Abstract
Purpose
Hyperglycemia damages the retinal mitochondria, and the mitochondrial damage plays a central role in the development of diabetic retinopathy. Patients with diabetes also have higher homocysteine levels, and abnormalities in homocysteine metabolism result in decreased levels of hydrogen sulfide (H2S), an endogenous gasotransmitter signaling molecule with antioxidant properties. This study aimed to investigate the role of H2S in the development of diabetic retinopathy.
Methods
Streptozotocin-induced diabetic mice were administered a slow releasing H2S donor GYY4137 for 6 months. The retina was used to measure H2S levels, and their retinal vasculature was analyzed for the histopathology characteristic of diabetic retinopathy and oxidative stress, mitochondrial damaging matrix metalloproteinase-9 (MMP-9), and mitochondrial integrity. These parameters were also measured in the isolated retinal endothelial cells incubated in high glucose medium containing GYY4137.
Results
Administration of GYY4137 to diabetic mice ameliorated decrease in H2S and prevented the development of histopathology, characteristic of diabetic retinopathy. Diabetes-induced increase in oxidative stress, MMP-9 activation, and mitochondrial damage were also attenuated in mice receiving GYY4137. Results from isolated retinal endothelial cells confirmed the results obtained from diabetic mice.
Conclusions
Thus, supplementation of H2S donor prevents the development of diabetic retinopathy by ameliorating increase in oxidative stress and preserving the mitochondrial integrity. H2S donors may provide a novel therapeutic strategy to inhibit the development of diabetic retinopathy.
Keywords: antioxidant, diabetic retinopathy, homocysteine, hydrogen sulfide, mitochondria, MMP-9
Diabetic retinopathy is the leading cause of acquired blindness, and despite extensive research in the field, its etiology remains elusive. In the early stage of diabetic retinopathy, retinal capillary cells undergo accelerated apoptosis resulting in pericyte ghosts and acellular capillaries.1–3 A growing body of evidence supports the hypothesis that oxidative stress mitochondrial damage contributes to accelerated apoptosis of retinal capillary cells. Reactive oxygen species (ROS), by damaging the mitochondrial membranes, alter their potential, and allow cytochrome c to leak out into the cytosol, leading to cell apoptosis.4,5 One of the molecular consequences of oxidative stress is the activation of matrix metalloproteinases (MMPs); ROS oxidize sulfide bond in their pro-domains, and also reduce their tissue inhibitors of MMPs (TIMPs).6,7 Diabetes transcriptionally and functionally activates gelatinase metalloproteinase-9 (MMP-9), and suppresses its tissue inhibitor Timp1 in the retina and its capillary cells.3,8,9 In the early stages of diabetic retinopathy, activated gelatinase MMPs act as apoptotic inducer, and damages the mitochondria. Our previous study has documented a direct role of MMP-9 in the development of diabetic retinopathy, and have shown that diabetic mice with the MMP-9 gene knocked out are protected from the development of histopathology characteristic of diabetic retinopathy.3,7
Patients with diabetes also have elevated levels of homocysteine, a nonproteinogenic, sulfur-containing amino acid, and elevated homocysteine is now recognized as an independent risk factor of diabetic retinopathy.10,11 Homocysteine is metabolized to cysteine by transsulfuration process using cystathionine-β synthase (CBS) and cystathionine γ-lyase (CSE), and cysteine is also a substrate for CBS and CSE to generate a gasotransmitter hydrogen sulfide (H2S).12,13 An imbalance between homocysteine and H2S increases oxidative stress, inflammation, and ischemic injury.13,14 H2S is generally considered as a toxic gas, which can affect the central nervous system, but recently it is also recognized as a signaling molecule with significant cytoprotective effects.15 Our recent study has shown that retina from human donors with established diabetic retinopathy although have increased homocysteine compared to their age-matched nondiabetic human donors, H2S levels in the same diabetic donors is significantly lower.16 Accumulating evidence has suggested various physiological functions of H2S, including neuromodulation, vasodilation, inflammation, and apoptosis.17–19 However, the role of H2S in diabetic retinopathy remains in its incipient stages.
The goal of this study is to investigate the role of H2S in diabetic retinopathy, focusing on its role in increasing oxidative stress MMP-9 activation. Using retinal microvessels from streptozotocin-induced diabetic mice, receiving a slow-releasing pharmacological donor of H2S, we have investigated the effect of regulation of H2S on ROS-MMP-9-mitochondrial damage. The in vivo results were confirmed in the human retinal endothelial cells (HRECs), exposed to high glucose.
Methods
Mice
Diabetes was induced in 7 to 9-week-old C57BL/6J mice (both male and female; Jackson Laboratory, Bar Harbor, ME, USA) by administration streptozotocin (55 mg/kg BW, intraperitoneal) for four consecutive days between 4 PM and 5 PM. The mice presenting blood glucose > 250 mg/dL, 2 days after the last injection, were considered diabetic. We routinely induce hyperglycemia by streptozotocin administration in both male and female mice, and can maintain similar severity of hyperglycemia by adjusting their insulin regimen.3,20,21 Diabetic mice were divided into 4 groups, group 1 mice received a slow-releasing H2S donor GYY4137 (GY; Cat. No. SML0100 Sigma-Aldrich, St. Louis, MO, USA; DMSO solution diluted in normal saline, 0.25 mg/Kg, intraperitoneal)22 every other day (Diab/GY); group 2 mice received intraperitoneally 100 mg/kg BW DL-homocysteine (Cat. No. 44925; Sigma-Aldrich),23 dissolved in PBS (Diab/H), and group 3 mice received both of DL-homocysteine and GY (Diab/H + GY). Mice in group 4 remained diabetic, without any supplementation (Diab); each group had a total of 10 to 15 mice. Supplementation of GY, homocysteine, or homocysteine and GY was initiated soon after the establishment of hyperglycemia (2 days after the last streptozotocin injection). Neither GY or homocysteine had any effect on the body weight and blood glucose of these diabetic mice, and the values obtained at the initiation, and at 6 months were similar in all of the four groups of diabetic mice (P < 0.05 versus normal and P > 0.05 versus diabetes; Table 1). Six months after induction of diabetes, the animals were euthanized, one eye was immediately fixed in 4% paraformaldehyde for histopathology, and the other eye was used to isolate the retina. Age-matched normal mice (male and female) were used as controls (Norm), and each study had retina from similar numbers of male and female mice. The treatment of animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and was approved by the Wayne State University's Institutional Animal Care and Use Committee.
Table 1.
