Abstract
Background
Elevated levels of circulating microparticles (MPs) and molecules of the complement system have been reported in patients with systemic lupus erythematosus (SLE). Moreover, microparticles isolated from patients with SLE (SLE-MPs) contain higher levels of damage-associated molecular patterns (DAMPs) than MPs from healthy controls (CMPs). We hypothesize that the uptake of MPs by monocytes could contribute to the chronic inflammatory processes observed in patients with SLE. Therefore, the aim of this study was to evaluate the expression of activation markers, production of proinflammatory mediators, and activation of the NF-κB signaling pathway in monocytes treated with CMPs and SLE-MPs.
Methodology
Monocytes isolated from healthy individuals were pretreated or not with pyrrolidine dithiocarbamate (PDTC) and cultured with CMPs and SLE-MPs. The cell surface expression of CD69 and HLA-DR were evaluated by flow cytometry; cytokine and eicosanoid levels were quantified in culture supernatants by Cytokine Bead Array and ELISA, respectively; and the NF-κB activation was evaluated by Western blot and epifluorescence microscopy.
Results
The cell surface expression of HLA-DR and CD69, and the supernatant levels of IL-6, IL-1β, PGE2, and LTB4 were higher in cultures of monocytes treated with SLE-MPs than CMPs. These responses were blocked in the presence of PDTC, a pharmacological inhibitor of the NF-κB pathway, with concomitant reduction of IκBα and cytoplasmic p65, and increased nuclear translocation of p65.
Conclusions
The present findings indicate that significant uptake of SLE-MPs by monocytes results in activation, production of inflammatory mediators, and triggering of the NF-κB signaling pathway.
Keywords: Autoimmunity, Inflammation, NF-κB pathway, Lupus
Autoimmunity, Inflammation, NF-κB pathway, Lupus
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder of unknown etiology with a broad spectrum of clinical manifestations, mainly characterized by the presence of multiple circulating antibodies against a variety of self-antigens [1]. Several studies have shown that an increased rate of apoptosis and diminished removal of apoptotic cells favor the development of autoimmunity [2, 3]. During apoptosis, cells release diverse subcellular vesicles, including apoptotic bodies and microparticles (MPs), which differ in size, origin, and composition [4, 5]. These vesicles act as modulators of the immune response and represent a primary source of autoantigens in patients with SLE [6].
Microparticles are a heterogeneous population of membrane vesicles (0.1–1 μm diameter) primarily derived from the plasma membrane of different cells during processes of cell activation and death. These vesicles contain different cellular components and play essential roles in the intercellular communication involved in processes, such as inflammation, coagulation, angiogenesis, and apoptosis [7, 8] Microparticles can act through different mechanisms, including the release of molecules into the extracellular environment, the triggering of enzymatic cascades by molecules exposed on the surface, direct interactions with target cells, and the transfer of intracellular molecules and surface receptors to acceptor cells by membrane fusion or internalization [8]. Also, MPs can bind to circulating antibodies to form immune complexes, which, in turn, interact with target cells through complement and Fc receptors [9].
High levels of circulating MPs have been reported in patients with SLE [10, 11, 12]; moreover, these MPs have a higher content of immunoglobulins, complement components, and damage-associated molecular patterns (DAMPs), in comparison with MPs from healthy controls [13, 14, 15]. Thus, the recognition of these extracellular vesicles by cells of the immune system could contribute to the chronic inflammatory processes observed in the course of SLE. However, this hypothesis has not been extensively studied, and many proinflammatory mechanisms induced by MPs in patients with SLE remain to be elucidated.
Monocytes are considered critical players in the development of SLE. Patients with SLE have monocytes with different phenotypic and functional alterations [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26] that produce inflammatory mediators and perpetuate the autoimmune response through antigenic presentation and co-stimulation of self-reactive T- and B-cells. When compared to monocytes from healthy controls, monocytes from patients with SLE overexpress different molecules: Fc gamma receptor I (FcγRI, CD64) [16-18], adhesion, and costimulatory molecules such as CD40L [19], and the Intercellular Adhesion Molecule-1 (ICAM-1, CD154) [18]. These molecules could reflect the activation state of monocytes, and suggest they might be more efficient antigen-presenting cells in patients with SLE. Additionally, in in vitro experiments, monocytes from patients with SLE overproduce inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) [21, 22], interleukin 6 (IL-6) [22,23], IL-1β [22], and BAFF [26]. Moreover, the accumulation of CD16+ monocytes in renal tissues correlate with impaired renal function in lupus nephritis [27, 28]. Monocytes could also play a key role in vesicle removal as expected from their potent phagocytic activity that allows them to clear circulating apoptotic bodies, microorganisms, and immune complexes.
The NF-κB (nuclear factor kappa-light-chain-enhancer of activated B-cells) proteins comprise a family of transcription factors that regulate the expression of different genes, including those coding for pro-inflammatory cytokines, chemokines, and adhesion molecules [29]. This family of transcription factors play an essential role in the survival of lymphocytes, activation of innate immune cells, and therefore, the initiation of a normal immune response. The canonical NF-κB pathway involves various steps, including the phosphorylation, ubiquitination, and degradation of the NF-κB Inhibitor alpha (IκBα) that leads to the nuclear translocation of the NF-κB p50 and p65 subunits. Constitutive activation of the NF-κB pathway is frequently associated with inflammatory diseases, such as rheumatoid arthritis and SLE [30]; also, in response to TNF-α, the monocytes of patients with SLE show greater NF-κB nuclear translocation and lower p-IκBα levels than monocytes from healthy individuals [31]. The variety of molecules contained in MPs of patients with SLE could contribute to greater NF-κB activation in cells interacting with the vesicles.
Considering the role of monocytes in the development of SLE and their ability to clear dead cells components [32], as well as the constitutive activation of the NF-κB pathway observed in inflammatory diseases [30] and the higher nuclear translocation of NF-κB in TNF-α treated-monocytes from patients with SLE [31], this study aimed to evaluate the activation, production of inflammatory mediators, and the triggering of the NF-κB signaling pathway in monocytes exposed to microparticles isolated from the plasma of patients with SLE.
2. Materials and methods
2.1. Patients and controls
Thirty patients diagnosed with SLE, according to the American College of Rheumatology criteria [33, 34], were enrolled in the present study. They were evaluated by the Grupo de Reumatología (Rheumatology Group) de la Universidad de Antioquia (GRUA, by its Spanish acronym) at Hospital Universitario San Vicente Fundación (HUSVF, by its Spanish acronym), in Medellín, Colombia. Also, 15 gender-matched healthy individuals who were similar to patients in age participated in the study as healthy controls (HCs). Additionally, blood samples were drawn from 10 healthy individuals (age range = 24–31 years) for isolating peripheral mononuclear cells (PBMCs) and monocytes. All HCs declared not to be taking any medication or having any active infectious disease. All individuals agreed to participate in the study voluntarily by signing an informed consent approved by the Ethics Committee of the Institute of Medical Research in the Faculty of Medicine at Universidad de Antioquia.
