Abstract
Background
Statistical models are increasingly being used to estimate and project the prevalence and burden of asthma. Given substantial variations in these estimates, there is a need to critically assess the properties of these models and assess their transparency and reproducibility. We aimed to critically appraise the strengths, limitations and reproducibility of existing models for estimating and projecting the global, regional and national prevalence and burden of asthma.
Methods
We undertook a systematic review, which involved searching Medline, Embase, World Health Organization Library and Information Services (WHOLIS) and Web of Science from 1980 to 2017 for modelling studies. Two reviewers independently assessed the eligibility of studies for inclusion and then assessed their strengths, limitations and reproducibility using pre-defined quality criteria. Data were descriptively and narratively synthesised.
Results
We identified 108 eligible studies, which employed a total of 51 models: 42 models were used to derive national level estimates, two models for regional estimates, four models for global and regional estimates and three models for global, regional and national estimates. Ten models were used to estimate the prevalence of asthma, 27 models estimated the burden of asthma – including, health care service utilisation, disability-adjusted life years, mortality and direct and indirect costs of asthma – and 14 models estimated both the prevalence and burden of asthma. Logistic and linear regression models were most widely used for national estimates. Different versions of the DisMod-MR- Bayesian meta-regression models and Cause Of Death Ensemble model (CODEm) were predominantly used for global, regional and national estimates. Most models suffered from a number of methodological limitations – in particular, poor reporting, insufficient quality and lack of reproducibility.
Conclusions
Whilst global, regional and national estimates of asthma prevalence and burden continue to inform health policy and investment decisions on asthma, most models used to derive these estimates lack the required reproducibility. There is a need for better-constructed models for estimating and projecting the prevalence and disease burden of asthma and a related need for better reporting of models, and making data and code available to facilitate replication.
Resources are limited and health system planners need to make investment decisions based on available evidence of disease prevalence, associated morbidity and health care utilisation, mortality and costs, how these are likely to have the potential to change over time and their amenability to interventions [1-8]. Modelling studies have the potential to inform such important deliberations [1,9-11], hence, they have been prioritised by inter-governmental organisations such as the World Health Organization (WHO) and funders such as Bill and Melinda Gates Foundation [3,5,8,12-17]. The Institute for Health Metrics and Evaluation (IHME) [18] and The Child Health Epidemiology Reference Group (CHERG) [19] are two prominent examples of initiatives that have extensively used modelling approaches to generate estimates of disease epidemiology and morbidity.
Focusing specifically on estimates of asthma prevalence and burden, we observe that varying estimates have been reported in the published literature at national, regional and global levels, which are not consistent over the years (as shown in Table 1). Given the range of models used to estimate prevalence and burden of asthma, it is a challenging task to determine an appropriate model that can be applied in a new data set. Furthermore, there is a lack of a robust critical evidence base to help guide decisions on which model(s) are most appropriate for different contexts. There is, therefore, a need for a systematic appraisal of the merits and limitations of available models. In this study, we sought to systematically describe and critique existing models for prevalence and burden of asthma in relation to their strengths, limitations and reproducibility.
Table 1.
Global prevalence and burden (mortality) of asthma
| Study | Year | Prevalence in thousands (uncertainty interval) | Burden: mortality in thousands (uncertainty interval) |
|---|---|---|---|
| GBD [12,13] |
2017 |
272 677 (242 295-304 699) |
495 (338-641) |
| GBD [3,17] |
2016 |
339 440 (319 582-360 796) |
420 (338.8-517.7) |
| GBD [14] |
2015 |
358 198 (323 134-393 466) |
397 (363-439) |
| GBD [5,8] |
2013 |
241 695 (238 151-245 465) |
489 (397.7-676.8) |
| GBD [1,9] |
2010 |
334 247 (Not available) |
345.7 (282.6–529.1) |
| WHO [20] |
2004 |
234 900 (Not available) |
287 (Not available) |
*GBD – Global Burden of Disease, WHO – World Health Organization
METHODS
Protocol
We described and documented the methods employed in this systematic review in detail in a previously published protocol [21] and therefore confine ourselves here to a summary of the methods employed.
Eligibility criteria
We included any study that applied models for estimating and projecting prevalence or disease burden of asthma. We included original research and review articles, including systematic reviews, meta-analyses and meta-syntheses of observational studies in human populations of any age and sex. We included research articles from any country and any setting (urban/rural) and published in any language. Our outcomes of interest were the prevalence of asthma and different components of the disease burden of asthma. The components of disease burden were direct and indirect costs of asthma (costs due to primary care utilisation, hospitalisation, ambulatory care, emergency visit, drug cost, absenteeism, presentism), disability-adjusted life years (DALYs), years lived with disability (YLDs), years of life lost (YLLs), potential years of life lost, healthy years of life lost, active life expectancy, disability-free life expectancy, disability-adjusted life expectancy, and healthy life expectancy (HALE).
Information sources
We identified relevant published and unpublished studies through searching electronic databases, hand-searching of pertinent journals and checking reference lists of all the eligible papers for studies published between January 1980 and September 2017. The following electronic databases were searched: Medline, Embase, World Health Organization Library and Information Services (WHOLIS library catalogue of books and reports) and Web of Science Core Collection. Journals that were hand-searched included The Lancet, BMJ (1980-2017), European Respiratory Journal (1988-2017), Lancet Respiratory Medicine (2013-2017), Lancet Global Health (2013-2017) and Journal of Global Health (2011-2017). We ran the last searches on 16 September 2017. We included papers published in any language and translated the papers that were not in English with the help of fluent or native speakers.
Search strategy
Comprehensive search strategies were developed for all the aforementioned databases in consultation with a senior medical librarian at The University of Edinburgh to identify both published and unpublished (grey literature) primary studies as well as reviews. The search terms used included but were not limited to asthma, wheeze, epidemiology, prevalence, burden, morbidity, mortality, DALY, QALY, HALE, primary health care, emergency service, hospitalisation, absenteeism, cost of illness, model, estimate, projection. The detailed search strategies used to search each database are given in Appendix S1 in the Online Supplementary Document.
Study selection
Two reviewers independently checked and screened the titles and abstracts of identified articles against the inclusion criteria. Full-text copies of potentially eligible studies were obtained and assessed by two independent reviewers (MRB and MAI) on the basis of the inclusion criteria. Discussion between the two reviewers resolved the majority of discrepancies; a third reviewer (BIN) arbitrated in the case of any disagreements.
Data collection process
We developed a data extraction form and used it to extract relevant data from included studies. The data extraction form was piloted on 10 included studies and was then refined accordingly prior to full use in the review. Data extraction was performed independently by two reviewers (MRB and MAI). Any discrepancies in data extraction were resolved through discussion between the reviewers, with arbitration by a third reviewer (BIN) if a decision could not be reached.
Data items
The following data items were extracted from each paper: 1) study identification (authors’ name, study/publication year, title); 2) aims and methods of the study (context of the study; country or region; study outcomes; burden type; case definitions; type of estimation; data and study population: data sources, age, sex, study area; estimation/study period; study design; data type; response variables; and data level); 3) model information (model name; model purpose; model structure; appropriateness of the model; model assumptions; model building; model fitting; model diagnosis; goodness-of-fit; robustness; missing data; uncertainty estimation; validation; sensitivity analysis; adequate model presentation; adequate reporting of estimates; reproducibility: availability of input data, computer coding and model fitting manual).
Summary measures
As this review mainly focused on the properties of models rather than quantitative measures, we did not perform any quantitative synthesis of the data.
Methods of analysis
We produced a tabular summary of the data to summarise the overall evidence. Descriptive statistics and graphical presentation were used to summarise the results. A detailed critical narrative synthesis of the models was undertaken regarding their strengths, limitations and reproducibility.
Quality appraisal of the included models
To our knowledge, there is no existing quality appraisal tool to assess the quality of the components of models for estimating the prevalence and burden of diseases. We therefore developed our own model evaluation checklist by adapting relevant sections from pertinent critical appraisal checklists [22,23], reporting guidelines [24] and other guidelines for good practice in modelling studies [25-28]. Prior to finalising this checklist, we consulted three experts (two medical statisticians and an epidemiologist) in modelling studies.
