Abstract
Therapeutic approaches for musculoskeletal tissue regeneration commonly employ growth factors (GFs) to influence neighboring cells and promote migration, proliferation, or differentiation. Despite promising results in preclinical models, the use of inductive biomacromolecules has achieved limited success in translation to the clinic. The field has yet to sufficiently overcome substantial hurdles such as poor spatiotemporal control and supraphysiological dosages, which commonly result in detrimental side effects. Physiological presentation and retention of biomacromolecules is regulated by the extracellular matrix (ECM), which acts as a reservoir for GFs via electrostatic interactions. Advances in the manipulation of extracellular proteins, decellularized tissues, and synthetic ECM-mimetic applications across a range of biomaterials have increased the ability to direct the presentation of GFs. Successful application of biomaterial technologies utilizing ECM mimetics increases tissue regeneration without the reliance on supraphysiological doses of inductive biomacromolecules. This review describes recent strategies to manage GF presentation using ECM-mimetic substrates for the regeneration of bone, cartilage, and muscle.
Keywords: Growth factor, Extracellular matrix, Spatiotemporal control, Tissue engineering, Affinity
Graphical abstract
Highlights
-
•
The contribution of growth factors during endogenous formation and repair of bone, cartilage, and muscle is reviewed.
-
•
The application and challenges of growth factors in clinical applications is discussed.
-
•
Carriers for growth factors are described, with an emphasis on the native extracellular matrix (ECM) and its constituents.
-
•
Frontiers in growth factor delivery are highlighted.
1. Introduction
Musculoskeletal (MSK) disease affects more than half of people aged 18 and over, and nearly three out of four over the age of 65 [1]. Given that more than 20% of Americans will be over 65 by 2030 [2], the magnitude and impact of MSK disease is profound. The occurrence far outstrips the penetration of circulatory or respiratory diseases, and the costs of treatment are enormous. Recent data reveal the financial toll of MSK disease on the American economy. In 2016, MSK disorders were the aggregated health category with the highest modeled spending at an estimated $380.9 billion [3]. These costs were greater than spending on diabetes, urogenital, blood, and endocrine disorders ($309.1 billion) and cardiovascular diseases ($255.1 billion).
Tissue engineering is an exciting approach to treat MSK disorders and address the shortcomings of existing clinical approaches such as tissue grafting. Tissue engineering requires effective strategies to influence the behavior of cells to migrate, proliferate, and differentiate toward a desired phenotype to construct a functional tissue subunit or structure. Cell behavior can be regulated using an array of methods including controlling the biophysical properties of the substrate [4,5], co-culture with accessory cells [6,7], genetic manipulation [8,9], and acute, intermittent, or sustained presentation of inductive macromolecules such as growth factors (GFs) [10,11].
GFs are naturally occurring proteins that stimulate cell division and differentiation and are important to tissue development and repair [12]. Delivery methods for GFs have been under investigation for more than three decades to capture the potent effect of these stimuli on tissue regeneration, particularly for MSK applications. However, the translational success of GFs for clinical application has been underwhelming, primarily driven by the high cost of synthesis using recombinant technologies, limited spatiotemporal control, and the supraphysiological doses necessary to overcome protein instability that leads to detrimental side effects [[13], [14], [15]]. In the context of this review, spatiotemporal control is defined as the engineered regulation of the location and effect time of a pharmacological agent or bioactive material. Indeed, many current approaches are hindered by low affinity materials used to deliver the GF payload, providing an opportunity to improve upon the efficacy of pharmacological approaches in tissue engineering.
Endogenous extracellular matrix (ECM) plays a key role in tissue formation and repair, serving as a scaffold for cell adhesion and proliferation, providing structure and mechanical robustness to the tissue, and also binding and presenting GFs secreted by neighboring cells to regulate cell survival, proliferation, and differentiation [[16], [17], [18]]. Among other roles, the ECM regulates the retention and presentation of GFs through electrostatic interactions while also controlling other events such as integrin clustering that regulates GF signaling. Thus, many tissue engineering platforms seek to mimic the characteristics of the ECM using synthetic materials or ECM-derived proteins and tissues combined with other biomaterials to deliver GFs for MSK tissue regeneration (Fig. 1). In this review, we will highlight various strategies used to deliver GFs for MSK tissue repair with a particular focus on methods that mimic or incorporate ECM-based interactions. We describe the use of engineered ECMs and dynamic control systems to regulate the release of GFs. Finally, we will identify major challenges with current approaches and potential future directions for investigation by the field.
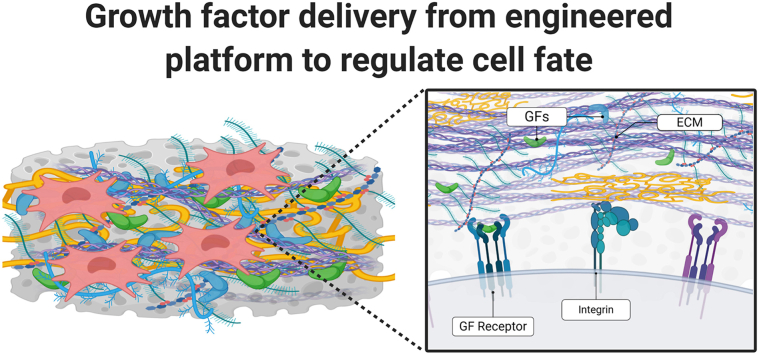
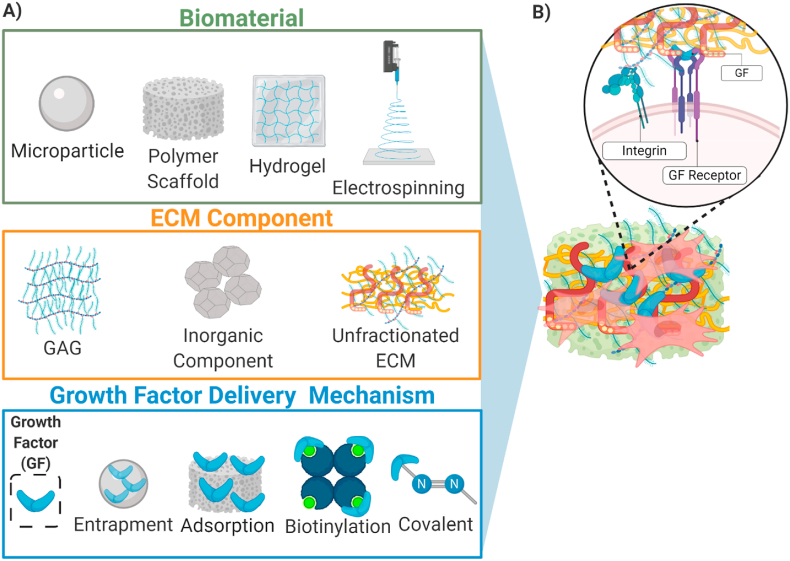
Fig. 1.
(A) A sampling of methods for GF delivery used to influence transplanted or host cells for tissue engineering. Various biomaterials have been utilized for tissue engineering applications, driven by application or tissue type. ECM components are commonly utilized for their cell instructive nature and capacity for GF retention. Materials can be further altered to tune the presentation of GFs and increase their effectiveness. (B) Combinations of these biomaterials, either synthetic or naturally derived, coupled with an ECM component regulate spatiotemporal GF presentation. Inclusion of ECM improves molecular feedback between cells and the matrix by engaging integrin signaling which may potentiate GF signaling.
1.1. Impact of musculoskeletal injuries
The MSK organ system includes bone, muscle, cartilage, tendon, ligament, and accessory tissues that support the body. MSK organs are comprised of a tissue-specific ECM that is essential for proper function. Both injury and age-related matrix degeneration significantly impact mobility and the capacity for pain free articulation of joints. Bone fracture, osteoarthritis and muscle injury are among the most prevalent MSK conditions contributing to disability across the world.
Nearly 8 million fractures occur each year in the United States, with 3.7 million traumatic fractures reported in 2017 [19,20]. While bone tissue has a remarkable capacity for regeneration, many bone defects such as slow-healing fractures or non-unions require surgical intervention to bridge the fracture site and enable tissue repair. In the elderly, fractures are highly susceptible to delayed union due to the compromised bone structure, reduced vascularity, dysfunctional state of chronic inflammation, reduced number of progenitor cells in the periosteum, and the decreased production and responsiveness of tissues to growth factors [[21], [22], [23], [24], [25], [26]].
Similar to age-related bone loss that occurs in osteoporosis, cartilage tissue is naturally lost as we age. This degradation can be accelerated through high impact blows experienced in some sports or trauma and through excessive joint loading, which is common in obese individuals [27]. Osteoarthritis, a functional outcome of reduced cartilage tissue, was diagnosed in more than 52 million Americans in 2012, and the number of diagnosed arthritis cases in the US is projected to balloon to 78 million by 2040 [28].
Skeletal muscle injury commonly results from high impact traumatic collisions, surgical procedures, or sports-related muscle contusions. The robust healing capacity of skeletal muscle is capable of regenerating tears and contusions without intervention. The loss of 20% or more of skeletal muscle mass exhausts this natural healing capacity and requires intervention, typically in the form of an autologous tissue graft [29]. Sarcopenia, the natural loss of muscle mass due to age, is associated with an annual cost of $18.5 billion in the United States alone [30].
Taken together these MSK tissue injuries and diseases represent a significant burden to the individual and the US healthcare system. There remains an unmet clinical need to improve outcomes by discovering methodologies to promote MSK tissue regeneration. Currently there are few clinically-approved treatments that employ locally applied recombinant GFs to stimulate repair or regeneration despite their critical role in the endogenous spatiotemporal cascade of tissue regeneration [12].
1.2. Contribution of growth factors toward musculoskeletal tissue development and repair
The temporal cascade of MSK repair has been thoroughly investigated and detailed in previous reviews [[31], [32], [33]]. A brief synopsis of the MSK tissue healing process post-injury can be broadly divided into five stages: development of a hematoma, inflammation, progenitor cell recruitment, matrix deposition, and matrix remodeling [34,35]. Development of a hematoma and inflammation results in rapid infiltration of macrophages into the injury site, driven by the increased expression of monocyte chemotactic proteins, platelet-derived growth factor (PDGF-BB), and inflammatory factors such as interleukin-1 (IL-1) and IL-6 [[36], [37], [38]].
