Abstract
Purpose
Outline the association between age-related macular degeneration (AMD) and functional difficulty using novel item response theory (IRT) psychometric techniques, and highlight populations particularly at risk of functional impairment.
Methods
This cross-sectional study included 5604 US adults. Primary outcomes were item response theory–adjusted visual and physical difficulty scores. Secondary analyses of AMD populations at highest risk of reporting greater functional difficulty were undertaken.
Results
In total, there were 386 participants with early AMD (mean presenting visual acuity [pVA], 0.12) and 55 with late AMD (mean pVA, 0.35). Those with late AMD reported substantially higher item visual difficulty, whereas those with both early/late AMD reported significantly higher item physical difficulty versus those with no AMD (P < .05). In univariate regression, only those with late AMD reported significantly higher visual difficulty versus those with no AMD (10.1 points [95% confidence interval (CI), 8.2–12.1 points] vs 7.1 points [95% CI, 7.0–7.2 points]; P = .003). Both early/late AMD reported higher physical difficulty versus those with no AMD (11.6 points [95% CI, 11.1–12.1 points; P = .005]; 13.4 points [95% CI, 11.8–15.0 points; P = .03], respectively, versus 11.0 points [95% CI, 10.9–11.1 points]. After adjustment for sociodemographic and medical variables (excluding pVA), only those with late AMD reported significantly greater visual and physical difficulty versus those with no AMD (10.0 points [95% CI, 8.2–11.9 points] vs 7.1 [95% CI, 7.0–7.2 points; P = .002]; and 12.7 points [95% CI, 11.3–14.0 points] vs 11.0 [95% CI, 10.9–11.1 points; P = .02], respectively); greater visual difficulty in those with late AMD persisted after additionally adjusting for pVA versus those with no AMD (9.1 points [95% CI, 7.6–10.6 points] vs 7.1 points [95% CI, 7.0–7.2 points; P = .01]). Among individuals with AMD, lower income, higher medical comorbidities, depression, and pVA predicted greater visual and physical difficulties.
Conclusions
AMD confers significant functional difficulty among US adults with sociodemographic characteristics influencing dysfunction; highlighting the value of alternatives to Snellen visual acuity in assessing visual characteristics. With aging populations and the increasing prevalence of AMD, health care professionals should be aware of the functional burden of AMD and recognize those at higher risk of functional difficulty.
Translational Relevance
Contemporary psychometric validation techniques can be effective in accurately describing the level of functional impairment for those with visual impairment.
Keywords: age-related macular degeneration, macular degeneration, function
Introduction
Age-related macular degeneration (AMD) is a leading cause of vision loss worldwide,1–7 with an estimated global prevalence of 200 million, which is expected to rise to approximately 300 million by 2040.8 In the United States, AMD is the leading cause of central vision impairment among older adults and affects approximately 3 million people,9,10 with more than 7 million more at risk.10 Although early AMD is often asymptomatic with up to 84% of patients unaware of their condition,11 AMD-related central vision loss is associated with difficulty with daily tasks such as reading and driving.10 As a larger proportion of the US population is living well beyond the age of 60 years and are at risk of developing AMD,12 an understanding of the impact of AMD on visual and physical functioning in this population is of paramount importance.
AMD is known to adversely affect visual and physical functioning, as well as quality of life. Prior studies have shown the presence of late AMD to be associated with up to a 50% decrease in moderate to vigorous physical activity,13 significant impairment performing activities of daily living,14 decreased social engagement,15 poor quality of life,16–19 and a greater risk of all-cause mortality.20 Furthermore, direct medical care for AMD costs an estimated $575 million dollars each year,21 not including associated losses in patient and caregiver productivity, representing a substantial burden to patients and society.22 An association between AMD and impaired visual or physical function has been reported in smaller studies using German, Turkish, and Australian cohorts,14,16,23 as well as in larger European studies.24 However, reports of AMD and visual function in US-based cohorts25,26 have not compared visual function between AMD stages and are not necessarily generalizable in the entire US population. To date, the impact of AMD on visual and physical function in a large US cohort representative of the national population has not been described.
In this study, we use the National Health and Nutrition Examination Survey (NHANES) questionnaire and examination database from 2005 to 2008 to understand the association between AMD presence and severity and self-reported visual and physical difficulty, outlining populations of patients with AMD who are particularly at risk. We used item response theory (IRT) to psychometrically validate and adjust item difficulty scores for both visual and physical difficulty questionnaires. This technique improves the measurement properties of questionnaire data by adjusting individual and sum question scores based on calculated difficulty and discrimination properties, thus, providing a more accurate representation of the association between AMD severity and self-reported visual or physical difficulty.
Methods
Study Population
The NHANES is an on-going biennial cross-sectional survey representing a noninstitutionalized civilian US population, performed by the National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention.27 NHANES has been a continuous survey program that has provided health statistics for the United States since 1999 and examines a nationally representative sample of about 5000 people each year, located in counties across the United States. For each cycle, NHANES participants were invited to undergo comprehensive health examination in a mobile examination center, which included retinal photography for those over the age of 40 in the 2005 to 2008 cycles. Self-reported sociodemographic information, medical history, and visual and physical difficulty symptoms were attained through home interview. The 2005 to 2006 and 2007 to 2008 NHANES protocols were approved by the NCHS research ethics review board and written informed consent was obtained from all study participants. All data are de-identified and made publicly available by the NCHS.
Evaluation of Macular Degeneration
An ophthalmic digital imaging system (CR6-45NM; Canon USA Inc, Melville, NY) and digital camera (EOS 10D; Canon USA Inc) were used to capture retinal images for participants aged 40 and older.27 Fundus images were graded at the University of Wisconsin, Madison, according to the modified Wisconsin Age-Related Maculopathy Grading Classification Scheme.20,28,29 All retinal images were graded by at least 2 experienced specialists, with a third specialist clarifying any disagreements. Early AMD was defined as signs of drusen with a grid area of greater than 500-µm circle and/or pigmentary abnormalities.27 Late AMD was defined as the presence of exudative or geographic atrophy.27 In cases where retinal images were available for both eyes, the severity of AMD in the worse eye was used for AMD classification.
Evaluation of Other Covariates
Sociodemographic and medical history data, including age, sex, ethnicity, education (less than high school completion, high school completion, or education above the level of high school), poverty index ratio (below the poverty level), within 1 to 2 times (above) the poverty level, or more than 2 times above the poverty level), and history of comorbidities, were collected during home interview. Individual medical comorbidity scores were calculated out of 6 points by self-reported history of (1) hypertension, (2) diabetes, (3) heart disease (congestive heart failure, coronary artery disease, or heart attack), (4) lung disease (emphysema or chronic bronchitis), (5) cancer (any), and (6) stroke. Other examination measurements, including the Patient Health Questionnaire-9 depression score, and presenting VA (pVA), were conducted in the mobile examination center. Depression was classified using the self-administered Patient Health Questionnaire-9 questionnaire, widely used in clinical and research settings, with a score of 10 or higher serving as a recommended cut-point for screening for major depression, with 88% sensitivity and 88% specificity.30,31 VA was measured using a Nidek ARK-760 autorefractor containing built-in Snellen VA charts, measuring the presenting distance VA in each eye with whatever form of correction (i.e. glasses, contact lenses), if any, the participant was wearing at the time of the examination. We converted pVA measurements from Snellen to logarithm of the minimum angle of resolution measurements, using a continuous logarithm of the minimum angle of resolution VA scale.32
Evaluation of Visual Difficulty Symptoms
Functional visual difficulty was assessed by self-report using the following 6 questions: (1) reading small print, (2) up-close housework, (3) seeing curbs or stairs in dim light, (4) noticing objects in peripheries, (5) finding things on a crowded shelf, and (6) daytime driving in a familiar place. Each of the 6 questions was reported on a 5-point ordinal Likert scale ranging from (1) no difficulty, (2) a little difficulty, (3) moderate difficulty, (4) extreme difficulty and (5) unable to do because of eyesight. Options (6) don't do this for other reasons, (77) refused, and (99) don't know, were considered as missing variables.