2.2. Isolation and characterization of microparticles
Microparticles from patients with SLE (SLE-MPs) and controls (CMPs) were isolated from 4 mL of citrated venous blood (BD-Vacutainer® 3.8% sodium citrate tubes; Becton Dickinson, San Jose, CA) as previously described [35]. Briefly, the blood sample was centrifuged at 1,800 x g for 10 min at 21 °C to obtain total plasma. This plasma was then centrifuged twice at 3,000 x g for 20 min at 21 °C to remove the platelets and obtain platelet-poor plasma (PPP). Finally, 1 mL of PPP was centrifuged at 16,900 x g for 1 h to allow the sedimentation of MPs. These vesicles were suspended in filtered Dulbecco's Phosphate-buffered saline (1X DPBS; Gibco, Grand Island, NY), taking care not to disturb them, and kept at −70 °C until use.
The isolated vesicles were confirmed to be MPs (0.1 μm–1 μm in diameter) by flow cytometry analysis using reference latex microspheres with diameters of 0.1 μm, 0.5 μm, 1.0 μm, and 2.0 μm (Polysciences, Inc. Warrington, PA). The acquisition threshold for size (FSC-A) and granularity (SSC-A) parameters were set with filtered sheath fluid. Samples were acquired on a FACSCanto™ II flow cytometer (Becton Dickinson) with FACSDiva™ Software version 6.0 (Becton Dickinson).
The protein content of the MPs was quantified by the bicinchoninic acid (BCA) method (Thermo Scientific, Waltham, MA) following the manufacturer's instructions. The absorbance of samples was read at 562 nm using a Universal Microplate Reader BiotekELx800NB spectrophotometer (Biotek Instruments. Winooski, VT). Protein concentrations were calculated using a standard curve made with known concentrations of bovine serum albumin (BSA).
2.3. Isolation of peripheral blood mononuclear cells
PBMCs were isolated from EDTA-anticoagulated venous blood (BD-Vacutainer® tubes; Becton Dickinson) after centrifugation at 900 x g for 30 min at 21 °C on a density gradient (1.077 g/mL) of Histopaque (Sigma Aldrich, St. Louis, MO). The cells were washed twice with PBS at 300 x g for 10 min and suspended in RPMI (Gibco, Grand Island, NY) supplemented with penicillin (100 U/mL)/streptomycin (100 μg/mL) (Cambrex-Bioscience, Walkersville, MD), and 10% inactivated fetal bovine serum (iFBS). Cell viability was evaluated by trypan blue (Sigma Aldrich, St. Louis, MO) dye exclusion in Neubauer chamber: percentages ≥95% were observed.
2.4. Labeling of microparticles
Microparticles (1000 μL) obtained from blood samples of HCs were mixed with 1 μM carboxyfluorescein succinimidyl ester (CFSE; Thermo scientific, Waltham, MA) and incubated at 37 °C for 10 min; then 100 μL of iFBS were added, and vesicles were incubated at room temperature for 5 additional minutes. Finally, the vesicles were washed twice with RPMI-1640 supplemented with 10% iFBS at 16,900 x g for 1 h. The supernatant was removed, the pellet was suspended in 1 mL filtered PBS and the CFSE-labeled CMPs were acquired on a FACSCanto™ II flow cytometer.
2.5. Binding and uptake of microparticles
PBMCs (1 × 105) from healthy controls were cultured for 1 h with or without CFSE-labeled CMPs (30, 50, and 100 μg/mL of protein). The cells were then stained with anti-CD14-MY4-RD1 (clone 322A-1; Beckman Coulter, Brea, CA), anti-CD3-V510 (clone OKT3; Biolegend, San Diego, CA), and anti-CD19-V450 (clone HIB19; BD Pharmingen, San Diego, CA) mAbs, incubated in darkness at room temperature for 20 min, washed with PBS, and acquired on the flow cytometer. An initial acquisition was run in the FACSCanto™ II flow cytometer to evaluate the percentage of binding/uptake of MPs by the PBMCs. Subsequently, 0.01% v/v trypan blue was added to the acquisition tubes to quench the fluorescence of the MPs bound to cell surfaces, and a second acquisition was run on the cytometer to evaluate the percentage of uptake of MPs by the cells. The percentages of both, binding/uptake and uptake of MPs by different leukocyte populations were determined with the Overton subtraction algorithm (FlowJo™ Software version X; Ashland, OR: Dickinson and Company 2019).
2.6. Enrichment of monocytes and effect of microparticles on the expression of cell surface markers
Monocytes were enriched from the peripheral blood samples of healthy individuals by negative selection using the RosetteSep™ Human Monocyte Enrichment Cocktail (StemCell Technologies, Vancouver, Canada) according to the manufacturer's instructions. The purity of monocytes was ≥90%. Approximately 1 × 105 monocytes were pretreated with different doses (0, 1, 5, and 25 μM) of pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB activation [36, 37]. Monocytes were then stimulated for 6 h, under adherent conditions, with CMPs or 5 μg/mL Lipopolysaccharide (LPS from Escherichia coli, serotype O111:B4; InvivoGen, San Diego, CA) (positive control). All cultures were performed at 37 °C and 5% CO2 in 96-well flat-bottom plates in a final volume of 200 μL of RPMI-1640 supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% iFBS previously filtered through a 0.20 μm-Acrodiscs® syringe filter. After incubation, the cell surface expression of activation markers (HLA-DR and CD69) was evaluated by flow cytometry.
The viability of PDTC-treated cells was assessed with propidium iodide (PI) and DIOC6 staining. Additionally, to rule out any effect of 25 μM PDTC on the cellular ability to bind and uptake MPs, PBMCs treated or not with PDTC were incubated with CFSE-labeled CMPs for 1 h to evaluate the percentages of binding and uptake of MPs by cells.
2.7. Effect of microparticles on the production of cytokines and eicosanoids by monocytes
Monocytes (1 × 105) isolated from healthy individuals and pretreated with or without 25 μM PDTC for 1 h were stimulated with 30 μg/mL vesicles (SLE-MPs or CMPs). Culture supernatants were collected after 6 and 24 h incubation and stored at 20 °C to evaluate the concentrations of cytokines and eicosanoids.
The concentration of TNF-α, IL-1β, and IL-6 was evaluated in 6 h-supernatants by CBA (Human Inflammatory Cytokine kit; BD Biosciences, San Diego, CA) following the manufacturer's instructions. Additionally, the levels of PGE2 (6-hour supernatants) and BAFF and LTB4 (24-hour supernatants) were quantified by ELISA following the manufacturers' instructions (PGE2 and LTB4 kits; Abcam, Cambridge, United Kingdom. BAFF kit; R&D Systems, Minneapolis, MN). The PDTC-mediated inhibition of the production of these inflammatory mediators was expressed in terms of percentage of inhibition in relation to the PDTC-unexposed control.
2.8. Effect of microparticles on the activation of the NF-κB signaling pathway
PBMCs (2 × 106) from healthy individuals pretreated or not with PDTC for 1 h were cultured with 30 μg/mL vesicles (CMPs or SLE-MPs) or 5 μg/mL LPS for 30 min. Then, cells were collected and washed with cold PBS for preparing cell lysates, as described below, to evaluate the levels of p65 and IκBα.