Effect of GY on Body Weight and Glucose of Diabetic Mice
| Group | Body Weight (g) Initial → Final | Blood Glucose (mg/dl) Initial → Final |
|---|---|---|
| Normal | 18.9 ± 1.5 → 36 ± 4.7 | 130 ± 17 → 124 ± 21 |
| Diabetes | 19.8 ± 1.8 → 25 ± 3.1* | 378 ± 97* → 457 ± 85* |
| Diabetes + GY | 20.2 ± 1.4 → 24 ± 4.1* | 378 ± 108* → 463 ± 112* |
| Diabetes + homocysteine | 20.7 ± 1.7 → 26 ± 3.7* | 366 ± 106* → 419 ± 108* |
| Diabetes + homocysteine + GY | 19.6 ± 2.1 → 25 ± 2.3* | 405 ± 117* → 434 ± 118* |
Each group had 10 or more mice, and body weight and blood glucose are the mean ± SD of the values obtained in the beginning (initial) and at the end of the experiment (final, 6 months).
P < 0.05 versus normal.
Microvessels were prepared by incubating the retina at 37°C in 5 to 6 mL deionized water for 60 minutes in a shaking water bath. The nonvascular tissue was gently removed under a dissecting microscope using a Pasteur pipette, and the microvasculature was largely (> 95%) devoid of nonvascular tissue.16
Retinal Endothelial Cells
Primary human retinal endothelial cells (HRECs) were purchased from Cell Systems Corporation (Cat. No. ACBRI 181; Cell Systems Corp., Kirkland, WA, USA), and were cultured in Dulbecco's modified Eagle medium (DMEM)-F12, as described previously.23,24 Cells from the sixth to ninth passage, with 80 to 90% confluency, were incubated in medium containing normal glucose (NG; 5 mM D-glucose) or high glucose (HG; 20 mM D-glucose) for 96 hours in the presence or absence of 100 µM L-homocysteine thiolactone hydrochloride (HG/H; Cat. No. S784036; Sigma-Aldrich). To investigate the effect of regulation of H2S, 150 µM GY22 was supplemented in high glucose (HG/GY), and in high glucose + homocysteine (HG/H + GY) incubations. To rule out the effect of osmolarity, each experiment included HRECs incubated in 20 mM L-glucose (L-Glu), instead of 20 mM D-glucose.
Hydrogen Sulfide
H2S was measured in the cell media or retinal homogenate using methylene blue assay, as previously reported.16 Briefly, to trap H2S, 350 µl of the incubation media or 30 µg retinal homogenate was transferred directly into a cryovial tube containing 1% (w/v) zinc acetate. Following incubation for 10 to 20 minutes at room temperature, 20 mM N-dimethyl-p-phenylenediamine sulfate (in 7.2 M HCl) and 30 mM FeCl3 (in 1.2 M HCl) were added, and the mixture was incubated in dark at 37°C (retina = 1–2 hours; culture media = 15–30 minutes). Protein was removed by 10% trichloroacetic acid, and the absorbance in the supernatant was measured at 670 nm, using sodium hydrosulfide (NaHS) as a standard.
Endogenous H2S in cells was measured by in situ fluorescence microscopy using the fluorescence probe azido-4-methylcoumarin.25 HRECs grown on coverslips were incubated with 50 µM 7-azido-4-methylcoumarin (Cat. No. 802409; Sigma-Aldrich) for 30 minutes, washed with PBS, and the fluorescence response of 7-Azido-4-Methylcoumarin to H2S was visualized using Zeiss ApoTome fluorescence microscope (Carles Zeiss Inc., Chicago, IL, USA) at 20 times magnification.
Homocysteine
Homocysteine levels were quantified in 25 µg retinal protein using an ELISA kit from Cell Bio Labs Inc. (Cat. No. STA-670; San Diego, CA, USA), as described previously.23 Homocysteine-BSA was as used as a standard, and homocysteine content was normalized to the total protein in the sample.
Activities of CBS and CSE
CBS activity was measured using 40 to 60 µg retinal protein using cystathionine β synthase activity assay kit (Cat. No. K998; Bio Vision, Milpitas, CA, USA) and cysteine and homocysteine as substrates, as reported previously.16 The specificity of CBS activity was measured by using 7-amino-4-methylcoumarin as a standard.
CSE activity was measured according to the published methods.26 Briefly, approximately 50 µg protein was incubated with 10 mM L-cysteine and the cofactor pyridoxal-5′-phosphate (2 mM) at 37°C in a shaking water bath for 2 hours. The reaction mixture was then transferred directly into a cryovial tube containing 1% (w/v) zinc acetate, and 20 mM N-dimethyl-p-phenylenediamine sulfate (in 7.2 M HCl) and 30 mM FeCl3 (in 1.2 M HCl) were then added. After incubating for 15 to 30 minutes at 37°C in the dark, the proteins were precipitated by 10% trichloroacetic acid, and absorbance of the resulting supernatant was measured at 670 nm. The amount of H2S produced/mg protein/hour was calculated.
Histopathology
Whole retina was carefully isolated from the paraformaldehyde fixed eye, and incubated at 37°C for 45 to 75 minutes in 3% crude trypsin (Gibco, Grand Island, NY, USA) solution containing 200 mM sodium fluoride. The neuroretinal tissue was gently brushed away under a microscope, and the vasculature was stained with periodic acid Schiff–hematoxylin to count the acellular capillaries by light microscopy, and imaged using the Zeiss ApoTome microscope using a 40× objective.3,20
Vascular Permeability and Optical Coherence Tomography
Micron IV (Phoenix Research Labs, Pleasanton, CA, USA) retinal imaging system was used to quantify retinal thickness. After anesthetizing mice with Ketamine-Xylazine, their pupils were dilated using 1% tropicamide ophthalmic solution, and the cornea was lubricated with Goniovisc. After photographing the fundus using fundus camera for small animals, the animals were injected with AK-FLUOR (0.5% solution, 0.01 mL/g BW, intraperitoneally), and their fundus was photographed 10 minutes after fluorescein injection using a barrier filter for fluorescein angiography.20 The images were converted into 8 bit using imageJ 1.53a and the threshold was set equally in each group of mice with the setting adjusted to dark background. Zeiss imaging software analysis module was used to mark the vessels “Red,” and the leakage was quantified by region of interest in the visible white area.27 The value obtained from normal mice was considered as 100%.
For optical coherence tomography (OCT), after lubricating the pupils with Goniovisc, the eyes were positioned in front of the OCT camera to obtain high-resolution b-scan of the retinal cross-sections, as described previously.20 Thickness of total retinal layers, ganglion cell layer + inner plexiform layer (GCL + IPL) and of inner nuclear layer (INL) was measured at 200 to 400 µm distance on either side of the optic disc using the caliper tool in the InSight software.
