wall suction for negative pressure, sponge and adhesive transparent sheet for dressing and normal saline for irrigation.
Methods
All patients with extremity ulcers initially underwent surgical debridement. They were then allotted into two groups, group 1 (NPWT with normal saline instillation- NPWTi) including 25 patients and group 2 (NPWT) including 23 patients. Tissue-bit samples taken on day1 and day 10 were used for bacteriology and for assessing histology. The wound surface-area was measured using the software ImageJ on day 1 and day 10.
Results
Median log difference in colony-count between day1 and day10 was 0.6 (0.2–1.4) in group1 and 0.13 (0.04–0.6) in group 2 (p < 0.05). Mean percentage reduction in wound size was 28.82 and 19.80 in group 1 and group 2 respectively (p < 0.05). Histological parameters of wound healing assessed as surface epithelium, granulation, inflammatory cells, proliferative blood-vessels and fibroblasts were significantly better in group1. A drawback observed with NPWTi was skin maceration around the ulcer which was successfully managed.
Conclusion
Our findings suggest that wound healing is significantly better when saline instillation is combined with NPWT. It can aid in complex extremity ulcers management by reducing the size of the wound with healthier looking granulation tissue.
Keywords: Bacterial load, Granulation, Negative pressure wound therapy, Saline solution, Ulcer
Highlights
-
•
Wound healing requires adequate infection control, improved microcirculation and healthy granulation as seen with NPWT.
-
•
NPWT with saline instillation (NPWTi) has gained popularity in past few years for management of extremity ulcers.
-
•
NPWTi reduces bacterial bioburden, promotes granulation tissue and reduces wound size which leads to better wound healing.
-
•
This is the first prospective randomized controlled trial comparing conventional NPWT to NPWTi using saline irrigation.
1. Introduction
Extremity wounds are common problems in surgical practice. Delayed wound healing is an important aspect in management as it results in increased morbidity, prolonged hospital stay, loss of time to work and increased financial burden. Apart from conventional moist saline gauze dressings, various modalities of local wound therapy described to improve wound healing are ultrasound therapy [1], infrared therapy [2], platelet rich plasma. Negative pressure wound therapy (NPWT) remains an established means to manage acute and chronic extremity wounds, especially challenging wounds, as an adjunct to debridement, systemic antibiotic therapy, topical antiseptics/antimicrobials [[3], [4], [5]].
The primary effects of NPWT on wound healing include wound contracture and size reduction; stabilization of wound environment and protection from contaminating microorganisms; reduction of edema and improvement in perfusion; removal of exudates, and cellular proliferation with improved granulation tissue [6]. Topical irrigation solutions like normal saline (NS) have been used in wounds for cleansing of wound beds, removing exudate/debris and controlling microbial growth. Combining NPWT with instillation (NPWTi) of topical solution influences wound healing owing to cyclic cleansing, dilution of wound debris, disruption of biofilm, accelerated granulation tissue and thus earlier reduction in wound size [5,7,8].
Surgical debridement is an important aspect in management of extremity ulcers. Despite serial debridement, wound remains to be contaminated with pathogenic microbes. Microbes generate metabolic toxins and inflammatory mediators contributing to cytolytic enzymes and free oxygen radicals production, compete with host cells for nutrients and oxygen, contributing to tissue hypoxia. These factors make the granulation tissue fragile, reduce fibroblast number and collagen production which ultimately impair wound healing [9]. To attenuate these effects, NS, antimicrobials and antiseptics have been proposed as potentially effective instillation solution for treating heavily infected wounds and when combined with NPWT promote better wound healing [8,10,11].
Owing to above mentioned benefits of NPWTi, reduced wound size, earlier wound closure and reduced hospital stay are other observed advantages emphasizing its cost-effectivness as well [5,7,12].
The advantages of NPWTi over NPWT however have been evaluated in numerous retrospective studies. Only one prospective randomized study has compared NPWT with NPWTi using 0.1% polyhexanide-betaine solution in which addition of irrigation showed no change in clinical outcomes of diabetic foot infections [13]. We undertook this prospective randomized control trial (RCT) to study whether addition of saline instillation to conventional NPWT results in better wound healing.
Our hypotheses are NPWT with saline is better for healing of wound as measured by reduction in wound size, reduction in bacterial burden and improvement in histological appearance of the wound instillation as assessed by changes in surface epithelium, histological appearance of granulation tissue, inflammatory cells, proliferating blood vessels and fibroblastic reaction and changes in adnexal structures.
2. Methodology
2.1. CTRI: CTRI/2020/05/025,410http://ctri.nic.in/Clinicaltrials/advsearch.php
We conducted a prospective, parallel arm, randomized controlled study in the department of Surgery at Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India from March 2016 to December 2017. Institute Ethics Committee approval was obtained prior to commencement of study. The study was conducted in collaboration with Department of Microbiology and Pathology for objective assessment of the benefits of NPWTi over conventional NPWT. All patients aged ≥18 years with wound in one of the extremities admitted in the department of Surgery were included in the study. The exclusion criteria was patients with uncontrolled hyperglycaemia (HbA1c > 12%), peripheral vascular disease, malignancy, active necrotizing fasciitis, wounds with open joint capsules or those on concomitant drug therapy like immunosuppressant/corticosteroids. Block randomization was done by a faculty not part of this study for allocation of patients to two groups – Group 1 [Negative Pressure Wound Therapy with Normal Saline instillation (NPWTi)] and Group 2 (conventional NPWT). Serially numbered opaque sealed envelopes (SNOSE) were used for allocation concealment. Blinding of the surgeons and patients was not possible as the visual differences in the two groups (NPWT vs NPWTi) were obvious and difficult to conceal. Written and informed consent was obtained from all participants included in the study.