2.8.1. Nuclear and cytoplasmic fractionation
Microparticles-treated PBMCs (2 × 106) were lysate to obtain cytoplasmic and nuclear fractions as described elsewhere [38]. Briefly, cells were centrifuged at 1,000 x g for 5 min at 4 °C, and the cell pellet was suspended in 200 μL of buffer A (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5% (v/v) IGEPAL, 0.5 mM DTT, and protease inhibitor), incubated at 4 °C for 20 min, and centrifuged at 6,000 x g for 10 min at 4 °C to collect the supernatant (cytoplasmic fraction). The cell pellet was then washed twice with buffer A, suspended in 200 μL of buffer C (20 mM HEPES pH 7.9, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% (v/v) glycerol, 0.5 mM DTT, 0.5 mM PMSF, and protease inhibitor), incubated at 4 °C for 30 min, and centrifuged at 14,500 x g for 10 min at 4 °C to collect the supernatant (nuclear fraction). The cytoplasmic and nuclear lysates were stored at -70 °C. Immediately before use, 20 μL of buffer D (20 mM HEPES pH 7.9, 0.2 mM EDTA, 20% (v/v) glycerol, 0.5 mM DTT, 0.5 mM PMSF, and protease inhibitor) were added to cell lysates and their protein content were quantified by the BCA method.
2.8.2. Total cell lysate
Microparticles-treated PBMCs (2 × 106) were suspended in 100 μL of lysis buffer (25 mM Tris-HCl, 120 Mm NaCl, 0.5% IGEPAL, 50 mM NaF, 1 mM Na3VO4, and protease inhibitor), incubated at 4 °C for 20 min, and centrifuged at 14,500 x g for 10 min at 4 °C. The cell lysate supernatant was collected and stored at -70 °C until use. Before use, the protein content of the total cell lysates were quantified by the BCA method.
2.8.3. Protein electrophoresis and western blotting
Proteins in cell lysates were separated by electrophoresis (7 μg protein per lane) in 15% polyacrylamide gel with sodium dodecyl sulfate (SDS-PAGE) under reducing conditions at 140 V for 1 h. A wet protein transfer onto polyvinylidene fluoride (PVDF) membranes was performed at 40 V, 4 °C, for one hour using a Mini Trans-Blot®Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA). After transfer, membranes were covered with blocking buffer (5% skim milk, 0.05% Tween-20 in 1X PBS) for 1 h at room temperature. The membranes were then incubated at 4 °C overnight with mouse anti-human p65, IκBα, and β-actin antibodies (IgG1 isotypes, Santa Cruz Biotechnology, Dallas, TX) diluted 1:200, 1:1000, and 1:8000 in blocking buffer, respectively. After, the membranes were incubated with RDye®800CW-conjugated goat anti-mouse IgG polyclonal antibody (1:7000 in blocking buffer, LI-COR Biosciences, Lincoln, NE) at room temperature for 1 h. Finally, fluorescencent bands were evaluated using the Li-Cor Odyssey®9120 Infrared Imaging System with the ODYSSEY® application software (LI-COR Biosciences; Lincoln, NE). The relative protein band expression was calculated after densitometry analysis and normalization to the β-actin signal that was used as a loading control.
2.9. Epifluorescence microscopy
Monocytes (300 × 105) isolated from peripheral blood of healthy individuals were cultured on 12 mm glass coverslips (Marienfeld, Lauda-Königshofen, Germany), previously conditioned with a poly-L-lysine solution (Sigma Aldrich) for 2 h at 37 °C. These cells were then treated for 30 min with 30 μg/mL of vesicles (CMPs or SLE-MPs) or 5 μg/mL LPS (positive control), fixed with 4% paraformaldehyde for 20 min at 37 °C, incubated with 50 mM NH4Cl for 10 min, permeated with 0.25% Triton X-100 for 5 min, and blocked with 2% FBS for 1 h at room temperature. After, the cells were incubated for 1 h at room temperature with a primary anti-p65 mAb (isotype IgG1, 1:150; Santa Cruz biotechnology, Dallas, TX), washed with PBS, and then incubated for 1 h with a secondary goat anti-mouse IgG (1:2500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) polyclonal antibody, Alexa fluor 488™ Phalloidin (1:1000; Thermo Scientific), and Hoechst 33342 (1:5000; Invitrogen, Carlsbad, CA). Finally, the cells were preserved on the glass coverslips with FluorSave™ solution (Calbiochem, San Diego, CA). Slides were analyzed with an inverted epifluorescence microscope (IX 81 Olympus, Tokyo, Japan) using Image-Pro Plus 2D Image Analysis software. At least six different fields were evaluated for each experimental condition. The images were analyzed using ImageJ software (WS Rasband, National Institute of Health. Bethesda, MD, USA, http://imagej.nih.gov/ij). Cell localization of p65 was analyzed by merging nucleus/p65 and cytoplasmic/p65 images and calculating the Pearson's correlation coefficients.
2.10. Statistical analysis
The expression per cell of HLA-DR and CD69 induced by CMPs and SLE-MPs in monocytes was compared with the Kruskal-Wallis test. The expression of the surface activation markers, the levels of cytokines and eicosanoids, the IκBα degradation, and the p65 cytoplasmic in monocytes pretreated or not with PDTC and stimulated or not with MPs, were compared using the two-way Analysis of Variance (ANOVA) with Bonferroni post hoc test. In the case of microscopy assays, the cell localization of p65 was assessed with Pearson's correlation coefficient. Then, the correlation coefficients derived from different treatments were compared with one-way ANOVA, with Bonferroni post hoc test for contrasting means. We use ranks including the minimal and maximal value as the criterion to show the variability to make evident the most relevant and real differences and to avoid bordering differences. A residual normality test was performed to validate the statistical model. In all cases, a p-value ≤ 0.05 was considered statistically significant. The statistical analysis was run in GraphPad Prism version 6 (GraphPad Software; La Jolla, CA).
3. Results
3.1. Microparticles are mainly ingested by monocytes
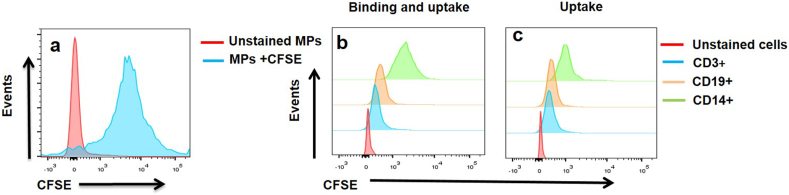
Because different types of cells can interact with vesicles, through several mechanisms including receptor-mediated interactions and even membrane fusion, the ability to bind and uptake microparticles was compared between monocytes, T- and B-cells. To this purpose, PBMCs from 10 healthy individuals were incubated for 1 h with CFSE-labeled microparticles (CMPs) at different concentrations (30, 50, and 100 μg/mL protein). The size and characteristics of these MPs have been previously reported by our group [35]. The CFSE labeling of the MPs used in the present study was highly efficient and reached 86.3 ± 2.6% (Figure 1a). The comparative analysis of flow cytometry histograms showed that MPs were mainly bound and ingested by monocytes, followed by B-cells, and to a lesser degree, by T-cells (Figures 1b and 1c).
Figure 1.
Microparticles from healthy controls are mainly bound and ingested by monocytes. Representative histogram of CMPs (MPs obtained from blood cells of healthy controls) stained with CFSE (a). Representative histograms of binding and uptake of CMPs (100 μg/mL) by CD14+, CD19+, and CD3+ cells from a patient with SLE (b). Representative histograms of uptake of CMPs (100 μg/mL) by CD14+, CD19+, and CD3+ cells from a patient with SLE. Similar results were observed in additional experiments (n = 10) and with different concentrations of MPs.
