Abstract
Retinoblastoma management remains complex, requiring individualized treatment based on International Classification of Retinoblastoma (ICRB) staging, germline mutation status, family psychosocial factors and cultural beliefs, and available institutional resources. For this 2020 retinoblastoma review, PubMed was searched for articles dated as early as 1931, with an emphasis on articles from 1990 to the present day, using keywords of retinoblastoma, chemotherapy, intravenous chemotherapy, chemoreduction, intra-arterial chemotherapy, ophthalmic artery chemosurgery, intravitreal chemotherapy, intracameral chemotherapy, cryotherapy, transpupillary thermotherapy, laser, radiation, external beam radiotherapy, plaque radiotherapy, brachytherapy, and enucleation. We discuss current treatment modalities as used in the year 2020, including intravenous chemotherapy (IVC), intra-arterial chemotherapy (IAC), intravitreal chemotherapy (IvitC), intracameral chemotherapy (IcamC), consolidation therapies (cryotherapy and transpupillary thermotherapy [TTT]), radiation-based therapies (external beam radiotherapy [EBRT] and plaque radiotherapy), and enucleation. Additionally, we present a consensus treatment algorithm based on the agreement of three North American retinoblastoma treatment centers, and encourage further collaboration amongst the world's most expert retinoblastoma treatment centers in order to develop consensus management plans and continue advancement in the identification and treatment of this childhood cancer.
Keywords: Algorithm, eye, oncology, pediatric, retinoblastoma, treatment
Retinoblastoma, the most common ocular malignancy in childhood, is lethal if left untreated. In high-income countries (HICs), retinoblastoma is considered a curable cancer with a near 100% disease-free survival rate.[1] However, the prognosis in low-and-middle-income countries (LMICs) is often somber, where more than 80% of global cases occur.[2,3] Predictions indicate that most retinoblastoma cases arise in Asia (53%), followed by Africa (29%), Latin America (8%), North America (3%), and Europe (6%).[4] Given this distribution, global retinoblastoma patient survival is calculated to be <30%.[5,6,7] This contrast is supported by published data from developing countries, where survival is reported to be 40% (23-70%) in low-income countries and 79% (54-93%) in upper-middle-income countries.[8] With regards to advanced retinoblastoma, enucleation has historically been the standard of care, especially in LMICs.[9] However, over the last three decades, major centers have decreased their enucleation rates in favor of globe-salvaging techniques.[9,10,11,12]
Management of retinoblastoma remains in constant evolution and treatment can vary among different centers worldwide. However, the same primary goals of protecting life and preventing metastatic disease, followed by globe preservation, and finally optimization of vision are commonly shared among retinoblastoma specialists. The currently used therapies maintain excellent survival rates when disease is identified in the localized intraocular stage, while newer therapies have been focusing on additional improvement in globe preservation and providing the best possible visual acuity outcome. The refinement of these curative strategies has led to unprecedented cure rates and globe salvage in centers where a complete armamentarium of treatment options is available.
Herein we present a comprehensive review of the current treatment modalities for retinoblastoma, along with their suggested indications and most common toxicities. PubMed was searched for articles dating back to 1931, with particular emphasis on articles published from 1990 to present day 2020. Keywords searched included retinoblastoma, chemotherapy, intravenous chemotherapy, chemoreduction, intra-arterial chemotherapy, ophthalmic artery chemosurgery, intravitreal chemotherapy, intracameral chemotherapy, cryotherapy, transpupillary thermotherapy, laser, radiation, external beam radiotherapy, plaque radiotherapy, brachytherapy and enucleation. The authors collate the current available literature and present a treatment algorithm for intraocular retinoblastoma based on the expert consensus between three different retinoblastoma centers in North America, designed to provide referring physicians with a concise guideline for decision making, thus shortening referral times. This model is intended for use by the retinoblastoma multidisciplinary team as a means to guide and organize resources.
Pretreatment Protocol
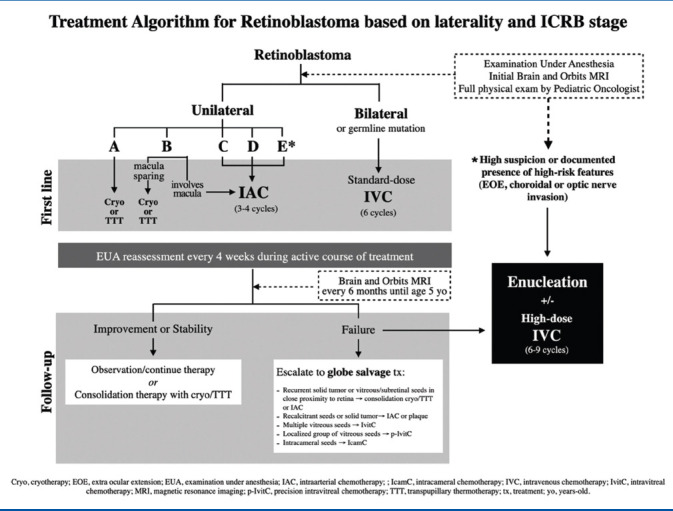
The treatment of choice for retinoblastoma depends largely on the International Classification of Retinoblastoma (ICRB) staging [Table 1], the presence or absence of extraocular clinical factors, germline testing results, the family psychosocial situation, and available institutional resources.[13] An in-depth initial evaluation of the disease is important in order to decide the extent of the desired treatment and avoid unnecessary side effects. Even before examining the patient, a complete history is of upmost importance. For example, a positive family history should raise suspicion for a germline mutation and might require the child to undergo systemic chemotherapy to prevent pineoblastoma, even if the disease presents unilaterally. Genetic testing is advisable in all cases of retinoblastoma, both for the patient and for the rest of his/her nuclear family if germline disease is confirmed. All patients should undergo a baseline high-resolution simple and contrast-enhanced magnetic resonance imaging (MRI) of the brain and orbits with careful attention for pineoblastoma or any features of optic nerve invasion. Typically, complete blood count, urine sample, and general physical examination are performed by the pediatric oncologist. The first office visit is usually complemented with a careful examination under anesthesia, where ICRB staging is confirmed and the first treatment can be applied.
Table 1.
Modern treatment of retinoblastoma: A 2020 Review. International classification of retinoblastoma (ICRB)
| Group | Mnemonic | Features |
|---|---|---|
| A | Small tumor | Retinoblastoma ≤3 mm in basal diameter or thickness |
| B | Bigger tumor Beside the macula or optic nerve | Retinoblastoma >3 mm in basal diameter or thickness OR tumor location ≤3 mm from foveola tumor location ≤1.5 mm from optic disc tumor-associated subretinal fluid ≤3 mm from tumor margin |
| C | Contiguous seeds | Retinoblastoma with subretinal seeds ≤3 mm from tumor vitreous seeds ≤3 mm from tumor subretinal and vitreous seeds ≤3 mm from tumor |
| D | Diffuse seeds | Retinoblastoma with subretinal seeds >3 mm from tumor vitreous seeds >3 mm from tumor subretinal and vitreous seeds >3 mm from tumor |
| E | Extensive tumor | Retinoblastoma occupying >50% of the globe OR neovascular glaucoma opaque media from hemorrhage in subretinal space, vitreous, or anterior chamber invasion of postlaminar optic nerve, choroid (>2 mm), sclera, orbit, anterior chamber |
The response to the first treatment can guide long-term outcomes. Hence, this might be the most important decision made by the ocular oncologist, with the goal of delivering a potent therapy with the needed strength while avoiding unnecessary toxicity. A simplified consensus of three retinoblastoma centers on treatment protocol based on ICRB staging and laterality is presented in Table 2. The specific treatment modalities are discussed in detail below.
