Summary
Epidural electrical stimulation of the spinal cord is an emergent strategy for the neurological recovery of lower-extremity motor function. Motoneuron pools are thought to be recruited by stimulation of posterior roots. Here, we linked electromyographic data of epidurally evoked lower-extremity responses of 34 individuals with upper motoneuron disorders to a population model of the spinal cord constructed using anatomical parameters of thousands of individuals. We identified a relationship between segmental stimulation sites and activated spinal cord segments, which made spinal motor mapping from epidural space possible despite the complex anatomical interface imposed by the posterior roots. Our statistical approach provided evidence for low-threshold sites of posterior roots and effects of monopolar and bipolar stimulation previously predicted by computer modeling and allowed us to test the impact of different upper motoneuron disorders on the evoked responses. Finally, we revealed a statistical association between intraoperative and postoperative mapping of the spinal cord.
Subject areas: Nervous System Anatomy, Neuroscience, Neuroanatomy, Clinical Neuroscience
Graphical Abstract

Highlights
-
•
Spinal motor maps are created by epidural lumbosacral posterior root stimulation
-
•
Muscle-specific stimulation sites coincide with segmental motoneuron pool locations
-
•
Lower-extremity motor recruitment is alike in different upper motoneuron disorders
-
•
Intraoperative mapping predicts postoperative selectivity of epidural stimulation
Nervous System Anatomy; Neuroscience; Neuroanatomy; Clinical Neuroscience
Introduction
Epidural electrical stimulation (EES) of the spinal cord is broadly known as a treatment for chronic intractable pain of the trunk and limbs (Krames et al., 2009; Rock et al., 2019; Shealy et al., 1967). For this indication, EES is generally applied at C2–C3 vertebral levels for neck and upper-extremity pain (Rock et al., 2019; Schoen et al., 2017) and at T8–T10 for lower-back and lower-extremity coverage (Air et al., 2012; Shils and Arle, 2018) and is associated with the stimulation of ascending fiber branches of cutaneous afferents in the spinal cord dorsal columns (Holsheimer, 1998; Shils and Arle, 2012; Tulgar et al., 1993). Its use in motor disorders has a history nearly as long as in pain (Cook and Weinstein, 1973; Illis et al., 1976; Minassian et al., 2012), with neuromodulation of spasticity having been the initial interest in spinal cord injury (SCI) (Barolat et al., 1995; Dimitrijevic et al., 1986; Pinter et al., 2000; Richardson et al., 1979). Recent studies of EES demonstrated unprecedented improvements of lower-extremity motor function thought to be irreversibly lost due to chronic SCI and signaled a new era of application in motor disorders (Calvert et al., 2019b). Following the observation that EES can enable volitionally initiated activation of otherwise paralyzed muscles (Angeli et al., 2014; Harkema et al., 2011), the facilitation of overground walking was the next breakthrough finding (Angeli et al., 2018; Gill et al., 2018; Wagner et al., 2018). These first advances in demonstrating efficacy have partially preceded the understanding of the underlying principles. The vast neuroprosthetics, neuroanatomical, and physiological knowledge gained from the application in pain (Barolat et al., 1993; Gildenberg, 2009; He et al., 1994; Shils and Arle, 2018) cannot be translated to the use for motor function. All contemporary studies had placed the epidural electrodes at T11–L1 vertebral levels guided by monitoring of evoked responses, based on the assumption that specific spinal cord segments innervating lower-extremity muscles could be targeted with electrodes directly overlying them (Calvert et al., 2019a; Harkema et al., 2011; Murg et al., 2000; Wagner et al., 2018). However, no study so far was designed to demonstrate whether such functional monitoring would identify the anatomical stimulation site relative to the activated lumbosacral spinal cord segments. Motor effects of EES are associated with the stimulation of proprioceptive fibers within posterior roots (Capogrosso et al., 2013; Dimitrijevic et al., 1980; Formento et al., 2018; Minassian et al., 2016; Wagner et al., 2018). There is a dissociation between the segmental anatomy of the lumbosacral spinal cord and the longitudinally running posterior roots, which have a complex topographic anatomy (Wall et al., 1990) and can be stimulated from a wide range of rostrocaudal electrode positions (Ladenbauer et al., 2010; Rattay et al., 2000) due to their intrathecal lengths of up to 16 cm (Lang and Geisel, 1983) and the current spread within the cerebrospinal fluid (Capogrosso et al., 2013). In addition, imaging techniques cannot identify segmental electrode positions.
Here, we sought to map the human lumbosacral spinal cord by implementing a relationship between the statistically estimated anatomical, segmental site of a stimulating epidural cathode and the segments of activated motoneuron pools. To achieve this goal, we linked vertebral cathode sites and evoked responses of multiple lower-limb muscles involved in locomotion to an anatomical model of the vertebral locations of the lumbosacral spinal cord and segmental innervation probabilities, constructed using parameters of thousands of subjects from literature. Electromyographic (EMG) responses evoked by low-frequency EES applied from vertebral locations ranging from T9 to L1 were analyzed. Data were derived from 34 individuals with upper motoneuron disorders, a sample size that, for the first time, made sound statistical analysis possible. We hypothesized that—despite the anatomically complex interface imposed by the posterior roots—threshold stimulation would precisely map the rostrocaudal locations of the anatomically separate motoneuron pools innervating the rectus femoris (L2–L4) and the triceps surae muscle group (L5–S2), based on theoretically predicted low-threshold sites of proprioceptive root fibers at their segmental entries (Capogrosso et al., 2013; Ladenbauer et al., 2010; Rattay et al., 2000; Struijk et al., 1993). We extended our analysis to multiple lower-extremity muscles with overlapping segmental innervations and tested whether EMG amplitudes of evoked responses would reflect the segmental spinal cord organization. Our further goals were to inquire the impact of the type and severity of upper motoneuron disorder as well as of mono- and bipolar EES on the recruitment of the evoked responses. Finally, we statistically evaluated the association between intra- and postoperative mapping of the spinal cord.
Results
Spinal cord model and segmental positions of stimulating cathodes
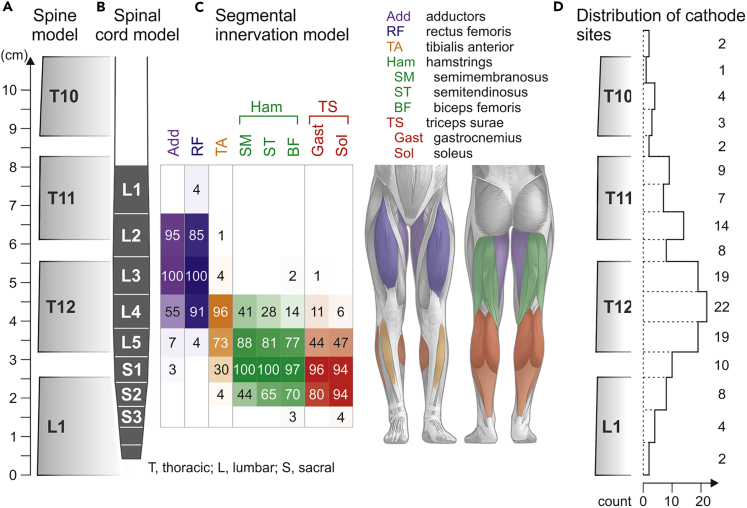
We interfaced EMG data of epidurally evoked lower-extremity responses of 34 subjects with upper motoneuron disorders (Table S1) with the segmental organization of the lumbosacral spinal cord pursuing a statistical approach. We constructed a straight-line geometrical model of the spine from the lower endplate of the L1- to the upper endplate of the T10-vertebral body from anatomical dimensions (Figure 1A; Tables S2 and S3). Vertebral body and intervertebral disc heights increased monotonically from T10 to L1. The total height of the model was 108.7 mm. We incorporated a straight-line model of the lumbosacral spinal cord (Figure 1B; Tables S4 and S5). The segmental heights decreased monotonically in caudal direction. The total heights of the lumbar and sacral spinal cord amounted to 49.8 mm and 26.5 mm, respectively. The termination of the conus medullaris was defined at the lower third of the L1-vertebral body (median value derived from a population of n = 4,797 samples from literature, IQR from the L1/L2-intervertebral disc to the upper third of the L1-vertebral body; Table S6). We estimated that the mean termination level of our 34 subjects would be within ±5.7 mm of the model's value, i.e., of the population, with a confidence of 95% (see Transparent Methods). We added segmental innervation probabilities of the lower-extremity muscles of interest (Figure 1C). Motoneuron pools of the medial and anterior thigh muscles (adductors, Add; rectus femoris, RF; L2–L4 spinal cord segments) were segmentally separate from the posterior lower-leg muscles (triceps surae muscle group, TS; L5–S2). The tibialis anterior (TA) motoneuron pool overlapped partially with the segmental locations of the other motoneuron pools. The segmental innervation of the hamstrings muscle group (Ham) was broader than that of the other muscles studied. We identified the vertebral positions of all tested cathodes of the midline-placed epidural linear leads from X-rays and transformed them into longitudinal coordinates in the straight-line spine model (Figure S1). This was a necessary step to link evoked responses to the segmental stimulation site, which cannot be deduced from imaging techniques. The distribution of tested cathode sites (n = 134, 34 subjects) ranged from the T9/T10-intervertebral disc to the lower endplate of the L1-vertebral body, covering the low-thoracic as well as all lumbar and sacral spinal cord segments (Figure 1D).
