Abstract
Background
Our study aimed to compare the clinical outcomes of patients with and without diabetes admitted to hospital with COVID-19.
Methods
This retrospective multicentre cohort study comprised 24 tertiary medical centres in France, and included 2851 patients (675 with diabetes) hospitalized for COVID-19 between 26 February and 20 April 2020. A propensity score-matching (PSM) method (1:1 matching including patients’ characteristics, medical history, vital statistics and laboratory results) was used to compare patients with and without diabetes (n = 603 per group). The primary outcome was admission to an intensive care unit (ICU) and/or in-hospital death.
Results
After PSM, all baseline characteristics were well balanced between those with and without diabetes: mean age was 71.2 years; 61.8% were male; and mean BMI was 29 kg/m2. A history of cardiovascular, chronic kidney and chronic obstructive pulmonary diseases were found in 32.8%, 22.1% and 6.4% of participants, respectively. The risk of experiencing the primary outcome was similar in patients with or without diabetes [hazard ratio (HR): 1.16, 95% confidence interval (CI): 0.95–1.41; P = 0.14], and was 1.29 (95% CI: 0.97–1.69) for in-hospital death, 1.26 (95% CI: 0.9–1.72) for death with no transfer to an ICU and 1.14 (95% CI: 0.88–1.47) with transfer to an ICU.
Conclusion
In this retrospective study cohort of patients hospitalized for COVID-19, diabetes was not significantly associated with a higher risk of severe outcomes after PSM.
Trial registration number
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; PSM, propensity score-matching; RAS, renin–angiotensin system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: Covid-19, Diabetes, Mortality, Propensity score-matching
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly spread around the world and been declared a pandemic by the World Health Organization (WHO). So far, the COVID-19 pandemic has resulted in more than one million deaths across 200 countries. From the first Chinese published reports, epidemiological features have rapidly identified diabetes as one of the leading comorbidities associated with a worse COVID-19 prognosis [1]. In addition, the studies published so far have provided consistent results, with a two- to threefold greater prevalence of diabetes in patients in intensive care units (ICUs) compared with less severe cases, and a dramatic increase in mortality in patients with diabetes [1], [2], [3], [4]. Other comorbidities frequently associated with diabetes, such as obesity, hypertension, chronic kidney disease and cardiovascular disease, have also been shown to be associated with a higher risk of severe outcomes for COVID-19 [5], [6], [7], [8], [9]. However, whether diabetes is associated with poorer COVID-19 outcomes independently of diabetes-related comorbidities has remained unclear. Thus far, only a few published studies have directly compared patients with and without diabetes to address this issue [10], [11], [12], [13]. Moreover, despite multiple adjustments, comparisons between patients with and without diabetes have remained potentially biased by important differences in terms of clinical characteristics and medical history, but also by their varied clinical and biological presentations at admission. The aim of the present study, therefore, is to investigate the potential association between diabetes and clinical outcomes in patients hospitalized for COVID-19 by using a propensity score-matching (PSM) approach to account for a wide range of comorbidities.
Methods
Study settings and population
The present Critical COVID-19 France (CCF) study is a retrospective, observational, multicentre study initiated by the French Society of Cardiology that includes all consecutive adult patients admitted to hospital with a diagnosis of SARS-CoV-2 infection between 26 February and 20 April 2020 at 24 clinical centres across France (NCT04344327). Its overall protocol has been partially described in a previous report [14]. Briefly, according to WHO criteria, SARS-CoV-2 infection is defined as a positive result on real-time reverse transcription–polymerase chain reaction (rRT–PCR) tests of nasal and pharyngeal swabs or lower respiratory tract aspirates, or the presence of typical imaging characteristics on chest computed tomography (CT) when laboratory test results are inconclusive. Patients directly admitted to ICUs were not included in this cohort (the number of such patients are not available).
The CCF study has been declared to and authorized by the French Data Protection Committee [Commission nationale de l’informatique et des Libertés (CNIL), authorization no. 2207326v0], and has been conducted in accordance with the ethical standards laid down by the 1964 Declaration of Helsinki and its later amendments.
Data collection
All data were collected by local investigators using an electronic case-report form available on REDCap (Research Electronic Data Capture) software (Vanderbilt University, Nashville, TN, USA) and hosted by a secure server from the French Institute of Health and Medical Research at the INSERM Paris Cardiovascular Research Centre. General patients’ characteristics included their demographic characteristics, coexisting medical conditions and medications for chronic diseases. Other detailed data, including clinical parameters, blood test results and chest CT characteristics (when performed), were also recorded at admission. The degree of lung injury on CT scans was categorized as low (<25% involvement), moderate (25–50% involvement) or severe (>50% involvement), and only scans performed within the first 24 h were considered. Data on pharmacological therapies, mode of respiratory support, any complications or associated diagnoses and vital statistics were also gathered during the hospital stay. Any therapies delivered during the patients’ stay in hospital (including pharmacological agents to treat SARS-CoV-2) were left to the discretion of the referring medical team.