3.2. SLE-MPs induce increased expression of HLA-DR and CD69 in monocytes through a mechanism inhibited by PDTC
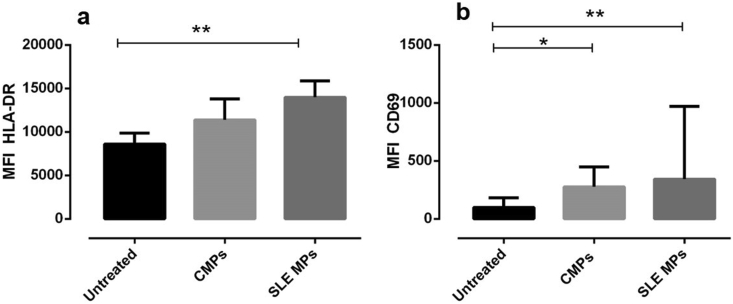
The highest capacity of monocytes to bind and uptake MPs could induce cellular activation; therefore, the expression of cell activation antigens (HLA-DR and CD69) was evaluated in monocytes of healthy individuals exposed to CMPs and SLE-MPs. The expression (Mean Fluorescence Intensity/MFI) of both markers increased in monocytes stimulated with SLE-MPs (p ≤ 0.01) in comparison with unstimulated monocytes (Figures 2a and b). Similarly, the CD69 MIF also increased in monocytes exposed to CMPs (p ≤ 0.05) in comparison with unstimulated monocytes (Figure 2b); CMPs also induced an increase in the expression of HLA-DR, but it was not statistically significant (Figure 2a).
Figure 2.
Microparticles from patients with SLE increase HLA-DR and CD69 expression on monocytes. MFI of HLA-DR (a) and CD69 (b) on monocytes treated with or without 30 μg/mL of CMPs (MPs from controls) and SLE MPs (MPs from patients with SLE). Comparisons among groups were performed using the Kruskal–Wallis test; ∗p ≤ 0.05; ∗∗p ≤ 0.01. Results are shown as mean ± range, n = 7. The asterisks indicate statistically significant differences.
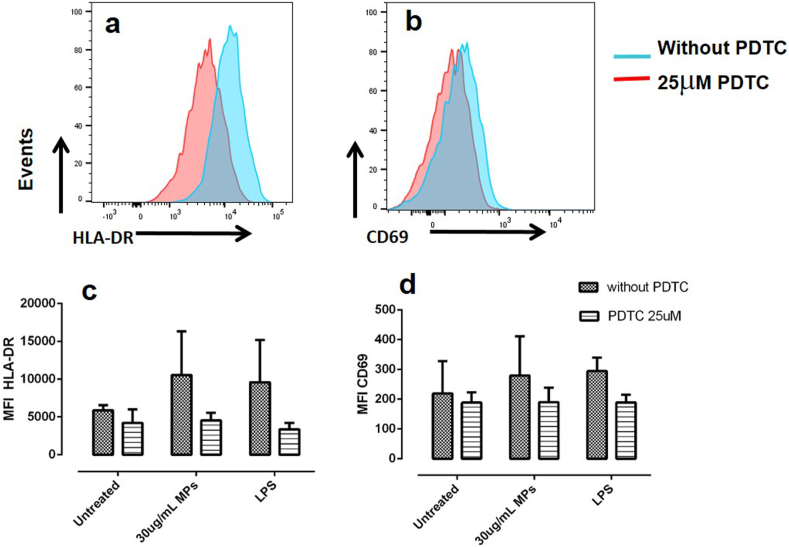
Then, to establish whether the MP-induced activation of monocytes depended on the activation of the NF-κB pathway, monocytes were pretreated with different concentrations of PDTC (1, 5, and 25 μM). This molecule inhibited the MP-induced increase of HLA-DR and CD69 in monocytes in a dose-dependent manner (data not shown), suggesting that at least partially, the activation of NF-κB pathway was involved. As PDTC (25 μM) induced the highest percentages of inhibition for HLA-DR and CD69 (55.8% and 32.0%, respectively) (Figures 3a, 3b, and 3c), it was the concentration of the inhibitor used in the subsequent experiments.
Figure 3.
PDTC antagonizes the expression of HLA-DR and CD69 induced by MPs on monocytes. Representative histograms of HLA-DR and CD69 expression in monocytes stimulated with MPs in the presence (red histogram) and absence of PDTC (blue histogram) (a). MFI of HLA-DR (b) and CD69 (c) on monocytes treated with or without CMPs (MPs from controls) in the presence and absence of PDTC. Results are shown as media ± range. Data were compared using two-way ANOVA; n = 3. The asterisks indicate statistically significant differences.
An additional experiment was carried out to discard any deleterious effect of PDTC on cell viability. To this purpose, monocytes were treated with PDTC for 6 h; then, cells were stained with DIOC6 and PI to evaluate the mitochondrial membrane potential and the plasma membrane integrity, respectively. The PDTC did not significantly affect any of those parameters (data not shown). Additionally, the inhibitor did not affect the cellular ability to bind and uptake the microparticles.
3.3. SLE-MPs induce increased levels of cytokines and eicosanoids through a mechanism inhibited by PDTC
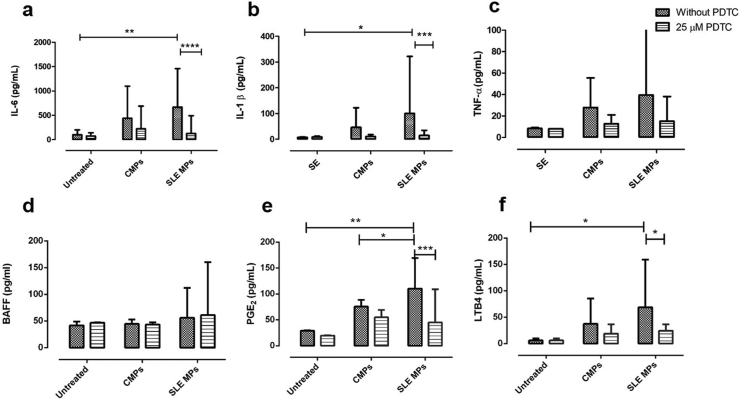
Monocytes from healthy individuals were pretreated or not with PDTC and cultured in the presence of CMPs or SLE-MPs; as positive controls, PDTC-treated monocytes were exposed to IFN-γ or LPS. The levels of cytokines and eicosanoids were measured in culture supernatants. Compared to unstimulated monocytes, cultures treated with SLE-MPs showed a significant increase of IL-6 (p ≤ 0.01) (Figure 4a), IL-1β (p ≤ 0.05) (Figure 4b), PGE2 (p ≤ 0.01) (Figure 4e), and LTB4 (p ≤ 0.05) (Figure 4f), while CMPs induced non-significant increases of those inflammatory mediators. The exposure of monocytes to SLE-MPs and CMPs caused a non-significant increase in the TNF-α levels (Figure 4c) and did not increase the levels of BAFF (Figure 4d).
Figure 4.