Table 2.
Modern treatment of retinoblastoma: A 2020 Review. Treatment algorithm for retinoblastoma based on laterality and International Classification of Retinoblastoma (ICRB) stage
Intravenous Chemotherapy (IVC)
Introduced in the early 1990s, systemic IVC remains an essential tool for retinoblastoma treatment. IVC usually consists of 2, 3 or 4 chemotherapeutic agents administered monthly through a central or peripheral catheter for a total of 6-9 consecutive cycles.[14] The most frequently used regimen consists of three drugs, including vincristine, etoposide, and carboplatin (VEC).1413 In Monterrey, Mexico, vincristine is sometimes replaced with cyclophosphamide by the pediatric oncologist when concern for neurotoxicity is present, however, the former is more likely to induce myelosuppression and hemorrhagic cystitis.[15] Given the reduction in tumor size, IVC is sometimes referred to as 'chemoreduction'.[14] Focal consolidation with thermotherapy (cryotherapy or transpupillary thermotherapy) often aids in tumor control. Cryotherapy administration immediately preceding chemotherapy has been reported to enhance drug availability to the intraocular spaces when administered within 48 hours of the thermal disruption.[16]
Current indications for IVC include patients with bilateral disease [Fig. 1], confirmed germline mutation, family history of retinoblastoma, or cases with suspected optic nerve or choroidal invasion.[14] Additionally, IVC plays a protective role in the prevention of long-term second cancers, metastases, and pineoblastoma.[17,18,19] Other indications for IVC include patients weighing less than 6 kg awaiting intra-arterial chemotherapy (IAC), referred to as 'bridge therapy'.[20] While in some centers IVC might still be employed for unilateral retinoblastoma, a study of 91 patients demonstrated superiority of IAC compared with IVC for globe salvage, including superior control for solid tumor, sub retinal and vitreous seeding.[21] Hence, the authors prefer IAC over IVC for unilateral retinoblastoma.
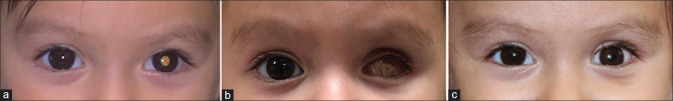
Figure 1.
Modern treatment of retinoblastoma. The role of intravenous chemotherapy (IVC) in bilateral disease. A 4-month-old patient was diagnosed with a (a) Group B retinoblastoma in the right eye, and was treated with 6 cycles of standard-dose IVC, (b) achieving a complete regression of the tumor. Consolidation therapy with TTT was required during the course of the treatment, leaving flat scars (black arrows) and completely regressed tumors. The (c) left eye was diagnosed with Group D retinoblastoma, regressing to a (d) smaller calcified scar in the macular region after treatment
As with most systemic chemotherapies, transient alopecia, cytopenia, and fever can occur.[11] However, systemic toxicity from IVC for retinoblastoma is usually mild. While transfusion of blood components might be occasionally required, granulocyte colony-stimulating factor is generally not required with standard VEC doses, but is advisable with cyclophosphamide. Patients receive routine prophylaxis for Pneumocystis jirovecii pneumonia. Chemotherapy-related nausea, emesis, and constipation can be medically managed. Ophthalmic toxicities from IVC have not been observed. Long-term renal toxicity is rare when chemotherapeutic agents are appropriately dosed. Infertility rarely occurs with recommended doses of IVC, however, the addition of melphalan can risk infertility in males, especially when a cumulative dose of 140 mg/m2 is reached. Secondary acute myelogenous leukemia following IVC for retinoblastoma is also rare, and has been associated with higher doses of chemotherapy, concomitant external beam radiotherapy (EBRT) and other predisposing conditions.[19,22] Long-term, real-world outcomes at 20 years in a large cohort of 964 eyes with retinoblastoma revealed lasting tumor control with avoidance of enucleation and/or EBRT for Group A (96%), Group B (91%), Group C (91%), Group D (71%), and Group E (32%).[23]
Following enucleation, a detailed analysis of the globe's histopathological features is of utmost importance. Histopathology reports provide useful information to the ocular oncologist that can guide the course of treatment. In the presence of high risk features, including post-laminar optic nerve invasion, massive choroidal invasion (>3 mm diameter), or extraocular extension, adjuvant IVC is required for prevention of metastases.[24,25,26] On the contrary, if the ocular pathologist reports absence of said features, enucleation alone could be curative and additional chemotherapy might not be necessary.
Intra-Arterial Chemotherapy (IAC)
In 1990, Akihiro Kaneko pioneered targeted chemotherapy of intraocular retinoblastoma.[4] Since then, this modality has earned a pivotal role in the modern treatment of retinoblastoma, especially for unilateral tumors.[27,28,29,30] IAC is a complex and usually costly procedure ideally performed in an angiography suite by an experienced neurosurgeon or interventional neuroradiologist, in which a microcatheter is guided by fluoroscopy to deliver chemotherapeutic agents supraselectively into the ophthalmic artery. Given the expense and specialized training required, IAC may not be a feasible option in developing countries.[31] Compared with IVC, IAC results in 10 times the chemotherapy dose delivered directly to the eye.[31,32] Chemotherapy generally consists of one, two, or three drugs, typically delivered once a month for a mean of three sessions.[14,31,33] ICRB stages B and C usually require no more than single-drug therapy with melphalan dosed at 5 mg.[34] However, more advanced disease with extensive vitreous or sub retinal seeding observed in ICRB stages D and E, or refractory tumors might require dose escalation or the addition of topotecan or carboplatin.[31] The latter (carboplatin) has been falling into disuse as a first-line drug due to high rates of ophthalmic toxicity but is still used in tandem therapy of the fellow eye, discussed later in this section, as an alternative to melphalan when the accumulative dose surpasses 0.4 mg/kg.[14]
Given the success of IAC for globe salvage in advanced cases and refractory tumors, this treatment modality has become more widely used over the past decade.[35,36,37,38] Main indications for IAC include both first-line and globe salvage therapies. IAC is employed as primary therapy for non-germline, unilateral, group B, C, D, or E retinoblastoma [Fig. 2] or as a secondary therapy for unilateral or bilateral advanced recalcitrant disease facing enucleation.[14,39,40] IAC is effective against sub retinal and vitreous seeds, especially when in close proximity to the retina.[41,31]
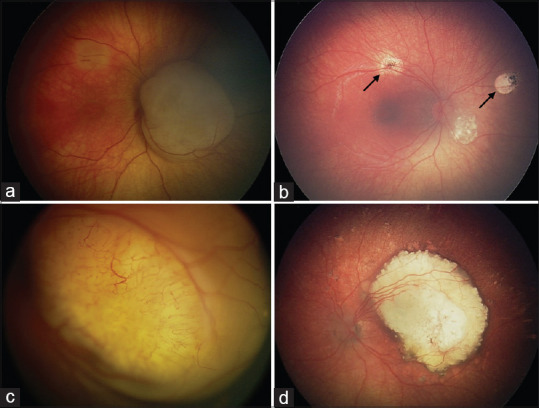
Figure 2.
