Highlights
-
•
ZG16 is negatively correlated to PD-L1 expression in patient with CRC.
-
•
ZG16 blocks PD-L1 expression to modulate immune response in colon cancer.
-
•
Overexpression of ZG16 promotes NK cells survival and proliferation.
-
•
ZG16 overexpression inhibits in vivo SW480 xenograft tumor growth.
Keywords: Zg16, Pd-l1, Immune system, Colon carcinoma, Nk cell
Abbreviations: zg16, Zymogen granule protein 16; crc, Colorectal cance; ihc, immunohistochemistry; MSI, microsatellite instability
Abstract
CRC is a heterogeneous disease due to global molecular alterations, including mismatch-repair-deficient (dMMR) and microsatellite instability-high (MSI-H). Immunotherapy has achieved durable responses in a subset of patients with dMMR-MSI-H metastatic CRC. It has been showed that Loss of ZG16 is highly associated with colorectal cancer. However, whether ZG16 modulates tumor immunity in colorectal cancer is unclear. In this study, we demonstrated that the expression of ZG16 is associated with distant metastasis and lymphatic invasive in colorectal cancer. Besides, ZG16 is negatively correlated to PD-L1 expression in patient with CRC and overexpression of ZG16 blocks PD-L1 expression in colorectal cancer cells. In addition, overexpression of ZG16 promotes NK cells survival and proliferation, which is dependent on NKG2D expression. Our data demonstrate that ZG16 plays a role in modulation of immune response in colorectal cancer. The strong correlation between ZG16 and PD-L1 suggests that ZG16 may serve a biomarker to stratify patient who will benefit from immunotherapy.
Introduction
Colorectal cancer (CRC) is by far the second leading cause of cancer-related deaths. CRC is a heterogeneous disease due to global molecular alterations, such as mismatch-repair-deficient (dMMR) and microsatellite instability-high (MSI-H) [1]. Immunotherapy has been approved for the treatment of a subset of CRCs. Two programmed cell death 1 (PD1) inhibitors, pembrolizumab and nivolumab, have shown great efficacy in CRC patients harboring dMMR and MSI-H alterations. Recent study demonstrated that multiple immune checkpoints, including PD-1, PD-L1, CTLA-4, LAG-3 and IDO, are highly expressed in MSI-H tumors [2], which explains why immunotherapy achieves durable responses in a subset of patients with dMMR-MSI-H metastatic CRC [3]. While these data support that dMMR or MSI-H are associated with tumor sensitivity to immunotherapy in some cases, they also highlight the need to define other biomarkers that can stratify patients who would benefit from immunotherapy.
Human zymogen granule protein 16 (ZG16) has a Jacalin-like lectin domain, which is mainly expressed by mucus-secreting cells, including goblet cells in the intestine [4]. We previous show that ZG16 expression was significantly decreased in colorectal cancer compared to normal tissue [5]. ZG16 gene expression and copy number variations (CNV) were associated with multiple molecular and clinicopathological features of CRC including MSI, MLH1 silencing and so on [2]. In addition, it has been demonstrated that the expression of ZG16 is regulated by miR-196a and loss of ZG16 induces stemness and progression of CRC, suggesting that ZG16 functions as a tumor suppressor [6]. Besides, overexpression of ZG16p significantly supressed proliferation of Caco-2 cells [7].
Lectins play important roles in immune regulation [8]. Jacalin, a lectin from jackfruit, has been reported to induce T cell mitosis and activate B cells [9]. Considering that ZG16 expression was dramatically decreased in tumor area compared to adjacent normal tissue and ZG16 contains a lection domain, we hypothesized that ZG16 may function as a new immune response regulator in colorectal cancer and a potential biomarker for immunotherapy.
In this study, we investigated whether ZG16 plays a role in modulation of immune response and how ZG16 modulates tumor immunity in colorectal cancer. We performed immunohistochemistry (IHC) analysis and qRT-PCR in a cohort of CRC patients, and found that ZG16 is negatively correlated with lymphatic invasive and distant metastasis. Besides, overexpression of ZG16 blocks PD-L1 expression in colorectal cancer, and promotes NK cells survival and proliferation. Very importantly, ZG16 suppresses colorectal tumor growth via the immune system. Our work suggests that ZG16 serves as a new immune response regulator and a potential biomarker for immunotherapy.
Material and methods
Cell culture
CRC cell lines (SW480 and HCT116) were obtained from American Type Culture Collection (ATCC, Manassas, VA). All cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were maintained in a humidified incubator adjusted with 5% CO2 at 37 °C. All cells were authenticated by DNA fingerprinting using highly-polymorphic short tandem repeat (STR) analysis and confirmed free from mycoplasma contamination.
Vectors and lentivirus production
Lentiviral vector for ZG16 overexpression or control vector were constructed by Gibson assembly (NEB). The lentivirus was produced as described [10]. Briefly, H293T cells were plated in 10 cm dish at 30% confluence in DMEM plus 10% FBS. For each dish, 9 µg of lentiviral vector, 0.9 µg of pVSVg (Addgene, Cat. #8584), 9 µg of psPAX2 (Addgene, Cat. #12,260) were mixed and transfected to the H293T cells. Lentivirus was collected 48 h later by filtering through a 0.45 µm strainer.
