Abstract
Objective
To evaluate change in bone mineral density (BMD) during development of knee osteoarthritis (OA) in elderly Chinese community residents. Further, to monitor disease progression by recording speed of sound (SOS), one parameter of BMD provided by quantitative ultrasound measurement.
Methods
A total of 4173 community residents of the Chinese mainland were organized to complete questionnaires and relevant measurements, including anthropometry, radiology and quantitative ultrasound (QUS). SOS measurements of the distal radius were acquired using QUS measurements. The Kellgren-Lawrence (KL) grade of knee OA was evaluated by two experienced radiographers using X-rays. Finally, a general linear models analysis was performed to determine potential relationships. Further, the area under the receiver operating characteristic curve (ROC AUC) was applied to assess the distinction model.
Results
The SOS score in the OA group was significantly lower than that in the control group (p < 0.001). However, after adjustment for age and body mass index (BMI), no significant difference was observed in the male population (p = 0.841), while a significantly lower SOS score presented in knee OA participants in the female population (p = 0.033). A turning point in SOS scores, from increasing to decreasing trends, occurred around KL grade 2; the SOS score gradually increased with progression in participants from KL grades 0 to 2, whereas the SOS score presented a significant decrease in participants with KL grades 3 and 4. The AUC for the model to distinguish OA progression was 0.891.
Conclusion
There was a non-linear and stage-specific association between SOS score and knee OA, which presented a positive relationship in early stages, but a negative relationship in advanced stages. A decline of SOS score in knee OA patients in early stages should alert clinicians to the possibility of disease progression.
The Translational potential of this article
In the present study, the relationship between OA and BMD had established by SOS. The results suggested that close monitoring of SOS in elderly Chinese communities residents with knee OA could alert disease progression involvement by an easily accessible method, and help early referral to orthopedist consultation for further examination and treatment.
Keywords: Age, Body mass index, Bone mineral density, Osteoarthritis, Speed of sound
Introduction
Osteoarthritis (OA) is a common age-related joint disorder characterized by mobility limitations and chronic pain [1]. Up to 10% of males and 18% of females over 60 years of age suffer from OA, resulting in a tremendous socioeconomic burden that costs 1%–2.5% of the gross domestic product of developed countries [2]. Because of a deficiency of efficient disease-modifying treatments, it is still very meaningful to gain a deeper insight into the pathogenesis of OA. Various research endeavours have gradually revealed that OA is a whole joint disease, involving structural alterations in the articular cartilage, subchondral bone and synovium during progression [3]. Furthermore, advances in research have shown that subchondral bone remodeling induced by abnormal stress is a major driver of OA development [4]. Subsequently, changes in bone mineral density (BMD) are detected in these pathological activities. However, the relationship between OA and BMD has long been a controversial issue [5,6]. An inverse relationship between BMD and OA has been reported by some studies; their results indicated that the higher the BMD the less probability there is to develop OA [[7], [8], [9]]. However, other studies have shown that an elevated BMD presented in patients with OA [[10], [11], [12]].
Historically, dual energy X-ray absorptiometry (DXA) is considered an essential measurement of BMD for rheumatological diseases [13]. Indeed, DXA measurements were commonly used in exploring the relationship between OA and BMD in previous studies [8,14]. However, a definite correlation between OA and BMD has not yet been established. The use of quantitative ultrasound (QUS) is increasing and has shown promising utility in bone health evaluation [15], particularly in community health service centers. One of the major parameters of QUS, speed of sound (SOS), could well reflect the heterogeneity of bone material and be used to evaluate bone density [16]. It has also been reported that SOS has shown characteristics of acceptable precision and stability over time to perform an effective follow-up study [17]. Moreover, compared with DXA measurements, QUS is flexible to use, cheap to access and has no radiation, which makes it appropriate for a potentially wider use in large community-based screening studies [[18], [19], [20]].
Additionally, to our knowledge, little is known about the association between BMD and knee OA that has been estimated by SOS. Prospective outcomes with greater clinical and public health relevance of the preliminary studies are significantly important to the seminar. We hypothesized that BMD has a stage-specific relationship with knee OA; thus, monitoring BMD to realize the progression of knee OA is achievable. Therefore, the primary objective of the present study was to make the correlation between BMD and OA clearer to Chinese community members by the use of SOS.
Materials and methods
Participants
Participants were recruited from physical examination centers in community health service centers in the Zhejiang area. Subjects aged 50–79 years were included. A general health questionnaire was conducted to complete the prescreen of all participants. Exclusion criteria included diabetes mellitus, renal illness, endocrine disease, cancer, severe cardiovascular disease, a specific arthritis (caused by hemophilia, infection, autoimmunity, or congenital aplasia), a history of significant trauma of the knee(s), contraindication to X-ray, and a history of taking glucocorticoids, estrogens, thyroid hormone, calcium preparations and other drugs that could affect bone metabolism. Finally, a total of 4173 Chinese mainland inhabitants composed of 1752 men and 2421 women were included. All participants offered informed consent and this study was approved by the Institutional Review Board of The First Affiliated Hospital of Zhejiang Chinese Medical University (IRB No. 2018-ZX-026-01).
