Abstract
Cicatrizing conjunctivitis constitutes a group of chronic local and systemic disorders that cause conjunctival scarring. A systematic approach is required to sift through the clinical history, examination, and laboratory investigations of patients to arrive at the correct diagnosis of the underlying cause. Establishing the etiology is critical, as the therapeutic approach changes based on the cause of conjunctival inflammation. Effective management of patients with the condition requires knowledge of multiple modalities such as systemic immunosuppressive therapy, use of scleral contact lenses, and surgery for ocular surface and vision improvement. We review the clinical features of this condition and present diagnostic and treatment algorithms to help simplify the complexities in its management. This review attempts to place all the relevant information on chronic cicatrizing conjunctivitis together in one place for the benefit of cornea and ocular surface specialists, general ophthalmologists, and ophthalmology residents.
Keywords: Cicatrizing conjunctivitis, mucous membrane pemphigoid, ocular cicatricial pemphigoid, Stevens–Johnson syndrome
The word cicatrix originates from Latin and means the scar of a healed wound. Chronic cicatrizing conjunctivitis (CCC), therefore, refers to conditions characterized by inflammation and scarring of the conjunctiva. The clinical spectrum of cicatrization ranges from barely discernible subconjunctival fibrosis to gross distortion of the ocular surface anatomy such as ankyloblepharon. A host of local insults and systemic diseases can cause CCC. Although some of these are innocuous and self-limiting, others can be progressive and adversely affect not just the eye but other organ systems in the body as well.[1]
Reliable epidemiological data for cicatrizing conjunctivitis are available only from two large prospective studies in the United Kingdom and Australia/New Zealand.[2,3] These estimate the minimum incidence of CCC to be 1.3 per million and 1.5 per million, respectively, highlighting the rarity of the condition. We believe that the complexity in arriving at the diagnosis and lower index of suspicion among the treating clinicians could contribute to it being underdiagnosed. In this review, we attempt to simplify the clinical approach to CCC by providing diagnostic and management algorithms based on available evidence as well as our own experience.
Establishing the Diagnosis
Conditions such as ocular surface burns, Stevens–Johnson Syndrome (SJS) or toxic epidermal necrolysis (TEN), atopic keratoconjunctivitis (AKC) or vernal keratoconjunctivitis (VKC), and mucous membrane pemphigoid (MMP), can lead to CCC and are leading causes of unilateral and bilateral limbal stem-cell deficiency (LSCD) as well.[4] The causes of CCC can be diverse and can be classified as primarily an ocular disease without systemic involvement or as a part of a complex of multisystem disorders. Fig. 1 illustrates the various differential diagnoses of CCC. Clinical signs of CCC depend on the periodicity, severity, and cause of conjunctival inflammation. The clinician needs to be on the lookout for the tell-tale signs of CCC along with the other signs of any underlying primary pathology in the eye. These early and subtle signs may include caruncular or subconjunctival fibrosis, which may progress to forniceal foreshortening and symblepharon formation. More advanced cases present with a dry ocular surface, loss of plica semilunaris, keratin deposits on the ocular surface or lid margin, loss of the normal architecture of the lid margin and mucocutaneous junction, entropion, trichiasis, distichiasis, ankyloblepharon, and dermalization of the ocular surface [Fig. 2]. LSCD is often a feature of long-standing cases of CCC. It presents with loss of corneal luster, persistent corneal epithelial defects, or fibrovascular pannus formation across the limbus on the cornea.[5] The presence of clinical signs of CCC should prompt the clinician to systematically look for the underlying cause. We have summarized our diagnostic approach to patients with CCC in a simple algorithm mentioned in Fig. 3.
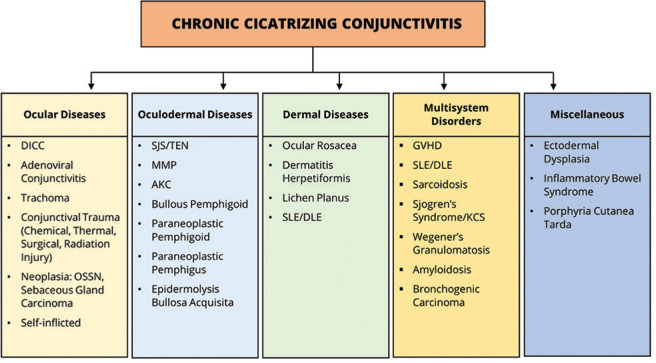
Figure 1.