Figure 1.
Spinal cord and segmental innervation model
(A) Straight-line anatomical model of the spine, defined by vertebral body and intervertebral disc heights.
(B) Straight-line model of the lumbosacral spinal cord aligned with the spine model.
(C) Segmental innervation probabilities (0%–100%) of Add, RF, TA, SM, ST, BF, Gast, and Sol, reflected by the opacity of the respective colors. Sources and values considered in the spine model are specified in Tables S2 and S3, those of the spinal cord model in Tables S4–S6.
(D) Distribution of vertebral cathode positions tested; numbers are counts per bin (intervertebral disc or upper, middle, or lower third of vertebral body). Cathode positions tested in different monopolar or bipolar setups in a given subject were counted only once.
The epidurally evoked responses are posterior root-muscle reflexes
We had previously demonstrated the reflex nature of responses evoked with midline-placed cylindrical electrodes as used here (Hofstoetter et al., 2018; Minassian et al, 2004, 2016). Post-stimulation depression of responses to double stimuli and unchanged onset latencies with increasing stimulation amplitude affirmed that, across stimulation sites and amplitudes, this was also the case for the present dataset (see Data S1 and Figure S2). EES would hence map the segmental anatomy of the lumbosacral spinal cord through the electrical stimulation of proprioceptive afferents.
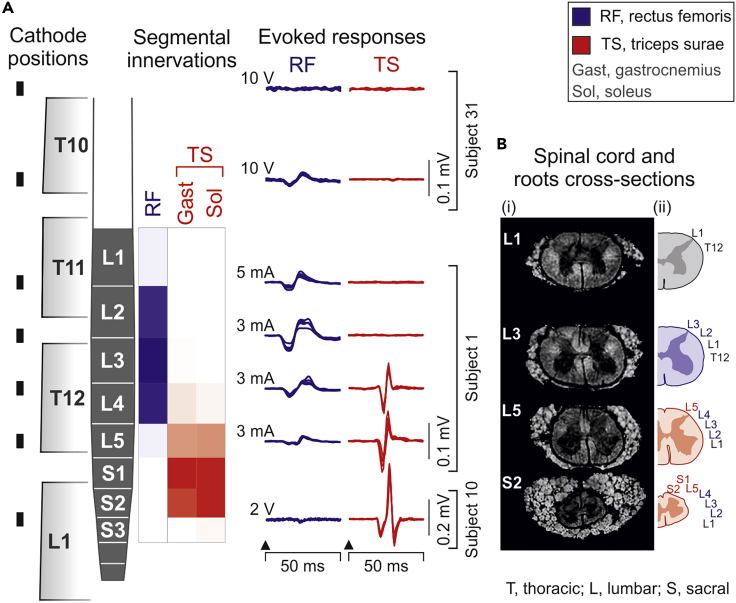
PRM reflexes evoked from a wide range of epidural cathode sites
EMG responses of RF and TS evoked by EES with threshold amplitude are illustrated in Figure 2A. The responses reflected the segmental anatomy of the lumbosacral spinal cord despite their elicitation through an anatomically complex interface (Figure 2B). Although responses could be evoked by stimulation applied from a wide rostrocaudal range, the largest EMG potentials were elicited when the estimated segmental cathode sites were located over the segmental locations of the respective motoneuron pools in the anatomical model.
Figure 2.
Mapping the segmental lumbosacral spinal cord anatomy by epidurally evoked muscle responses—exemplary results
(A) EMG responses of RF and TS evoked by epidural stimulation with threshold amplitude, aligned with the respective rostrocaudal cathode positions (black rectangles) and segmental innervations. Neither muscle was recruited from the most rostral site with maximum stimulation (10 V). EMG response derived from three subjects as indicated.
(B) (i) Magnetic resonance microscopy images of the spinal cord and the complex peripheral rim composed of posterior and anterior roots shown in cross-sections at spinal cord segmental levels as indicated (Calabrese et al., 2018). (ii) Estimated positions of the left T12–S2 posterior roots reflecting their complex anatomical arrangement (Wall et al., 1990).
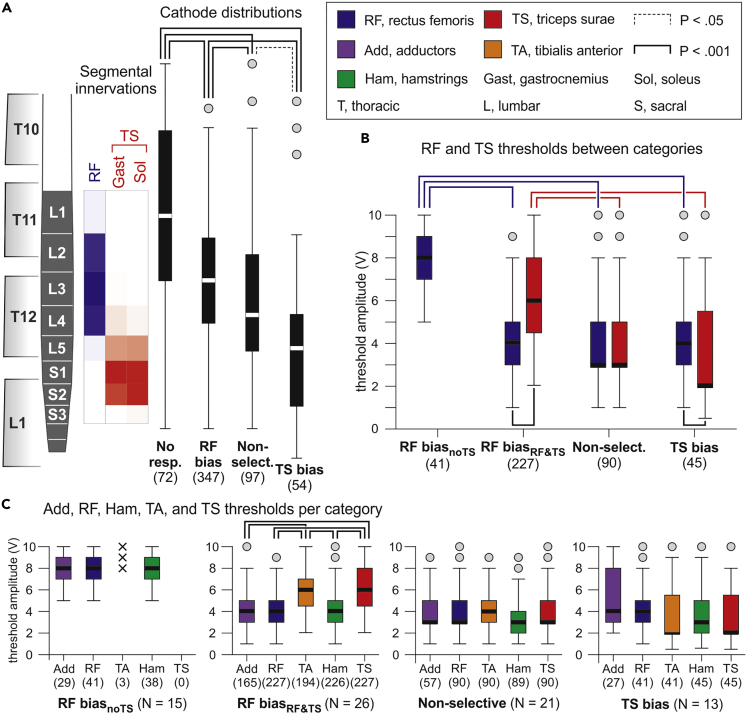
Mapping of the lumbosacral spinal cord based on PRM-reflex thresholds
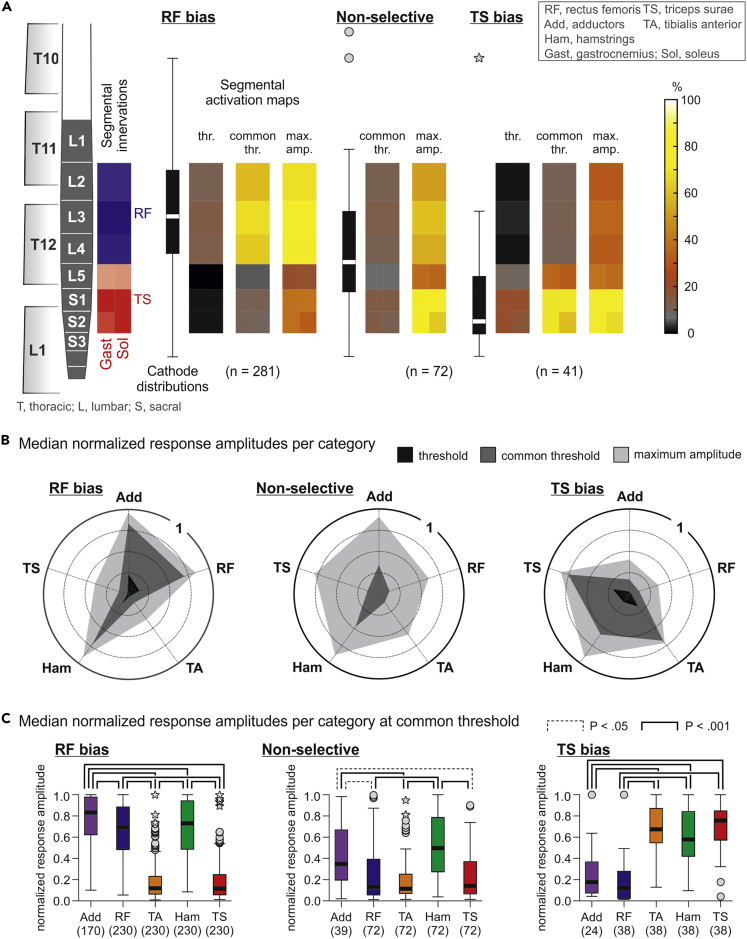
We classified the vertebral cathode positions of 570 EMG datasets (34 subjects, two legs, multiple electrode setups; monopolar stimulation, n = 72; bipolar stimulation, n = 498) into four categories based on the elicitation of RF- and TS-posterior root-muscle (PRM) reflexes and their relative thresholds (no responses, RF bias, non-selective, TS bias; see Figure 3 and Transparent Methods). RF and TS were chosen as the key muscles in this analysis because of their largely separate segmental innervations. We found a significant difference between the rostrocaudal cathode distributions (registered coordinates in the model) of the four categories (Kruskal-Wallis test, χ2(3) = 97.688, p < .001, r = .414; Figure 3A). Post-hoc comparisons revealed differences between each of the categories (non-selective vs. TS bias, p = .016; all other p < .001). The no-responses category was associated with the most rostral cathode positions, with the median at the L1 spinal cord segment. The RF-bias category had a median cathode site at the L3 segment with an IQR covering the L2–L4 segments. In 28.0% of the datasets within this category, TS responses were not evoked even with maximum stimulation amplitude. Non-selective recruitment of RF and TS resulted from a cathode site distribution with its median at the L4 segment. The TS-bias category was associated with the most caudal cathode sites, with the median at the L5 segment and an IQR covering the L4–S2 segments. Thus, the categorization based on the thresholds of the L2–L4 innervated RF and the L5–S2 innervated TS produced statistically separate rostrocaudal distributions of cathode positions with dispersions that almost perfectly matched the respective rostrocaudal segmental innervations in the anatomical model.