Immunofluorescence Staining
Retinal cryosections (8 µm) were incubated with homocysteine or MMP-9 antibodies (Cat. No. ab15154 and Cat. No. ab119906, respectively; Abcam, Cambridge, MA, USA; each at 1:200 dilution). Fluorescence labeled secondary antibodies included DyLight-488 labeled (green) for homocysteine and Texas Red (red) for MMP-9 (Cat. Nos. DI-1488 and TI200, respectively, Vector Laboratories, Burlingame, CA, USA; each at 1:500 dilution). The sections mounted with DAPI (blue) containing medium were photographed by ZEISS ApoTome fluorescence microscope using a 20× objective.20
Reactive Oxygen Species
Total ROS were quantified using 2′,7′-dichlorofluorescein diacetate (DCFDA; Cat. No. D6883; Sigma-Aldrich) method in cell lysate or retinal microvessel (5 µg protein).21 Percentage change was calculated considering the values obtained from cells in normal mice, or normal glucose, as 100%.
Mitochondrial ROS were quantified using MitoSOX Red (Cat. No. M36008; Thermo Fisher Scientific, Waltham, MA, USA). Briefly, the nonadherent cells were removed by washing with DMEM, and incubated with 5 µM MitoSOX red for 20 minutes at 37°C.24 The fluorescence was visualized using Zeiss ApoTome fluorescence microscope using a 20× objective.
Protein Carbonyls
Protein carbonyls were quantified in 15 to 25 µg protein using 2,4-dinitrophenylhydrazine derivatization, according to the manufacturer's instructions (Protein Carbonyl Assay Kit, Cat. No. ab126287; Abcam, Cambridge, MA, USA). Protein carbonyls concentration was calculated using BSA as a standard.28
MMP-9 Activity
Enzyme activity was quantified by fluorescence kit using a specific anti-MMP-9 monoclonal antibody and a fluorogenic substrate (SensoLyte Plus 520 MMP-9 Assay Kit; AnaSpec, Inc., Fremont, CA, USA), as described previously.29 MMP-9 induced cleavage of the fluorogenic peptide was measured at 490 nm excitation and 520 nm emission wavelengths.
Gene Transcripts
RNA extracted by TRIZOL (Invitrogen, Carlsbad, CA, USA) was used to prepare cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR (qRT-PCR) was performed using SYBR green master mix (Applied Biosystems) and the gene- and species-specific primers (Table 2). Specific products were confirmed by SYBR green single melt curve analysis.21,23 Housekeeping genes included 18S rRNA for mice and β-actin for HRECs. Relative fold change was calculated using the delta-delta Ct method.
Table 2.
Primer Sequences
| Primer | Size (bp) | Sequence |
|---|---|---|
| Mouse | ||
| mtDNA-short | 117 | Fwd-CTA GCA GAA ACA AAC CGG GCRev-CCG GCT GCG TAT TCT ACG TT |
| mtDNA-long | 10,090 | Fwd-GCC AGC CTG ACC CAT AGC CAT AAT ATRev- GAG AGA TTT TAT GGG TGT AAT GCG G |
| Cytb | 75 | Fwd-AGA CAA AGC CAC CTT GAC CCG ATRev-ACG ATT GCT AGG GCC GCG AT |
| ND6 | 173 | Fwd-CCC AGC TAC TAC CAT CAT TCA AGTRev-GAT GGT TTG GGA GAT TGG TTG ATG T |
| 18S | 149 | Fwd-GCC CTG TAA TTG GAA TGA GTC CAC TTRev-CTC CCC AAG ATC CAA CTA CGA GCT TT |
| Human | ||
| mtDNA-short | 223 | Fwd-CCC CAC AAA CCC CAT TAC TAA ACC CARev-TTT CAT CAT GCG GAG ATG TTG GAT GG |
| mtDNA-long | 8,842 | Fwd-TCT AAG CCT CCT TAT TCG AGC CGARev-TTT CAT CAT GCG GAG ATG TTG GAT GG |
| Cyt b | 138 | Fwd-TCA CCA GAC GCC TCA ACC GCRev-GCC TCG CCC GAT GTG TAG GA |
| β-Actin | 237 | Fwd-AGC CTC GCC TTT GCC GAT CCGRev-TCT CTT GCT CTG GGC CTC GTC G |
Mitochondrial DNA Damage
Damage of mitochondrial DNA (mtDNA) was quantified by extended length PCR, as detailed previously.30 In brief, long and short mtDNA regions (10.0 kb and 117 bp for mice and 8.8 kb and 223 bp for HRECs) were amplified using semiquantitative PCR, and the amplified products were resolved on an agarose gel. Relative amplification was quantified by normalizing the intensity of the long product to the short product; the degree of the damage was inversely proportional to the ratio.
Mitochondrial Membrane Potential
Changes of mitochondrial membrane potential were measured by staining cells with a mitochondrial binding dye, JC-1 (Cat. No. MP03168;, Molecular Probes, Carlsbad, CA, USA). As reported previously,31 the cells were washed with PBS and incubated with DMEM containing 5 µM JC-1 for 30 minutes at 37°C. Cells were again washed with PBS, and visualized under Zeiss ApoTome fluorescence microscope at 20 times magnification.
Cell Apoptosis
Cell death was measured by a photometric enzyme immunoassay using the Cell Death Detection ELISA PLUS kit from Roche Diagnostics (Cat. No. 11774425001; Indianapolis, IN, USA), as described previously.23 The values obtained from cells in normal glucose were considered as 100%.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). SigmaStat software (Systat, San Jose, CA, USA) was used to perform statistical analyses, and comparison between groups was made using 1-way ANOVA followed by a post hoc Bonferroni test; P < 0.05 was considered significant.