A tissue bit sample from edge of the wound was sent for aerobic culture and sensitivity at admission. All participants underwent surgical debridement initially, either under regional block in minor procedure room, or under general anaesthesia in operation theatre. Intravenous Ceftriaxone (1 g twice daily) and Metronidazole (500 mg thrice daily) were started empirically for all patients at admission and it was changed to appropriate antibiotic therapy based on the antimicrobial culture-sensitivity pattern. Blood profile including complete blood count, blood-sugar profile, albumin were recorded at admission. Patients were given high-protein diet and those with haemoglobin <8 g m/dl were given packed red-blood cell transfusion. Once the wound was fit for initiation of NPWT, participants were allotted into one of the treatment groups.
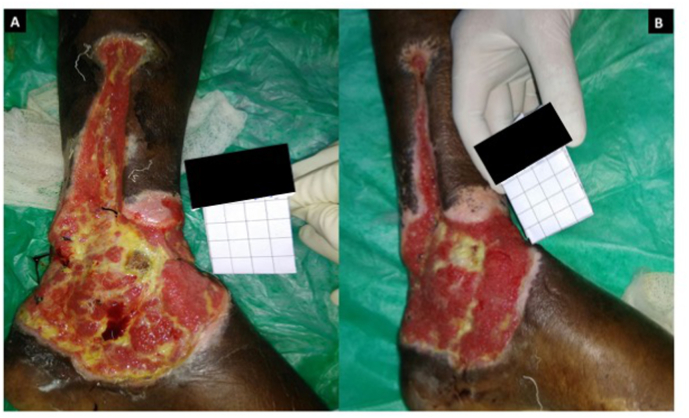
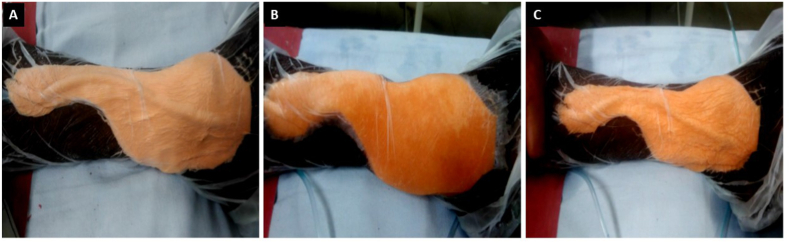
A wedge of tissue of approximately 2–3 mm was collected from the wound edge for assessing histopathological parameters as well as for qualitative and quantitative (Colony Forming Units – CFU) bacterial cultures on day 1 of initiating NPWT. A clinical photograph of the wound was taken on day 1 (Fig. 2A) alongside a 4 × 4 cm grid paper and the wound surface area was measured using the software ImageJ version 1.51t software. Then NPWT was applied over the wound using sterile foam cut in the shape of the wound, arranged in 2 layers with a drain tube placed in between the layers (Fig. 1). This system was sealed using a sterile transparent plastic sheet having adhesive surface on one side. Drain tube was connected to suction device and pressure was set at −125 mm/Hg. Change of dressing was done once every 48 h in both the groups (see Fig. 3).
Fig. 2.
Images of the wound.
A. Day 1 picture showing the wound over left leg (medial aspect) at the initiation of NPWTi. B. Day 10 picture of the same wound at the end of intervention period following NPWTi.
Fig. 1.
Images showing procedure of negative pressure wound therapy with normal saline instillation (NPWTi).
A. NPWTi showing foam covering the wound in two layers with a drain tube placed between the two layers of foam and connected to suction device and system sealed with adhesive transparent sheet. B. Instillation of normal saline till the foam is visibly saturated. C. Following 10 min of dwell time, drain tube re-connected to the negative suction.
Fig. 3.
Consort diagram.
Patients in group1 received intermittent instillation of NS four times a day at an interval of 4 h with a dwell time of 10 min. Volume for instillation was determined by amount just enough to visibly saturate the foam indicated by a darker colour change (Fig. 1B) and after a dwell time of 10 min, drain tube was re-connected to the suction device (Fig. 1C). A biopsy was repeated from the wound edge on day 10 of initiating NPWT/NPWTi and sent for histopathology and bacteriology. A photograph of the wound was taken again on day 10 (Fig. 2B) to measure wound-surface area and assess percentage reduction in wound size.
The patients could not be blinded to the treatment group allocation as the use of NS for instillation was evident and required co-operation on their part as well. The investigators, data collectors and the statistician were not blinded. The microbiologist and the pathologist assessing the tissue bit specimen were blinded and were not aware of treatment group allocation.
Data collected included demographics, parameters affecting wound healing, such as BMI, haemoglobin, albumin and smoking status, wound location and etiology. The primary outcome measured was reduction in wound size and secondary outcomes were quantitative change in bacterial burden and change in histological appearance of granulation tissue.
Granulation tissue was assessed on histopathology in terms of changes on day 10 compared to day 1. The parameters studied were surface epithelium, histological appearance of granulation tissue, inflammatory cells, proliferating blood vessels and fibroblastic reaction and changes in adnexal structures. These entities were semi-quantitatively expressed as absent (no presence of any of the considered parameters); scarcely present: 1+(present on < 33% of the lesions' preparations), present: 2+ (present in 33–66% of lesions' preparations) and extensively present: 3+ (present in >66% of lesions’ preparations) based on the percentage of parameters seen on histology. These grades were further classified as no improvement, mild improvement and moderate improvement when difference in the grades between day 1 and day 10 was 0, 1 and 2 respectively in order to measure the significance of the difference following intervention.