Bone tissue regeneration occurs through one of two mechanisms: intramembranous (direct) or endochondral (indirect) ossification. Both mechanisms rely upon the initial recruitment and differentiation of mesenchymal stromal cells (MSCs), a multipotent progenitor cell population capable of differentiating into cartilage forming chondrocytes and bone forming osteoblasts. MSCs migrate from the periosteum and endosteum of the bone to the fracture site upon stimulation by PDGF-BB, fibroblast growth factor-2 (FGF-2), transforming growth factor-α (TGF-α), stem cell-derived factor 1 (SDF1), and hypoxia inducible factor-1 (HIF-1) [[39], [40], [41], [42]]. Intramembranous repair, characterized by the direct differentiation of MSCs into osteoblasts, is the pathway of healing in fractures fixed with absolute rigidity and for cranial bones in the skull. The majority of fractures heal predominantly through endochondral ossification, in which MSCs differentiate first to chondrocytes prior to transforming to osteoblasts that form the new bone [43]. There are a number of GFs critical to defining the differentiation patterns of these MSCs during repair. Osteogenic differentiation is regulated by transcriptional activation of Runx2 and Sp7 (Osterix), which are direct downstream targets of bone morphogenetic proteins (BMPs) and canonical Wnt GFs [[44], [45], [46]]. Fate specification of MSCs into chondrocytes occurs through transcriptional activation of Sox 9, which can be robustly stimulated by transforming growth factor-β1 (TGF-β1) [47,48]. In order for chondrocytes to mature to osteoblasts, they must undergo hypertrophic differentiation, marked by the expression of the matrix molecule collagen X and ~20-fold increase in size [49]. Hypertrophic chondrocytes are highly bioactive and secrete both angiogenic, vascular endothelial growth factor (VEGF) [50,51] and placental derived growth factor (PlGF) [52] and osteogenic proteins (BMPs) [53] that bind to the matrix and regulate vascular invasion into the cartilage callus and mineralization of the matrix.
Articular cartilage lacks sufficient vascularization and progenitor cell content to facilitate robust tissue regeneration after damage. The generation of articular cartilage is dependent upon the chondrogenic differentiation of MSC populations into chondrocytes that are identified by their high expression of Noggin, TGF-β receptor 2, and growth differentiation factor 5 (GDF-5) [33]. Several members of the TGF-β superfamily are highly expressed by mesenchymal condensations during cartilage formation, which are responsive to TGF-β1 [47,48]. Following chondrogenic differentiation, FGFs retained in the cartilage matrix play a critical role in regulating the balance between a catabolic or anabolic cellular state. Upon cartilage damage, FGF-2 is released from the ECM and induces catabolic degradation of the matrix [55]. Conversely, the release of FGF-18 from the ECM promotes generation of cartilage ECM by chondrocytes through FGF receptor 3 signaling [55,56].
Skeletal muscle generation is initiated by the activation and proliferation of satellite stem cells, the primary progenitor population for muscular tissue. Hepatocyte growth factor (HGF) released from muscle tissue post-injury induces migration and proliferation of satellite stem cells to the site of tissue damage [57]. The sequential expression of insulin like growth factor 1 (IGF-1) and −2 by myocytes and macrophages stimulate myoblast proliferation and differentiation in damaged muscular tissue, while TGF-β1 inhibits differentiation [58,59]. The modification and remodeling of the matrix proteins of regenerated muscle tissue is coordinated primarily by matrix metalloproteinases (MMPs), namely MMP-2 and -9 [60]. The repair process is concluded by remodeling of the deposited ECM toward a functional tissue architecture for homeostasis.
As described above, MSK tissue formation and regeneration is a highly complex process that is coordinated by the presentation and availability of numerous GFs produced by neighboring cells (Table 1). As this sequence of events is well-characterized, tissue engineers seek to capitalize on this knowledge and present these cues using engineered materials to accelerate or enhance MSK tissue regeneration.
Table 1.
Growth factors implicit in regeneration of MSK tissue. Regeneration is dependent on the temporal cascade of GFs along with other cytokines, macromolecules, and enzymes
Key: bone morphogenetic protein (BMP), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), placental growth factor (PlGF), growth differentiation factor 5 (GDF-5), fibroblast growth factor 18 (FGF-18), hepatocyte growth factor (HGF), insulin like growth factor 1,2 (IGF-1,2).
1.3. Clinical application of growth factors for musculoskeletal repair
Cytokines and GFs induce chemotaxis of responsive cells, regulate differentiation, and play a critical role in tissue development and repair. Due to the potency of these factors, there is an emphasis on the development of effective strategies to use GFs when tissue cannot be healed through other established methods. Several GFs have been approved by the United States Food and Drug Administration (FDA) to facilitate tissue regeneration. Regranex® (Novartis) utilizes recombinant PDGF-BB to accelerate the healing of diabetic foot ulcers [61,62]. Leukine® (Partner Therapeutics, Inc.) and Leucomax® (Schering-Plough) release granulocyte-macrophage colony stimulating factor (GM-CSF) to stimulate the immune system of patients receiving chemotherapy but have also accelerated the healing of diabetic ulcers when applied topically [57]. BMP-2 is the only FDA-approved GF for adjuvant MSK regeneration and has elicited broad investigation for use in tissue engineering applications (Table 2) [63,64]. With annual sales of over $750 million, INFUSE® Bone Graft (Medtronic) is widely used by orthopedic surgeons to stimulate bone formation for spinal fusion and promote fracture repair in patients with a high risk for non-union. The BMP-2 loaded collagen carrier of the INFUSE® system can be used alone or in combination with other treatment techniques (e.g., bone marrow aspirate) to increase bone regeneration and lower failure rates in spinal fusion and tibial fracture repair [65,66]. However, high dosages of BMP-2 (12–30 mg per implant) can result in significant complications including inflammation, heterotopic ossification, hematoma, and myelopathy that require additional revision procedures [15,67]. These adverse effects are attributable to the supraphysiological levels of BMP-2 required and the low affinity of proteins for collagen, with as much as 80% of the BMP-2 released from the collagen sponge in 6 days [68,69]. Thus, delivery methods possessing higher affinity for GFs may decrease these adverse effects. ECM mimetics that retain spatiotemporal control while increasing bioavailability of GFs can increase the effectiveness of treatments and reduce the supraphysiological dose of GFs currently used.
Table 2.
Biomaterial systems presenting bone morphogenetic protein 2 (BMP-2) for musculoskeletal tissue regeneration. Applications in vivo resulted in increased tissue generation, mechanical properties, or both, while applications in vitro lead to enhanced osteogenesis.
| Carrier | Delivery Method | Dose | Model (species/cell line) | Citation |
|---|---|---|---|---|
| Porous titanium oxide | Surface adsorption | 5–20 μg | Subcutaneous (rat) | [70] |
| PLGA microspheres | Entrapment | 1 mg | Femoral defect (rat) | [71] |
| Alginate hydrogel | Entrapment | 5 μg | Femoral defect (rat) | [72] |
| Electrospun silk/poly(ethylene oxide) | Entrapment | N/A | In vitro (MSC) | [73] |
| Sintered PLA microparticles | Entrapment | N/A | In vitro (C2C12) | [74] |
| Laponite-loaded alginate/methylcellulose hydrogel | Surface adsorption and entrapment. | 200 ng/mL | Femoral defect (rat) | [75] |
| PLGA scaffold | Entrapment | 100 μg | Osteochondral defect (rabbit) | [76] |
| PLL polyelectrolyte capsules in alginate gels | Electrostatic interaction (ionic) | 5.2 mg/m2 | Subcutaneous (mouse) | [77] |
| PAA polyelectrolyte coated PCL/TCP scaffolds | Electrostatic interaction (ionic) | 6 μg | Femoral defect (rat) | [78] |
| Heparin-conjugated fibrinogen | Electrostatic interaction (ECM) | 1 μg | Intramuscular (rat) | [79] |
| Heparin-functionalized alginate | Electrostatic interaction (ECM) | 1 μg | Subcutaneous (mouse) | [80] |
| Heparin methacrylamide microparticles in alginate | Electrostatic interaction (ECM) | 30 μg | Femoral defect (rat) | [81] |
| PEA coated PCL tubes coated with fibronectin | Electrostatic interaction (ionic) | 15 ng | Segmental radius defect (mouse) | [82] |
| Biotinylated BMP-2 in collagen sponge | Electrostatic interaction (ionic) | 0.5 μg | Subcutaneous (mouse) | [83] |
| Decellularized ECM-coated PCL | Electrostatic interaction (ECM) | 50 μg/mL | Subcutaneous (mouse) | [84] |
| Heparin-coated PCL microthreads | Covalent immobilization | 5.1 ng/cm2 | In vitro (MSC) | [85] |
1.4. Generation and content of musculoskeletal ECM
The ECM is a complex mixture of matricellular and structural proteins secreted by cells into the extracellular space that provides the supportive architecture on which cells adhere, migrate, and regulate tissue development [86]. The composition of the ECM is tissue dependent, and each tissue has components that interact with tissue-specific GFs [87,88].
Collagens are the most prevalent structural protein, comprising 30% of all proteins, which imbue tensile strength and facilitate cellular adhesion to the ECM [89]. There are 16 identified fibrillar collagen subtypes, with collagen I as the most prevalent collagen in bone and muscle tissue, and cartilage tissue being enriched in collagen II [90,91]. The use of collagenous materials in tissue engineering has been bolstered by its biocompatibility and the capacity to tune the degradation mechanics of the material [92,93].