Modern treatment of retinoblastoma. The role of intra-arterial chemotherapy (IAC) in unilateral disease. (a) Unilateral group B retinoblastoma with macular involvement. Following 4 cycles of IAC, (b) the majority of the macula had been spared without the need for additional consolidation therapies. (c) Unilateral group D retinoblastoma with macular involvement and serous retinal detachment. After 4 cycles of IAC, the retina completely reattached leaving a (d) smaller calcified macular scar and scattered calcified subretinal seeds
Other applications for IAC include tandem therapy for advanced bilateral cases, minimal exposure (<2 cycles) and rescue IAC for recurrence after previous IAC. Tandem therapy remains controversial due to the concern for increased vascular toxicity in the better seeing eye, unknown effect on pineoblastoma prevention, and limited effect on pre-existing metastases that could lead to increased child mortality.[14] In the three centers authoring this report, adjuvant IVC is often considered as front-line therapy for patients with known or suspected germline mutation for prevention of metastasis, pineoblastoma, and second cancers and front-line IAC is reserved for those with unilateral, somatic mutation.[17,18,42] Unfortunately, genetic mutation is not known at presentation so a surrogate of age is used with youngest children (<6 months) at highest risk for germline mutation. Likewise, caution is recommended, especially for group D or E eyes with suspicion for high risk features. High risk retinoblastoma warrants enucleation and additional 6-9 cycles of high-dose IVC to prevent metastatic disease.[25,43,44] Due to small caliber vessels, use of IAC is typically reserved for patients older than 3 or 4 months.[19] In younger patients, bridge therapy with IVC is administered until weight reaches 6 kg.[14]
Despite localized delivery of chemotherapeutic agents, systemic toxicity has been observed following IAC. Transient neutropenia has been observed in 12% of patients.[20] Femoral artery occlusion together with blue toe syndrome can be managed and reversed with anticoagulation.[30,45] More severe complications like carotid artery dissection, stroke, and death, are seldom reported but can occur.[46] Patient selection is critical, as undetected extraocular extension, optic nerve or massive choroidal invasion can lead to metastasis when patients are managed with IAC alone without systemic chemotherapy.
Periocular side effects are often self-limited and include periorbital edema, cutaneous hyperemia, madarosis, blepharoptosis, scalp hair loss, and extraocular dysmotility.[31,47,48] Serious ophthalmic vascular events include choroidal occlusive vasculopathy, branch or central retinal artery occlusion, ophthalmic artery spasm or occlusion, vitreous hemorrhage, and others. Rhegmatogenous retinal detachment, possibly secondary due to accelerated tumor regression of endophytic tumors has been reported in 8-16% of cases treated with primary IAC.[49] Vascular events do not correlate with decreased globe salvage but can limit visual acuity.[4,42] Risk for vascular events is similar when IAC is used as primary or following other therapies.[35]
Intraocular Chemotherapy
Intravitreal chemotherapy
Despite significant improvements in survival, tumor control, and globe salvage in the IVC and IAC eras, several group D and E eyes often required enucleation for vitreous seed recurrence.[14,34] Intravitreal chemotherapy (IvitC), first introduced by Kaneko and Suzuki in 2003, was found useful in combination with IAC for many eyes that otherwise would have been lost. Current indications for IvitC include the presence of refractory or recurrent vitreous seeds following other treatments [Fig. 3]. It is noteworthy to highlight that IvitC is almost never used as primary therapy, but mostly as globe salvage therapy, given the limited efficacy on the primary tumor. Contraindications for IvitC include presence of tumor or vitreous seeds at the planned site of needle entry, tumor invasion of the pars plana, and anterior chamber seeding. Careful clinical examination with the aid of ultrasound biomicroscopy (UBM) can help administer IvitC safely.
Figure 3.
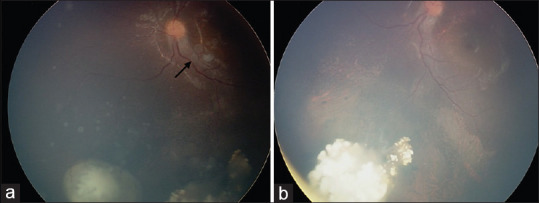
Modern treatment of retinoblastoma. The role of intraocular chemotherapy. Diffuse vitreous seeding from retinoblastoma managed with intravitreal chemotherapy (IvitC). (a) Active vitreous seeds surrounding the tumor and overlying the macula (black arrow), with (b) resolution after two cycles of IvitC with melphalan and one cycle of IvitC with topotecan
The most commonly used drugs in IvitC are melphalan and topotecan, either alone or in combination. The recommended doses of 20-30 μg every 2-4 weeks have been found to efficiently control vitreous seeds while avoiding toxic side effects.[50,51] When the intended injection volume surpasses 0.1 mL, especially when injecting more than one drug, an anterior chamber paracentesis is performed prior to the intravitreal injection. Following injection, the needle is withdrawn while simultaneous triple-freeze-thaw cryotherapy is delivered at the entry site. The eye is gently jiggled for 30 seconds to achieve homogenous distribution throughout the entire vitreous cavity, and copious irrigation of the ocular surface is performed with sterile saline. Parents are instructed to avoid drop instillation, rubbing, or other manipulation of the eye for 7 days following the procedure. These measures are termed 'anti-reflux mechanisms' and avoid undesired spreading of tumor into the extraocular space. Prior to the implementation of anti-reflux safety measures, extraocular extension was reported in 0.4% of cases, but studies reported a considerable decrease in risk which ranges from 0–0.08% with current injection techniques.[52,53,54,55] Serious ocular adverse events can be associated with IvitC, including cataract, vitreous and sub retinal hemorrhage, ocular hypotony, phthisis bulbi, salt-and-pepper retinopathy, anterior segment toxicity, conjunctival chemosis and congestion, injection-site episcleral pigmentation, iris and scleral thinning, iris heterochromia, posterior synechiae, anterior uveitis, optic disc edema, and hemorrhagic retinal necrosis.[54,56,60] The risk for such events can vary with injection technique and ocular pigmentation.[60]
Precision intravitreal chemotherapy
First described in 2018, precision intravitreal chemotherapy (p-IvitC) was introduced to treat localized vitreous seeding.[61] Modified from the standard technique which treats diffuse vitreous seeds, p-IvitC was designed to inject the chemotherapeutic drug(s) in close proximity to a single or localized group of vitreous seeds under indirect ophthalmoscopy, rather than directing the needle toward the center of the globe and dispersing the agent(s) throughout the vitreous cavity.[62] In p-IvitC, the eye is not jiggled following the injection in order to avoid unwanted dispersion of the injected drug(s). Instead, the eye is kept still and the head is positioned with the vitreous seed(s) located inferiorly, using gravity as an aid to minimize exposure to the macula or other unwanted sites.[62] This modality seems to improve drug functionality, translating into a reduction of mean 4-5 injections down to 2.6 injections. With prolonged tumor control observed at 10 months follow-up, retinal pigment epithelial mottling was observed in 13% of cases, and occurred distant from the foveola.[62]
Intracameral chemotherapy
Introduced in 2017 by Munier et al., intracameral chemotherapy (IcamC) was designed to provide sufficient drug availability in the anterior chamber.[62] Previously, aqueous seeding remained an indication for immediate enucleation or anterior chamber plaque radiotherapy given that conventional routes of chemotherapy administration failed to reach tumoricidal doses in the anterior chamber.[63] The original technique describes administration of oral acetazolamide 5 mg/kg prior to injection in order to suppress aqueous humor secretion and prevent drug dilution.[63] Aqueous humor was then aspirated from the anterior and posterior chambers through a transcorneal approach with a 34-gauge long needle. Without removing the needle, a syringe exchange was then performed to replace a comparable volume of aqueous with melphalan (15-20 μg/0.05 mL) or topotecan (7.5 μg/0.015 mL).[63] The dose was fragmented, distributing 1/3 of the dose to the anterior chamber, and the remaining 2/3 to the posterior chamber via a transiridal approach. Following the injection, cryotherapy was applied to the entry site at the time of needle removal. IcamC has also been used in combination with plaque radiotherapy, with complete tumor control in one case after 3 years follow-up.[63,64] Known side effects include iris heterochromia and progressive cataract formation in the treated eye, with stable corneal endothelial cell density after 5-years follow-up.[65] Use of topotecan rather than melphalan might result in fewer adverse effects and could be similarly efficacious. In such cases, a transcorneal approach with infusion into the anterior chamber aqueous is sufficient and repeated monthly as necessary.[63] A similar technique with intracameral topotecan is employed by the authors.