Assessing strengths, limitations and reproducibility of the included models
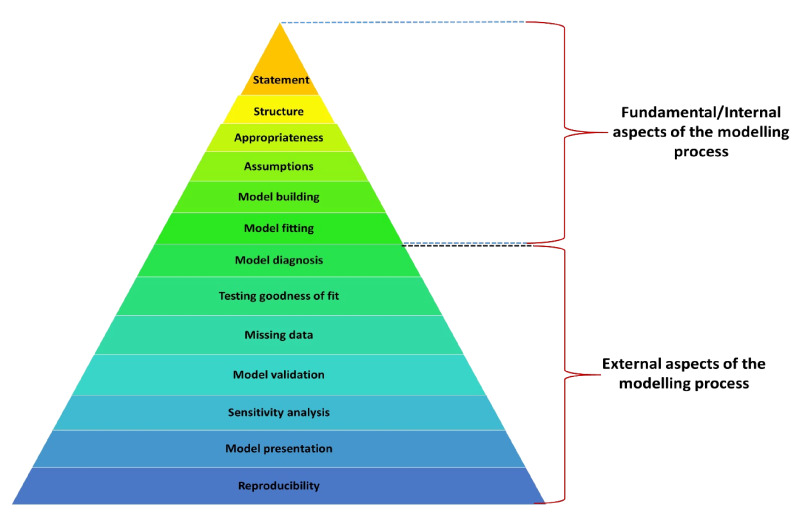
Our checklist consists of 13 quality criteria that a model should possess. We present the checklist by a pyramid in Figure 1 in terms of the hierarchy of the model quality criteria. Table S1 in the Online Supplementary Document provides a description of these criteria. We classified the model quality criteria into two groups: 1) fundamental or internal aspects of the modelling process; and 2) external aspects. The fundamental or internal aspects are: model statement, model structure, model appropriateness, model assumptions, model building and model fitting. The external aspects are: model diagnosis, testing goodness-of-fit, addressing missing data, model validation, sensitivity analysis, adequate presentation and reporting of model to ensure reproducibility.
Figure 1.
Checklist for assessing the quality of models.
Reporting
This systematic review is reported following the guidelines of the Preferred Reporting Items for Systematic review and Meta Analysis (PRISMA) checklist [29].
RESULTS
Study selection
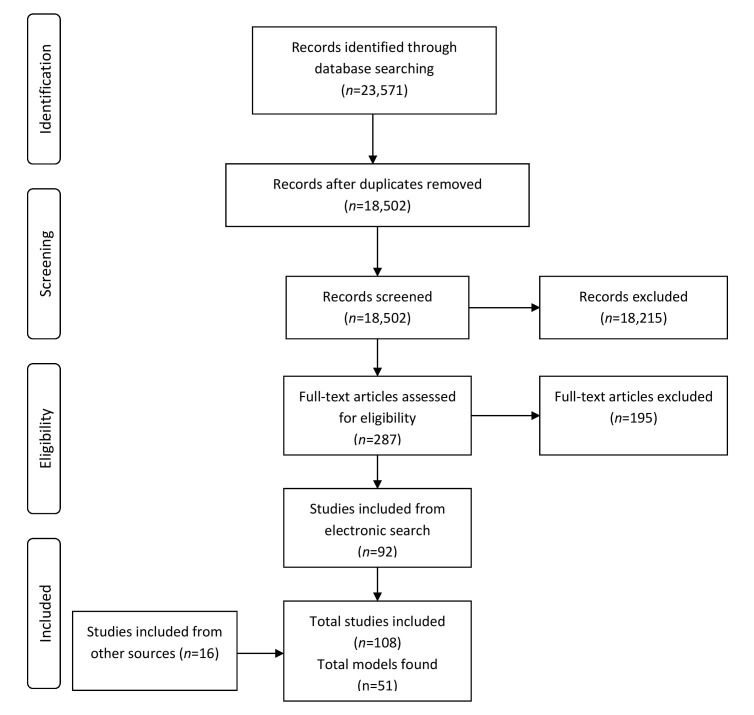
Our electronic search of the databases yielded a total of 23 571 references. After removing duplicates, 18 502 study titles and abstracts were screened against our inclusion criteria. Among these, 287 full-text articles were assessed in detail. Finally, 92 papers met the inclusion criteria. An additional 16 papers identified from hand-searching of journals and scrutinising reference lists of included studies met the inclusion criteria. Therefore, a total of 108 studies were included in the systematic review. The PRISMA flow diagram of the selected papers is presented in Figure 2. We included five non-English papers: two Dutch papers, one German paper, one French paper and one Spanish paper.
Figure 2.
PRISMA flow diagram of selected papers.
Study characteristics
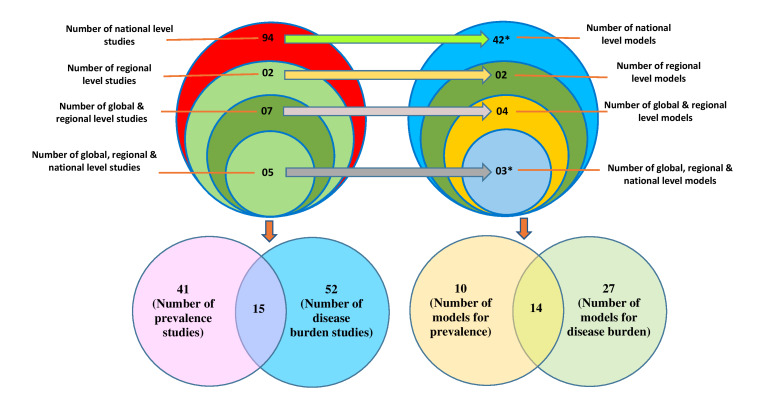
We identified 94 studies that estimated the prevalence and burden of asthma at a national level; two at a regional level; seven at both global and regional levels; and five at global, regional and national levels (Figure 3). Among the 108 included studies, 41 studies estimated the prevalence of asthma, 52 studies estimated the burden of asthma and 15 studies estimated both prevalence and burden of asthma (Figure 3). Most of the included studies originated from Europe (n = 33, 30.6%); followed by North America (n = 29, 26.9%), worldwide or multi-country (n = 19, 17.6%), Asia (n = 15, 13.9%), South America (n = 7, 6.5%) and Australia (n = 4, 3.7%); and only one study was from Africa (Table 2).
Figure 3.
Distribution of included studies and models. *One model (linear regression model) was used in both national-level and global-and-regional-level studies which we counted as national level model due to its high uses in national level studies; and one model (CODEm) was used in both global-and-regional-level and global-regional-and national-level studies which we counted as global-regional-and national-level model due to its high uses in global-regional-and national-level studies.
Table 2.
Distribution of included studies by region
| Region | Number of studies (%) |
|---|---|
| Africa |
1 (1.0) |
| Asia |
15 (13.9) |
| Australia |
4 (3.7) |
| Europe |
33 (30.6) |
| North America |
29 (26.9) |
| South America |
7 (6.5) |
| Worldwide or multi-country |
19 (17.6) |
| Total | 108 (100.0) |
Models for estimating and projecting prevalence and burden of asthma
A total of 51 models were used in the 108 included studies: 42 models were used to derive national level estimates (41 national level models plus one model common with global-and-regional level); two models were used to derive regional level estimates; four were used for global and regional estimates; and three models were used to derive global, regional and national estimates (Figure 3). Among these 51 models, ten models were used to estimate the prevalence of asthma, 27 models were used to estimate the burden of asthma, and 14 models were used to estimate both the prevalence and burden of asthma (Figure 3). Distribution of all the models is presented by study level and type of measurement in Table 3.
Table 3.
Distribution of models by study level and type of measurement
| Study level |
Type of measurement |
||
|---|---|---|---|
|
Prevalence |
Burden |
Both prevalence and burden |
|
|
National |
1. Meta-analysis: random effect model |
1. Two-part models |
1. Logistic regression model |
| 2. Logistic regression model with regression splines/restricted cubic splines |
2. Generalised linear models with gamma distribution and logarithmic link function |
2. Linear regression model* |
|
| 3. Exponential regression model |
3. Log transformed linear model |
3. Poisson regression model |
|
| 4. General linear predictive model |
4. LOESS (locally weighted regression) model |
4. Negative binomial regression model |
|
| 5. Hierarchical logistic regressions model |
5. Bootstrapped prevalence-based cost of illness model |
5. Generalised estimating equations (GEE) |
|
| 6. Survey weighted logistic regression model |
6. Box-Jenkins regression-ARIMA model |
6. Generalised linear models |
|
| 7. Conditional Autoregressive (CAR) model |
7. Generalised linear mixed effect model |
||
| 8. Cost assessment model |
8. Computer simulation model |
||
| 9. Economic model |
9. Double exponential smoothing model |
||
| 10. Exchangeable (EX) Model – Poisson-Gamma Model |
10. Epidemiological model based on a dynamic multi-state lifetable |
||
| 11. First degree homogeneous Markov model |
11. RIVM Chronic disease model |
||
| 12. Generalised additive model (GAM) |
|||
| 13. Heckman selection model |
|||
| 14. Joinpoint regression model |
|||
| 15. Log-linear autoregression model |
|||
| 16. Log-linear regression model |
|||
| 17. Machine learning based prediction model |
|||
| 18. Multiplicative models for rates (Beslow and Day method) |
|||
| 19. Multivariate regression model with weighted least squares |
|||
| 20. Polynomial regression model |
|||
| 21. Quadratic regression model |
|||
| 22. Quantile regression model |
|||
| 23. Seasonal autoregressive integrated moving average (SARIMA) model |
|||
| 24. Weighted linear regression model |
|||
| 25. Zero-inflated negative binomial regression model |
|||
|
Regional |
1. Nonlinear exponential regression model |
- |
- |
| 2. Meta-analysis: random effects Bayesian model | |||
|
Global and regional |
1. DisMod-MR |
1. Cause Of Death Ensemble modeling (CODEm)† |
1. DisMod |
| 2. Cause-of-death modeling (CodMod) |
2. DisMod II |
||
| 3. Linear regression model* |
|||
| Global, regional and national | 1. DisMod-MR 2.0 | 1. Cause Of Death Ensemble modeling (CODEm)† | 1. DisMod-MR 2.1 |
*Linear regression model was used in both national-level and global-and-regional-level studies which we counted as national level model due to its high uses in national level studies.