Results
Diabetic Mice
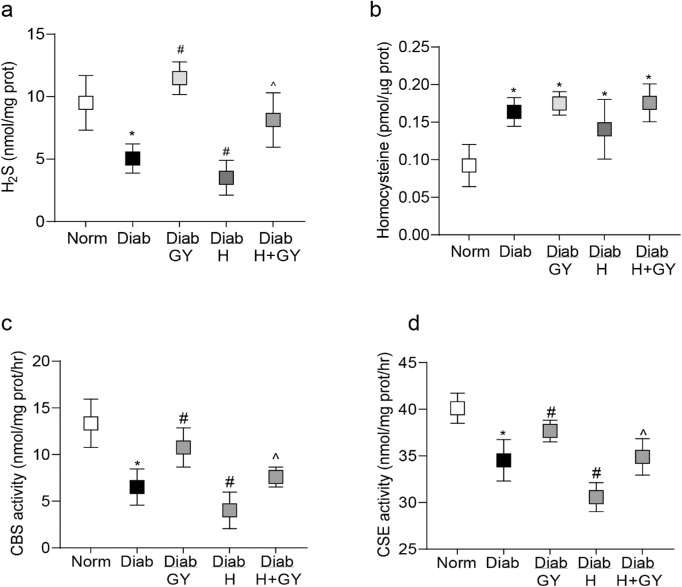
In accordance with the results from human disease samples,16 retina from diabetic mice had approximately 45% less H2S and approximately 2 fold higher homocysteine compared to their age-matched normal mice (both, P < 0.05 versus normal group; Figs. 1a, 1b). Because both CBS and CSE are involved in the transsulfuration process to produce H2S,12,13 their enzyme activities were quantified. As shown in Figures 1c, 1d, both CBS and CSE were decreased significantly in the retina from diabetic mice. Administration of GY soon after induction of diabetes (Diab/GY group), although failed to attenuate increase in retinal homocysteine, it ameliorated decrease in H2S content and the activities of CBS and CSE. H2S levels and the enzyme activities in diabetic mice receiving GY were significantly higher compared to diabetic mice without GY supplementation (P < 0.05 versus Diab group; Figs. 1a–d).
Figure 1.
Effect of hydrogen sulfide donor on diabetes-induced alterations in H2S and its enzymatic machinery. Retina from diabetic mice, with or without homocysteine and GY supplementation, were analyzed for (a) H2S and (b) homocysteine levels using their respective colorimetric methods. (c) CBS activity was quantified using cysteine and homocysteine as substrates, and (d) CSE activity using L-cysteine as a substrate and pyridoxal-5′-phosphate as a cofactor. Each measurement was made in duplicate in 5 to 7 mice/group, and the values are represented as mean ± SD. Norm, normal; Diab and Diab/GY, diabetic mice without or with GY respectively; Diab/H and Diab/H + GY, homocysteine supplemented diabetic mice, without or with GY. *P < 0.05 versus normal, #P < 0.05 versus diabetes, and ^P < 0.05 versus diabetic mice receiving homocysteine.
The effect of restoration of H2S on the development of diabetic retinopathy was determined by quantifying the numbers of degenerative capillaries and pericyte ghosts in the trypsin digested retina. As shown in Figure 2a, diabetes-induced increase in the number of degenerative capillaries and pericyte ghosts was prevented in Diab/GY group, and the values from mice in the Norm and Diab/GY groups were not different from each other (P > 0.05 versus normal and P < 0.05 versus Diab group).
Figure 2.
Hydrogen sulfide donor and retinal damage in diabetic mice. Representative images from 5 to 7 mice/group showing (a) trypsin-digested retinal microvessels, stained with PAS showing acellular capillaries (thin arrow) and pericyte ghosts (thick arrowhead); (b) fluorescein angiography. The insert shows vascular leakage, and the histogram represents permeability intensity (c) OCT by the OCT module of Micron IV showing thickness of the total retinal layers and GCL + IPL at 200 to 400 µm away from the optic disc using the caliper tool in the InSight software; the histograms represent mean ± SD of the values from those mice at 300 µm. *P < 0.05 and #P < 0.05 compared to normal and diabetes, respectively.
The effect of GY administration on vascular integrity was further confirmed by fluorescence angiography. Compared with normal control mice, diabetic mice had significant leakage of fluorescein in the retinal vasculature 10 minutes after its administration. However, GY administration significantly attenuated vascular permeability in diabetic mice (Fig. 2b). Fundus OCT data from the same mice receiving GY showed amelioration of diabetes-induced thinning of the retina and of GCL + IPL (P < 0.05 versus Diab group; Fig. 2c), but compared with normal mice, thickness of INL was not changed observed in diabetic mice, and GY had no effect on this layer.
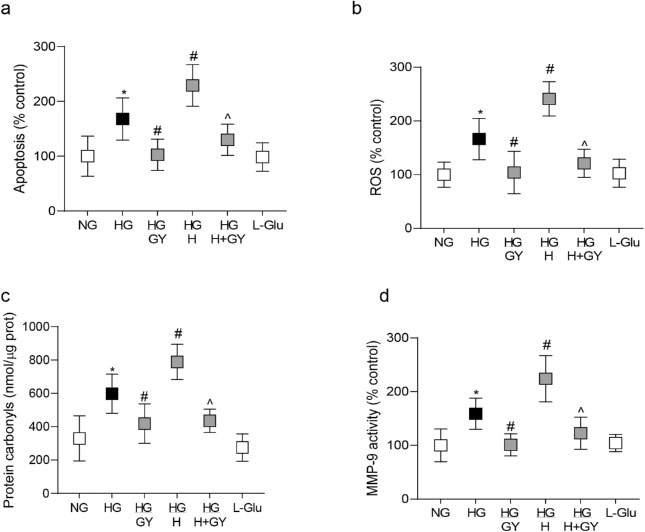
H2S is considered to act as an antioxidant13,14; the effect of GY on oxidative stress was determined in retinal microvasculature. GY administration ameliorated diabetes-induced increase in ROS and protein carbonyls; the values in Diab/GY group were significantly lower than those in the Diab group (P < 0.05 versus Diab group; Figs. 3a, 3b). Increase in ROS is shown to contribute to the activation of redox-sensitive MMP-98,32; GY administration ameliorated MMP-9 activation (P < 0.05 versus Diab group), and the co-localization of MMP-9 and homocysteine in retinal microvasculature was also decreased (Figs. 3c, 3d).
Figure 3.
GY supplementation and oxidative stress and MMP-9 activation. Mice retinal microvessels prepared by osmotic shock were analyzed for oxidative stress by quantifying (a) ROS levels using DCFDA and (b) protein carbonyls employing 2,4-dinitrophenylhydrazine. (c) MMP-9 activity was measured using its monoclonal antibody and a fluorogenic substrate. (d) Representative image of the co-localization of homocysteine and MMP-9 using DyLight-488 (green) and Texas Red (red) conjugated secondary antibodies for homocysteine and MMP-9, respectively. Each measurement was made in duplicate in 6 to 7 mice/group, and the values are represented as mean ± SD. Norm, normal; Diab and Diab/GY, diabetic mice without or with GY respectively; Diab/H and Diab/H + GY, homocysteine supplemented diabetic mice, without or with GY. *P < 0.05 versus normal, #P < 0.05 versus diabetes, and ^P < 0.05 versus diabetic mice receiving homocysteine.