Diabetes was determined by a self-reported medical history of diabetes, previous medical records indicative of diabetes or ongoing treatment with glucose-lowering medications. However, no information regarding type of diabetes was available for our cohort.
Outcomes
The primary study outcome was a composite of transfer to an ICU or in-hospital death. Secondary outcomes were each component of the primary outcome on its own, death in an ICU and death with no transfer to an ICU. The date of the final follow-up for patients who remained hospitalized was 21 April 2020.
Statistical analysis
The present report was prepared in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist [15]. As most patients’ characteristics—whether covariates prior to admission or those related to medical presentation at admission—were dramatically different between those with and without diabetes, a PSM approach was used to better account for such differences. This involved 1:1 matching based on characteristics, using a ‘nearest neighbour matching’ algorithm and a caliper at 0.1 [16]. Characteristics used for matching included age, gender, body mass index (BMI), and ‘prior to admission’ characteristics [history of hypertension, dyslipidaemia, cardiovascular disease, heart failure and chronic obstructive pulmonary disease (COPD), smoking status, use of renin–angiotensin system (RAS) blockers] and ‘at admission’ characteristics (decision to withdraw life-sustaining therapy, oxygen saturation, creatinine, aspartate aminotransferase, leucocyte counts, C-reactive protein). Ultimately, the study matched 603 participants (89.5% and 27.8% of patients with and without diabetes before matching, respectively). After PSM, all absolute standardized differences were <10%, indicating robust matching [16] (Fig. S1; see Supplementary materials associated with this article online). Cox’s models were fitted for endpoints with diabetes used as covariates in the matched cohort.
As for sensitivity analysis, two other propensity scores were also constructed: (i) one using, in addition to age, gender and BMI, all personal characteristics and comorbidities included in the main propensity score (history of hypertension, dyslipidaemia, cardiovascular disease, heart failure and COPD, smoking status, RAS blocker use); and (ii) another using, in addition to age, gender and BMI, all admission vital statistics and laboratory findings (decision to withdraw life-sustaining therapies, oxygen saturation, creatinine, aspartate aminotransferase, leucocyte counts, C-reactive protein).
Continuous data are reported as means ± standard deviation (SD) for normally distributed data and as medians [25th, 75th percentiles] for non-normally distributed data. Categorical data are reported as counts (n) and percentages (%). Comparisons used the chi-square or Fisher’s exact tests for categorical variables and Student’s t test or Mann–Whitney–Wilcoxon test, as appropriate, for continuous variables. Kaplan–Meier survival curves for patients with and without diabetes at hospital admission were plotted and compared by log-rank test. A two-tailed P value <0.05 was considered statistically significant. All data were analyzed using R software, version 3.6.3 (R Project for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
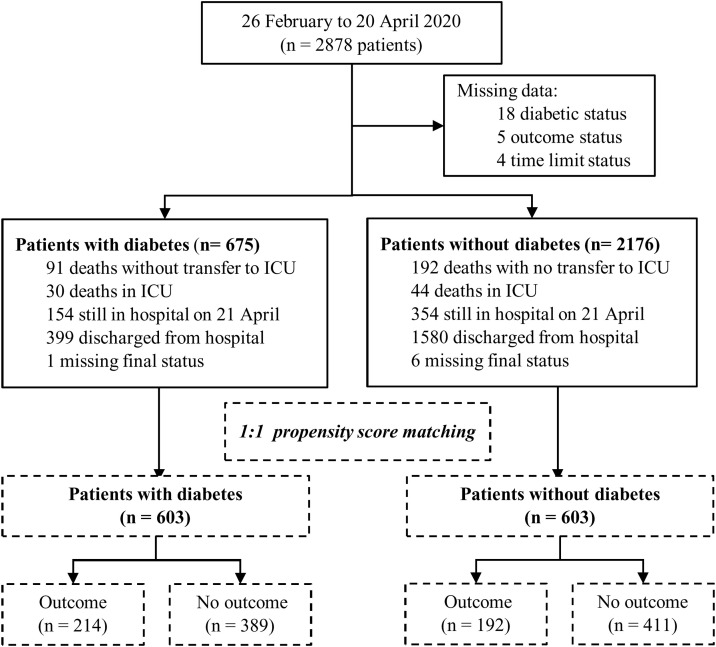
Overall, 2878 patients hospitalized for COVID-19 at 24 French clinical centres were included between 26 February and 20 April 2020 (all participating centres are listed in Table S1; see Supplementary materials associated with this article online). Of these patients, 27 were excluded from analysis because of missing data for outcomes or diabetes status (Fig. 1 ). Baseline characteristics and outcomes for the 2851 unmatched patients are presented in Table S2 and Table S3 (see Supplementary materials associated with this article online).