Anthropometry
Weight and height data were obtained from each participant by stadiometer (Mahr GmbH, Gottingen, Germany) and standardized balance-beam scale (Tanita, Tokyo, Japan), respectively. All participants were required to be lightly dressed and barefoot for this process. Finally, the computational formula of Body Mass Index (BMI) was calculated by dividing weight in kilograms by height in meters squared.
Radiological measurement
Radiographs of bilateral knees were taken by SD3000 Synchro Stand (Accele Ray, Switzerland). The posteroanterior and lateral X-ray projections during weight-loading were then taken to evaluate the degree of arthritis according to the Kellgren-Lawrence (KL) grading scale [21]. This radio-diagnosis was executed by two experienced radiologists independently. If one knee had a more serious condition, the higher grade was recorded. A third senior radiologist would make a judgment when there was a discrepancy. Knee OA patients were defined as those with KL grade≥2.
BMD measurement
The BMD of the distal radius was assessed using The Sun-light Mini-Omni Ultrasound Bone Sonometer (Sunlight; BeamMed Ltd., Israel). Participants were asked to remove any metal ornaments, such as watchbands or bracelets, when executing the measurement. The measurements were performed by well-trained medical personnel and repeated three times for each person. To compute the vitro precision of the study, 2 probes performed in 10 consecutive measurements of the phantom from the manufacturer. The intra-operator and inter-operator precision of SOS measurements were also assessed. Nine healthy volunteers participated over a period of 10 consecutive days in five successive assessments of the distal radius by three experienced operators. The results were similar with those of a previous study: the short-term in vitro precision was 0.06% for the distal radius and intra-operator and inter-operator precision was 0.76% and 0.77%, respectively [22]. Finally, two computer operators took charge of the digital data input throughout the study. After that, the senior researchers would check the information to ensure the veracity and reliability.
Statistical analysis
The participants were divided into two groups: the knee OA group and the control group (without knee OA). The Student’s t-test and the Chi-square test were applied to analyze the continuous variables and categorical variables, respectively, among the two groups. The contrast of continuous variables between subgroups arranged in KL grades was done by analysis of variance (ANOVA) and that of categorical variables was done by the Chi-square test. The mean SOS scores of the two groups were analyzed using general linear models (GLM) with adjustment for covariates (age, sex, and BMI). KL grade 2 was considered as a reference for the Post hoc analyses by GLM. Based on the GLM, by setting the SOS score as the responding variable and age, sex, and BMI as covariates, the discriminative ability of OA progression was assessed using the area under the receiver operating characteristic curve (ROC AUC) value. All of these operations were performed using SPSS software (version 20; IBM/SPSS Inc., Armonk, NY, USA). The WHO criterion was set to categorize individual BMD in this study, with a T-score less than 2.5 standard deviations (SD) below the normal defined as osteoporosis (OP) and a T-score between - 2.5 and −1.0 SD defined as osteopenia. A P-value ˂0.05 was considered significant for all testing in this study.
Results
Table 1 presents an overview of subject characteristics, showing a significant difference in age, sex and BMI between the control group and the knee OA group. The mean age was 58.50 years in the control group and 65.58 years in the knee OA group. The mean BMI in the control group was significantly lower than that in the knee OA group. The ratio of females to males in the control group was 51%, while it rose to 68% in the knee OA group. A similar result occurred when comparing the osteoporosis ratio (18% in the control group and 27% in the knee OA group).
Table 1.
Characteristics of subjects by the presence of knee OA.
| group | Control |
Knee OA |
P∗ |
|---|---|---|---|
| (n = 2463) | (n = 1710) | ||
| Age (years) | 58.50 ± 3.21 | 65.58 ± 3.82 | <0.001 |
| Female sex (n) | 1263(0.51) | 1158(0.68) | <0.001 |
| BMI (kg/m2) | 23.48 ± 1.25 | 24.59 ± 1.28 | <0.001 |
| Status of osteoporosis | |||
| Osteopenia (n) | 1000 (0.41) | 622 (0.36) | <0.001 |
| Osteoporosis (n) | 445 (0.18) | 458 (0.27) | <0.001 |
| Mean SOS | |||
| Unadjusted | 4031.65 ± 195.35 | 3980.89 ± 200.69 | <0.001 |
| Model 1 | 4019.97 ± 4.71 | 4003.17 ± 6.04 | 0.055 |
| Model 2 | 4019.37 ± 4.76 | 4004.04 ± 6.12 | 0.086 |
Values are the mean ± standard error (SE) or n (%), as appropriate.
Model 1, adjusted for age and sex.
Model 2, adjusted for age, sex and BMI.
∗P values by Student’s T tests for continuous variables and Chi square test for categorical variables.
OA, osteoarthritis; BMI, body mass index; SOS, speed of sound.
According to the results of the GLM analysis, SOS scores within the two groups were significantly different: the unadjusted SOS score in the knee OA group was lower than that in the control group. After adjusting for sex and age (Model 1 in Table 1), the knee OA group SOS score was lower than that in the control group. Even when adjusted further for BMI (Model 2 in Table 1), that relationship remained; however, there was no longer a statistically significant difference between the two groups.