Sample size was calculated to be 23 in each group using “OpenEpi version 3.03”. It was calculated using an alpha level (type 1 error) of 5% and power of study (1-β) of 80% assuming mean reduction in wound size in NPWT group as 20.4 cm2 and associated mean reduction in NPWT with normal saline instillation to be 25.5 cm2 (25% further reduction) [14]. Data analysis was done using IBM SPSS statistics software version 19.0 (Chicago, USA). The continuous variables were summarized as mean ± SD, discrete variables were summarized as median (IQR- Interquartile Range) and categorical variables were summarized as proportions. Difference in outcome for normally distributed variables was assessed using independent samples t-test and for non-normally distributed variables using Mann-Whitney test. Association between treatment group and the categorical variables were assessed using Pearson's Chi-square test. A p-value of less than 0.05 was considered statistically significant.
3. Results
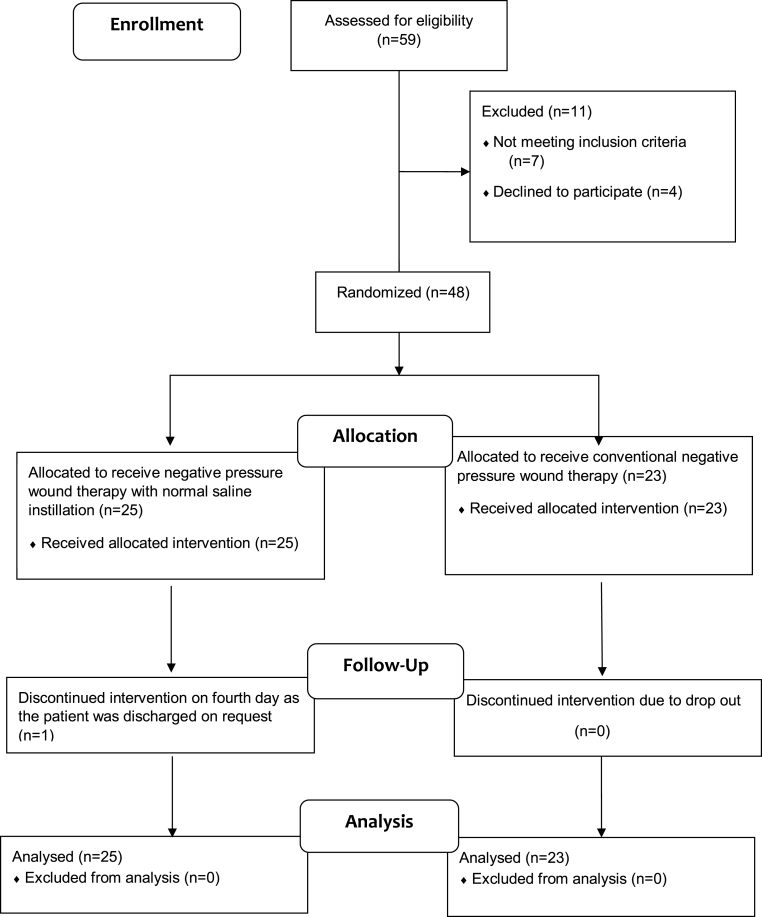
A total of 48 patients with ulcer in one of the extremities, eligible according to the inclusion criteria, were included in the study. All patients included in the study had ulcer in one of the lower limbs. Block randomization was done for allocation of the 48 patients included in the study into the two groups, group 1 and group 2. Group 1 included 25 participants who underwent NPWTi and group 2 included 23 participants who underwent conventional sNPWT. One of the participants for NPWTi group discontinued the therapy on fourth day following the initiation of the intervention. This participant was considered as a “drop-out” during the analysis. Therefore, for the analysis, parameters requiring comparison of day 1 and day 10 data such as, change in colony forming unit (CFU) counts, percentage reduction in the surface area and change in the histopathological, total number of participants who received treatment in NPWTi group was taken as 24 with one participant being regarded as a “drop-out”.
There were no differences in demographic parameters, factors affecting wound healing (haemoglobin, albumin, HbA1c) in both the groups (Table 1). Both groups were similar in distribution of co-morbidities (p = 0.07) and smoking habits (p = 0.39) (Table 2). Diabetic foot was the most common etiology observed and were similarly distributed in both the groups while other causes of ulcers were post traumatic, post insect or snake bite and some spontaneous onset/unknown which were also similarly distributed in groups 1 and 2.
Table 1.
Demographics and parameters affecting wound healing.
| NPWTi | NPWT | p value | |
|---|---|---|---|
| Age (yrs) [Median (IQR)] | 56 (35–66.5) | 56 (44–66) | 0.98∗ |
| Male:Female n (%) | 23 (92):2(8) | 16 (69.6):7(30.4) | 0.07∗∗ |
| BMI (kg/m2) (Mean ± SD) |
22.83 ± 2.44 | 22.87 ± 2.33 | 0.90∗∗∗ |
| Haemoglobin (gm/dl) (Mean ± SD) |
10.14 ± 1.66 | 8.57 ± 1.63 | 0.71∗∗∗ |
| Albumin (g/mL) (Mean ± SD) |
3.028 ± 0.42 | 2.70 ± 0.50 | 0.21∗∗∗ |
| Median HbA1C (%) [Median (IQR)] | 7.1 (6.1–9.6) | 7.1 (6.6–10.0) | 0.37∗ |
BMI- Body Mass Index.
Mann-Whitney test.
Fischer's exact test.
Unpaired t-test.
Table 2.