Both SW480 and HCT116 cells were transfected with 1 × 106 IFU/ml lentivirus 24 h. Transduced cells were selected using 2 µg/ml puromycin (Sigma-Aldrich) for 7 days, and the expression efficiency was detected by western blotting.
Western blot analysis
equal amounts of protein lysates (5 μg/lane) were run on 4%−20% gradient SDS-polyacrylamide gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and then were transferred to Immobilon-P nitrocellulose membranes (Millipore, Billerica, MA, USA). The membranes were probed with rabbit anti-PD-L1 antibody (Proteintech; 17,952–1-AP; polyclonal; 1:1000; CD274: B7-H1 PD-L1; Rosemont; USA) and mouse anti-β-actin (Sigma-Aldrich; 100,242- MM05; 1: 5000) in 4 °C overnight after being blocked with 5% non-fat milk. The membranes were then incubated with species-specific conjugated secondary antibodies (GE Dharmacon) at room temperature for 1 hour. An ECL blotting analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, USA) was used for detecting protein expression.
Cell viability assay
SW480 or SW480 cells with ZG16 overexpression (SW480-ZG16) were seeded at 5 × 104 cells/well in 6 well plate for 1–5 days. Cell viability was determined by CCK8 reagent according to the manufacturer (Dojindo Molecular Technologies, Inc., Shanghai, China). Effect on cell growth was normalized to untreated control. Each data point was generated in triplicate and each experiment was done three times (n = 3). Unless otherwise stated, a representative result is presented.
Large scale expansion of NK cells from peripheral blood
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donor by density gradient centrifugation using Ficoll-Paque media (GE Healthcare Life Sciences, Uppsala, Sweden) [11]. PBMCs were cultured using BINKIT (Biotherapy Institute of Japan, Japan). For NK cell expansion, the medium contained 0.01 KE/ml OK-432 (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) and 700 IU/ml recombinant human interleukin-2 (rhIL-2; Kexin biotechnology Corp, Beijing, China) supplemented with 5% heat-inactivated autologous plasma. The culture medium was changed every three days and NK cells were sub-cultured whenever it's confluent.
NK cell co-culture and proliferation
SW480 cells were grown at 37° in 5% CO2 humidified air in RPMI-1640 medium that contained 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. NK cells were grown in the same conditions in RPMI-1640 medium that contained 20% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 10 IU/ml IL-2. In co-cultures the cells were grown in a mixed medium (1: 1) of SW480 and NK cells. SW480-ZG16, or SW480 cells were first seeded at 5 × 103 cells per plate, and then cultured several days. Then, the NK cells (5 × 103 cells/well) were added to the plate.
For co-culture with medium from SW480 cells, NK cells were plated for 24 h. Then cell culture medium collected from SW480 or SW480-ZG16 were added to the plate. Cell viability was evaluated by CCK8 assay for different time points.
T cell co-culture and proliferation
Fresh blood was collected from 4 health donors and CD8+T cells were isolated. We therefore investigated whether there is a correlation between ZG16 overexpression and CD8+ T cell survival and proliferation. We then performed co-culture assay similar as in NK cells.
Detection of proliferation using flow cytometry
CellTrace stock solution was prepared immediately prior to use by adding the appropriate volume of DMSO to CellTrace violet reagent and mixing. 1 μl of CellTrace stock solution (5 μM) in DMSO was added to 1 ml of cell suspension in PBS for a final working solution. The cells were incubated for 20 min at 37 °C and protected from light. Five folds (5x) the original staining volume of culture medium (containing at least 1% protein) was then added to the cells and incubated for 5 min to remove any free dye remaining in the solution. The cells were pelleted by centrifugation and resuspended in freshly pre-warmed complete culture medium. The cells were incubated for at least 10 min to allow the CellTrace reagent to undergo acetate hydrolysis. The proliferation cells were determined using BD FACScan flow cytometry. For each sample of NK cells, the fluorescence of 100,000 cells was gated and counted.
ELISA to quantify NKG2D type ii integral membrane protein
NK cells were washed twice in and diluted to 100 million cells/ml 1x PBS (pH7.2–7.4). After two freeze-thaw cycles to break up the cell membranes, the cell lysates were centrifuged for 5 min and the supernatant was collected. Quantification of NKG2D protein by ELISA followed the protocol provided by manufacturer (Cusabio, Huamei biotechnology Co., Ltd., Wuhan, China). Optical density of each well was determined using a microplate reader at 450 nm.
Patients
56 surgically resected adenocarcinomas of colon between 2012 and 2018 were retrospectively reviewed at Department of Pathology, First Affiliated Hospital of Zhengzhou University. All cases were free of chemotherapy. The uses of human tissues and patients’ clinical data were approved by Institute Review Board of Zhengzhou University. All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Real-Time quantitative PCR
Total RNA was extracted from Paraffined tissues using the RNeasy FFPE Kit (QIAGEN) and cDNA was synthesized using the SuperScript VILO Master Mix (ThermoFisher Scientific) following the manufacturer's instructions. We used 5 μl cDNA in the PCR with ZG16 or GAPDH primers mixed with Power SYBR Green PCR mix at a final volume of 20 μl in triplicate (Applied Biosystems). qPCR analysis was done using the ABI StepOne plus Software Real-Time PCR system. Gene expression of target genes was normalized against ACTB and compared among different groups by the ΔΔCT method.