Evaluation of Physical Difficulty Symptoms
Functional physical difficulty was assessed by self-report using 13 questions pertaining to problems with (1) managing money, (2) walking a quarter mile, (3) walking up 10 steps, (4) crouching, stooping or kneeling, (5) performing household chores, (6) preparing meals, (7) walking between rooms (same floor), (8) using cutlery, (9) getting dressed, (10) grasping small objects, (11) going to the movies, (12) attending social events, and (13) doing leisure activities at home. Each of the 13 questions was reported on a 4-point ordinal Likert scale ranging from (1) no difficulty, to (2) some difficulty, (3) much difficulty, and (4) unable to do. Options (5) do not do this activity, (7) refused, and (9) don't know, were considered as missing variables.
Factor Analysis and IRT
Factor analysis and IRT techniques were applied to 6 visual difficulty-related items (questions), and 13 physical difficulty-related questions. Factor analysis is a psychometric method used to identify the underlying latent traits (unobservable characteristics) being tested in a questionnaire, and determine which questions most strongly represent each latent trait.33,34 IRT is a psychometric validation method used to outline how well each question can discriminate between participants of differing ability, and how well those differences are reflected by question responses (usually on a Likert scale).35,36
The initial correlation matrices (using pairwise deletion methods for missing data) and unrotated factor analyses were used to identify the number of latent traits tested by the constellation of questions pertaining to visual difficulty and by those pertaining to physical difficulty. Latent traits were considered substantive if the calculated eigenvalue (essentially factor variances) was greater than 1.0 (above the point of inflection at which the scree plot of eigenvalues asymptotes to a floor), and questions were considered to be associated with those traits if their individual loading values were greater than 0.5. The shortlisted latent traits and their associated questions were subsequently specified in a rotated (Promax oblique) factor analysis.37,38 Questions that did not load strongly onto the identified latent traits (poorly associated) were considered spurious and subsequently discarded from further evaluation.
IRT, using a graded response model (GRM), was next undertaken to ensure each question could adequately discriminate between differing levels of participant ability (considered substantive if discrimination >2.0) and how well those differences were reflected by individual question responses (reported as the beta-threshold for each of the 4 or 5 options on the Likert scale). Questions with poor discrimination (where a difference in difficulty is not well reflected by a difference in score) and poor ability differentiation were considered unable to accurately discriminate participants of differing ability and were subsequently discarded. The GRM then used the adjusted discrimination and differentiation calculations of the retained items to impute new modified visual or physical difficulty scores for each question and to calculate modified sum scores for each participant. These modified, rescaled scores (on a continuous scale) are used for between-group comparisons.
Factor analysis and IRT analyses were used to identify which of the original 6 and 13 questions to retain for the “visual difficulty” and “physical difficulty” questionnaires respectively. Modified sum “visual difficulty” and “physical difficulty” scores were then calculated for individual participants based on the difficulty and discrimination of each question. IRT-adjusted question scores were used for subsequent analyses. Further detail regarding factor analysis and IRT can be found elsewhere.35,37,38
Statistical Analyses
All analyses were restricted to those aged 40 years or older who underwent fundus imaging. Given the NHANES complex probability sampling design, interview and examination weights are computed by the NCHS for each 2-year cycle. For the present study, combined 4-year weights standardized to the 2000 US Census population as recommended by the NCHS were used to provide valid estimates for all analyses. Differences in demographic characteristics by AMD diagnosis and severity were compared using t tests and χ2 tests. Differences in each questionnaire item score after IRT adjustment were compared using univariate regression models. Multivariate linear regression models were used to estimate the association between AMD severity and sum visual or physical difficulty scores. Multivariate linear regression models were then used to determine sociodemographic predictors of greater visual and physical difficulty among participants with AMD. Results at a P value of less than the .05 level were considered statistically significant. Statistical analysis was performed using Stata version 16.0 (StataCorp LP, College Station, TX).
Results
Study Population
A total of 20,497 individuals participated in the 2005 to 2008 NHANES survey. Of those, 6797 were over 40 years old and eligible for retinal imaging, 5604 (82.4%) of whom had nonmissing retinal imaging data (Table 1). Those with missing retinal imaging data were significantly more likely to be older than 70 years old, to be of Black or other ethnicity, to have less than high school education, to be living below the poverty level, to have 3 or more comorbidities, to not have depression, and to have a worse pVA (P < .05 for all). Men and women were equally likely to have missing retinal imaging data (P = .36).
Table 1.
Demographic Characteristics of Participants with Complete Data: NHANES 2005–2008
| No AMD (n = 5163)a | Early AMD (n = 386) | Late AMD (n = 55) | |
|---|---|---|---|
| Age category (%, CI)b | |||
| 40–49 | 36.3 (33.5–39.2)c | 13.9 (8.6–21.8) | None |
| 50–59 | 31.1 (29.2–33.0) | 17.7 (11.6–26.0) | 1.5 (1.9–10.4) |
| 60–69 | 18.7 (16.9–20.6) | 22.1 (16.7–28.7) | 10.1 (3.8–24.4) |
| ≥70 | 14.0 (12.5–15.5) | 46.3 (39.4–53.2)* | 88.4 (74.6–95.2)* |
| Female (%, CI) | 52.6 (51.3–53.9) | 49.9 (44.9–54.8) | 67.9 (51.2–81.0) |
| Ethnicity (%, CI) | |||
| Non-Hispanic White | 76.5 (71.9–80.6) | 85.8 (80.8–89.6)* | 93.9 (85.3–97.6)* |
| Non-Hispanic Black | 10.0 (7.7–13.0) | 4.0 (2.4–6.5) | 3.0 (0.8–10.6) |
| Hispanic/Mexican | 8.7 (6.9–10.8) | 6.8 (4.9–9.5) | 0.6 (0.0–0.5) |
| Other | 4.8 (3.7–6.3) | 3.4 (1.5–7.7) | 2.4 (0.5–10.9) |
| Education | |||
| <High school | 17.5 (15.3–20.1) | 22.5 (16.5–29.9) | 21.2 (12.0–34.7) |
| High school | 26.3 (24.3–28.5) | 26.2 (21.5–31.4) | 31.3 (19.3–46.5) |
| >High school | 56.2 (52.5–59.8) | 51.4 (44.8–57.9) | 47.5 (33.7–61.3) |
| Poverty (%, CI) | |||
| >2× PL | 74.7 (71.6–77.5) | 69.3 (64.3–73.8)* | 55.4 (41.3–68.7)* |
| 1–2× PL | 16.6 (14.6–18.7) | 22.4 (18.5–26.7) | 31.0 (18.1–47.7) |
| <PL | 8.8 (7.5–10.2) | 8.4 (6.1–11.4) | 13.6 (7.1–24.6) |
| Comorbid score (%, CI)d | |||
| 0 | 38.3 (35.7–41.0) | 21.4 (16.3–27.5) | 15.9 (6.4–34.1) |
| 1 | 36.9 (34.8–39.0) | 41.6 (32.9–50.9) | 28.4 (17.1–43.3) |
| 2 | 17.2 (15.8–18.7) | 23.5 (18.2–29.8) | 35.8 (21.1–53.9)* |
| 3–6 | 7.6 (6.5–8.9) | 13.5 (9.6–18.5)* | 19.8 (11.8–31.7)* |
| Depression (%, CI)e | 7.1 (5.8–8.7) | 6.7 (3.6–12.2) | 4.1 (0.9–16.3) |
| pVA logMAR (mean, SD)f | 0.15 (0.12–0.17) | 0.12 (0.10–0.14) | 0.35 (0.26 to 0.42) |
Reported n value is unweighted, for reference only.
Percentages are computed using NHANES 4-year interview or examination weights, with 95% CIs.
Interpretation of this cell: of those with no AMD, 36.3% were aged 40–49 years, 31.1% were aged 50–59 years, and so on (using 4-year interview weighted NHANES percentages).
Comorbidity score out of 6 sum points, allocated for self-reported hypertension, diabetes, lung disease, heart disease, stroke, or cancer.