SLE-MPs increase the levels of IL-6, IL-1β, PGE2, and LTB4 in cultures of monocytes, and PDTC inhibits this effect. Levels of IL-6, IL-1β, TNF-α, and BAFF (a–d) and eicosanoids PGE2 (e) and LTB4 (f) in culture supernatants of PDTC-treated monocytes exposed to CMPs (MPs from controls) and SLE-MPs (MPs from patients with SLE). Results are shown as media ± range. Data were compared using two-way ANOVA ∗p ≤ 0.05, ∗p ≤ 0.01, ∗∗∗p ≤ 0.00; n = 10. The asterisks indicate statistically significant differences.
The cultures of PDTC-treated monocytes exposed to SLE-MPs showed decreased concentrations (percentage respect to the PDTC-unexposed monocytes) of IL-6 (86,5 %; p ≤ 0.001), IL-1β (77.3%; p ≤ 0.001), PGE2 (57.4%; p ≤ 0.001), and LTB4 (50.7%; p ≤ 0.05) (Figures 4a, 4b, 4e, and 4f). PDTC induced a non-significant reduction in the levels of IL-6 and IL-1β in cultures with CMPs (Figures 4a and 4b).
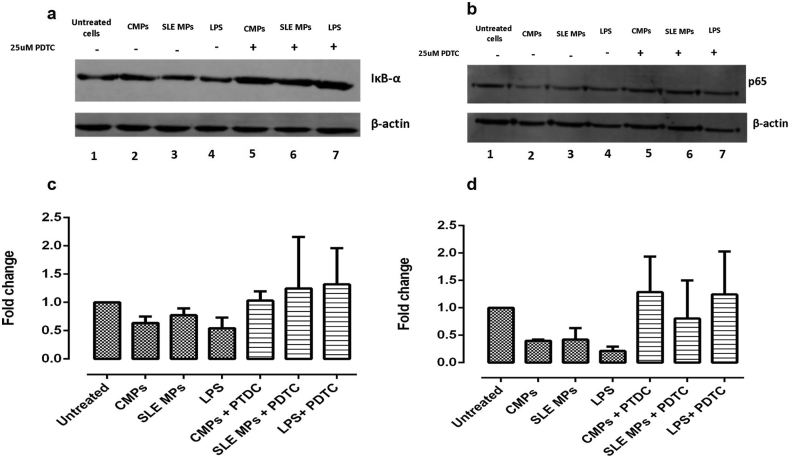
3.4. Microparticles induced decreased levels of IκBα and cytoplasmic p65 in PBMCs
As PDTC inhibited the proinflammatory effects of SLE-MPs on monocytes, the effect of microparticles on the activation of the NF-κB pathway was evaluated. PBMCs from healthy individuals were treated with or without PDTC for 1 h and stimulated with CMPs, SLE-MPs, or LPS (positive control) for 30 min. Supplementary Figure in comparison with unstimulated cells, the total lysate from PBMCs treated with CMPs an SLE-MPs showed reduced levels of the IκBα protein (Figure 5a: lanes 2 and 3), although there was no significant difference between exposure to SLE-MPs and CMPs. The pretreatment of cells with PDTC inhibited the degradation of IκBα induced by MPs (Figure 5a: lanes 5, 6, and 7). The consolidated densitometry analysis for the IκBα levels, expressed as the fold change with respect to unstimulated cells, is shown in Figure 5c.
Figure 5.
PBMCs treated with SLE-MPs and CMPs show reduced levels of IκBα and cytoplasmic p65, and PDTC inhibits this reduction. Representative immunoblots are shown for IκBα (a) and p65 (b). Densitometry analysis of the levels of the IκBα (c) and the cytoplasmic p65 protein (d) expressed as the fold change with respect to unstimulated cells. β-actin protein immunoblot was performed to ensure equal sample loading. Results are shown as media ± range. Data were compared using two-way ANOVA; n = 5.
On the other hand, in comparison with untreated cells, PBMCs cultured with CMPs and SLE-MPs showed a reduced cytoplasmic level of p65 (Figure 5b: lanes 2 and 3); moreover, this change was prevented in the presence of PDTC (Figure 5b: lanes 5 and 6). There were no significant differences between treatments with CMPs and SLE-MPs. The consolidated densitometry analysis for the cytoplasmic p65 protein, expressed in terms of fold change with respect to unstimulated cells, is shown in Figure 5d.
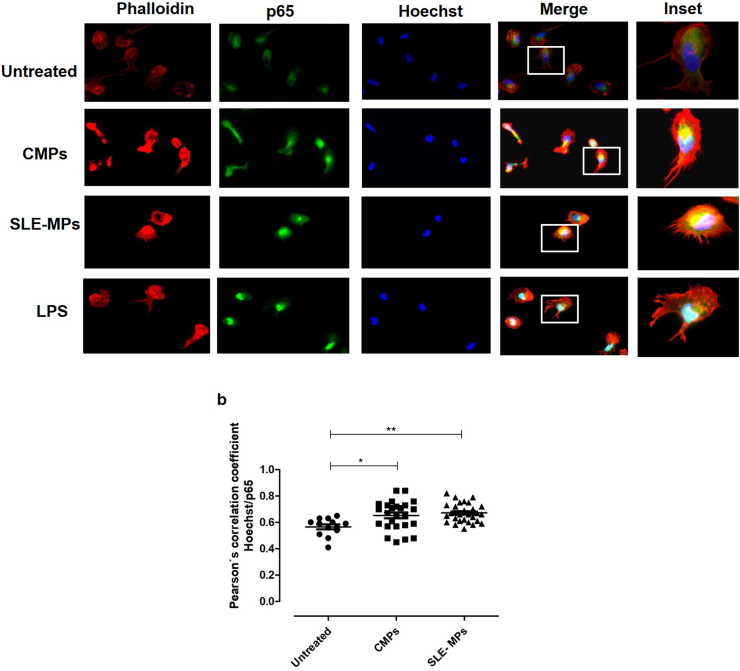
3.5. Microparticles induced the nuclear translocation of p65 in monocytes
After, to confirm the findings observed on western blotting, monocytes from peripheral blood of healthy individuals were treated with CMPs, SLE-MPs, or LPS (positive control) to evaluate the localization of p65 in the nucleus and cytoplasm by epifluorescence microscopy (Figure 6a). The analysis of individual and merged fluorescent images of the nucleus, cytoskeleton and p65 showed that p65 was located mainly in the cytoplasm of unstimulated monocytes while it increased in the nucleus after treatment with CMPs (p < 0.05), SLE-MPs (p < 0.01), and LPS (p < 0.001) (Figure 6b). There was no significant difference in the nuclear translocation of p65 between treatments with CMPs and SLE-MPs.
Figure 6.
Microparticles induce the nuclear translocation of p65 in monocytes. The nuclear translocation of the NF-κB subunit p65 was analyzed through epifluorescence microscopy. Individual images from the nucleus (blue), phalloidin (red), and p65 (green), and image overlapping (merge) (a). Colocalization of Green and blue fluorescent signals was evaluated in all cells found in six random fields using the Pearson's correlation coefficient (b); n = 5. The asterisks indicate statistically significant differences.