Focal Therapies
Focal therapies are often used for tumor consolidation in conjunction with IVC or IAC.[64] Currently used focal therapies mainly include cryotherapy and transpupillary thermotherapy (TTT). Regardless of choice, all focal therapies result in chorioretinal scarring to some extent and can lead to reduction in visual field or visual acuity if lesions are treated inside the macula. Consideration should be given to alternative chemotherapy-based treatment regimens for tumors involving the fovea, especially if both eyes are involved.
Cryotherapy
Cryotherapy remains a reliable and regularly used treatment in the management of retinoblastoma. Indications include treatment of small tumors and foci of sub retinal or preretinal seeds. A modality termed 'chemo-cryo' describes the application of cryotherapy to the peripheral ora serrata on the same day as IVC in order to improve drug concentration to the intraocular space.[14] Treatment is performed under indirect ophthalmoscopy, placing the cryotherapy probe on the conjunctiva for peripheral lesions or directly on the sclera following a conjunctival incision for more posteriorly located lesions. A triple-freeze-thaw technique is preferably employed, visualizing the tumor becoming entirely encased in an ice ball and then waiting for a complete thaw prior to applying the following freeze cycle. Presently, cryotherapy is rarely used as standalone therapy, and is more frequently used in combination with some sort of chemotherapy, most commonly IVC but sometimes IAC. Exudative and rhegmatogenous retinal detachment have been reported following extensive cryotherapy.[14,66]
Transpupillary thermotherapy (TTT)
Transpupillary thermotherapy with diode laser has largely supplanted laser photocoagulation in the modern armamentarium of retinoblastoma treatment. As with cryotherapy, TTT can be used in combination with chemotherapy as primary treatment for small tumors less than 3 mm in diameter and 2 mm in thickness [Fig. 4].[67] TTT is usually administered through indirect ophthalmoscopy, using a 810 nm diode laser on continuous mode. Multiple spots are often required to cover the entire tumor. The goal is to provide sufficient application time until a grey-white uptake is achieved. Multiple TTT sessions, ranging from 2-6, are usually required at 4 week intervals, to achieve the endpoint of a flat scar or completely calcified tumor. Indocyanine green (ICG) can be used to enhance the effects of TTT in cases with suboptimal response, tumor recurrence, or lightly pigmented fundus. ICG is usually infused at a dose of 0.3-0.5 mg/kg approximately one minute prior to TTT application.[14]
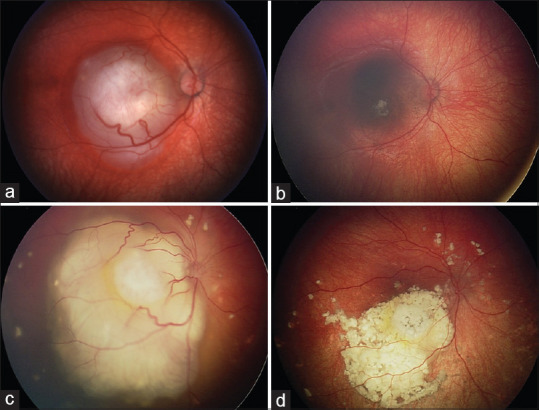
Figure 4.
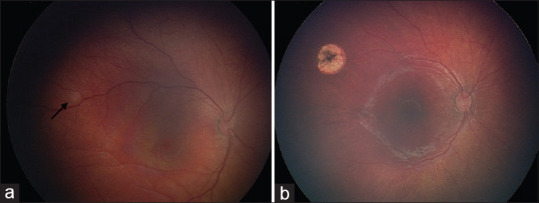
Modern treatment of retinoblastoma. The role of consolidation therapies. Group A retinoblastoma managed with transpupillary thermotherapy (TTT). (a) Subtle tumor (black arrow) temporal to the macula, with (b) regression 1 month after treatment
Complications associated with TTT include iris atrophy, anterior or posterior synechiae, and focal cataract. More severe, sight-threatening complications are rare with appropriate use and include retinal vein occlusion, vitreous hemorrhage, retinal neovascularization, vitreoretinal traction, and retinal detachment.[68,69,70]
External Beam Radiotherapy
Prior to the introduction to IVC, external beam radiotherapy (EBRT) was used as globe salvage therapy. Today, EBRT is mostly of historical significance in most developed nations, due to the many associated side effects and improved outcomes following introduction of effective chemotherapy for retinoblastoma. However, EBRT still maintains a role in the setting of extraocular tumor extension, orbital recurrence, and positive optic nerve margin following enucleation.[71] The combination of EBRT and IVC for treatment of orbital retinoblastoma has been reported to achieve tumor control in 71% of patients.[72] Radiation side effects associated with EBRT include tear deficiency, dry eye syndrome, filamentary keratopathy, cataract, radiation retinopathy, optic neuropathy, and orbital growth retardation causing facial deformity.[73,74] The most serious side effects of EBRT are the subsequent development of second primary tumors in the field of radiation, especially in patients with germline retinoblastoma. This risk has been reported to be as high as 53% by age 50, making patients with germline mutation more likely to die from second cancers than from retinoblastoma itself.[75,76,77] The most common second primary tumor is osteosarcoma, followed by other bone tumors, soft tissue sarcoma, melanoma, and epithelial tumors (bladder, breast, colorectal, kidney, lung, nasal cavity, prostate, retroperitoneum, thyroid, tongue, uterus). Due to these side effects, we recommend avoiding treatment with EBRT if other effective treatment methods are available.
Plaque Radiotherapy
First described in 1929, plaque radiotherapy, also called brachytherapy, was initially used as globe salvage therapy for recurrent tumor following EBRT.[78,79] In the present era, brachytherapy is typically used as secondary treatment for medium sized (≤16 mm in largest basal diameter and > 3 to ≤ 9 mm in thickness) chemoresistant tumors with or without localized vitreous or sub retinal seeding, following recurrence after IVC or IAC.[80] Plaque radiotherapy can also be used to manage diffuse anterior segment retinoblastoma with or without IVC in the absence of choroidal or retinal tumors. Typically, a 2 mm safety margin is added to the largest basal diameter for optimal tumor coverage. Tumors within 2 mm of the optic nerve require a notched plaque, with deep notch used for 3 or more clock hours of tumor around the nerve. Iodine-125 is the most commonly used isotope in the United States and the dose is customized to deliver 35-40 Gy to the tumor apex. When possible, secondary plaque radiotherapy is delivered 1-2 months following IVC in order to minimize side effects.