Comparison of comorbidities and smoking habits among the participants of both the groups.
| NPWTi n (%) | NPWT n (%) | p value | ||
|---|---|---|---|---|
| Comorbidities | No | 11 (44) | 4 (17.4) | 0.07∗∗ |
| Diabetes mellitus (DM) | 8 (32.0) | 8 (34.8) | ||
| Hypertension (HTN) | 0 (0) | 2 (8.7) | ||
| Coronary Artery Disease (CAD) | 0 (0) | 1 (4.3) | ||
| Chronic Kidney Disease (CKD) | 0 (0) | 0 (0) | ||
| DM & HTN | 5 (20) | 3 (13) | ||
| DM & CAD | 0 (0) | 1 (4.3) | ||
| DM & CKD | 0 (0) | 3 (13) | ||
| HTN & CKD | 1 (4) | 1 (4.3) | ||
| Smoker | Yes | 14 (56) | 10 (43.5) | 0.39∗∗∗ |
| No | 11 (44) | 13 (56.5) |
Fischer's exact test.
Chi-sqre test.
3.1. Bacterial burden
Median log difference in Colony Forming Unit counts between day1 and day10 among group1 and group 2 was 0.6 (0.2–1.4) and 0.13 (0.04–0.6) respectively. The reduction of biofilm in group1 compared to group2 was statistically significant (p = 0.003) (Table 3). The different organisms grown in both the groups were Pseudomonas aeruginosa, Acinetobacter baumanii, Proteus mirabilis, Escherichia coli, Klebsiella pneumoniae, Providencia species, Enterobacter and Staphylococcus aureus. In both the groups the most frequently observed bacteria on day1 of initiation of the intervention were Pseudomonas aeruginosa. Following intervention in NPWTi group, there was a reduction in the isolation of Pseudomonas while Acinetobacter showed a higher rate of isolation. In NPWT group there was reduction in isolation of Pseudomonas while E. coli showed a higher rate of isolation. However, most of these infections were polymicrobial making it difficult to assign the significance of these findings.
Table 3.
Comparison of CFU and percentage reduction in the size of the wound on day 1 and day 10 among the two groups.
| NPWTi | NPWT | p value | |
|---|---|---|---|
| Median log difference in CFU between day 1 and day 10 | 0.6 (0.2–1.4) | 0.13 (0.04–0.6) | 0.003∗ |
| Percentage reduction | 28.82 | 19.80 | 0.03∗ |
Mann-Whitney U test.
3.2. Reduction in wound size
In this study, the average size (in mm2) of wound at the initiation of intervention was 81 (63–114) in group 1 and 78 (62–98) in group 2 which was comparable. The average size of wound (in mm2) following intervention on day 10 was 66 (45–92) in group 1 and 60 (50–88) in group 2. The mean percentage reduction in size of the wound between day 1 and day 10 in group 1 was 28.82% and 19.80% in group 2 which was statistically significant (p = 0.03) (Table 3).
The median number of days from day of admission to day of initiation of therapy was 10 (6.5–19.5) in NPTWi group and 11 (8.0–27.0) in NPWT group which was not statistically significant (p = 0.570). Similarly, there was no statistically significant difference in days from initiation of therapy to discharge between the two groups, group 1 and group 2 (24.88 Vs 24.09) (p = 0.84). The number of patients with wound fit for closure at the time of discharge was 22 (88%) in group 1 and 17 (73.9) in group 2 which was not statistically significant (p = 0.21).
3.3. Wound histology
As regards to the histopathological examination, significant improvements were observed in instillation group (Table 4). The parameters were graded as no improvement, mild improvement or moderate improvement. Improvement in group 1 with regards to surface epithelium was no-improvement in 1 (4.2%) out of 24 patients, mild in 13 (54.2%) and moderate in 10 (41.7%) compared to 7(30.4%), 16(69.6%) and 0 respectively in group2. This observed difference between two groups was significant (p < 0.001). Similarly significant improvement in granulation tissue (p = 0.003), improvement in inflammatory cells (p = 0.001) and improvement in proliferating blood vessels and fibroblastic reaction (p = 0.001) were observed in group1. However, difference in improvement in adnexal structure was not statistically significant in both the groups (p = 0.58).
Table 4.
Comparison of change in histological parameters between day 1 and day 10 among the two groups.
| Comparison of change in | No improvement n (%) | Mild improvement n (%) | Moderate improvement n (%) | p value∗∗ |
|---|---|---|---|---|
| Surface Epithelium | ||||
| NPWTi | 1 (4.2) | 13 (54.2) | 10 (41.7) | <0.001 |
| NPWT | 7 (30.4) | 16 (69.6) | 0 | |
| Granulation tissue | ||||
| NPWTi | 3 (12.5) | 6 (25) | 15 (62.5) | 0.003 |
| NPWT | 3 (13) | 16 (69.6) | 4 (17.4) | |
| Inflammatory cells | ||||
| NPWTi | 2 (8) | 8 (32) | 14 (56) | 0.001 |
| NPWT | 9 (39.1) | 11 (47.8) | 3 (13) | |
| Proliferating blood vessels and fibroblastic reaction | ||||
| NPWTi | 1 (4.2) | 11 (45.8) | 12 (50) | 0.001 |
| NPWT | 5 (21.7) | 17 (73.9) | 1 (4.3) | |
| adnexal structure | No improvement n (%) | Improvement n (%) |
p value 0.58∗∗∗ |
|
| NPWTi | 19 (79.2) | 5 (20.8) | ||
| NPWT | 18 (78.3) | 5 (21.7) | ||
Fisher's exact test.
1-sideFischer's exact test.