4. Discussion
This study showed that binding or uptake of SLE-MPs by monocytes induced cell activation as evidenced by the increased expression of the surface activation markers, HLA-DR and CD69, and the higher production of inflammatory mediators (IL-6, IL-1β, PGE2, and LTB4). Additionally, the treatment of monocytes with MPs induced the nuclear translocation of p65, suggesting the activation of the NF-κB signaling pathway. Therefore, we proposed that in patients with SLE, the binding or uptake of MPs by monocytes could trigger signaling pathways that drive the chronic inflammatory process underlying the pathogenesis of the disease. In the present study, MPs were mainly bound and internalized by monocytes, which could be explained by their potent phagocytic activity and critical role in the removal of material released from dying cells [28]. The inefficient uptake of MPs by T-cells suggests the process is mainly mediated by endocytic mechanisms and not merely by membrane fusion.
Previous studies have shown that MPs have several effects on the immune response; for example, MPs induce increased surface expression of CD80, CD86, CD83, and HLA-DR in plasmacytoid dendritic cells (pDCs) [39]; and augment the synthesis of inflammatory mediators, such as cytokines (IL-6, IL-1β, IL-8, TNF-α) and eicosanoids (PGE2, prostacyclin/PGI2, thromboxane B2/TXB2) by different types of cells [40, 41, 42, 43, 44]. Additionally, circulating MPs isolated from patients with SLE have known inflammatory effects: they induce the production of IFN-α, IFN-γ, and TNF-α in pDCs; and IL-6 and TNF-α in myeloid dendritic cells [45]. That is to say, the uptake of MPs by monocytes could alter their cell phenotype and function, as previously described in patients with SLE.
The uptake of CMPs by monocytes increased the CD69 expression while the uptake of SLE-MPs induced an increase even higher of CD69; and moreover, of HLA-DR. The higher expression of those molecules reflect the activated state of monocytes and point their higher cell ability for antigen presentation, an event that could be critical in autoimmunity.
Also, SLE-MPs increased the levels of IL-6, IL-1β, PGE2, and LTB4 (Figure 4), inflammatory mediators that play an important role in the pathogenesis of SLE. IL-6 is found in high concentration in the plasma of patients with SLE in whom promotes the production of IgG and the differentiation of plasma cells to antibody-secreting cells [46, 47, 48]. Similarly, elevated levels of IL-1β have been reported in the serum and urine of patients with SLE [49]. Additionally, PGE2 modulates many immune processes at inflamed tissues, including edema, fever, and the production of inflammatory cytokines [50].
Chae and colleagues demonstrated that PGE2 induces a higher synthesis of IL-6, IL-10, IFN-γ, and NO in the murine model of pristane-induced lupus [51]. Moreover, the treatment of human monocytes with LTB4 raised the production of IL-1 and IL-6 [52,53]. Curiously, various cytokines promote the production of proinflammatory eicosanoids, e.g., IL-1 and TNF-α induce the synthesis of PGE2 [54]; meaning that the production of proinflammatory eicosanoids and cytokines is interrelated. Therefore, it is possible to suggest that PGE2 and LTB4, induced by MPs, might contribute to the deregulated production of IL-6 and IL-1β in patients with SLE; and in turn, cytokines would induce the synthesis of eicosanoids, contributing this way to the amplification of the inflammatory response in patients with SLE. Furthermore, the pretreatment of macrophages with PGE2 induces a reduced removal of apoptotic cells [54], pointing that the high levels of PGE2 induced by SLE-MPs could also contribute to the decreased ability of monocytes to remove microvesicles and apoptotic cells. The persistence of MPs in the extracellular space exposes the immune system to their self-antigen content and also favor the appearance of neoantigens as a result of post-translational modifications [55]. PDTC is a potent NF-κB inhibitor that blocks the IκBα release from NF-κB in the cytoplasm. The pretreatment of monocytes with PDTC inhibited the effect of microparticles, as evidenced by the lower levels of IL-6, IL-1β, PGE2, and LTB4, and suggested the NF-κB pathway was involved in the production of those inflammatory mediators (Figure 4). The effect of PDTC was more significant in monocytes exposed to SLE-MPs than to CMPs; that means that the synthesis of inflammatory mediators could be activated through different signaling pathways, depending on the source of MPs (patients vs. controls).
Therefore, it is plausible to hypothesize that compared to CMPs, SLE-MPs contain more components for the activation of different signaling pathways, including the NF-κB one. Previous evidence obtained from our Group [35, 56] and other authors [14] shown that SLE-MPs contain higher levels of HMGB1 and CD40L [11] than their normal counterparts. Our previous observations also support that circulating MPs from SLE patients are not only found in a higher proportion [10, 11, 12], but also vary in composition with respect to the MPs of healthy individuals. Phenotypic analyzes of these vesicles reveal that different molecules, especially immunoglobulins and complement components [15], are found in a higher proportion in the MPs of patients with SLE. Additionally, an increase in the frequency of C1q + MPs and immune complexes with IgM and IgG has been described in patients with SLE; the latter were positively correlated with disease activity and IgM + negatively [35]. DAMPs such as HGMB1 [56] and other molecules such as tissue factor, CD40L, vascular cell adhesion molecule 1 (VCAM-1) [14], glycolytic enzymes and apoptogenic proteins [13], are found in a higher proportion in the MPs of patients with SLE. Therefore, these vesicles could induce a more pronounced effect on monocytes compared to the MPs of healthy individuals, helping to perpetuate the inflammatory process in SLE.
HMGB1 and CD40L could interact with TLR2/4 and CD40 in the target cells and trigger the NF-κB signaling pathway [57, 58]. Depending on the activation state of the cell they come from, microparticles can have different contents of mRNA, proteins, receptors, and enzymes which will define the effect of vesicles on the recipient cells [59]. In this study, the inhibitory effect of PDTC on the synthesis of cytokines and eicosanoids induced by MPs on monocytes showed that the proinflammatory effect was related to an NF-κB mediated mechanism. PBMCs treated with MPs showed lower levels of cytoplasmic p65 and IκBα in the total cellular lysate; and, in addition, that monocytes treated with the microvesicles exhibited a higher nuclear translocation of p65, especially after treatment with SLE-MPs. Consistent with the present results, previous studies showed that following treatment with MPs, the nuclear translocation of NF-κB increased in neutrophils, monocytes, and macrophages, as well as synovial fibroblasts from patients with rheumatoid arthritis; what is more, the inhibition of the NF-κB pathway reduced the production of cytokines [43, 60]. Although previous studies had suggested the role of NF-κB in the proinflammatory effect induced by MPs, the present study provides the first evidence that SLE-MPs induce the activation of monocytes by a mechanism dependent at least partially on the triggering of the NF-κB signaling pathway. This route probably contribute to the constitutive activation of the NF-κB pathway observed in patients with SLE.
Previous studies have proposed that in addition to having a role in the inflammatory process, the NF-κB pathway can modulate the phenotype of extracellular vesicles. For example, the inhibition of the NF-κB signaling pathway reduces the levels of tissue factor in MPs derived from endothelial cells after stimulation with Chlamydia pneumoniae [55]. Additionally, exosomes isolated from the plasma of NFκB-knockout mice have an altered protein composition when compared to exosomes from wild type mice [56]. Then, it is possible to consider that an activated NF-κB pathway could contribute to the higher number and altered composition of circulating MPs in patients with SLE.