†CODEm was used in both global-and-regional-level and global-regional-and national-level studies which we counted as global-regional-and national-level model due to its high uses in global-regional-and national-level studies.
Outcome measures and statistical methods used by the included studies
A tabular summary of the data is presented in Table S2 in the Online Supplementary Document to summarise the overall evidence. The included studies mainly used models for deriving annual estimates, trends, changes of estimates over a period, projections, predicted estimates and forecasted estimates of prevalence and various components of the disease burden of asthma. In addition to estimates of prevalence, the components of disease burden that were estimated were mortality; health care service utilisation (health care provider visit, family practitioner visits, specialist visits, emergency room visits/emergency department visits, hospitalisation/hospital admission, re-admission); productivity loss (due to absenteeism, presenteeism and overall work impairment); cost of asthma – direct medical expenditure (costs of physician office visits, emergency department visits, outpatient visits, inpatient visits, medications, and other medical visits, asthma exacerbations and readmissions), indirect costs (costs of absenteeism, costs of parents’ productivity loss and work-time loss, children’s loss of lifetime earnings because of premature death), societal costs; incidence; asthma exacerbation; YLDs; and quality of life.
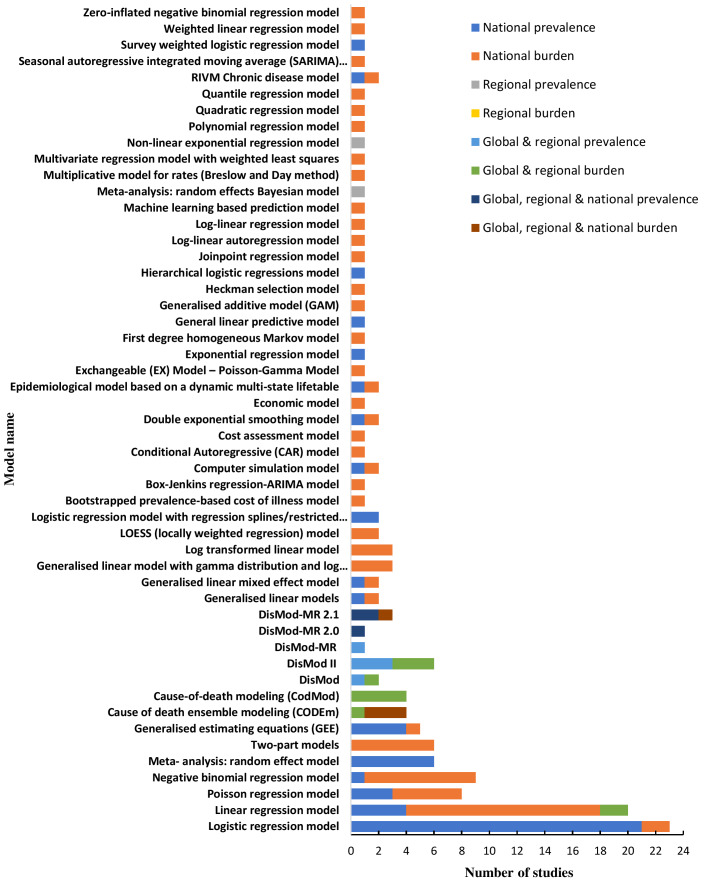
The frequencies of uses of each model by type of study are shown in Figure 4. Logistic regression modelling was the most commonly used approach for estimating the national prevalence of asthma, which was used in 21 studies [30-50] (out of 46 studies that estimated national prevalence). This was followed by linear regression, which was used in 14 studies [51-64] (out of 57 studies) to estimate and project the national burden of asthma. DisMod, DisMod II and cause of death modelling (CodMod) were used to estimate global and regional prevalence and burden of asthma in four studies [10,20,65,66] (out of seven studies). Different versions of DisMod-MR-Bayesian meta-regression models (DisMod-MR, DisMod-MR 2.0 and DisMod-MR 2.1) and Cause Of Death Ensemble modelling (CODEm - mixed effects linear/nonlinear models and/or spatial-temporal Gaussian process regression models) were used in all the five studies [3,5,8,14,17] for estimating the global, regional and national prevalence and burden of asthma.
Figure 4.
Frequencies of uses of each model by type of study. NB. Sum of the frequencies of uses of a model may not be equal to the number of studies used that model, because many studies used more than one model and some studies used same model for estimating both prevalence and more than one component of burden.
Strengths, limitations and reproducibility of the included models
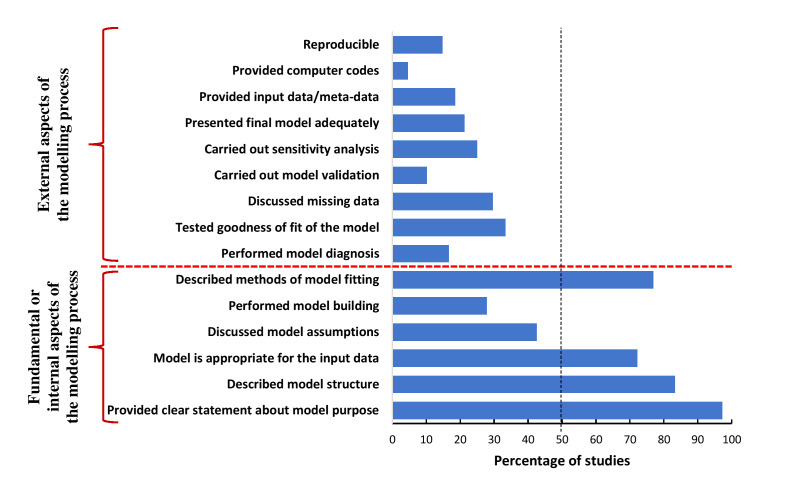
Figure 5 presents the results emanating from the application of the checklist we developed to appraise the quality of models.
Figure 5.
Percentage of studies fulfilled each model quality criteria.
Strengths of the models
More than half of the studies fulfilled four of the six internal model quality criteria. One hundred and five (97.2%) studies [1,3,5,8-10,14,17,20,30,32,33,35-127] provided a clear statement about the questions that the models aimed to answer. Four in five studies (n = 90; 83.3%) [1,3,5,8,10,14,17,20,30,33,34,36-44,46-51,53-55,57,59-70,72,73,75-78,81-84,87,89-111,113-120,122-127] explicitly described the structure of the models, and nearly three-quarters of studies (n = 78; 72.2%) [1,3,5,8-10,14,17,20,30,33-44,46-50,65-67,70-72,75-77,81,82,84,85,87-94,96-114,116-120,123-128] used appropriate modelling tools to deal with the nature, distribution and type of input data. Adequate description of the process of model fitting was provided by 83 (76.9%) studies [1,3,5,8-10,14,17,20,34-44,46-51,53-55,57,60-66,68-70,72,76,78,81-84,87,89-111,113-119,122,124-127] (Figure 5).