Fig. 1.
Flow chart of patients included in the study. ICU: intensive care unit.
After PSM, 603 patients in each group (with and without diabetes) were compared for outcomes (Fig. 1); their baseline characteristics according to diabetes status are presented in Table 1 . Mean ± SD age was 71 ± 15 years, and 745 (61.8%) were male. The prevalence of comorbidities at admission was: hypertension (76.3%); cardiovascular diseases (32.8%); chronic heart failure (15.7%); chronic kidney disease (22.1%); and COPD (6.4%). As for biological findings, levels of C-reactive protein were elevated, whereas most of the other laboratory findings at admission, such as haemoglobin, platelets, white cell counts, liver enzymes and estimated glomerular filtration rates (eGFRs), were within normal ranges. Abnormalities on chest CT scans were considered severe in 211 (22.4%) patients. Median time from symptom onset to hospital admission was 6.5 [3.0, 10.0] days, and duration of hospital stay was 8.0 [5.0, 13.0] days (Table 1, Table 2 ). Antibiotic drugs were the most prescribed therapy (75.9%) while in hospital. Overall, anticoagulation therapy was prescribed during hospitalization in 90.5% of patients, including prophylactic doses (66.6%) and therapeutic doses (23.9%).
Table 1.
Patients’ characteristics in the propensity score-matched cohort at hospital admission.
| All | No diabetes | Diabetes | P | n | |
|---|---|---|---|---|---|
| Demographic data | n = 1206 | n = 603 | n = 603 | ||
| Age, years | 71.2 ± 14.5 | 71.3 ± 15.9 | 71.0 ± 13.0 | 0.65 | 1203 |
| Male gender, n (%) | 745 (61.8) | 377 (62.5) | 368 (61.0) | 0.64 | 1206 |
| Body mass index, kg/m2 | 29.0 ± 5.98 | 29.1 (6.29) | 29.0 ± 5.68 | 0.93 | 1063 |
| Previous comorbidities, n (%) | |||||
| Hypertension | 914 (76.3) | 456 (76.1) | 458 (76.5) | 0.95 | 1198 |
| Dyslipidaemia | 552 (46.0) | 265 (44.0) | 287 (48.1) | 0.18 | 1199 |
| Smoking | 164 (14.0) | 83 (14.1) | 81 (13.8) | 0.92 | 1175 |
| Cardiovascular disease | 390 (32.8) | 192 (32.3) | 198 (33.4) | 0.73 | 1188 |
| Coronary artery disease | 246 (20.4) | 116 (19.2) | 130 (21.6) | 0.35 | 1206 |
| Peripheral arterial disease | 98 (8.22) | 40 (6.69) | 58 (9.76) | 0.07 | 1192 |
| Stroke | 147 (12.3) | 80 (13.4) | 67 (11.3) | 0.31 | 1192 |
| Chronic heart failure | 186 (15.7) | 89 (15.0) | 97 (16.5) | 0.54 | 1182 |
| Chronic kidney disease | 263 (22.1) | 127 (21.3) | 136 (22.9) | 0.57 | 1191 |
| GFR <60 mL/min/1.73 m2 | 185 (15.5) | 90 (15.1) | 95 (16.0) | 0.74 | 1191 |
| GFR <30 mL/min/1.73 m2 | 48 (4.03) | 23 (3.86) | 25 (4.20) | 0.88 | 1191 |
| Dialysis | 30 (2.52) | 14 (2.35) | 16 (2.69) | 0.85 | 1191 |
| COPD | 77 (6.38) | 34 (5.64) | 43 (7.13) | 0.35 | 1206 |
| Cancer | 166 (13.8) | 92 (15.3) | 74 (12.3) | 0.16 | 1206 |
| Active | 59 (4.89) | 36 (5.97) | 23 (3.81) | 0.11 | 1206 |
| In remission | 107 (8.87) | 56 (9.29) | 51 (8.46) | 0.69 | 1206 |
| Treatment before hospitalization, n (%) | |||||
| Beta-blockers | 419 (34.7) | 205 (34.0) | 214 (35.5) | 0.63 | 1206 |