Differential diagnosis of chronic cicatrizing conjunctivitis. The causes are classified as ocular diseases without systemic involvement, oculodermal diseases, dermal diseases with variable ocular involvement, multisystem disorders, and other miscellaneous congenital and acquired diseases (modified from the classification proposed by Dr. JK Dart[1]). DICC = Drug-induced cicatrizing conjunctivitis, OSSN = ocular surface squamous neoplasia, SJS = Stevens–Johnson syndrome, TEN = toxic epidermal necrolysis, MMP = mucous membrane pemphigoid, AKC = atopic kerato-conjunctivitis, SLE = systemic lupus erythematosus, DLE = discoid lupus erythematosus, GVHD = graft-versus-host disease, KCS = keratoconjunctivitis sicca
Figure 2.
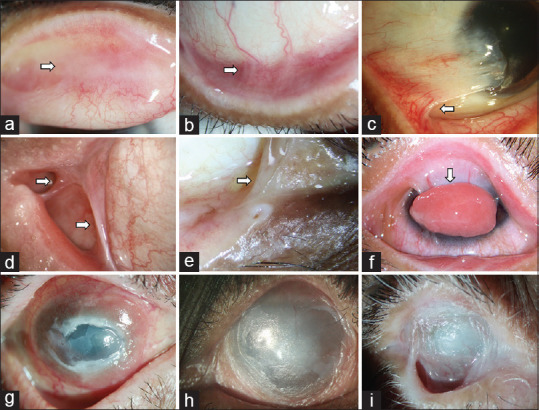
Clinical signs of conjunctival cicatrization. Upper tarsal scarring and granulomas (a), forniceal foreshortening (b), partial limbal stem-cell deficiency (LSCD) with symblebharon (c), lateral canthal fibrosis (d), medial canthal fibrosis and loss of plica semilunaris (e), severe symblepharon with ocular surface granuloma (f), total LSCD with conjunctival granulation and persistent epithelial defect (g), total LSCD with forniceal closure and ocular surface dermalization (h), near-total forniceal loss with ankyloblepharon and dermalization (i)
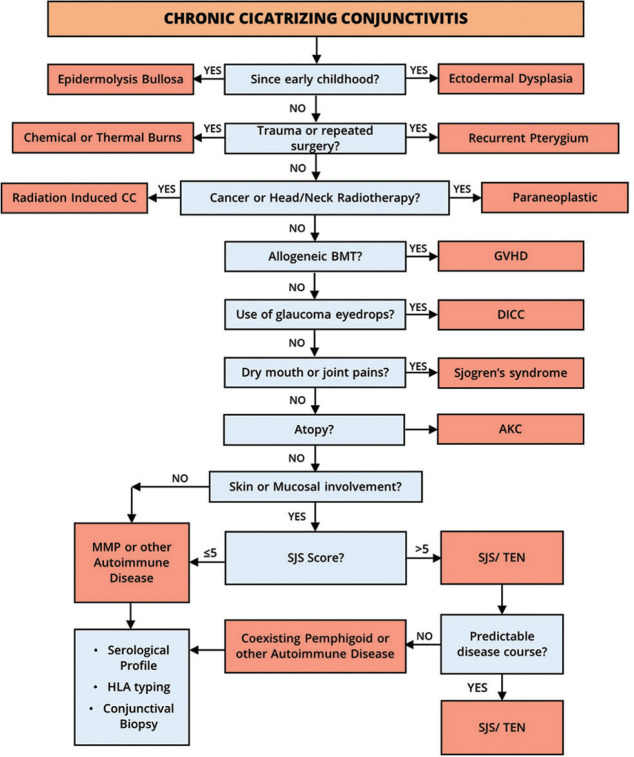
Figure 3.
Algorithm to establish the etiological diagnosis of chronic cicatrizing conjunctivitis. Detailed history taking and clinical examination can help narrow down the possible differential diagnosis. CC = Cicatrizing conjunctivitis, BMT = bone-marrow transplantation, GVHD = graft-versus-host disease, DICC = drug-induced cicatrizing conjunctivitis, AKC = atopic keratoconjunctivitis, MMP = mucous membrane pemphigoid, SJS = Stevens–Johnson syndrome, TEN = toxic epidermal necrolysis, HLA = human leucocyte antigen
Conditions such as epidermolysis bullosa and ectodermal dysplasia usually present in childhood and are associated with specific findings involving the hair, teeth, skin, and other mucosal structures [Fig. 4].[6,7] Infections such as trachoma, adenoviral keratoconjunctivitis, and mycoplasma-induced rash and mucositis are known to cause conjunctival scarring that is nonprogressive and can generally be identified based on history and specific clinical signs.[8] Insults such as ocular surface chemical or thermal burns, ocular surface surgery, and radiotherapy to the head/neck region can be ruled in or out by a detailed history. A history of allogeneic bone marrow transplantation followed by dry-eye disease and CCC points to a diagnosis of graft-versus-host disease.[9]
Figure 4.