To better understand this association, as shown in Table 2, participants in this study were divided into five subgroups based on the degree of knee OA according to KL grades. The unadjusted and adjusted mean SOS scores were significantly different between each subgroup. In KL grades 0, 1, and 2, SOS scores tended to increase gradually, while in KL grades 3 and 4, they tended to decrease sharply compared to lower KL grades. The sex-age-adjusted SOS scores showed similar inverted U-shaped associations (Model 1 in Table 2). Although after further adjusting (Model 2 in Table 2), this association did not change. According to the post hoc analyses, the differences between each subgroup were significant in each model; the adjusted SOS scores were particularly significantly different between KL grades 2, 3 and 4.
Table 2.
Mean SOS (m/s) score according to the severity of radiographic knee OA.
| KL grades | 0 |
1 |
2 |
3 |
4 |
P for trend‡ |
|---|---|---|---|---|---|---|
| (n = 1357) | (n = 1106) | (n = 611) | (n = 697) | (n = 402) | ||
| Unadjusted | 4025.79 ± 5.28 | 4038.83 ± 5.85† | 4050.18 ± 7.87 | 3954.86 ± 7.37† | 3920.71 ± 9.70† | <0.001 |
| Model 1 | 4022.91 ± 8.16∗ | 4038.77 ± 5.87 | 4051.05 ± 7.98 | 3962.14 ± 9.12† | 3932.87 ± 15.17† | <0.001 |
| Model 2 | 4023.31 ± 8.36∗ | 4038.88 ± 5.89 | 4050.94 ± 7.99 | 3961.70 ± 9.33† | 3932.12 ± 15.54† | <0.001 |
Values are the means ± standard error (SE).
‡P for trend values by general linear models.
∗P < 0.05, †P < 0.01 by post hoc analyses, KL grade 2 subgroup as reference.
Model 1, adjusted for age and sex.
Model 2, adjusted for age, sex and BMI.
SOS, speed of sound; OA, osteoarthritis; KL, Kellgren-Lawrence; BMI, body mass index.
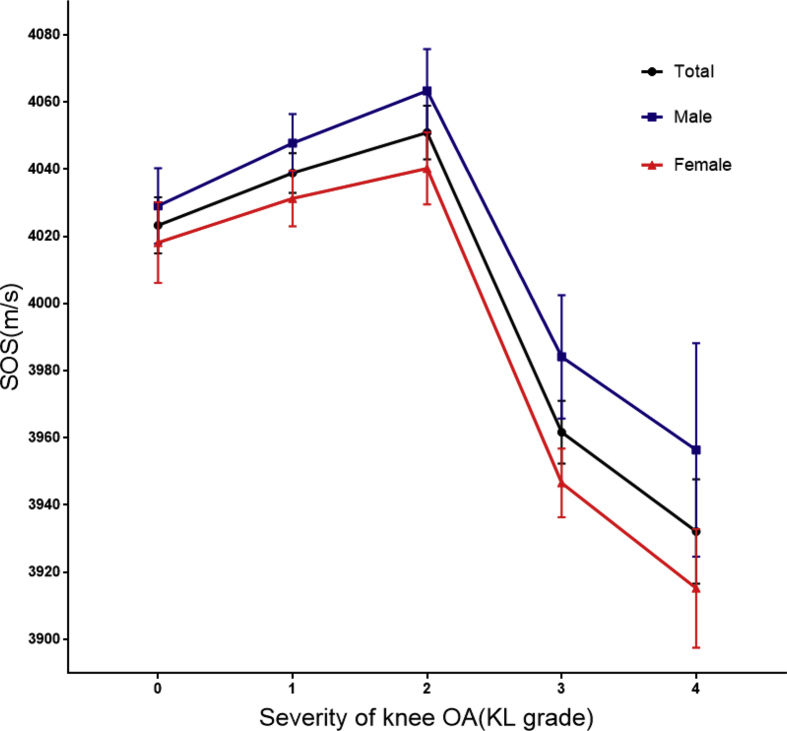
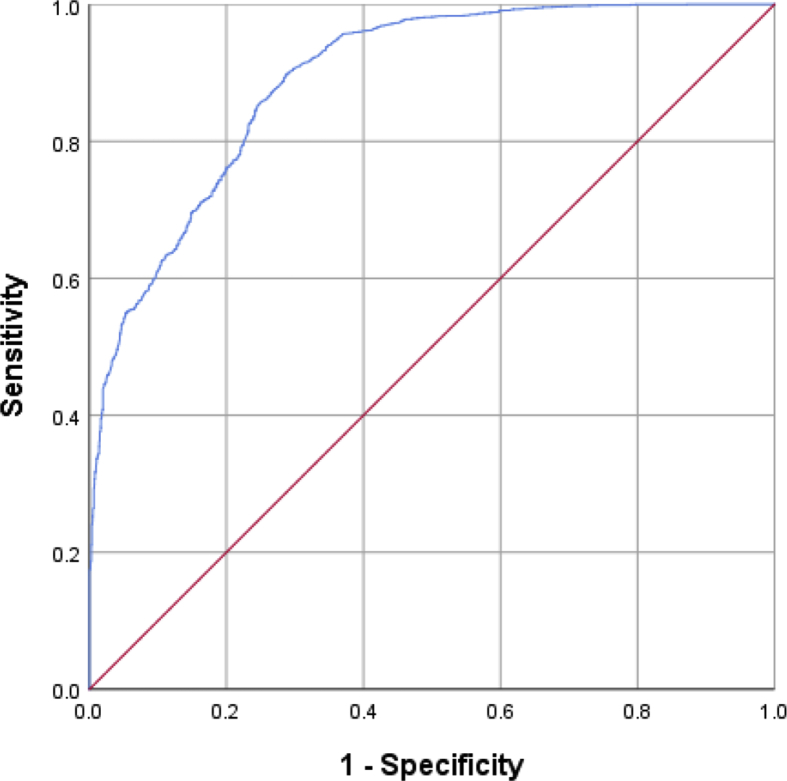
The association between SOS and knee OA are presented in Table 3, Table 4 stratified by gender. The unadjusted SOS scores showed significant differences between the groups whether male or female. However, the male age-adjusted SOS score became nonsignificant between the control group and the knee OA group. Meanwhile, the female SOS scores were significantly difference between the two groups, even after further adjusting for age and BMI. The same trend was observed within the two genders in that SOS scores showed an inverted U-shaped association (Fig. 1) with the degree of severity of knee OA. After adjusting for age and BMI, this similar trend continued. Finally, the ROC was calculated to evaluate the cutoff for distinguishing OA progression: the AUC achieved a value of 0.891 (89.8% sensitivity and 71.1% specificity) (Fig. 2).