| ACEis or ARBs | 621 (51.5) | 305 (50.6) | 316 (52.4) | 0.57 | 1206 |
| Diuretics | 356 (29.5) | 177 (29.4) | 179 (29.7) | 0.95 | 1206 |
| Statins | 446 (37.0) | 202 (33.5) | 244 (40.5) | 0.01 | 1206 |
| Antiplatelet agents | 405 (33.6) | 179 (29.7) | 226 (37.5) | 0.005 | 1206 |
| Anticoagulation | 224 (18.6) | 123 (20.4) | 101 (16.7) | 0.12 | 1206 |
| Clinical characteristics at admission | |||||
| Time from illness onset to hospitalization, days | 6.5 ± 4.8 | 6.7 ± 4.8 | 6.3 ± 4.7 | 0.17 | 1160 |
| Fever, n (%) | 117 (9.89) | 69 (11.6) | 48 (8.15) | 0.057 | 1183 |
| Heart rate, bpm | 86.5 ± 18.6 | 85.9 ± 19.2 | 87.0 ± 17.9 | 0.31 | 1096 |
| Systolic blood pressure, mmHg | 134 ± 22.7 | 134 ± 22.4 | 135 ± 23.0 | 0.53 | 1186 |
| Diastolic blood pressure, mmHg | 74.9 ± 13.5 | 75.4 ± 13.3 | 74.4 ± 13.8 | 0.24 | 1186 |
| Respiratory frequency, cpm | 23.6 ± 6.4 | 23.8 ± 6.7 | 23.4 ± 6.1 | 0.32 | 892 |
| O2 saturation, % | 94.5 ± 3.7 | 94.5 ± 3.9 | 94.5 ± 3.4 | 0.91 | 1194 |
| FiO2, % | 28.9 ± 12.4 | 28.9 ± 12.5 | 29.0 ± 12.3 | 0.82 | 1162 |
| Withdrawal of life-sustaining therapy, n (%) | 287 (24.6) | 137 (23.7) | 150 (25.4) | 0.55 | 1169 |
| Laboratory findings at admission, median [25th, 75th percentiles]a | |||||
| Haemoglobin, g/dL | 13.1 [11.7, 14.3] | 13.3 [11.8, 14.5] | 13.0 [11.6, 14.2] | 0.089 | 1196 |
| Platelets, G/L | 195 [154, 264] | 188 [153, 257] | 203 [155, 275] | 0.028 | 1182 |
| White cell count, per mL | 6.50 [4.95, 9.00] | 6.46 [4.88, 9.10] | 6.60 [5.02, 8.95] | 0.399 | 1190 |
| Lymphocytes, per mL | 0.96 [0.69, 1.32] | 0.93 [0.69, 1.30] | 0.98 [0.70, 1.33] | 0.183 | 1170 |
| Creatinine, µmol/L | 85.0 [66.0, 119] | 85.0 [67.9, 116] | 84.0 [64.0, 122] | 0.598 | 1190 |
| GFR, mL/min/1.73 m2 | 76.0 [50.7, 98.6] | 75.4 [52.6, 94.9] | 77.1 [49.4, 101] | 0.556 | 1187 |
| Alanine aminotransferase, IU/L | 31.0 [21.0, 49.0] | 33.0 [22.0, 51.0] | 30.0 [21.0, 48.0] | 0.057 | 1101 |
| Aspartate aminotransferase, IU/L | 39.0 [27.0, 60.0] | 41.0 [27.0, 64.0] | 37.0 [26.0, 55.0] | 0.024 | 1097 |
| Bilirubinaemia, mg/L | 9.00 [6.80, 12.6] | 9.00 [6.80, 13.0] | 9.00 [6.70, 12.0] | 0.625 | 1018 |
| γ-Glutamyl transferase, IU/L | 54.0 [31.0, 100] | 56.0 [32.0, 112] | 52.0 [30.0, 90.0] | 0.217 | 978 |
| C-reactive protein, mg/L | 76.2 [35.2, 133] | 77.0 [38.1, 138] | 75.7 [34.0, 128] | 0.629 | 1159 |
| D-dimer, µg/L | 970 [416, 1860] | 980 [470, 1910] | 955 [363, 1754] | 0.314 | 463 |
| PaO2, mmHg | 73.0 [64.0, 88.0] | 74.0 [65.0, 89.0] | 72.0 [64.0, 88.0] | 0.312 | 873 |
| Parenchymal involvement on computed tomography, n (%) | |||||
| Low (<30%) | 382 (40.6) | 200 (42.9) | 182 (38.3) | 0.17 | 941 |
| Moderate (30–50%) | 348 (37.0) | 163 (35.0) | 185 (38.9) | 0.233 | 941 |
| Severe (>50%) | 211 (22.4) | 103 (22.1) | 108 (22.7) | 0.877 | 941 |
Data are presented as means ± SD unless otherwise specified; a data not normally distributed; GFR, glomerular filtration rate; COPD, chronic obstructive pulmonary disease; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
Table 2.