Glycosaminoglycans (GAGs) are highly negative, unbranched polysaccharide chains found in all MSK tissues, with the four primary GAGs being heparan sulfate, chondroitin sulfate, keratan sulfate, and hyaluronic acid [94]. These hydrophilic GAGs, which are highly expressed in cartilaginous tissues, generate significant structural support for tissue architecture and load distribution by drawing fluid into the matrix [[95], [96], [97]]. Due to the enriched presence of acidic sugars and sulfate groups, GAGs are highly electronegative and interact readily with circulating proteins [98]. Proteoglycans are GAGs covalently bound to a core protein. Proteoglycans achieve similar functional outputs depending on the associated GAGs and represent how most GAGs are presented physiologically [99].
Hydroxyapatite (HAp) constitutes 70% of bone tissue, is the prominent inorganic component of mature bone, and is precipitated by osteoblasts upon the incorporation of phosphoproteins [[100], [101], [102]]. When implanted ectopically, HAp exhibits osteoinductive and osteoconductive potential [[103], [104], [105]]. HAp is biocompatible and does not elicit an immunogenic response yet is mechanically brittle and must typically be incorporated with other materials in tissue engineered constructs [[106], [107], [108]].
These ECM constituents increase cell adhesion, differentiation, and host integration when evaluated for MSK tissue repair [[109], [110], [111], [112], [113]]. However, the regenerative potential of these materials is limited and incapable of repairing large tissue defects on their own, motivating the use of GFs to bridge the gap. Numerous biomaterials have been developed to leverage the endogenous interactions of GFs with native tissues and promote MSK regeneration. The presentation of GFs from various platforms is dependent upon the composition of the vehicle, as well as the affinity of GFs for the underlying substrate. In the sections that follow, we will highlight the utility and mechanism of common delivery platforms for the localized presentation of GFs for MSK tissue regeneration.
2. Monolithic delivery systems
Monolithic biomaterial delivery systems use a single biocompatible material or are complexed with other platforms to localize GFs at the target site for diffusion-based control over macromolecule presentation. Monolithic materials take many forms including bulk aggregates, microparticles, hydrogels, electrospun scaffolds, and even metals. Monolithic platforms have remained popular for four decades due to the relative ease to manufacture and simple chemistry. However, the simplicity of these devices results in limited spatiotemporal control necessary for the efficacious presentation of GFs. Through recent advances in controlling the mechanical and degradation characteristics of polymers, monolithic systems have increased efficacy for GF delivery.
Polymer microspheres and hydrogels remain some of the most popular biomaterial systems used for GF presentation intended for MSK repair [114,115]. Poly(lactic-co-glycolic acid) (PLGA) has been widely used to form numerous substrates such as microspheres, fibers, and scaffolds. The release of macromolecules from PLGA is dependent on the molecular weight and the relative amounts of lactic and glycolic components to influence degradation by hydrolysis [[116], [117], [118]]. GFs such as VEGF and BMP-2 can be readily incorporated into PLGA substrates to increase osteogenic phenotype and bone formation [119,120], while the release of IGF-1 from PLGA substrates improved cartilage generation in a rabbit growth plate model [121], thereby demonstrating the retention of bioactivity and spatiotemporal control. The formation of polymer nanofibers through electrospinning recapitulates the nanostructure of the ECM, which has broad tissue engineering applications. PLGA electrospun fibers exhibited sustained bFGF release over 14 days and were evaluated when inserted into a damaged site of a chronic rotator cuff tear model [122]. The insertion of bFGF-loaded fibers increased collagen and GAG content within repair tissue while also increasing the stiffness and ultimate load of regenerated tissue in the tear space, demonstrating the potential for this approach to restore the required biomechanics of damaged muscle [123].
Hydrogels are water swollen polymeric networks that become insoluble and mechanically stable upon ionic or covalent crosslinking. GFs can be efficiently incorporated into hydrogels while retaining bioactivity, yet hydrogels offer limited temporal control of GF release without alteration of degradation or mesh size to regulate diffusion from the scaffold [[124], [125], [126], [127]]. Alginate is a polysaccharide that is commonly used to form hydrogels for GF delivery due to its biocompatibility, ease of use, and tunability of mechanical properties through the addition of divalent cations [128,129]. However, alginate does not degrade naturally, necessitating alternative means to induce degradation for control over GF release or to give way to tissue formation. Gamma irradiation shortens the alginate polymer chains prior to crosslinking, while oxidation of the polymer generates acetal groups that facilitate hydrolysis, both of which accelerate disintegration of alginate hydrogels [130]. BMP-2 was entrapped in alginate irradiated with 5 Gy and exhibited sustained release of BMP-2 over 21 days, with nearly 99% of the total protein released in the first 7 days. The sustained presentation of BMP-2 increased bone generation and functional torque when used to treat a critically sized rat femoral defect [131]. Recently, published data confirm the necessity of facilitating reinnervation in muscle tissue for increasing functional output. Alginate hydrogels containing IGF-1, VEGF, or both were inserted into the tibialis anterior (TA) muscle of mice with transected hind limb sciatic nerves [132]. Animals treated with IGF-1 or VEGF exhibited increased toe spread, an indicator of improved sciatic nerve functionality, while animals treated with both GFs exhibited the best restoration of nerve function.
Collagen and gelatin, hydrolyzed collagen, are common biopolymers that have broad applications for protein delivery [133]. TGF-β1 released from gelatin microspheres induced chondrogenesis in periosteum cell micromasses better than free TGF-β1 [134]. IGF-1 and HGF released from gelatin microspheres in the TA muscle of rats increased the infiltration of cells positive for Pax 7, a marker for muscle progenitor cells [135]. Muscles treated with GFs exhibited increased infiltration and new muscle fibers over 2 weeks that far exceeded the number of Pax7-positive cells infiltrating control gelatin microspheres. Vitrigel, a high density collagen network containing 25–33% w/v collagen fibrils [136], is also effective as a TGF-β1 delivery platform for chondrogenesis [137]. Vitrigel constructs coated with TGF-β1 released GF over 14 days, with 40% released over the first 2 days. Upon implantation into the murine trochlear groove, tissues treated with TGF-β1-coated constructs exhibited more tissue and improved weight distribution compared to tissues treated with uncoated collagen.
Composites of monolithic platforms are useful to enable the presentation of multiple GFs with unique temporal requirements. For example, BMP-2 was loaded into PLGA microparticles that were subsequently loaded into a gelatin hydrogel containing VEGF [71]. Due to its sensitivity to temperature, the gelatin hydrogel degraded quickly, resulting in a rapid release of VEGF that was no longer detected in the implant by day 10. Due to the slower degradation rate of PLGA compared to gelatin, BMP-2 was retained at the implant site for 8 weeks. Sequential presentation of GFs (i.e., burst release of VEGF with sustained BMP-2 presentation) increased bone tissue volume and vessel density in a murine femoral defect model [71]. In another example, BMP-2 (2.5 or 5 μg) or TGF-β1 (50 ng) was entrapped in PLGA microspheres and then suspended in alginate for retention at the implantation site [138]. More than 20% of each GF was retained in the articular femoral defect of New Zealand rabbits over 14 days. Tissue generation was improved for both groups compared to unloaded microspheres, with defects treated with BMP-2 exhibiting a dose-dependence on cartilage formation at 2 weeks. Spatiotemporal control of GFs has been successfully employed to promote the repair of osteochondral tissues as well. TGF-β1 was released from a conical chitosan-gelatin hydrogel, while BMP-2 was released from PLGA to induce bone formation and generate osteochondral tissue [76]. The combined polymer constructs exhibited unique GF release profiles, with the faster degrading gelatin hydrogel initially releasing a higher percentage of encapsulated TGF-β1 until day 10, when BMP-2 concentration was greater. Upon implantation in a rabbit knee defect, the scaffolds induced a tissue gradient of positive staining for collagen type II to osteocalcin, indicating the expected tissue gradient of cartilage to bone found in the osteochondral knee tissue.
There is a need for materials that model the heterogeneous tissue gradients found within the body to promote repair of interfacial tissues [128]. The presentation of GFs using 3D printing offers precise control over material distribution and GF location. VEGF and BMP-2 were entrapped in 3D printed methylcellulose-irradiated alginate bioinks containing clay nanoparticles and then deposited into polycaprolactone (PCL) scaffolds. Bioinks were spatially organized within the scaffolds to create a VEGF loaded core surrounded by BMP-2 at increasing concentrations on the scaffold periphery. These composite scaffolds increased bone formation in a critically sized rat femoral bone defect compared to gradient scaffolds presenting either growth factor alone [75].
Collectively, these data demonstrate the utility of monolithic systems to promote repair of MSK tissues, even with relatively simple delivery devices. However, most of these approaches are limited by the rate of GF diffusion from the vehicle, which is dependent upon material degradation (Fig. 2). Hence, other approaches to present GFs in a more biomimetic manner are necessary to provide additional opportunities to improve tissue formation.
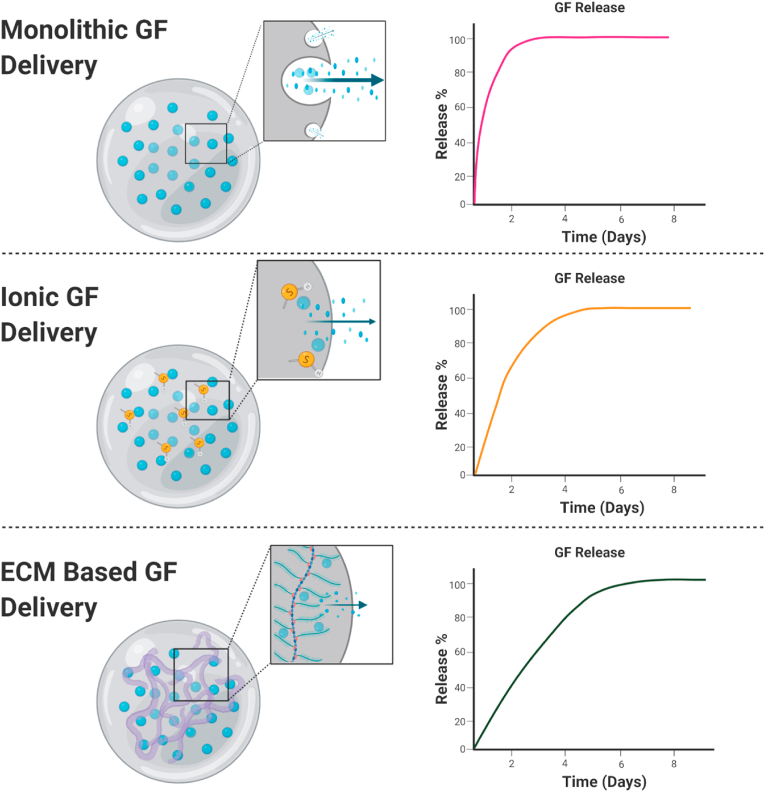
Fig. 2.