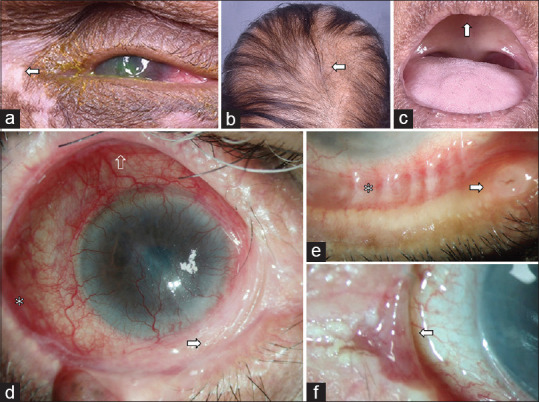
Clinical presentation of anhidrotic ectodermal dysplasia (AED) and drug-induced cicatrizing conjunctivitis (DICC). The top row (a-c) illustrates a case of AED with characteristic extraocular findings like peri-ocular vitiligo (a), alopecia (b), edentulism (c), and lack of sweating. The clinical signs of DICC include inferior forniceal shortening (d) with more severe fibrosis nasally (white arrow) as compared to temporally (white asterisk) and disproportionately lesser involvement of the superior fornix (transparent arrow), zipper-like symblepharon (white asterisk), and punctal edema (white arrow) with or without ulceration (e) or puntum to punctum fibrotic bands (f)
Long-term use of any topical medication can cause CCC due to either the drug itself or the preservatives used. This has been described as pseudopemphigoid or drug-induced cicatrizing conjunctivitis (DICC), which can either be progressive or nonprogressive and is commonly seen in patients using antiglaucoma medications for prolonged duration.[10] Clinical clues in such cases are involvement of the inferior lacrimal puncta (punctal edema, fibrosis or occlusion), periocular skin changes, zipper-like scarring, and symblepharon that are more prominent in the inferior and medial part of the eye compared to the superior and lateral parts [Fig. 4].
Cicatrizing conjunctivitis in a patient with dry-eye disease and dry mouth indicates a diagnosis of long-standing primary or secondary Sjogren's syndrome, which can be further elucidated by a focused systemic examination, serological tests, HLA typing, and if indicated, a salivary gland biopsy.[11] HLA typing is an underexplored diagnostic test that is not affected by the initiation of immunosuppression and, when used in conjunction with other tests, can help in identifying disease associations of CCC. Conditions such as long-standing ocular allergy (AKC/VKC) present with a history of itching and findings such as conjunctival papillae, whereas rosacea blepharo-conjunctivitis has typical skin features. Clinical features of rare causes of CCC such as bullous pemphigoid and linear IgA disease are presented in Fig. 5. A number of systemic autoimmune diseases and immunobullous disorders such as sarcoidosis, pemphigus vulgaris, discoid lupus erythematosus, lichen planus, Behcet's disease, and many others can cause CCC, but the diagnosis is usually self-evident due to prominent involvement of other systems.[12,13,14] Though systemic involvement is evident in most of these cases, some diseases like paraneoplastic pemphigus or pemphigoid can present with an ocular involvement before the onset of systemic manifestations.[15] Both the existing prospective epidemiological studies on CCC list the most frequent underlying causes of CCC to be MMP and SJS/TEN.[2,3] Patients with either disease would give a history of ulceration of the mouth and/or the skin. In our experience, the end-stage manifestations of CCC due to both MMP and SJS are incredibly similar, and it may be impossible to distinguish the two based on clinical examination alone. However, on careful examination of the ocular surface, subtle differences do exist in the earlier stages [Fig. 6]. A scoring system that takes into account medical history as well as systemic and clinical examination findings in such cases has been published recently. A score of >5 was found to be highly sensitive and specific in identifying SJS as the underlying cause of CCC.[16] We recommend that clinicians use this scoring system routinely, which would help them identify the likelihood of SJS being the underlying cause of CCC with a high degree of confidence. In our opinion, these cases do not need further diagnostic procedures. A score of ≤5 indicates the need for a further diagnostic workup to look for other underlying causes.
Figure 5.