Immunohistochemistry (IHC) staining and interpretation
For immunohistochemistry, paraffined tissues were sectioned at a thickness of 4 µm and subsequently stained with hematoxylin and eosin (H&E) and PD-L1 antibody (rabbit anti-human monoclonal, SP142 [12], [13], Spring Bio-science, Pleasanton, CA, USA) or NKG2D (rabbit anti-human monoclonal, bs-0938R, Bioss, Swiss 070,215). Stained slides were reviewed by two pathologists blinded to the clinicopathological features. The final IHC score was taken as the average score from all cores of each tumor. PD-L1 positive tumor-infiltrating immune cells were evaluated. The IHC was defined positive if more than 1% of immune cells showed membrane staining.
In vivo mouse study
All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee (First Affiliated Hospital of Zhengzhou university). Suspensions of 3 million SW480-ZG16 or SW480 cells (in PBS) were mixed 1:1 with BD Matrigel Basement Membrane Matrix (Cat. #356,231; Corning, NY, USA) were subcutaneously inoculated in right flank (n = 7/ group, Stock No: 002,019/nude). Animals were assessed for 4 weeks after the inoculation for tumor incidence and growth and then were sacrificed. Tumor volume was measured using the formula: Tumor volume = 1/2(length × width) [2].
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7.01 (GraphPad Software Inc.) unless otherwise indicated. Group allocation was performed randomly. In all studies, data represent biological replicates (n) and are depicted as mean ± s.d. or mean ± SEM as indicated in the figure legends. Comparison of mean values was conducted with unpaired, two-tailed Student's t-test, one-way ANOVA or two-way ANOVA with Tukey's multiple comparisons test as indicated in the figure legends. In all analyses, P values less than 0.05 were considered statistically significant.
Results
ZG16 is negatively correlated to PD-L1 expression in patient with CRC
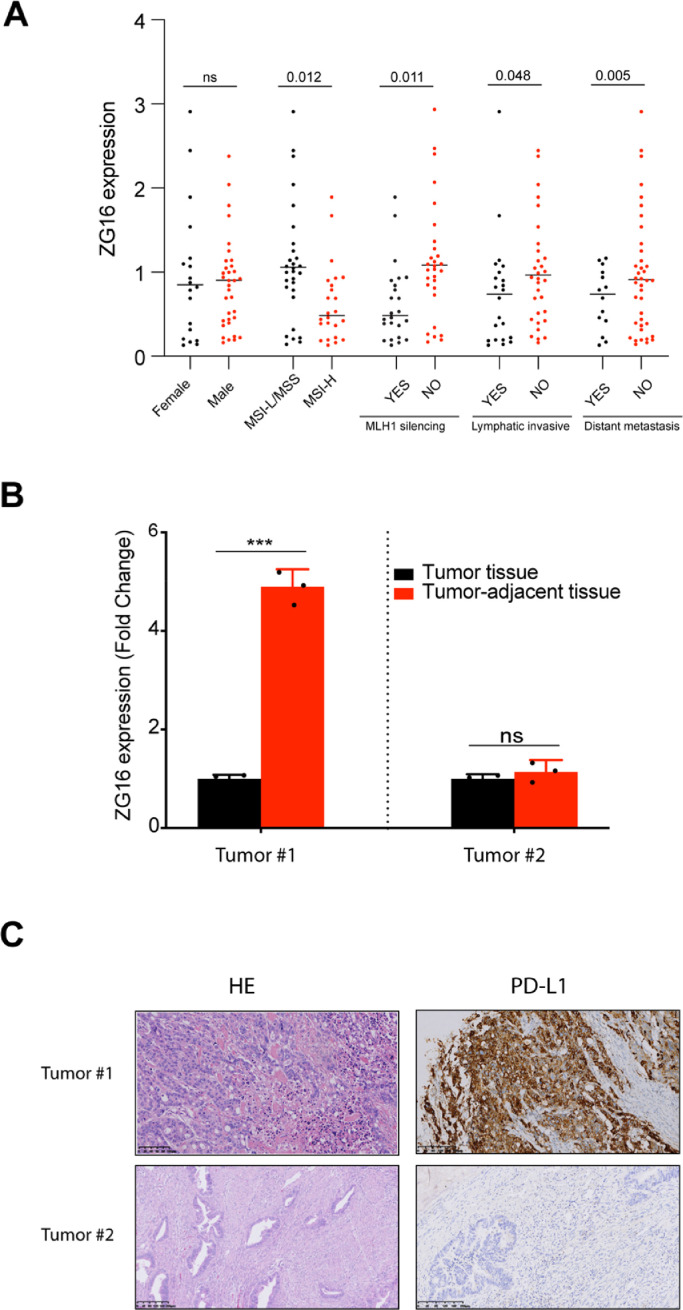
It has been showed that ZG16 expression is significantly decreased in CRC patients[5], and the expression of ZG16 is significantly correlated with overall survival and progression free survival based on The Cancer Genome Atlas (TCGA) COAD dataset (Figure S1). To further confirm the relationship between ZG16 and clinicopathological features, we quantified the expression of ZG16 in 56 colon cancer patients, including 24 patients with MSI-H CRC, 1 patient with MSI-L CRC and 31 patients with MSS CRC (Table 1). All 56 cases included in this study did not receive chemotherapy. 19 patients at stage Ⅲ, 3 patients at stage IV did not receive chemotherapy because those patients have advanced or metastatic disease at the time of diagnose. In consistent with previous finding, the expression of ZG16 is significantly decreased in patients with MSI-H CRC and MLH1 silencing CRC (Fig. 1A). Besides, we found that the expression of ZG16 is negatively correlated to distant metastasis and lymphatic invasive in colorectal cancer (Fig. 1A). Therefore, these data suggest that ZG16 represents a novel potential biomarker for prognosis of CRC.