Limitations of the models
Most studies failed to fulfil two internal model quality criteria and all the external criteria. Sixty-two (57.4%) studies [30,32-34,36-38,40,41,44-59,61-64,67,68,71,73,74,79,81,83,85,87,88,92,93,95,96,98-101,104,106,109-115,117,122,127-129] did not provide any information about the model assumptions, while, only 30 (27.8%) studies [1,3,8,14,33,36,38,44,50,55,57,67,70,72,75-77,82,84,90-92,94,96,97,100,103,110,116,124] performed model building to select necessary variables for the models. The model diagnosis (model adequacy checking) was performed by 18 (16.7%) studies [49,68,69,72,75,76,82,86,89-91,93,97,101,103,105,107,118], whereas 36 (33.3%) studies [1,3,5,8,14,46,49-51,54-56,60,61,63,64,67,69,72,81,82,84,89-91,94,96,97,103,107,110,117-119,122,124] tested and reported goodness-of-fit of the models. Very few studies discussed missing data (n = 32; 29.6%) [1,3,5,8-10,14,17,20,32,33,44,46,48,58,65,66,70,76,78,85,86,89,92,94,97,109,114,117,118,124,126], carried out model validation (n = 11; 10.2%) [1,3,5,8,14,41,80,84,96,97,123], performed sensitivity analysis (n = 27; 25%) [1,10,14,20,41,43,45,55,65,72,75,76,78-80,84,88,93,104,108,112,118-121,123,124] and presented final model adequately (n = 23; 21.3%) [40,51,53-56,62,69,75,81,84,89,90,93-95,97,99,101,106,107,117,124] (Figure 5).
Reproducibility
We found only 20 (18.5%) studies [3,14,17,49,51,52,54,62,69,81,90,93,98,99,101,104,110,114,117,122] that provided final input data or meta-data. Very few studies (n = 5; 4.6%) [3,14,17,90,117] provided the computer code used to fit the models, and very few models (n = 16; 14.8%) [49,51,54,62,69,81,90,93,98,99,101,104,110,114,117,122] were therefore judged to be reproducible (Figure 5).
Further analysis, assessing whether the included studies adhered the new Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) [24] showed that most of the included studies (n = 105; 97%) did not adhered the GATHER guidelines to report their derived estimates of prevalence and burden of asthma; nevertheless, 82% (n = 88) of these studies were published before the publication of GATHER guidelines. Among the 20 studies, that were published after the availability of GATHER guidelines, only three studies (15%) [3,14,18] adhered these guidelines to report their estimates.
DISCUSSION
Statement of principal findings
This systematic review of the international literature has found that a variety of models are used to estimate disease prevalence and burden; most models, however, suffer from methodological limitations, in particular, lack of reproducibility and sub-optimal reporting.
Almost all the studies provided a clear statement about the questions that the models aimed to answer. The majority of the studies described the structure of the models explicitly; mentioned the methods used to fit the models or used to estimate model parameters; and applied appropriate modelling tools to deal with the nature, distribution and type of input data. These were the strengths of the models. However, these models had substantial limitations. We observed a lack of clarity in reporting the models. The majority of the studies did not provide any information on the model assumptions, methods of model building, methods of model diagnosis, methods of handling missing data, model validation and sensitivity analysis. In addition, many studies did not adequately present the final model parameter estimates, standard errors and confidence intervals. Moreover, most of the models could not be reproduced as the studies did not provide input data (or alternatively meta-data, synthetic data or simulated data if input data could not be published due to confidentiality), computer coding, a model fitting manual or complete information about the process of model formulation.
Strengths and limitations
The main strengths of this review include the comprehensive search strategy employed, the use of established methodology (two independent reviewers for screening, full-text assessment and data extraction) and the inclusion of studies from all over the world including studies published in any language. Critical appraisal of identified models and expert involvement in developing model assessment tools further strengthened the credibility of this review. However, this review has a number of potential limitations. As with any systematic review, we may have missed some studies. Although risk factor based models or association models are particularly essential to assess determinants of a disease, we did not include studies that used models to estimate risk factors or to assess association rather than estimating prevalence and disease burden using model. The use of a self-developed customised model evaluation tools, due to the lack of appropriate critical appraisal checklist, may be considered to be a further limitation of this review. There is a need for the development of an internationally standard tool that will be used for the purpose.
Interpretation in the context of the wider literature
This systematic review is the first to synthesise models for prevalence and burden of asthma in the context of national, regional and global estimates and projections. Previous systematic reviews on disease modelling studies were mainly undertaken on other domains, such as prediction models [130-139], economic models [140] and decision analytic models [141-143]. Most of the findings of our review are in line with these previous reviews, suggesting that inadequate model development and poor reporting quality are the key issues in modelling studies that chiefly affect the quality of model-derived estimates and hinder the assessment of the usability of the models. A systematic review on projection models for prevalence and burden of chronic obstructive pulmonary disease (COPD) [144] argued that there was no consensus on the best model structure as models varied depending on the purpose and contexts of modelling. Another review on coronary heart disease policy models [25] emphasised introducing standard reporting guidelines to improve the reporting quality of models.
Implications for policy, practice and future research
Implication for asthma policy
Existing estimates are heavily reliant on modelling studies due to lack of data on direct measurements of asthma cases in many countries and regions. Whilst these modelling studies have advanced better understanding and appreciation of the burden of different diseases, the lack of reproducibility of the models, as highlighted in this review, requires concerted effort from researchers and decision makers to set in place platforms that will ensure that estimates of disease burden produced can be reproduced. Policymakers should thus be aware of the transparency of modelling processes and the reliability of the input data when making decisions on the basis of these model-based estimates.
Implications for model developers
The findings of this review suggest that models should be carefully designed to incorporate all the necessary methodological components required to develop a robust model, including an explicit statement about the purpose and structure of the model; statement of necessary model assumptions; variable selection applying appropriate techniques; model diagnostic accuracy checking; assessing goodness-of-fit; addressing missing data by applying suitable techniques; applying optimum methods of parameter estimation; carrying out sensitivity analysis; and performing both internal and external model validation. Besides, a highly complex model usually lacks understanding, usability, reproducibility and, hence, credibility. While publishing models, sufficient information about the complete modelling process, therefore, should be reported to facilitate its understanding and usability for non-technical audiences. For example, a model development manual should be made publicly available, including input data and necessary computer code, to describe the step-by-step process of model development with illustrative examples. Although the perspectives of this review are prevalence and disease burden of asthma, these recommendations also apply to the modelling prevalence and burden of other chronic diseases.
Implication for future research
Future research could potentially be undertaken to develop consensus guidelines for developing or fitting and reporting models for prevalence and burden of diseases. Moreover, developing a critical appraisal checklist for assessing the quality of models for prevalence and burden of diseases is another key area for future research.
Of the available approaches, we found the Bayesian meta-regression method- DisMod-MR, DisMod-MR 2.0, DisMod-MR 2.1 and CODEm [1,3,5,8,9,14,17] modelling tools faired best as they fulfilled most of our model quality criteria and were specially designed to deal with the diversity of data (multiple sources and designs) [11,145] needed to derive national, regional and global, level estimates. However, these modelling methods lack usability for the general user because of unavailability of sufficient technical detail and customised packages in standard statistical software such as R, SAS, and STATA. Therefore, more work needs to be done with these models to improve their usability. Moreover, DisMod-MR and CODEm are used as generic models by the Global Burden of Disease (GBD) collaborators to derive health estimates for numerous diseases and injuries. Therefore, the potential added value of well-constructed asthma-specific models should be considered.
Conclusions
Amidst data types and their sources, modelling remains indispensable for estimating the prevalence and burden of disease. This evidence synthesis has shown that existing models that have been applied to estimate the prevalence and burden of asthma suffer from methodological limitations, in particular, suboptimal reporting and lack of reproducibility. There is a need to enhance the reportage of models used for estimating and projecting the prevalence and disease burden of asthma and making data and code available to facilitate replication. Moreover, there is also a need for developing better-constructed asthma-specific models in an attempt to produce more accurate and consistent estimates. In the interim, we suggest using Bayesian meta-regression models and cause of death ensemble models for estimating national, regional and global prevalence and burden of asthma, and Box-Jenkins regression- autoregressive integrated moving average (ARIMA) model to make projections in relation to these estimates. We also suggest to validate the Bayesian meta-regression models against their alternative frequentist or classical models to check which modelling approaches generate better estimates of prevalence and burden of asthma than the others.
Additional material
Acknowledgements
We are grateful to Professor Jackie Price, Professor Steff Lewis and Dr Niall Anderson for their support as experts to develop the checklist for assessing the quality of the models included in our review. We are also grateful to Marshall Dozier, Senior Liaison Librarian for the College of Medicine and Veterinary Medicine, The University of Edinburgh, for her support in developing the search strategies. Finally, we express our gratitude to Fuentes Pacheco Andrea Carolina, Cameron Werner, Dewi Peerlings and Sumonkanti Das for helping with the translation of papers to English from other languages.
