Abstract
Purpose of review
This article provides an overview of protein biomarkers for acute respiratory distress syndrome (ARDS) and their potential use in future clinical trials.
Recent findings
The protein biomarkers studied as indices of biological processes involved in the pathogenesis of ARDS may have diagnostic and/or prognostic value. Recently, they also proved useful for identifying ARDS phenotypes and assessing heterogeneity of treatment effect in retrospective analyses of completed clinical trials.
Summary
This article summarizes the current research on ARDS biomarkers and provides insights into how they should be integrated as prognostic and predictive enrichment tools in future clinical trials.
Keywords: Acute respiratory distress syndrome, biomarker, pathogenesis, prognostic enrichment, predictive enrichment
Introduction
Acute respiratory distress syndrome (ARDS), a severe form of acute inflammatory lung injury and alveolar edema, is associated with high mortality and has important physical and cognitive consequences in survivors (1–4). ARDS is characterized by marked clinical and pathophysiologic heterogeneity (5,6), contributing to both underdiagnosis and undertreatment. Therefore, a key challenge in ARDS management, treatment, and prevention remains the establishment of a consensus clinical definition for ARDS that addresses this heterogeneity. The use of biomarkers can provide major insights into the pathophysiologic mechanisms underlying ARDS and can be helpful for diagnosis, risk stratification, and identification of candidate therapeutic targets (7–10). In addition, biomarkers have been crucial in identifying subgroups of patients (or phenotypes) with shared biological features that have prognostic and therapeutic implications in retrospective analyses and in providing a better pathophysiologic understanding of ARDS heterogeneity.
In this article, we review the current use of protein biomarkers for diagnostic, prognostic, and phenotype evaluation in patients with or at risk for ARDS and propose strategies for using biomarkers for predictive and/or prognostic enrichment in future precision ARDS trials.
Biomarkers in ARDS: from pathogenesis insights to diagnosis and risk stratification
Recent reviews summarize how the study of protein biomarkers has provided important insights into the pathophysiologic mechanisms of ARDS (7,8,11–15). These include disruption of the alveolar–capillary barrier (as assessed by elevated protein levels in pulmonary edema fluid) (16,17), exaggerated inflammatory responses (18,19), and lung endothelial (20–22) and epithelial injury determined by measurements of impaired alveolar fluid clearance (23,24) or the presence of specific markers of alveolar epithelial cell injury (e.g., surfactant protein D [SP-D] (25,26) and the soluble form of the receptor for advanced glycation end-products [sRAGE] (27–29)). In this article, we will focus on how plasma biomarkers can inform diagnosis and risk stratification in patients, with or at risk of ARDS, while also reducing heterogeneity and providing a tool for assessing heterogeneity of treatment effects in ARDS clinical trials.
Biomarkers for ARDS diagnosis
RAGE is abundantly expressed on alveolar epithelial type 1 cells; the extracellular domain of this multiligand receptor is released in the setting of lung epithelial injury (27). Plasma levels of sRAGE are elevated in patients with ARDS and are associated with the severity of lung injury and the degree of impairment of alveolar fluid clearance (27–30). Plasma sRAGE also increases in trauma patients who develop ARDS (31) and is associated with ARDS diagnosis (9,32). A high plasma sRAGE at intensive care unit (ICU) admission may also identify patients likely to develop ARDS among those with at least one clinical ARDS risk factor (33). Plasma levels of SP-D, another marker of lung epithelial injury, are also increased in patients with ARDS (26,34).
Elevated plasma angiopoietin-2 (Ang-2), a marker of lung endothelial barrier dysfunction, has predictive value for ARDS development in ICU patients under mechanical ventilation (35,36) or admitted for trauma (31). Notably, the predictive performance of plasma Ang-2 is improved when combined with the clinical Lung Injury Prediction Score (LIPS) (36,37).
Other plasma biomarkers with potential value for ARDS diagnosis include von Willebrand factor (vWF) (another marker of endothelial injury) and proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-8 (31,32,36,38).
Combining multiple biomarkers also has diagnostic value, as panels that include sRAGE, Ang-2, IL-8, IL-10, TNF-α, procollagen peptide III, and brain natriuretic peptide (31) or sRAGE, SP-D, IL-6, IL-8, and club cell secretory protein (26) show better performance for ARDS diagnosis than each biomarker measured alone.
