Abstract
Ethylene glycol monomethyl ether (EGME) has been used in many products usually handled by humans including inks, paints, polishes, brake fluids and so on. This present study therefore, investigated its effect on lung, in a time-course study in male Wistar rats. Animals were orally administered 50 mg/kg body weight of EGME for a period of 7, 14, and 21 days. Following 7 days of oral exposure to EGME, activities of GPx and SOD were significantly increased, as well as levels of K-Ras, c-Myc, p53, caspase-3, TNF-α and, IL-6, while NO level and GST activity were significantly reduced compared with control. At the end of 14 days exposure, GSH level was significantly decreased, while levels of K-Ras, c-Myc, p53, caspase-3, TNF-α, IL-6, NO and the activities of SOD and GPx were significantly elevated with respect to control. After 21 days of EGME administration, levels of Bcl-2, IL-10, GSH and NO as well as GST activity were significantly decreased, while levels of K-Ras, c-Myc, p53, Bax, caspase-3, IL-6, IL-1β, TNF-α, as well as GPx, CAT, and SOD activities were significantly elevated compared with control. Lung histopathology revealed chronic disseminated alveolar inflammation, bronchiolitis, severe alveolar and bronchi hyperplasia, severe disseminated inflammation, thrombosis, and thickened vessels as a result of EGME exposures. Exposures to EGME could trigger lung damage via the disorganization of the antioxidant system, eliciting the up-regulation of inflammatory, apoptotic, and oncogenic markers in rats.
Keywords: Ethylene glycol monomethyl ether, Oxidative stress, Inflammation, Apoptosis, Oncogenes, Histopathology, Lung
Abbreviations: MDA, malondialdehyde; GSH, reduced glutathione; NO, nitric oxide; CAT, catalase; GST, glutathione S-transferase; SOD, superoxide dismutase; GPx, glutathione peroxidase; IL-6, interleukin-6; IL-1β, interleukin-1 beta; TNF-α, tumor necrosis factor alpha; p53, tumor suppressor protein; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2 associated X; c-myc, myelocytomatosis; K-Ras, Kirsten rat sarcoma viral oncogene
1. Introduction
Ethylene glycol monomethyl ether (EGME) is a derivative of dimethoxyethilphatalate (DMEP) derivatives, a class of chemicals used in plastics manufacturing. These plastic materials are used for the production of children’s toys, materials for packaging food and drinks, household appliances, water channels, and different types of medical equipment [1]. Majorly, EGME serves as an organic solvent in the production of different industrial products including paints, resins, varnish, acetate cellulose, nail polish, and wood paints [2].
Particularly vulnerable tissues and organs to EGME are those with high metabolic and swift proliferation rate like cells of the thymus and testis [[3], [4], [5]]. Occupationally, exposure to EGME has been linked with neurological, reproductive and hematological abnormalities in human studies [[6], [7], [8], [9]]. Damage to reproductive organs, such as alteration of fertility in female and testicular atrophy, is the major result of EGME exposure in laboratory animals [10]. Also, EGME can attack cells involved in immunity, leading to low weight of thymus, thymus cell atrophy, and low splenic cell count [11].
EGME is formed via the metabolism of DMEP inside a mammalian body to form EGME, which in turn, is oxidized by alcohol dehydrogenase to form 2-methoxyacetaldehyde (2-MALD). Subsequently, oxidation of 2-MALD yields methoxyacetic acid (MAA), the teratogenic and toxic product [12]. Hence, EGME by itself is not toxic or teratogenic, but its metabolic product called MAA [5]. Methoxyacetaldehyde (MALD) was reported to induce hepatic- and testicular toxicity [13]. Accumulation of MALD in the hepatocytes triggers the generation of reactive oxygen species (ROS), leading to the oxidization of lipids, altering the membrane functions and reducing fluidity of membrane. Apart from the liver, ovarian and renal cells have been reported to be attacked by ROS generated by MALD. Hepatic and renal cells play an active role in the elimination of toxic substance including EGME [14]. The kidney is majorly involved in the excretion of toxic substances. As blood circulates in large volume, lethal substances are localized in the filtrate and activated, making the kidney a major target for toxic substances [15].
EGME-induced hepatic [16], renal [17], and testicular [18] oxidative stress, inflammation, and apoptosis, in a time-course study have been recently reported in rats, but little or no information existed on the effect of EGME on pulmonary cells. Thus, we checked the time course effect of EGME on pulmonary markers of lipid peroxidation (MDA), oxidative stress (CAT, SOD, GPx, GST, GSH, and NO), inflammation (IL-1β, IL-6, IL-10, and TNF-α), apoptosis (caspase-3, p53, Bax, and Bcl-2) and proto-oncogenic markers (c-Myc and K-Ras) in rats.
2. Materials and methods
2.1. Chemicals and kits
EGME (C3H8O2; CAS# 109-84-4; 99.5% purity), is a product of BDH Laboratory Supplies, Poole, BH15 1TD, England. Rats enzyme-linked immunosorbent assay (ELISA) kits for IL-10, IL-6, IL-1β, TNF-α, caspase-3, p53, Bax, Bcl-2, c-Myc, and K-Ras were manufactured by Cusabio Technology Llc, Houston, TX, USA. Reagents and chemicals were of quality and good grade and were procured from recognized chemical companies in the United States (Sigma) and United Kingdom (BDH).
2.2. Animals and experimental design
Male albino Wistar Unilever rats (150 g; 8 weeks old; n = 20) were bought from a breeding farm at the Federal University of Agriculture, Abeokuta (FUNAAB), Nigeria. Rats were housed in experimental rat cages in the animal house facility of our institution, where they have unrestricted access to water and food. Research procedures were handled following stipulated rule the Animal Care and Use Committee of our institution, approved by the Animal Ethical Committee of our department (FUNAAB), Nigeria. At the end of 1 week of adjustment to the new environment, the rats were grouped into four, having five animals each. Group 1 rats were designated as normal control and sacrificed (day 0) before the commencement of the administration of EGME. Groups 2, 3 and 4 animals were exposed orally to 50 mg/kg (1/20th of LD50) EGME for 7, 14, and 21 days respectively.
2.3. Collection and preparation of samples
Following EGME administration, rats were sacrificed by cervical dislocation, and were handled under the international rules for the handling and use of experimental animals [19]. We harvested the lung, washed in normal saline, dried, and weighed. Lung sections were suspended in 0.1 M (pH 7.4) phosphate buffer for homogenization. The homogenized tissue was centrifuged for 10 min at 5000 rpm, and the supernatant was used for the estimations of parameters investigated in triplicates.