Footnotes
Funding: MRB received PhD fellowship from the Bangabandhu Science & Technology Fellowship Trust, Bangladesh and was supported by the College of Medicine & Veterinary Medicine, The University of Edinburgh, and the Farr Institute, UK. BIN and AS were also supported by the Farr Institute, UK. The Farr Institute is funded by a consortium of funders led by the Medical Research Council (MRC). CJW is supported by NHS Lothian via the Edinburgh Clinical Trials Unit.
Authorship contributions: AS conceived the idea for this review. MRB conducted the literature search. MRB and MAI independently reviewed the studies under the supervision of AS, BIN and CJW. All authors contributed equally in designing methods, analysing data, interpreting results, developing model quality appraisal framework, writing the manuscript, and critical review and final approval of the manuscript.
Competing interests: The authors have completed the ICMJE Unified Competing Interest form (available on request from the corresponding author) and declare no competing interest.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staruch RM, Beverly A, Sarfo-Annin JK, Rowbotham S.Calling for the next WHO Global Health Initiative: the use of disruptive innovation to meet the health care needs of displaced populations. J Glob Health. 2018;8:010303. 10.7189/jogh.08.010303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151-210. 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schütte S, Acevedo PNM, Flahault A.Health systems around the world - a comparison of existing health system rankings. J Glob Health. 2018;8:010407. 10.7189/jogh.08.010407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudan I, Yoshida S, Chan KY, Cousens S, Sridhar D, Bahl R, et al. Setting health research priorities using the CHNRI method: I. Involving funders. J Glob Health. 2016;6:010301. 10.7189/jogh.06.010301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Incardona B, Qazi SA, Stenberg K, Campbell H, Nair H.Cost-effectiveness analysis of revised WHO guidelines for management of childhood pneumonia in 74 Countdown countries. J Glob Health. 2017;7:010409. 10.7189/jogh.07.010409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-71. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163-96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathers CD, Lopez AD, Murray CJL. The Burden of Disease and Mortality by Condition: Data, Methods, and Results for 2001. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global Burden of Disease and Risk Factors. Washington (DC): The World Bank; 2006. p. 45-93. [PubMed] [Google Scholar]
- 11.Foreman KJ, Lozano R, Lopez AD, Murray CJ.Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. 10.1186/1478-7954-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GBD 2017 Mortality Collaborators Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1684-735. 10.1016/S0140-6736(18)31891-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691-706. 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430-40. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 16.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE.Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. 10.1371/journal.pone.0072788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IHME. Institute for Health Metrics and Evaluation (IHME). 2007. Available: http://www.healthdata.org/about. Accessed: 10 November 2018.
- 19.CHERG. Child Health Epidemiology Reference Group 2001. Available: http://cherg.org/about/background.html. Accessed: 10 November 2018.
- 20.World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. [Google Scholar]
- 21.Bhuia MR, Nwaru BI, Weir CJ, Sheikh A.Models for estimating and projecting global, regional and national prevalence and disease burden of asthma: Protocol for a systematic review. BMJ Open. 2017;7:e015441. 10.1136/bmjopen-2016-015441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11:e1001744. 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Critical Appraisal Skills Programme. CASP Economic Evaluation Checklist. 2018. Available: https://casp-uk.net/casp-tools-checklists/. Accessed: 19 June 2018.
- 24.Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. PLoS Med. 2016;13:e1002056. 10.1371/journal.pmed.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unal B, Capewell S, Critchley JA.Coronary heart disease policy models: a systematic review. BMC Public Health. 2006;6:213. 10.1186/1471-2458-6-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003;6:9-17. 10.1046/j.1524-4733.2003.00234.x [DOI] [PubMed] [Google Scholar]
- 27.Kirsch F.Economic Evaluations of Multicomponent Disease Management Programs with Markov Models: A Systematic Review. Value Health. 2016;19:1039-54. 10.1016/j.jval.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 28.Weinstein MC, Toy EL, Sandberg EA, Neumann PJ, Evans JS, Kuntz KM, et al. Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001;4:348-61. 10.1046/j.1524-4733.2001.45061.x [DOI] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardal S, Smith A, Luo HA, Zhang T, Groeneweg G, Jimenez Mendez R, et al. Asthma in British Columbia: Are we finally breathing easier? A population-based study of the burden of disease over 14 years. J Asthma. 2017;54:308-17. 10.1080/02770903.2016.1208223 [DOI] [PubMed] [Google Scholar]
- 31.James AL, Knuiman MW, Divitini ML, Hui J, Hunter M, Palmer LJ, et al. Changes in the prevalence of asthma in adults since 1966: the Busseltion health study. Eur Respir J. 2010;35:273-8. 10.1183/09031936.00194308 [DOI] [PubMed] [Google Scholar]
- 32.Malik G, Tagiyeva N, Aucott L, McNeill G, Turner SW.Changing trends in asthma in 9-12 year olds between 1964 and 2009. Arch Dis Child. 2011;96:227-31. 10.1136/adc.2010.189175 [DOI] [PubMed] [Google Scholar]
- 33.Akinbami LJ, Simon AE, Rossen LM.Changing Trends in Asthma Prevalence Among Children. Pediatrics. 2016;137:e20152354. 10.1542/peds.2015-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kausel L, Boneberger A, Calvo M, Radon K.Childhood Asthma and Allergies in Urban, Semiurban, and Rural Residential Sectors in Chile. ScientificWorldJournal. 2013;2013:937935. 10.1155/2013/937935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burney PGJ, Chinn S, Rona RJ.Has the prevalence of asthma increased in children? Evidence from the national study of health and growth 1973-86. BMJ. 1990;300:1306-10. 10.1136/bmj.300.6735.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brozek G, Lawson J, Szumilas D, Zejda J.Increasing prevalence of asthma, respiratory symptoms, and allergic diseases: Four repeated surveys from 1993-2014. Respir Med. 2015;109:982-90. 10.1016/j.rmed.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 37.Venn A, Lewis S, Cooper M, Hill J, Britton J.Increasing prevalence of wheeze and asthma in Nottingham primary schoolchildren 1988-1995. Eur Respir J. 1998;11:1324-8. 10.1183/09031936.98.11061324 [DOI] [PubMed] [Google Scholar]
- 38.Verlato G, Corsico A, Villani S, Cerveri I, Migliore E, Accordini S, et al. Is the prevalence of adult asthma and allergic rhinitis still increasing? Results of an Italian study. J Allergy Clin Immunol. 2003;111:1232-8. 10.1067/mai.2003.1484 [DOI] [PubMed] [Google Scholar]
- 39.Miller GF, Coffield E, Leroy Z, Wallin R.Prevalence and Costs of Five Chronic Conditions in Children. J Sch Nurs. 2016;32:357-64. 10.1177/1059840516641190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Silvarrey-Varela A, Pertega-Diaz S, Rueda-Esteban S, Sanchez-Lastres JM, San-Jose-Gonzalez MA, Sampedro-Campos M, et al. Prevalence and geographic variations in asthma symptoms in children and adolescents in Galicia (Spain). Arch Bronconeumol. 2011;47:274-82. 10.1016/j.arbr.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 41.Linneberg A, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T.Secular trends of allergic asthma in Danish adults. The Copenhagen Allergy Study. Respir Med. 2001;95:258-64. 10.1053/rmed.2001.1031 [DOI] [PubMed] [Google Scholar]