Table 3.
Mean SOS (M/S) according to the presence of radiographic knee OA stratified by sex.
| Group | Control | Knee OA | P∗ |
|---|---|---|---|
| Male | (n = 1200) | (n = 552) | |
| Unadjusted | 4040.03 ± 5.84 | 4018.12 ± 8.62 | 0.035 |
| Model 1 | 4034.03 ± 6.44 | 4031.15 ± 10.44 | 0.831 |
| Model 2 |
4033.99 ± 6.49 |
4031.24 ± 10.58 |
0.841 |
| Female | (n = 1263) | (n = 1158) | |
| Unadjusted | 4023.69 ± 5.42 | 3963.15 ± 5.66 | <0.001 |
| Model 1 | 4007.80 ± 6.74 | 3980.47 ± 7.15 | 0.017 |
| Model 2 | 4006.68 ± 6.83 | 3981.70 ± 7.26 | 0.033 |
Values are the means ± standard error (SE).
∗P for trend values by general linear models.
Model 1, adjusted for age.
Model 2, adjusted for age and BMI.
SOS, speed of sound; OA, osteoarthritis; BMI, body mass index.
Table 4.
Mean SOS (m/s) according to the severity of radiographic knee OA stratified by sex.
| KL grades | 0 | 1 | 2 | 3 | 4 | P for trend‡ |
|---|---|---|---|---|---|---|
| Male | (n=661) | (n=539) | (n=300) | (n=182) | (n=70) | |
| Unadjusted | 4034.42 ± 7.81 | 4046.90 ± 8.64 | 4060.44 ± 11.59 | 3977.30 ± 14.88† | 3942.87 ± 23.99† | <0.001 |
| Model 1 | 4028.30 ± 10.95 | 4047.76 ± 8.71 | 4063.83 ± 12.34 | 3985.33 ± 17.97† | 3958.58 ± 31.05† | <0.001 |
| Model 2 |
4029.09 ± 11.22 |
4047.76 ± 8.71 |
4063.33 ± 12.44 |
3984.14 ± 18.33† |
3956.43 ± 31.75† |
<0.001 |
| Female | (n=696) | (n=567) | (n=311) | (n=515) | (n=332) | |
| Unadjusted | 4017.60 ± 7.18 | 4031.16 ± 7.96 | 4040.28 ± 10.74 | 3946.94 ± 8.35† | 3916.04 ± 10.40† | <0.001 |
| Model 1 | 4018.15-±11.75 | 4031.28 ± 8.20 | 4040.24 ± 10.76 | 3946.61 ± 10.02† | 3915.22 ± 17.28† | <0.001 |
| Model 2 | 4018.16 ± 12.03 | 4031.29 ± 8.28 | 4040.24 ± 10.76 | 3946.60 ± 10.25† | 3915.21 ± 17.68† | <0.001 |
Values are the means ± standard error (SE).
‡P for trend values by general linear models.
∗P < 0.05, †P < 0.01 by post hoc analyses, KL grade 2 subgroup as reference.
Model 1, adjusted for age.
Model 2, adjusted for age and BMI.
SOS, speed of sound; OA, osteoarthritis; KL, Kellgren-Lawrence; BMI, body mass index.
Fig. 1.
Adjusted SOS by the severity of knee OA. Values are adjusted for age, BMI. ∗P < 0.05, †P < 0.01 by post hoc analyses with KL grade 2 subgroup as reference. SOS, speed of sound; OA, osteoarthritis; BMI, body mass index; KL, Kellgren-Lawrence
Fig. 2.
Receiver operating characteristics curves (ROC) for distinguishing knee osteoarthritis (OA) progression.
Discussion
This study focused on SOS to explore the association between knee OA and BMD in an aging Chinese community population. The association based on this novel parameter was confirmed by the results of the established DXA measurement of BMD [23]. SOS shows a negative association with the presence of knee OA, and a positive association with the severity of knee OA of KL grades 0, 1 and 2.