Representative GF release profiles from monolithic, ionic, and ECM-based delivery systems. Monolithic carriers release GFs by diffusion, while ionic and ECM-based GF delivery leverage the interaction of positively charged proteins with negatively charged substrates for sustained presentation. Release profiles are drawn to reflect the relative release profile of each mechanism.
3. Growth factor delivery controlled by ionic interactions
The endogenous tissue ECM regulates the retention and presentation of native GFs primarily through electrostatic interactions. Electronegative components within the ECM (e.g., GAGs, HAp, etc.) have a high affinity for the positively charged amine groups of GFs. In an effort to model endogenous interactions and achieve improved spatiotemporal control, the field has prioritized the generation of biomaterials with increased electronegative charge to mimic the high affinity of GFs for the endogenous ECM.
Polyelectrolyte (PE) films have been used to model the charge-based interactions of ECM and proteins. PE coatings arranged on composite systems impart a high negative electrostatic charge that retain macromolecules [139]. PE films deposited on the surface of polylactic acid (PLA) and PLGA were used to form a six-layer construct through layer-by-layer (LBL) deposition of electrostatically retained materials for implantation [77]. The presentation of BMP-2 and TGF-β1 from multilayer LBL scaffolds induced osteogenic differentiation of human embryonic bodies upon implantation. In another example, BMP-2 and VEGF were released from LBL constructs of PCL/β-tricalcium phosphate (TCP) [78]. Upon subcutaneous implantation, constructs releasing both GFs induced greater bone mineral density and trabecular thickness compared to those releasing only BMP-2. This approach leverages the ionic interactions of proteins with other substrates in a spatially controlled manner to enable the controlled release of multiple GFs.
Biomaterial systems that are amenable to alterations in surface charge facilitate tuning of the affinity of GFs for the substrate, thereby improving spatiotemporal control and retention of these instructive biomacromolecules. Carboxymethyl cellulose (CMC) is a synthetic plant-derived polymer that is readily sulfated, increasing the surface electronegativity that mimics the electrostatic properties of GAGs [140]. Sulfation increased retention of TGF-β1 in peroxide-gelled CMCs. Control CMC hydrogels released 20–30% of TGF-β1 within 2 days, while sulfated CMC hydrogels released only 1–2% of TGF-β1 over the same duration. Local TGF-β1 delivery using sulfated CMC hydrogels induced similar collagen II, aggrecan, and chondroitin sulfate production by MSC populations as free protein, highlighting the retained bioactivity of GFs with this delivery mechanism.
In contrast to traditional hydrogels, self-assembling peptide amphiphiles (PA) possess increased affinity to GFs such as TGF-β1 [141]. PA hydrogels formed from peptides with asparagine-to-aspartic acid mutations released TGF-β1 25% slower than groups without the aspartic acid substitution. The delayed presentation time was due to increased fibrillar formations with the asparagine that reduced the binding availability of TGF-β1, while the aspartic acid mutation reduced fibrillar formations. The prolonged presentation of GF increased GAG production and expression of chondrogenic genes by ATDC5 cells.
PEGylation of highly branched amine terminal polyamidoamine (PAMAM) dendrimers resulted in a cationic construct that could quickly penetrate anionic cartilage tissue without toxicity [142]. IGF-1 covalently conjugated to this PEG/PAMAM construct retained bioactivity and maintained extended residence in the articular space. Moreover, the application of IGF-1 functionalized to PEG/PAMAM dendrimers rescued cartilage from degeneration in an induced osteoarthritis model. This nanocarrier-based ionic interaction is an exciting approach to overcome previous challenges in delivering therapeutics to cartilage with a dense matrix that impairs drug delivery. Unfortunately, ionic tethering often involves complex chemistries to generate charged species that are non-specific, which enable endogenous proteins to bind to available sites and compete with the GF of interest. The retention of charge and bioactivity over long periods of time is unlikely and reduces the likelihood of many of these methods to be used as an off-the-shelf solution for clinical application unless new methods can be developed to stabilize the formulations.
While ionic interactions are an effective approach to transiently link GFs to biomaterials for local delivery, long-term presentation and stability of GFs may be better achieved by direct conjugation to matrices. The biotin-avidin interaction is among the strongest naturally occurring non-covalent bonds and has been used to immobilize proteins on multiple surfaces [143]. Biotinylation of BMP-2 and FGF-2 to gelatin nanofibers increased GF retention and osteogenic gene expression of adipose-derived stromal cells [144,145]. In another approach, PDGF was covalently conjugated to fibrin via an activated Factor XIII (FXIII) transglutaminase. PDGF was released over 71 h in response to plasmin cleavage from the fibrin gel. When evaluated in a murine ischemic epigastric flap model, defects treated with PDGF-conjugated fibrin exhibited increased muscle tissue production and perfusion compared to PDGF-loaded control fibrin [146].
The development of fully synthetic mimetics of GF adhesion sites and GF receptors provide another opportunity to link GFs to underlying materials. FGF-2 was covalently functionalized to block copolymers of styrene-sulfonate/PEG/vinyl-sulfonate, exhibiting similar retention of FGF-2 as that of a heparan sulfate control [147]. Interestingly, the conjugation of low concentrations of FGF-2 to the block copolymer generated a higher number of fibrillar nodes and fibrillar structures in human umbilical vein endothelial cells and human dermal fibroblasts than the FGF-2 presented by heparan sulfate, indicating the cell populations exhibited increased responsiveness to the synthetic presentation of FGF-2.
As these data illustrate, charged substrates that mimic the electronegative interactions of the native ECM facilitate increased spatiotemporal control of GFs for MSK tissue applications. However, many of these methods require complex chemical interactions and extended material synthesis times, reducing the possibility of translation to the clinic. Therefore, techniques that utilize the ECM to direct cellular response and increase spatiotemporal GF control may be more widely available and have been identified as an avenue to translation.
4. Extracellular matrix-based scaffolds
The ECM is a complex mixture of matricellular proteins and GAGs that provide complementary sites for cell adhesion, GF retention, and activation of cell signaling pathways (Fig. 3). Many GFs are characterized as heparan-binding, in which GFs associate with the heparan sulfate proteoglycans of the endogenous ECM with high affinity [148]. Such interactions promote GF retention, enabling the ECM to serve as an endogenous depot of GFs for on-demand release for tissue homeostasis or to participate in tissue repair. In an effort to capture tissue specific matrix microenvironments, ECM has been decellularized and used to functionalize monolithic substrates [[149], [150], [151]]. ECM derived from porcine pericardia was decellularized and used as a hydrogel for bFGF delivery [152]. The sulfate and sugar groups in the ECM retained bFGF, releasing 27.8% of bFGF by the fifth day versus 73.5% of bFGF released from a collagen gel control. These data provide evidence that the ECM derived from native tissues can be harnessed for GF delivery.
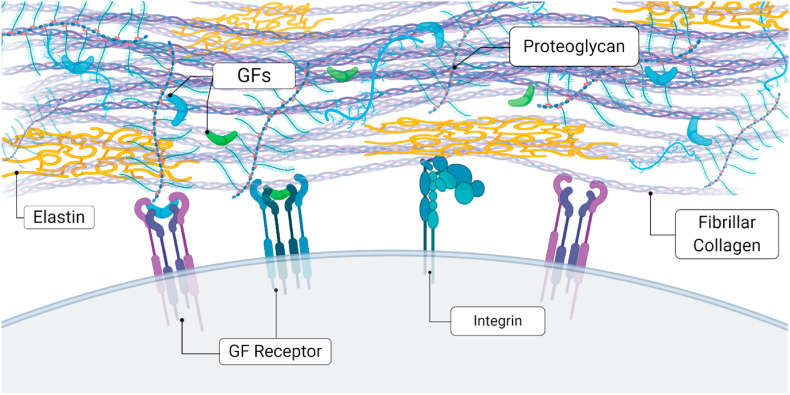
Fig. 3.
The ECM acts as a reservoir for the retention of GFs necessary for musculoskeletal tissue repair. Endogenous GFs are commonly sequestered by heparan-binding proteoglycans within the ECM. Cells engage the surrounding ECM via integrin engagement with specific ligands present in matricellular proteins. This interaction may also enable GF receptor clustering on the cell surface, further promoting activation of the targeted cell signaling pathway and resultant changes in cell phenotype.
Decellularized cell-secreted ECMs are generated by controlling aspects of the microenvironment during cell culture including cell density, duration of culture, and inclusion of inductive cues in the culture media [87,[153], [154], [155]]. Dermagraft® (Organogenesis) is an FDA-approved substrate used clinically for healing of diabetic foot ulcers and is generated by human neonatal dermal fibroblasts on a bioresorbable mesh [156]. The therapeutic efficacy of this product is due to the sequestered endogenous GFs from fibroblasts that are retained in the ECM upon removal of the cells [157]. This approach represents an exciting strategy for GF retention that may influence the differentiation of multipotent cell populations for MSK tissue repair. The retention of exogenous GFs within decellularized cell secreted matrices can be modulated through refinement of the culture conditions. MSCs cultured in osteogenic media (OM) prior to decellularization formed an ECM with more HAp but lower GAG content [84]. The resulting ECM increased retention of exogenous BMP-2, likely due to ionic adsorption to HAp. When examined in vivo, bone volume was higher in the OM-induced ECM group with adsorbed BMP-2 than BMP-2 adsorbed to ECM produced in growth media [84]. These data demonstrate the importance of the culture microenvironment on ECM composition and resultant GF affinity.