Activation of MMP-9 is implicated in the mitochondrial damage3,33; the effect of GY on retinal mitochondrial damage was investigated. Compared to diabetic mice, Gy receiving diabetic mice had significantly lower ratio of amplification of 10 kb/117 bp amplicons (P < 0.05 versus Diab group; Fig. 4a), and higher gene transcripts of Cytb and ND6 (Figs. 4b, 4c).
Figure 4.
Mitochondrial damage and GY supplementation. Mouse retinal microvessels were employed to quantify (a) mtDNA damage by extended length PCR by quantifying relative amplification of 10 kb and 117 bp products; lower ratio represents more damage. The gene transcripts of (b) Cytb) and (c) ND6 were quantified by real time qRT-PCR using 18sRNA as a housekeeping gene. Values are represented as mean ± SD from six mice/group, with each measurement made in duplicate. *P < 0.05 versus normal and #P < 0.05 vs. diabetes.
Many patients with diabetes also have high homocysteine levels, and hyperglycemia and homocysteine can produce synergistic detrimental effects on the vasculature.34 Effect of homocysteine supplementation on H2S levels was determined in a hyperglycemic milieu. Homocysteine supplementation, soon after induction of diabetes in mice, despite producing no additional increase in retinal homocysteine (P > 0.05 versus Diab group), further decreased H2S levels and reduced CBS and CSE activities; H2S levels and enzyme activities were 20 to 30% lower in mice in the Diab/H group compared to mice in the Diab group (P < 0.05 versus Diab; see Fig. 1). However, despite significant increase in oxidative stress and MMP-9 activity (P < 0.05 versus Diab group), supplemental homocysteine had no significant effect on the mitochondrial damage and retinal histopathology (P > 0.05 versus Diab grop; Figs. 23–4); the values from diabetic mice with, or without, homocysteine was similar, but were significantly different from those obtained from normal mice (P < 0.05 versus Normal group).
Mice receiving simultaneous administration of homocysteine and GY had significantly higher retinal H2S and CBS and CSE activities, compared to diabetic mice receiving homocysteine alone (P < 0.05 versus Diab + Homocysteine group; see Fig. 1), but GY administration failed to provide any beneficial effect on their retinal histopathology (acellular capillaries and pericyte ghosts) and oxidative stress-MMP-9-mitochondrial damage (Figs. 23–4).
Retinal Endothelial Cells
Compared to cells in normal glucose, high glucose decreased extracellular and intracellular H2S by approximately 50% (Figs. 5a, 5b), increased homocysteine levels by twofold and inhibited CBS and CSE activities (Figs. 5c–e). Addition of 150 µM GY in high glucose medium significantly ameliorated decrease in H2S, however, increasing GY concentration to 300 µM had no additional beneficial effect on H2S levels; H2S values from high glucose incubated cells with 150 µM GY or 300 µM GY were not significantly different from each other (P > 0.05). As a control, 15 µM GY failed to restore glucose-induced decrease in H2S levels (Fig. 5f); subsequent experiments utilized 150 µM GY. Values obtained from cells incubated in 20 mM L-glucose, instead of 20 mM D-glucose, were significantly higher (P < 0.05), and were similar to those obtained from cells in normal glucose. HRECs incubated in high glucose, with or without 150 µM GY, had similar morphology as seen in the cells incubated in normal glucose (Fig. 5g), suggesting that GY supplementation did not damage the cell phenotype.
Figure 5.
Hydrogen sulfide donor and glucose-induced alterations in H2S and its enzymatic machinery in retinal endothelial cells. HRECs, incubated in high glucose, in the absence or presence of homocysteine, and without or with GY for 96 hours were analyzed for (a) extracellular H2S level in their culture medium using NaHS as a standard, and (b) endogenous H2S by in situ fluorescent microscopy using azido-4-methylcoumarin fluorescence probe. The accompanying histogram represents mean ± SD from three different experiments, with each measurement performed in six or more cells. (c) Homocysteine levels were quantified using an ELISA kit, and (d, e) the activities of CBS and CSE were measured using spectrophotometric methods. Each measurement was made in duplicate or triplicate in four to five different cell preparations. (f) Concentration curve of GY on extracellular H2S in the culture medium of HRECs incubated in high glucose. (g) HRECs incubated with 150 µM GY visualized under an Olympus BX50 microscope and imaged at 10 times magnification. NG, cells in 5 mM glucose; HG and HG/GY, cells in 20 mM glucose without and with GY, respectively; HG/H and HG/H + GY, cells in high glucose + homocysteine, in the absence and presence of GY, respectively; L-Glu, cells in 20 mM L-glucose. *P < 0.05 versus NG; #P < 0.05 versus HG, and ^P < 0.05 versus HG/H.
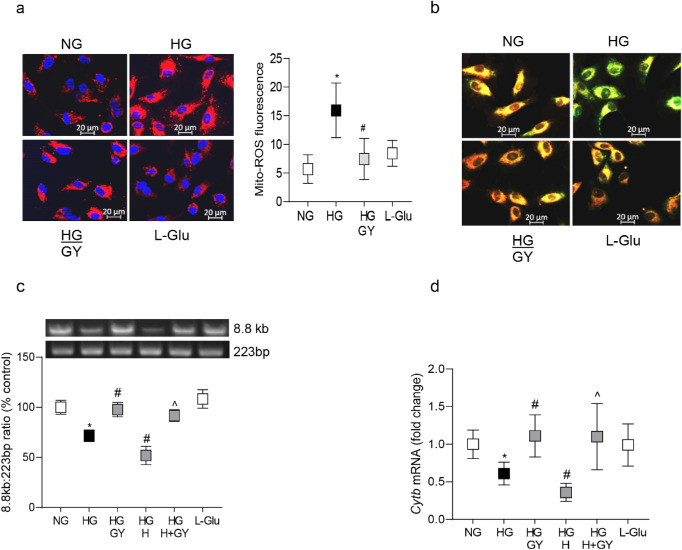
Consistent with H2S, GY supplementation also attenuated increase in cell apoptosis (Fig. 6a), oxidative stress (Figs. 6b, 6c) and MMP-9 activation (Fig. 6d), and prevented increase in mitochondrial ROS and membrane permeability (Figs. 7a, 7b) and mtDNA damage and transcription of Cytb (Figs. 7c, 7d). Compared to the HG group, while cell apoptosis, oxidative stress (protein carbonyls and ROS), MMP-9 activity, and mitochondrial damage (permeability and mtDNA damage) were significantly reduced in the HG/GY group, transcripts of Cytb were increased. Values from cells in 20 mM L-glucose were similar to those in normal glucose.
Figure 6.