The findings in the present study provide evidence that MPs could contribute to the inflammation observed in patients with SLE. When circulating monocytes bind and uptake the microparticles, the cell surface expression of HLA-DR and CD69 increase, as well as the production of IL-6, IL- 1β, PGE2, and LTB4. The MP-induced activation of monocytes, at least partially mediated by the NF-κB pathway, could favor the cell migration to peripheral tissues, enhance the antigen presentation to autoreactive T-cell in an immunogenic environment, and promote the activation of autoreactive B-cells, favoring this way, the perpetuation of the inflammatory state in patients with SLE.
Declarations
Author Contribution Statement
K. Álvarez, M. Rojas and G. Vásquez: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
J. Villar-Vesga and B. Ortiz-Reyes: Performed the experiments; Analyzed and interpreted the data.
A. Vanegas-Garcia: Contributed reagents, materials, analysis tools or data.
D. Castaño: Analyzed and interpreted the data; Wrote the paper.
Funding Statement
G. Vásquez was supported by Fondo Colombiano de Investigaciones Científicas y Proyectos Especiales “Francisco José de Caldas” (1115- 569-33389). K. Álvarez was supported by Fondo Colombiano de Investigaciones Científicas y Proyectos Especiales “Francisco José de Caldas” (Joven Investigador 2015). This work was supported by Universidad de Antioquia (Sostenibilidad).
Data Availability Statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Marta Mesa for her English Language Wording review and correction.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary figure.
PBMCs treated with SLE-MPs and CMPs show reduced levels of IκBα and cytoplasmic p65, and PDTC inhibits this reduction. Representative full immunoblots are shown for and p65 (a), IκBα (b) and ß-actin (c). The (∗) indicate the presence of two lanes that are not relevant for the current experiment, since they were used for other no relative to this assay. n=5 independent experiments.
References
- 1.Tsokos G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365(22):2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Schiller M., Bekeredjian-Ding I., Heyder P., Blank N., Ho A.D., Lorenz H.M. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15(1):183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann M., Voll R.E., Zoller O.M., Hagenhofer M., Ponner B.B., Kalden J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41(7):1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Beyer C., Pisetsky D.S. The role of microparticles in the pathogenesis of rheumatic diseases. Nat. Rev. Rheumatol. 2010;6(1):21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 5.Burbano C., Rojas M., Vásquez G., Castaño D. Microparticles that form immune complexes as modulatory structures in autoimmune responses. Mediat. Inflamm. 2015;2015:267590. doi: 10.1155/2015/267590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisetsky D.S., Lipsky P.E. Microparticles as autoadjuvants in the pathogenesis of SLE. Nat. Rev. Rheumatol. 2010;6(6):368–372. doi: 10.1038/nrrheum.2010.66. [DOI] [PubMed] [Google Scholar]
- 7.Distler J.H., Pisetsky D.S., Huber L.C., Kalden J.R., Gay S., Distler O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005;52(11):3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- 8.Mause S.F., Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 2010;107(9):1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 9.Krajewska-Włodarczyk M., Owczarczyk-Saczonek A., Żuber Z., Wojtkiewicz M., Wojtkiewicz J. Role of microparticles in the pathogenesis of inflammatory joint diseases. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen C.T., Ostergaard O., Johnsen C., Jacobsen S., Heegaard N.H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011;63(10):3067–3077. doi: 10.1002/art.30499. [DOI] [PubMed] [Google Scholar]
- 11.Sellam J., Proulle V., Jungel A., Ittah M., Miceli Richard C., Gottenberg J.E. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 2009;11(5):R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen C.T. Circulating microparticles in systemic lupus erythematosus. Dan. Med. J. 2012;59(11):B4548. [PubMed] [Google Scholar]
- 13.Ostergaard O., Nielsen C.T., Tanassi J.T., Iversen L.V., Jacobsen S., Heegaard N.H.H. Distinct proteome pathology of circulating microparticles in systemic lupus erythematosus. Clin. Proteonomics. 2017;14:23. doi: 10.1186/s12014-017-9159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mobarrez F., Vikerfors A., Gustafsson J.T., Gunnarsson I., Zickert A., Larsson A. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci. Rep. 2016;6:36025. doi: 10.1038/srep36025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostergaard O., Nielsen C.T., Iversen L.V., Tanassi J.T., Knudsen S., Jacobsen S. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum. 2013;65(10):2680–2690. doi: 10.1002/art.38065. [DOI] [PubMed] [Google Scholar]
- 16.Szucs G., Kavai M., Suranyi P., Kiss E., Csipo I., Szegedi G. Correlations of monocyte phagocytic receptor expressions with serum immune complex level in systemic lupus erythematosus. Scand. J. Immunol. 1994;40(5):481–484. doi: 10.1111/j.1365-3083.1994.tb03493.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Lee P.Y., Sobel E.S., Narain S., Satoh M., Segal M.S. Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res. Ther. 2009;11(1):R6. doi: 10.1186/ar2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hepburn A.L., Mason J.C., Davies K.A. Expression of Fcgamma and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology (Oxford, England) 2004;43(5):547–554. doi: 10.1093/rheumatology/keh112. [DOI] [PubMed] [Google Scholar]
- 19.Katsiari C.G., Liossis S.N., Souliotis V.L., Dimopoulos A.M., Manoussakis M.N., Sfikakis P.P. Aberrant expression of the costimulatory molecule CD40 ligand on monocytes from patients with systemic lupus erythematosus. Clin. Immunol. (Orlando, Fla) 2002;103(1):54–62. doi: 10.1006/clim.2001.5172. [DOI] [PubMed] [Google Scholar]
- 20.Funauchi M., Ohno M., Minoda M., Horiuchi A. Abnormal expression of intercellular adhesion molecule-1 on peripheral blood mononuclear cells from patients with systemic lupus erythematosus. J. Clin. Lab. Immunol. 1993;40(3):115–124. [PubMed] [Google Scholar]
- 21.Steinbach F., Henke F., Krause B., Thiele B., Burmester G.R., Hiepe F. Monocytes from systemic lupus erythematous patients are severely altered in phenotype and lineage flexibility. Ann. Rheum. Dis. 2000;59(4):283–288. doi: 10.1136/ard.59.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriques A., Ines L., Carvalheiro T., Couto M., Andrade A., Pedreiro S. Functional characterization of peripheral blood dendritic cells and monocytes in systemic lupus erythematosus. Rheumatol. Int. 2012;32(4):863–869. doi: 10.1007/s00296-010-1709-6. [DOI] [PubMed] [Google Scholar]
- 23.Yuan W., DiMartino S.J., Redecha P.B., Ivashkiv L.B., Salmon J.E. Systemic lupus erythematosus monocytes are less responsive to interleukin-10 in the presence of immune complexes. Arthritis Rheum. 2011;63(1):212–218. doi: 10.1002/art.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongan A.E., Ramdahin S., Warrington R.J. Interleukin-10 response abnormalities in systemic lupus erythematosus. Scand. J. Immunol. 1997;46(4):406–412. doi: 10.1046/j.1365-3083.1997.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 25.Llorente L., Richaud-Patin Y., Wijdenes J., Alcocer-Varela J., Maillot M.C., Durand-Gasselin I. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur. Cytokine Netw. 1993;4(6):421–427. [PubMed] [Google Scholar]