Biomarkers for Risk Stratification in ARDS
Elevated levels of plasma SP-D (25), vWF (38), soluble tumor necrosis factor receptor I and II (sTNFrI; sTNFr2) (18), soluble intercellular adhesion molecule-1 (sICAM-1) (39), and plasminogen activator inhibitor-1 (PAI-1) (40) are all independently associated with worse outcomes in ARDS.
High plasma sRAGE levels are associated with increased lung injury severity, fewer ventilator-free days, and increased mortality in patients with ARDS (41). A meta-analysis of individual patient data from eight studies confirmed an independent association between high baseline plasma sRAGE and high 90-day ARDS mortality (42). Other mortality-associated ARDS biomarkers include Ang-2, IL-6, IL-8, IL-4, IL-2, and Krebs von den Lungen-6 (32,43,44). Low levels of the endogenous anticoagulant protein C were also associated with increased mortality and fewer ventilator-free days (40), whereas high plasma levels of IL-2 receptor and of procalcitonin were associated with increased mortality in unselected patients with ARDS (45) and patients with ARDS due to community-acquired pneumonia (46), respectively.
Models that combine multiple clinical variables and biomarkers have been computed to improve risk stratification. For example, one model combining six clinical variables and eight biomarkers was better at predicting mortality than a model based only on clinical variables or only on biomarkers in a secondary analysis of patients previously enrolled in the ARDS Network ALVEOLI trial (47). A simplified model combining two clinical variables (age, APACHE III) and two biomarkers (SP-D, IL-8) also had good performance and was subsequently validated in three additional cohorts (48).
Enriching future clinical trials with biomarkers
ARDS is a clinically and biologically complex, heterogeneous syndrome with a variety of underlying etiologies including etiologies that are pulmonary and directly injure the lung (such as viral or bacterial pneumonia, aspiration of gastric contents) and etiologies that are non-pulmonary and indirectly injure the lung (such as non-pulmonary sepsis, severe traumatic injuries, transfusion of blood products). The failure of most randomized clinical trials in ARDS to improve patient outcomes may be explained by such heterogeneity; better identification of appropriate subsets of patients to target with novel therapies is still needed (49). In this context, biomarkers could be used both to understand heterogeneity in clinical trial populations and as enrichment strategies in future trials (4,43). Determining how biomarker-derived approaches should be integrated to improve future ARDS research remains a major challenge.
Identification of ARDS phenotypes
The use of biomarkers has already been crucial in addressing ARDS heterogeneity (5,6,14,50). For example, a secondary clinical analysis revealed that patients with ARDS from direct lung injury had higher lung epithelial injury (as assessed by plasma sRAGE and SP-D) and lower lung endothelial injury (as assessed by plasma Ang-2) and inflammation (as assessed by plasma vWF, IL-6, and IL-8) compared to patients with ARDS arising from indirect lung injury (43).
Latent class analysis (LCA) has also been used to study ARDS heterogeneity. This novel approach is unbiased, making no a priori assumptions as to whether there are distinct biological subsets among groups of heterogeneous patients with ARDS arising from different underlying conditions. LCA has consistently identified distinct phenotypes of ARDS characterized by specific combinations of biomarkers and clinical characteristics in secondary analyses of multiple clinical trial cohorts (51–53). A “hyperinflammatory” phenotype, with elevated serum levels of inflammatory markers, was identified in approximately one-third of the patients, while a “hypoinflammatory” phenotype was identified in the remaining patients. Mortality was higher and ventilator-free days were higher in the hyperinflammatory phenotype, as further validated in secondary analyses of three additional clinical trials (54–56). These molecular phenotypes could be reliably identified with a three-biomarker model (plasma IL-8, sTNFr, and serum bicarbonate) (54). Parsimonious three-variable (IL-8, protein C, and bicarbonate) and four-variable (three-variable plus vasopressor use) models were also recently validated for phenotype classification (57). Preliminary studies also suggest that these phenotypes can be identified among patients with ARDS due to SARS-CoV-2 infection (58). Bos et al. confirmed the presence of two molecular phenotypes of ARDS using cluster analysis of data from the MARS cohort (59); these phenotypes could be distinguished by distinct serum IL-6, Ang-1/2, PAI-1, and interferon-gamma levels. Gene expression profiles in peripheral blood leukocytes were used to evaluate the distinct biology underlying these phenotypes including upregulation of pathways of oxidative phosphorylation or mitochondrial dysfunction in the hyperinflammatory (or “reactive”) subgroup (60).