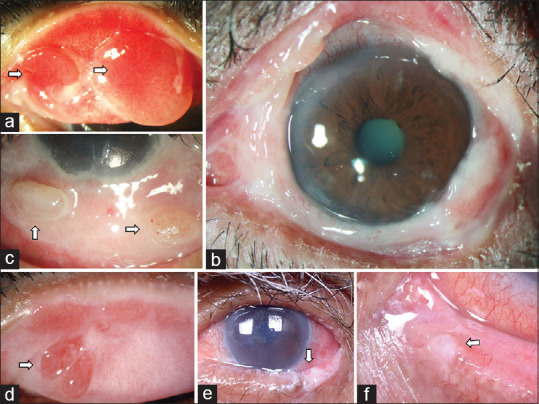
Clinical presentations of other rare causes of chronic cicatrizing conjunctivitis (CCC). These include bullous pemphigoid, which presents with exuberant granulation tissue and large conjunctival granulomas on the tarsus (a), bulbar (b), and forniceal conjunctiva (c). Linear IgA disease, which also presents with flat tarsal granulomas (d). Pagetoid variant of sebaceous gland carcinoma which shows keratinization and irregularity of the lid-margin and tarsal conjunctiva (e) and can also develop punctal fibrosis with the use of topical antimetabolite therapy (f)
Figure 6.
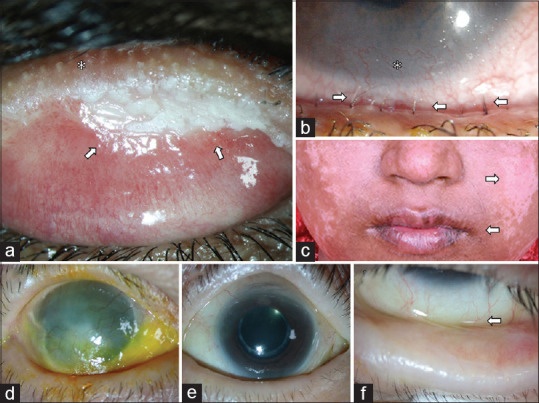
Clinical presentation of Stevens–Johnson syndrome (SJS) and mucous membrane pemphigoid (MMP). The clinical signs suggestive of SJS as the etiology of chronic cicatrizing conjunctivitis include lid-margin keratinization (white arrows, a), distichiatic lashes (white arrows, b) causing corneal scarring both of which cause lid-wiper keratopathy in the corresponding areas of the corneal surface (white asterisk, b) and extraocular sequelae of the acute episode like pigmentary changes on the skin (white arrows, c) and mucocutaneous junction changes in the lips (c). Unlike SJS, MMP usually presents as a disease of insidious onset and gradual progression with asymmetrical involvement, typically with more advanced damage in one eye (d) and early involvement in the other eye (e), with subtle forniceal foreshortening as the earliest sign (f)
Other important differential diagnoses that should be considered, especially when a unilateral involvement is noted, are a diffuse variant of ocular surface squamous neoplasia and sebaceous gland carcinomas that can clinically present with features of CCC [Fig. 5].[17,18] Conditions like trauma and DICC can also present with unilateral involvement.
Role of Conjunctival Biopsy
We strongly recommend that all cases with a clinical suspicion of ocular MMP should undergo a conjunctival biopsy for both histopathology and direct immunofluorescence (DIF). This not only helps to confirm the diagnosis in a significant proportion of cases but also helps to identify other causes of CCC such as ocular surface squamous neoplasia, sarcoidosis, and AKC, which are differentiated based on histopathology. Two biopsies measuring 2–4 mm each are harvested from the clinically uninvolved parts of the bulbar conjunctiva. It is crucial to avoid areas that are already scarred or that have other coexistent pathologies like pinguecula or pterygium. One biopsy is fixed in formalin for histopathology, and the other is transferred in an appropriate transport medium, such as Michel's medium, for DIF. A DIF study is considered positive for MMP if linear deposits of immunoglobulins or complement are found in the basement membrane (subepithelial zone). The reported rate of conjunctival biopsies being positive on DIF varies widely from 50% to 86%.[19] Taking biopsies simultaneously from both the bulbar conjunctiva and the buccal mucosa is recommended, as it may improve the diagnostic yield.[20] Immunoperoxidase techniques as well as indirect immunofluorescence studies and detection of specific autoantibodies from serum may additionally help, but the lack of widespread availability of these tests and transport media remain its limitation.[21]
Approach to Treatment
Goals of treatment in cases of CCC include arresting progressive cicatrization, correction of lid abnormalities, preventing complications such as keratopathy and corneal perforation, providing relief from symptoms as well as improving vision. In the context of CCC, prevention has a very critical role. In conditions such as SJS and chemical burns, there is an initial window of opportunity during the acute stage of the disease, where prompt and appropriate management can significantly contain the extent and severity of cicatrizing sequelae.[22,23] In SJS, several studies have demonstrated that early amniotic membrane grafting can dramatically reduce chronic ocular complications.[24,25] Acute chemical burns are more complex because of the diversity of factors that affect the clinical course, including but not restricted to the type of chemical (pH and concentration), duration of exposure, mode of injury, amount of intra-ocular penetration, the extent of adnexal damage, etc.[26] Nevertheless, urgent and appropriate treatment with irrigation and lavage, intensive topical steroids, and amniotic membrane grafting can go a long way in limiting the conjunctival fibrosis and reduce the need for further reconstructive surgery.