2.4. Estimation of oxidative stress parameters
Buege and Aust [20] was used for the estimation of MDA, NO concentration in the lung was estimated using Griess Reagent [21], GSH concentration was determined following the procedure of Moron et al. [22], GPx activity was determined by the procedure of Rotruck et al. [23], lung GST activity was determined by the procedure of Habig et al. [24], the SOD activity was estimated using the procedure of Misra and Fridovich [25], while CAT activity was determined using the procedure of Sinha [26].
2.5. Estimations of lung levels of IL-10, IL-6, IL-1β, TNF-α, caspase-3, p53, Bax, Bcl-2, c-Myc, and K-Ras
These were done following the protocols described in the purchased Cusabio ELISA kits (Cusabio Technology Llc, Houston, TX, USA).
2.6. Estimation of total protein concentration
Lung level of total protein was estimated using the procedure of Gornall et al. [27], and was used for the determination of SOD, GST, GPx, and CAT activities.
2.7. Lung histopathology
Excised lung sections were preserved in 10% buffered-formalin solution for 48 h, dehydration in ascending concentrations of alcohol concentration and double-washed in xylene. Tissue sections were paraffinized, excised, and then stained with haematoxylin and eosin dye. Slides were viewed at a magnification of 400 x under a microscope.
2.8. Statistical analyses
Data were examined using one-way analysis of variance (ANOVA), and then Tukey test for multiple comparisons among the groups of rats using Graph Pad Prism 6.0. Results were written as mean ± SEM, and P value below 0.05 was taken to be significant.
3. Results
3.1. Lung relative weight of EGME-administered rats
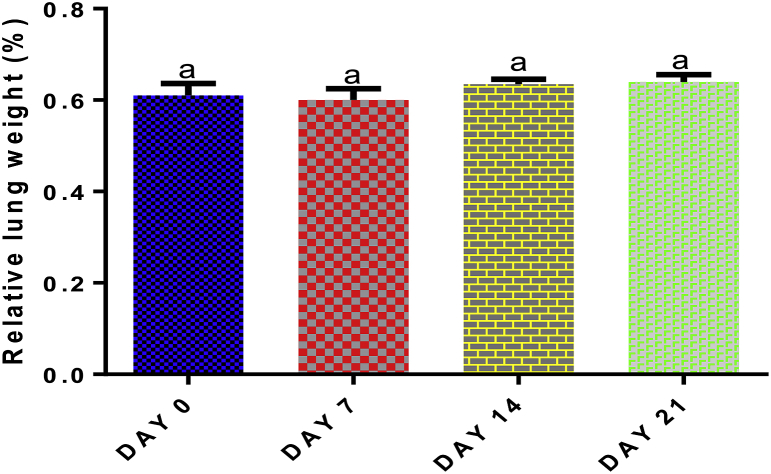
The lung weight was measured to check the effect of EGME on the pulmonary cell content, whether the cells are rapidly dividing (increasing cell content) or dying (decreasing cell content and function) compared with control. The calculated lung weight was then used to calculate the relative lung weight of the animals. With respect to control, no significant effect (p > 0.05) was seen in relative lung weight following 7, 14, or 21 days of EGME administration (Fig. 1).
Fig. 1.
Time course effect of EGME on relative lung weight. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
3.2. Time course effect of EGME on lung MDA level
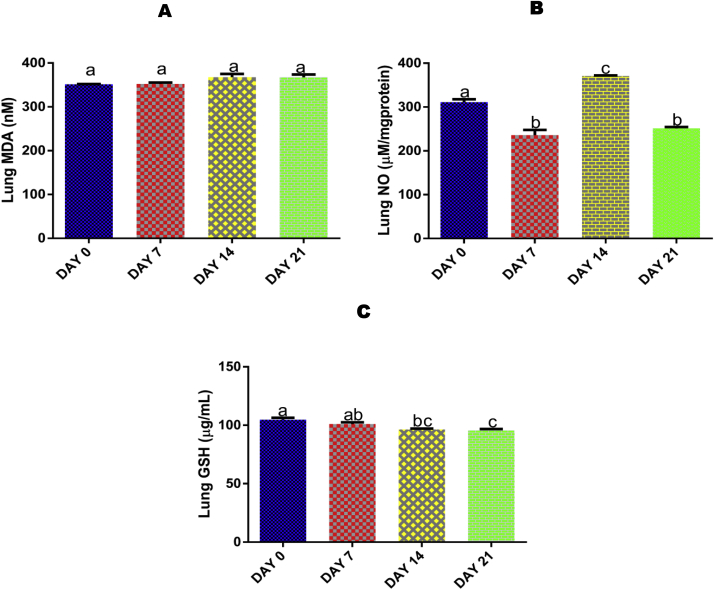
No significant (p > 0.05) effect was seen in lung MDA concentration after 7, 14, and 21 days of EGME administrations compared with control (Fig. 2A).
Fig. 2.
Time course effect of EGME on lung MDA (2A), NO (2B), and GSH (2C) concentrations. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
3.3. Time course effect of EGME on lung NO level
Administrations of EGME for 7 and 21 days lowered significantly (p < 0.05) the pulmonary level of NO compared with control and 14 days of administrations, while administrations for 14 days significantly (p < 0.05) elevated lung NO level with respect to control, 7, and 21 days of exposure (Fig. 2B).
3.4. Time course effect of EGME on lung GSH level
Administrations of EGME for 14 and 21 days significantly (p < 0.05) reduced the lung level of GSH with respect to day 0 (Fig. 2C).