Table 1.
Clinical-pathological features of all patients with colorectal cancer included in this study.
| Clinical-pathological features | Case number | Percent)%( |
|---|---|---|
| Gender | ||
| Male | 36 | 35.71 |
| Female | 20 | 64.29 |
| Age | ||
| <60 | 38 | 67.86 |
| ≥60 | 18 | 32.14 |
| Histotype | ||
| adenocarcinoma | 50 | 89.29 |
| mucous adenocarcinoma | 6 | 10.71 |
| Lymphatic invasive | ||
| yes | 22 | 39.29 |
| no | 34 | 60.71 |
| Distant metastasis | ||
| yes | 15 | 12.5 |
| no | 41 | 87.5 |
| stages | ||
| Ⅰ+Ⅱ | 34 | 68.82 |
| Ⅲ+Ⅳ | 22 | 31.18 |
| Microsatellite instability status | ||
| MSI-High | 24 | 42.86 |
| MSS+MSI-Low | 32 | 57.14 |
Fig. 1.
ZG16 is negatively correlated to PD-L1 expression in patient with CRC.
A, Association of different clinicopathological features with ZG16 expression in 56 CRC patients. The p-value was calculated by unpaired, two-tailed Student's t-test.
B, qPCR-based transcriptional profiles of ZG16 in different CRC tumors. Data are mean ± s.d. of three biological replicates (n = 3). ***p < 0.001 by unpaired, two-tailed Student's t-test. ns, not significant.
C, IHC analysis of PD-L1 expression in tumors with CRC.
In attempt to determine whether ZG16 plays a role in modulation of immune response, we did IHC staining to quantify Programmed death-ligand 1 (PD-L1) expression in CRC tumors. We noticed that the basic expression of ZG16 was largely different in different patients, such as tumor #1 and tumor #2. Very surprisingly, low expression of ZG16 in the tumor area compared to the adjacent tissue in the same patient (tumor #1, define low ZG16 expression in the tumor) lead to the increased expression of PD-L1, which allows CRC cancers to evade the host immune system. In sharp contrast, if the expression of ZG16 in tumor area was similar compared to adjacent tissue in the same patient (tumor #2, define high ZG16 expression in the tumor), the expression of PD-L1 was not differentially expressed between tumor and adjacent normal tissue (Fig. 1B, 1C). The strong correlation between ZG16 and PD-L1 suggests that ZG16 is involved in modulation of immune response, and may serve a biomarker to stratify patient who will benefit from immunotherapy.
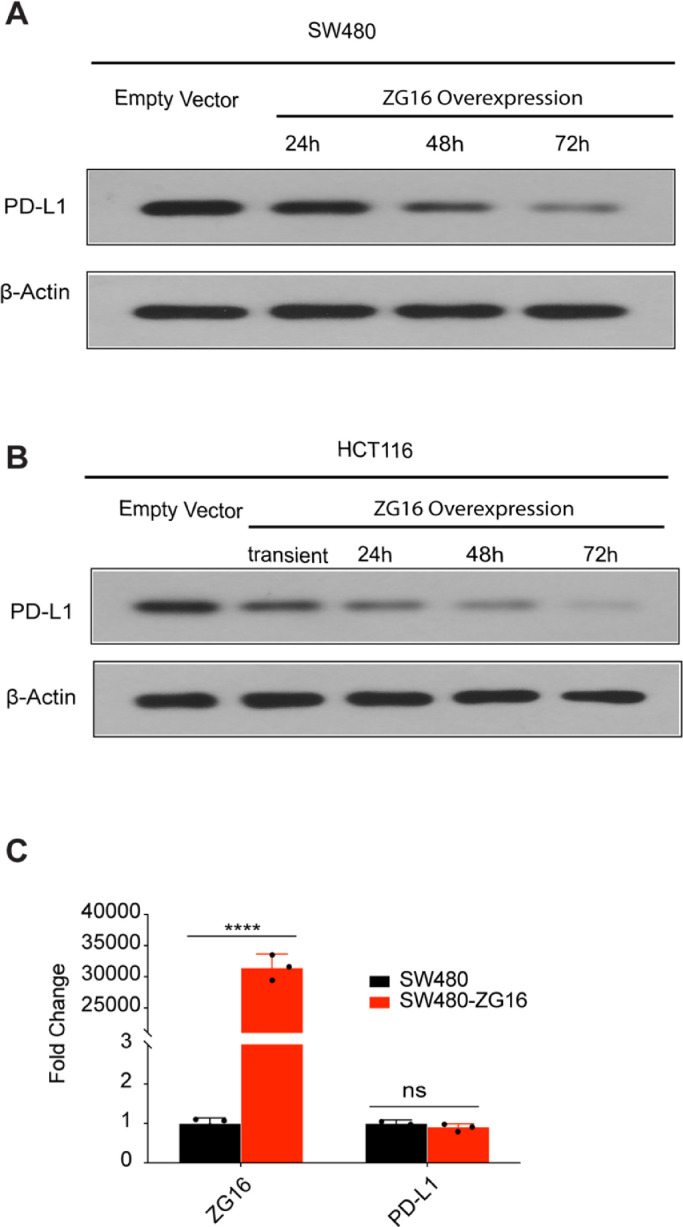
Overexpression of ZG16 blocks PD-L1 expression in colorectal cancer
To evaluate how PD-L1 is regulated by ZG16 in colon cancer, we tested the PD-L1 expression in two different colon cancer cell lines (SW480 and HCT116) after ZG16 overexpression. Very importantly, the expression of PD-L1 was markedly decreased in ZG16-overexpressed SW480 cells, indicating that PD-L1 is directly regulated by ZG16 in colon cancer (Fig. 2A). Consistently, similar results were observed in another colon cell line HCT116 after ZG16 overexpression (Fig. 2B). We then determined how PD-L1 expression is regulated by ZG16. Notably, the mRNA level of PD-L1 was not affected by ZG16 overexpression, suggesting that PD-L1 is not controlled by transcriptional regulation (Fig. 2C). Thus, ZG16 blocks PD-L1 expression to modulate immune response in colon cancer.