PLGA and PLLA alone are electrically neutral and do not support ionic interactions. To enhance the affinity of GFs for these commonly used aliphatic polyesters, heparin was covalently conjugated to decellularized ECM (dECM) secreted by human fibroblasts [158]. When deposited on PLGA/PLLA meshes, heparinized dECM increased retention of TGF-β1 nearly three-fold compared to control scaffolds, resulting in increased MSC aggregation and collagen II staining when implanted in a rabbit articular cartilage defect. Similarly, TGF-β3 was adsorbed to multilayers of decellularized porcine chondrocyte-generated ECM. TGF-β3 was effectively retained within the ECM, and rabbit articular cartilage defects treated with TGF-β3-loaded constructs exhibited the greatest cartilage regeneration compared to free protein [159]. Similarly, heparinized decellularized bovine meniscus (DBM) demonstrated increased retention of PDGF-BB [160]. PDGF-BB was retained for over 2 weeks on heparinized DBM, promoting cellular infiltration and increased meniscus integration compared unmodified DBM.
Utilization of decellularized matrices for MSK tissue regeneration leverages the diverse protein constituents of the ECM, increasing GF retention, cellular adhesion, and synergistic integrin activation. The complexity of the ECM recapitulates the physiological environment during cellular proliferation and differentiation. However, the difficulty in defining the ECM content and generation of consistent materials for repeated studies limits the translation of this method to clinical application. The lack of GF specificity impedes the expansion of ECM-based materials for the temporal expression of multiple GFs, as occurs during native tissue regeneration. Isolated ECM components that have high affinity for GFs and modulate the cellular response are a tantalizing mechanism for increasing MSK tissue regeneration.
4.1. Natural substrates to present growth factors
As described above, native ECM retains GFs through ionic interactions and presents these GFs in a physiologically relevant manner while also facilitating cellular adhesion. Unfortunately, naturally derived materials suffer from substantial batch-to-batch variability, thereby limiting widespread adoption and reproducibility of this approach. As with most natural biomaterials, the differences in biologically sourced tissues are not readily overcome, and the use of cultured cells to generate ECM is limited by the extended culture time for matrix generation, high reagent costs, and potential for contamination. Additionally, differences in decellularization methods translate to variability in DNA and GF retention, as well as structural integrity that confounds efficacy during clinical evaluation [161]. Due to these challenges, the utilization of individual ECM proteins with high GF affinity and beneficial cellular interactions have been extensively investigated.
Matricellular proteins and proteoglycans are transiently present in the ECM of MSK tissues but are critical to development and tissue regeneration [162]. Biomacromolecules such as tenascin C, decorin, heparan sulfate, and chondroitin sulfate bind GFs and provide an environment to direct cellular phenotype [163,164]. Chondroitin sulfate has high affinity for heparin binding GFs such as PDGF-BB, TGF-β1, and TGF-β3 and retains the bioactivity of these GFs when complexed with other biomaterials [165,166]. The inclusion of chondroitin sulfate into PEG hydrogels increased the retention of TGF-β3, increasing the expression of chondrogenic markers from MSCs and generating higher amounts of collagen when compared to the non-chondroitin sulfate gels [167]. Decorin, a leucine-rich proteoglycan containing a single GAG that regulates GF activity and collagen fibrillogenesis [168], was incorporated into collagen hydrogels to increase retention and efficacy of BMP-2 [169]. BMP-2 retention was moderately extended, with 28% of the loaded BMP-2 released by day 20 from the decorin-collagen composite compared to 35% released from the collagen hydrogel. The BMP-2 loaded decorin-collagen composite hydrogel successfully bridged a critically sized rat composite bone-muscle injury by 12 weeks. Additionally, ECM proteins have been engineered to modulate the GF response of cell populations. A synthetic decorin mimetic, termed DS-SILY, exhibited comparable affinity to collagen I as native decorin. DS-SILY has high affinity and retention for IFN-γ and PDGF, GFs with known inflammatory effects that induce hyperplasia in smooth muscle cells. The retention of IFN-γ and PDGF by DS-SILY in vitro reduced the inflammatory phenotype of smooth muscle cells by sequestering them from cellular interactions [170,171].
Heparin is a large, electronegative protein with high affinity for many GFs and can be conjugated to materials through carbodiimide chemistry [79,172]. Heparin was conjugated to fibrinogen to create fibrin gels for BMP-2 delivery, releasing 90% of BMP-2 over 13 days compared to a similar quantity over only 3 days from unmodified fibrin hydrogels, which resulted in improved calcium deposition when implanted ectopically [79]. This work showed the potential for covalently linking heparin without requiring an intermediate peptide linker [173]. Heparin can also be chemically modified for more efficient inclusion into implantable constructs for improved tissue regeneration. Heparin-methacrylate microspheres displayed a dose-dependent retention of BMP-2 at a subcutaneous injection site compared to free BMP-2 post-injection [81]. When implanted in a femoral bone defect, heparin-methacrylate microspheres loaded with BMP-2 and suspended in RGD-modified alginate hydrogels improved bone tissue regeneration with decreased heterotopic bone volume compared to alginate gels containing free BMP-2.
Hydroxyapatite (HAp) is an osteoconductive constituent of the bone ECM that facilitates rapid adsorption of GFs to its negatively charged surface [174]. BMP-2 adsorbed to HAp granules was encapsulated in MSC spheroids, resulting in a more robust osteogenic response compared to exogenous BMP-2 presented in solution [175]. Importantly, the spatial distribution of osteogenic markers within MSC spheroids was dramatically improved by incorporation of BMP-2-adsorbed HAp, while expression of osteogenic markers was restricted to the spheroid periphery when treated with free BMP-2. In another example, incorporation of HAp into 3D printed methylcellulose/alginate bioinks increased the retention of BMP-2 and VEGF, resulting in increased bone tissue generation [75].
Other techniques capitalize upon the ability of surfaces to preferentially organize matricellular proteins for increased growth factor retention and integrin activation. In one such study, fibronectin (FN) was deposited on poly (ethyl acrylate) (PEA) films, resulting in FN spreading and connected networks of this matricellular protein, whereas PEA-free surfaces resulted in globular FN deposition [82]. The spread networks of FN increased BMP-2 adsorption and integrin binding sites via fibronectin III9-10 modules, synergistically increasing the bioactivity of BMP-2 and generating more bone tissue in rat femoral and radial defect models [82,176]. Muscle repair is enhanced by using substrates with isotropic alignment to guide cell morphology and arrangement. Myoblasts seeded on HGF-loaded crosslinked fibrin microthreads exhibited improved infiltration and cell proliferation compared to non-crosslinked microthreads and fibrin controls [177]. When applied to the treatment of TA muscle defects, HGF-loaded crosslinked fibrin microthreads resulted in increased twitch force, decreased defect size, and increased myogenin-positive nuclei compared to non-crosslinked and non-loaded controls.
5. Conclusions and future directions
GF-based treatments are promising for MSK tissue regeneration, and advances in the design of systems that can achieve local and sustained GF release hold enormous potential in improving the efficacy of this approach. Clinical GF applications rely upon supraphysiological doses of the biomacromolecules and low affinity carriers that result in rapid release and undesirable detrimental effects. In order to address this challenge, biomaterial systems that mimic the ECM architecture, affinity, or charge distribution have been developed to increase the binding efficiency and efficacy of associated GFs. Many of the technologies highlighted herein depend upon passive biomaterial characteristics for the control of GFs that cannot be altered post-fabrication. As an alternative, we posit that there is enormous promise in improving the affinity of GFs for the delivery vehicle. For example, the insertion of the 123–144 domain of PlGF-2 into VEGF-A, BMP-2, and PDGF-BB increased the affinity of those GFs for an array of matrix proteins [178]. Similarly, functionalization of PDGF-BB and VEGF-A with the globular domain of the laminin-α1 chain, which has high affinity for syndecan, enhanced growth factor binding and morphogenesis [179]. This increased affinity resulted in greater tissue generation from the altered GFs outperforming as compared to the non-altered controls.
Strategies to tailor GF release post-implantation would provide a substantial advantage compared to current approaches that are dependent upon diffusion or degradation of the carrier. In one approach, light was used to initiate payload release by degradation or polarity changes, with near infrared light inducing the release of entrapped molecules from hyaluronic acid hydrogels containing gold nanorods [146,180]. Self-healing hydrogels, hydrogels that can recover from damage, offer another opportunity for sustained GF presentation [84]. Self-healing PLGA hydrogels were used to release an antimicrobial agent while retaining the controlled release of bFGF [181]. Yet another approach includes the dynamic release of payloads from force sensitive materials. PLGA microparticles sensitive to osmotic annealing were used to release payloads upon mechanical stimulation [182].
Upon injury, MMPs are secreted by invading somatic and progenitor cells and macrophages during tissue regeneration and are an effective mechanism for inducing localized and cell-mediated GF release from biomaterial systems [183]. IGF-1 conjugated to PEG with an MMP-sensitive peptide linker (PL) was formulated to respond to increased MMP activity in wound sites [184]. The release of IGF-1 from the PEG-PL was induced by multiple MMPs. However, released IGF-1 diminished cell proliferation and intracellular internalization, suggesting that IGF-1 bioactivity was impaired. MMP-sensitive crosslinkers in transglutaminase crosslinked chondroitin sulfate (CS) PEG hydrogels retained BMP-2 through electrostatic interactions with CS, while MMP-sensitive crosslinker concentration influenced cellular response [185]. MSC viability and speed of migration in the CS-PEG hydrogel was increased with higher concentrations of the MMP-sensitive crosslinker. In another example, BMP-2-loaded acrylamide nanoparticles fabricated with MMP-sensitive crosslinkers increased bone tissue volume in a rat tibial fracture model [186]. Collectively, these data provide evidence that cell-mediated degradation of substrates is a key parameter for cellular activity, and this activity can be leveraged to maximize GF availability for responsive cells.