3.5. Time course effect of EGME on lung activity of GPx
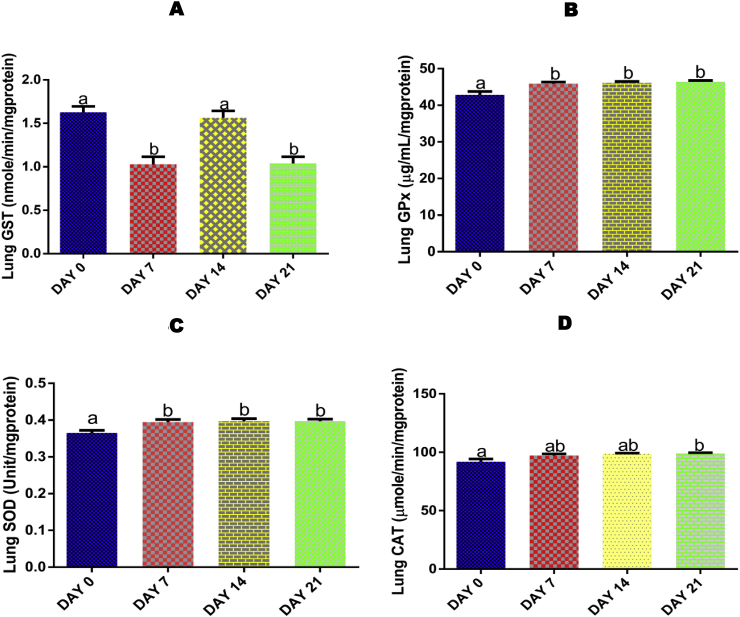
Administrations of EGME for 7, 14, and 21 days resulted in a significant (p < 0.05) increase in lung GPx activity with reference to day 0 (Fig. 3A).
Fig. 3.
Time course effect of EGME on lung GPx (3A), GST (3B), SOD (3C), and CAT (3D) activities. Values are expressed as mean ± standard error of the mean (n = 5). Bars labeled with different letters are statistically significant (p < 0.05).
3.6. Time course effect of EGME on lung GST activity
For GST, both 7 and 21 days of exposure to EGME significantly (p < 0.05) decreased the pulmonary activity of the antioxidant enzyme when compared with day 0 and 14 days of exposure (Fig. 3B).
3.7. Time course effect of EGME on lung SOD activity
Lung SOD activity was significantly (p < 0.05) increased following 7, 14, and 21 days of EGME administrations compared with control (Fig. 3C).
3.8. Time course effect of EGME on lung CAT activity
Only exposure for 21 days significantly (p < 0.05) increased the lung CAT activity when compared with control (Fig. 3D).
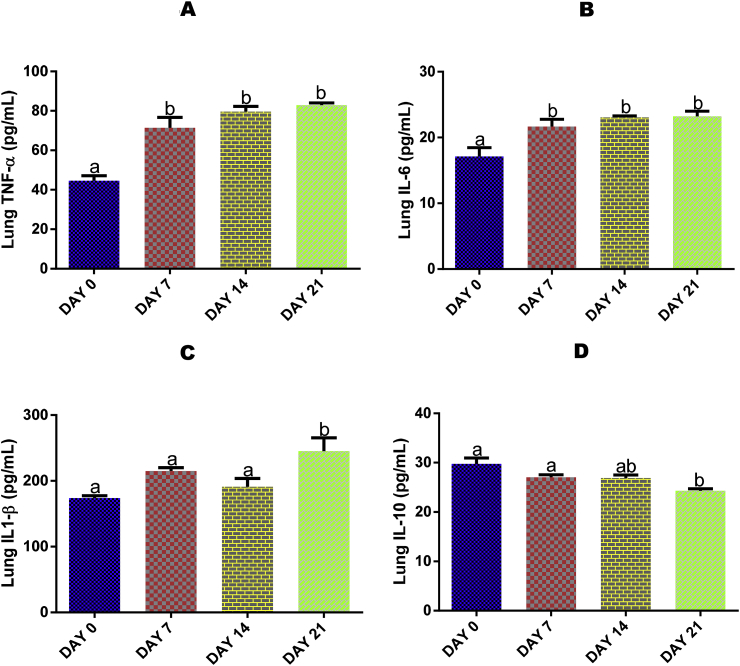
3.9. Time course effect of EGME on lung IL-10, IL-1β, IL-6, and TNF-α levels
Lung TNF-α (Fig. 4A) and IL-6 (Fig. 4B) levels were significantly (p < 0.05) elevated after 7, 14 and 21 days, while IL-1β (Fig. 4C) and IL-10 (Fig. 4D) levels were significantly (p < 0.05) increased and reduced respectively by 21 days of EGME administrations with respect to day 0.
Fig. 4.
Time course effect of EGME on lung TNF-α (4A), IL-6 (4B), IL-1β (4C), and IL-10 (4D) levels. Values are expressed as mean ± standard error of the mean. Bars labeled with different letters are statistically significant (p < 0.05).
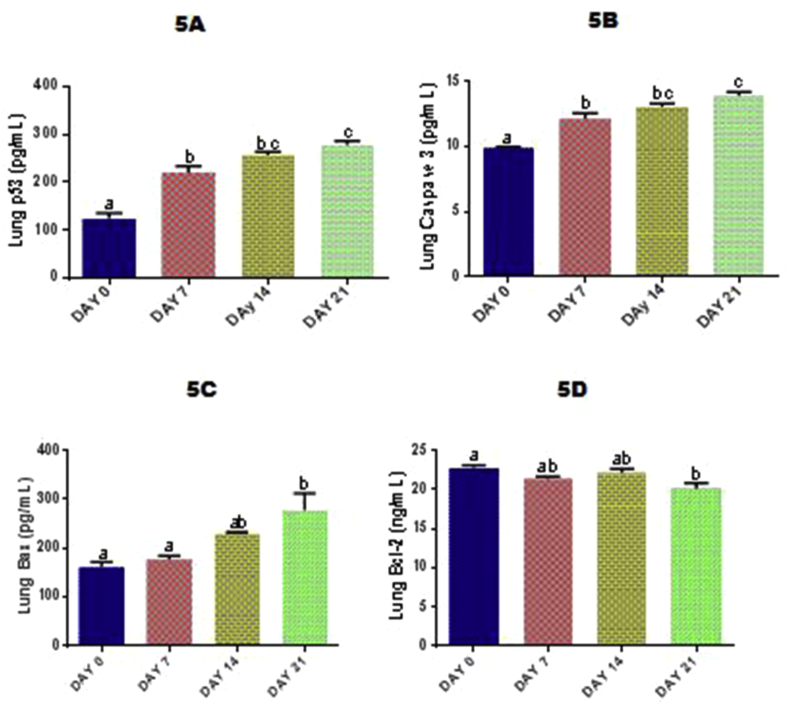
3.10. Time course effect of EGME on lung p53, Bcl-2, Bax, and caspase-3 levels
Exposure to EGME for 7, 14 and 21 days significantly (p < 0.05) raised the lung levels of p53 (Fig. 5A) and caspase-3 (Fig. 5B) in a time-dependent manner compared with day 0. Lung Bax (Fig. 5C) and Bcl-2 (Fig. 5D) concentrations were significantly (p < 0.05) increased and lowered respectively after 21 days of EGME administrations compared with day 0.