- 26.Harigai M., Kawamoto M., Hara M., Kubota T., Kamatani N., Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J. Immunol. (Baltimore, Md : 1950) 2008;181(3):2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 27.Olaru F., Döbel T., Lonsdorf A.S., Oehrl S., Maas M., Enk A.H. Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis. JCI Insight. 2018;3(11) doi: 10.1172/jci.insight.96492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrera García A., Gómez-Puerta J.A., Arias L.F., Burbano C., Restrepo M., Vanegas A.L. Infiltrating CD16. Autoimmune Dis. 2016;2016:9324315. doi: 10.1155/2016/9324315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma I. Nuclear factor (NF)-κB proteins: therapeutic targets(∗) Ann. Rheum. Dis. 2004;63(Suppl 2):ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tak P.P., Firestein G.S. NF-κB: a key role in inflammatory diseases. J. Clin. Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi X., Qian T., Li M., Chen F., Chen Y., Hao F. Aberrant low expression of A20 in tumor necrosis factor-α-stimulated SLE monocytes mediates sustained NF-κB inflammatory response. Immunol. Invest. 2015;44(5):497–508. doi: 10.3109/08820139.2015.1037957. [DOI] [PubMed] [Google Scholar]
- 32.Hirose S., Lin Q., Ohtsuji M., Nishimura H., Verbeek J.S. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int. Immunol. 2019;31(11):687–696. doi: 10.1093/intimm/dxz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan E.M., Cohen A.S., Fries J.F., Masi A.T., McShane D.J., Rothfield N.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40 doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 35.Burbano C., Villar-Vesga J., Orejuela J., Muñoz C., Vanegas A., Vásquez G. Potential involvement of platelet-derived microparticles and microparticles forming immune complexes during monocyte activation in patients with systemic lupus erythematosus. Front. Immunol. 2018;9:322. doi: 10.3389/fimmu.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreck R., Meier B., Mannel D.N., Droge W., Baeuerle P.A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 1992;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock H.W., Sternsdorf T., Liese J., Belohradsky B., Weber C., Wedel A. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J. Immunol. 1993;151(12):6986–6993. [PubMed] [Google Scholar]
- 38.Stadanlick J.E., Kaileh M., Karnell F.G., Scholz J.L., Miller J.P., Quinn W.J., 3rd Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat. Immunol. 2008;9(12):1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angelot F., Seilles E., Biichle S., Berda Y., Gaugler B., Plumas J. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica. 2009;94(11):1502–1512. doi: 10.3324/haematol.2009.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mesri M., Altieri D.C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 1999;274(33):23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 41.Nomura S., Tandon N.N., Nakamura T., Cone J., Fukuhara S., Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158(2):277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 42.Distler J.H., Jungel A., Huber L.C., Seemayer C.A., Reich C.F., 3rd, Gay R.E. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. U. S. A. 2005;102(8):2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neri T., Armani C., Pegoli A., Cordazzo C., Carmazzi Y., Brunelleschi S. Role of NF-kappaB and PPAR-gamma in lung inflammation induced by monocyte-derived microparticles. Eur. Respir. J. 2011;37(6):1494–1502. doi: 10.1183/09031936.00023310. [DOI] [PubMed] [Google Scholar]
- 44.Niessen A., Heyder P., Krienke S., Blank N., Tykocinski L.O., Lorenz H.M. Apoptotic-cell-derived membrane microparticles and IFN-alpha induce an inflammatory immune response. J. Cell Sci. 2015;128(14):2443–2453. doi: 10.1242/jcs.162735. [DOI] [PubMed] [Google Scholar]
- 45.Dieker J., Tel J., Pieterse E., Thielen A., Rother N., Bakker M. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheum. 2016;68 doi: 10.1002/art.39417. [DOI] [PubMed] [Google Scholar]
- 46.Grondal G., Gunnarsson I., Ronnelid J., Rogberg S., Klareskog L., Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin. Exp. Rheumatol. 2000;18(5):565–570. [PubMed] [Google Scholar]
- 47.Linker-Israeli M., Deans R.J., Wallace D.J., Prehn J., Ozeri-Chen T., Klinenberg J.R. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J. Immunol. 1991;147(1):117–123. [PubMed] [Google Scholar]
- 48.Kitani A., Hara M., Hirose T., Harigai M., Suzuki K., Kawakami M. Autostimulatory effects of IL-6 on excessive B cell differentiation in patients with systemic lupus erythematosus: analysis of IL-6 production and IL-6R expression. Clin. Exp. Immunol. 1992;88(1):75–83. doi: 10.1111/j.1365-2249.1992.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brugos B., Vincze Z., Sipka S., Szegedi G., Zeher M. Serum and urinary cytokine levels of SLE patients. Pharmazie. 2012;67(5):411–413. [PubMed] [Google Scholar]
- 50.Korotkova M., Jakobsson P.J. Persisting eicosanoid pathways in rheumatic diseases. Nat. Rev. Rheumatol. 2014;10(4):229–241. doi: 10.1038/nrrheum.2014.1. [DOI] [PubMed] [Google Scholar]
- 51.Chae B.S., Shin T.Y., Kim D.K., Eun J.S., Leem J.Y., Yang J.H. Prostaglandin E2-mediated dysregulation of proinflammatory cytokine production in pristane-induced lupus mice. Arch Pharm. Res. 2008;31(4):503–510. doi: 10.1007/s12272-001-1185-6. [DOI] [PubMed] [Google Scholar]
- 52.Poubelle P.E., Stankova J., Grassi J., Rola-Pleszczynski M. Leukotriene B4 up-regulates IL-6 rather than IL-1 synthesis in human monocytes. Agents Actions. 1991;34(1-2):42–45. doi: 10.1007/BF01993233. [DOI] [PubMed] [Google Scholar]
- 53.Rola-Pleszczynski M., Lemaire I. Leukotrienes augment interleukin 1 production by human monocytes. J. Immunol. 1985;135(6):3958–3961. [PubMed] [Google Scholar]
- 54.Rossi A.G., McCutcheon J.C., Roy N., Chilvers E.R., Haslett C., Dransfield I. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J. Immunol. 1998;160(7):3562–3568. [PubMed] [Google Scholar]
- 55.Dieker J., Muller S. Post-translational modifications, subcellular relocation and release in apoptotic microparticles: apoptosis turns nuclear proteins into autoantigens. Folia Histochem. Cytobiol. 2009;47(3):343–348. doi: 10.2478/v10042-009-0068-1. [DOI] [PubMed] [Google Scholar]
- 56.Burbano C., Gómez-Puerta J.A., Muñoz-Vahos C., Vanegas-García A., Rojas M., Vásquez G. HMGB1+ microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur. J. Immunol. 2019;49(2):323–335. doi: 10.1002/eji.201847747. [DOI] [PubMed] [Google Scholar]
- 57.Yu M., Wang H., Ding A., Golenbock D.T., Latz E., Czura C.J. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26(2):174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 58.Gillespie E.F., Raychaudhuri N., Papageorgiou K.I., Atkins S.J., Lu Y., Charara L.K. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-kappaB. Invest. Ophthalmol. Vis. Sci. 2012;53(12):7746–7753. doi: 10.1167/iovs.12-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Distler J.H.W., Huber L.C., Gay S., Distler O., Pisetsky D.S. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39(8):683–690. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 60.Salanova B., Choi M., Rolle S., Wellner M., Luft F.C., Kettritz R. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J. Biol. Chem. 2007;282(38):27960–27969. doi: 10.1074/jbc.M704039200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.