We propose a simplified algorithm for treating cases of CCC illustrated in Fig. 7. Different etiologies of CCC have different approaches to treatment. In progressive CCC, due to DICC, withdrawing the offending drug and replacing it with a preservative-free alternative can arrest the progression of cicatrization in most cases. Additionally, topical steroids can be used in tapering doses to control the surface inflammation. However, in some cases of DICC, cicatrization can continue to progress despite the withdrawal of the inciting agent. It is important to bear in mind that the established cicatricial changes in these cases are not expected to regress even after stopping the inciting agent/drug, and this in itself is not an indication for immunosuppressive therapy (IMT). It is very critical to effectively manage DICC in early stages as most of these patients have long-standing glaucoma, not tolerating topical antiglaucoma medications. They would need early glaucoma filtration surgeries, the success of which mainly depends on the health of the conjunctiva.[27]
Figure 7.
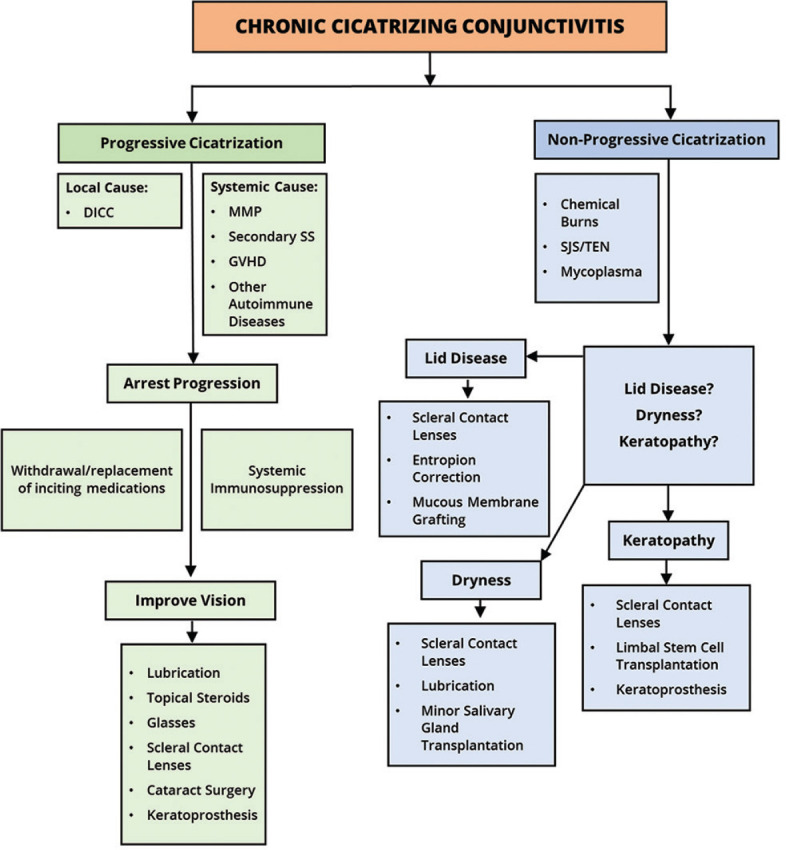
Algorithm for deciding the line of management in patients with cicatrizing conjunctivitis. DICC = Drug-induced cicatrizing conjunctivitis, SS = Sjogren's syndrome, MMP = mucous membrane pemphigoid, GVHD = graft-versus-host disease, SJS = Stevens–Johnson syndrome, TEN = toxic epidermal necrolysis
Systemic autoimmune disorders such as MMP lead to progressive CCC that, if unchecked, can and usually does lead to blinding complications in the eye. Besides, the disease may also have extraocular manifestations such as asymptomatic oral and nasopharyngeal involvement in a significant proportion of patients.[19] The clinical features and outcomes in patients with positive DIF findings on biopsy do not vary from those with a negative biopsy.[28] Therefore, once other causes of progressive CCC such as DICC, neoplasia, and atopy have been ruled out, a clinical diagnosis of MMP warrants treatment with systemic IMT. A number of drugs have been used for this purpose, and a detailed description is beyond the scope and purpose of this review. In our experience, severe disease usually requires treatment with intravenous or oral steroids along with cyclophosphamide or rituximab for control of inflammation. Less severe cases or cases where inflammation has been adequately controlled can be treated with maintenance doses of drugs such as mycophenolate mofetil, azathioprine, and methotrexate.[29] Although dapsone has been described in the treatment of MMP, in our view, the clinical utility may be restricted to very early or mild cases.