Fig. 5.
Time course effect of EGME on lung p53 (5A), caspase-3 (5B), Bax (5C) and Bcl-2 (5D) levels. Values are expressed as mean ± standard error of the mean. Bars labeled with different letters are statistically significant (p < 0.05).
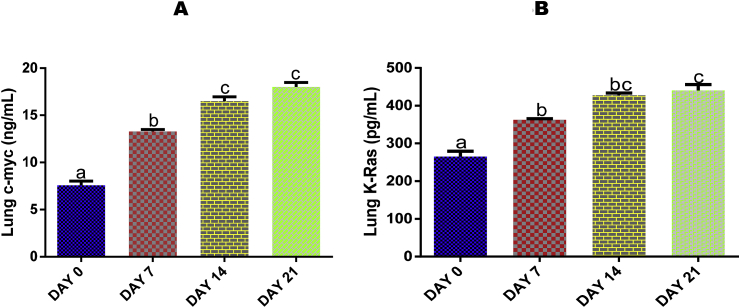
3.11. Time course effect of EGME on lung levels of c-Myc and K-Ras
Both lung levels of c-Myc (Fig. 6A) and K-Ras (Fig. 6B) were significantly (p < 0.05) elevated by EGME after 7, 14 and 21 days of administrations with respect to day 0.
Fig. 6.
Time course effect of EGME on lung c-myc (6A) and K-Ras (6B) levels. Values are expressed as mean ± standard error of the mean. Bars labeled with different letters are statistically significant (p < 0.05).
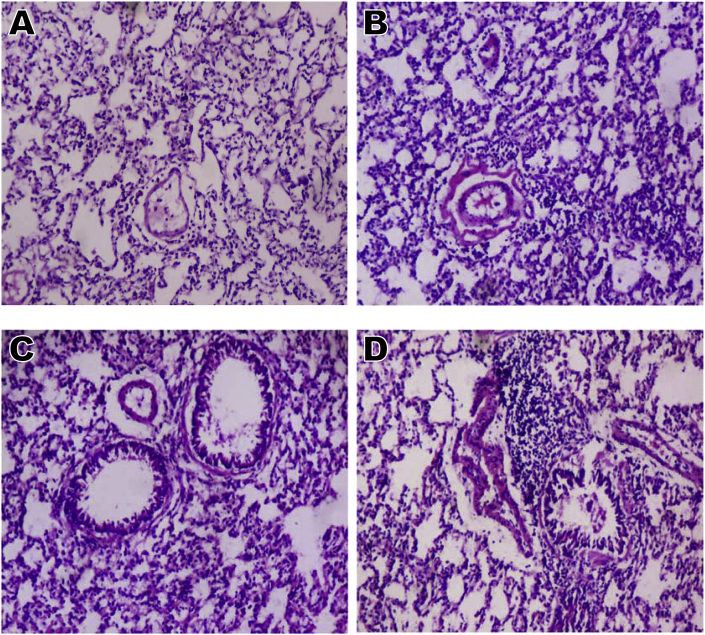
3.12. Time course effect of EGME on lung histopathology
Lung histopathology (Fig. 7) revealed chronic disseminated alveolar inflammation, bronchiolitis, severe alveolar and bronchi hyperplasia, severe disseminated inflammation, thrombosis, and thickened vessels after 7, 14, and 21 days of EGME administrations compared with the control that revealed normal architecture.
Fig. 7.
Lung microphotographs (x 100) showing (A) normal architecture; (B) disseminated alveolar inflammation, peribronchiolar inflammation, thickened vessels, thrombosis, and alveolar hyperplasia; (C) bronchiolitis, severe alveolar hyperplasia, severe disseminated inflammation, thrombosis, and thickened vessels; and (D) chronic disseminated alveolar inflammation, bronchiolitis, severe alveolar and bronchi hyperplasia, severe disseminated inflammation, thrombosis, and thickened vessels. A = Day 0; B = Day 7; C = Day 14; D = Day 21.
4. Discussion
Although the utilization of EGME has diminished recently as a result of control procedures and provisions of alternatives to it, the fact remains that EGME is still utilized in some parts of the world. The yearly utilization of EGME by the Chinese and in Taiwan is estimated at over 3000 tonnes [28]. Sales for use in some household products, consumer goods, pharmaceutical preparations, medicines, and pesticide formulations have been prohibited in the Europe. Overall deposits of EGME documented by the Canadian National Pollutant Release Inventory (NPRI) in 1994 are summed at 17 tonnes [29], and were deposited into the environment from a facility in Southern Ontario. Overall transfer of EGME for off-site elimination into the incinerators is summed at 2.12 tonnes in 1994. Total documentation of about 0.07 tonne of EGME was released for energy production in 1994 [29]. As reported by NPRI, overall on-site and off-site disposal of EGME to the environment in Southern Ontario were summed at 6.3 and 33.9 tonnes respectively in 1995 [29]. It is therefore pertinent to investigate the time-course effect of EGME administrations on some pulmonary biochemical parameters of lipid peroxidation, oxidative stress, apoptosis, inflammation, and cell proliferation in male Wistar rats.
Estimating the thiobarbituric acid reactive substance (TBARS) is employed to determine the level of lipid peroxidation, and also as an indirect method of assessing oxidative stress within and outside the cell [30,31]. Although free radicals are products of normal metabolic pathways, they can also be generated via exposures to toxic biological and chemical agents in cells. The generated free radicals are very reactive and unstable because they possess unpaired electrons, enabling them to attack electron-rich polyunsaturated fatty acids of the phospholipid bilayer of biological membranes. These lead to destruction of the membrane structure and the cell as a whole, causing the migration of cell components directly into the bloodstream [32,33]. From our findings, EGME administrations to rats for the period investigated did not have any significant effect on lung MDA (a marker of lipid peroxidation) concentration (Fig. 2). The reason for this may be due to the ability of the endogenous enzymatic and non-enzymatic antioxidant systems to withstand and overcome the harmful cellular effects of reactive oxygen and nitrogen species.