4. Discussion
Wounds in extremities pose a great challenge to the treating surgeon. NPWT is established as an important adjunct in the management of extremity wound. The benefits of NPWT include reduction of tissue edema thus improving tissue perfusion [8]; removal of wound exudates and debris [8,15]; and inhibition of bacterial growth and reduction of wound infection [8].
The use of various irrigation solutions in an open wound has been practiced in order to reduce bacterial bioburden, remove exudates and improve granulation tissues which accelerate wound healing process. Normal saline (NS) soaked moist dressing is widely used method of dressing in surgical practice including an extremity wound. To our best knowledge, this study is the only prospective, randomized controlled study (human study) comparing the effects of NPWTi using normal saline with NPWT in the management of extremity wounds barring one recently published prospective RCT comparing clinical outcomes following NPWT with and without antiseptic irrigation in patients with diabetic foot infections using 0.1% polyhexanide-betaine as irrigation solution [13].
Instillation solution used should be compatible with NPWTi foam dressings. These include normal saline, hypochlorous acid solution, sodium hypochlorite solution (dilute Dakin's solution 0.125% or quarter strength), acetic acid solution (0.25%–1.0%), and polyhexamethylene biguanide (0.1%) + betaine (0.1%) (8). Mafenide acetate or povidone iodine solution and silver containing dressings are other topical antiseptic/antimicrobial solutions, however, incompatibility with NPWTi foam dressings limits their usage as instillation solution. The choice of instillation solution also relies on its biocompatibility (antiseptic efficacy relative to its cytotoxicity). Most of the topical antimicrobial agents have cytotoxic effects, impair cellular microcirculation, increase microvascular permeability and vessel leakage, all of which negatively impact wound healing [[16], [17], [18]]. Instillation of topical antibiotics is also discouraged because of contact sensitisation and potential local resistance [8]. NS on the other hand has outcomes comparative to recommended antiseptic solutions including Prontosan (0.1% polyhexamethylenebiguanide+0.1% betaine) and Lavasept (0.04%polyhexanide [8]. It also has the advantage of being universally available and physiological. Various case series have shown successful outcomes with saline with regards to improved granulation tissue [15,[19], [20], [21]], mean duration of therapy, proportion of wounds closed and time to final surgical procedure [5,11,[22], [23], [24]]. There is lack of controlled clinical evidence in the literature demonstrating superiority of any of the above listed instilled topical antiseptics compared with saline as instillation solution [11] and in a recently published randomized study, addition of 0.1% polyhexanide-betaine solution for irrigation of wounds in diabetic foot infection patients showed no added benefit in terms of clinical outcomes [13]. NS as the first choice is now recommended by consensus guidelines as opposed to topical antiseptic solutions which were previously recommended as first-line solutions [8]. This was therefore the rationale for using NS as instillation solution in our study and we observed positive effect in terms of improved granulation tissue, reduced wound surface area and reduced bacterial load.
Dwell time refers to time period that the instillation solution remains in contact with wound bed while negative pressure is disconnected. According to previous consensus guideline [8], appropriate dwell time was 10–20 min. Longer dwell time reduces the efficacy of negative pressure therapy and risks leakage of instillation solution and surrounding skin maceration while shorter dwell time leads to reduced benefits of instillation. A prospective study that used continuous irrigation of 0.1% polyhexanide-betaine observed no added benefits of the irrigation solution [13]. We used 10 min as dwell time for NPWTi group which is supported by recent consensus guideline and observed favourable results [8].
As per the consensus guideline the volume of NS to be instilled per sitting is till the foam became visibly saturated, as indicated by darkening of the foam and we observed better wound healing with this practice [25].
The pressure to be applied for NPWTi is between −125mm/Hg to −150 mm/Hg according to the consensus guideline [8]. At negative pressure beyond this, formation of granulation tissue is retarded and bacterial flora proliferates which hinders wound healing [[26], [27], [28]]. We used −125 mm/Hg supported by recent consensus guideline and found positive results [8].
A recommended period of negative pressure application following instillation of the irrigating solution is 2–6 h [4] and 2–3 h as per consensus guidelines [8]. We applied negative pressure for 4 h with intermittent irrigation of the wound bed every 4 h for a total of four times daily, withheld instillation overnight and connected negative pressure in continuous manner while patient was asleep. This was practically feasible and avoided inconvenience to the patients, their care-givers, resident doctors as well as nursing staff as compliance in monitoring NPWTi system overnight with respect to identification of system leak cannot be ensured, especially in setting like ours with limited availability of nursing-staffs, more so in the night shifts. A practical problem faced due to application of continuous negative pressure was that patient had to be bedridden for most part of the day. So we made arrangements for bedside physiotherapy and educated patients about the same.
In our study, there was significant percentage reduction in size of the wound in group1 (p = 0.03). NPWT with NS instillation enhances wound healing owing to cyclic cleansing, dilution of wound debris, disruption of biofilm, accelerated granulation tissue leading to improved wound healing and thus cause wound contracture [5,7,8]. No human study assessing the effect of NPWTi or comparing NPWT vs NPWTi in terms of reduction of size of the wound has been published so far. In a previous study, mean reduction of wound size following conventional NPWT was 28.4% compared to 9.5% increase in control group undergoing saline-moistened gauze dressing [29]. Measurement of reduction in wound surface area may not be the ideal way of evaluation of wound healing due to differences in the distribution and the size of wounds among the patients. In our study, however, there was homogeneity in the size and the distribution of wounds in all study participants, i.e., patients with wounds of comparable size in the lower extremities were taken in the study.