Treatments and clinical outcomes during follow-up according to diabetes status in propensity score-matched cohort.
| All | No diabetes | Diabetes | P | n | |
|---|---|---|---|---|---|
| Treatment introduced during hospitalization, n (%) | |||||
| Chloroquine or hydroxychloroquine | 197 (16.3) | 97 (16.1) | 100 (16.6) | 0.88 | 1206 |
| Antivirals | 148 (12.3) | 70 (11.6) | 78 (12.9) | 0.54 | 1206 |
| Corticosteroids | 93 (7.71) | 53 (8.79) | 40 (6.63) | 0.2 | 1206 |
| Antibiotics | 915 (75.9) | 453 (75.1) | 462 (76.6) | 0.59 | 1206 |
| Anticoagulation | 1020 (90.5) | 503 (90.1) | 517 (90.9) | 0.76 | 1127 |
| Prophylactic doses | 751 (66.6) | 359 (64.3) | 392 (68.9) | 0.12 | 1127 |
| Curative doses | 269 (23.9) | 144 (25.8) | 125 (22.0) | 0.15 | 1127 |
| Respiratory support, n (%) | |||||
| Low-dose oxygen | 804 (66.7) | 407 (67.5) | 397 (65.8) | 0.58 | 1206 |
| High-flow oxygen delivery | 210 (17.4) | 102 (16.9) | 108 (17.9) | 0.7 | 1206 |
| High-flow nasal cannula | 69 (5.72) | 42 (6.97) | 27 (4.48) | 0.08 | 1206 |
| Non-invasive ventilation | 35 (2.90) | 17 (2.82) | 18 (2.99) | 1 | 1206 |
| Invasive mechanical ventilation | 169 (14.0) | 74 (12.3) | 95 (15.8) | 0.1 | 1206 |
| Outcomes | |||||
| Hospital stay, daysa | 8.00 [5.00, 13.0] | 8.00 [5.00, 13.0] | 8.00 [5.00, 13.0] | 0.47 | 745 |
| Primary outcome, n (%) | 406 (33.7) | 192 (31.8) | 214 (35.5) | 0.2 | 1206 |
| All deaths, n (%) | 202 (16.7) | 91 (15.1) | 111 (18.4) | 0.14 | 1206 |
| Transfer to ICU, n (%) | 244 (20.2) | 118 (19.6) | 126 (20.9) | 0.62 | 1206 |
| Death in ICU, n (%) | 40 (3.3) | 17 (2.8) | 23 (3.8) | 0.42 | 1206 |
| Death with no transfer to ICU, n (%) | 162 (13.4) | 74 (12.3) | 88 (14.6) | 0.27 | 1206 |
Data not normally distributed are presented as medians [25th, 75th percentiles].
Outcomes
During a median follow-up duration of 17.0 [5.0, 26.0] days, the primary outcome was recorded in 406 (33.7%) patients, including 162 (13.4%) who died with no transfer to an ICU and 244 (20.2%) who were transferred to an ICU. The overall rate of in-hospital death was 16.7% (202 patients) including 40 (3.3%) deaths in an ICU. At the end of follow-up, 749 (62.3%) patients were discharged and 252 (20.9%) were still in hospital. As shown in Table S4 (see Supplementary materials associated with this article online), patients experiencing the primary outcome, compared with those who did not, were more often male, more often had chronic kidney disease and heart failure, and also had higher levels of inflammatory expression in biological findings at admission.
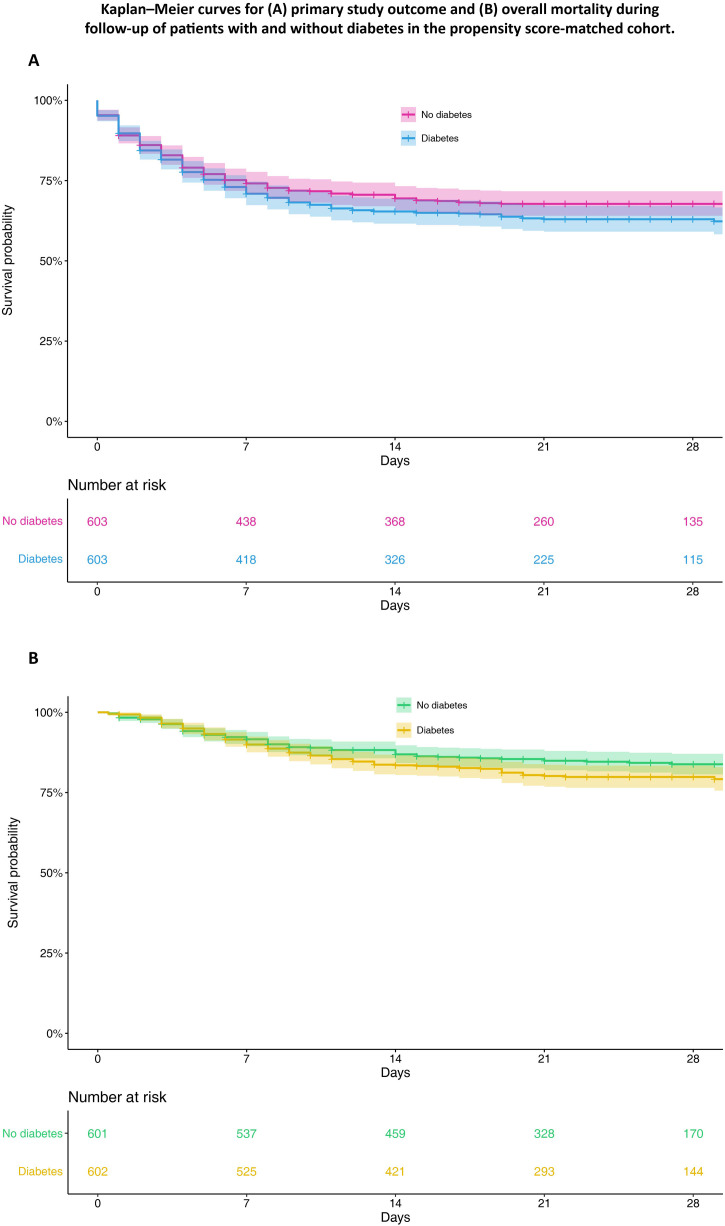