NO is a vital signaling molecule formed within the cells, playing a major role in many important physiological processes, including immune response, blood pressure and neural communication [34]. Overproduction of cellular NO may cause the generation of peroxynitrite that can eventually destroy tissues [35,36]. The significant elevation of pulmonary NO level (Fig. 2B) after 14 days of EGME administrations compared with control may be due to EGME-induced lung oxidative tissue damage in the animals. EGME-induced oxidative stress may have resulted in the overproduction of reactive species that subsequently caused the significant decrease in NO (Fig. 2B) after 7 and 21 days of EGME administrations. Free radicals have important roles in NO-coordinated cellular signaling [34]. Free radicals alter NO availability, resulting to different vascular problems that ensue following loss of NO function [34]. ROS concentrations can chemically inactivate and lower bioactive NO concentrations. It is referred to as NO mopping and considered as a major outcome of oxidative stress [34]. NO in cooperation with other free radicals has been reported to elicit oxidative stress by the vasculature, in inflammatory state [37].
Oxidative stress characterized by elevated generation of ROS promotes the processes of vascular dysfunction [38]. This oxidative stress is majorly as a result of an imbalance between the endogenous antioxidant systems and the production of ROS, in favor of the latter [34]. We recorded a significant decrease in lung GSH (Fig. 2C) concentration and GST (Fig. 3A) activity, as well as a significant elevation in lung activities of GPx (Fig. 3B), SOD (Fig. 3C) and CAT (Fig. 3D), all of these suggesting the involvement of EGME in the production of ROS, causing oxidative stress. SOD and CAT function synergistically. While the former catalyzes the detoxification of superoxide radicals to hydrogen peroxide (H2O2), the latter decompose H2O2 to water and oxygen [39]. GST is a phase two drug-metabolizing enzyme and catalyzes the release and transfer of GSH to xenobiotic for their detoxification. GSH is the chemical substrate for GPx, a process that concomitantly detoxifies H2O2 to water and oxygen [40].
The cytokine family is made up of different multifunctional proteins that are involved in the regulation of immunity and tissue inflammation [41,42]. Every biological organs are under the regulatory control of cytokines, including the cardiovascular [43], respiratory [44] and nervous [45,46] systems, as well as renal [47] and gastrointestinal tracts [48]. Cytokines are produced majorly by the immune cells when toxic or harmful chemical and biological agents are sensed within the cells [41,42]. Cytokines that stimulate inflammation are IL-6, IL-1β, TNF-α, IL-1β, interferon–(IFN–) γ, and IL-17 [49,50], and are called pro-inflammatory cytokines. On the other hand, examples of anti-inflammatory cytokines are IL-10 and IL-4 [49,51]. The significant increase in the concentrations of lung TNF-α (Fig. 4A), IL-6 (Fig. 4B), IL-1β (Fig. 4C), and lowered concentration of IL-10 (Fig. 4D) after 7, 14 and 21 days of EGME administrations, indicate EGME-induced lung damage, leading to their secretion and mobilization by the immune cells to the affected areas where they initiate the inflammatory process [52]. Macrophages extend response to inflammation via the promotion cytokine formation in cells that lack direct response to injury by toxic chemicals or biological agents [53]. It has been reported that IL-8, by responding to TNF-α and IL-1β signals, is secreted by diverse lung epithelial cells, pleural mesothelial cells and pulmonary fibroblasts [54,55]. Every nucleus containing cell possesses an active receptor for TNF-α and IL-1, indicating the importance of both as important cytokines in the intensification of inflammation [53].
p53 gene acts as an emergency gene in the initiation of cell cycle arrest or programmed cell death [56,57]. p53 can suppress malignant transformation by initiating cell cycle arrest in G1 phase or via apoptosis. Loss of p53 gene functions may explain the uncontrolled proliferation of cells and the resistance of the transformed cells to genotoxic anticancer drugs [57,58]. In this study, the significant increase in lung p53 (Fig. 5A) level after 7, 14 and 21 days of administrations is an indication of EGME-induced pulmonary damage. This could be attributed to EGME-induced lung oxidative stress and inflammation recorded in this study, which may have resulted in p53 activation. Activated p53 may have elicited cell cycle arrest and activation of apoptotic genes to initiate apoptosis. Bcl-2 and Bax are respectively, anti-apoptotic and pro-apoptotic proteins regulating the initiation of apoptosis via the regulation of mitochondrial function [59,60]. Bcl-2 and Bax have the potential to compete with each other by forming heterodimers, indicating an inverse affair, where Bcl-2 homodimers support cell proliferation and Bax homodimers support apoptosis [61]. Combined activities of Bax and Bcl-2 coordinate programmed cell death by stimulating mitochondrial membrane leakage, leading to the release of cytochrome c out of the mitochondrion into the cytosol. Overproduction of Bcl-2 hinders the pore formation and out flow of cytochrome c from the mitochondrion to the cytosol [59,62]. Therefore, the inhibition of Bcl-2 triggers the release of cytochrome c from the mitochondrion. It is also noteworthy that Bcl-2-Bax heterodimerization can negatively elicit and inhibit pro-apoptotic Bax activity [61]. Thus, minimal expressions of Bcl-2 concentration and sustained Bax expressions can make homodimers of Bax to be formed, leading to the up-regulation of apoptosis [63]. In this study, the significant increase in lung Bax (Fig. 5C) and decrease in Bcl-2 (Fig. 5D) levels after 21 days of EGME administrations suggest p53-induced apoptosis, since the apoptotic players, Bax and Bcl-2 are the key targets of p53. In response to lung toxicity, up-regulated p53 recorded in the study, may have up-regulated Bax and down-regulated Bcl-2 expressions. The resulting increase in the cytosolic level of free Bax may eventually attack the mitochondrial membrane; creating pores in it, leading to the flow of cytochrome c into the cytoplasm that later propagates cellular apoptosis. Previous studies have documented that the proportionality of anti- and pro-apoptotic proteins dictate how cells are exposed to death stimulus [64]. p53 inhibits Bcl-2 through an association with negative regulatory element beyond the promoter of Bcl-2 gene [65]. Immediately out of the mitochondrion, cytochrome c associates with Apaf-1, and then favors procaspase-9 activation [66,67]. Activated caspase-9 stimulates the activation of caspases involved in executing apoptosis such as caspase-3 and -7, via proteolytic cleavage, and propagating the apoptotic stimulus to the execution stage [68]. The above explanations will therefore, explain and justify the increased level of lung caspase-3 (Fig. 5B) recorded after 7, 14 and 21 days of EGME administrations. The released cytochrome c following Bax attack on the mitochondrial membrane may have interacted with downstream mediators like caspase-9 and Apaf-1, resulting in the formation of apoptosome that cleaved the executioner caspases including caspase-3 and facilitate the programmed cell death.