- 42.Nurmagambetov T, Khavjou O, Murphy L, Orenstein D.State-level medical and absenteeism cost of asthma in the United States. J Asthma. 2017;54:357-70. 10.1080/02770903.2016.1218013 [DOI] [PubMed] [Google Scholar]
- 43.Kolokotroni O, Middleton N, Nicolaou N, Pipis S, Priftis KN, Milton DK, et al. Temporal changes in the prevalence of childhood asthma and allergies in urban and rural areas of Cyprus: results from two cross sectional studies. BMC Public Health. 2011;11:858. 10.1186/1471-2458-11-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Morrison-Carpenter T, Holt JB, Callahan DB.Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000-2009. BMC Public Health. 2013;13:1156. 10.1186/1471-2458-13-1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Marco R, Cappa V, Accordini S, Rava M, Antonicelli L, Bortolami O, et al. Trends in the prevalence of asthma and allergic rhinitis in Italy between 1991 and 2010. Eur Respir J. 2012;39:883-92. 10.1183/09031936.00061611 [DOI] [PubMed] [Google Scholar]
- 46.Kim JL, Brisman J, Aberg MA, Forslund HB, Winkvist A, Toren K.Trends in the prevalence of asthma, rhinitis, and eczema in 15 year old adolescents over an 8 year period. Respir Med. 2014;108:701-8. 10.1016/j.rmed.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 47.Uphoff EP, Bird PK, Anto JM, Basterrechea M, von Berg A, Bergstrom A, et al. Variations in the prevalence of childhood asthma and wheeze in MeDALL cohorts in Europe. ERJ Open Res. 2017;3:00150-2016. 10.1183/23120541.00150-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Sharif NA, Nemery B, Barghuthy F, Mortaja S, Qasrawi R, Abdeen Z.Geographical variations of asthma and asthma symptoms among schoolchildren aged 5 to 8 years and 12 to 15 years in Palestine: the International Study of Asthma and Allergies in Childhood (ISAAC). Ann Allergy Asthma Immunol. 2003;90:63-71. 10.1016/S1081-1206(10)63616-2 [DOI] [PubMed] [Google Scholar]
- 49.Luyt DK, Burton PR, Simpson H.Epidemiological study of wheeze, doctor diagnosed asthma, and cough in preschool children in Leicestershire. BMJ. 1993;306:1386-90. 10.1136/bmj.306.6889.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goren AI, Hellmann S.Changing prevalence of asthma among schoolchildren in Israel. Eur Respir J. 1997;10:2279-84. 10.1183/09031936.97.10102279 [DOI] [PubMed] [Google Scholar]
- 51.Prietsch SOM, Zhang L, Catharino AR, Vauchinski L, Rodrigues FE.Asthma mortality among Brazilian children up to 19 years old between 1980 and 2007. J Pediatr (Rio J). 2012;88:384-8. 10.2223/JPED.2215 [DOI] [PubMed] [Google Scholar]
- 52.Oganov RG, Maslennikova GY.Asthma mortality in Russia between 1980 and 1989. Eur Respir J. 1999;13:287-9. 10.1034/j.1399-3003.1999.13b11.x [DOI] [PubMed] [Google Scholar]
- 53.Pesut DP, Bulajic MV, Nagomi-Obradovic LM, Grgurevic AD, Gledovic ZB, Ponomarev DR, et al. Asthma mortality in Serbia: A 30-year analysis. Respir Med. 2011;105:S50-3. 10.1016/S0954-6111(11)70011-7 [DOI] [PubMed] [Google Scholar]
- 54.Chatkin G, Chatkin JM, Fritscher CC, Cavalet-Blanco D, Bittencourt HR, Sears MR.Asthma mortality in southern Brazil: Is there a changing trend? J Asthma. 2007;44:133-6. 10.1080/02770900601182483 [DOI] [PubMed] [Google Scholar]
- 55.Thanh NX, Ohinmaa A, Yan C.Asthma-related productivity losses in Alberta, Canada. J Asthma Allergy. 2009;2:43-8. 10.2147/JAA.S5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Woensel JB, van Aalderen WM, Kneyber MC, Heijnen ML, Kimpen JL.Bronchiolitis hospitalisations in the Netherlands from 1991 to 1999. Arch Dis Child. 2002;86:370-1. 10.1136/adc.86.5.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tual S, Godard P, Bousquet J, Annesi-Maesano I.The decrease in asthma-related mortality in France. Rev Mal Respir. 2010;27:e1-5. 10.1016/j.rmr.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 58.Wang LY, Zhong Y, Wheeler L.Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2:A11. [PMC free article] [PubMed] [Google Scholar]
- 59.Severien C, Friedrich HJ.Entwicklung der Hospitalisationsraten für das Asthma bronchiale im Kindesalter. Monatsschr Kinderheilkd. 1998;146:951-6. Germany. 10.1007/s001120050349 [DOI] [Google Scholar]
- 60.Jang J, Gary Chan KC, Huang H, Sullivan SD.Trends in cost and outcomes among adult and pediatric patients with asthma: 2000-2009. Ann Allergy Asthma Immunol. 2013;111:516-22. 10.1016/j.anai.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 61.Antunes FP, Costa MDN, Paim JS, Vieira-da-Silva LM, Santos C, Cruz AA, et al. Trends in hospitalizations for respiratory diseases in Salvador, Bahia State, Brazil, 1998-2009. Cad Saude Publica. 2012;28:869-77. 10.1590/S0102-311X2012000500006 [DOI] [PubMed] [Google Scholar]
- 62.Chatkin JM, Barreto SM, Fonseca NA, Gutierrez CA, Sears MR.Trends in asthma mortality in young people in southern Brazil. Ann Allergy Asthma Immunol. 1999;82:287-92. 10.1016/S1081-1206(10)62610-5 [DOI] [PubMed] [Google Scholar]
- 63.Cohen S, Berkman N, Avital A, Springer C, Kordoba L, Haklai Z, et al. Decline in asthma prevalence and severity in Israel over a 10-year period. Respiration. 2015;89:27-32. 10.1159/000368613 [DOI] [PubMed] [Google Scholar]
- 64.Morris RD, Munasinghe RL.Geographic variability in hospital admission rates for respiratory disease among the elderly in the United States. Chest. 1994;106:1172-81. 10.1378/chest.106.4.1172 [DOI] [PubMed] [Google Scholar]
- 65.Mathers CD, Bernard C, Iburg KM, Inoue M, Ma Fat D, Shibuya K, et al. Global burden of disease in 2002: data sources, methods and results. Geneva: World Health Organization; 2003. [Google Scholar]
- 66.Mathers CD, Stein C, Ma Fat D, Rao C, Inoue M, Tomijima N, et al. Global Burden of Disease 2000: Version 2 methods and results. Geneva: World Health Organization; 2002. [Google Scholar]
- 67.Ding B, DiBonaventura M, Karlsson N, Ling X.A cross-sectional assessment of the prevalence and burden of mild asthma in urban China using the 2010, 2012, and 2013 China National Health and Wellness Surveys. J Asthma. 2017;54:632-43. 10.1080/02770903.2016.1255750 [DOI] [PubMed] [Google Scholar]
- 68.Schleicher NC, Koziol JA, Christiansen SC.Asthma mortality rates among California youths. J Asthma. 2000;37:259-65. 10.3109/02770900009055448 [DOI] [PubMed] [Google Scholar]
- 69.Hassanzadeh J, Mohammadbeigi A, Mousavizadeh A, Akbari M.Asthma prevalence in Iranian guidance school children, a descriptive meta-analysis. J Res Med Sci. 2012;17:293-7. [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan PW, Ghushchyan VH, Slejko JF, Belozeroff V, Globe DR, Lin SL.The burden of adult asthma in the United States: Evidence from the medical expenditure panel survey. J Allergy Clin Immunol. 2011;127:363.e1. 10.1016/j.jaci.2010.10.042 [DOI] [PubMed] [Google Scholar]
- 71.James AL, Knuiman MW, Divitini ML, Hui J, Hunter M, Palmer LJ, et al. Changes in the prevalence of asthma in adults since 1966: the Busselton health study. Eur Respir J. 2010;35:273-8. 10.1183/09031936.00194308 [DOI] [PubMed] [Google Scholar]
- 72.Lincoln D, Morgan G, Sheppeard V, Jalaludin B, Corbett S, Beard J.Childhood asthma and return to school in Sydney, Australia. Public Health. 2006;120:854-62. 10.1016/j.puhe.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 73.Nath JB, Hsia RY.Children’s emergency department use for asthma, 2001-2010. Acad Pediatr. 2015;15:225-30. 10.1016/j.acap.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rezvanfar MA, Kebriaeezadeh A, Moein M, Nikfar S, Gharibnaseri Z, Abdollahi-Asl A.Cost analysis of childhood asthma in Iran: A cost evaluation based on referral center data for asthma & allergies. J Res Pharm Pract. 2013;2:162-8. 10.4103/2279-042X.128149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chouaid C, Vergnenegre A, Vandewalle V, Liebaert F, Khelifa A, Galiponet al. The costs of asthma in France: an economic analysis by a Markov model. [In French]. Rev Mal Respir. 2004;21:493-9. 10.1016/S0761-8425(04)71353-4 [DOI] [PubMed] [Google Scholar]