Compared with patients without diabetes, those with diabetes had shorter hospital durations from admission to occurrence of the primary outcome (15.0 days [5.0, 25.0] vs 18.0 days [6.0, 27.0], respectively; P < 0.03), but not from admission to death (7.0 days [4.0, 11.0] vs 6.0 days ([4.0, 11.0]; P = 0.37). Primary study outcomes were recorded in 214 (35.5%) patients with diabetes compared with 192 (31.8%) without diabetes (P = 0.20). A similar rate of secondary outcomes (overall mortality, transfer to an ICU, invasive mechanical ventilation) was also observed in patients with vs without diabetes (P > 0.05 for all). In-hospital death during follow-up was reported in 111 (18.4%) patients with compared with 91 (15.1%) patients without diabetes (P = 0.14; Table 2). Kaplan–Meier survival curves for the primary outcome and overall mortality according to diabetes status are presented in Fig. 2 . Cox’s proportional-hazards survival regression analyses showed no significant association between diabetes and incidence of either the primary outcome [HR: 1.16, 95% confidence interval (CI): 0.95–1.41; P = 0.14] or any of the secondary outcomes (Table 3 ).
Fig. 2.
Kaplan–Meier curves for (A) primary study outcome and (B) overall mortality during follow-up of patients with and without diabetes in the propensity score-matched cohort.
Table 3.
Hazard ratios (HRs) for outcomes during follow-up in patients with vs without diabetes in the propensity score-matched cohort.
| HR (95% CI) | P | |
|---|---|---|
| Primary outcome | 1.16 (0.95–1.41) | 0.14 |
| In-hospital mortality | 1.29 (0.97–1.69) | 0.08 |
| Death in intensive care unit (ICU) | 1.42 (0.75–2.70) | 0.28 |
| Death with no transfer to ICU | 1.26 (0.93–1.72) | 0.14 |
| Transfer to ICUa | 1.14 (0.88–1.47) | 0.31 |
CI, confidence interval.
Patients with a decision to withdraw life-sustaining therapy were excluded from the analysis.
Sensitivity analyses
In these analyses, the incidence of outcomes in the diabetes and non-diabetes groups were compared with two other PSM cohorts (one including personal characteristics and comorbidities, the other including admission vital statistics and laboratory findings) to better assess the impact of each type of covariate on the association between diabetes and outcome events. In the first PSM cohort wherein patients with and without diabetes were matched according to their personal characteristics and comorbidities, no differences were found in either vital statistics or laboratory or radiological findings between the two groups (Table S5; see Supplementary materials associated with this article online). In the second PSM cohort, which included only those with matching vital statistics and biological findings, patients with diabetes were older, more frequently male, had higher BMI scores and a greater prevalence of comorbidities than noted in the unmatched cohort. Cox’s regression analysis revealed that, in both PSM cohorts, any associations between diabetes status and risk of severe outcomes were non-significant (Table S6; see Supplementary materials associated with this article online).