Fig. 2.
ZG16 blocks PD-L1 expression in colorectal cancer.
A, Western blot analysis of PD-L1 expression in SW480 or SW480-ZG16 cells.
B, Western blot analysis of PD-L1 expression in HCT116 or HCT116-ZG16 cells.
C, qPCR-based transcriptional profiles of ZG16 or PD-L1 in SW480 or SW480-ZG16 cells. Data are mean ± s.d. of three biological replicates (n = 3). ****p < 0.0001 by unpaired, two-tailed Student's t-test. ns, not significant.
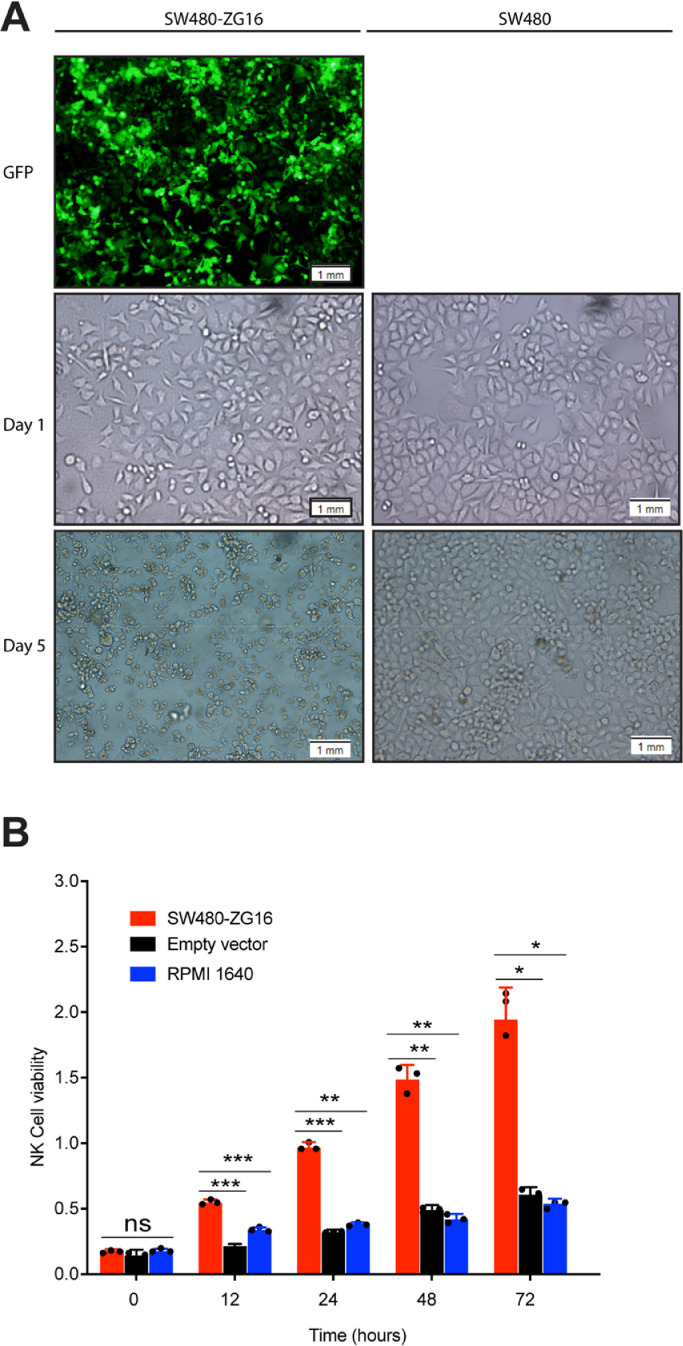
Overexpression of ZG16 promotes NK cells survival and proliferation
As a secret protein, ZG16 may also be involved in regulation of other immune cells. To determine whether overexpression of ZG16 in colon cancer cells affects the survival and proliferation of NK cells, we co-cultured NK cells with ZG16 over-expressed SW480 cells (SW480-ZG16) or control vector transfected SW480 cells for different timepoints. We noticed that there were much more NK cells (aggregation) in SW480-ZG16 group than control group (Fig. 3A). Similar results were also seen in other two colon cell lines (Figure S2). In addition, overexpression of ZG16 significantly increased the cell number of NK cells after co-culture for different timepoints, leading to enhanced proliferation of NK cells compared to control group (Fig. 3B). Together, these results suggest a role for ZG16 to regulate NK cell survival and proliferation.