Figure 3.
PRM-reflex thresholds of RF and TS reflect the segmental lumbosacral spinal cord anatomy
(A) Statistical comparison (Kruskal-Wallis test) of cathode distributions separated according to the elicitation of RF- and TS-PRM reflexes and their thresholds (Th). Categories are as follows: No RF and TS responses; RF bias, ThRF < ThTS; Non-selective, ThRF = ThTS; TS bias, ThRF > ThTS. Segmental innervation probabilities are illustrated by the opacity of blue (RF) and red (TS) boxes aligned with the spinal cord model. Data derived from all 34 subjects.
(B) ThRF and ThTS of the different categories; the RF-bias category was sub-divided into RF biasnoTS, RF but not TS recruited, and RF biasRF&TS, RF and TS recruited. Statistical comparisons between categories were performed using Kruskal-Wallis test and within categories, using separate Wilcoxon tests.
(C) Response thresholds of Add, RF, TA, Ham, and TS per category. In the RF-biasnoTS category, TA responded in three cases only (x) and was not considered in the statistical comparison (linear mixed model). Numbers in parentheses are available datasets per category and muscle; N is the number of subjects per analysis. Boxplots illustrate median cathode locations (A) and response thresholds (B and C), respectively, as bold horizontal lines within boxes spanning the IQR, and whiskers extending to the smallest and largest values that are not outliers (values 1.5–3 times the IQR; plotted as circles). Brackets indicate statistical significance, dotted lines, p < .05, and solid lines, p < .001.
We further divided the RF-bias category into two sub-categories: RF biasnoTS, RF but not TS recruited even with maximum stimulation amplitude; and RF biasRF&TS, RF and TS recruited. Between categories (Figure 3B), thresholds of RF and TS responses, respectively, differed significantly (datasets obtained with voltage-controlled stimulators, see Transparent Methods; Kruskal-Wallis tests; RF, χ2(3) = 88.643, p < .001, r = .471; TS, χ2(2) = 84.299, p < .001, r = .483). Post-hoc comparisons revealed significantly higher RF thresholds in the RF-biasnoTS category compared with any other category as well as significantly higher TS thresholds in the RF-biasRF&TS category compared with the non-selective and the TS-bias categories (all p < .001). Response thresholds hence increased when progressively shifting cathode sites rostrally from the respective segmental innervations. Thresholds of RF and TS differed both within the RF biasRF&TS category (Wilcoxon test; Z = −13.223, p < .001, r = .878) as well as within the TS bias category (Z = −7.696, p < .001, r = .555).
We expanded the analysis of response thresholds to all five studied muscles by fitting separate linear mixed models to the data obtained for each category (Figure 3C). No differences between thresholds were found for the RF-biasnoTS (F(2,105) = .141, p = .869, = .003; only thigh muscles compared), the non-selective (F(4,411) = .924, p = .450, = .009), nor the TS-bias categories (F(4,194) = 2.237, p = .066, = .044). In the RF-biasRF&TS category, thresholds differed significantly (F(4,1034) = 66.706, p < .001, = .205). Post-hoc comparisons revealed lower thresholds for the thigh than the lower leg muscles (all p < .001). Notably, responses of Ham did not reflect the segmental innervation of this muscle group. Their thresholds differed neither from the RF thresholds in the two RF-bias categories nor from the TS threshold in the TS-bias category.
Finally, we compared the datasets obtained in the SCI (N = 26; number of datasets n = 319; see Transparent Methods) and the non-SCI subjects (N = 8; n = 179). There was a significant association between category (no responses, RF bias, non-selective, TS bias) and pathology (SCI, non-SCI), χ2(3) = 11.113, p = .011, Cramer's V = .149, with the SCI subjects contributing more (adjusted residuals of 2.2) than expected under the null hypothesis to the non-selective category and less (−2.6) to the no-responses category. Yet, there was also a significant association between rostrocaudal cathode distribution (categorized into intervertebral disc or upper, middle, or lower third of vertebral body) and pathology (χ2(14) = 88.344, p < .001, Cramer's V = .421). When analyzing reduced datasets (SCI, n = 232 datasets; non-SCI, n = 127) with no statistical difference in cathode distribution between subject groups (χ2(7) = 12.198, p = .094, Cramer's V = .184), there was no association between category and pathology (χ2(3) = 3.517, p = .319, Cramer's V = .099).
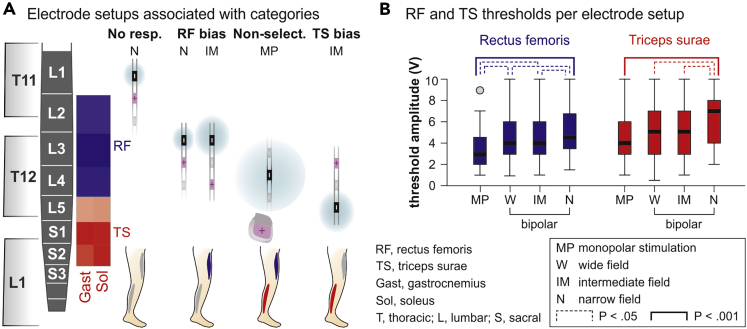
Influence of cathode-anode setup
We found a significant association between electrode setup (bipolar stimulation with cathodes either rostral or caudal to anodes and with wide, intermediate, and narrow fields, or monopolar stimulation; see Transparent Methods) and the four RF- and TS-threshold-based categories (χ2(24) = 61.491, p < .001, Cramer's V = .173). Narrow fields with rostral cathodes contributed more (adjusted residual of 2.7) and monopolar setups less (−2.2) than expected under the null hypothesis to the no-responses category. Narrow and intermediate fields with rostral cathodes contributed more than expected to the RF-bias category (2.9 and 2.2, respectively), whereas intermediate fields with caudal cathodes contributed less (−2.7). Monopolar setups contributed more (2.2) and wide fields with rostral cathodes less (−2.1) than expected to the non-selective category. Intermediate fields with caudal cathodes contributed more than expected (2.4) to the TS-bias category. Figure 4A illustrates all electrode setups that contributed more than expected to a specific category. Thresholds of RF and TS, respectively, differed significantly between bipolar wide, intermediate, and narrow fields as well as monopolar stimulation (Kruskal-Wallis tests; RF, χ2(3) = 25.701, p < .001, r = .241; TS, χ2(3) = 21.403, p < .001, r = .243). Post-hoc comparisons revealed lower RF-thresholds for monopolar than for bipolar stimulation, as well as lower TS-thresholds for monopolar than bipolar stimulation with narrow fields; further significant differences are highlighted in Figure 4B.