Depression classified as Patient Health Questionnaire-9 score of ≥10.
LogMAR in 0.1 increments, where logMAR 0 = 20/20.
Significant difference (P < .05) versus those with no AMD. Ethnicity (other), Asian or mixed ethnicity; logMAR, logarithm of the minimum angle of resolution; PL, poverty level.
Of the 5604 remaining participants, 441 had features of AMD on fundus photographs, 386 of which were classified as early and 55 as late AMD (Table 1). Compared with those without photographic evidence of AMD, those with signs of early AMD were significantly more likely to be older than 70 years of age (P < .001), more likely to be non-Hispanic White (P < .001), less likely to be more than 2 times above the poverty level (P = .03), more likely to have 3 or more comorbidities (P < .001), and more likely to have a worse pVA (P < .001). Compared with those without photographic evidence of AMD, those with signs of late AMD were significantly more likely to be older than 70 years of age (P < .001) and of non-Hispanic White ethnicity (P < .001), less likely to be more than 2 times above the poverty level (P = .04), more likely to have 2 or more comorbidities (P = .04), and more likely to have a worse pVA (P < .001). There was no significant difference in gender, education or depression between those with either early or late AMD and those without AMD (P > .05 for all) (Table 1).
Individual Visual Difficulty Question Analysis
Of the 6 questions pertaining to visual difficulty, one latent trait was identified in the unrotated factor analysis (eigenvalue 3.3), and the rotated (Promax oblique) factor analysis confirmed that all 6 questions corresponded well with that latent trait (all loading >0.65). All 6 questions demonstrated good ability to discriminate between participants of differing ability (discrimination >2.5) and good differentiation of individual level of ability. None of the original 6 visual difficulty questions were removed (Table 2).
Table 2.
Mean Item Visual Difficulty Scores (Out of 5 Points Each) Among Participants With and Without AMD After FA and IRT Adjustment
| No AMD | Early AMD | Late AMD | |
|---|---|---|---|
| Q1. Difficulty reading ordinary newsprint? | 1.3 (1.3–1.3)a,b | 1.3 (1.2–1.4)a,b P = .78 | 1.9 (1.5–2.3)a,b P = .002 |
| Q2. Difficulty with up-close housework? | 1.2 (1.2–1.2) | 1.2 (1.2–1.3) P = .71 | 1.8 (1.4–2.1) P = .003 |
| Q3. Difficulty seeing curb or stairs in dim light? | 1.2 (1.2–1.3) | 1.2 (1.2–1.3) P = .73 | 1.8 (1.4–2.1) P = .003 |
| Q4. Difficulty noticing objects on the side? | 1.1 (1.1–1.1) | 1.1 (1.1–1.2) P = .56 | 1.6 (1.3–1.9) P = .004 |
| Q5. Difficulty finding things on crowded shelf? | 1.1 (1.1–1.2) | 1.2 (1.1–1.2) P = .60 | 1.6 (1.3–1.9) P = .003 |
| Q6. Difficulty driving (daytime) in familiar places? | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) P = .46 | 1.5 (1.2–1.7) P = .005 |
Univariable linear regression results for individual item questions, with ordinal AMD independent variable. Corresponding P values shown for early/late AMD, versus no AMD.
All values listed are mean scores (on a continuous scale from 1 to 5) after individual item IRT-adjustment (each item out of 5 points, where 1 = no difficulty, and 5 = cannot do because of my sight).
In the univariate regression analyses, the mean modified visual difficulty scores for each of the 6 visual difficulty questions for those without AMD were between 1.1 and 1.3 out of 5.0 points (Table 2). There was no significant difference in individual question scores for those with early AMD compared with those without AMD (P > .05 for all). However, individuals with late AMD reported significantly greater visual difficulty, with modified visual difficulty scores ranging from 1.5 to 1.9 points, substantially higher than those from participants without AMD for all 6 questions (P < .05 for all) (Table 2).
Individual Physical Difficulty Question Analysis
Of the 13 questions pertaining to physical difficulty, 1 latent trait was identified in the unrotated factor analysis (eigenvalue 6.2). The unrotated and rotated (Promax oblique) factor analysis confirmed that only 10 questions corresponded well with that latent trait (“difficulty with money,” “difficulty using cutlery,” and “difficulty with leisure at home” loaded poorly (<0.5) onto the latent trait, and were subsequently removed from further analyses). Of the 10 remaining physical difficulty questions, 9 demonstrated good ability to discriminate between participants of differing ability (discrimination of >2.2) but “difficulty grasping small objects” discriminated poorly (<2.0) and was subsequently removed. The remaining 9 questions demonstrated good differentiation of individual level of ability (Table 3).
Table 3.
Mean Item Physical Difficulty Scores (Out of 4 Points Each) Among Participants With and Without AMD After FA and IRT Adjustment
| No AMD | Early AMD | Late AMD | |
|---|---|---|---|
| Q1. Difficulty managing money (removed) | — | — | — |
| Q2. Difficulty walking a quarter milec | 1.4 (1.3–1.4)a,b | 1.4 (1.3–1.5)a,b P = .04 | 1.7 (1.5–2.0)a,b P = .005 |
| Q3. Difficulty walking up 10 stepsc | 1.2 (1.2–1.2) | 1.3 (1.2–1.4) P = .02 | 1.5 (1.3–1.8) P = .003 |
| Q4. Difficulty crouching, stooping or kneelingc | 1.7 (1.7–1.7) | 1.7 (1.7–1.8) P = .25 | 2.0 (1.7–2.2) P = .03 |
| Q5. Difficulty performing household choresc | 1.2 (1.2–1.2) | 1.3 (1.2–1.4) P = .02 | 1.5 (1.3–1.7) P = .003 |
| Q6. Difficulty preparing mealsc | 1.1 (1.1–1.1) | 1.1 (1.1–1.2) P = .03 | 1.3 (1.1–1.4) P = .008 |
| Q7. Difficulty walking between rooms (same floor)c | 1.1 (1.1–1.1) | 1.1 (1.1–1.1) P = .03 | 1.2 (1.1–1.3) P = .008 |
| Q8. Difficulty using knife, fork, or cup (removed) | — | — | — |
| Q9. Difficulty dressing yourselfc | 1.1 (1.1–1.1) | 1.1 (1.1–1.2) P = .03 | 1.2 (1.1–1.3) P = .005 |
| Q10. Difficulty grasping small objects (removed) | — | — | — |
| Q11. Difficulty going to the movies or eventsc | 1.1 (1.1–1.2) | 1.2 (1.1–1.3) P = .02 | 1.5 (1.2–1.7) P = .002 |
| Q12. Difficulty attending social eventsc | 1.1 (1.1–1.1) | 1.2 (1.1–1.2) P = .02 | 1.4 (1.2–1.6) P = .003 |
| Q13. Difficulty doing home leisure activities (removed) | — | — | — |
Univariable linear regression results for individual item questions, with ordinal AMD independent variable. Corresponding P values shown for early/late AMD, versus no AMD.
All values listed are mean scores (on a continuous scale from 1 to 4) after individual item IRT-adjustment (each item out of 4 points, where 1 = no difficulty, and 4 = unable to do.
The nine remaining questions after psychometric validation and item reduction.
In the univariate regression analyses, the mean modified physical difficulty scores for the remaining 9 physical difficulty questions for those without AMD were between 1.1 and 1.7 out of 4.0 points (Table 3). There was a significant difference in score for most questions for those with early AMD compared with those without AMD, although scores were not substantially higher (P < .05 for all excluding question 4; P = .25). Those with late AMD reported significantly greater difficulty with substantially higher modified physical difficulty scores versus those without any AMD for all 9 remaining questions (P < .05 for all); physical difficulty scores for those with late AMD ranged from 1.2 to 2.0 out of 4.0 points (Table 3).
Sum Visual Difficulty Regression Analysis
In univariate regression analysis of the modified sum visual difficulty score, those with no AMD reported on average 7.1 sum points of difficulty (95% confidence interval [CI], 7.0–7.2 points). Although there was no significant difference in the modified sum visual difficulty score between those with early AMD versus those without AMD (0.1 points higher; P = .66), individuals with late AMD experienced significantly higher visual difficulty versus those with no AMD (3.0 sum points higher; P = .003) (Fig. 1A).