c-Myc belongs to the b/HLH/LZ proteins of Myc group that mediate programmed cell death and cell proliferation [69]. Expression of Myc protein is altered in about 33% of cancers in humans via different mechanisms [70]. mRNA of c-Myc is highly short-lived and lack of positive regulatory signals causes its transcription to decrease, resulting to low c-Myc protein concentrations, and lack of proliferative stimulus [71]. c-Myc function in tumor cells is usually raised, at times by self-gene mutation, but often via the induction of the expression of c-Myc via upstream oncogenic pathways [71]. Oncogenic activities of c-Myc are counterbalanced by its ability to also induce apoptosis via numerous pathways [72,73]. K-Ras is another proto-oncogene whose mutations are seen in approximately 20–25% of lung adenocarcinomas in Western nations [[74], [75], [76]] and approximately 10–15% of incidences in Asia [77,78]. Accepted globally, K-Ras-mutant tumors are the most frequent and potentially targetable molecular subtype of non-small-cell lung cancer (NSCLC) [79]. As it is the case with the vast majority of potentially actionable genetic alterations in NSCLC, K-Ras mutations are almost exclusively seen in lung adenocarcinomas and are uncommonly found in squamous-cell cancers [80,81]. Pulmonary c-Myc (Fig. 6A) and K-Ras (Fig. 6B) levels after 7, 14 and 21 days of EGME administrations were significantly increased, which may be an indication of EGME-induced mutations in these oncogenes by amplification or translocation to areas of high transcriptional activities, resulting into their activations and subsequent generation of reactive oxygen species that may have elicited DNA damage. Also, activation of these oncogenes may therefore, triggers the significant increase in the levels of apoptotic mediators (p53, Bax, caspase-3) recorded in this study that facilitated apoptosis and prevented tumor initiation and progression.
Lung histopathology further confirmed our findings in this study, by revealing chronic disseminated alveolar inflammation, bronchiolitis, severe alveolar and bronchi hyperplasia, severe disseminated inflammation, thrombosis, and thickened vessels throughout the period investigated.
We therefore concluded that exposures to EGME over time could pose health risk by triggering lung damage via the disorganization of the antioxidant system, eliciting the up-regulation of inflammatory, apoptotic, and oncogenic markers in rats. We therefore advised based on the outcomes of this study that exposure to EGME should be totally avoided or reduced to the barest minimum.
Funding
None received.
CRediT authorship contribution statement
Oluwatobi T. Somade: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration. Babajide O. Ajayi: Methodology, Investigation, Resources, Supervision, Project administration. Olubisi E. Adeyi: Methodology, Investigation, Resources, Supervision, Project administration. Anuoluwapo A. Adeshina: Investigation, Resources. Mary O. Adekoya: Methodology, Investigation, Resources, Project administration. Ridwan O. Abdulhameed: Methodology, Investigation, Resources, Project administration.
Declaration of competing interest
None to declare.
References
- 1.Darmanto W., Wahyuningsih S.P.A., Husein S.A., Aminah N.S., Firdaus A.N., Sajidah E.S., Izzatin M., Khaleyla F. Effect of 2-methoxyethanol induction on mice (Mus musculus) liver, kidney and ovary. J Phys Conf Ser. 2018;1116 [Google Scholar]
- 2.International Programme on Chemical Safety (Ipcs) World Health Organization; Geneva: 1990. Environmental health criteria 115. 2–Methoxyethanol, 2–ethoxyethanol, and their acetates, international programme on chemical safety. [Google Scholar]
- 3.Bagchi G., Waxman D.J. Toxicity of ethylene glycol monomethyl ether: impact on testicular gene expression. Int J Androl. 2008;31:269–274. doi: 10.1111/j.1365-2605.2007.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boatman R.J. International industry initiatives to improve the glycol ether health effects knowledge base. Toxicol Lett. 2005;156:39–50. doi: 10.1016/j.toxlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Johanson G. Toxicity review of ethylene glycol monomethyl ether and its acetate ester. Crit Rev Toxicol. 2000;30:307–345. doi: 10.1080/10408440091159220. [DOI] [PubMed] [Google Scholar]
- 6.El-Zein R.A., Abdel-Rahman S.Z., Morris D.L., Legator M.S. Exposure to ethylene glycol monomethyl ether: clinical and cytogenetic findings. Arch Environ Health. 2002;57:371–376. doi: 10.1080/00039890209601424. [DOI] [PubMed] [Google Scholar]
- 7.Larese F., Fiorito A., De Zotti R. The possible haematological effects of glycol monomethyl ether in a frame factory. Br J Ind Med. 1992;49:131–133. doi: 10.1136/oem.49.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch L.S., Cullen M.R. Effect of exposure to ethylene glycol ethers on shipyard painters: III. Hematologic effects. Am J Ind Med. 1988;14:527–536. doi: 10.1002/ajim.4700140504. [DOI] [PubMed] [Google Scholar]
- 9.Welch L.S., Schrader S.M., Turner T.W., Cullen M.R. Effects of exposure to ethylene glycol ethers on shipyard painters: II. Male reproduction. Am J Ind Med. 1988;14:509–526. doi: 10.1002/ajim.4700140503. [DOI] [PubMed] [Google Scholar]
- 10.Dodo T., Taketa Y., Sugiyama M., Inomata A., Sonoda J., Okuda Y., Mineshima H., Hosokawa S., Aoki T. Collaborative work on evaluation of ovarian toxicity. 11) Two- or four-week repeated-dose studies and fertility study of ethylene glycol monomethyl ether in female rats. J Toxicol Sci. 2009;34(1):SP121–S128. doi: 10.2131/jts.34.s121. [DOI] [PubMed] [Google Scholar]
- 11.Exon J.H., Mather G.G., Bussiere J.L., Olson D.P., Talcott P.A. Effects of subchronic exposure of rats to 2-methoxyethanol or 2- butoxyethanol: thymic atrophy and immunotoxicity. Fund Appl Toxicol. 1991;16:830–840. doi: 10.1016/0272-0590(91)90168-4. [DOI] [PubMed] [Google Scholar]
- 12.Rumanta M., Tien W.S., Sri S. Proseding Institut Teknologi Bandung. Bandung; Indonesia: 2001. Pengaruh asam metoksiasetat terhadap organ reproduksi mencit. [Google Scholar]
- 13.Moslen M.T., Kaphalia L., Balasubramanian H., Yin Y.M., William W.A. Species differences in testicular and hepatic biotransformation of 2-methoxyethanol. Toxicology. 1995;96:217–224. doi: 10.1016/0300-483x(94)02921-g. [DOI] [PubMed] [Google Scholar]