Figure 4.
Impact of mono- and bipolar stimulation on selectivity and threshold of PRM-reflex elicitation
(A) Electrode setups contributing more than expected under the null hypothesis to the no-responses, RF-bias, non-selective, and TS-bias categories. Cathode (−) positions represent the medians of distributions within the four different categories, cf. Figure 3A, shown with respect to the major segmental innervations of RF and TS. Anodes (+) shield the distribution of the cathodic field (symbolized by blue areas).
(B) Dependence of RF- and TS-response thresholds on the electrode setup. Boxplots illustrate median thresholds as bold horizontal lines within boxes spanning the IQR and whiskers extending to the smallest and largest values that are not outliers (values 1.5–3 times the IQR; plotted as circle). Data derived from, monopolar stimulation, ten subjects; wide field, 30 subjects; intermediate field, 18 subjects; and narrow field, 14 subjects. Brackets indicate statistical significance (Kruskal-Wallis tests), dotted lines, p < .05, and solid lines, p < .001.
Mapping of the lumbosacral spinal cord based on PRM-reflex amplitudes
We analyzed the peak-to-peak amplitudes of PRM reflexes at threshold (lowest stimulation amplitude evoking either RF or TS responses), common threshold (evoking RF and TS responses), as well as the maximum stimulation applied. For each electrode setup and stimulation amplitude, we obtained mean peak-to-peak amplitudes for each muscle per leg from all available responses and normalized them to the maximum mean peak-to-peak amplitude in the respective muscle obtained across all tested electrode setups in the same recording. Median values per muscle were then determined separately for the RF-bias, the non-selective, and TS-bias categories and within each category for the three stimulation levels (394 datasets derived from 25 subjects; see Transparent Methods). Rostrocaudal cathode distributions differed significantly between categories also for this reduced number of datasets (Kruskal-Wallis test, χ2(4) = 203.663, p < .001, r = .439, all post-hoc pairwise comparisons p < .001).
Spinal cord maps of motoneuron pool activation derived from the RF- and TS-response amplitudes and segmental innervation probabilities showed a clear dependence on the rostrocaudal cathode sites (Figure 5A). A median cathode site at the L3 segmental level led to the selective activation of the L2–L4 spinal cord segments at threshold, with gradual spread to L5–S2 at common threshold and maximum stimulation amplitude, but the rostral segments remained predominantly activated. Conversely, a median cathode site at the S2 segmental level led to the selective activation of the L5–S2 spinal cord segments at threshold and recruited rostral segments with increased stimulation amplitudes. A median cathode site right between the segmental innervations of RF and TS resulted in a non-selective activation of L2–S2. Thus, threshold responses reflected the anatomical locations of the key segments of RF and TS, but increasing the stimulation amplitude led to a dissociation between the segmental cathode site and the activated spinal cord segments.
Figure 5.
PRM-reflex amplitudes reflect the segmental lumbosacral spinal cord anatomy
(A) Spinal cord maps of spatial motoneuron pool activation for three cathode distributions (RF bias, non-selective, TS bias) and three stimulation-amplitude levels (threshold, common threshold, maximum) derived from normalized response amplitudes and segmental innervation probabilities. Only major segmental innervations of RF and TS (innervation probabilities ≥44%; Figure 1C) were considered, illustrated by the opacity of blue and red boxes. Data derived from 25 subjects.
(B) Polar plots of muscle activation for the three categories and stimulation levels. Radial axes are muscles and polar coordinates are median normalized peak-to-peak amplitudes.
(C) Normalized response amplitudes of all muscles studied per category at common threshold and compared using separate linear mixed models. Numbers in parentheses are available datasets per category and muscle. Boxplots illustrate median cathode locations (A) and response thresholds (C), respectively, as bold horizontal lines within boxes spanning the IQR, and whiskers extending to the smallest and largest values that are not outliers (values 1.5–3 times the IQR; plotted as circles) or extreme values (values >3 times the IQR; asterisks). Brackets indicate statistical significance, dotted lines, p < .05, and solid lines, p < .001.
Polar plots of normalized response amplitudes reveal the preferential activation of the medial and anterior thigh muscles with a median cathode site at the L3 segmental level and of the lower-leg muscles with a median cathode site at S2 (Figure 5B). At maximum stimulation, the polygon function approached the shape of a regular pentagon in the non-selective category, indicating near equal recruitment of all muscles with a median cathode site between the L4 and L5 segmental levels. Ham was strongly recruited by all three cathode distributions.
We compared the normalized response amplitudes of the five muscles at common threshold and found significant differences in all three categories (linear mixed models; RF bias, F(4,1085) = 366.725, p < .001, = .575; non-selective, F(4,322) = 18.488, p < .001, = .187; and TS bias, F(4,171) = 29.681, p < .001, = .410). Significant results of post-hoc comparisons are shown in Figure 5C. The segmental anatomy of the lumbosacral spinal cord was well reflected by the normalized EMG amplitudes of the PRM reflexes of Add, RF, TA, and TS, but not of Ham.
To investigate whether the type of upper motoneuron disorder would impact the PRM-reflex recruitment, we compared the EMG amplitudes obtained in the SCI (N = 19) and the non-SCI subjects (N = 6) within the RF-bias category, separately for threshold, common threshold, and maximum stimulation (statistical comparisons in other categories not possible due to sample sizes). At threshold and common threshold, no differences between the two subgroups were detected for any of the five muscles studied. At maximum stimulation, the EMG amplitudes of the Ham responses were larger in the SCI (1579.6 ± 163.2 μV) than the non-SCI subjects (889.4 ± 289.7 μV). Notably, absolute stimulation amplitudes did not differ between SCI and non-SCI subjects at threshold, common threshold, and maximum stimulation. The same analyses conducted for the severity of SCI (motor-complete, AIS A or B, N = 13; versus motor-incomplete, AIS C or D, N = 6) revealed no differences at threshold and common threshold. At maximum stimulation, EMG amplitudes of TA (motor-complete SCI: 451.6 ± 72.0 μV; motor-incomplete SCI: 154.2 ± 103.6 μV) and Ham responses (1858.2 ± 195.1 μV; 1023.9 ± 280.8 μV) differed significantly. Again, stimulation amplitudes did not differ between subgroups. All values and results of the statistical testing are specified in Tables S7 and S8.
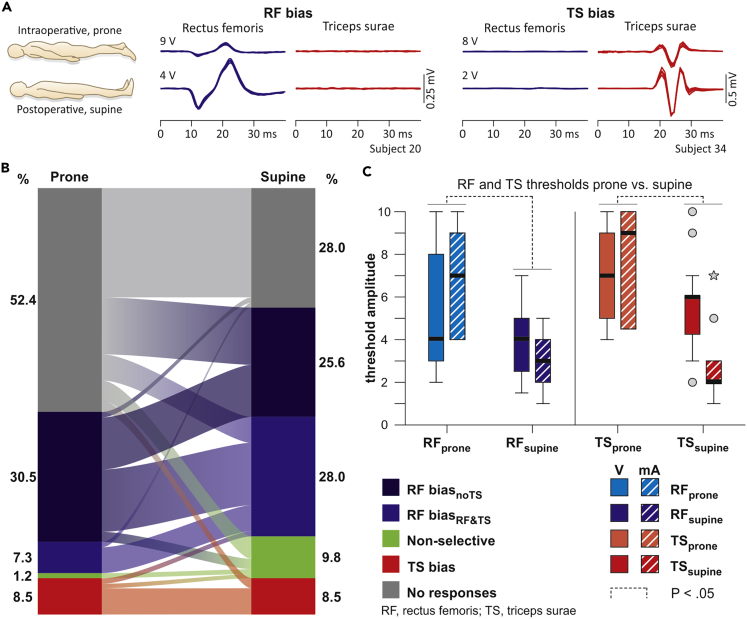
Intraoperative monitoring predicts postoperative segmental cathode position
The surgical placement of an epidural lead is guided by neurophysiological monitoring with the patient in the prone position, whereas postoperatively, the active electrodes along the epidural array are selected in the supine position (Harkema et al., 2011; Wagner et al., 2018). We therefore tested whether segmental cathode positions and motoneuron pool activation identified intraoperatively would be maintained despite the considerable anterior-to-posterior migration of the spinal cord and roots within the dural sac accompanying the change in body position (Holsheimer et al., 1994; Ranger et al., 2008). We analyzed 82 available pairs of datasets with the same electrode setups tested both intra- and postoperatively in a given subject (Figure 6A). Statistical testing revealed a significant association between categories (χ2(16) = 79.975, p < .001, Cramer's V = .494; Figure 6B). The following transitions occurred more frequently than expected under the null hypothesis: RF biasnoTS, prone to RF biasRF&TS, supine (adjusted residual: 2.7); RF biasRF&TS, prone to RF biasRF&TS, supine (3.1); non-selectiveprone to non-selectivesupine (3.1); TS biasprone to TS biassupine (6.2); and no responsesprone to no responsessupine (4.4). Transitions from RF biasnoTS, prone to no responsessupine (−3.2) and no responsesprone to RF biasRF&TS, supine (−3.5) occurred less frequently than expected.