Hydrogen sulfide donor and glucose-induced cell apoptosis-oxidative stress and MMP-9 activation. HRECs were analyzed for (a) cell apoptosis using an ELISA method, (b) total ROS by DCFDA method, (c) protein carbonyls by employing 2,4-dinitrophenylhydrazine, and (d) MMP-9 activity using its fluorogenic substrate. Values obtained from cells in normal glucose are considered as 100%, and are represented as mean ± SD from three different cell preparations, with each measurement performed in duplicate. *P < 0.05 versus NG; #P < 0.05 versus HG, and ^P < 0.05 versus HG/H.
Figure 7.
Glucose-induced mitochondrial damage and H2S donor. (a) Mitochondrial ROS were quantified by MitoSOX Red and imaged at 20 times magnification using Apotome microscope, and the histogram represent the fluorescence intensity quantified by ImageJ, from 5 to 8 images/group. (b) Mitochondrial membrane potential was determined by cationic dye JC-1, and while green fluorescence represents the depolarized (monomer) mitochondria, orange represents hyperpolarized (J aggregates) mitochondria. (c) Damage to the mtDNA was quantified by extended-length PCR using long mtDNA (8.8 kb) and short (223 bp) amplicons of the mtDNA. (d) Gene transcripts of Cytb were quantified by qRT-PCR using β-actin as the housekeeping gene. Each measurement was made in duplicate/triplicate in four to five different cell preparations, and the histograms represent values mean ± SD. NG, cells in 5 mM glucose; HG and HG/GY, cells in 20 mM glucose without and with GY, respectively; HG/H and HG/H + GY, cells in high glucose + homocysteine, in the absence and presence of, respectively; LGlu, cells in 20 mM L-glucose. *P < 0.05 versus NG; #P < 0.05 versus HG; and ^P < 0.05 versus HG/H.
Compared to glucose alone, cells in high glucose medium, supplemented with homocysteine (HG/H group), had significantly lower H2S and CBS and CSE activities (P < 0.05 versus HG; Fig. 5). Their cell death and oxidative stress-MMP-9-mitochondrial damage (membrane and DNA) were also worsened (Fig. 6, Fig. 7). Addition of GY in the high glucose medium containing homocysteine attenuated increase in homocysteine, and ameliorated decrease in H2S and CBS-CSE activities (Fig. 5). Their cell apoptosis and oxidative stress MMP-9 activation-mitochondrial damage were also attenuated (Fig. 6, Fig. 7); the values from cells in the HG/GY and the HG/H + GY groups were similar with each other, but were different from those obtained from cells without GY.
Discussion
Hydrogen sulfide is recognized as a crucial gasotransmitter, which exerts many biological actions in various tissues including anti-inflammation and vasoregulation. It is synthesized endogenously from L-cysteine, and its generation is closely associated with homocysteine metabolism.35,36 Our previous work has shown that human donors with diabetic retinopathy have significantly reduced retinal H2S levels and CBS activity, compared to their age-matched nondiabetic donors.16 H2S displays significant antioxidant properties, and is now also considered as a signaling molecule.15 Here, using both in vitro and in vivo models of diabetic retinopathy, we have provided exciting results showing that a slow-releasing H2S donor prevents the development of histopathology characteristic of diabetic retinopathy. We propose a possible mechanism that, by restoring H2S levels, increase in oxidative stress-activation of MMP-9 is ameliorated. This protects mitochondrial integrity, and prevents damage to the mtDNA and its transcription, protecting cells from undergoing accelerated apoptosis, and the development of diabetic retinopathy.
Normal plasma homocysteine levels range from 4 to 15 µmols/L, but 5% to 12% of the general population has mildly elevated levels (hyperhomocysteinemia). High homocysteine is considered as a risk factor for several vascular diseases, including heart disease, and is also implicated in retinal disorders, including retinal vein occlusion, endothelial cell dysfunction,37 and disruption of retinal pigment epithelium.38,39 Patients with diabetes generally present high plasma homocysteine levels, and the levels can go up to 50 to 100 µmols/L,11,40 and clinical studies have shown a strong correlation between hyperhomocysteinemia and diabetic retinopathy.40,41 In physiological conditions, homocysteine and H2S regulate each other,13 however, in patients with diabetes and animal models, plasma H2S levels are significantly lower.42,43 Our previous study has shown greater than threefold elevation in retinal homocysteine in human diabetic donors with documented retinopathy compared to without retinopathy, and approximately twofold decrease in H2S levels.16 Here, we show that retinal H2S levels are significantly lower in diabetic mice, and administration of a slow releasing H2S donor, in addition to preventing decrease in H2S, prevents the development of histopathology characteristic of diabetic retinopathy. In addition, GY also inhibits diabetes-induced retinal vascular leakage in these mice. In support, GY is shown to prevent retinal vascular leakage and abnormalities in retinal function in mice lacking CBS,44 and its intravitreal administration preserves ganglion cells from acute ischemic injury and optic nerve crush, and by dilating retinal vessels, improves their perfusion.45 In diabetes, GY protects accelerated atherosclerosis and inhibits inflammasome activation.46 Supplementation with H2S donor, sodium hydrosulfide (NaHS), in diabetic rat model improves retinal vascular permeability, neuronal dysfunction, and thickness,47 and in the rat glaucoma model, provides neuroprotection and attenuates ganglion cell apoptosis.48
Hydrogen sulfide is endogenously produced during homocysteine metabolism by CBS and CSE,13,49 and although both of these enzymes are expressed ubiquitously, CBS expression is mainly in the nervous system and CSE in the endothelial and vascular smooth muscle cells.50 Our results clearly document that diabetes inhibits both of these enzymes in the retinal vasculature, and GY prevents their inhibition. Consistent with these, GY is shown to upregulate CBS in cardiomyocytes,13 and upregulates CSE in mice lacking CBS.44
Hydrogen sulfide acts as an antioxidant; it can scavenge ROS directly, inhibit the biological activity of peroxynitrite, and enhance the levels of GSH.13,14 In diabetic retinopathy, oxidative stress mitochondrial damage plays a major role.5,51 The data presented here show that GY inhibits diabetes-induced increase in oxidative stress and mitochondrial damage. These results are supported by others showing that NaHS protects elevation of oxidative stress and mitochondrial damage in an in vitro model of Parkinson's disease.52 In the pathogenesis of diabetic retinopathy, activation of MMP-9 damages the mitochondria, and MMP-9 itself is an oxido-sensitive enzyme.8 Here, we show that GY also ameliorates activation of MMP-9 in retinal microvasculature. In support, treatment of mice lacking CBS with NaHS ameliorates MMP-9 activation and microvascular permeability in the brain, in part, by normalizing the MMP/TIMP ratio.18 Addition of homocysteine in a diabetic milieu is shown to have synergistic detrimental vascular effects, and our recent study has shown that supplementation with homocysteine in hyperglycemic conditions exacerbates increase in ROS and MMP-9 activation.23 Now, we show that hyperglycemia and homocysteine, by exacerbating inhibition of CBS and CSE, although further decrease H2S levels and increase oxidative stress-MMP-9 activation, do not aggravate mitochondrial damage, and the severity of retinopathy in diabetic mice with, or without, homocysteine is similar. This could be that the decrease in H2S, induced by supplemental homocysteine, might not be sufficient to further damage the mitochondria. Diabetic retinopathy is also considered as a low-grade inflammatory disease.53 H2S also affects inflammation, and GY is shown to inhibit inflammation-induced adipocyte dysfunction.54 The possibility that GY could be ameliorating the development of retinal histopathology via inhibiting diabetes-induced retinal inflammatory mediators cannot be ruled out. In addition, we recognize that the results presented here are from both male and female mice; the influence of sex hormones on any of the parameters investigated in this manuscript cannot be ruled out.