Compared to EBRT, plaque radiotherapy is convenient, given that it only takes 2-4 days to deliver the complete dose, with the minor disadvantage of requiring two surgeries to place and remove the plaque.[81,82] When compared to EBRT, many serious side effects are avoided with plaque radiotherapy, particularly ipsilateral orbit and facial hypoplasia, and most importantly, second cancers.[83] Success of plaque radiotherapy as secondary treatment following IAC has been reported to be 79%, even in the presence of localized vitreous seeding.[84]
Despite excellent tumor control following plaque radiotherapy, side effects can occur and include cataract (20-43%), radiation maculopathy (25%), radiation papillopathy (26%), and vitreous hemorrhage (54%).[82,84,85] A comparison between primary and secondary plaque radiotherapy revealed a higher incidence of radiation retinopathy (27% vs. 40%) and cataract formation (33% vs. 43%) with the latter.[81,83,85] Intravitreal anti-vascular endothelial growth factor (anti-VEGF) medications can be employed to treat macular edema following plaque treatment. However, prior to confirmed tumor regression, intravitreal injections of non-chemotherapeutic agents should be avoided to prevent extraocular tumor extension. A more conservative approach to prevent or treat macular edema in such cases is the use of sub-Tenon's triamcinolone. Sector retinal laser photocoagulation can be used in combination with prophylactic sub-Tenon's triamcinolone to prevent macular edema or proliferative radiation retinopathy following plaque radiotherapy.[82]
Enucleation
Despite great advances in retinoblastoma management, globe enucleation still remains a current treatment in the modern era [Fig. 5]. It is usually reserved for massive group E tumors, poor tumor visualization (e.g., due to vitreous hemorrhage), presence of extraocular extension, suspected invasion of the optic nerve or choroid, or recalcitrant tumors that have failed previous globe salvage therapies (e.g., IAC, IvitC, plaque radiotherapy, etc.).[11,86,87,88]
Figure 5.
Modern treatment of retinoblastoma. The role of enucleation and prosthetic rehabilitation. (a) Unilateral Group E retinoblastoma that required (b) enucleation, with a dermo-lipid graft placed for economic reasons. (c) On follow-up 6 weeks later, a custom-made prosthesis was adjusted
Known complications include chemosis, conjunctival cysts, pyogenic granuloma, blepharoptosis, lagophthalmos, superior sulcus defect, enophthalmos, symblepharon, implant exposure, and infection.[89] Orbital implant exposure requires urgent wound repair. Infection can be managed topically or systemically with antibiotics but implant removal can be necessary in severe cases. Giant papillary conjunctivitis secondary to continuous contact of the prosthesis can be managed with antibiotic-steroid ointments and copious amounts of lubricants.
Eye removal can lead to functional, physical, and psychological effects.[90] Hence, prosthetic rehabilitation is a crucial event. Cosmetic rehabilitation following enucleation is generally advised with a conformer during the initial 6 weeks.[90] Following the sixth week, once risk for dehiscence, bleeding or infection has diminished, molds are taken for a custom ocular prothesis. Some centers report that early prosthesis insertion has been found to improve the quality of life.[90,91]
Follow-Up Protocol
After the first treatment has been instated, follow-up visits are generally scheduled every 4 weeks to evaluate response to therapy, identify side effects, and make decisions accordingly. The algorithm [Table 2] summarizes some situations where patients might be simply observed, globe salvage treatment continued, adjusted, escalated, or replaced with enucleation.
At any time during the course of the treatment, as genetic results for the RB1 mutation become available, the family should be advised of the impact results will have for the long-term follow-up, and in vitro fertilization with preimplantation diagnosis can be discussed as part of family planning. Patients and their families are offered psychotherapy alongside medical treatment, where alarm signs are acted upon, and the grieving process is normalized. Additional ancillary tests will be required by the pediatric oncologist routinely, especially if standard-dose or high-dose IVC is being administered. A high-resolution simple and contrasted MRI of the brain and orbits should be rigorously repeated every 6 months until age 5 years old, and occasionally more extensive workup, including blood samples, a lumbar puncture, or a full-body osseous gammagram may be required as directed by the pediatric oncologist.
Long-Term Monitoring of the Cancer-Free Patient
A retinoblastoma survivor should ideally be monitored for life. This is particularly true for patients with germline mutation where second cancers can appear at times remote from primary cancer treatment. After complete tumor control has been achieved, patients are followed with frequent eye exams until age 7, and then less frequently throughout the rest of their lives. It is reassuring to know that most patients manifest recurrences by 3 years after treatment with little recurrence thereafter.[92] However, very-late onset recurrences can occur, as far as 11 years after initial treatment.[93] Therefore, visits every 1-2 years with the pediatric oncologist are warranted. Ophthalmology visits should be focused on monitoring long-term effects secondary to the cancer treatment (e.g. amblyopia, glaucoma, cataract, vitreous hemorrhage, retinal detachment, etc.), preservation of the fellow unaffected eye (if such is the case), as well as the usual prevention for a patient of his/her age group (e.g. correction of refractive errors).
Conclusion
Proper management of retinoblastoma is complex. Each case is unique, and treatment regimens must be carefully customized for varying disease presentations, available equipment, and regional culture or traditions. Close cooperation between the treating ocular oncologist and the multidisciplinary team is critical to achieve treatment success, with all parties prioritizing patient safety and preservation of life.
This is a comprehensive review of the current global standard of care of retinoblastoma. While management varies among different retinoblastoma centers depending on availability of resources and level of experience, an algorithm is presented to visually summarize the most up-to-date literature. The authors encourage further collaboration towards the creation of a unifying treatment model based upon the agreement of the most renowned and state-of-the-art retinoblastoma centers throughout the world.