- 76.Barnett SB, Nurmagambetov TA.Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127:145-52. 10.1016/j.jaci.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 77.Trogdon JG, Murphy LB, Khavjou OA, Li R, Maylahn CM, Tangka FK, et al. Costs of Chronic Diseases at the State Level: The Chronic Disease Cost Calculator. Prev Chronic Dis. 2015;12:E140. 10.5888/pcd12.150131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadatsafavi M, Lynd L, Marra C, Carleton B, Tan WC, Sullivan S, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17:74-80. 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zannetos S, Zachariadou T, Zachariades A, Georgiou A, Talias MA.The economic burden of adult asthma in Cyprus; a prevalence-based cost of illness study. BMC Public Health. 2017;17:262. 10.1186/s12889-017-4184-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukherjee M, Stoddart A, Gupta RP, Nwaru BI, Farr A, Heaven M, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14:113. 10.1186/s12916-016-0657-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adeloye D, Chan KY, Rudan I, Campbell H.An estimate of asthma prevalence in Africa: a systematic analysis. Croat Med J. 2013;54:519-31. 10.3325/cmj.2013.54.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendez-Luck CA, Yu H, Meng YY, Jhawar M, Wallace SP.Estimating health conditions for small areas: asthma symptom prevalence for state legislative districts. Health Serv Res. 2007;42:2389-409. 10.1111/j.1475-6773.2007.00793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention (CDC) Forecasted state-specific estimates of self-reported asthma prevalence–United States, 1998. MMWR Morb Mortal Wkly Rep. 1998;47:1022-5. [PubMed] [Google Scholar]
- 84.Soyiri IN, Reidpath DD, Sarran C.Forecasting peak asthma admissions in London: an application of quantile regression models. Int J Biometeorol. 2013;57:569-78. 10.1007/s00484-012-0584-0 [DOI] [PubMed] [Google Scholar]
- 85.Suruki RY, Daugherty JB, Boudiaf N, Albers FC.The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74. 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lozano P, Fishman P, VonKorff M, Hecht J.Health care utilization and cost among children with asthma who were enrolled in a health maintenance organization. Pediatrics. 1997;99:757-64. 10.1542/peds.99.6.757 [DOI] [PubMed] [Google Scholar]
- 87.Chinn S, Jarvis D, Burney P, Luczynska C, Ackermann-Liebrich U, Anto JM, et al. Increase in diagnosed asthma but not in symptoms in the European Community Respiratory Health Survey. Thorax. 2004;59:646-51. 10.1136/thx.2004.021642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Backman H, Raisanen P, Hedman L, Stridsman C, Andersson M, Lindberg A, et al. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin Exp Allergy. 2017;47:1426-35. 10.1111/cea.12963 [DOI] [PubMed] [Google Scholar]
- 89.Kamble S, Bharmal M.Incremental direct expenditure of treating asthma in the United States. J Asthma. 2009;46:73-80. 10.1080/02770900802503107 [DOI] [PubMed] [Google Scholar]
- 90.Wijesinghe M, Weatherall M, Perrin K, Crane J, Beasley R.International Trends in Asthma Mortality Rates in the 5-to 34-Year Age Group A Call for Closer Surveillance. Chest. 2009;135:1045-9. 10.1378/chest.08-2082 [DOI] [PubMed] [Google Scholar]
- 91.To T, Stanojevic S, Feldman R, Moineddin R, Atenafu EG, Guan J, et al. Is asthma a vanishing disease? A study to forecast the burden of asthma in 2022. BMC Public Health. 2013;13:254. 10.1186/1471-2458-13-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brogger J, Bakke P, Eide GE, Johansen B, Andersen A, Gulsvik A.Long-term changes in adult asthma prevalence. Eur Respir J. 2003;21:468-72. 10.1183/09031936.03.00056103 [DOI] [PubMed] [Google Scholar]
- 93.Alvarez-Alvarez I, Niu H, Guillen-Grima F, Aguinaga-Ontoso I.Meta-analysis of prevalence of wheezing and recurrent wheezing in infants. Allergol Immunopathol (Madr). 2018;46:210-7. 10.1016/j.aller.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 94.Ungar WJ, Coyte PC, Chapman KR, MacKeigan L.The patient level cost of asthma in adults in south central Ontario. Pharmacy Medication Monitoring Program Advisory Board. Can Respir J. 1998;5:463-71. 10.1155/1998/362797 [DOI] [PubMed] [Google Scholar]
- 95.Dai H, Lee BR, Hao J.Predicting Asthma Prevalence by Linking Social Media Data and Traditional Surveys. Ann Am Acad Pol Soc Sci. 2017;669:75-92. 10.1177/0002716216678399 [DOI] [Google Scholar]
- 96.Ram S, Zhang W, Williams M, Pengetnze Y.Predicting asthma-related emergency department visits using big data. IEEE J Biomed Health Inform. 2015;19:1216-23. 10.1109/JBHI.2015.2404829 [DOI] [PubMed] [Google Scholar]
- 97.Rosychuk RJ, Youngson E, Rowe BH.Presentations to Alberta emergency departments for asthma: a time series analysis. Acad Emerg Med. 2015;22:942-9. 10.1111/acem.12725 [DOI] [PubMed] [Google Scholar]
- 98.Mohammadbeigi A, Hassanzadeh J, Mousavizadeh A.Prevalence of asthma in elementary school age children in Iran–a systematic review and meta analysis study. Pak J Biol Sci. 2011;14:887-93. 10.3923/pjbs.2011.887.893 [DOI] [PubMed] [Google Scholar]
- 99.Varmaghani M, Farzadfar F, Sharifi F, Rashidian A, Moin M, Moradi-Lakeh M, et al. Prevalence of Asthma, COPD, and Chronic Bronchitis in Iran: A Systematic Review and Meta-analysis. Iran J Allergy Asthma Immunol. 2016;15:93-104. [PubMed] [Google Scholar]
- 100.Solis Soto MT, Patino A, Nowak D, Radon K.Prevalence of asthma, rhinitis and eczema symptoms in rural and urban school-aged children from Oropeza Province - Bolivia: a cross-sectional study. BMC Pulm Med. 2014;14:40. 10.1186/1471-2466-14-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.So SY, Ng MMT, Ip MSM, Lam WK.Rising asthma mortality in young males in Hong Kong, 1976-85. Respir Med. 1990;84:457-61. 10.1016/S0954-6111(08)80109-6 [DOI] [PubMed] [Google Scholar]
- 102.Sullivan P, Ghushchyan VG, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. School absence and productivity outcomes associated with childhood asthma in the USA. J Asthma. 2018;55:161-8. 10.1080/02770903.2017.1313273 [DOI] [PubMed] [Google Scholar]
- 103.Tzeng JY, Hsiao CK, Chen CJ.Spatial model selection using Bayes factor and ratio of variabilities for asthma mortality data. Chin J Publ Health. 1998;17:158-69. [Google Scholar]
- 104.Entezari A, Mehrabi Y, Varesvazirian M, Pourpak Z, Moin M.A systematic review of recent asthma symptom surveys in Iranian children. Chron Respir Dis. 2009;6:109-14. 10.1177/1479972309103884 [DOI] [PubMed] [Google Scholar]
- 105.Frank PI, Wicks PD, Hazell ML, Linehan MF, Hirsch S, Hannaford PC, et al. Temporal change in the prevalence of respiratory symptoms and obstructive airways disease 1993-2001. Br J Gen Pract. 2005;55:596-602. [PMC free article] [PubMed] [Google Scholar]
- 106.Ng TP, Tan WC.Temporal trends and ethnic variations in asthma mortality in Singapore, 1976-1995. Thorax. 1999;54:990-4. 10.1136/thx.54.11.990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.López-Campos JL, Cayuela A, Rodriguez-Dominguez S, Vigil E.Temporal trends in asthma mortality over 30 years. J Asthma. 2008;45:611-4. 10.1080/02770900802127006 [DOI] [PubMed] [Google Scholar]
- 108.Tavakoli H, FitzGerald JM, Chen W, Lynd L, Kendzerska T, Aaron S, et al. Ten-year trends in direct costs of asthma: a population-based study. Allergy. 2017;72:291-9. 10.1111/all.12993 [DOI] [PubMed] [Google Scholar]
- 109.Chew FT, Goh DY, Ooi BC, Lee BW.Time trends and seasonal variation in acute childhood asthma in tropical Singapore. Respir Med. 1998;92:345-50. 10.1016/S0954-6111(98)90119-6 [DOI] [PubMed] [Google Scholar]
- 110.Graudenz GS, Carneiro DP, Vieira RP.Trends in asthma mortality in the 0- to 4-year and 5- to 34-year age groups in Brazil. J Bras Pneumol. 2017;43:24-31. 10.1590/s1806-37562015000000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vicendese D, Abramson MJ, Dharmage SC, Tang ML, Allen KJ, Erbas B.Trends in asthma readmissions among children and adolescents over time by age, gender and season. J Asthma. 2014;51:1055-60. 10.3109/02770903.2014.936447 [DOI] [PubMed] [Google Scholar]