Figure 1.
Unadjusted sum visual and physical difficulty score by AMD severity (with 95% CI). (A) Unadjusted sum visual difficulty score, point estimate and 95% CI. Unadjusted sum point estimate and 95% CIs for (i) no AMD (7.1 [7.0–7.2]; vertical dashed line), (ii) early AMD (7.2 [6.9–7.5]), and (iii) late AMD (10.1 [8.2–12.1]). (B) Unadjusted sum physical difficulty score, point estimate and 95% CI. Unadjusted sum point estimate and 95% CIs for (i) no AMD (11.0 [10.9–11.1]; vertical dashed line), (ii) early AMD (11.6 [11.1–12.1]), and (iii) late AMD (13.4 [11.8–15.0]).
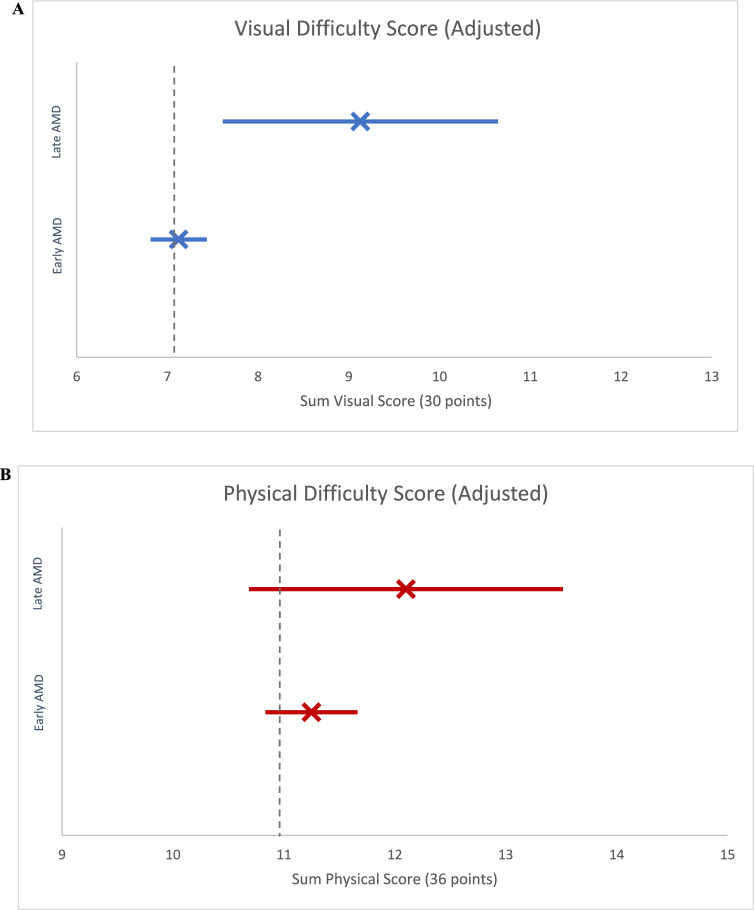
In multivariate regression analysis not accounting for pVA, those with early AMD did not report significantly greater visual difficulty versus those without AMD (0.1 sum points higher; P = .76); however, those with late AMD reported significantly greater visual difficulty than those without AMD (2.9 sum points higher; P = .002). After adjustment for pVA, late AMD was similarly associated with significantly greater visual difficulty (2.0 sum points higher vs no AMD; P = .01). Older age, education below high school level, living within 1 to 2 times or below the poverty level, 2 or more medical comorbidities, depression, and worse pVA were all independently associated with significantly higher self-reported visual difficulty (P < .05 for all) (Table 4) (Fig. 2A).
Table 4.
Multivariate Linear Regression Predicting IRT-Adjusted Sum Visual Difficulty Score
| Change in IRT-Adjusted Sum Visual Difficulty Scoreg | ||||
|---|---|---|---|---|
| Model 1a | P Value | Model 2b | P Value | |
| AMD status | ||||
| None | Reference | — | Reference | — |
| Early AMD | 0.1 (−0.3 to 0.4)c | .76 | 0.0 (−0.3 to 0.3) | .96 |
| Late AMD | 2.9 (1.1 to 4.7) | .002 | 2.0 (0.5 to 3.5) | .01 |
| Age category (years) | ||||
| 40–49 | Reference | — | Reference | — |
| 50–59 | 0.1 (−0.1 to 0.3) | .16 | 0.1 (−0.1 to 0.3) | .17 |
| 60–69 | −0.1 (−0.3 to 0.1) | .27 | −0.2 (−0.3 to 0.0) | .09 |
| ≥70 | −0.1 (−0.4 to 0.1) | .23 | −0.3 (−0.5 to −0.1) | .008 |
| Gender | ||||
| Male | Reference | — | Reference | — |
| Female | 0.1 (−0.1 to 0.2) | .43 | 0.0 (−0.1 to 0.2) | .58 |
| Ethnicity | ||||
| Non-Hispanic White | Reference | — | Reference | — |
| Hispanic/Mexican | −0.2 (−0.4 to 0.0) | .10 | −0.2 (−0.4 to 0.0) | .03 |
| Non-Hispanic Black | 0.1 (−0.1 to 0.3) | .47 | 0.0 (−0.2 to 0.3) | .75 |
| Other | 0.2 (−0.2 to 0.6) | .30 | 0.2 (−0.2 to 0.5) | .43 |
| Education | ||||
| >High school | Reference | — | Reference | — |
| High school | 0.0 (−0.2 to 0.2) | .92 | 0.0 (−0.2 to 0.2) | .89 |
| <High school | 0.4 (0.2 to 0.5) | .001 | 0.3 (0.1 to 0.5) | .002 |
| Poverty level | ||||
| >2× PL | Reference | — | Reference | — |
| 1–2× PL | 0.4 (0.2 to 0.5) | <.001 | 0.3 (0.1 to 0.5) | .001 |
| <PL | 0.8 (0.5 to 1.1) | <.001 | 0.7 (0.4 to 1.0) | <.001 |
| Comorbidity scored | ||||
| 0 | Reference | — | Reference | — |
| 1 | 0.0 (−0.1 to 0.2) | .61 | 0.0 (−0.1 to 0.2) | .54 |
| 2 | 0.2 (0.0 to 0.4) | .04 | 0.2 (0.0 to 0.4) | .04 |
| 3–6 | 0.7 (0.4 to 1.1) | <.001 | 0.7 (0.3 to 1.0) | <.001 |
| Depressione | ||||
| No depression | Reference | — | Reference | — |
| Depression | 1.8 (1.2 to 2.3) | <.001 | 1.7 (1.2 to 2.3) | <.001 |
| pVA (logMAR)f | 0.2 (0.2 to 0.3) | <.001 | ||
Model 1 is partially adjusted for age, gender, ethnicity, education, poverty, comorbidities, depression.
Model 2 is fully adjusted for age, gender, ethnicity, education, poverty, comorbidities, depression and pVA (logMAR).
Coefficient and 95% CI (computed using interview weights to provide estimates for the total US population and age-standardized to the 2000 US census population), representative of difference in sum difficulty score versus those with no AMD, where a positive coefficient corresponds with higher reported difficulty.
Comorbidity score out of 6 sum points, allocated for self-reported hypertension, diabetes, lung disease, heart disease, stroke, or cancer.
Depression classified as Patient Health Questionnaire-9 score of ≥10.
LogMAR in 0.1 increments, where logMAR 0 = 20/20.
Sum visual difficulty score out of 30 points. Ethnicity (other), Asian or mixed ethnicity; logMAR, logarithm of the minimum angle of resolution; PL, poverty level.
Figure 2.