Interestingly, application of these analytic approaches to patients at risk of developing ARDS has revealed the presence of similar inflammatory phenotypes among ICU patients with a clinical risk factor for ARDS (61) and those with an increased risk of postoperative pulmonary complications after elective abdominal surgery (62).
Heterogeneity of treatment effect and enrichment strategies
The concept of “heterogeneity of treatment effects” is attributable to the variability in therapeutic responses among distinct phenotypes. Protein biomarkers could be helpful to overcome this issue and facilitate biomarker-based selection of patients to target in “enriched” trials. Prospective enrichment strategies can include both predictive and prognostic enrichment. The overall aim of predictive enrichment is to personalize treatments for subjects with shared biologic profiles rather than searching for treatments that are applicable to everyone (63,64). Trials that utilize predictive enrichment enroll a smaller and more homogeneous subgroup more likely to respond to an intervention targeting a specific biologic mechanism (50,64,65).
The overall aim of prognostic enrichment is to identify a subset of patients that is more likely to develop an outcome of interest (such as mortality) who can be selected to increase the power to detect a benefit from a therapeutic intervention (50,64,65). These enrichment strategies can be used to guide the enrollment of selected patients such as those most likely to develop the outcome or to respond to a given therapy.
Biomarkers may be useful for prognostic, predictive, or both forms of enrichment. For example, severe hypoxemia can have value for both prognostic (higher risk of death) and predictive (better response to prone position) enrichment in patients with ARDS (1,66).
Treatment responsive subgroups have been reported within the context of completed clinical trials through retrospective analysis of LCA-identified phenotypes.
A secondary analysis of the ALVEOLI trial of two levels of positive end-expiratory pressure (PEEP) for treatment of ARDS (52) showed a decreased 90-day mortality (from 50% to 40%) in patients with the hyperinflammatory phenotype exposed to higher versus lower PEEP (53). By contrast, mortality was higher when higher levels of PEEP were used in the hypoinflammatory phenotype (53), although the original trial found no difference. Similarly, ARDS mortality was lower in the hyperinflammatory phenotype with a liberal rather than a conservative fluid strategy in the Fluid and Catheter Treatment Trial (FACTT) for ARDS (54), whereas the original trial found no effect of fluid strategy on mortality and more ventilator-free days with the conservative fluid strategy (67). Similar distinct effects have also been reported for simvastatin in a secondary analysis of the HARP-2 trial of simvastatin in ARDS (68). The original trial found no difference in clinical outcomes between simvastatin and placebo, whereas a secondary analysis showed better survival in the hyperinflammatory phenotype with simvastatin but no difference in therapeutic response among patients with the hypoinflammatory phenotype. To summarize, inflammatory ARDS phenotypes have been consistently identified in secondary analyses of clinical trial cohorts and hold great promise for application as tools for both predictive and prognostic enrichment. However, they should now be prospectively validated and rapid biomarker measurement methods are still needed to allow practical incorporation into future enriched trials (see the Challenges and limitations section below).
Other ARDS phenotypes have also been described, such as radiographic phenotypes of focal and nonfocal ARDS based on the extent of loss of aeration apparent in lung CT scans (69). Radiographic phenotypes are thought to identify patients with different lung physiology who may have distinct responses to mechanical ventilation (5). A French prospective observational multicenter study showed higher plasma sRAGE and PAI-1 levels in radiographically nonfocal compared to focal ARDS (70). Nonfocal ARDS was also associated with increased mortality (70) and with greater impairment of alveolar fluid clearance (71), implicating sRAGE as a useful correlate of radiographic ARDS phenotypes (72). The multicenter randomized controlled Lung Imaging for Ventilator Setting in ARDS (LIVE) trial evaluated different mechanical ventilation strategies tailored to radiographic phenotypes in 400 patients with moderate-to-severe ARDS (73). No between-group differences in 90-day mortality were seen in the intention-to-treat analysis of the primary endpoint; however, a prespecified post-hoc analysis revealed misclassification of 21% of the radiographic phenotypes assigned at the time of randomization (73,74). It would now be interesting to evaluate whether measurement of plasma sRAGE, reported as higher in nonfocal compared to focal ARDS (28,70,72), might improve “real-time” radiographic phenotyping in future trials.