Our data showed that the control group was 51% female, whereas the knee OA group was 68% female, indicating that females are susceptible to knee OA [24]. Furthermore, our results also showed that age and BMI were higher in the knee OA group than those in the control group. Consistent with previous conclusions, obesity has been considered a moderate risk factor and age has been deemed one of the most high-impact risk factors for knee OA [24]. Likewise, it is highly accepted that female sex is one of the evident risk factors for knee OA [25,26]. Interestingly, in males in our study, the significant difference in SOS between the control group and the knee OA group became insignificant after adjusting for age and BMI. The findings from our results stratified by gender indicated the morbidity of knee OA was mainly influenced by age and BMI in the male cohort.
Evidence of causality has been observed for high BMD on knee OA [10,11]. However, a prospective study has demonstrated that severe knee OA may be associated with lower BMD [27]. These inconsistent results regarding BMD may be caused by leaving the grades of knee OA out of the account. Inverted U-shaped trends were found in the present study. Within the range of KL 2, SOS presented a positive correlation with the severity of knee OA. However, this became a negative correlation in advanced KL grades 2, 3 and 4. Furthermore, this inverted U-shaped trend remains after adjusting for different variants, not only in women but also in men. A similar relationship was mentioned between BMD and KL grades in a cross-sectional study [23]. A rational explanation for the inverted U-shaped relationship might be caused by different pathological changes in the different stages of knee OA. Findings from cross-sectional studies of a middle-aged population without clinical symptoms demonstrated a positive relationship between systemic BMD and tibial cartilage volume, as well as prevalence of tibiofemoral cartilage defects [28]. During the early stages of OA, the defining characteristics are cartilage loss and cartilage composition changes [29]. These pathological changes result in susceptibility of articular cartilage to erosion by mechanical forces [30]. Repetitive stress on the articular surface, increasing with increasing BMD, alters the property of the subchondral bone. This is consistent with previous reports that subchondral bone has shown a strong relationship with cartilage lesions [31,32]. This process is accompanied by increased bone turnover in the subchondral bone, which has recently been associated with development of OA [33]. Meanwhile, increased bone turnover was observed in OA patients with bone marrow lesions in that high expression of bone biochemical markers and cytokines were detected in their samples [31,34]. For example, the bone-alkaline phosphatase (BAP) level reflects the underlying function of the active“bone formation”system in the subchondral bone in early stages of OA [35].
OA animal models have greatly mimicked many characteristics of human OA development, including progressive articular cartilage degradation, subchondral bone resorption and sclerosis, and osteophyte formation [36]. Histomorphometry analysis showed significant subchondral bone loss in late post-surgery stages after anterior cruciate ligament transection (ACLT) [37]. Moreover, a clinical study also found that, in severe stages of knee OA, longitudinal BMD loss is particularly strongly associated with progressive cartilage loss [27,[38], [39], [40]]. Therefore, outcomes in the KL 3 and 4 groups in the present study can be well explained. Meanwhile, there may be other factors making great contributions to decreased BMD in advanced stages of knee OA. Subchondral cysts could be a pathological characteristic of late OA, which also mirrors the resorption phase in the process of this disease [41]. In addition, studies on another feature of serious OA, osteophytes and joint space narrowing (JSN), showed that the former was positively associated with BMD, but the latter had a negative association with BMD [14]. In conclusion, in the early stages, a series of pathological changes triggers progression of arthritis, mainly including matrix degradation and progressive cartilage defects, microfracture and secondary remodeling in the subchondral bone [[42], [43], [44], [45]]. As a result, BMD increases with the progression of OA. The pathological process eventually results in an imbalance between repair and destruction of the joint. Subsequently, an accelerated generalized BMD loss is accompanied with progressive knee OA over time.
The results of the present study estimated by SOS have provided some insights into the association between OA and BMD. In addition, the BMD trends provided by SOS obtained from distal radius using QUS in this study have been well documented in studies using DXA [23]. There are some possible explanations for the feasibility for using SOS to estimate the relationship between OA and BMD in the present study. On one hand, QUS assessment can identify the properties of bone tissue as reliably as BMD measurements [46]. On the other hand, knee OA has been considered a syndrome, rather than a single disease; it is a heterogeneous disease involving a wide range of underlying pathways [47]. Consequently, local changes could influence generalized BMD through some cytokine and signaling pathways. In previous studies, it has also been recognized that generalized BMD could be influenced by pathological cartilage degradation [[48], [49], [50]].
This study includes a general community population with a modest sample size and a limited age range. As far as we know, it is novel to estimate OA and BMD by SOS in the general public. Although DXA of the lumbar spine and femoral neck are widely used in BMD evaluation, the use of QUS is practical and advisable in developing countries. Therefore, rather than exploring the relationships between knee OA and the BMD of a specific anatomical location, the analysis of a relationship between radiographic OA and SOS is more functional and revealing. There is another reason that this study is appropriate for community-based general screening use. In the limited space of community health services centers, it is a challenge to gather multi-position BMD, including the hip, femoral neck and spine. Thus, portable equipment is suitable and the forearm is used to measure BMD. Additionally, the AUC value has demonstrated the SOS monitoring would be useful for distinguishing knee OA progression.