Fully adjusted sum visual and physical difficulty score by AMD severity (with 95% CI). (A) Fully adjusted sum visual difficulty score, point estimate and 95% CI. Unadjusted sum point estimate and 95% CIs for (i) no AMD (7.1 [7.0–7.2]; vertical dashed line), (ii) early AMD (7.1 [6.8–7.4]), and (iii) late AMD (9.1 [7.6–10.6]). (B) Fully adjusted sum physical difficulty score, point estimate and 95% CI. Unadjusted sum point estimate and 95% CIs for (i) no AMD (11.0 [10.9–11.1]; vertical dashed line), (ii) early AMD (11.2 [10.8–11.7]), and (iii) late AMD (12.1 [10.7–13.5]).
To understand the risk factors associated with poorer functioning among individuals with AMD, we restricted our multivariate regression model to individuals with AMD. Participants living below the poverty level, with 3 or more medical comorbidities, depression, or worse pVA reported significantly higher modified sum visual difficulty scores (P < .05 for all). There was no association between age, gender, ethnicity, or education, and a higher self-reported modified sum visual difficulty score among those with AMD (P > .05 for all) (Table 6).
Table 6.
Multivariate Linear Regression Analysis of the Association Between Baseline Sociodemographic Factors and IRT-Adjusted Sum Visual and Physical Difficulty Scores Among Those With AMD
| Change in Sum Visual Difficulty Scoree | Change in Sum Physical Difficulty Scoref | |||
|---|---|---|---|---|
| Any AMD (n = 441) | P Value | Any AMD (n = 441) | P Value | |
| Age category | ||||
| 40–49 | Reference | — | Reference | — |
| 50–59 | 0.5 (0.0 to 1.1)a | 0.07 | 0.3 (−0.3 to 1.0) | 0.29 |
| 60–69 | 0.3 (−0.3 to 0.8) | 0.30 | 0.6 (−0.4 to 1.6) | 0.25 |
| ≥70 | 0.1 (−0.4 to 0.6) | 0.56 | 0.5 (−0.1 to 1.1) | 0.09 |
| Gender | ||||
| Male | Reference | — | Reference | — |
| Female | 0.4 (−0.1 to 0.8) | 0.11 | 0.4 (0.0 to 0.9) | 0.07 |
| Ethnicity | ||||
| Non-Hispanic White | Reference | — | Reference | — |
| Hispanic/Mexican | −0.2 (−0.8 to 0.4) | 0.44 | −0.4 (−1.1 to 0.4) | 0.32 |
| Non-Hispanic Black | −0.2 (−0.8 to 0.3) | 0.36 | −0.4 (−1.0 to 0.3) | 0.25 |
| Other | 0.1 (−0.9 to 1.0) | 0.89 | 0.4 (−0.8 to 1.6) | 0.52 |
| Education | ||||
| >High school | Reference | — | Reference | — |
| High school | −0.2 (−0.7 to 0.3) | 0.36 | 0.1 (−0.5 to 0.8) | 0.62 |
| <High school | 0.1 (−0.6 to 0.8) | 0.72 | 0.4 (−0.3 to 1.1) | 0.22 |
| Poverty level | ||||
| >2× PL | Reference | — | Reference | −0.03 |
| 1–2× PL | 0.2 (−0.4 to 0.7) | 0.59 | 0.7 (0.1 to 1.4) | |
| <PL | 0.9 (0.3 to 1.5) | 0.008 | 2.1 (0.8 to 3.3) | 0.002 |
| Comorbidity scoreb | ||||
| 0 | Reference | — | Reference | — |
| 1 | 0.1 (−0.5 to 0.6) | 0.83 | 0.5 (0.0 to 0.9) | 0.04 |
| 2 | 0.1 (−0.7 to 0.9) | 0.81 | 1.1 (0.2 to 1.9) | 0.02 |
| 3–6 | 1.1 (0.5 to 1.8) | 0.002 | 3.4 (2.4 to 4.3) | <0.001 |
| Depressionc | ||||
| No depression | Reference | — | Reference | — |
| Depression | 1.5 (0.1 to 2.9) | 0.04 | 2.3 (0.9 to 3.7) | 0.002 |
| pVA (logMAR)d | 0.8 (0.6 to 0.9) | <0.001 | 0.3 (0.2 to 0.4) | <0.001 |
Coefficient and 95% CI (computed using interview weights to provide estimates for the total US population and age-standardized to the 2000 US census population), representative of difference in sum difficulty score versus those with no AMD, where a positive coefficient corresponds with higher reported difficulty.
Comorbidity score out of 6 sum points, allocated for self-reported hypertension, diabetes, lung disease, heart disease, stroke, or cancer.
Depression classified as Patient Health Questionnaire-9 score of ≥10.
LogMAR in 0.1 increments, where logMAR 0 = 20/20.
Sum visual difficulty score out of 30 points.
Sum physical difficulty score out of 36 points. Ethnicity (other), Asian or mixed ethnicity; logMAR, logarithm of the minimum angle of resolution; PL, poverty level.
Sum Physical Difficulty Regression Analysis
In a univariate regression analysis of the modified sum physical difficulty score, those with no AMD reported on average 11.0 sum points of difficulty (95% CI, 10.9–11.1). Those with both early and late AMD reported significantly higher difficulty versus those with no AMD (0.5 sum points higher [P = .03] and 2.3 sum points higher [P = .005], respectively) (Fig. 1B).
In a multivariate regression analysis not accounting for pVA, those with early AMD did not report significantly higher physical difficulty versus those without AMD (0.3 sum points higher; P = .20); however, those with late AMD reported significantly greater physical difficulty than those without AMD (1.7 sum points higher; P = .02). This association did not persist after adjustment for pVA (P = .21 and P = .12 for early and late AMD vs no AMD, respectively). Female gender, living within 1 to 2 times of or below the poverty level, having 1 or more medical comorbidities, depression, and worse pVA were independently associated with significantly higher reported sum physical difficulty scores (P < .05 for all) (Table 5) (Fig. 2B).
Table 5.
Multivariate Linear Regression Predicting IRT-Adjusted Sum Physical Difficulty Score
| Change in IRT-Adjusted Sum Physical Difficulty Scoreg | ||||
|---|---|---|---|---|
| Model 1a | P Value | Model 2b | P Value | |
| AMD status | ||||
| None | Reference | — | Reference | — |
| Early AMD | 0.3 (−0.2 to 0.7)c | .20 | 0.3 (−0.2 to 0.7) | .21 |
| Late AMD | 1.7 (0.3 to 3.0) | .02 | 1.1 (−0.3 to 2.6) | .12 |
| Age category | ||||
| 40–49 | Reference | — | Reference | — |
| 50–59 | 0.1 (−0.2 to 0.3) | .63 | 0.0 (−0.2 to 0.3) | .64 |
| 60–69 | −0.5 (−0.8 to −0.2) | .002 | −0.5 (−0.8 to −0.2) | .001 |
| ≥70 | 0.0 (−0.2 to 0.3) | .79 | −0.1 (−0.4 to 0.1) | .27 |
| Gender | ||||
| Male | Reference | — | Reference | — |
| Female | 0.3 (0.1 to 0.4) | .004 | 0.2 (0.1 to 0.4) | .009 |
| Ethnicity | ||||
| Non-Hispanic White | Reference | — | Reference | — |
| Hispanic/Mexican | −0.3 (−0.6 to 0.0) | .04 | −0.3 (−0.6 to −0.1) | .02 |
| Non-Hispanic Black | 0.1 (−0.1 to 0.3) | .21 | 0.1 (−0.1 to 0.3) | .38 |
| Other | 0.0 (−0.5 to 0.5) | .90 | −0.1 (−0.6 to 0.4) | .76 |
| Education | ||||
| >High school | Reference | — | Reference | — |
| High school | 0.2 (0.0 to 0.3) | .03 | 0.2 (0.0 to 0.3) | .03 |
| <High school | 0.2 (−0.1 to 0.5) | .12 | 0.2 (−0.1 to 0.4) | .14 |
| Poverty level | ||||
| >2× PL | Reference | — | Reference | — |
| 1–2× PL | 0.7 (0.4 to 1.0) | <.001 | 0.7 (0.4 to 0.9) | <.001 |
| <PL | 1.1 (0.8 to 1.4) | <.001 | 1.1 (0.7 to 1.4) | <.001 |
| Comorbidity scored | ||||
| 0 | Reference | — | Reference | — |
| 1 | 0.2 (0.1 to 0.4) | .002 | 0.2 (0.1 to 0.4) | .001 |
| 2 | 0.7 (0.5 to 1.0) | <.001 | 0.7 (0.5 to 0.9) | <.001 |
| 3–6 | 2.6 (2.1 to 3.1) | <.001 | 2.5 (2.0 to 3.0) | <.001 |
| Depressione | ||||
| No depression | Reference | — | Reference | — |
| Depression | 2.7 (2.0 to 3.3) | <.001 | 2.6 (2.0 to 3.3) | <.001 |
| pVA (logMAR)f | 0.2 (0.1 to 0.3) | <.001 | ||
Model 1 is partially adjusted for age, gender, ethnicity, education, poverty, comorbidities, and depression.