Financial support and sponsorship
Support provided in part by the Eye Tumor Research Foundation, Philadelphia, PA (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, and in the preparation, review or approval of the manuscript. Carol L. Shields, M.D. has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Global Retinoblastoma Study Group. Global retinoblastoma presentation and analysis by national income level. JAMA Oncol. 2020;6:685–95. doi: 10.1001/jamaoncol.2019.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chantada G, Fandiño A, Manzitti J, Urrutia L, Schvartzman E. Late diagnosis of retinoblastoma in a developing country. Arch Dis Child. 1999;80:1714. doi: 10.1136/adc.80.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla B, Hasan F, Azad R, Seth R, Upadhyay AD, Pathy S, et al. Clinical presentation and survival of retinoblastoma in Indian children. Br J Ophthalmol. 2016;100:1728. doi: 10.1136/bjophthalmol-2015-306672. [DOI] [PubMed] [Google Scholar]
- 4.Munier FL, Beck-Popovic M, Chantada GL, Cobrinik D, Kivelä TT, Lohmann D, et al. Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace “Alive, with good vision and no comorbidity”. Prog Retin Eye Res. 2019;73:100764. doi: 10.1016/j.preteyeres.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Dimaras H, Corson TW, Cobrinik D, White A, Zhao J, Munier FL, et al. Retinoblastoma. Nat Rev Dis Prim. 2015;1:15021. doi: 10.1038/nrdp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyawira G, Kahaki K, Kariuki-Wanyoike M. Survival among retinoblastoma patients at the Kenyatta National Hospital. Kenya J Ophthalmol East Cent South Afr. 2013;17(1) [Google Scholar]
- 7.Dean M, Bendfeldt G, Lou H, Giron V, Garrido C, Valverde P, et al. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guatemala. Cancer Lett. 2014;351:59–63. doi: 10.1016/j.canlet.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naseripour M. “Retinoblastoma survival disparity”: The expanding horizon in developing countries. Saudi J Ophthalmol. 2012;26:157–161. doi: 10.1016/j.sjopt.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scelfo C, Francis JH, Khetan V, Jenkins T, Marr B, Abramson DH, et al. An international survey of classification and treatment choices for group D retinoblastoma. Int J Ophthalmol. 2017;10:961–7. doi: 10.18240/ijo.2017.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson DH, Daniels AB, Marr BP, Francis JH, Brodie SE, Dunkel IJ, et al. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma. PLoS One. 2016;11:1–13. doi: 10.1371/journal.pone.0146582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields JA, Shields CL, Sivalingam V. Decreasing frequency of enucleation in patients with retinoblastoma. Am J Ophthalmol. 1989;108:185–8. doi: 10.1016/0002-9394(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 12.Gündüz K, Günalp I, Yalçindaǧ N, Unal E, Taçyildiz N, Erden E, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004;111:1917–24. doi: 10.1016/j.ophtha.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113:2276–80. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Lally SE, Leahey AM, Jabbour PM, Caywood EH, Schwendeman R, et al. Targeted retinoblastoma management: When to use intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Curr Opin Ophthalmol. 2014;25:374–85. doi: 10.1097/ICU.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 15.Shahsavari M, Mashayekhi A. Pharmacotherapy for retinoblastoma. J Ophthalmic Vis Res. 2009;4:169–73. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson TW, Chan HS, Moselhy GM, Heydt DD, Jr, Frey CM, Gallie BL. Penetration of chemotherapy into vitreous is increased by cryotherapy and cyclosporine in rabbits. Arch Ophthalmol. 1996;114:1390–5. doi: 10.1001/archopht.1996.01100140590011. [DOI] [PubMed] [Google Scholar]
- 17.Shields CL, Meadows AT, Shields JA, Carvalho C, Smith AF. Chemoreduction for retinoblastoma may prevent intracranial neuroblastic malignancy (trilateral retinoblastoma) Arch Ophthalmol. 2001;119:1269–72. doi: 10.1001/archopht.119.9.1269. [DOI] [PubMed] [Google Scholar]
- 18.Ramasubramanian A, Kytasty C, Meadows AT, Shields JA, Leahey A, Shields CL. Incidence of pineal gland cyst and pineoblastoma in children with retinoblastoma during the chemoreduction era. Am J Ophthalmol. 2013;156:825–9. doi: 10.1016/j.ajo.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Turaka K, Shields CL, Meadows AT, Leahey A. Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer. 2012;59:121–5. doi: 10.1002/pbc.23278. [DOI] [PubMed] [Google Scholar]
- 20.Gobin YP, Dunkel IJ, Marr BP, Francis JH, Brodie SE, Abramson DH. Combined, sequential intravenous and intra-arterial chemotherapy (Bridge Chemotherapy) for young infants with retinoblastoma. PLoS One. 2012;7:e44322. doi: 10.1371/journal.pone.0044322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields CL, Jorge R, Say EAT, Magrath G, Alset A, Caywood E, et al. Unilateral retinoblastoma managed with intravenous chemotherapy versus intra-arterial chemotherapy. Outcomes based on the international classification of retinoblastoma. Asia-Pacific J Ophthalmol (Phila) 2016;5:97–103. doi: 10.1097/APO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 22.Friedman DL, Himelstein B, Shields CL, Shields JA, Needle M, Miller D, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. 2000;18:12–7. doi: 10.1200/JCO.2000.18.1.12. [DOI] [PubMed] [Google Scholar]
- 23.Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: Is chemotherapy a factor? Ophthalmology. 2007;114:1378–83. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 24.Shields CL, Bas Z, Tadepalli S, Dalvin LA, Rao R, Schwendeman R, et al. Long-term (20-year) real-world outcomes of intravenous chemotherapy (chemoreduction) for retinoblastoma in 964 eyes of 554 patients at a single centre. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2019-315572. bjophthalmol-2019-315572. doi: 101136/bjophthalmol-2019-315572 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Honavar SG, Singh AD, Shields CL, Meadows AT, Demirci H, Cater J, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–31. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 26.Uusitalo MS, Van Quill KR, Scott IU, Matthay KK, Murray TG, O'Brien JM. Evaluation of chemoprophylaxis in patients with unilateral retinoblastoma with high-risk features on histopathologic examination. Arch Ophthalmol. 2001;119:41–8. [PubMed] [Google Scholar]
- 27.Kaliki S, Shields CL, Shah SU, Eagle RC, Jr, Shields JA, Leahey A. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol. 2011;129:1422–7. doi: 10.1001/archophthalmol.2011.289. [DOI] [PubMed] [Google Scholar]
- 28.Yamane T, Kaneko A, Mohri M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int J Clin Oncol. 2004;9:69–73. doi: 10.1007/s10147-004-0392-6. [DOI] [PubMed] [Google Scholar]
- 29.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115:1398–404. doi: 10.1016/j.ophtha.2007.12.014. 1404e1. [DOI] [PubMed] [Google Scholar]
- 30.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol. 2011;129:732–7. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 31.Manjandavida FP, Stathopoulos C, Zhang J, Honavar SG, Shields CL. Intra-arterial chemotherapy in retinoblastoma-A paradigm change. Indian J Ophthalmol. 2019;67:740–54. doi: 10.4103/ijo.IJO_866_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE, Griffin GC, et al. Intra-arterial chemotherapy for retinoblastoma: report No.1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129:1399–406. doi: 10.1001/archophthalmol.2011.150. [DOI] [PubMed] [Google Scholar]