Unfortunately, some discrepancies could be caused by parameters obtained from the non-load-bearing joint used in the current study. Our findings are also limited because the study is cross-sectional and lacks long-term follow-up data. Although the importance of the influence of sex, age and BMI on knee OA has been highlighted, other variables, such as joint motion, joint pain scores, smoking or drinking status, and dietary habits are not integrated into our volunteer information. Overall, although these deficiencies may partly mask the relationship between knee OA and systemic BMD, general trends should not be altered. Our study offers an important option for knee OA patients to monitor their BMD. This study also lends support to the use of QUS to gain information on BMD beneficial to estimate early knee OA progression. Multi-center and longitudinal studies are urgently required for further exploration of this diagnostic option.
Conclusion
The current study provides strong evidence that female gender, high BMI and advanced age are independent risk factors for knee OA. Dependent on different radiologic stages, SOS shows an approximate inverted U-shaped association with knee OA, with SOS increasing in early stages and dropping in late stages. Our data also suggest that closely monitoring SOS in knee OA patients could alert health care personnel to disease progression and help in obtaining early referral to orthopedist consultation for further examination and treatment.
Statement of ethics
All participants offered informed consent and this study was approved by the Institutional Review Board of The First Affiliated Hospital of Zhejiang Chinese Medical University (IRB No. 2018-ZX-026-01).
Author contributions
L.F. and C.X. conceived and designed the study, performed the acquisition and interpretation of data, and were involved in drafting the manuscript. H.X, Q.G, Z.S, P.Z, R.X. and Z.Z. were involved in the acquisition and interpretation of data. L.K, H.J, and P.W. were involved in the acquisition and statistical analysis of data. P.T. was involved in the interpretation of data, and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding Sources
This work was supported by Chinese National Natural Science Foundation [grant numbers 81774332, 81774346, 81873324, 81873325, 81973869, 81904221, 81904223 and 81904219], Natural Science Foundation of Zhejiang Province [grant numbers LQ16H270007 and LY18H270004]; Traditional Chinese Medical Administration of Zhejiang Province [grant numbers, 2018ZA034, 2019ZQ018 and 2018ZZ011]; Health Commission of Zhejiang Province [grant numbers 2019RC225]; Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Chinese Traditional Medicine), Zhejiang Chinese Medical University [grant numbers ZYX2018001 and ZYX2018004], Youth Foundation of Zhejiang Chinese Medical University [grant numbers KC201932 and Q2019Y01].
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
The authors have no acknowledgements to disclose.
Contributor Information
Hongting Jin, Email: hongtingjin@163.com.
Peijian Tong, Email: peijiantongzjtcm@163.com.
References
- 1.Mazzotti E., Teti G., Falconi M., Chiarini F., Barboni B., Mazzotti A. Age-related alterations affecting the chondrogenic differentiation of synovial fluid mesenchymal stromal cells in an equine model. Cells. 2019;8(10) doi: 10.3390/cells8101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H. Osteoarthritis Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Ondrésik M., Azevedo Maia F.R., da Silva Morais A., Gertrudes A.C., Dias Bacelar A.H., Correia C. Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng. 2017;114(4):717–739. doi: 10.1002/bit.26182. [DOI] [PubMed] [Google Scholar]
- 4.Aizah N., Chong P.P., Kamarul T. Cartilage; 2019. Early alterations of subchondral bone in the rat anterior cruciate ligament transection model of osteoarthritis. 1947603519878479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herrero-Beaumont G., Roman-Blas J.A., Largo R., Berenbaum F., Castañeda S. Bone mineral density and joint cartilage: four clinical settings of a complex relationship in osteoarthritis. Ann Rheum Dis. 2011;70(9):1523–1525. doi: 10.1136/ard.2011.151233. [DOI] [PubMed] [Google Scholar]
- 6.Glowacki J., Tuteja M., Hurwitz S., Thornhill T.S., LeBoff M.S. Discordance in femoral neck bone density in subjects with unilateral hip osteoarthritis. J Clin Densitom. 2010;13(1):24–28. doi: 10.1016/j.jocd.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q., Xu J., Wang K., Cai J., Wu J., Ren J. Associations between systemic bone mineral density, knee cartilage defects and bone marrow lesions in patients with knee osteoarthritis. Int J Rheum Dis. 2018;21(6):1202–1210. doi: 10.1111/1756-185X.13148. [DOI] [PubMed] [Google Scholar]