Fig. 3.
Overexpression of ZG16 promotes NK cells survival and proliferation.
A, NK cells were co-cultured with SW480 or SW480-ZG16 cells for 1day or 5days.
B, NK cells were co-cultured with SW480 or SW480-ZG16 cells for different timepoints. Cell viability of NK cells was measured by CCK8 assay. Data are mean ± s.d. of three biological replicates (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by two-way ANOVA with Tukey's multiple comparisons test. ns, not significant.
To investigate whether overexpression of ZG16 affects the survival and proliferation of CD8+ T cells, we co-cultured primary CD8+ T cells with ZG16 over-expressed colon cells or control vector transfected colon cells for different timepoints. We observed that ZG16 overexpression dramatically promotes CD8+ T survival and proliferation (Figure S3).
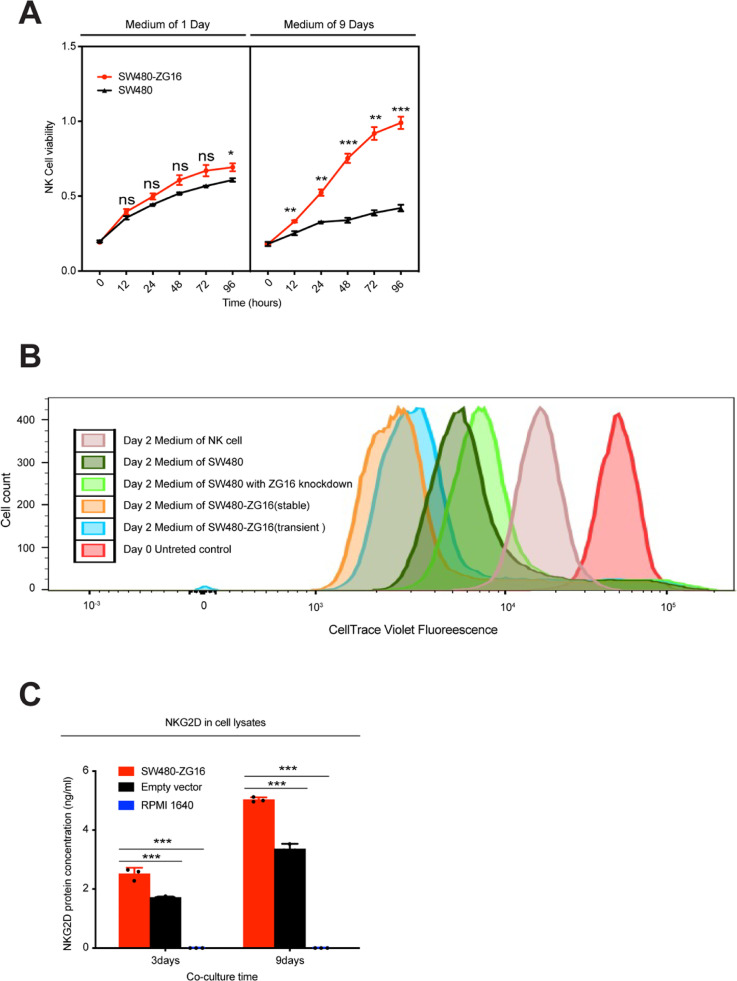
NK cell proliferation induced by ZG16 overexpression is dependent on NKG2D
Given that ZG16 is a secret protein, we speculate that co-culturing NK cells with medium from SW480-ZG16 may also enhance NK cell proliferation. We then collected medium from both SW480-ZG16 and SW480 cells, and co-cultured them with NK cell for different timepoints. In consistent with previous results, medium from SW480-ZG16 (9 days) considerably boosted the proliferation of NK cells compared to control medium from SW480 (Fig. 4A).
Fig. 4.
ZG16 stimulates NK cell proliferation through upregulation of NKG2D.
A, NK cells were co-cultured with medium from SW480 or SW480-ZG16 cells for different timepoints. Cell viability of NK cells was measured by CCK8 assay. Data are mean ± s.d. of three biological replicates (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 by two-way ANOVA with Tukey's multiple comparisons test. ns, not significant.
B, NK cells were co-cultured with medium from SW480 or SW480-ZG16 cells for 2days. Cell viability of NK cells was measured by CellTrace™ Cell Proliferation Kit.
C, Protein level of NKG2D was quantified by ELISA. NK cells were co-cultured with medium from SW480 or SW480-ZG16 cells for indicated time points. NK cells were then washed in PBS and lysed. Protein level of NKG2D was then quantified by ELISA. ***p < 0.001 by two-way ANOVA with Tukey's multiple comparisons test.