As an alternative to allowing cells mediate localized GF release, targeting the biomaterial to a specific tissue is another underexplored strategy for overcoming potential ectopic effects of the biomacromolecule. Strategies that modify GFs or drugs themselves with bone-targeting ligands to enable systemic delivery were recently reviewed [187] and suggest similar approaches could be taken to increase specificity of biomaterials carrying GF payloads. In one successful example, a peptide with high affinity for tartrate-resistant acid phosphatase (TRAP), a protein deposited by osteoclasts on bone resorptive surfaces, was applied to the surface of poly (styrene-alt-maleic anhydride)-b-poly (styrene) nanoparticles to achieve preferential delivery of a Wnt activating drug to fractured bones [188]. Other strategies to direct nanoparticles towards diseased or injured blood vessels by adding vascular “zip codes” could also help achieve a tissue specific delivery of GFs [189].
An emerging alternative to delivering GFs themselves is delivering the messenger RNA (mRNA) that encodes the desired GF [190]. Therapeutically, this approach transfects single stranded mRNA into a population of cells that will then be transcribed to temporarily synthesize the protein encoded by the mRNA without the risk of genomic integration. This technology has recently, and famously, been utilized to develop a highly effective and safe COVID19 vaccine that utilizes mRNA to encode for the coronavirus spike protein that is found on the surface of the SARS-CoV-2 virus [191]. In addition to its application for vaccines, mRNA-mediated production of lipidated proteins may be particularly effective for therapeutic production of these GFs in a desired cell population, as hydrophobic proteins are both costly to manufacture and difficult to deliver. A potential downside of mRNA therapies is that they are typically short lived. Thus, it is particularly important to utilize biomaterials for sustained and local delivery for regenerative applications [192]. Interestingly, mineral-based particles are effective at prolonging and enhancing delivery of both cDNA and mRNA [193,194]. As such, mineralization of ECM-based biomaterials could potentiate mRNA-based approaches to stimulate localized GF production.
Increased spatiotemporal control of GFs is necessary to elevate their application as MSK tissue repair solutions out of the lab and into the clinic. Biomaterial systems that incorporate both the spatiotemporal control of the ECM and interactive functionality to induce GF release may offer the best mechanism to translate GF-based solutions for treating MSK tissue injuries.
Declaration of competing interest
The authors have no conflicts to report.
Acknowledgements
This work was supported by the National Institutes of Health under award number R01 DE025475 and R01 DE025899 to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript. All schematics in this work were created using BioRender.com.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Weinstein S., Yelin E., Watkins-Castillo S.I. Prevelance of select medical conditions. The burden of musculoskeletal diseases in the united states. 2020;(3) [Google Scholar]
- 2.States United. C.B. 2017 national population projections tables: main series. 2017 (census.gov) [Google Scholar]
- 3.Dieleman J.L. US health care Spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri O. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler A.J. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Han Q. Dermal mesenchymal stem cells promoted adhesion and migration of endothelial cells by integrin in psoriasis. Cell Biol. Int. 2020:1–10. doi: 10.1002/cbin.11492. [DOI] [PubMed] [Google Scholar]
- 7.Vorwald C.E., Joshee S., Leach J.K. Spatial localization of endothelial cells in heterotypic spheroids influences notch signaling. J. Mol. Med. 2020;98(3):425–435. doi: 10.1007/s00109-020-01883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Y. Enhanced chondrogenesis in a coculture system with genetically manipulated dedifferentiated chondrocytes and ATDC5 cells. Biotechnol. Bioeng. 2020;117(10):3173–3181. doi: 10.1002/bit.27482. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Fernandez T. Gene delivery of TGF-beta3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. 2016;22(9–10):776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 10.Kempen D.H. Enhanced bone morphogenetic protein-2-induced ectopic and orthotopic bone formation by intermittent parathyroid hormone (1-34) administration. Tissue Eng. 2010;16(12):3769–3777. doi: 10.1089/ten.tea.2010.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed S., Wu B. Sustained growth factor delivery in tissue engineering applications. Ann. Biomed. Eng. 2014;42(7):1528–1536. doi: 10.1007/s10439-013-0956-6. [DOI] [PubMed] [Google Scholar]
- 12.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 13.McKay W.F., Peckham S.M., Badura J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007;31(6):729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiller K.L., Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv Transl Res. 2015;5(2):101–115. doi: 10.1007/s13346-013-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein N.E. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg. Neurol. Int. 2013;4:S343–S352. doi: 10.4103/2152-7806.114813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker A., Turnbull J.E., Gallagher J.T. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J. Biol. Chem. 1994;269(2):931–935. [PubMed] [Google Scholar]
- 17.Flaumenhaft R., Rifkin D.B. Extracellular matrix regulation of growth factor and protease activity. Curr. Opin. Cell Biol. 1991;3(5):817–823. doi: 10.1016/0955-0674(91)90055-4. [DOI] [PubMed] [Google Scholar]
- 18.Bassat E. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547(7662):179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einhorn T.A. Enhancement of fracture-healing. J bone joint surg Am. 1995;77(6):940–956. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Rui P.K.K. 2017. Emergency department summary tables. 2017, centers for disease control and prevention: national center for health statistics. National hospital ambulatory medical care survey. [Google Scholar]
- 21.Clark D. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell. 2020;(3):19. doi: 10.1111/acel.13112. : p. e13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josephson A.M. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2019;116(14):6995–7004. doi: 10.1073/pnas.1810692116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yukata K. Aging periosteal progenitor cells have reduced regenerative responsiveness to bone injury and to the anabolic actions of PTH 1-34 treatment. Bone. 2014;62:79–89. doi: 10.1016/j.bone.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C. Effect of age on vascularization during fracture repair. J. Orthop. Res. 2008;26(10):1384–1389. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppe J.P. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao J.J. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;22(8):1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- 27.King L.K., March L., Anandacoomarasamy A. Obesity & osteoarthritis. Indian J med res. 2013;138:185–193. [PMC free article] [PubMed] [Google Scholar]
- 28.Hootman J.M. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US Adults, 2015-2040. Arthritis Rheum. 2016;68(7):1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner N.J., Badylak S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- 30.Janssen I. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 31.Grefte S. Skeletal muscle development and regeneration. Stem Cell. Dev. 2007;16(5):857–868. doi: 10.1089/scd.2007.0058. [DOI] [PubMed] [Google Scholar]
- 32.Einhorn T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 33.Tiku M.L., Sabaawy H.E. Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention. Ther Adv Musculoskelet Dis. 2015;7(3):76–87. doi: 10.1177/1759720X15576866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjorn R., Olsen, Anthony M., Reginato, Wang W. Bone development. Annu. Rev. Cell dev. Biol. 2000;16(1):191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 35.Ciciliot S., Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. 2010;16(8):906–914. doi: 10.2174/138161210790883453. Curr Pharm Des. [DOI] [PubMed] [Google Scholar]
- 36.Rundle C.H. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38(4):521–529. doi: 10.1016/j.bone.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Bourque W.T., Gross M., Hall B.K. Expression of four growth factors during fracture repair. Int. J. Dev. Biol. 1993;37(4):573–579. [PubMed] [Google Scholar]
- 38.Chazaud B. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc. Sport Sci. Rev. 2009;37(1):18–22. doi: 10.1097/JES.0b013e318190ebdb. [DOI] [PubMed] [Google Scholar]
- 39.Wang X. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int. Orthop. 2013;37(12):2491–2498. doi: 10.1007/s00264-013-2059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozaki Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cell. Dev. 2007;16(1):119–129. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 41.Ceradini D.J. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 42.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahney C.S. Cellular biology of fracture healing. J. Orthop. Res. 2019;37(1):35–50. doi: 10.1002/jor.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Secreto F.J., Hoeppner L.H., Westendorf J.J. Wnt signaling during fracture repair. Curr. Osteoporos. Rep. 2009;7(2):64–69. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho T.J., Gerstenfeld L.C., Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J. Bone Miner. Res. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 46.Marsell R., Einhorn T.A. The biology of fracture healing. Injury. 2011;42(6):551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortier L.A. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011;469(10):2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W., Rigueur D., Lyons K.M. TGFbeta signaling in cartilage development and maintenance. Birth Defects Research Part C - embryo Today. 2014;102(1):37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper K.L. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature. 2013;495(7441):375–378. doi: 10.1038/nature11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colnot C.I., Helms J.A. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100(2):245–250. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 51.Gerber H.P. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 52.Maes C. Placental growth factor mediates mesenchymal cell development, cartilage turnover, and bone remodeling during fracture repair. J. Clin. Invest. 2006;116(5):1230–1242. doi: 10.1172/JCI26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bostrom M.P. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J. Orthop. Res. 1995;13(3):357–367. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 54.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bus. Manag. Res. 2009;24(2):274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson D. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J. Biol. Chem. 2005;280(21):20509–20515. doi: 10.1074/jbc.M410148200. [DOI] [PubMed] [Google Scholar]
- 56.Moore E.E. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13(7):623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Miller K.J. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. Cell Physiol. 2000;(1):278. doi: 10.1152/ajpcell.2000.278.1.C174. p. C174-81. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi S. Sequence of IGF-I, IGF-II, and HGF expression in regenerating skeletal muscle. Histochem Cell Biol. 2004;122(5):427–434. doi: 10.1007/s00418-004-0704-y. [DOI] [PubMed] [Google Scholar]