Model 2 is fully adjusted for age, gender, ethnicity, education, poverty, comorbidities, depression, and pVA (logMAR).
Coefficient and 95% CI (computed using interview weights to provide estimates for the total US population and age-standardized to the 2000 US census population), representative of difference in sum difficulty score versus those with no AMD, where a positive coefficient corresponds with higher reported difficulty.
Comorbidity score out of 6 sum points, allocated for self-reported hypertension, diabetes, lung disease, heart disease, stroke, or cancer.
Depression classified as Patient Health Questionnaire-9 score of ≥10.
LogMAR in 0.1 increments, where logMAR 0 = 20/20.
Sum physical difficulty score out of 36 points. Ethnicity (other), Asian or mixed ethnicity; logMAR, logarithm of the minimum angle of resolution; PL, poverty level.
In a multivariate regression analysis restricted to participants with AMD, those living within 1 to 2 times of or below the poverty level, with any medical comorbidities, with depression, or with worse pVA reported significantly higher modified sum physical difficulty scores (P < .05 for all). There was no association between age, gender, ethnicity, or education, and a higher self-reported modified sum physical difficulty score for those with AMD (P > .05 for all) (Table 6).
Discussion
In this study, we found that US adults with early and late AMD experience significantly greater visual and physical difficulty across a variety of daily tasks. The association between late AMD and self-reported visual difficulty persisted even after adjustment for sociodemographic characteristics, medical comorbidities, and pVA. However, the association between late AMD and self-reported physical difficulty appeared to be driven primarily by level of VA impairment. Among participants with AMD, those with lower income, medical comorbidities, or depression represented a subset of the population more likely to report higher levels of visual and physical difficulty. Our results reinforce the negative impact of AMD-related vision impairment on not only visual functioning but also physical functioning in older US adults and identify groups particularly at risk of functional difficulty.
AMD-related vision loss has been associated with poor visual function and vision-related quality of life,17,39 consistent with present findings. As expected, late AMD in particular was associated with substantially worse pVA (compared with early or no AMD) and conferred greater visual difficulty, consistent with previous findings outlining impaired pVA as a risk factor for visual difficulty.23–26,40 However, although impaired pVA may in part explain the association between late AMD and vision-related difficulty, the association persisted even after accounting for pVA. This finding may highlight the importance of using multiple visual assessment tools to fully capture the visual symptoms experienced by patients with late AMD. For example, visual symptoms like poor contrast sensitivity, low luminesce VA, and critical print size are not necessarily well-captured by a Snellen chart alone, but are demonstrably influential in determining the severity of visual functional difficulty for those with AMD.25,26 A lack of other measurement tools in the NHANES data was a limitation to the present study.
The association between AMD and poor physical function has also been previously described, although inconsistently. Although some studies have found that those with late AMD had significantly lower mobility versus those with early to intermediate AMD23 and that patients with AMD (late AMD in particular) were more likely to experience difficulty performing activities of daily living, even when adjusting for pVA,14 other investigators have found no significant association between AMD and physical difficulty when adjusting for pVA.13 We similarly found that, although the association between self-reported physical difficulty and late AMD persisted after adjustment for known covariates like age and comorbid illness, this effect seemed to be driven mostly by pVA. This finding highlights that VA may be an independent driver of physical difficulty experienced by those with AMD.
In a secondary analysis restricted to participants with AMD, we found that those living below the poverty line, with greater medical comorbidities, or with depression experienced greater visual and physical difficulties. This analysis highlights a group of patients with AMD particularly susceptible to experiencing functional difficulty, and it is important to monitor them closely. There have also been strong links between poverty and functional difficulty demonstrated elsewhere, particularly in low to middle income countries.41 However, associations remain relatively unstudied in high-income countries like the United States. Poverty and functional difficulty are believed to operate in a cycle, each reinforcing the other.41 Indeed, we presently demonstrate that, among those with any AMD, poverty is independently associated with greater visual and physical difficulties, even when accounting for sociodemographic features and medical comorbidities. Our findings from a nationally representative population in a high-income countries add to the current body of literature, outlining a group of AMD patients susceptible to experiencing greater functional difficulty.
The association between medical comorbidities and functional impairment is also well-described42,43; however, it is usually attributed to older age. Here we found that, even when accounting for age, medical comorbidities conferred greater visual and physical difficulties among those with AMD. This finding highlights that specific consideration for functional impairment should be given when assessing AMD patients with medical comorbidities, irrespective of age. Although we were unable to analyze early and late AMD groups separately, we found that those with late AMD were more likely to have more medical comorbidities, as has been described elsewhere,44–46 suggesting that this group may be at even greater risk of functional difficulty. Equally, there is a well-described association between depression and functional impairment,47,48 although this association was not previously described for AMD patients to our knowledge. Interestingly, the prevalence of depression in our cohort was substantially lower than has been previously reported for those with AMD and visual impairment.49,50 This finding may be incidental or indicative of a sampling limitation (those with depression may be less likely to agree to NHANES participation), which may be representative of the significance of nonparticipation as a defining characteristic of depression.51 Either way, the association between depression and AMD and visual impairment is well-described, and an integrative management approach for those with low vision should always be considered. Additionally, although we did not find higher rates of depression among our AMD cohort in contrast with prior studies,50,52,53 depression was independently associated with functional difficulty among those with AMD. With the potential additive and multiplicative effects of medical comorbidities and depression on functional difficulty and quality of life,54,55 our findings suggest that patients with AMD with either medical comorbidities and/or depression are at particular risk of experiencing functional difficulty.
Here we applied IRT to allow for more realistic reflection of what each questionnaire is measuring, and how reliably each question contributes to that measurement.56 Although only 1 dimension was found in factor analysis for both questionnaires here, making the use of alternative psychometric validation techniques like Rasch possible, we believe IRT is particularly suited for a number of reasons. IRT is especially well-suited for analyzing questionnaire data measuring clinical latent traits, such as visual difficulty and physical difficulty.57 The GRM (the IRT model chosen for the current study) is further suited for validation of ordinal-scaled Likert-type questionnaires for a number of reasons. GRMs are less constrained than other psychometric validation techniques used in previous studies (i.e., Rasch models or partial credit models) in that they have fewer assumptions (such as assuming equal question discrimination [imposed by Rasch models]), impose fewer restrictions on the data, and are appropriately robust to handle larger datasets with distributions slightly deviated from normal if required.58,59 For example, whereas Rasch models may be mathematically similar, they constrain item discrimination (assuming equality between items), whereas the IRT GRM used presently does not assume equal discrimination between items, and strives to model data to better fit each item.35,60 By subsequently imputing modified questionnaire scores, we believe the psychometric models used here provides more accurate comparisons between groups. Further, the GRM is also unique in that it has been demonstrated as particularly appropriate as a robust model for large datasets with distributions deviated from normal.58,59 Importantly, here we found that only 9 of the original 13 physical difficulty questions reliably measured that latent trait. And notably, by ascribing unique weights to the remaining visual and physical difficulty questions (based on imputed discrimination and difficulty properties) and calculating rescaled, modified scores using GRM validation techniques, we could demonstrate participants’ true level of experienced visual and physical difficulties with a greater degree of accuracy than using nonpsychometrically validated questionnaire scores.