- 33.Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumontv, Chitale R, et al. Pearls and pitfalls of intraarterial chemotherapy for retinoblastoma. J Neurosurg Pediatr. 2012;10:175–81. doi: 10.3171/2012.5.PEDS1277. [DOI] [PubMed] [Google Scholar]
- 34.Ancona-Lezama D, Dalvin LA, Lucio-Alvarez JA, Jabbour P, Shields CL. Ophthalmic vascular events after intra-arterial chemotherapy for retinoblastoma: Real-world comparison between primary and secondary treatments. Retina. 2019;39:2264–72. doi: 10.1097/IAE.0000000000002315. [DOI] [PubMed] [Google Scholar]
- 35.Dalvin LA, Ancona-Lezama D, Lucio-Alvarez JA, Masoomian B, Jabbour P, Shields CL. Ophthalmic vascular events after primary unilateral intra-arterial chemotherapy for retinoblastoma in early and recent eras. Ophthalmology. 2018;125:1803–11. doi: 10.1016/j.ophtha.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Schaiquevich P, Ceciliano A, Millan N, Taich P, Villasante F, Fandino AC, et al. Intra-arterial chemotherapy is more effective than sequential periocular and intravenous chemotherapy as salvage treatment for relapsed retinoblastoma. Pediatr Blood Cancer. 2013;60:766–70. doi: 10.1002/pbc.24356. [DOI] [PubMed] [Google Scholar]
- 37.Grigorovski N, Lucena E, Mattosinho C, Parareda A, Ferman S, Catalá J. Use of intra-arterial chemotherapy for retinoblastoma: Results of a survey. Int J Ophthalmol. 2014;7:726–30. doi: 10.3980/j.issn.2222-3959.2014.04.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramson DH, Fabius AWM, Francis JH, Marr BP, Dunkel IJ, Brodie SE, et al. Ophthalmic artery chemosurgery for eyes with advanced retinoblastoma. Ophthalmic Genet. 2017;38:16–21. doi: 10.1080/13816810.2016.1244695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abramson DH, Marr BP, Francis JH, Dunkel IJ, Fabius AWM, Brodie SE, et al. Simultaneous bilateral ophthalmic artery chemosurgery for bilateral retinoblastoma (tandem therapy) PLoS One. 2016;11:1–11. doi: 10.1371/journal.pone.0156806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Leahey A, Griffin G, et al. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina. 2013;33:2103–9. doi: 10.1097/IAE.0b013e318295f783. [DOI] [PubMed] [Google Scholar]
- 41.Shields CL, Shields JA. Intra-arterial chemotherapy for retinoblastoma. JAMA Ophthalmol. 2016;134:1201. doi: 10.1001/jamaophthalmol.2016.2712. [DOI] [PubMed] [Google Scholar]
- 42.Shields CL, Say EAT, Pefkianaki M, Regillo CD, Caywood EH, Jabbour PM, et al. Rhegmatogenous retinal detachment after intraarterial chemotherapy for retinoblastoma: The 2016 founders award lecture. Retina. 2017;37:1441–50. doi: 10.1097/IAE.0000000000001382. [DOI] [PubMed] [Google Scholar]
- 43.Kaliki S, Shields CL. Retinoblastoma: Achieving new standards with methods of chemotherapy. Indian J Ophthalmol. 2015;63:103–9. doi: 10.4103/0301-4738.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanık Ö, Gündüz K, Yavuz K, Taçyıldız N, Ünal E. Chemotherapy in retinoblastoma: Current approaches. Turkish J Ophthalmol. 2015;45:259–67. doi: 10.4274/tjo.06888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson EC, Elhammady MS, Quintero-Wolfe S, Murray TG, Aziz-Sultan MA. Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: Initial experience with 17 tumors. J Neurosurg. 2011;114:1603–8. doi: 10.3171/2011.1.JNS10466. [DOI] [PubMed] [Google Scholar]
- 46.Sarici A, Kizilkilic O, Celkan T, Gode S. Blue toe syndrome. JAMA. 2017;57:801–2. doi: 10.1001/jamaophthalmol.2013.1458. [DOI] [PubMed] [Google Scholar]
- 47.Shields CL, Bianciotto CG, Jabbour P, Griffin GC, Ramasubramanian A, Rosenwasser R, et al. Intra-arterial chemotherapy for retinoblastoma: Report no.2, treatment complications. Arch Ophthalmol. 2011;129:1407–15. doi: 10.1001/archophthalmol.2011.151. [DOI] [PubMed] [Google Scholar]
- 48.Marr B, Gobin P, Dunkel I, Brodie SE, Abramson DH. Spontaneously resolving periocular erythema and ciliary madarosis following intra-arterial chemotherapy for retinoblastoma. Middle East Afr J Ophthalmol. 2010;17:207–9. doi: 10.4103/0974-9233.65492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vajzovic LM, Murray TG, Aziz-Sultan MA, Schefler AC, Wolfe SQ, Hess D, et al. Supraselective intra-arterial chemotherapy: Evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–6. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields CL, Alset AE, Say EAT, Caywood E, Jabbour P, Shields JA. Retinoblastoma control with primary intra-arterial chemotherapy: Outcomes before and during the intravitreal chemotherapy era. J Pediatr Ophthalmol Strabismus. 2016;53:275–84. doi: 10.3928/01913913-20160719-04. [DOI] [PubMed] [Google Scholar]
- 51.Shields CL, Shields JA. Retinoblastoma management: Advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol. 2010;21:203–12. doi: 10.1097/ICU.0b013e328338676a. [DOI] [PubMed] [Google Scholar]
- 52.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130:1268. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 53.Shimoda Y, Hamano R, Ishihara K, Shimoda N, Hagimura N, Akiyama H, et al. Effects of intraocular irrigation with melphalan on rabbit retinas during vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2008;246:501–8. doi: 10.1007/s00417-007-0685-3. [DOI] [PubMed] [Google Scholar]
- 54.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: Preliminary results. JAMA Ophthalmol. 2014;132:319–25. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 55.Munier FL. Classification and management of seeds in retinoblastoma ellsworth lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35:193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francis JH, Abramson DH, Ji X, Shields CL, Teixeira LF, Schefler AC, et al. Risk of extraocular extension in eyes with retinoblastoma receiving intravitreous chemotherapy. JAMA Ophthalmol. 2017;135:1426–9. doi: 10.1001/jamaophthalmol.2017.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013;27:147–50. doi: 10.1016/j.sjopt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shields CL, Douglass AM, Beggache M, Say EAT, Shields JA. Intravitreous chemotherapy for active vitreous seeding from retinoblastoma: Outcomes after 192 consecutive injections. The 2015 Howard Naquin lecture. Retina. 2016;36:1184–90. doi: 10.1097/IAE.0000000000000903. [DOI] [PubMed] [Google Scholar]
- 59.Francis JH, Schaiquevich P, Buitrago E, Del Sole MJ, Zapata G, Croxatto JO, et al. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: A preclinical and clinical study. Ophthalmology. 2014;121:1810–7. doi: 10.1016/j.ophtha.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Rishi P, Sharma T, Agarwal V, Maitray A, Sharma M, Bansal N, et al. Complications of intravitreal chemotherapy in eyes with retinoblastoma: See editorial on pg.359. Ophthalmol Retin. 2017;1:448–50. doi: 10.1016/j.oret.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 61.Francis JH, Marr BP, Brodie SE, Abramson DH. Anterior ocular toxicity of intravitreous melphalan for retinoblastoma. JAMA Ophthalmol. 2015;133:1459. doi: 10.1001/jamaophthalmol.2015.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu MD, Dalvin LA, Welch RJ, Shields CL. Precision intravitreal chemotherapy for localized vitreous seeding of retinoblastoma. Ocul Oncol Pathol. 2019;5:284–9. doi: 10.1159/000491432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munier FL, Gaillard M-C, Decembrini S, Bongiovanni M, Beck-Popovic M. Intracameral chemotherapy (Melphalan) for aqueous seeding in retinoblastoma: Bicameral injection technique and related toxicity in a pilot case study. Ocul Oncol Pathol. 2017;3:149–55. doi: 10.1159/000453617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paez-Escamilla M, Bagheri N, Teira LE, Corrales-Medina FF, Harbour J. Intracameral topotecan hydrochloride for anterior chamber seeding of retinoblastoma. JAMA Ophthalmol. 2017;135:1453–4. doi: 10.1001/jamaophthalmol.2017.4603. [DOI] [PubMed] [Google Scholar]