- 59.Kollias H.D., McDermott J.C. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J. Appl. Physiol. 1985;104(3):579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- 60.Chen X., Li Y. Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adhes. Migrat. 2009;3(4):337–341. doi: 10.4161/cam.3.4.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamakawa S., Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7:10. doi: 10.1186/s41038-019-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barrientos S. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22(5):569–578. doi: 10.1111/wrr.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim D.H. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009;9(11):886–892. doi: 10.1016/j.spinee.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Copuroglu C., Calori G.M., Giannoudis P.V. Fracture non-union: who is at risk? Injury. 2013;44(11):1379–1382. doi: 10.1016/j.injury.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Burkus J.K. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg Am. 2009;91(5):1181–1189. doi: 10.2106/JBJS.G.01485. [DOI] [PubMed] [Google Scholar]
- 66.Starr A.J. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures. J Bone Joint Surg Am. 2003;85(10):2049. doi: 10.2106/00004623-200310000-00027. author replies 2049-50. [DOI] [PubMed] [Google Scholar]
- 67.Woo E.J. Adverse events after recombinant human BMP2 in nonspinal orthopaedic procedures. Clin. Orthop. Relat. Res. 2013;471(5):1707–1711. doi: 10.1007/s11999-012-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H.S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp. Mol. Med. 2012;44(5):350–355. doi: 10.3858/emm.2012.44.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olthof M.G.L. Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J Tissue Eng Regen Med. 2018;12(6):1339–1351. doi: 10.1002/term.2664. [DOI] [PubMed] [Google Scholar]
- 70.Hall J. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J. Clin. Periodontol. 2007;34(5):444–451. doi: 10.1111/j.1600-051X.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 71.Kempen D.H. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 72.Kolambkar Y.M. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone. 2011;49(3):485–492. doi: 10.1016/j.bone.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li C. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27(16):3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 74.Suciati T. Zonal release of proteins within tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2006;17(11):1049–1056. doi: 10.1007/s10856-006-0443-9. [DOI] [PubMed] [Google Scholar]
- 75.Freeman F.E. 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Adv. Sci. 2020;(33):6. doi: 10.1126/sciadv.abb5093. p. eabb5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han F. A pilot study of conically graded chitosan-gelatin hydrogel/PLGA scaffold with dual-delivery of TGF-beta1 and BMP-2 for regeneration of cartilage-bone interface. J Biomed Mater Res B Appl Biomater. 2015;103(7):1344–1353. doi: 10.1002/jbm.b.33314. [DOI] [PubMed] [Google Scholar]
- 77.Facca S. Active multilayered capsules for in vivo bone formation. Proc. Natl. Acad. Sci. U. S. A. 2010;107(8):3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah N.J. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32(26):6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H.S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. 2010;16(4):1225–1233. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 80.Jeon O. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Contr. Release. 2011;154(3):258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hettiaratchi M.H. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci Adv. 2020;(1):6. doi: 10.1126/sciadv.aay1240. p. eaay1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng Z.A. Nanoscale coatings for ultralow dose BMP-2-driven regeneration of critical-sized bone defects. Adv. Sci. 2019;6(2):1800361. doi: 10.1002/advs.201800361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Uludag H. Biotinated bone morphogenetic protein-2: in vivo and in vitro activity. Biotechnol. Bioeng. 1999;65(6):668–672. doi: 10.1002/(sici)1097-0290(19991220)65:6<668::aid-bit7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 84.Larochette N. Osteogenic-differentiated mesenchymal stem cell-secreted extracellular matrix as a bone morphogenetic protein-2 delivery system for ectopic bone formation. Acta Biomater. 2020;116:186–200. doi: 10.1016/j.actbio.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Gadalla D., Goldstein A.S. Improving the osteogenicity of PCL fiber substrates by surface-immobilization of bone morphogenic protein-2. Ann. Biomed. Eng. 2020;48(3):1006–1015. doi: 10.1007/s10439-019-02286-1. [DOI] [PubMed] [Google Scholar]
- 86.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123(Pt 24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harvestine J.N. Cell-secreted extracellular matrix, independent of cell source, promotes the osteogenic differentiation of human stromal vascular fraction. J Mater Chem B. 2018;6(24):4104–4115. doi: 10.1039/C7TB02787G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaviani R. Changes in growth plate extracellular matrix composition and biomechanics following in vitro static versus dynamic mechanical modulation. J Musculoskelet Neuronal Interact. 2018;18(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- 89.Patino M.G. Collagen: an overview. Implant dent. 2002;11(3):280–285. doi: 10.1097/00008505-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 90.Burgeson R.E., Nimni M.E. Collagen types. molecular structure and tissue distribution. Clin. Orthop. Relat. Res. 1992:250–272. [PubMed] [Google Scholar]
- 91.Lodish H., Berk A., Zipursky S. W.H. Freeman; New York: 2000. Collagen: the Fibrous Proteins of the Matrix in Molecular Cell Biology. [Google Scholar]
- 92.Marinucci L. Biocompatibility of collagen membranes crosslinked with glutaraldehyde or diphenylphosphoryl azide: an in vitro study. J. Biomed. Mater. Res. 2003;67(2):504–509. doi: 10.1002/jbm.a.10082. [DOI] [PubMed] [Google Scholar]
- 93.Everts V. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J. Cell. Physiol. 1992;150(2):221–231. doi: 10.1002/jcp.1041500202. [DOI] [PubMed] [Google Scholar]
- 94.Prydz K. Determinants of glycosaminoglycan (GAG) structure. Biomolecules. 2015;5(3):2003–2022. doi: 10.3390/biom5032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gillard G.C. A comparison of the glycosaminoglycans of weight-bearing and non-weight-bearing human dermis. J Invest Dermatol. 1977;69(2):257–261. doi: 10.1111/1523-1747.ep12506406. [DOI] [PubMed] [Google Scholar]
- 96.Casale J., Crane J.S. Biochemistry, glycosaminoglycans, in. StatPearls. 2020 Treasure Island (FL) [PubMed] [Google Scholar]
- 97.Glant T.T. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J. Immunol. 1998;160(8):3812–3819. [PubMed] [Google Scholar]
- 98.Gandhi N.S., Mancera R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008;72(6):455–482. doi: 10.1111/j.1747-0285.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- 99.Varki A., Cummings R., Esko J. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. Proteoglycans and Sulfated Glycosaminoglycans, in Essentials of Glycobiology. [PubMed] [Google Scholar]
- 100.Sommerfeldt D.W., Rubin C.T. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 2001:S86–S95. doi: 10.1007/s005860100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hunter G.K., Goldberg H.A. Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. Unit. States Am. 1993;90(18):8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reznikov N., Shahar R., Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014;10(9):3815–3826. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 103.Yamasaki H., Sakai H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials. 1992;13(5):308–312. doi: 10.1016/0142-9612(92)90054-r. [DOI] [PubMed] [Google Scholar]
- 104.Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17(1):31–35. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 105.Chang B.S. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials. 2000;21(12):1291–1298. doi: 10.1016/s0142-9612(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 106.Ishihara K. Adhesive bone cement containing hydroxyapatite particle as bone compatible filler. J. Biomed. Mater. Res. 1992;26(7):937–945. doi: 10.1002/jbm.820260708. [DOI] [PubMed] [Google Scholar]
- 107.Yamamura K., Iwata H., Yotsuyanagi T. Synthesis of antibiotic-loaded hydroxyapatite beads and in vitro drug release testing. J. Biomed. Mater. Res. 1992;26(8):1053–1064. doi: 10.1002/jbm.820260807. [DOI] [PubMed] [Google Scholar]
- 108.He J., Genetos D.C., Leach J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng. 2010;16(1):127–137. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee D.J. Decellularized bone matrix grafts for calvaria regeneration. J. Tissue Eng. 2016;7 doi: 10.1177/2041731416680306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soicher M.A. Remineralized bone matrix as a scaffold for bone tissue engineering. J. Biomed. Mater. Res. 2014;102(12):4480–4490. doi: 10.1002/jbm.a.35118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang J. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials. 2016;89:114–126. doi: 10.1016/j.biomaterials.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 112.Wolf M.T. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials. 2012;33(10):2916–2925. doi: 10.1016/j.biomaterials.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Coppi P. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng. 2006;12(7):1929–1936. doi: 10.1089/ten.2006.12.1929. [DOI] [PubMed] [Google Scholar]
- 114.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem. Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 115.Katti K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces. 2004;39(3):133–142. doi: 10.1016/j.colsurfb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 116.Cohen S. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. (N. Y.) 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 117.Mooney D.J. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17(14):1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 118.Richardson T.P. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 119.Leach J.K. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27(17):3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 120.Kirby G.T.S. PLGA-based microparticles for the sustained release of BMP-2. Polymers. 2011;3(1):571–586. [Google Scholar]
- 121.Sundararaj S.K.C. Treatment of growth plate injury using IGF-I-loaded PLGA scaffolds. J. Regen. Med. Tissue Eng. 2015;(12):9. doi: 10.1002/term.1670. p. E202-E209. [DOI] [PubMed] [Google Scholar]
- 122.Zhao S. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int. J. Nanomed. 2014;9:2373–2385. doi: 10.2147/IJN.S59536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ingavle G.C., Leach J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. B Rev. 2014;20(4):277–293. doi: 10.1089/ten.TEB.2013.0276. [DOI] [PubMed] [Google Scholar]
- 124.Jain E. Sustained release of multicomponent platelet-rich plasma proteins from hydrolytically degradable PEG hydrogels. J. Biomed. Mater. Res. 2017;105(12):3304–3314. doi: 10.1002/jbm.a.36187. [DOI] [PubMed] [Google Scholar]
- 125.Burdick J.A. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J. Contr. Release. 2002;83(1):53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 126.Alsberg E. Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003;82(11):903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 127.Drury J.L., Dennis R.G., Mooney D.J. The tensile properties of alginate hydrogels. Biomaterials. 2004;25(16):3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 128.Lowen J.M., Leach J.K. Functionally graded biomaterials for use as model systems and replacement tissues. Adv. Funct. Mater. 2020;30(44) doi: 10.1002/adfm.201909089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leach J.K., Whitehead J. Materials-directed differentiation of mesenchymal stem cells for tissue engineering and regeneration. ACS Biomater. Sci. Eng. 2018;4(4):1115–1127. doi: 10.1021/acsbiomaterials.6b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kong H.J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5(5):1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 131.Kolambkar Y.M. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Raimondo T.M. Combined delivery of VEGF and IGF-1 promotes functional innervation in mice and improves muscle transplantation in rabbits. Biomaterials. 2019;216:119246. doi: 10.1016/j.biomaterials.2019.119246. [DOI] [PubMed] [Google Scholar]