In our study, we observed statistically significant decrease in bacterial bioburden and CFU counts in NPWTi group compared to NPWT at the end of day10 of therapy (p = 0.003). In a study by Gabriel et al. there was significant reduction in the bacterial bioburden (p=<0.001) and the time taken to control wound infection (6.0 ± 1.5 days) with NPWTi [30]. Studies using NPWTi have shown that instillation reduces bioburden by removing nonviable tissue and thick exudate harbouring bacteria [15]. The patients in our study had polymicrobial infections and antibiotics were changed according to culture-sensitivity pattern in both the groups. Thus, the reduction in bacterial load following NPWTi compared to NPWT was taken to be significant and not merely due to antibiotic therapy. The biofilms are difficult to eradicate owing to the extracellular substances they secrete and to combat this, requires multimodal strategies such as debridement of the wound, antimicrobials and continuous disruption. NPWTi effectively lowers the bacterial bioburden and disrupts these biofilms [7].
Piaggesi et al. assessed various histopathological parameters such as keratosis, fibrosis, inflammatory cells, granulation tissue, neo-capillary formation to study the effect of total contact-cast in neuropathic foot ulcers [31]. These parameters have not been studied in relation to NPWT or NPWTi and we extrapolated them to compare the effects of NPWTi vs conventional NPWT on wound healing.
In our study, the wounds treated with NPWTi showed a significant improvement in granulation tissue and significant reduction in inflammation in NPWTi group compared to NPWT (p = 0.004 and 0.005 respectively). The effect of NPWT to stimulate granulation tissue is well established [21,32]. Lessing et al. in a porcine wound model showed significant improvement in granulation tissue and reduction in inflammation with NPWTi suggesting this improvement was not merely due to edema [33]. But this result needed confirmation in human studies.
Similar findings of improved granulation tissue with NPWTi was observed macroscopically, over areas of wounds directly exposed to the foam compared to undermined areas lacking foam cover which reduced the access to instillation solution [19]. This study lacked histological examination of tissue bits to assess granulation tissue.
Other significant results of our study were improvement in surface epithelium (p = 0.005) and increase in proliferating blood vessels and fibroblastic reaction (p = 0.001) in NPWTi group. There was however no significant improvement in adnexal structures with the use of NPWTi. To our knowledge, ours is the only human study assessing benefits of NPWTi compared to NPWT in terms of these histological parameters.
We also studied other parameters of wound healing such as the percentage of participants with wounds fit for closure at the end of the intervention and the percentage of fit patients undergoing wound closure in form of split skin grafting (SSG).
In previous studies, significantly reduced morbidity, higher percentage of wound closure and decreased length of hospital stay was observed with NPWTi [34,35]. In our study, the number of patients undergoing SSG was significantly higher in group 1 compared to group 2. However, this observation has its limitation as number of patients fit for closure of wounds in both the groups was similar despite which many patients did not undergo SSG due to logistic reasons.
5. Perceived barriers and limitations to this study
In NPWTi group skin maceration was observed in two patients, on day 4 and on day 5 of NPWTi. This was attributed to improper contact between the skin and the adhesive area of transparent sheet used to create vacuum in a bigger sized wound. Instillation was stopped for a day and regular dressing was applied during which time maceration subsided. NPWTi therapy was re-initiated the next day using adhesive sheet along with tincture benzoin over the skin around the margin of the ulcer to provide proper seal and prevent seepage of NS during instillation.
JIPMER is a governmental, tertiary care centre that serves a large number of population, especially form below poverty lines background, and provides quality service free of cost. Use of commercial devices that delivers negative suction and instillation solution at pre-set time and volume or provides irrigation in a continuous manner is not practically feasible due to financial constraints. Similarly, due to requirement of beds for high patient load, all patients could not be kept admitted till complete healing of wound or till wound closure. Hence, ten-days period of intervention was set based on previous experience on wound management and patients with wound fit for closure had to be discharged after completion of intervention due to long waiting list and/or due to pending anaesthesia fitness. Hence parameters such as proportions of healed wounds or time to wound healing in each group could not be studied.
Similarly, uncontrolled diabetes and peripheral vascular disease (PVD), though major causes of leg ulcer, were excluded from this study. Randomized controlled studies comparing NPWT with NPWTi using normal saline with a larger sample size and subgroup stratification based on presence or absence of diabetes mellitus or PVD or studies with inclusion criteria as only diabetes mellitus or PVD may be undertaken in future.
6. Conclusion
In this study addition of saline instillation to NPWT has shown significant improvement in the clinical results compared to conventional NPWT. The use of NS instillation led to reduced bacterial bioburden, improved granulation tissue, angiogenesis and fibroblast proliferation. This resulted in an effective and faster contraction of the wound and improved wound healing. This was reflected as reduction in wound surface area. We conclude that NPWTi using normal saline can be used as a cost-effective adjunct following debridement in management of extremity wounds especially in low and lower middle income countries where hospitals cater to large number of patients and patients belonging to lower income classes.
Research Registration Unique Identifying Number (UIN)
Name of the registry: Clinical Trials Registry of India
Unique Identifying number or registration ID: CTRI/2020/05/025410
Hyperlink to your specific registration (must be publicly accessible and will be checked): http://ctri.nic.in/Clinicaltrials/advsearch.php
Author contribution
Author 1 (Prakriti Giri) was the primary investigator, responsible for preparing
protocol, collecting data, analysing data„ and preparing manuscript.
Author 2 (Balamourougan Krishnaraj) and Author 8 (Sarath Chandra Sistla) designed, closely monitored the study, attended IEC meetings, provided guidance and suggestions throughout, and checked the manuscript.