Another approach to predictive enrichment is currently under investigation in patients with moderate-to-severe ARDS unresolved between day 5 and day 14 after onset. Here, elevated BAL procollagen III, a marker of lung fibroproliferation, is used as an entry criteria to the Procollagen-3 Driven Corticosteroids for Persistent Acute Respiratory Distress Syndrome (ProCoCo) multicenter randomized controlled trial of methylprednisolone versus placebo (ClinicalTrials.gov Identifier: NCT03371498) (75,76).
In summary, both predictive and prognostic enrichment strategies may improve the efficiency of randomized controlled trials by increasing the likelihood of detecting a beneficial effect from a targeted therapeutic intervention in patients who are more likely to develop the outcome of interest (50,77,78). The main plasma protein biomarkers that have been evaluated in patients at risk of developing ARDS and those with ARDS, and their potential applications to enrich future clinical trials are summarized in the Table and the Figure.
Table. Selected plasma protein biomarkers that have been evaluated in patients at risk of developing and those with ARDS, and their potential applications to enrich future clinical trials.
ARDS: acute respiratory distress syndrome. Ang: angiopoietin. sRAGE: soluble receptor for advanced glycation end-products. IL: interleukin. sTNFr: soluble tumor necrosis factor receptor. TNF: tumor necrosis factor. ICU: intensive care unit. SP-D: surfactant protein D. PEEP: positive end-expiratory pressure. ST: suppression of tumorigenicity. PAI: plasminogen activator inhibitor.
| Plasma biomarker(s) | Potential application(s) | Reference(s) |
|---|---|---|
| Patients at risk of ARDS | ||
| Ang-2 |
|
(36) |
| sRAGE | (33) | |
| IL-6, IL-8, IL-10, sTNFr1, ST-2, fractalkine, sRAGE, Ang-2, procalcitonin, pentraxin-3 |
|
(59) |
| sRAGE, Ang-2, IL-8, IL-10, TNF-α, procollagen peptide III, and brain natriuretic peptide |
|
(31) |
| sRAGE, SP-D, IL-6, IL-8, and club cell secretory protein |
|
(26) |
| TNF-α, IL-6, IL-8 |
|
(60*) |
| Patients with ARDS | ||