- 65.Munier FL, Moulin A, Gaillard M-C, Bongiovanni M, Decembrini S, Houghton S. Intracameral chemotherapy for globe salvage in retinoblastoma with secondary anterior chamber invasion. Ophthalmology. 2018;125:615–7. doi: 10.1016/j.ophtha.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Gallie BL, Budning A, DeBoer G, Thiessen JJ, Koren G, Verjee Z, et al. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch Ophthalmol. 1996;114:1321–8. doi: 10.1001/archopht.1996.01100140521001. [DOI] [PubMed] [Google Scholar]
- 67.Anagnoste SR, Scott IU, Murray TG, Kramer D, Toledano S. Rhegmatogenous retinal detachment in retinoblastoma patients undergoing chemoreduction and cryotherapy. Am J Ophthalmol. 2000;129:817–9. doi: 10.1016/s0002-9394(00)00407-4. [DOI] [PubMed] [Google Scholar]
- 68.Hasanreisoglu M, Saktanasate J, Schwendeman R, Shields JA, Shields CL. Indocyanine green-enhanced transpupillary thermotherapy for retinoblastoma: Analysis of 42 tumors. J Pediatr Ophthalmol Strabismus. 2015;52:348–54. doi: 10.3928/01913913-20150929-17. [DOI] [PubMed] [Google Scholar]
- 69.Lumbroso L, Doz F, Urbieta M, Levy C, Bours D, Asselain B, et al. Chemothermotherapy in the management of retinoblastoma. Ophthalmology. 2002;109:1130–6. doi: 10.1016/s0161-6420(02)01053-9. [DOI] [PubMed] [Google Scholar]
- 70.Shields CL, Santos MC, Diniz W, Gündüz K, Mercado G, Cater JR, et al. Thermotherapy for retinoblastoma. Arch Ophthalmol. 1999;117:885–93. doi: 10.1001/archopht.117.7.885. [DOI] [PubMed] [Google Scholar]
- 71.Shields CL, Shields JA, Kiratli H, De Potter PV. Treatment of retinoblastoma with indirect ophthalmoscope laser photocoagulation. J Pediatr Ophthalmol Strabismus. 1995;32:317–22. doi: 10.3928/0191-3913-19950901-12. [DOI] [PubMed] [Google Scholar]
- 72.Kim JY, Park Y. Treatment of retinoblastoma: The role of external beam radiotherapy. Yonsei Med J. 2015;56:1478–91. doi: 10.3349/ymj.2015.56.6.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pradhan DG, Sandridge AL, Mullaney P, Abboud E, Karcioglu ZA, Kandil A, et al. Radiation therapy for retinoblastoma: A retrospective review of 120 patients. Int J Radiat Oncol Biol Phys. 1997;39:3–13. doi: 10.1016/s0360-3016(97)00156-9. [DOI] [PubMed] [Google Scholar]
- 74.Imhof SM, Hofman P, Tan KE. Quantification of lacrimal function after D-shaped field irradiation for retinoblastoma. Br J Ophthalmol. 1993;77:482–4. doi: 10.1136/bjo.77.8.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karp LA, Streeten BW, Cogan DG. Radiation-induced atrophy of the Meibomian gland. Arch Ophthalmol. 1979;97:303–5. doi: 10.1001/archopht.1979.01020010155013. [DOI] [PubMed] [Google Scholar]
- 76.Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: A possible age effect on radiation-related risk. Ophthalmology. 1998;105:573–80. doi: 10.1016/S0161-6420(98)94006-4. [DOI] [PubMed] [Google Scholar]
- 77.Wong FL, Boice JDJ, Abramson DH, Tarone RE, Kleinerman RA, Stovall M, et al. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278:1262–7. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 78.Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, et al. Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: An extended follow-up. J Clin Oncol. 2005;23:2272–9. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 79.Moore RF, Stallard HB, Milner JG. Retinal Gliomata treated by radon seeds. Br J Ophthalmol. 1931;15:673–96. doi: 10.1136/bjo.15.12.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shields CL, Shields JA, De Potter P, Minelli S, Hernandez C, Brady LW, et al. Plaque radiotherapy in the management of retinoblastoma. Use as a primary and secondary treatment. Ophthalmology. 1993;100:216–24. doi: 10.1016/s0161-6420(93)31667-2. [DOI] [PubMed] [Google Scholar]
- 81.Shields CL, Mashayekhi A, Sun H, Uysal Y, Friere J, Komarnicky L, et al. Iodine 125 plaque radiotherapy as salvage treatment for retinoblastoma recurrence after chemoreduction in 84 tumors. Ophthalmology. 2006;113:2087–92. doi: 10.1016/j.ophtha.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 82.Shields CL, Shields JA, Cater J, Othmane I, Singh AD, Micaily B. Plaque radiotherapy for retinoblastoma: Long-term tumor control and treatment complications in 208 tumors. Ophthalmology. 2001;108:2116–21. doi: 10.1016/s0161-6420(01)00797-7. [DOI] [PubMed] [Google Scholar]
- 83.Shields CL, Meadows AT, Leahey AM, Shields JA. Continuing challenges in the management of retinoblastoma with chemotherapy. Retina. 2004;24:849–62. doi: 10.1097/00006982-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Shields CL, Shields JA, Minelli S, De Potter P, Hernandez C, Cater J, et al. Regression of retinoblastoma after plaque radiotherapy. Am J Ophthalmol. 1993;115:181–7. doi: 10.1016/s0002-9394(14)73922-4. [DOI] [PubMed] [Google Scholar]
- 85.Francis JH, Barker CA, Wolden SL, McCormick B, Segal K, Cohen G, et al. Salvage/adjuvant brachytherapy after ophthalmic artery chemosurgery for intraocular retinoblastoma. Int J Radiat Oncol Biol Phys. 2013;87:517–23. doi: 10.1016/j.ijrobp.2013.06.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Materin MA, Bianciotto CG, Wu C, Shields CL. Sector laser photocoagulation for the prevention of macular edema after plaque radiotherapy for uveal melanoma: A pilot study. Retina. 2012;32:1601–7. doi: 10.1097/IAE.0b013e3182437e70. [DOI] [PubMed] [Google Scholar]
- 87.De Potter P. Current treatment of retinoblastoma. Curr Opin Ophthalmol. 2002;13:331–6. doi: 10.1097/00055735-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Shields CL, Uysal Y, Marr BP, Lally SE, Rodriques E, Kharod B, et al. Experience with the polymer-coated hydroxyapatite implant after enucleation in 126 patients. Ophthalmology. 2007;114:367–73. doi: 10.1016/j.ophtha.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 89.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: Review of current management. Oncologist. 2007;12:1237–46. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 90.Shah V, Yadav L, Singh M, Kharbanda S. Custom ocular prosthesis in rehabilitation of a child operated for retinoblastoma. Natl J Maxillofac Surg. 2015;6:232–6. doi: 10.4103/0975-5950.183871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chin K, Margolin CB, Finger PT. Early ocular prosthesis insertion improves quality of life after enucleation. Optometry. 2006;77:71–5. doi: 10.1016/j.optm.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 92.Shah SU, Shields CL, Lally SE, Shields JA. Hydroxyapatite orbital implant in children following enucleation: Analysis of 531 sockets. Ophthal Plast Reconstr Surg. 2015;31:108–14. doi: 10.1097/IOP.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 93.Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120:460–4. [PubMed] [Google Scholar]