- 14.Grant D.M. Detoxification pathways in the liver. J Inherit Metab Dis. 1991;14:421–430. doi: 10.1007/BF01797915. [DOI] [PubMed] [Google Scholar]
- 15.Lu F.C. UI Press; Jakarta: 1995. Toksikologi dasar, asas, organ sasaran, dan penilaian resiko edisi ke-2. [Google Scholar]
- 16.Somade O.T., Ajayi B.O., Olunaike O.E., Jimoh L.A. Hepatic oxidative stress, up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers following 2-methoxyethanol administrations in rats. Biochem Biophys Rep. 2020;24:100806. doi: 10.1016/j.bbrep.2020.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somade O.T., Ajayi B.O., Olushola M.O., Omoseebi E.O. Methyl cellosolve-induced renal oxidative stress and time-dependent up-regulation of pro-inflammatory cytokines, apoptotic, and oncogenic markers in rats. Toxicol Rep. 2020;7:779–787. doi: 10.1016/j.toxrep.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somade O.T., Ajayi B.O., Adeyi O.E., Adeshina A.A., James A.S., Ayodele P.F. Ethylene glycol monomethyl ether-induced testicular oxidative stress and time-dependent up-regulation of apoptotic, pro-inflammatory, and oncogenic markers in rats. Metabol Open. 2020;7:100051. doi: 10.1016/j.metop.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nrc . National Academy Press; Washington, DC: 1996. Guide for the Care and use of laboratory animals. [Google Scholar]
- 20.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 21.Green L.C., Wagner D.A., Glogowski J., Skiper P.L., Wishnock J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 23.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 24.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 25.Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 26.Sinha A.K. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 27.Gornall A.G., Bardawill C.J., David M.M. Determination of serum protein by biuret method. J Biol Chem. 1949;117:751–766. [PubMed] [Google Scholar]
- 28.Shih T.S., Liou S.H., Chen C.Y., Chou J.S. Correlation between urinary 2-methoxy acetic acid and exposure of 2-methoxy ethanol. Occup Environ Med. 1999;56(10):674–678. doi: 10.1136/oem.56.10.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Npri . National Pollutant Release Inventory; 1996. Summary report 1994. Canadian environmental protection act. Ottawa, Ontario, environment Canada; p. 240.http://www.ec.gc.ca/inrpnpri/ [Google Scholar]
- 30.Beltowski J., Wojcicka G., Gorny D., Marciniak A. The effect of dietary-induced obesity on lipid peroxidation, antioxidant enzymes and total plasma antioxidant capacity. J Physiol Pharmacol. 2000;51:883–896. [PubMed] [Google Scholar]
- 31.Akinloye O.A., Somade O.T., Akindele A.S., Adelabu K.B., Elijah F.T., Adewumi O.J. Anticlastogenic and hepatoprotective properties of ginger (Zingiber officinale) extract against nitrobenzene-induced toxicity in rats. Rom J Biochem. 2014;51(1):3–15. [Google Scholar]
- 32.Hamed A.N.E., Wahid A. Hepatoprotective activity of Borago officinalis extract against CCl4-induced hepatotoxicity in rats. J Nat Prod. 2015;8:113–122. [Google Scholar]
- 33.Somade O.T., Olorode S.K., Olaniyan T.O., Faokunla O. Quercetin, a polyphenolic phytochemical prevents sodium azide-induced extrahepatic oxidative stress in rats. Cognit Biol. 2016;2:1200798. [Google Scholar]
- 34.Pierini D., Bryan N.S. Nitric oxide availability as a marker of oxidative stress. Methods Mol Biol. 2014;1208:63–71. doi: 10.1007/978-1-4939-1441-8_5. [DOI] [PubMed] [Google Scholar]
- 35.Ajayi B.O., Adedara I.A., Farombi E.O. Benzo(a)pyrene induces oxidative stress, pro-inflammatory cytokines, expression of nuclear factor-kappa B and deregulation of wnt/beta-catenin signaling in colons of BALB/c mice. Food Chem Toxicol. 2016;95:42–51. doi: 10.1016/j.fct.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Ignarro L.J., Cirino G., Casini A., Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Lubos E., Handy D.E., Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2009;13:5323–5344. doi: 10.2741/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflügers Archiv. 2010;459(6):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 39.Tomlin C.D. tenth ed. The British Crop Protection Council; Surrey, UK: 1994. The E-pesticide manual. [Google Scholar]
- 40.Somade O.T., Adeniji K.D., Adesina A.A., Olurinde O.J. Oral acute toxicity study as well as tissues oxidative stress and histopathological disorders in edible camphor administered rats. Exp Toxicol Pathol. 2017;69:99–108. doi: 10.1016/j.etp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Feghali C.A., Wright T.M. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 42.Dinarello C.A. Historical insights into cytokines. Eur J Immunol. 2007;37(1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehra V.C., Ramgolam V.S., Bender J.R. Cytokines and cardiovascular disease. J Leukoc Biol. 2005;78(4):805–818. doi: 10.1189/jlb.0405182. [DOI] [PubMed] [Google Scholar]
- 44.Kimura H., Yoshizumi M., Ishii H., Oishi K., Ryo A. Cytokine production and signaling pathways in respiratory virus infection. Front Microbiol. 2013;4:276. doi: 10.3389/fmicb.2013.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafers M., Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437(3):188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 46.Allan S.M., Tyrrell P.J., Rothwell N.J. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5(8):629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 47.Morrell E.D., Kellum J.A., Hallows K.R., PastorSoler N.M. Epithelial transport during septic acute kidney injury. Nephrol Dial Transplant. 2014;29(7):1312–1319. doi: 10.1093/ndt/gft503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akiho H., Ihara E., Motomura Y., Nakamura K. Cytokine-induced alterations of gastrointestinal motility in gastrointestinal disorders. World J Gastrointest Pathophysiol. 2011;2(5):72–81. doi: 10.4291/wjgp.v2.i5.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su D.L., Lu Z.M., Shen M.N., Li X., Sun L.Y. Roles of pro- and anti-inflammatory cytokines in the pathogenesis of SLE. J Biomed Biotechnol. 2012;2012:347141. doi: 10.1155/2012/347141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinto L.M.O., Oliveira S.A., Braga E.L.A., Nogueira R.M.R., Kubelka C.F. Increased proinflammatory cytokines (TNF-α and IL-6) and anti-inflammatory compounds (sTNFRp55 and sTNFRp75) in Brazilian patients during exanthematic dengue fever. Mem Inst Oswaldo Cruz. 1999;94(3):387–394. doi: 10.1590/s0074-02761999000300019. [DOI] [PubMed] [Google Scholar]