Figure 6.
Intraoperative monitoring predicts postoperative segmental motoneuron pool activation
(A) EMG recordings of PRM reflexes evoked in RF and TS at threshold, intraoperatively in the prone and postoperatively in the supine position; five superimposed responses each. RF- versus TS-selectivity of the stimulation intraoperatively was maintained postoperatively, with decreased thresholds.
(B) Transitions between categories from the prone to the supine position. Eighty-seven percent of the intraoperative datasets that were classified into either of the two RF-bias categories and 71% of those of the TS-bias category remained within the respective categories postoperatively. Data derived from 16 subjects.
(C) Thresholds of RF and TS responses evoked in the prone and supine positions. Data derived from 12 subjects. Thresholds for voltage- and current-controlled stimulators were pooled for the statistical testing, because pairwise comparisons were based on signed ranks (Wilcoxon test). Boxplots illustrate median thresholds as bold horizontal lines within boxes spanning the IQR and whiskers extending to the smallest and largest values that are not outliers (values 1.5–3 times the IQR; plotted as circles) or extreme values (values >3 times the IQR; asterisks). Brackets (dotted lines) indicate statistical significance, p < .05.
In 37 of the 82 data pairs, RF and TS were recruited in the prone as well as the supine position and allowed the statistical comparison of response thresholds between body positions (Figure 6C). Pairwise comparisons revealed higher thresholds in the prone than the supine position for the recruitment of both muscles (Wilcoxon tests; RF, Z = −3.336, p = .001, r = .599; TS, Z = −2.524, p = .012, r = .700). Intraoperative segmental mapping hence predicted the segmental motoneuron pool activation obtained postoperatively with reduced response thresholds in the supine position.
Laterally located electrodes can lead to a dissociation between segmental cathode sites and activated spinal cord segments
We had hypothesized that midline-located electrodes would map the segmental organization of the lumbosacral spinal cord by preferentially activating posterior roots at their segmental entries (Capogrosso et al., 2013; Ladenbauer et al., 2010; Rattay et al., 2000; Struijk et al., 1993) and hence recruit motoneuron pools at the location of the stimulating cathode. Laterally placed electrodes could be located closer to posterior roots of passage and therefore activate more rostral spinal cord segments at threshold (Figure S3A). We explored this assumption by comparing pairs of datasets with midline and lateral electrode leads with same rostrocaudal anode and cathode positions (available from three subjects; see Data S1). For all midline-located bipolar electrode setups at the T12 vertebral level (n = 27), 66.7% of the datasets were classified into either of the two RF-bias categories, all of which remained within these categories when stimulation was shifted laterally (Figure S3B). Additional contributions from the non-selective and no-responses categories resulted in overall 77.8% of the datasets to belong to the RF-bias categories for lateral stimulation, hence increasing L2–L4 selectivity. For all midline-located bipolar electrode setups at the L1 vertebral level (n = 22), 27.3% of the datasets were classified into the TS-bias category, of which 83.3% changed to one of the two RF-bias categories and another 16.7% to the no-responses category with laterally located electrodes. None of the datasets were classified into the TS-bias category with lateral stimulation.
Discussion
We mapped the spinal cord through EES of a complex interface—the lumbosacral posterior roots floating in the cerebrospinal fluid. Classifying cathode locations according to relative thresholds of RF and TS responses resulted in separate rostrocaudal spatial dispersions that near-perfectly matched the statistically predicted anatomical locations of the respective motoneuron pools. EMG amplitudes of Add, RF, TA, and TS responses reflected the segmental organization of the spinal cord, but Ham was an exception. Theories on low-threshold sites of posterior root fibers and the impact of mono- and bipolar stimulation were confirmed. Motor recruitment by EES was directly compared between subjects with traumatic SCI and other upper motoneuron disorders for the first time. Differences were present at maximum stimulation amplitudes. Postoperative selectivity of motoneuron pool activation by EES in supine was predictable from intraoperative recordings in the prone position.
To estimate segmental cathode positions, we constructed the—to our knowledge—most comprehensive statistical model of the spine and spinal cord published to date. To mitigate inaccuracies in our interpretations resulting from the variability of anatomical parameters, our model integrated data from thousands of subjects and 59 sources. In addition, our cohort of 34 subjects was the largest in contemporary studies of EES in motor disorders. The termination level of the conus medullaris was an essential parameter, because its variation directly results in a shift of the vertebral positions of spinal cord segments (Wall et al., 1990). The deviation of the mean termination level of our sample from the population median was estimated to be less than 6 mm, with a confidence of 95%. The goodness of representation of our sample by the population model was reflected by the near-perfect match of the mapping results of RF and TS and the estimated anatomical locations of the L2–L4 and L5–S2 spinal cord segments. Our population model constructed by stacking spinal cord segmental heights is in good agreement with the vertebral positions of the lumbosacral spinal cord found in anatomical studies of human cadavers, which identified the rostral border of the L1 segment at the upper third of the T11-vertebral body and that of the S1 segment at the lower endplate of the T12 or the upper endplate of the L1-vertebral body (Canbay et al., 2014; Hintzsche and Gisler, 1935; Lang and Geisel, 1983; Wall et al., 1990). The major variations were the exact locations of the individual lumbar spinal cord segments. A recent study constructed an anatomical model of the C2–L5 spinal cord segments in relationship to bony landmarks using multiple parameters measured in nine human cadavers (Mendez et al., 2020). Compared with our model, the rostral border of the L1 spinal cord segment was near-identical, but the entire lumbar spinal cord was stretched in caudal direction by approximately 20%. This difference may stem from the different assumptions used to construct the models, e.g., the correlation between vertebral bone height and the intervertebral foramen-to-dorsal root entry zone distance used in (Mendez et al., 2020).
The vast majority of EES applications is in pain control and is firmly associated with the antidromic activation of sensory dorsal column fibers (Gildenberg, 2009). Sensory effects and evoked muscle responses might however be initiated separately in dorsal columns and posterior roots, respectively (He et al., 1994; North et al., 1997). In cats and non-human primates, the majority of long dorsal column fibers ascending from the lumbosacral to the thoracic and cervical spinal cord come from cutaneous mechanoreceptors (Davidoff, 1989). The ascending projections from hindlimb muscle spindle afferents largely terminate in the upper lumbar and lower thoracic segments, supposedly occupying deep positions in the dorsal columns to make synaptic contact with the posterior gray column/Clarke's column (Brodal, 1981; Lloyd and McIntyre, 1950; Whitsel et al., 1969). This may be the case in humans as well (York, 1985). Neural structures activated by EES are limited to the largest-diameter myelinated fibers in the outermost layer of the dorsal columns and to the posterior roots (Holsheimer, 1998, 2002). Posterior root fibers have lower thresholds (Capogrosso et al., 2013; Rattay et al., 2000; Struijk et al., 1993), largely because of the electrical conductivity of the cerebrospinal fluid (Geddes and Baker, 1967), which channels 80%–90% of the injected current flow (Holsheimer, 1998; Ladenbauer et al., 2010). The lumbar and upper sacral posterior roots hold all muscle spindle fibers from the lower extremities (Brodal and Rinvik, 1981; Lloyd, 1943). Our results strongly support that the evoked responses were not related to dorsal column stimulation, because statistically, response thresholds increased when stimulating cathode locations were rostral to the respective segmental posterior root entries. By contrast, responses could be evoked in a muscle when cathodes were located over the longitudinal extent of the associated posterior roots, i.e., at the segments of their homonymous motoneuron pools and caudally along the entire length of the terminal spinal cord (note that the vertebral exit levels of the L2–S2 roots are all caudal to the spinal cord termination level). Together with the demonstration of their reflex nature (see Data S1 and Figure S2), as in previous studies (Hofstoetter et al., 2018; Minassian et al, 2004, 2016), we confirmed that all evoked responses analyzed here were PRM reflexes.