Discussion
In this cohort of 1206 PSM patients hospitalized for COVID-19, no greater risk for worse outcomes was observed in patients with vs without diabetes. Yet, from the beginning of the COVID-19 pandemic in January 2020, there had been growing evidence from both descriptive and epidemiological studies of a greater prevalence of diabetes in severe COVID-19 patients. Indeed, diabetes prevalence varied from 17% to 37% in the most recent case series of hospitalized patients in the US and Europe [2], [3], [17], [18], [19]. In the present study, a similar rate of 23.6% patients with diabetes was reported among patients hospitalized for COVID-19. Likewise, a high rate of associated comorbidities, with hypertension being the most frequent one, followed by cardiovascular diseases, chronic respiratory disease and chronic kidney disease, was also observed. Most of these comorbidities (hypertension, cardiovascular diseases, chronic kidney disease) are known to be commonly found in those living with diabetes [20]. It was also revealed that, in the unmatched cohort, patients with diabetes were more prone to receive invasive mechanical ventilation and intensive care, and to face greater mortality, than those without diabetes [10], [11], [12], [13].
Despite several case series of patients hospitalized with COVID-19 worldwide, only a few studies have been specifically focused on the prognosis of patients with and without diabetes. In contrast to our present findings, those studies found that diabetes was associated with a higher risk of severe outcomes. Zhu et al. [12] reported a significant 1.49-fold higher risk of all-cause mortality between subgroups in a retrospective study of 7337 patients [952 with type 2 diabetes (T2D)] hospitalized in China for COVID-19. However, the authors failed to adjust for comorbidities closely related to T2D, such as hypertension, cardiovascular disease and chronic kidney disease, all of which have proved to be major risk factors in COVID-19 prognoses [17]. Another Chinese retrospective study of a small number of subjects (193 patients, 48 with diabetes) found that patients with diabetes had lower survival rates than those without diabetes, with an HR of 1.53 (P = 0.041) after adjusting for age, gender, hypertension, cardiovascular disease and cerebrovascular disease [11]. Similarly, in a preprint version of a nationwide study of 23,804 COVID-19-related deaths in England, the odds ratio (OR) for dying in hospital with COVID-19 in patients with T2D was 1.81-fold higher than in the population not known to have diabetes [21]. However, despite the large number of participants in that study, some important potential confounding comorbidities, such as hypertension and chronic kidney disease, were ignored. Moreover, time-to-event data were also not available in this preprint, which could negatively impact the robustness of their results.
Unlike the above-mentioned studies, our present study used PSM analysis to avoid the confounding effects of the comorbidities frequently associated with both diabetes and poorer outcomes with COVID-19, and also failed to find that diabetes was associated with a higher risk of severe outcomes. In accordance with our results, a recent study of 20,133 UK patients in hospital for COVID-19 found that, even though diabetes was commonly seen (28.1%) in this population, the association of diabetes with mortality risk was attenuated to the point of non-significance after multiple adjustments on Cox’s analysis (HR: 1.06) [22]. Similarly, in a US study of 5279 subjects in New York City, Petrilli et al. [23] found a 3.6-fold greater prevalence of diabetes in patients with COVID-19 admitted to hospital compared with those not admitted. However, after multiple adjustments, the risk of critical illness among inpatients with diabetes was similar to that of those without diabetes.
Taken together, these findings suggest that the increased risk of severe outcomes reported in patients with diabetes is ameliorated after adjusting for diabetes-related comorbidities. Indeed, the diabetes-associated risk of severe outcomes with COVID-19 could be more driven by the associated comorbidities than by the diabetes itself.
In sensitivity analyses where only vital statistics and laboratory findings were used to construct the propensity scores for matching, the risk of negative outcomes in both diabetes and non-diabetes groups proved to be similar, even though diabetes patients were older, had higher BMI scores and higher rates of associated comorbidities. The interpretation of these findings, however, is not obvious and subject to biases. Nevertheless, it could be argued that the differential risk between patients with and without diabetes might be driven by vital signs and biological findings rather than clinical characteristics and comorbidities. In two previous studies comparing patients with and without diabetes, Cox’s regression analyses found a higher rate of outcomes in patients with diabetes after adjusting for age, gender, hypertension, cardiovascular disease and cerebrovascular disease, although no adjustments were made for biochemical values despite a significant difference in inflammatory markers between groups [10], [11]. Moreover, in the study by Petrilli et al. [23], the risk of severe outcomes was significantly greater when adjusted only for clinical characteristics and medical history, but no longer significant after adjusting for both previous comorbidities and biological findings at admission. Taken together, these data suggest that the severity of infection at admission (based on, for example, clinical presentation or expression of inflammatory markers) instead of previous comorbidities might better for assessing risk for worse outcomes.