- 8.Barbour K.E., Murphy L.B., Helmick C.G., Hootman J.M., Renner J.B., Jordan J.M. Bone mineral density and the risk of hip and knee osteoarthritis: the Johnston county osteoarthritis project. Arthritis Care Res. 2017;69(12):1863–1870. doi: 10.1002/acr.23211. (Hoboken) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Z.H., Zeng C., Li Y.S., Yang T., Li H., Wei J. Relation between phalangeal bone mineral density and radiographic knee osteoarthritis: a cross-sectional study. BMC Muscoskel Disord. 2016;17:71. doi: 10.1186/s12891-016-0918-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funck-Brentano T., Nethander M., Movérare-Skrtic S., Richette P., Ohlsson C. Causal factors for knee, hip, and hand osteoarthritis: a mendelian randomization study in the UK biobank. Arthritis Rheum. 2019;71(10):1634–1641. doi: 10.1002/art.40928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergink A.P., Rivadeneira F., Bierma-Zeinstra S.M., Zillikens M.C., Ikram M.A., Uitterlinden A.G. Are bone mineral density and fractures related to the incidence and progression of radiographic osteoarthritis of the knee, hip, and hand in elderly men and women? The Rotterdam study. Arthritis Rheum. 2019;71(3):361–369. doi: 10.1002/art.40735. [DOI] [PubMed] [Google Scholar]
- 12.Hackinger S., Trajanoska K., Styrkarsdottir U., Zengini E., Steinberg J., Ritchie G.R.S. Evaluation of shared genetic aetiology between osteoarthritis and bone mineral density identifies SMAD3 as a novel osteoarthritis risk locus. Hum Mol Genet. 2017;26(19):3850–3858. doi: 10.1093/hmg/ddx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maricic M. Use of DXA-based technology for detection and assessment of risk of vertebral fracture in rheumatology practice. Curr Rheumatol Rep. 2014;16(8):436. doi: 10.1007/s11926-014-0436-5. [DOI] [PubMed] [Google Scholar]
- 14.Wen L., Shin M.H., Kang J.H., Yim Y.R., Kim J.E., Lee J.W. The relationships between bone mineral density and radiographic features of hand or knee osteoarthritis in older adults: data from the Dong-gu Study. Rheumatology. 2016;55(3):495–503. doi: 10.1093/rheumatology/kev377. (Oxford) [DOI] [PubMed] [Google Scholar]
- 15.Zha X.Y., Hu Y., Pang X.N., Chang G.L., Li L. Diagnostic value of osteoporosis self-assessment tool for Asians (OSTA) and quantitative bone ultrasound (QUS) in detecting high-risk populations for osteoporosis among elderly Chinese men. J Bone Miner Metabol. 2015;33(2):230–238. doi: 10.1007/s00774-014-0587-5. [DOI] [PubMed] [Google Scholar]
- 16.Ishimoto T., Suetoshi R., Cretin D., Hagihara K., Hashimoto J., Kobayashi A. Quantitative ultrasound (QUS) axial transmission method reflects anisotropy in micro-arrangement of apatite crystallites in human long bones: a study with 3-MHz-frequency ultrasound. Bone. 2019;127:82–90. doi: 10.1016/j.bone.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmi G., Scalzo G., de Terlizzi F., Peh W.C. Quantitative ultrasound in osteoporosis and bone metabolism pathologies. Radiol Clin. 2010;48(3):577–588. doi: 10.1016/j.rcl.2010.02.013. North Am. [DOI] [PubMed] [Google Scholar]
- 18.Adamczyk P., Szczepanska M., Pluskiewicz W. Skeletal status assessment by quantitative ultrasound and bone densitometry in children with different renal conditions. Osteoporos Int. 2018;29(12):2667–2675. doi: 10.1007/s00198-018-4659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grocholewicz K., Janiszewska-Olszowska J., Aniko-Włodarczyk M., Preuss O., Trybek G., Sobolewska E. Panoramic radiographs and quantitative ultrasound of the radius and phalanx III to assess bone mineral status in postmenopausal women. BMC Oral Health. 2018;18(1):127. doi: 10.1186/s12903-018-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Costoso A., Vlachopoulos D., Ubago-Guisado E., Ferri-Morales A., Cavero-Redondo I., Martínez-Vizcaino V. Agreement between dual-energy X-ray absorptiometry and quantitative ultrasound to evaluate bone health in adolescents: the PRO-BONE study. Pediatr Exerc Sci. 2018;30(4):466–473. doi: 10.1123/pes.2017-0217. [DOI] [PubMed] [Google Scholar]
- 21.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damilakis J., Papadokostakis G., Vrahoriti H., Tsagaraki I., Perisinakis K., Hadjipavlou A. Ultrasound velocity through the cortex of phalanges, radius, and tibia in normal and osteoporotic postmenopausal women using a new multisite quantitative ultrasound device. Invest Radiol. 2003;38(4):207–211. doi: 10.1097/01.RLI.0000057031.21810.F4. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.H., Lee J.S., Park J.H. Association between bone mineral density and knee osteoarthritis in Koreans: the fourth and fifth Korea national health and nutrition examination surveys. Osteoarthritis Cartilage. 2018;26(11):1511–1517. doi: 10.1016/j.joca.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Silverwood V., Blagojevic-Bucknall M., Jinks C., Jordan J.L., Protheroe J., Jordan K.P. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(4):507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Bultink I.E., Lems W.F. Osteoarthritis and osteoporosis: what is the overlap. Curr Rheumatol Rep. 2013;15(5):328. doi: 10.1007/s11926-013-0328-0. [DOI] [PubMed] [Google Scholar]
- 26.Geusens P.P., van den Bergh J.P. Osteoporosis and osteoarthritis: shared mechanisms and epidemiology. Curr Opin Rheumatol. 2016;28(2):97–103. doi: 10.1097/BOR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 27.Linde K.N., Puhakka K.B., Langdahl B.L., Søballe K., Krog-Mikkelsen I., Madsen F. Bone mineral density is lower in patients with severe knee osteoarthritis and attrition. Calcif Tissue Int. 2017;101(6):593–601. doi: 10.1007/s00223-017-0315-y. [DOI] [PubMed] [Google Scholar]