Previous electrophysiological studies had employed stimulation of posterior or anterior roots with the aim to identify the segmental innervation probabilities of lower-extremity muscles (Phillips and Park, 1991; Schirmer et al., 2011; Thage, 2009). Anatomically isolated roots exposed during surgery were directly stimulated, with current spread to other roots effectively avoided. In the present study, we mapped the segmentally organized motoneuronal pool activity of the L2–S2 spinal cord indirectly through electrical stimulation applied from the epidural space with inevitable current spread within the cerebrospinal fluid (Capogrosso et al., 2013) and concomitant depolarization of multiple posterior roots (Rattay et al., 2000) with complex topographic anatomy (Wall et al., 1990). The L3 spinal cord segment is flanked by the L3–T12 roots, and the S2 segment by the S2–L1 roots (Figure 2B). Posterior roots of passage from rostral segments are located laterally and, at sacral spinal cord levels, they additionally start to overlap in a posteroanterior fashion so that the S1 posterior roots overlay the other sacral roots (Wall et al., 1990). The posterior roots may thus pose a barrier and reduce the current flow not only to the dorsal columns but also to other roots. Despite this complexity and the fact that posterior roots can be stimulated along their entire length, muscles with separate segmental innervations were selectively recruited by cathodes located over the respective spinal cord segments. This selectivity can be explained by low-threshold sites of posterior root fibers at their entries into the spinal cord proposed by computer modeling studies (Ladenbauer et al., 2010; Rattay et al., 2000; Struijk et al., 1993). The present results may be the first practical demonstration of this long-assumed theory. Increased stimulation amplitudes caused a dissociation between the segmental cathode sites and the activated spinal cord segments. For cathodes over the lumbar spinal cord, this dissociation likely resulted from longitudinal current spread additionally recruiting the caudally located sacral roots and for cathodes over the sacral spinal cord, from transversal current spread around the spinal cord additionally recruiting the laterally located lumbar roots (Minassian et al., 2007; Rattay et al., 2000; Wall et al., 1990).
Computational modeling had predicted that monopolar stimulation would result in lower thresholds for posterior root stimulation, whereas bipolar setups would have a higher spatial selectivity (Struijk et al., 1993). Here, we demonstrated that thresholds of RF- and TS-PRM reflexes were lower for monopolar than bipolar stimulation. Concurrently, monopolar stimulation contributed more than statistically expected to the non-selective recruitment of RF and TS, whereas bipolar setups were more likely to recruit either of these muscles selectively. Computer simulations had also described an influence of the relative anode position in bipolar setups, with the anode shielding neural structures from cathodal stimulation (Rattay et al., 2000; Struijk et al., 1993). Our data showed that bipolar stimulation with caudal anodes contributed more than expected to the selective recruitment of RF, whereas the opposite polarity contributed more to a TS-biased recruitment.
There is a common consensus that epidural electrode arrays implanted for enhancing lower-extremity motor function need to overlay the lumbar and upper sacral spinal cord segments (Angeli et al., 2018; Gill et al., 2018; Wagner et al., 2018). Importantly, our data revealed that stimulation that was selective for either the mid-lumbar or upper sacral spinal cord during intraoperative monitoring in the prone position remained selective during postoperative studies in the supine position despite the considerable anterior-to-posterior migration of the spinal cord and roots when changing body position (Holsheimer et al., 1994; Ranger et al., 2008). This is relevant, because optimization of electrode configurations and stimulation parameters postoperatively is conducted in the supine position. In line with the spinal cord and roots moving closer to the posteriorly located epidural electrodes (Holsheimer et al., 1994), stimulation thresholds dropped in the supine position.
Our mapping study based on the use of midline-located, percutaneous linear electrode leads. Recent studies applying EES for the recovery of lower-extremity motor function had employed surgical paddle arrays with 16 electrodes arranged in three columns (Calvert et al., 2019a; Grahn et al., 2017; Harkema et al., 2011), although few investigations have capitalized on lateral cathodes to enhance motor function in the ipsilateral lower extremity (Gill et al., 2018; Wagner et al., 2018). Our results recommend that the identification of segmental cathode locations should be primarily performed using midline-located electrode setups. Laterally located electrodes may activate posterior roots of passage and activate spinal segments that do not correspond to the rostrocaudal stimulation site.
We used the lowest programmable EES frequency to ensure a relationship between segmental stimulation sites and activated spinal cord segments given by homonymous monosynaptic connections (Capogrosso et al., 2013; Minassian et al., 2004). The resulting spinal mapping represents an essential step toward establishing a framework of the causal relationship between epidural stimulation site, electrode setup, stimulation amplitude, and the recruited posterior roots. The impact of EES frequency requires an in-depth analysis to complement this framework. Functionally, variation of EES frequency may be exploited to alter the balance of activation between recruited motoneuron pools. An increase of EES frequency from 5–16 Hz to 21 Hz and beyond was suggested to shift the motor output patterns to flexor muscles (Jilge et al., 2004; Wagner et al., 2018). Indeed, mid-lumbar posterior-root stimulation at frequencies of 60 to 120 Hz could evoke a synergistic activation of flexor muscles that was used to facilitate the swing phase during overground locomotion in individuals with SCI (Wagner et al., 2018).
Limitations of the study
Our analysis involved the pooling of EMG data obtained by different electrode setups. We stratified for monopolar and bipolar stimulation and within the bipolar subset of data, for different setups. Differences in impedance across electrodes and subjects could have additionally influenced PRM-reflex recruitment. Electrode dimensions and materials of all lead models used were the same, leaving the conductor wire resistance as the only difference between models (Device specifications for lead models, Medtronic). Excluding this resistance, the median bipolar tissue impedance (Model 3487A) was previously measured to be 547 Ω (IQR: 453–652 Ω) (Alò et al., 2006). An increase in tissue impedance caused by fibrous encapsulation is a late phenomenon, not occurring within 18 days post-implantation (Alò et al., 2006), and was likely not relevant here. Tissue impedance can also increase should the stimulating electrodes not directly contact the dura mater (Manola and Holsheimer, 2004). Using voltage-controlled stimulators and monopolar or bipolar EES, alterations in impedance will change the current amplitudes in an inversely proportional way but will have little influence on the current flow directions within the dural sac (Manola and Holsheimer, 2004). Thus, these factors may shift the absolute response thresholds rather than change the recruitment order of spinal cord segments.
We focused parts of our analysis on RF and TS responses because among the tested muscles, their motoneuron pools show the least segmental overlap, which was essential for our threshold-based approach to identify segmental cathode locations. We might have used Add instead of RF; however, data of Add responses were available only in 25 subjects. Add was not considered in other EES studies (Angeli et al., 2018; Gill et al., 2018; Harkema et al., 2011; Wagner et al., 2018). An electrode lead placed to cover the L2–S2 segments can be configured to recruit all muscles essential for locomotion. Optimally targeting the hip flexor muscles may require electrodes located more rostrally by one segment (Wagner et al., 2018). As opposed to the noninvasive assessment employed here, selective EMG recordings from the deeply located iliopsoas muscle require fine-wire electrodes (Angeli et al., 2014; Harkema et al., 2011), or intraoperatively, intramuscular needle electrodes (Wagner et al., 2018).
All data were collected from participants being treated for spasticity, which could have led to excitability changes of monosynaptic reflex connections and thus impact the recruitment of PRM reflexes. PRM reflexes share some physiological characteristics with the H reflex (Minassian et al., 2020). Conflicting information exists regarding changes in the H reflex threshold and gain (slope of the H-reflex recruitment curve) in chronic SCI, proposed to be lower, equal, as well as higher compared with controls (Hilgevoord et al., 1994; Knikou et al., 2009; Schindler-Ivens and Shields, 2004). In the case of increased reflex excitability, the activation of a relatively small fraction of afferents could be sufficient to elicit a PRM reflex. Thus, the effective range of an epidural cathode would be increased and the segmental specificity reduced. Should such excitability changes be muscle specific, they could partially explain the behavior of Ham, as threshold stimulation did not reflect its segmental innervation. Our data suggest that PRM reflexes of Ham would not provide useful information for intraoperative monitoring to guide epidural lead placement.
We had included data of subjects with heterogeneous types of upper motoneuron disorders and severities of traumatic SCI in order to map the lumbosacral spinal cord from a wide range of available rostrocaudal cathode positions and to increase the sample size for intra- and postoperative comparisons with appropriate statistical power. This heterogeneity had no confounding impact on the major results of our study because there were no statistical differences in PRM recruitment between the subgroups at threshold and common threshold. Our results may suggest that individuals with upper motoneuron disorders other than SCI could be recruited for future studies of EES for motor recovery with similar effects on lower-extremity motor recruitment to be expected.