The strengths of the present study include its use of a large, nationally representative sample and the objective measurement of AMD severity. Sociodemographic factors, medical comorbidities, and VA were well-controlled for because of the comprehensive and standardized questionnaire and examination techniques used to collect NHANES data, which allowed for associations to be described in well-adjusted models. The strength of the potential association between AMD and visual and physical difficulties has been highlighted here, given the significance of the results despite the relatively small effect size. Additionally, psychometric validation methods allowed for more accurate, rescaled, modified visual and physical difficulty scores to be compared between patient groups and by AMD severity, which, to the knowledge of the authors, has not been previously used for comparable studies.
Our study is subject to a number of limitations. First, our results may have been influenced by nonparticipation. There were significant demographic differences between subjects with and without missing retinal imaging data (e.g., those with missing data were more likely to have impaired pVA). Because some of these factors are known to (and shown here to) influence visual and physical difficulty, this may have led to over or underestimation of functional difficulty in these subpopulations. For example, the surprisingly low individual item scores (even for those with late AMD) may be reflective of the fact that those with impaired pVA were more likely to have missing retinal imaging data and thus not were included in mean item difficulty score calculations. Such low item scores may also be a reflection of the fact NHANES only documented AMD grading of the worse eye, even though the better-seeing eye (with less severe AMD) usually determines functional vision; as participants compensate with it. We adjusted for this factor presently by using pVA in the better-seeing eye. The low scores may also reflect an under-reporting of symptoms in such a population, although the reasons for this pattern are not able to be determined presently. Interestingly, responses for “noticing objects in peripheries” (presumably associated with peripheral vision) loaded strongly onto the same factor as the other (central vision) visual items. This finding may outline a limitation of using so few questions (i.e., only 6 total visual questions in the dataset); a more comprehensive visual difficulty questionnaire including items relating to peripheral vision may have yielded a second strong factor. Moreover, we relied on self-reported data, and reporter bias may not have affected all demographics equally. Subjective measures of function may not correlate well with objective outcomes such as reading speed or falls and other adverse outcomes. Finally, the relatively small number of participants with late AMD meant that exploratory analyses of subpopulations at increased risk of reporting visual and physical functional difficulty were underpowered when stratified by AMD severity, resulting in us combining early and late AMD categories for these analyses. To mitigate this problem, we used pVA in these models to partially account for AMD severity.
In conclusion, we found that US adults over 40 years of age with late AMD experience substantially greater visual and physical difficulties compared with those without AMD, independent of sociodemographic characteristics and medical comorbidity status. Although self-reported visual difficulty seems to be independent of pVA, the association between AMD and physical difficulty seems to be driven largely by the level of VA impairment. Individuals with AMD with a lower income, more medical comorbidities, and depression experienced significantly greater visual and physical functional difficulties. With an aging US population and the increasing global prevalence of AMD, an awareness of the functional burden of AMD and those particularly at risk, as well as an assessment of visual characteristics other than Snellen pVA alone, will facilitate a comprehensive approach to the assessment and management of patients with AMD.
Acknowledgments
Disclosure: W. Mitchell, None; H. Resnick, None; N. Zebardast, None
References
- 1. Sommer A, Tielsch JM, Katz J, et al.. Racial differences in the cause-specific prevalence of blindness in East Baltimore. N Engl J Med. 1991; 325(20): 1412–1417, doi: 10.1056/nejm199111143252004. [DOI] [PubMed] [Google Scholar]
- 2. Klaver CCW. Age-specific prevalence and causes of blindness and visual impairment in an older population. Arch Ophthalmol. 1998; 116(5): 653, doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 3. Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: The Blue Mountains Eye Study. Clin Exp Ophthalmol. 2000; 28(4): 268–273, doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 4. Muñoz B. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000; 118(6): 819, doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 5. Weih LM. Age-specific causes of bilateral visual impairment. Arch Ophthalmol. 2000; 118(2): 264, doi: 10.1001/archopht.118.2.264. [DOI] [PubMed] [Google Scholar]
- 6. Bourne RRA, Stevens GA, White RA, et al.. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Global Health. 2013; 1(6): e339–e349, doi: 10.1016/s2214-109x(13)70113-x. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Age-related macular degeneration. Priority eye diseases. Geneva, Switzerland: World Health Organization; 2020. Available at: www.who.int/blindness/causes/priority/en/index7.html . Accessed June 2020. [Google Scholar]
- 8. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014; 2(2): e106–e116, doi: 10.1016/s2214-109x(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 9. Prevalence of age-related macular degeneration in the united states. Arch Ophthalmol. 2004; 122(4): 564, doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Common eye disorders. Vision Health Initiative (VHI). 2015; Available at: www.cdc.gov/visionhealth/basics/ced/index.html . Accessed May 2020. [Google Scholar]
- 11. Gibson D. Diabetic retinopathy and age-related macular degeneration in the U.S. Am J Prev Med. 2012; 43(1): 48–54, doi: 10.1016/j.amepre.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 12. Yu F, Silva Croso G, Kim TS, et al.. Assessment of automated identification of phases in videos of cataract surgery using machine learning and deep learning techniques. JAMA Network Open. 2019; 2(4): e191860, doi: 10.1001/jamanetworkopen.2019.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loprinzi PD, Swenor BK, Ramulu PY. Age-related macular degeneration is associated with less physical activity among us adults: cross-sectional study. PLoS One. 2015; 10(5): e0125394, doi: 10.1371/journal.pone.0125394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golpinath B, Liew G, Burlutsky G, Mitchell P. Age-related macular degeneration and 5-year incidence of impaired activities of daily living. Maturitas. 2014; 77(3): 263–266. [DOI] [PubMed] [Google Scholar]
- 15. Bennion AE, Shaw RL, Gibson JM. What do we know about the experience of age related macular degeneration? A systematic review and meta-synthesis of qualitative research. Soc Sci Med. 2012; 75(6): 976–985, doi: 10.1016/j.socscimed.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 16. Inan S, Cetinkaya E, Duman R, Dogan I, Inan U. Quality of life among patients with age-related severe macular degeneration assessed using the NEI-VFQ, HADS-A, HADS-D and SF-36 tests. A cross-sectional study. Sao Paulo Med J. 2019; 137(1): 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman AL, Yu F, Ensrud KE, et al.. Impact of age-related macular degeneration on vision-specific quality of life: follow-up from the 10-year and 15-year visits of the study of osteoporotic fractures. Am J Ophthalmol 2010; 150(5): 683–691, doi: 10.1016/j.ajo.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams RA. The psychosocial impact of macular degeneration. Arch Ophthalmol. 1998; 116(4): 514, doi: 10.1001/archopht.116.4.514. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell J, Bradley C.. Quality of life in age related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006, doi: 10.1186/1477-7525-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Z, Wang W, Keel S, Zhang J, He M. Association of age-related macular degeneration with risk of all-cause and specific-cause mortality in the National Health and Nutrition Examination Survey, 2005 to 2008. JAMA Ophthalmol. 2019; 137(3): 248, doi: 10.1001/jamaophthalmol.2018.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rein DB. The economic burden of major adult visual disorders in the united states. Arch Ophthalmol. 2006; 124(12): 1754, doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 22. Spooner K, Mhlanga C, Hong T, Broadhead G, Chang A. The burden of neovascular age-related macular degeneration: a patient's perspective. Clin Ophthalmol. 2018; 12: 2483–2491, doi: 10.2147/opth.s185052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pondorfer SG, Terheyden JH, Heinemann M, Wintergerst MWM, Holz FG, Finger RP. Association of vision-related quality of life with visual function in age-related macular degeneration. Sci Rep. 2019; 9(1): 15326, doi: 10.1038/s41598-019-51769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seland JH, Vingerling JR, Augood CA, et al.. Visual impairment and quality of life in the older European population, the Eureye study. Acta Ophthalmol. 2011; 89(7): 608–613, doi: 10.1111/j.1755-3768.2009.01794.x. [DOI] [PubMed] [Google Scholar]
- 25. Maguire. Baseline characteristics, the 25-item national eye institute visual functioning questionnaire, and their associations in the Complications of Age-related Macular Degeneration Prevention Trial (CAPT). Ophthalmology. 2004; 111(7): 1307–1316. [DOI] [PubMed] [Google Scholar]
- 26. Roh M, Selivanova A, Shin HJ, Miller JW, Jackson ML. Visual acuity and contrast sensitivity are two important factors affecting vision-related quality of life in advanced age-related macular degeneration. PLoS One. 2018; 13(5): e0196481, doi: 10.1371/journal.pone.0196481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uddin S, Khan A, Hossain ME, Moni MA. Comparing different supervised machine learning algorithms for disease prediction. BMC Med Informat Decision Making. 2019; 19(1): 281, doi: 10.1186/s12911-019-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein R, Davis M, Magli R, Segal P, Klein B, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991; 98(7): 1128–1134. [DOI] [PubMed] [Google Scholar]
- 29. Klein R, Klein BEK, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial united states population. Ophthalmology. 1999; 106(6): 1056–1065, doi: 10.1016/s0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X, Bullard KM, Cotch MF, et al.. Association between depression and functional vision loss in persons 20 years of age or older in the united states, NHANES 2005-2008. JAMA Ophthalmol. 2013; 131(5): 573, doi: 10.1001/jamaophthalmol.2013.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kroenke K, Spitzer R.. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002; 32(9): 509–515. [Google Scholar]
- 32. Snellen and logMAR acuity testing. 2015. Available at: www.rcophth.ac.uk/wp-content/uploads/2015/11/LogMAR-vs-Snellen.pdf. Accessed July 2020.