Author 3 (Jigish Ruparelia) assisted in carrying out the study by helping with dressing (NPWT and instillation)
Author 6 (Gomathi Shankar) assisted in getting relevant articles, checking the protocol, attending IEC meetings and smooth conduct of the study
Author 4 (Sujatha Sistla) assisted in analysis of CFU/quantitative culutres of the bacteria as well as the qualitative cultures
Author S (Debdatta Basu) assisted in analysis of various hisotopathological parameters of wound healing.
Author 7 (Sujiv Akkilagunta) assisted in final data analysis of the results/outcomes.
Provenance and peer review
Not commissioned, externally peer reviewed.
Not commissioned or externally peer reviewed.
Funding
None.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgement
None.
Contributor Information
Prakriti Giri, Email: prakriti.com@gmail.com.
Balamourougan Krishnaraj, Email: drbalak@gmail.com.
Sarath Chandra Sistla, Email: sarathsistla@hotmail.com.
Sujatha Sistla, Email: sujathasistla@gmail.com.
Debdatta Basu, Email: ddbasu@gmail.com.
Gomathi Shankar, Email: drgomathishankar@gmail.com.
Sujiv Akkilagunta, Email: sujiv.oh231@gmail.com.
Jigish Ruparelia, Email: jigish.ruparelia@gmail.com.
References
- 1.Kavros S.J., Liedl D.A., Boon A.J., Miller J.L., Hobbs J.A., Andrews K.L. Expedited wound healing with noncontact, low-frequency ultrasound therapy in chronic wounds: a retrospective analysis. Adv. Skin Wound Care. 2008 Sep;21(9):416–423. doi: 10.1097/01.ASW.0000323546.04734.31. [DOI] [PubMed] [Google Scholar]
- 2.Lin C.-C., Chang C.-F., Lai M.-Y., Chen T.-W., Lee P.-C., Yang W.-C. Far-infrared therapy: a novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. J Am Soc Nephrol JASN. 2007 Mar;18(3):985–992. doi: 10.1681/ASN.2006050534. [DOI] [PubMed] [Google Scholar]
- 3.A N., Khan W.S., J P. The evidence-based principles of negative pressure wound therapy in trauma & orthopedics. Open Orthop. J. 2014;8:168–177. doi: 10.2174/1874325001408010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim P.J., Attinger C.E., Crist B.D., Gabriel A., Galiano R.D., Gupta S. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds Compend Clin Res Pract. 2015;27(12):S2–S19. [PubMed] [Google Scholar]
- 5.Müller C.S.L., Burgard B., Zimmerman M., Vogt T., Pföhler C. On the significance of negative-pressure wound therapy with instillation in dermatology. J Dtsch Dermatol Ges J Ger Soc Dermatol JDDG. 2016 Aug;14(8):786–795. doi: 10.1111/ddg.13038. [DOI] [PubMed] [Google Scholar]
- 6.Singh D., Chopra K., Sabino J., Brown E. Practical things you should know about wound healing and vacuum-assisted closure management. Plast. Reconstr. Surg. 2020 Apr;145(4) doi: 10.1097/PRS.0000000000006652. 839e–54e. [DOI] [PubMed] [Google Scholar]
- 7.Bradley B.H., Cunningham M. Biofilms in chronic wounds and the potential role of negative pressure wound therapy: an integrative review. J Wound Ostomy Cont Nurs Off Publ Wound Ostomy Cont Nurses Soc. 2013 Apr;40(2):143–149. doi: 10.1097/WON.0b013e31827e8481. [DOI] [PubMed] [Google Scholar]
- 8.Kim P.J., Attinger C.E., Constantine T., Crist B.D., Faust E., Hirche C.R. Negative pressure wound therapy with instillation: international consensus guidelines update. Int. Wound J. 2020 Feb;17(1):174–186. doi: 10.1111/iwj.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surgical management of necrotizing soft tissue infections - UpToDate [Internet]. [cited 2020 Apr 30]. Available from: https://www.uptodate.com/contents/surgical-management-of-necrotizing-soft-tissue-infections.