Consistent with our in vivo results, supplementation of high glucose with GY in HRECs prevents increase in homocysteine, and decrease in H2S and activities of CBS and CSE. Furthermore, GY also prevents increase in the oxidative stress-MMP-9 activation-mitochondrial damage, protecting endothelial cells from accelerated apoptosis, which, in animal models, is documented to precedes the development histopathology characteristic of diabetic retinopathy.2 Addition of both homocysteine and GY in high glucose medium, normalizes H2S levels, oxidative stress-MMP-9-mitochondrial damage, and prevents increase in cell apoptosis.
However, in contrast to our in vitro results, simultaneous supplementation of GY and homocysteine, soon after induction of diabetes in mice, despite increasing retinal H2S levels, fails to provide any beneficial effect on retinal histopathology, and the numbers of acellular capillaries and pericyte ghosts continue to be high. One reason for the failure of GY to produce any effect on the retinopathy could be that the amount of H2S failed to attain a threshold level to protect increase in oxidative stress-mitochondrial damage, and the damaged mitochondria continues to accelerate cell apoptosis. Our study has focused mainly on the retinal endothelial cells and the vasculature, similar detrimental effects of homocysteine on other retinal cell types that are also affected in diabetes, including Muller cells,55 cannot be ruled out.
The preferable ocular drug delivery routes are the topical or systemic delivery, however, a well-organized blood-retinal barrier makes it challenging for the dugs to reach to the posterior segment of the eye. Intravitreal administration is routinely preferred for retinal diseases, but this invasive technique carries some unwanted hidden long-term drawbacks.56 Although we did not perform any pharmacokinetics of GY in the eye, the results presented here clearly document that the systemic administration of GY (at 0.25 mg/Kg) has beneficial effect in preventing the development of histopathology characteristic of diabetic retinopathy, suggesting that this slow-releasing small molecule can cross the blood-retinal barrier, reaching to the posterior segment of the eye.
In summary, we have identified a novel regulatory mechanism involved in the pathogenesis of diabetic retinopathy, specifically at the level of regulation of H2S. The diabetic environment, by inhibiting CBS and CSE activities, increases homocysteine and reduces H2S. This contributes to increased ROS accumulation, ROS, via activating MMP-9, damage the mitochondria, and the damaged mitochondria, in turn, continues to produce ROS. Retinal capillary cell apoptosis is accelerated, ultimately leading to the development of retinopathy (Fig. 8). H2S donors ameliorate reduction in H2S levels and protect MMP-9-mitochondrial damage, and this prevents/retards the development of diabetic retinopathy. Our results raise a possibility of the use of H2S releasing/stimulating compounds for patients with diabetes to protect retinal damage, and prevent them from losing their vision from this debilitating disease. With recent technical advances, the field of H2S releasing compounds has made great progress,49,57 and the future for developing controllable H2S donors with different H2S releasing mechanisms looks optimistic for patients with diabetes.
Figure 8.

Schematic showing a possible mechanism by which decrease in H2S in diabetes could result in diabetic retinopathy.
Acknowledgments
The authors thank Gina Polsinelli for her help in maintaining the animal colony. The study was supported in part by grants from the National Institutes of Health (EY014370, EY017313, and EY022230), and from The Thomas Foundation to RAK, and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, Wayne State University.
Data availability: RAK is the guarantor of this work and, as such, has full access to all the data in the study. RAK takes responsibility for the integrity of the data and the accuracy of the data analyses.
Disclosure: G. Mohammad, None; R. Radhakrishnan, None; R.A. Kowluru, None
References
- 1. Mizutani M, Kern TS, Lorenzi M.. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996; 97: 2883–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kowluru RA, Chan PS.. Metabolic memory in diabetes - from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2010; 81: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kowluru RA, Mohammad G, dos Santos JM, Zhong Q.. Abrogation of mmp-9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011; 60: 3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 5. Kowluru RA, Mishra M.. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015; 1852: 2474–2483. [DOI] [PubMed] [Google Scholar]
- 6. Farina AR, Cappabianca L, DeSantis G, et al. . Thioredoxin stimulates mmp-9 expression, de-regulates the MMP-9/TIMP-1 equilibrium and promotes mmp-9 dependent invasion in human mda-mb-231 breast cancer cells. FEBS Lett. 2011; 585: 3328–3336. [DOI] [PubMed] [Google Scholar]
- 7. Mohammad G, Kowluru RA.. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 3832–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowluru RA, Shan Y.. Role of oxidative stress in epigenetic modification of mmp-9 promoter in the development of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mishra M, Flaga J, Kowluru RA.. Molecular mechanism of transcriptional regulation of matrix metalloproteinase-9 in diabetic retinopathy. J Cell Physiol. 2016; 231: 1709–1718. [DOI] [PubMed] [Google Scholar]
- 10. Dong N, Shi H, Tang X.. Plasma homocysteine levels are associated with macular thickness in type 2 diabetes without diabetic macular edema. Int Ophthalmol. 2018; 38: 737–746. [DOI] [PubMed] [Google Scholar]
- 11. Tawfik A, Mohamed R, Elsherbiny NM, DeAngelis MM, Bartoli M, Al-Shabrawey M. Homocysteine: a potential biomarker for diabetic retinopathy. J Clin Med. 2019; 8: pi: E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber GJ, Pushpakumar S, Tyagi SC, Sen U.. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res. 2016; 113: 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nandi SS, Mishra PK.. H2s and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci Rep. 2017; 7: 3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karmin O, Siow YL.. Metabolic imbalance of homocysteine and hydrogen sulfide in kidney disease. Curr Med Chem. 2018; 25: 367–377. [DOI] [PubMed] [Google Scholar]