- 33. Kim J-O, Mueller CW.. Introduction to Factor Analysis. Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage Publications; 1978. [Google Scholar]
- 34. Kim J-O, Mueller CW.. Factor Analysis: Statistical Methods and Practical Issues. Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage Publications; 1978. [Google Scholar]
- 35. Bonifay W. Multidimensional Item Response Theory. Thousand Oaks, CA: Sage Publications; 2019. [Google Scholar]
- 36. Edelen MO, Reeve BB. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Qual Life Res. 2007; 16(S1): 5–18, doi: 10.1007/s11136-007-9198-0. [DOI] [PubMed] [Google Scholar]
- 37. Gorsuch R. Factor Analysis. Philadelphia, PA: W. B. Saunders Company; 1974. [Google Scholar]
- 38. Harman HH. Modern Factor Analysis. Chicago: University of Chicago Press; 1976. [Google Scholar]
- 39. Willis JR, Doan QV, Gleeson M, et al.. Vision-related functional burden of diabetic retinopathy across severity levels in the united states. JAMA Ophthalmol. 2017; 135(9): 926, doi: 10.1001/jamaophthalmol.2017.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schippert AC, Jelin E, Moe MC, Heiberg T, Grov EK. The impact of age-related macular degeneration on quality of life and its association with demographic data: results from the NEI VFQ-25 questionnaire in a Norwegian population. Gerontol Geriatr Med. 2018; 4: 233372141880160, doi: 10.1177/2333721418801601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banks LM, Kuper H, Polack S. Poverty and disability in low- and middle-income countries: a systematic review. PLoS One. 2017; 12(12): e0189996, doi: 10.1371/journal.pone.0189996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryan A, Murphy C, Boland F, Galvin R, Smith SM. What is the impact of physical activity and physical function on the development of multimorbidity in older adults over time? A population-based cohort study. J Gerontol A. 2018; 73(11): 1538–1544, doi: 10.1093/gerona/glx251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bayliss EA, Bayliss MS, Ware JE, Steiner JF. Predicting declines in physical function in persons with multiple chronic medical conditions: what we can learn from the medical problem list. Health Qual Life Outcomes. 2004; 2(1): 47, doi: 10.1186/1477-7525-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng Q, Saadine J, Klein R, Rothenberg R, Chou C, Il'yasova D. Early age-related macular degeneration with cardiovascular and renal comorbidities: an analysis of the National Health and Nutrition Examination Survey, 2005-2008. Ophthalmic Epidemiol. 2017; 24(6): 413–419. [DOI] [PubMed] [Google Scholar]
- 45. Weiner DE, Tighiouart H, Reynolds R, Seddon JM. Kidney function, albuminuria and age-related macular degeneration in NHANES iii. Nephrol Dial Transplant. 2011; 26(10): 3159–3165, doi: 10.1093/ndt/gfr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zlateva GP, Javitt JC, Shah SN, Zhou Z, Murphy JG. Comparison of comorbid conditions between neovascular age–related macular degeneration patients and a control cohort in the Medicare population. Retina. 2007; 27(9): 1292–1299, doi: 10.1097/01.iae.0000300915.81866.b8. [DOI] [PubMed] [Google Scholar]
- 47. Hammer-Helmich L, Haro JM, Jönsson B, et al.. Functional impairment in patients with major depressive disorder: the 2-year perform study. Neuropsychiatr Dis Treat. 2018; 14: 239–249, doi: 10.2147/ndt.s146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gili M, Toro M, Armengol S, Garcia-Campayo J, Castro A, Roca M. Functional impairment in patients with major depressive disorder and comorbid anxiety disorder. Can J Psychiatry. 2013; 58(12): 697–686, doi: 10.1177/070674371305801205. [DOI] [PubMed] [Google Scholar]
- 49. Casten R, Rovner B.. Depression in age-related macular degeneration. J Vis Impair Blind. 2008; 102(10): 591–599. [PMC free article] [PubMed] [Google Scholar]
- 50. Casten RJ, Rovner BW.. Update on depression and age-related macular degeneration. Curr Opin Ophthalmol. 2013; 24(3): 239–243, doi: 10.1097/icu.0b013e32835f8e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hao G, Bishwajit G, Tang S, Nie C, Ji L, Huang R. Social participation and perceived depression among elderly population in South Africa. Clin Interv Aging. 2017; 12: 971–976, doi: 10.2147/cia.s137993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cimarolli V, Casten R, Rovner B, Heyl V, Sörensen S, Horowitz A. Anxiety and depression in patients with advanced macular degeneration: current perspectives. Clin Ophthalmol. 2015; 10: 55, doi: 10.2147/opth.s80489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jacob L, Spiess A, Kostev K. Prevalence of depression, anxiety, adjustment disorders, and somatoform disorders in patients with age-related macular degeneration in Germany. German Med Sci. 2017; 15: 2–7, doi: 10.3205/000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kang H-J, Kim S-Y, Bae K-Y, et al.. Comorbidity of depression with physical disorders: research and clinical implications. Chonnam Med J. 2015; 51(1): 8, doi: 10.4068/cmj.2015.51.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ho C, Feng L, Fam J, Mahendran R. Coexisting medical comorbidity and depression: multiplicative effects on health outcomes in older adults. Int Psychogeriatr. 2014; 26(7): 1221–1229. [DOI] [PubMed] [Google Scholar]
- 56. Embretson S, Reise S.. Item Response Theory for Psychologists. Mahwah, NJ: L. Erlbaum Associates; 2000. [Google Scholar]
- 57. Van Nispen RM, Knol DL, Langelaan M, Van Rens GH. Re-evaluating a vision-related quality of life questionnaire with item response theory (IRT) and differential item functioning (DIF) analyses. BMC Med Res Methodol. 2011; 11(1): 125, doi: 10.1186/1471-2288-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sass D, Schmitt T, Walker C. Estimating non-normal latent trait distributions within item response theory using true and estimated item parameters. Appl Meas Educ. 2008; 21: 65–88. [Google Scholar]
- 59. Skrondal A, Rabe-Hesketh S.. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Boca Raton, FL: Chapman and Hall/CRC; 2004. [Google Scholar]
- 60. Columbia Public Health. Item response theory. Population health methods. 2012. Available at: www.publichealth.columbia.edu/research/population-health-methods/item-response-theory. Accessed October 2020.