We also performed CellTrace assay to determine NK cell proliferation after co-culture with medium from five different conditions. A greater degree of signal reduction was observed in cells co-cultured with medium from SW480-ZG16, which further suggests that ZG16 promotes NK cell proliferation (Fig. 4B).
NKG2D represents the main activating receptor of NK cells, which can bind to a large number of cell ligands expressed on cancer cells and is increased in response to cellular stress [14], [15], [16], [17]. Tumor cells may escape NK cell recognition through reduction of these ligands or suppression of NKG2D on NK cells[18], [19]. Therefore, restoring the expression of NKG2D on NK cells or of its ligands on cancer cells could be an effective approach to cancer immunotherapy. To demonstrate that increased proliferation of NK cells was accompanied by up-regulation of NKG2D, we quantified NKG2D concentration by ELISA in co-culture medium and cell lysates. We found that protein concentration of NKG2D was greatly increased after co-culture with SW480-ZG16 both in culture medium and cell lysates, suggesting that NK cell proliferation is dependent on NKG2D (Fig. 4C).
In sum, overexpression of ZG16 promotes NK cell survival and proliferation, which is dependent on NKG2D expression.
ZG16 overexpression inhibits in vivo SW480 xenograft tumor growth
Finally, we investigated in vivo efficacy of ZG16 overexpression in CRC cancer xenografts. SW480-ZG16 or SW480 xenografts were monitored for 22 days. Importantly, tumor growth was significantly suppressed by ZG16 overexpression (Fig. 5A, 5B). IHC analysis of residual tumors revealed that the ZG16 overexpression resulted in significantly more pronounced NKG2D than control tumor (Fig. 5C), indicating that overexpression of ZG16 stimulated NK cell activation, which in turn contributes to the tumor inhibition.
Fig. 5.
ZG16 Overexpression Inhibits in vivo SW480 Xenograft Tumor Growth via activation of immune system.
A, Growth curve of SW480 and SW480-ZG16 xenografts. Data are shown as mean ± s.d. *p < 0.05 by two-way ANOVA with Tukey's multiple comparisons test.
B, Images of SW480 and SW480-ZG16 xenograft tumors
C, H&E and IHC analysis (NKG2D) of SW480 and SW480-ZG16 xenografts. Original overall magnification, × 400(G).
Collectively, our study reveals that ZG16 blocks PD-L1 expression in colorectal cancer and ZG16 promotes NK cells survival and proliferation. The relationship between ZG16 and PD-L1 suggests that ZG16 is a modulator of immune response, and may serve a biomarker to stratify patient who will benefit from immunotherapy.
Discussion
CRC is a heterogeneous disease due to global molecular alterations, including dMMR and MSI-H [1]. Early diagnosis has greatly improved patients’ survival, but around 25% of patients still have advanced or metastatic disease at the time of diagnosis. The prognosis for patients with metastatic CRC remains poor. Thus, the identification of more effective prognostic factor for patients with CRC is an urgent unmet need [1]. Human ZG16 has a Jacalin-like lectin domain which recognizes pathogenic fungi in the digestive system. We previous show that loss of ZG16 is associated with molecular phenotypes of colorectal cancer [5]. In this study, we demonstrated that the expression of ZG16 is negatively correlated to distant metastasis and lymphatic invasive in colorectal cancer of which are two major prognosis factors for metastatic CRC. Our data suggest that ZG16 represents a novel potential biomarker for prognosis of CRC, especially for metastatic CRC.
Immunotherapy has achieved durable responses in a subset of patients with dMMR-MSI-H metastatic CRC [3]. Tumor cells may increase PD-L1 expression on their surface to escape immune surveillance [20]. We discovered that ZG16 is negatively correlated to PD-L1 expression in patient with CRC and overexpression of ZG16 blocks PD-L1 expression in colorectal cancer cells. Besides, we did not see any correlation between ZG16 and PD-L2 (Figure S4). It's very surprising we did not observe any difference on the RNA level of PD-L1 after overexpression of ZG16. Recent reports demonstrate that the activity of PD-L1 is regulated by N-glycosylation, and targeting glycosylated PD-L1 (gPD-L1) by monoclonal antibody blocks PD-L1/PD-1 interaction resulting in PD-L1 degradation [21],[22]. ZG16 has a lectin domain and lectins can interact with and bind to carbohydrate structures [23]. Based on these findings, it is highly possible that ZG16 could direct bind to glycosylated PD-L1 through its lectin domain, leading to PD-L1 degradation. Further work is required to validate this hypothesis and determine potential mechanisms.
As a effector lymphocytes in the innate immune system, NK cell manipulation promotes antitumor immunotherapy and controls inflammatory and autoimmune disorders [24]. We demonstrated that overexpression of ZG16 promotes NK cell survival and proliferation, which is dependent on NKG2D expression. Further study is required to determine key regulators by which ZG16 contributes to NK cell proliferation.
It has been shown that ZG16 is mainly expressed by goblet cells in the intestine [25]. The epithelial goblet cells have been positioned to take a leading role in controlling the function of the intestinal immune system, but how goblet cells regulate the function of the intestinal immune system is unclear [25]. We demonstrated that ZG16 is negatively correlated to PD-L1 expression and promotes NK cells survival and proliferation. Together, these data suggest that goblet cells may regulate the function of intestinal immune system through suppression of PD-L1 expression and activation of NK cells.