- 15. Kimura H, Shibuya N, Kimura Y.. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antiox Redox Signal. 2012; 17: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kowluru RA, Mohammad G, Sahajpal N.. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis (Lond). 2020; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ.. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008; 295: H801–H806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tyagi N, Moshal KS, Sen U, et al. . H2s protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009; 11: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pushpakumar S, Kundu S, Sen U.. Endothelial dysfunction: the link between homocysteine and hydrogen sulfide. Curr Med Chem. 2014; 21: 3662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mishra M, Duraisamy AJ, Kowluru RA.. Sirt1 – a guardian of the development of diabetic retinopathy. Diabetes. 2018; 67: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duraisamy AJ, Mishra M, Kowluru A, Kowluru RA.. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018; 59: 4831–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. John AMSP, Kundu S, Pushpakumar S, et al. . Gyy4137, a hydrogen sulfide donor modulates mir194-dependent collagen realignment in diabetic kidney. Sci Rep. 2017; 7: 10924–10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohammad G, Kowluru RA.. Homocysteine disrupts balance between mmp-9 and its tissue inhibitor in diabetic retinopathy: the role of DNA methylation. Int J Mol Sci. 2020; 21(5): 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duraisamy AJ, Mohammad G, Kowluru RA.. Mitochondrial fusion and maintenance of mitochondrial homeostasis in diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis. 2019; 1865: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 25. Hammers MD, Taormina MJ, Cerda MM, et al. . A bright fluorescent probe for H2s enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J Am Chem Soc. 2015; 137: 10216–10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin S, Teng X, Xiao L, et al. . Hydrogen sulfide ameliorated l-name-induced hypertensive heart disease by the akt/enos/no pathway. Exp Biol Med (Maywood, N.J.). 2017; 242: 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lückoff A, Caramoy A, Scholz R, Prinz M, Kalinke U, Langmann T.. Interferon-beta signaling in retinal mononuclear phagocytes attenuates pathological neovascularization. EMBO Mol Med. 2016; 8: 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohammad G, Alam K, Nawaz MI, Siddiquei MM, Mousa A, Abu El-Asrar AM. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem. 2015; 71: 359–372. [DOI] [PubMed] [Google Scholar]
- 29. Duraisamy AJ, Mishra M, Kowluru RA.. Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Invest Ophthalmol Vis Sci. 2017; 58: 6440–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA.. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010; 13: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohammad G, Kowluru RA.. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010; 90: 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH.. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic Biol Med. 2008; 44: 635–645. [DOI] [PubMed] [Google Scholar]
- 33. Mohammad G, Kowluru RA.. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinase-9. J Cell Physiol. 2012; 227: 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brazionis L, Rowley K Sr, Itsiopoulos C, Harper CA, O'Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008; 31: 50–56. [DOI] [PubMed] [Google Scholar]
- 35. Tyagi N, Mishra PK, Tyagi SC.. Homocysteine, hydrogen sulfide (h2s) and nmda-receptor in heart failure. Indian J Biochem Biophys. 2009; 46: 441–446. [PubMed] [Google Scholar]
- 36. Beard RS Jr, Bearden SE. Vascular complications of cystathionine beta-synthase deficiency: future directions for homocysteine-to-hydrogen sulfide research. Am J Physiol Heart Circ Physiol. 2011; 300: H13–H26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kundi H, Kiziltunc E, Ates I, et al. . Association between plasma homocysteine levels and end-organ damage in newly diagnosed type 2 diabetes mellitus patients. Endocr Res. 2017; 42: 36–41. [DOI] [PubMed] [Google Scholar]
- 38. Tsen CM, Hsieh CC, Yen CH, Lau YT.. Homocysteine altered ROS generation and no accumulation in endothelial cells. Chin J Physiol. 2003; 46: 129–136. [PubMed] [Google Scholar]
- 39. Ibrahim AS, Mander S, Hussein KA, et al. . Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget. 2016; 7: 8532–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Malaguarnera G, Gagliano C, Giordano M, et al. . Homocysteine serum levels in diabetic patients with non proliferative, proliferative and without retinopathy. Biomed Res Int. 2014; 2014: 191497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J.. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014; 9: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whiteman M, Gooding KM, Whatmore JL, et al. . Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010; 53: 1722–1726. [DOI] [PubMed] [Google Scholar]
- 43. Jain SK, Bull R, Rains JL, et al. . Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010; 12: 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. George AK, Homme RP, Majumder A, et al. . Hydrogen sulfide intervention in cystathionine-beta-synthase mutant mouse helps restore ocular homeostasis. Int J Ophthalmol. 2019; 12: 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu H, Anders F, Thanos S, et al. . Hydrogen sulfide protects retinal ganglion cells against glaucomatous injury in vitro and in vivo. Invest Ophthalmol Vis Sci. 2017; 58: 5129–5141. [DOI] [PubMed] [Google Scholar]
- 46. Zheng Q, Pan L, Ji Y.. H 2s protects against diabetes-accelerated atherosclerosis by preventing the activation of nlrp3 inflammasome. J Biomed Res. 2019; 34: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J.. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol. 2013; 169: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang S, Huang P, Lin Z, et al. . Hydrogen sulfide supplement attenuates the apoptosis of retinal ganglion cells in experimental glaucoma. Exp Eye Res. 2018; 168: 33–48. [DOI] [PubMed] [Google Scholar]
- 49. Powell CR, Dillon KM, Matson JB.. A review of hydrogen sulfide (h2s) donors: chemistry and potential therapeutic applications. Biochem Pharmacol. 2018; 149: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang S, Li H, Ge J.. A cardioprotective insight of the cystathionine γ-lyase/hydrogen sulfide pathway. Int J Cardiol. 2015; 7: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kowluru RA. Mitochondrial stability in diabetic retinopathy: lessons learned from epigenetics. Diabetes. 2019; 68: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tabassum R, Jeong NY.. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int J Med Sci. 2019; 16: 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007; 2007: 95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Comas F, Latorre J, Cussó O, et al. . Hydrogen sulfide impacts on inflammation-induced adipocyte dysfunction. Food Chem Tox. 2019; 131: 110543. [DOI] [PubMed] [Google Scholar]
- 55. Markand S, Tawfik A, Ha Y, et al. . Cystathionine beta synthase expression in mouse retina. Curr Eye Res 2013; 38: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nayak K, Misra M.. A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacotherapy. 2018; 107: 1564–1582. [DOI] [PubMed] [Google Scholar]
- 57. Citi V, Martelli A, Bucci M, et al. . Searching for novel hydrogen sulfide donors: the vascular effects of two thiourea derivatives. Pharmacol Res. 2020; 159: 105039. [DOI] [PubMed] [Google Scholar]