Resource availability
Lead contact
Further information and requests should be directed to and will be fulfilled by the Lead Contact, Ursula Hofstoetter (ursula.hofstoetter@meduniwien.ac.at).
Materials availability
This study did not generate new materials or new unique reagents.
Data and code availability
The published article includes all datasets analyzed during this study. Data were analyzed using MATLAB R2019a, The MathWorks, Inc., Natick, MA, USA, and IBM SPSS Statistics 26.0 for Windows, IBM Corporation, Armonk, NY, USA.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
The study was supported by the Austrian Science Fund (FWF), proj.nr. I 3837-B34. We wish to acknowledge the continuous support by Prof. Wolfgang Drexler, Head of Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Austria.
Author contributions
Conceptualization: K.M. and U.S.H.; Methodology: U.S.H., I.P., and K.M.; Software: U.S.H. and I.P.; Analysis: U.S.H., I.P., and A.B.; Resources: P.L., H.B., and B.F.; Data Curation: B.F. and U.S.H.; Writing—Original Draft: U.S.H., I.P., and K.M.; Review & Editing: all authors; Visualization: U.S.H., I.P., and A.B.; Supervision: K.M. and B.F.; Funding Acquisition: U.S.H.
Declaration of interests
The authors declare no competing interests.
Published: January 22, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101930.
Supplemental information
References
- Air E.L., Toczyl G.R., Mandybur G.T. Electrophysiologic monitoring for placement of laminectomy leads for spinal cord stimulation under general anesthesia. Neuromodulation. 2012;15:573–580. doi: 10.1111/j.1525-1403.2012.00475.x. [DOI] [PubMed] [Google Scholar]
- Alò K., Varga C., Krames E., Prager J., Holsheimer J., Manola L., Bradley K. Factors affecting impedance of percutaneous leads in spinal cord stimulation. Neuromodulation. 2006;9:128–135. doi: 10.1111/j.1525-1403.2006.00050.x. [DOI] [PubMed] [Google Scholar]
- Angeli C.A., Boakye M., Morton R.A., Vogt J., Benton K., Chen Y., Ferreira C.K., Harkema S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- Angeli C.A., Edgerton V.R., Gerasimenko Y.P., Harkema S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolat G., Massaro F., He J., Zeme S., Ketcik B. Mapping of sensory responses to epidural stimulation of the intraspinal neural structures in man. J. Neurosurg. 1993;78:233–739. doi: 10.3171/jns.1993.78.2.0233. [DOI] [PubMed] [Google Scholar]
- Barolat G., Singh-Sahni K., Staas W.E., Shatin D., Ketcik B., Allen K. Epidural spinal cord stimulation in the management of spasms in spinal cord injury: a prospective study. Stereotact. Funct. Neurosurg. 1995;64:153–164. doi: 10.1159/000098744. [DOI] [PubMed] [Google Scholar]
- Brodal A. Third edition. Oxford University Press; 1981. Neurological Anatomy in Relation to Clinical Medicine. [Google Scholar]
- Brodal P., Rinvik E. The somatic afferent pathways. In: Brodal A., editor. Neurological Anatomy in Relation to Clinical Medicine. Oxford University Press; 1981. pp. 46–147. [Google Scholar]
- Calabrese E., Adil S.M., Cofer G., Perone C.S., Cohen-Adad J., Lad S.P., Johnson G.A. Postmortem diffusion MRI of the entire human spinal cord at microscopic resolution. Neuroimage Clin. 2018;18:963–971. doi: 10.1016/j.nicl.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert J.S., Grahn P.J., Strommen J.A., Lavrov I.A., Beck L.A., Gill M.L., Linde M.B., Brown D.A., Van Straaten M.G., Veith D.D. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J. Neurotrauma. 2019;36:1451–1460. doi: 10.1089/neu.2018.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert J.S., Grahn P.J., Zhao K.D., Lee K.H. Emergence of epidural electrical stimulation to facilitate sensorimotor network functionality after spinal cord injury. Neuromodulation. 2019;22:244–252. doi: 10.1111/ner.12938. [DOI] [PubMed] [Google Scholar]
- Canbay S., Gürer B., Bozkurt M., Comert A., Izci Y., Başkaya M.K. Anatomical relationship and positions of the lumbar and sacral segments of the spinal cord according to the vertebral bodies and the spinal roots. Clin. Anat. 2014;27:227–233. doi: 10.1002/ca.22253. [DOI] [PubMed] [Google Scholar]
- Capogrosso M., Wenger N., Raspopovic S., Musienko P., Beauparlant J., Bassi Luciani L., Courtine G., Micera S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:19326–19340. doi: 10.1523/JNEUROSCI.1688-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A.W., Weinstein S.P. Chronic dorsal column stimulation in multiple sclerosis. Preliminary Report. N. Y. State J. Med. 1973;73:2868–2872. [PubMed] [Google Scholar]
- Davidoff R.A. The dorsal columns. Neurology. 1989;39:1377–1385. doi: 10.1212/wnl.39.10.1377. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M.M., Dimitrijevic M.R., Illis L.S., Nakajima K., Sharkey P.C., Sherwood A.M. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent. Nerv. Syst. Trauma. 1986;3:129–144. doi: 10.1089/cns.1986.3.129. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M.R., Faganel J., Sharkey P.C., Sherwood A.M. Study of sensation and muscle twitch responses to spinal cord stimulation. Int. Rehabil. Med. 1980;2:76–81. doi: 10.3109/09638288009163961. [DOI] [PubMed] [Google Scholar]
- Formento E., Minassian K., Wagner F., Mignardot J.B., Le Goff-Mignardot C.G., Rowald A., Bloch J., Micera S., Capogrosso M., Courtine G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018;21:1728–1741. doi: 10.1038/s41593-018-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes L.A., Baker L.E. The specific resistance of biological material--a compendium of data for the biomedical engineer and physiologist. Med. Biol. Eng. 1967;5:271–293. doi: 10.1007/BF02474537. [DOI] [PubMed] [Google Scholar]
- Gildenberg P. Neuromodulation: a historical perspective. In: Neuromodulation E.K., Peckham P., Rezai A., editors. Elsevier-Academic Press; 2009. pp. 9–20. [Google Scholar]
- Gill M.L., Grahn P.J., Calvert J.S., Linde M.B., Lavrov I.A., Strommen J.A., Beck L.A., Sayenko D.G., Van Straaten M.G., Drubach D.I. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018;24:1677–1682. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- Grahn P.J., Lavrov I.A., Sayenko D.G., Van Straaten M.G., Gill M.L., Strommen J.A., Calvert J.S., Drubach D.I., Beck L.A., Linde M.B. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin. Proc. 2017;92:544–554. doi: 10.1016/j.mayocp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y., Ferreira C., Willhite A., Rejc E., Grossman R.G. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Barolat G., Holsheimer J., Struijk J.J. Perception threshold and electrode position for spinal cord stimulation. Pain. 1994;59:55–63. doi: 10.1016/0304-3959(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Hilgevoord A.A.J., Koelman J.H.T.M., Bour L.J., Ongerboer de Visser B.W. Normalization of soleus H-reflex recruitment curves in controls and a population of spastic patients. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1994;93:202–208. doi: 10.1016/0168-5597(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Hintzsche E., Gisler P. Die Lage der Rückenmarksegmente im Wirbelkanal. Schweiz Arch. Neurol. Neurochir Psychiatr. 1935;35:287–294. [Google Scholar]
- Hofstoetter U.S., Freundl B., Binder H., Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PLoS One. 2018;13:e0192013. doi: 10.1371/journal.pone.0192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation. 2002;5:25–31. doi: 10.1046/j.1525-1403.2002._2005.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36:531–540. doi: 10.1038/sj.sc.3100717. [DOI] [PubMed] [Google Scholar]
- Holsheimer J., den Boer J.A., Struijk J.J., Rozeboom A.R. MR assessment of the normal position of the spinal cord in the spinal canal. AJNR. Am. J. Neuroradiol. 1994;15:951–959. [PMC free article] [PubMed] [Google Scholar]
- Illis L.S., Oygar A.E., Sedgwick E.M., Awadalla M.A. Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis. Lancet (London, England) 1976;1:1383–1386. doi: 10.1016/s0140-6736(76)93030-0. [DOI] [PubMed] [Google Scholar]