- 133.Foox M., Zilberman M. Drug delivery from gelatin-based systems. Expet Opin. Drug Deliv. 2015;12(9):1547–1563. doi: 10.1517/17425247.2015.1037272. [DOI] [PubMed] [Google Scholar]
- 134.Kudva A.K. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019;90:287–299. doi: 10.1016/j.actbio.2019.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ju Y.M. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater. 2014;10(10):4332–4339. doi: 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 136.Takezawa T. Collagen Vitrigel:a novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004;13(4):463–474. doi: 10.3727/000000004783983882. [DOI] [PubMed] [Google Scholar]
- 137.Maruki H. Effects of a cell-free method using collagen vitrigel incorporating TGF-beta1 on articular cartilage repair in a rabbit osteochondral defect model. J Biomed Mater Res B Appl Biomater. 2017;105(8):2592–2602. doi: 10.1002/jbm.b.33792. [DOI] [PubMed] [Google Scholar]
- 138.Reyes R. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFbeta1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med. 2014;8(7):521–533. doi: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- 139.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 140.Arora A., Mahajan A., Katti D.S. TGF-beta1 presenting enzymatically cross-linked injectable hydrogels for improved chondrogenesis. Colloids Surf B Biointerfaces. 2017;159:838–848. doi: 10.1016/j.colsurfb.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 141.Lewis J.A. Transforming growth factor β-1 binding by peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 2020;6(8):4551–4560. doi: 10.1021/acsbiomaterials.0c00679. [DOI] [PubMed] [Google Scholar]
- 142.Geiger B.C. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci. Transl. Med. 2018;(469):10. doi: 10.1126/scitranslmed.aat8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heyes C.D. Biocompatible surfaces for specific tethering of individual protein molecules. J. Phys. Chem. B. 2004;108(35):13387–13394. [Google Scholar]
- 144.Udomluck N. Surface functionalization of dual growth factor on hydroxyapatite-coated nanofibers for bone tissue engineering. Appl. Surf. Sci. 2020:520. [Google Scholar]
- 145.Lee H. Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int J Biol Macromol. 2016;93(Pt B):1559–1566. doi: 10.1016/j.ijbiomac.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 146.Mittermayr R. Controlled release of fibrin matrix-conjugated platelet derived growth factor improves ischemic tissue regeneration by functional angiogenesis. Acta Biomater. 2016;29:11–20. doi: 10.1016/j.actbio.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 147.Paluck S.J. A heparin-mimicking block copolymer both stabilizes and Increases the activity of fibroblast growth factor 2 (FGF2). Biomacromolecules. 2016;17(10):3386–3395. doi: 10.1021/acs.biomac.6b01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang F. Comparison of the interactions of different growth factors and glycosaminoglycans. Molecules. 2019;(18):24. doi: 10.3390/molecules24183360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Poel W.E. Preparation of acellular homogenates from muscle samples. Science. 1948;108(2806):390–391. doi: 10.1126/science.108.2806.390-a. [DOI] [PubMed] [Google Scholar]
- 150.Voytik-Harbin S.L. Small intestinal submucosa: a tissue-derived extracellular matrix that promotes tissue-specific growth and differentiation of cells in vitro. Tissue Engineering. 1998;4(2):157–174. [Google Scholar]
- 151.Hoganson D.M. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials. 2010;31(27):6934–6940. doi: 10.1016/j.biomaterials.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 152.Seif-Naraghi S.B. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8(10):3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Blache U., Stevens M.M., Gentleman E. Harnessing the secreted extracellular matrix to engineer tissues. Nat Biomed Eng. 2020;4(4):357–363. doi: 10.1038/s41551-019-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Decaris M.L., Leach J.K. Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann. Biomed. Eng. 2011;39(4):1174–1185. doi: 10.1007/s10439-010-0217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hoch A.I. Cell-secreted matrices perpetuate the bone-forming phenotype of differentiated mesenchymal stem cells. Biomaterials. 2016;74:178–187. doi: 10.1016/j.biomaterials.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marston W.A. The efficacy and safety of dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26(6):1701–1705. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 157.Marston W.A. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Exp Rev Med Devices. 2004;1(1):21–31. doi: 10.1586/17434440.1.1.21. [DOI] [PubMed] [Google Scholar]
- 158.Kim I.G. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials. 2016;85:18–29. doi: 10.1016/j.biomaterials.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 159.Yang S.S. Extracellular matrix (ECM) multilayer membrane as a sustained releasing growth factor delivery system for rhTGF-beta3 in articular cartilage repair. PLoS One. 2016;(6):11. doi: 10.1371/journal.pone.0156292. p. e0156292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee K.I. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 2018;76:126–134. doi: 10.1016/j.actbio.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gilbert T.W., Sellaro T.L., Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 162.Bornstein P., Sage E.H. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14(5):608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 163.Kjellen L., Lindahl U. Proteoglycans: structures and interactions. Annu rev biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- 164.De Laporte L. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS One. 2013;(4):8. doi: 10.1371/journal.pone.0062076. p. e62076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park Y.J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J. Contr. Release. 2000;67(2–3):385–394. doi: 10.1016/s0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 166.Hintze V. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-beta1 (TGF-beta1) Acta Biomater. 2012;8(6):2144–2152. doi: 10.1016/j.actbio.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 167.Park J.S. Chondrogenic differentiation of mesenchymal stem cells embedded in a scaffold by long-term release of TGF-β3 complexed with chondroitin sulfate. J. Biomed. Mater. Res. 2010:806–816. doi: 10.1002/jbm.a.32388. 92A(2) [DOI] [PubMed] [Google Scholar]
- 168.Boskey A.L., Robey P.G., Marcus R. fourth ed. Academic Press; San Diego: 2013. Chapter 11-The Regulatory Role of Matrix Proteins in Mineralization of Bone, in Osteoporosis; pp. 235–255. [Google Scholar]
- 169.Ruehle M.A. Decorin-supplemented collagen hydrogels for the co-delivery of bone morphogenetic protein-2 and microvascular fragments to a composite bone-muscle injury model with impaired vascularization. Acta Biomater. 2019;93:210–221. doi: 10.1016/j.actbio.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scott R.A., Panitch A. Decorin mimic regulates platelet-derived growth factor and interferon-gamma stimulation of vascular smooth muscle cells. Biomacromolecules. 2014;15(6):2090–2103. doi: 10.1021/bm500224f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Paderi J.E., Panitch A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules. 2008;9(9):2562–2566. doi: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- 172.Hannink G. Evaluation of collagen/heparin coated TCP/HA granules for long-term delivery of BMP-2. J Mater Sci Mater Med. 2013;24(2):325–332. doi: 10.1007/s10856-012-4802-4. [DOI] [PubMed] [Google Scholar]
- 173.Sakiyama-Elbert S.E., Hubbell J.A. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J. Contr. Release. 2000;69(1):149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 174.Gorbunoff M.J., Timasheff S.N. The interaction of proteins with hydroxyapatite: III. Mechanism. 1984;136(2):440–445. doi: 10.1016/0003-2697(84)90241-0. Anal Biochem. [DOI] [PubMed] [Google Scholar]
- 175.Whitehead J., Kothambawala A., Leach J.K. Morphogen delivery by osteoconductive nanoparticles instructs stromal cell spheroid phenotype. Adv Biosyst. 2019:3. doi: 10.1002/adbi.201900141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Llopis-Hernandez V. Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Sci Adv. 2016:2. doi: 10.1126/sciadv.1600188. p. e1600188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grasman J.M. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials. 2015;72:49–60. doi: 10.1016/j.biomaterials.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Martino M.M. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885–888. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- 179.Mochizuki M. Growth factors with enhanced syndecan binding generate tonic signalling and promote tissue healing. Nat Biomed Eng. 2020;4(4):463–475. doi: 10.1038/s41551-019-0469-1. [DOI] [PubMed] [Google Scholar]
- 180.Xiao P. Light-induced release of molecules from polymers. Prog. Polym. Sci. 2017;74:1–33. [Google Scholar]
- 181.Chen M. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019;373:413–424. [Google Scholar]
- 182.Peredo A.P. Mechano-activated biomolecule release in regenerating load-bearing tissue microenvironments. Biomaterials. 2020:120255. doi: 10.1016/j.biomaterials.2020.120255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paiva K.B., Granjeiro J.M. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch. Biochem. Biophys. 2014;561:74–87. doi: 10.1016/j.abb.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 184.Braun A.C. Bioresponsive release of insulin-like growth factor-I from its PEGylated conjugate. J. Contr. Release. 2018;279:17–28. doi: 10.1016/j.jconrel.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 185.Anjum F. Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials. 2016;87:104–117. doi: 10.1016/j.biomaterials.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 186.Qi H. Systemic administration of enzyme-responsive growth factor nanocapsules for promoting bone repair. Biomater Sci. 2019;7(4):1675–1685. doi: 10.1039/c8bm01632a. [DOI] [PubMed] [Google Scholar]
- 187.Nielsen J.J., Low S.A. Bone-targeting systems to systemically deliver therapeutics to bone fractures for accelerated healing. Curr. Osteoporos. Rep. 2020;18(5):449–459. doi: 10.1007/s11914-020-00604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang Y. Fracture-targeted delivery of beta-catenin agonists via peptide-functionalized nanoparticles augments fracture healing. ACS Nano. 2017;11(9):9445–9458. doi: 10.1021/acsnano.7b05103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jarvinen T.A.H., Pemmari T. Systemically administered, target-specific, multi-functional therapeutic recombinant proteins in regenerative medicine. Nanomaterials. 2020;(2):10. doi: 10.3390/nano10020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kowalski P.S. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019;27(4):710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Polack F.P. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guan S., Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133–143. doi: 10.1038/gt.2017.5. [DOI] [PubMed] [Google Scholar]
- 193.Fontana G. Mineral-coated microparticles enhance mRNA-based transfection of human bone marrow cells. Mol. Ther. Nucleic Acids. 2019;18:455–464. doi: 10.1016/j.omtn.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Krebs M.D. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J Biomed Mater Res A. 2010;92(3):1131–1138. doi: 10.1002/jbm.a.32441. [DOI] [PubMed] [Google Scholar]