In summary, our study is the first to demonstrate that ZG16 is negatively correlated to PD-L1 expression in patient with CRC and overexpression of ZG16 blocks PD-L1 expression in colorectal cancer cells. Besides, overexpression of ZG16 promotes NK cells survival and proliferation. These data suggest ZG16 plays a role in modulation of immune response in colorectal cancer. The strong correlation between ZG16 and PD-L1 suggests that ZG16 may serve a biomarker to stratify patient who will benefit from immunotherapy.
Author contributions statement
H. Meng designed and performed the experiments, analyzed data and wrote the manuscript. WC. Li performed the experiments and analyzed data. EJ. Liu and Y. Ding provided experimental materials.L. Wang analyzed the data and provided conceptual inputs.
Declaration of Competing Interest
None
Footnotes
Financial support: This study was supported by Advancing a Healthier Wisconsin fund (project#5,520,227) to LW. Henan Science and Technology Planning Project (142,300,410,204) to HM. Project of Henan Science and Technology 2018 (project#2,018,020,006) to HM.
Declaration of Competing Interest: The authors declare no potential conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.101003.
Appendix. Supplementary materials
References
- 1.Keum N., Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 2.Llosa N.J., Cruise M., Tam A. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesh K., Stadler Z.K., Cercek A. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tateno H., Yabe R., Sato T. Human ZG16p recognizes pathogenic fungi through non-self polyvalent mannose in the digestive system. Glycobiology. 2012;22:210–220. doi: 10.1093/glycob/cwr130. [DOI] [PubMed] [Google Scholar]
- 5.Meng H., Li W., Boardman L.A., Wang L. Loss of ZG16 is associated with molecular and clinicopathological phenotypes of colorectal cancer. BMC Cancer. 2018;18:433. doi: 10.1186/s12885-018-4337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Du P., She J., Cao L., Li Y., Xia H. Loss of ZG16 is regulated by miR-196a and contributes to stemness and progression of colorectal cancer. Oncotarget. 2016;7:86695–86703. doi: 10.18632/oncotarget.13435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mito A., Nakano Y., Saitoh T. Lectin ZG16p inhibits proliferation of human colorectal cancer cells via its carbohydrate-binding sites. Glycobiology. 2018;28:21–31. doi: 10.1093/glycob/cwx088. [DOI] [PubMed] [Google Scholar]
- 8.Ni Y., Tizard I. Lectin-carbohydrate interaction in the immune system. Vet Immunol Immunopathol. 1996;55:205–223. doi: 10.1016/s0165-2427(96)05718-2. [DOI] [PubMed] [Google Scholar]
- 9.Roque-Barreira M.C., Campos-Neto A. Jacalin: an IgA-binding lectin. J Immunol. 1985;134:1740–1743. [PubMed] [Google Scholar]
- 10.Shalem O., Sanjana N.E., Hartenian E. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salot S., Bercegeay S., Dreno B. Large scale expansion of Vgamma9Vdelta2 T lymphocytes from human peripheral blood mononuclear cells after a positive selection using MACS "TCR gamma/delta+ T cell isolation kit". J Immunol Methods. 2009;347:12–18. doi: 10.1016/j.jim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Cha Y.J., Kim H.R., Lee C.Y., Cho B.C., Shim H.S. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer. 2016;97:73–80. doi: 10.1016/j.lungcan.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K., Okita R., Saisho S., Maeda A., Nojima Y., Nakata M. Prognostic value of Cox-2 and PD-L1 expression and its relationship with tumor-infiltrating lymphocytes in resected lung adenocarcinoma. Cancer Manage Res. 2017;9:741–750. doi: 10.2147/CMAR.S146897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nausch N., Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 15.Zhu S., Denman C.J., Cobanoglu Z.S. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm. Res. 2015;32:779–792. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez L., Metais J.Y., Escudero A. Vol. 23. American Association for Cancer Research; 2017. pp. 5824–5835. (Memory T Cells Expressing an NKG2D-CAR Efficiently Target Osteosarcoma Cells). Clinical cancer research: an official journal of the. [DOI] [PubMed] [Google Scholar]
- 17.Duan S., Guo W., Xu Z. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. 2019;18:29. doi: 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundholm M., Schroder M., Nagaeva O. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez-Blanco A., De la Cruz-Hernandez E., Dominguez G.I. Upregulation of NKG2D ligands and enhanced natural killer cell cytotoxicity by hydralazine and valproate. Int J Oncol. 2011;39:1491–1499. doi: 10.3892/ijo.2011.1144. [DOI] [PubMed] [Google Scholar]
- 20.Chen G., Huang A.C., Zhang W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C.W., Lim S.O., Chung E.M. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell. 2018;33:187–201. doi: 10.1016/j.ccell.2018.01.009. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C.W., Lim S.O., Xia W. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Damme E.J. Lectins as tools to select for glycosylated proteins. Methods Mol Biol. 2011;753:289–297. doi: 10.1007/978-1-61779-148-2_19. [DOI] [PubMed] [Google Scholar]
- 24.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 25.Pelaseyed T., Bergstrom J.H., Gustafsson J.K. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.