- 28.Brennan S.L., Pasco J.A., Cicuttini F.M., Henry M.J., Kotowicz M.A., Nicholson G.C. Bone mineral density is cross sectionally associated with cartilage volume in healthy, asymptomatic adult females: Geelong Osteoporosis Study. Bone. 2011;49(4):839–844. doi: 10.1016/j.bone.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felson D.T. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21(1):10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muratovic D., Findlay D.M., Cicuttini F.M., Wluka A.E., Lee Y.R., Edwards S. Bone marrow lesions in knee osteoarthritis: regional differences in tibial subchondral bone microstructure and their association with cartilage degeneration. Osteoarthritis Cartilage. 2019;27:1653–1662. doi: 10.1016/j.joca.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Qin H.J., Xu T., Wu H.T., Yao Z.L., Hou Y.L., Xie Y.H. SDF-1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone. 2019;125:140–150. doi: 10.1016/j.bone.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Lin C., Liu L., Zeng C., Cui Z.K., Chen Y., Lai P. Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12. Bone Res. 2019;7:5. doi: 10.1038/s41413-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdes A.M., Meulenbelt I., Chassaing E., Arden N.K., Bierma-Zeinstra S., Hart D. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthritis Cartilage. 2014;22(5):683–689. doi: 10.1016/j.joca.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Ota S., Chiba D., Sasaki E., Kumagai G., Yamamoto Y., Nakaji S. Symptomatic bone marrow lesions induced by reduced bone mineral density in middle-aged women: a cross-sectional Japanese population study. Arthritis Res Ther. 2019;21(1):113. doi: 10.1186/s13075-019-1900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiffel V., Rundle C.H., Sheng M.H., Das S., Lau K.W. A mouse noninvasive intraarticular tibial plateau compression loading-induced injury model of posttraumatic osteoarthritis. Calcif Tissue Int. 2019;106(2):158–171. doi: 10.1007/s00223-019-00614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayami T., Pickarski M., Zhuo Y., Wesolowski G.A., Rodan G.A., Duong L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Lee J.Y., Harvey W.F., Price L.L., Paulus J.K., Dawson-Hughes B., McAlindon T.E. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013;65(6):1541–1546. doi: 10.1002/art.37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding C., Cicuttini F., Boon C., Boon P., Srikanth V., Cooley H. Knee and hip radiographic osteoarthritis predict total hip bone loss in older adults: a prospective study. J Bone Miner Res. 2010;25(4):858–865. doi: 10.1359/jbmr.091012. [DOI] [PubMed] [Google Scholar]
- 40.Im G.I., Kwon O.J., Kim C.H. The relationship between osteoarthritis of the knee and bone mineral density of proximal femur: a cross-sectional study from a Korean population in women. Clin Orthop Surg. 2014;6(4):420–425. doi: 10.4055/cios.2014.6.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Audrey H.X., Abd Razak H.R., Andrew T.H. The truth behind subchondral cysts in osteoarthritis of the knee. Open Orthop J. 2014;8:7–10. doi: 10.2174/1874325001408010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu B., Cui G., Zhang Q., Cheng X., Tang S. Desumoylation of aggrecan and collagen II facilitates degradation via aggrecanases in IL-1β-mediated osteoarthritis. J Pain Res. 2019;12:2145–2153. doi: 10.2147/JPR.S194306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou W., He X., Chen Z., Wang Y., Feng H., Zhang G. LncRNA HOTAIR-mediated Wnt/β-catenin network modeling to predict and validate therapeutic targets for cartilage damage. BMC Bioinf. 2019;20(1):412. doi: 10.1186/s12859-019-2981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millerand M., Berenbaum F., Jacques C. Danger signals and inflammaging in osteoarthritis. Clin Exp Rheumatol. 2019;37(Suppl. 120):48–56. (5) [PubMed] [Google Scholar]
- 45.Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4(6):221–229. doi: 10.1302/2058-5241.4.180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frost M.L., Blake G.M., Fogelman I. Quantitative ultrasound and bone mineral density are equally strongly associated with risk factors for osteoporosis. J Bone Miner Res. 2001;16(2):406–416. doi: 10.1359/jbmr.2001.16.2.406. [DOI] [PubMed] [Google Scholar]
- 47.Deveza L.A., Loeser R.F. Is osteoarthritis one disease or a collection of many. Rheumatology. 2018;57(Suppl. 4):iv34–iv42. doi: 10.1093/rheumatology/kex417. (Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teichtahl A.J., Wang Y., Wluka A.E., Strauss B.J., Proietto J., Dixon J.B. Associations between systemic bone mineral density and early knee cartilage changes in middle-aged adults without clinical knee disease: a prospective cohort study. Arthritis Res Ther. 2017;19(1):98. doi: 10.1186/s13075-017-1314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S., Kim T.N., Kim S.H. Knee osteoarthritis is associated with increased prevalence of vertebral fractures despite high systemic bone mineral density: a cross-sectional study in an Asian population. Mod Rheumatol. 2014;24(1):172–181. doi: 10.3109/14397595.2013.854060. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y., Stannus O.P., Aitken D., Cicuttini F., Antony B., Jones G. Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann Rheum Dis. 2014;73(11):2003–2009. doi: 10.1136/annrheumdis-2013-203691. [DOI] [PubMed] [Google Scholar]