Nonprogressive cicatrization due to causes such as ocular surface burns and SJS/TEN does not need systemic immunosuppression. The focus of therapy in these cases should be on optimizing the ocular surface environment. Cases of SJS/TEN quite often have disturbances of the mucocutaneous junction at the eyelid margins, sometimes with keratinization. If left unaddressed, over months and years, this causes progressive keratopathy that can lead to corneal vascularization, opacification, and perforation. Early identification of lid margin changes and the corresponding keratopathy provides a window of opportunity for intervention in these cases. It has been demonstrated that measures such as lid margin mucous membrane grafting (MMG) and use of fluid-filled scleral lenses help in preventing progressive keratopathy and preserving good vision in the long term.[30,31]
In both progressive and nonprogressive cases of CCC, dry-eye disease may warrant treatment with frequent lubricant medication, autologous serum as well as the use of fluid-filled scleral lenses. Minor salivary gland transplantation to the eye may be a useful adjunctive measure in carefully selected cases of severe dry eye.[32] Procedures for vision restoration may be carried out after undertaking the measures mentioned above. Cataract surgery has been reported to be safe and effective in conditions with CCC such as MMP and SJS/TEN, provided quiescence of disease has been achieved before surgery.[33,34]
Strategies to deal with LSCD in cases of CCC must consider the status of the tear film, eyelids, fornices, conjunctiva, and extent of corneal stromal involvement. In cases with wet ocular surface and otherwise normal adnexa, allogeneic limbal stem-cell transplantation can help to restore vision in the setting of bilateral LSCD.[35] Various surgical techniques exist, such as simple limbal epithelial transplantation (SLET), which is an inexpensive, single-stage technique that allows for in vivo expansion of limbal epithelial stem cells on the recipient surface.[36] Good case selection and the use of systemic IMT to prevent allograft rejection are keys to achieving successful outcomes after allogeneic limbal transplantation.[37] An alternative approach in similar cases is the use of a type 1 Boston keratoprosthesis, which may be considered in cases where a suitable donor for limbal tissue is unavailable, there is extensive corneal stromal damage or disorganized anterior segment, and/or there are constraints in the use of systemic IMT.[38,39,40] Conditions causing CCC and bilateral LSCD quite often have a dry ocular surface with abnormal adnexal structure and function. These factors preclude the use of either limbal stem-cell transplantation or a type 1 Boston keratoprosthesis for vision restoration. Surgical options for improving vision in cases of CCC with bilateral LSCD with dry surface include devices such as the type 2 Boston keratoprosthesis, modified osteo-odonto keratoprosthesis, or the LVP keratoprosthesis.[41,42,43]
Conclusion
Cicatrizing conjunctivitis is not a homogenous entity, but a group of local or systemic disorders that cause conjunctival scarring and can potentially lead to blinding sequelae. This article first aims at sensitizing the readers to carefully look for signs of cicatrization in all patients presenting with chronic ocular surface inflammation like in the case of dry eyes, chronic red eyes, allergy, or LSCD. It would also guide the ophthalmologists to arrive at a likely diagnosis of the underlying cause of CCC by using our recommended algorithms. However, this does not mean every ophthalmologist should be familiar with IMT or be equipped to dispense scleral lenses but should be able to diagnose and refer the patient appropriately to those managing ocular surface diseases (OSD). Lastly, our review article emphasizes that it is critical for clinicians managing OSD, like general ophthalmologists or cornea/ocular surface specialists, to train themselves with skillsets such as: administering IMT or working closely with an internist or rheumatologist; dispensing scleral lenses; and performing ocular surface surgeries like MMG, SLET, and keratoprosthesis.