- Jilge B., Minassian K., Rattay F., Pinter M.M., Gerstenbrand F., Binder H., Dimitrijevic M.R. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp. Brain Res. 2004;154:308–326. doi: 10.1007/s00221-003-1666-3. [DOI] [PubMed] [Google Scholar]
- Knikou M., Angeli C.A., Ferreira C.K., Harkema S.J. Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int. J. Neurosci. 2009;119:2056–2073. doi: 10.1080/00207450903139747. [DOI] [PubMed] [Google Scholar]
- Krames E., Rezai A., Peckham P., Aboelsaad F. What is neuromodulation? In: Neuromodulation E.K., Peckham P., Rezai A., editors. Elsevier-Academic Press; 2009. pp. 3–8. [Google Scholar]
- Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010;18:637–645. doi: 10.1109/TNSRE.2010.2054112. [DOI] [PubMed] [Google Scholar]
- Lang J., Geisel U. [Lumbosacral part of the dural sac and the topography of its contents] Morphol. Med. 1983;3:27–46. [PubMed] [Google Scholar]
- Lloyd D. Neuron patterns controlling transmission of ipsilateral hind limb reflexes in cat. J. Neurophysiol. 1943;6:293–315. [Google Scholar]
- Lloyd D.P.C., McIntyre A.K. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke’s column. J. Neurophysiol. 1950;13:39–54. doi: 10.1152/jn.1950.13.1.39. [DOI] [PubMed] [Google Scholar]
- Manola L., Holsheimer J. Technical performance of percutaneous and laminectomy leads analyzed by modeling. Neuromodulation. 2004;7:231–241. doi: 10.1111/j.1094-7159.2004.04207.x. [DOI] [PubMed] [Google Scholar]
- Mendez A., Islam R., Latypov T., Basa P., Joseph O., Knudsen B., Siddiqui A., Summer P., Staehnke L., Grahn P. Segment-specific orientation of the dorsal and ventral roots for precise therapeutic targeting of human spinal cord. bioRxiv. 2020;31:928804. doi: 10.1016/j.mayocp.2020.07.039. [DOI] [PubMed] [Google Scholar]
- Minassian K., Freundl B., Hofstoetter U. The posterior root-muscle reflex. In: Deletis V., Shils J., Sala F., Seidel K., editors. Neurophysiology in Neurosurgery. A Modern Approach. Academic Press; 2020. pp. 239–253. [Google Scholar]
- Minassian K., Hofstoetter U., Tansey K., Mayr W. Neuromodulation of lower limb motor control in restorative neurology. Clin. Neurol. Neurosurg. 2012;114:489–497. doi: 10.1016/j.clineuro.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K., Jilge B., Rattay F., Pinter M.M., Binder H., Gerstenbrand F., Dimitrijevic M.R. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42:401–416. doi: 10.1038/sj.sc.3101615. [DOI] [PubMed] [Google Scholar]
- Minassian K., McKay W.B., Binder H., Hofstoetter U.S. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics. 2016;13:284–294. doi: 10.1007/s13311-016-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K., Persy I., Rattay F., Pinter M.M., Kern H., Dimitrijevic M.R. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 2007;26:275–295. doi: 10.1016/j.humov.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Murg M., Binder H., Dimitrijevic M.R. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches - a functional method to define the site of stimulation. Spinal Cord. 2000;38:394–402. doi: 10.1038/sj.sc.3101038. [DOI] [PubMed] [Google Scholar]
- North R., Lanning A., Hessels R., Cutchis P. Spinal cord stimulation with percutaneous and plate electrodes: side effects and quantitative comparisons. Neurosurg. Focus. 1997;2:e3. doi: 10.3171/foc.1997.2.1.4. [DOI] [PubMed] [Google Scholar]
- Phillips L.H., Park T.S. Electrophysiologic mapping of the segmental anatomy of the muscles of the lower extremity. Muscle Nerve. 1991;14:1213–1218. doi: 10.1002/mus.880141213. [DOI] [PubMed] [Google Scholar]
- Pinter M.M., Gerstenbrand F., Dimitrijevic M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord. 2000;38:524–531. doi: 10.1038/sj.sc.3101040. [DOI] [PubMed] [Google Scholar]
- Ranger M.R.B., Irwin G.J., Bunbury K.M., Peutrell J.M. Changing body position alters the location of the spinal cord within the vertebral canal: a magnetic resonance imaging study. Br. J. Anaesth. 2008;101:804–809. doi: 10.1093/bja/aen295. [DOI] [PubMed] [Google Scholar]
- Rattay F., Minassian K., Dimitrijevic M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000;38:473–489. doi: 10.1038/sj.sc.3101039. [DOI] [PubMed] [Google Scholar]
- Richardson R.R., Cerullo L.J., McLone D.G., Gutierrez F.A., Lewis V. Percutaneous epidural neurostimulation in modulation of paraplegic spasticity. Six case reports. Acta Neurochir. (Wien) 1979;49:235–243. doi: 10.1007/BF01808963. [DOI] [PubMed] [Google Scholar]
- Rock A.K., Truong H., Park Y.L., Pilitsis J.G. Spinal cord stimulation. Neurosurg. Clin. N. Am. 2019;30:169–194. doi: 10.1016/j.nec.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Schindler-Ivens S.M., Shields R.K. Soleus H-reflex recruitment is not altered in persons with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2004;85:840–847. doi: 10.1016/j.apmr.2003.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer C.M., Shils J.L., Arle J.E., Cosgrove G.R., Dempsey P.K., Tarlov E., Kim S., Martin C.J., Feltz C., Moul M., Magge S. Heuristic map of myotomal innervation in humans using direct intraoperative nerve root stimulation. J. Neurosurg. Spine. 2011;15:64–70. doi: 10.3171/2011.2.SPINE1068. [DOI] [PubMed] [Google Scholar]
- Schoen N., Chieng L.O., Madhavan K., Jermakowicz W.J., Vanni S. The use of intraoperative electromyogram during spinal cord stimulator placement surgery: a case series. World Neurosurg. 2017;100:74–84. doi: 10.1016/j.wneu.2016.12.077. [DOI] [PubMed] [Google Scholar]
- Shealy C.N., Mortimer J.T., Reswick J.B. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth. Analg. 1967;46:489–491. [PubMed] [Google Scholar]
- Shils J.L., Arle J.E. Intraoperative neurophysiologic methods for spinal cord stimulator placement under general anesthesia. Neuromodulation. 2012;15:560–572. doi: 10.1111/j.1525-1403.2012.00460.x. [DOI] [PubMed] [Google Scholar]
- Shils J.L., Arle J.E. Neuromonitoring for spinal cord stimulation lead placement under general anesthesia. J. Clin. Neurol. 2018;14:444–453. doi: 10.3988/jcn.2018.14.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijk J.J., Holsheimer J., Boom H.B.K. Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans. Biomed. Eng. 1993;40:632–639. doi: 10.1109/10.237693. [DOI] [PubMed] [Google Scholar]
- Thage O. The myotomes L2-S2 in man. Acta Neurol. Scand. 2009;41:241–244. doi: 10.1111/j.1600-0404.1965.tb01878.x. [DOI] [PubMed] [Google Scholar]
- Tulgar M., He J., Barolat G., Ketcik B., Struijk H., Holsheimer J. Analysis of parameters for epidural spinal cord stimulation. 3. Topographical distribution of paresthesiae-- a preliminary analysis of 266 combinations with contacts implanted in the midcervical and midthoracic vertebral levels. Stereotact. Funct. Neurosurg. 1993;61:146–155. doi: 10.1159/000100632. [DOI] [PubMed] [Google Scholar]
- Wagner F.B., Mignardot J.-B., Le Goff-Mignardot C.G., Demesmaeker R., Komi S., Capogrosso M., Rowald A., Seáñez I., Caban M., Pirondini E. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- Wall E.J., Cohen M.S., Abitbol J.J., Garfin S.R. Organization of intrathecal nerve roots at the level of the conus medullaris. J. Bone Joint Surg. Am. 1990;72:1495–1499. [PubMed] [Google Scholar]
- Whitsel B., Petrucelli L., Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969;15:67–78. doi: 10.1016/0006-8993(69)90310-2. [DOI] [PubMed] [Google Scholar]
- York D.H. Somatosensory evoked potentials in man: differentiation of spinal pathways responsible for conduction from the forelimb vs hindlimb. Prog. Neurobiol. 1985;25:1–25. doi: 10.1016/0301-0082(85)90021-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets analyzed during this study. Data were analyzed using MATLAB R2019a, The MathWorks, Inc., Natick, MA, USA, and IBM SPSS Statistics 26.0 for Windows, IBM Corporation, Armonk, NY, USA.