- 51.Marie C., Pitton C., Fitting C., Cavaillon J.M. Regulation by anti-inflammatory cytokines (IL-4, IL-10, IL-13, TGFβ) of interleukin-8 production by LPSand/or TNF-α-activated human polymorphonuclear cells. Mediat Inflamm. 1996;5(5):334–340. doi: 10.1155/S0962935196000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Somade O.T., Ajayi B.O., Adeyi O.E., Aina B.O., David B.O., Sodiya I.D. Activation of NF-kB mediates up-regulation of cerebellar and hypothalamic pro-inflammatory chemokines (RANTES and MCP-1) and cytokines (TNF-α, IL-1β, IL-6) in acute edible camphor administration. Sci Africa. 2019;5 doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toews G.B. Cytokines and the lung. Eur Respir J. 2001;18(34):3S–17S. doi: 10.1183/09031936.01.00266001. [DOI] [PubMed] [Google Scholar]
- 54.Thornton A.J., Strieter R.M., Lindley I., Baggiolini M., Kunkel S.L. Cytokine-induced gene expression of a neutrophil chemotactic factor/interleukin-8 by human hepatocytes. J Immunol. 1990;144:2609–2613. [PubMed] [Google Scholar]
- 55.Standiford T.J., Kunkel S.L., Basha M.A., Chensue S.W., Lynch J.P., Toews G.B., Westwick J., Strieter R.M. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.el-Deiry W.S., Tokino T., Velculescu V.E., Levy D.B., Parsons R., Trent J.M., Lin D., Mercer W.E., Kinzler K.W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 57.Hartwell L.H., Kastan M.B. Cell cycle control and cancer. Science (Wash D C) 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 58.Yao S.L., Akhtar A.J., McKenna K.A., Bedi G.C., Sidransky D., Mabry M., Ravi R., Collector M.I., Jones R.J., Sharkis S.J., Fuchs E.J., Bedi A. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med. 1996;2:1140–1143. doi: 10.1038/nm1096-1140. [DOI] [PubMed] [Google Scholar]
- 59.Hou Q., Cymbalyuk E., Hsu H.C., Xu M., Hsu Y.T. Apoptosis modulatory activities of transiently expressed Bcl-2: roles in cytochrome c release and Bax regulation. Apoptosis. 2003;8:617–629. doi: 10.1023/A:1026187526113. [DOI] [PubMed] [Google Scholar]
- 60.Degli E.M., Dive C. Mitochondrial membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun. 2003;304:455–461. doi: 10.1016/s0006-291x(03)00617-x. [DOI] [PubMed] [Google Scholar]
- 61.Oltvai Z.N., Milliman C.L., Korsmeyer S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 62.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 63.Teoh P.L., Azimahtol H.L.P. Effects of styrylpyrone derivative (SPD) on expression of Bcl-2 and Bax genes in human ovarian carcinoma cell line, Caov-3. Malays Appl Biol. 1999;28:107–111. [Google Scholar]
- 64.Zhang L., Yu J., Park B.H., Kinzler K.W., Vogelstein B. Role of Bax in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 65.Miyashita T., Krajewski S., Krajewska M., Wang H.G., Lin H.K., Liebermann D.A., Hoffman B., Reed J.C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 66.Budihardjo I., Oliver H., Lutter M., Luo X., Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 67.Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 68.Srinivasula S.M., Ahmad M., Fernandes-Alnemri T., Alnemri E.S. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998;1:949–957. doi: 10.1016/s1097-2765(00)80095-7. [DOI] [PubMed] [Google Scholar]
- 69.Prendergast G.C. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 70.Spencer C.A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Canc Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- 71.Miller D.M., Thomas S.D., Islam A., Muench D., Sedoris K. c-Myc and cancer metabolism. Clin Canc Res. 2012;18(20):5546–5553. doi: 10.1158/1078-0432.CCR-12-0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pelengaris S., Khan M., Evan G. c-MYC: more than just a matter of life and death. Nat Rev Canc. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 73.Kelly G.L., Rickinson A.B. Burkitt lymphoma: revisiting the pathogenesis of a virus-associated malignancy. Hematol Am Soc Hematol Educ Program. 2007:277–284. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 74.Dogan S., Shen R., Ang D.C., Johnson M.L., D’Angelo S.P., Paik P.K., Brzostowski E.B., Riely G.J., Kris M.G., Zakowski M.F., Ladanyi M. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Canc Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Osta B.E., Behera M., Kim S., Berry L.D., Sica G., Pillai R.N., Owonikoko T.K., Kris M.G., Johnson B.E., Kwiatkowski D.J., Bunn P.A., Khuri F.R., Ramalingam S.S. Characteristics and outcomes of patients (pts) with metastatic KRAS mutant lung adenocarcinomas: lung Cancer Mutation Consortium (LCMC) database. J Clin Oncol. 2017;35(15):9021. doi: 10.1016/j.jtho.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shepherd F.A., Domerg C., Hainaut P., Janne P.A., Pignon J.P., Graziano S., Douillard J.Y., Brambilla E., Le Chevalier T., Seymour L. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31:2173–2181. doi: 10.1200/JCO.2012.48.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dearden S., Stevens J., Wu Y.L., Blowers D. Mutation incidence and coincidence in non-small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap) Ann Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshizawa A., Sumiyoshi S., Sonobe M., Kobayashi M., Fujimoto M., Kawakami F., Tsuruyama T., Travis W.D., Date H., Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013:852–861. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 79.Jordan E.J., Kim H.R., Arcila M.E., Barron D., Chakravarty D., Gao J., Chang M.T., Ni A., Kundra R., Jonsson P. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Canc Discov. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clcgp Network Genomic Medicine (NGM), A genomics-based classification of human lung tumors. Sci Transl Med. 2013;5(209) doi: 10.1126/scitranslmed.3006802. 209ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cgarn Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]