Financial support and sponsorship
Hyderabad Eye Research Foundation (HERF), Hyderabad, Telangana, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dart JK. The 2016 Bowman Lecture Conjunctival curses: Scarring conjunctivitis 30 years on. Eye (Lond) 2017;31:301–32. doi: 10.1038/eye.2016.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radford CF, Rauz S, Williams GP, Saw VP, Dart JK. Incidence, presenting features, and diagnosis of cicatrising conjunctivitis in the United Kingdom. Eye (Lond) 2012;26:1199–208. doi: 10.1038/eye.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobba S, Devlin C, Di Girolamo N, Wakefield D, McCluskey P, Chan E, et al. Incidence, clinical features and diagnosis of cicatrising conjunctivitis in Australia and New Zealand. Eye (Lond) 2018;32:1636–43. doi: 10.1038/s41433-018-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazirani J, Nair D, Shanbhag S, Wurity S, Ranjan A, Sangwan V. Limbal stem cell deficiency-demography and underlying causes. Am J Ophthalmol. 2018;188:99–103. doi: 10.1016/j.ajo.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16:58–69. doi: 10.1016/j.jtos.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine JD, Johnson LB, Weiner M, Stein A, Cash S, Deleoz J, et al. Eye involvement in inherited epidermolysis bullosa: Experience of the National Epidermolysis Bullosa Registry. Am J Ophthalmol. 2004;138:254–62. doi: 10.1016/j.ajo.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Saw VP, Dart JK, Sitaru C, Zillikens D. Cicatrising conjunctivitis with anti-basement membrane autoantibodies in ectodermal dysplasia. Br J Ophthalmol. 2008;92:1403–10. doi: 10.1136/bjo.2007.130583. [DOI] [PubMed] [Google Scholar]
- 8.Shah PR, Williams AM, Pihlblad MS, Nischal KK. Ophthalmic manifestations of mycoplasma-induced rash and mucositis. Cornea. 2019;38:1305–8. doi: 10.1097/ICO.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 9.Nassar A, Tabbara KF, Aljurf M. Ocular manifestations of graft-versus-host disease. Saudi J Ophthalmol. 2013;27:215–22. doi: 10.1016/j.sjopt.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorne JE, Anhalt GJ, Jabs DA. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology. 2004;111:45–52. doi: 10.1016/j.ophtha.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Bjordal O, Norheim KB, Rødahl E, Jonsson R, Omdal R. Primary Sjögren's syndrome and the eye. Surv Ophthalmol. 2020;65:119–32. doi: 10.1016/j.survophthal.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Thorne JE, Jabs DA, Nikolskaia O, Anhalt G, Nousari HC. Discoid lupus erythematosus and cicatrizing conjunctivitis: Clinicopathologic study of two cases. Ocul Immunol Inflamm. 2002;10:287–92. doi: 10.1076/ocii.10.4.287.15595. [DOI] [PubMed] [Google Scholar]
- 13.Thorne JE, Jabs DA, Nikolskaia OV, Mimouni D, Anhalt GJ, Nousari HC. Lichen planus and cicatrizing conjunctivitis: Characterization of five cases. Am J Ophthalmol. 2003;136:239–43. doi: 10.1016/s0002-9394(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 14.Memar O, Jabbehdari S, Caughlin B, Djalilian AR. Ocular surface involvement in pemphigus vulgaris: An interdisciplinary review. Ocul Surf. 2020;18:40–6. doi: 10.1016/j.jtos.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 15.van Mook WNK, Fickers MM, Theunissen PH, van der Kley JA, Duijvestijn JA, Pas HH, et al. Paraneoplastic pemphigus as the initial presentation of chronic lymphocytic leukemia. Ann Oncol. 2001;12:115–8. doi: 10.1023/a:1008324929876. [DOI] [PubMed] [Google Scholar]
- 16.Shanbhag SS, Chanda S, Donthineni PR, Sane SS, Priyadarshini SR, Basu S. Clinical clues predictive of Stevens-Johnson syndrome as the cause of chronic cicatrising conjunctivitis. Br J Ophthalmol. 2019 doi: 10.1136/bjophthalmol-2019-314928. pii: bjophthalmol-2019-314928. doi: 101136/bjophthalmol-2019-314928 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Akpek EK, Polcharoen W, Chan R, Foster CS. Ocular surface neoplasia masquerading as chronic blepharoconjunctivitis. Cornea. 1999;18:282–8. doi: 10.1097/00003226-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Honavar SG, Shields CL, Maus M, Shields JA, Demirci H, Eagle RC. Primary intraepithelial sebaceous gland carcinoma of the palpebral conjunctiva. Arch Ophthalmol. 2001;119:764–7. doi: 10.1001/archopht.119.5.764. [DOI] [PubMed] [Google Scholar]
- 19.Ong HS, Setterfield JF, Minassian DC, Dart JK Mucous Membrane Pemphigoid Study Group 2009–2014. Mucous membrane pemphigoid with ocular involvement: The clinical phenotype and its relationship to direct immunofluorescence findings. Ophthalmology. 2018;125:496–504. doi: 10.1016/j.ophtha.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Grau AE, Setterfield J, Saw VP. How to do conjunctival and buccal biopsies to investigate cicatrising conjunctivitis: Improving the diagnosis of ocular mucous membrane pemphigoid. Br J Ophthalmol. 2013;97:530–1. doi: 10.1136/bjophthalmol-2012-302963. [DOI] [PubMed] [Google Scholar]