Study limitations
Our study has some limitations. Unfortunately, detailed data for diabetes characteristics that might influence outcomes, such as type of diabetes, HbA1c levels, diabetes duration, diabetic therapies and microvascular complications, were not available for the present cohort. Such a lack of information is a clear study limitation as it diminishes any confidence in our results. However, in the Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study, involving a well-documented French cohort of 1317 diabetes patients hospitalized for COVID-19, Cariou et al. [24] found that BMI scores, but not diabetes-related patterns before admission, were positively and independently associated with tracheal intubation and/or death within 7 days. Nevertheless, another obvious limitation is the lack of data on glycaemic control at admission or during hospitalization. Indeed, Wang et al. [25] observed that, in 605 patients with no previous diagnosis of diabetes, fasting blood glucose at admission was an independent predictor of 28-day mortality in patients with COVID-19. Furthermore, Zhu et al. [12] demonstrated that, in patients with T2D, those with well-controlled blood glucose during their hospital stay had better prognoses than those with poorly controlled glycaemia. Thus, these data suggest it may be glycaemic control at admission and during hospitalization that has an impact on COVID-19 prognoses in diabetes patients instead of their previous glycaemic control or other specific patterns of diabetes [26]. Third, the dataset used here came from 24 clinical centres where COVID-19 care strategies may have differed from one centre to another due to the urgent circumstances of the COVID-19 pandemic. Fourth, given the retrospective nature of the cohort, it is not possible to avoid classification biases. However, our data were collected by CCF investigators, all of whom were first-line doctors directly in charge of the study participants, and the amount of missing data was limited. Fifth, only data from patients hospitalized for COVID-19 were analyzed, which means that our findings cannot be generalized to COVID-19 patients with less severe forms of infection. Finally, as all HRs found by PSM analysis were >1 with wide CIs, no null effect of diabetes on COVID-19 outcomes can be claimed, especially as all HRs from Cox’s analyses were also >1.
Conclusion
Our present findings suggest that, despite the high prevalence of diabetes in patients hospitalized for COVID-19, the risk of severe outcomes was mainly driven by associated comorbidities or more severe clinical presentations at admission to hospital. These results provide new insights into risk stratification for patients with COVID-19. However, further studies on a larger scale and with better control of confounding biases, especially for glucose control before and during hospitalization, are still warranted to confirm these findings.
Ethics approval and consent to participate
The CCF study was declared to and authorized by the French Data Protection Committee [Commission nationale de l’informatique et des libertés (CNIL), authorization no. 2207326v0], and was conducted in accordance with the ethical standards laid down by the 1964 Declaration of Helsinki and its later amendments.
Authors’ contributions
L.P. and W.S. had full access to all of the study data, and take full responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: L.P., W.S.; acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: L.P., W.S. Critical revision of the manuscript for important intellectual content: all authors. Final approval of the version submitted for publication: all authors. The corresponding author attests that all listed authors have met authorship criteria and that no others meeting the criteria have been omitted.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Competing interests
L.P. reports grants, personal fees and non-financial support from Novo Nordisk, MSD and Sanofi, personal fees and non-financial support from Eli Lilly, and non-financial support from Servier. A.C. acknowledges a research grant from RESICARD (research nurses), and consultancy and lecture fees from Amgen, AstraZeneca, Bayer Pharma, Alliance BMS-Pfizer, Novartis and Sanofi-Aventis, which are not related to the present manuscript. The other authors have nothing to declare
Funding
This research received no specific grants from funding agencies in either the public, commercial or not-for-profit sectors.
Acknowledgements
We are grateful to the medical and paramedical staff involved in the care of patients during the study period as well as all members of the French Society of Cardiology.
Footnotes
Supplementary material (Tables S1–S6, Fig. S1) related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.diabet.2020.101222.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Caussy C., Pattou F., Wallet F., Simon C., Chalopin S., Telliam C., et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41:2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada T., Mikami T., Chopra N., Miyashita H., Chernyavsky S., Miyashita S. Patients with chronic kidney disease have a poorer prognosis of coronavirus disease 2019 (COVID-19): an experience in New York City. Int Urol Nephrol. 2020;52:1405–1406. doi: 10.1007/s11255-020-02494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pranata R., Lim M., Yonas E., Vania R., Lukito A., Siswanto B., et al. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. 2020;S1262–3636(July (20)) doi: 10.1016/j.diabet.2020.07.005. 30097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of Covid-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 11.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with Covid-19 and pre-existing type 2 diabetes. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.04.021. 311068-77 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335–337. doi: 10.1016/j.diabet.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauvel C., Weizman O., Trimaille A., Mika D., Pommier T., Pace N., et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 aAdmitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iglay K., Hannachi H., JosephvHowie P., Xu J., Li X., Engel S., et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Cur Med Res Opin. 2016;32:1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 21.Barron E.B.C., Kar P., Weaver A., Bradley D., Ismail H., Knighton P., et al. Type 1 and Type 2 diabetes and COVID-19 related mortality in England: a whole population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Ma P., Zhang S., Song S., Wang Z., Ma Y., et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63:2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheen A., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabet Med. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.