- 112.Bedouch P, Marra CA, FitzGerald JM, Lynd LD, Sadatsafavi M.Trends in Asthma-Related Direct Medical Costs from 2002 to 2007 in British Columbia, Canada: A Population Based-Cohort Study. PLoS One. 2012;7:e50949. 10.1371/journal.pone.0050949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chua KL, Ma S, Prescott S, Ho MHK, Ng DK, Lee BW.Trends in childhood asthma hospitalisation in three Asia Pacific countries. J Paediatr Child Health. 2011;47:723-7. 10.1111/j.1440-1754.2011.02040.x [DOI] [PubMed] [Google Scholar]
- 114.Ebmeier S, Thayabaran D, Braithwaite I, Benamara C, Weatherall M, Beasley R.Trends in international asthma mortality: Analysis of data from the WHO Mortality Database from 46 countries (1993-2012). Lancet. 2017;390:935-45. 10.1016/S0140-6736(17)31448-4 [DOI] [PubMed] [Google Scholar]
- 115.Manfreda J, Becker AB, Wang PZ, Roos LL, Anthonisen NR.Trends in physician-diagnosed asthma prevalence in Manitoba between 1980 and 1990. Chest. 1993;103:151-7. 10.1378/chest.103.1.151 [DOI] [PubMed] [Google Scholar]
- 116.Gonzalez-Barcala FJ, Aboal J, Carreira JM, Rodriguez-Alvarez MX, Puga A, Sanjose E, et al. Trends of asthma mortality in Galicia from 1993 to 2007. J Asthma. 2012;49:1016-20. 10.3109/02770903.2012.728272 [DOI] [PubMed] [Google Scholar]
- 117.Huang C, Liu W, Hu Y, Zou Z, Zhao Z, Shen L, et al. Updated prevalences of asthma, allergy, and airway symptoms, and a systematic review of trends over time for childhood asthma in shanghai, China. PLoS One. 2015;10:e0121577. 10.1371/journal.pone.0121577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newson R, Strachan D, Archibald E, Emberlin J, Hardaker P, Collier C.Acute asthma epidemics, weather and pollen in England, 1987-1994. Eur Respir J. 1998;11:694-701. [PubMed] [Google Scholar]
- 119.Arathimos R, Granell R, Henderson J, Relton CL, Tilling K.Sex discordance in asthma and wheeze prevalence in two longitudinal cohorts. PLoS One. 2017;12:e0176293. 10.1371/journal.pone.0176293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoogendoorn M, Feenstra TL, Rutten-Van Molken MPMH.Projections of future resource use and the costs of asthma and COPD in the Netherlands. [In Dutch]. Ned Tijdschr Geneeskd. 2006;150:1243-50. Dutch. [PubMed] [Google Scholar]
- 121.Suijkerbuijk AWM, De Wit GA, Wijga AH, Heijmans MJWM, Hoogendoorn M, Rutten-Van Molken MPMH, et al. Societal costs of asthma, COPD and respiratory allergy. [In Dutch]. Ned Tijdschr Geneeskd. 2013;157:A6562. [PubMed] [Google Scholar]
- 122.Bauman A.Has the prevalence of asthma symptoms increased in Australian children? J Paediatr Child Health. 1993;29:424-8. 10.1111/j.1440-1754.1993.tb03013.x [DOI] [PubMed] [Google Scholar]
- 123.Rutten-van Mölken MP, Postma MJ, Joore MA, Van Genugten ML, Leidl R, Jager JC.Current and future medical costs of asthma and chronic obstructive pulmonary disease in The Netherlands. Respir Med. 1999;93:779-87. 10.1016/S0954-6111(99)90262-7 [DOI] [PubMed] [Google Scholar]
- 124.Mathers CD, Loncar D.Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jarvis D, Newson R, Janson C, Corsico A, Heinrich J, Anto JM, et al. Prevalence of asthma-like symptoms with ageing. Thorax. 2018;73:37-48. 10.1136/thoraxjnl-2016-209596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nurmagambetov T, Kuwahara R, Garbe P.The Economic Burden of Asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348-56. 10.1513/AnnalsATS.201703-259OC [DOI] [PubMed] [Google Scholar]
- 127.Aguinaga Ontoso I, Arnedo Pena A, Bellido J, Guillen Grima F, Suarez Varela MM.The prevalence of asthma-related symptoms in 13-14-year-old children from 9 Spanish populations. The Spanish Group of the ISAAC Study (International Study of Asthma and Allergies in Childhood) [In Spanish]. Med Clin (Barc). 1999;112:171-5. [PubMed] [Google Scholar]
- 128.Ito Y, Tamakoshi A, Wakai K, Takagi K, Yamaki K, Ohno Y.Trends in asthma mortality in Japan. J Asthma. 2002;39:633-9. 10.1081/JAS-120014928 [DOI] [PubMed] [Google Scholar]
- 129.Sliwczynski A, Brzozowska M, Iltchew P, Czeleko T, Kucharczyk A, Jedrzejczyk T, et al. Epidemiology of asthma in Poland in urban and rural areas, based on provided health care services. Pneumonol Alergol Pol. 2015;83:178-87. [DOI] [PubMed] [Google Scholar]
- 130.Luo G, Nkoy FL, Stone BL, Schmick D, Johnson MD.A systematic review of predictive models for asthma development in children. BMC Med Inform Decis Mak. 2015;15:99. 10.1186/s12911-015-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smit HA, Pinart M, Anto JM, Keil T, Bousquet J, Carlsen KH, et al. Childhood asthma prediction models: a systematic review. Lancet Respir Med. 2015;3:973-84. 10.1016/S2213-2600(15)00428-2 [DOI] [PubMed] [Google Scholar]
- 132.Van Wyk SS, Lin HH, Claassens MM.A systematic review of prediction models for prevalent pulmonary tuberculosis in adults. Int J Tuberc Lung Dis. 2017;21:405-11. 10.5588/ijtld.16.0059 [DOI] [PubMed] [Google Scholar]
- 133.Lamain – de Ruiter M Kwee A, Naaktgeboren CA, Franx A, Moons KGM, Koster MPH. Prediction models for the risk of gestational diabetes: a systematic review. Diagn Progn Res. 2017;1:3 10.1186/s41512-016-0005-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Damen JA, Hooft L, Schuit E, Debray TP, Collins GS, Tzoulaki I, et al. Prediction models for cardiovascular disease risk in the general population: systematic review. BMJ. 2016;353:i2416. 10.1136/bmj.i2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walsh ME, Horgan NF, Walsh CD, Galvin R.Systematic review of risk prediction models for falls after stroke. J Epidemiol Community Health. 2016;70:513-9. 10.1136/jech-2015-206475 [DOI] [PubMed] [Google Scholar]
- 136.Luo G, Nkoy FL, Gesteland PH, Glasgow TS, Stone BL.A systematic review of predictive modeling for bronchiolitis. Int J Med Inform. 2014;83:691-714. 10.1016/j.ijmedinf.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 137.Collins GS, Mallett S, Omar O, Yu LM.Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med. 2011;9:103. 10.1186/1741-7015-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perel P, Edwards P, Wentz R, Roberts I.Systematic review of prognostic models in traumatic brain injury. BMC Med Inform Decis Mak. 2006;6:38. 10.1186/1472-6947-6-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van den Boorn HG, Engelhardt EG, van Kleef J, Sprangers MAG, van Oijen MGH, Abu-Hanna A, et al. Prediction models for patients with esophageal or gastric cancer: A systematic review and meta-analysis. PLoS One. 2018;13:e0192310. 10.1371/journal.pone.0192310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Squires H, Chilcott J, Akehurst R, Burr J, Kelly MP.A systematic literature review of the key challenges for developing the structure of public health economic models. Int J Public Health. 2016;61:289-98. 10.1007/s00038-015-0775-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McManus E, Sach T, Levell N.The Use of Decision-Analytic Models in Atopic Eczema: A Systematic Review and Critical Appraisal. Pharmacoeconomics. 2018;36:51-66. 10.1007/s40273-017-0564-7 [DOI] [PubMed] [Google Scholar]
- 142.Alsumali A, Al-Hawag A, Samnaliev M, Eguale T.Systematic assessment of decision analytic models for the cost-effectiveness of bariatric surgery for morbid obesity. Surg Obes Relat Dis. 2018;14:1041-59. 10.1016/j.soard.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 143.Goehler A, Geisler BP, Manne JM, Jahn B, Conrads-Frank A, Schnell-Inderst P, et al. Decision-analytic models to simulate health outcomes and costs in heart failure: a systematic review. Pharmacoeconomics. 2011;29:753-69. 10.2165/11585990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 144.McLean S, Barbour V, Wild S, Simpson C, Sheikh A.Models for estimating projections for disease prevalence and burden: a systematic review focusing on chronic obstructive pulmonary disease. J Health Serv Res Policy. 2015;20:246-53. 10.1177/1355819615579232 [DOI] [PubMed] [Google Scholar]
- 145.Flaxman AD, Vos DT, Murray CJ, editors. An integrative metaregression framework for descriptive epidemiology. Seattle: University of Washington Press; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.