- 21.Power WJ, Neves RA, Rodriguez A, Dutt JE, Foster CS. Increasing the diagnostic yield of conjunctival biopsy in patients with suspected ocular cicatricial pemphigoid. Ophthalmology. 1995;102:1158–63. doi: 10.1016/s0161-6420(95)30896-2. [DOI] [PubMed] [Google Scholar]
- 22.Shanbhag SS, Rashad R, Chodosh J, Saeed HN. Long-term effect of a treatment protocol for acute ocular involvement in Stevens-Johnson syndrome/Toxic epidermal necrolysis. Am J Ophthalmol. 2019;208:331–41. doi: 10.1016/j.ajo.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagoner MD. Chemical injuries of the eye: Current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 24.Gregory DG. Treatment of acute Stevens-Johnson syndrome and toxic epidermal necrolysis using amniotic membrane: A review of 10 consecutive cases. Ophthalmology. 2011;118:908–14. doi: 10.1016/j.ophtha.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 25.Sharma N, Thenarasun SA, Kaur M, Pushker N, Khanna N, Agarwal T, et al. Adjuvant role of amniotic membrane transplantation in acute ocular Stevens-Johnson syndrome: A randomized control trial. Ophthalmology. 2016;123:484–91. doi: 10.1016/j.ophtha.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;9:CD009379. doi: 10.1002/14651858.CD009379.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: A clinical approach. Eye Contact Lens. 2019;45:11–8. doi: 10.1097/ICL.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labowsky MT, Stinnett SS, Liss J, Daluvoy M, Hall RP, 3rd, Shieh C. Clinical implications of direct immunofluorescence findings in patients with ocular mucous membrane pemphigoid. Am J Ophthalmol. 2017;183:48–55. doi: 10.1016/j.ajo.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Saw VP, Dart JK, Rauz S, Ramsay A, Bunce C, Xing W, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid strategies and outcomes. Ophthalmology. 2008;115:253–61e1. doi: 10.1016/j.ophtha.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: Long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17–28. doi: 10.1016/j.ajo.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology. 2015;122:248–53. doi: 10.1016/j.ophtha.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Wakamatsu TH, Sant'Anna A, Cristovam PC, Alves VAF, Wakamatsu A, Gomes JAP. Minor salivary gland transplantation for severe dry eyes. Cornea. 2017;36(Suppl 1):S26–33. doi: 10.1097/ICO.0000000000001358. [DOI] [PubMed] [Google Scholar]
- 33.Puranik CJ, Murthy SI, Taneja M, Sangwan VS. Outcomes of cataract surgery in ocular cicatricial pemphigoid. Ocul Immunol Inflamm. 2013;21:449–54. doi: 10.3109/09273948.2013.819106. [DOI] [PubMed] [Google Scholar]
- 34.Narang P, Mohamed A, Mittal V, Sangwan VS. Cataract surgery in chronic Stevens-Johnson syndrome: Aspects and outcomes. Br J Ophthalmol. 2016;100:1542–6. doi: 10.1136/bjophthalmol-2015-308041. [DOI] [PubMed] [Google Scholar]
- 35.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–9. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123:1000–10. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 37.Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. 2019;67:1265–77. doi: 10.4103/ijo.IJO_117_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aravena C, Bozkurt TK, Yu F, Aldave AJ. Long-term outcomes of the boston Type I keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2016;35:1156–64. doi: 10.1097/ICO.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 39.Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: A systematic review. Ocul Surf. 2018;16:272–81. doi: 10.1016/j.jtos.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Basu S, Serna-Ojeda JC, Senthil S, Pappuru RR, Bagga B, Sangwan V. The Aurolab Keratoprosthesis (KPro) versus the Boston Type I Kpro: 5-year clinical outcomes in 134 cases of bilateral corneal blindness. Am J Ophthalmol. 2019;205:175–83. doi: 10.1016/j.ajo.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-term visual outcomes and complications of boston keratoprosthesis Type II implantation. Ophthalmology. 2017;124:27–35. doi: 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness: Long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123:1319–29. doi: 10.1001/archopht.123.10.1319. [DOI] [PubMed] [Google Scholar]
- 43.Basu S, Nagpal R, Serna-Ojeda JC, Bhalekar S, Bagga B, Sangwan V. LVP keratoprosthesis: Anatomical and functional outcomes in bilateral end-stage corneal blindness. Br J Ophthalmol. 2018 doi: 10.1136/bjophthalmol-2017-311649. pii: bjophthalmol-2017-311649. [DOI] [PubMed] [Google Scholar]