- 10.Fernandez L.G., Sibaja Alvarez P., Kaplan M.J., Sanchez-Betancourt A.A., Matthews M.R., Cook A. Application of negative pressure wound therapy with instillation and dwell time of the open abdomen: initial experience. Cureus. 2019 Sep 16;11(9) doi: 10.7759/cureus.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkert D., Ali M., Naud M., Maire N., Trial C., Téot L. Negative pressure wound therapy with saline instillation: 131 patient case series. Int. Wound J. 2013 Dec;10(Suppl 1):56–60. doi: 10.1111/iwj.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milcheski D.A., Portocarrero M.L., Alvarez D.M., Mazuca LG. de MP., Monteiro A.A., Gemperli R. Initial experience with negative-pressure wound therapy with instillation in complex wounds. Rev. Col. Bras. Cir. 2017 Aug;44(4):348–353. doi: 10.1590/0100-69912017004008. [DOI] [PubMed] [Google Scholar]
- 13.Lavery L.A., Davis K.E., La Fontaine J., Farrar J.D., Bhavan K., Oz O.K. Does negative pressure wound therapy with irrigation improve clinical outcomes? A randomized clinical trial in patients with diabetic foot infections. Am. J. Surg. 2020 Feb;220(4):1076–1082. doi: 10.1016/j.amjsurg.2020.02.044. S0002961020301227. [DOI] [PubMed] [Google Scholar]
- 14.Etoz A., Ozgenel Y., Ozcan M. The use of negative pressure wound therapy on diabetic foot ulcers: a preliminary controlled trial. Wounds. 2004 Aug;16(8):264–269. [Google Scholar]
- 15.Obst M.A., Harrigan J., Wodash A., Bjurstrom S. Early-stage management of complex wounds using negative pressure wound therapy with instillation and a dressing with through holes. Wounds Compend Clin Res Pract. 2019 May;31(5):E33–E36. [PubMed] [Google Scholar]
- 16.Paddle-Ledinek J.E., Nasa Z., Cleland H.J. Effect of different wound dressings on cell viability and proliferation. Plast. Reconstr. Surg. 2006 Jun;117(SUPPLEMENT):110S–118S. doi: 10.1097/01.prs.0000225439.39352.ce. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch T., Seipp H.-M., Jacobsen F., Goertz O., Steinau H.-U., Steinstraesser L. Antiseptics in surgery. Eplasty. 2010 May 27;10:e39. [PMC free article] [PubMed] [Google Scholar]
- 18.Langer S., Sedigh Salakdeh M., Goertz O., Steinau H.U., Steinstraesser L., Homann H.H. The impact of topical antiseptics on skin microcirculation. Eur. J. Med. Res. 2004 Sep 29;9(9):449–454. [PubMed] [Google Scholar]
- 19.Téot L., Boissiere F., Fluieraru S. Novel foam dressing using negative pressure wound therapy with instillation to remove thick exudate. Int. Wound J. 2017 Oct;14(5):842–848. doi: 10.1111/iwj.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupert P., Ochoa R.A., Punch L., Van Epps J., Gordon-Burroughs S., Martinez S. The use of NPWT-i technology in complex surgical wounds. Cureus. 2016 Dec 8;8(12) doi: 10.7759/cureus.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall K.D., Patterson J.S. Three cases describing outcomes of negative-pressure wound therapy with instillation for complex wound healing. J Wound Ostomy Cont Nurs Off Publ Wound Ostomy Cont Nurses Soc. 2019 Jun;46(3):251–255. doi: 10.1097/WON.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 22.Kim P.J., Attinger C.E., Oliver N., Garwood C., Evans K.K., Steinberg J.S. Comparison of outcomes for normal saline and an antiseptic solution for negative-pressure wound therapy with instillation. Plast. Reconstr. Surg. 2015 Nov;136(5) doi: 10.1097/PRS.0000000000001709. 657e-64e. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel A., Kahn K., Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 24.Fluieraru S., Bekara F., Naud M., Herlin C., Faure C., Trial C. Sterile-water negative pressure instillation therapy for complex wounds and NPWT failures. J. Wound Care. 2013 Jun;22(6):298–299. doi: 10.12968/jowc.2013.22.6.293. 293–4, 296. [DOI] [PubMed] [Google Scholar]
- 25.Kim P.J., Attinger C.E., Steinberg J.S., Evans K.K., Lehner B., Willy C. Negative pressure wound therapy with instillation: consensus guidelines. Plast. Reconstr. Surg. 2013 Sep:1. doi: 10.1097/PRS.0b013e3182a80586. [DOI] [PubMed] [Google Scholar]
- 26.Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann. Plast. Surg. 1997 Jun;38(6):563–576. discussion 577. [PubMed] [Google Scholar]
- 27.Ngo Q.D., Vickery K., Deva A.K. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2012 Feb;20(1):83–90. doi: 10.1111/j.1524-475X.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 28.Morykwas M.J., Faler B.J., Pearce D.J., Argenta L.C. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann. Plast. Surg. 2001 Nov;47(5):547–551. doi: 10.1097/00000637-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 29.McCallon S.K., Knight C.A., Valiulus J.P., Cunningham M.W., McCulloch J.M., Farinas L.P. Vacuum-assisted closure versus saline-moistened gauze in the healing of postoperative diabetic foot wounds. Ostomy/Wound Manag. 2000 Aug;46(8):28–32. 34. [PubMed] [Google Scholar]
- 30.Gabriel A., Shores J., Heinrich C., Baqai W., Kalina S., Sogioka N. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int. Wound J. 2008 Jun;5(3):399–413. doi: 10.1111/j.1742-481X.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piaggesi A., Viacava P., Rizzo L., Naccarato G., Baccetti F., Romanelli M. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: effects of pressure relief. Diabetes Care. 2003 Nov;26(11):3123–3128. doi: 10.2337/diacare.26.11.3123. [DOI] [PubMed] [Google Scholar]
- 32.Saxena V., Hwang C.-W., Huang S., Eichbaum Q., Ingber D., Orgill D.P. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast. Reconstr. Surg. 2004 Oct;114(5):1086–1096. doi: 10.1097/01.prs.0000135330.51408.97. discussion 1097-1098. [DOI] [PubMed] [Google Scholar]
- 33.Lessing C., Slack P., Hong K.Z., Kilpadi D., McNulty A. Negative pressure wound therapy with controlled saline instillation (NPWTi): dressing properties and granulation response in vivo. Wounds Compend Clin Res Pract. 2011 Oct;23(10):309–319. [PubMed] [Google Scholar]
- 34.Kim P.J., Attinger C.E., Steinberg J.S., Evans K.K., Powers K.A., Hung R.W. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast. Reconstr. Surg. 2014 Mar;133(3):709–716. doi: 10.1097/01.prs.0000438060.46290.7a. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez P.S., Betancourt A.S., Fernández L.G. Negative pressure wound therapy with instillation in the septic open abdomen utilizing a modified negative pressure therapy system. Ann Med Surg. 2012;36:246–251. doi: 10.1016/j.amsu.2018.10.007. 2018 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]