| IL-8, sTNFr, bicarbonate |
|
(53**) |
| IL-8, protein C, bicarbonate | (56) | |
| IL-6, Ang-1/2, PAI-1 | (57**) | |
| sRAGE |
|
(28*,29,42*,69*,71,88) |
Figure. Schematic representation of the value of selected protein biomarkers for diagnosis of acute respiratory distress syndrome and potential application for predictive and prognostic trial enrichment.
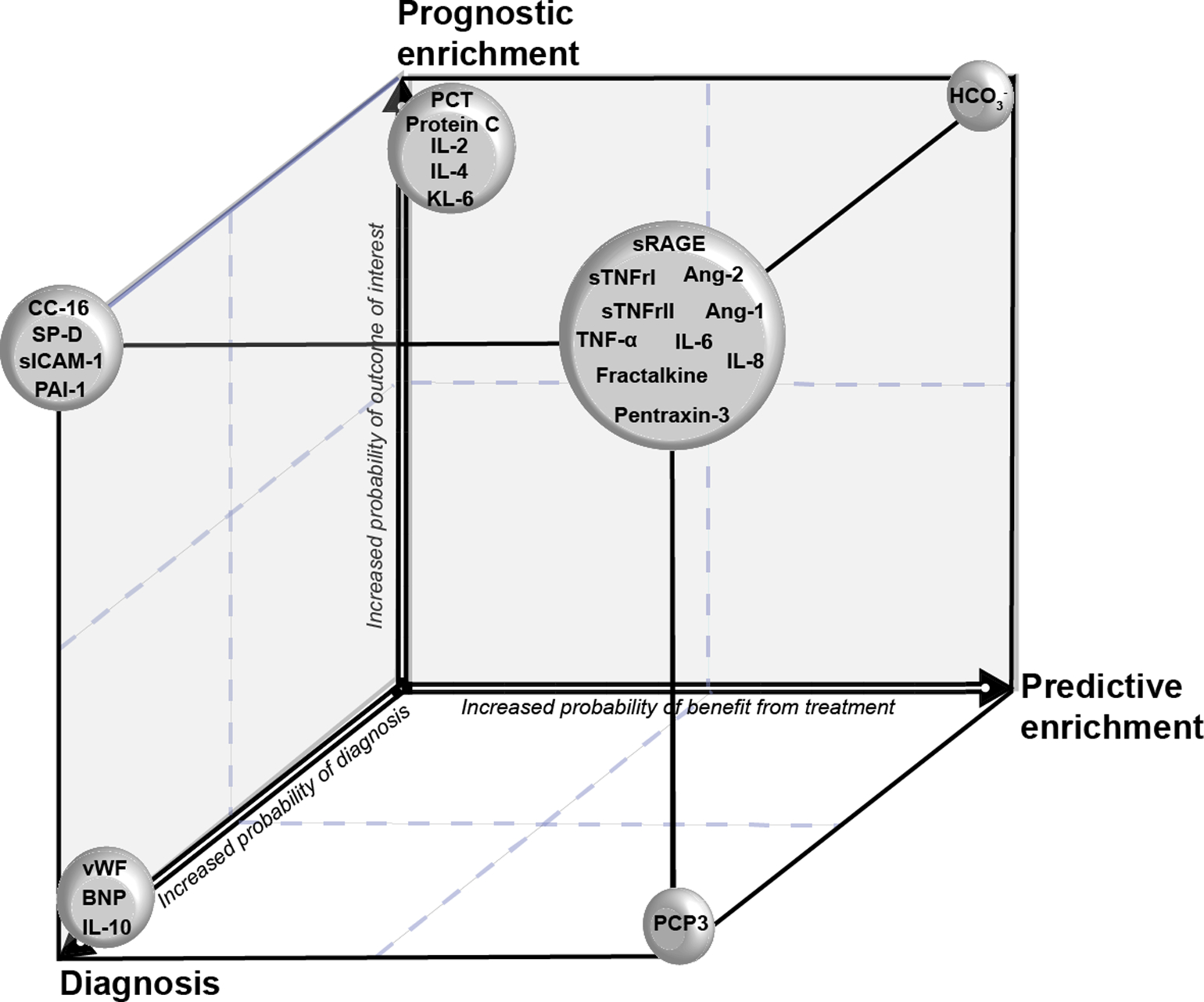
KL-6: Krebs von den Lungen 6. PCT: procalcitonin. IL: interleukin. SP-D: surfactant protein D. sICAM-1: soluble intercellular adhesion molecule-1. CC16: club cell secretory protein. PAI-1: plasminogen activator inhibitor-1. vWF: von Willebrand factor. BNP: brain natriuretic peptide. sRAGE: soluble receptor for advanced glycation end-products. sTNFr: soluble tumor necrosis factor receptor. Ang: angiopoietin. TNF: tumor necrosis factor. PCP3: procollagen peptide III.
Challenges and limitations
Studies incorporating biomarker-driven strategies for ARDS management are unfortunately scarce, but they should be further evaluated to assess the full potential of biomarkers, in addition to their use as enrichment tools. A major potential application of biomarkers is the identification of biologic pathways to target in future interventional trials (79). For example, measuring plasma sRAGE could be useful in selecting patients with increased lung epithelial injury who may benefit from epithelial-targeted therapies, such as beta-agonists, keratinocyte growth factor, or anti-RAGE therapies, to prevent or treat ARDS (28,29,42,80–85). Conversely, ARDS patients with pronounced lung endothelial injury (e.g., as assessed by plasma Ang-2) may benefit more from candidate therapies, such as recombinant Ang-1, that target the endothelium (35,43).
Ideally, biomarkers should be useful for monitoring the progression or repair of lung injury, as well as the therapeutic response in ARDS (86). For instance, the use of a lung-protective, low-tidal volume ventilation strategy was associated with a decrease (or smaller increase) in plasma lung epithelial injury markers SP-D and sRAGE (25,41,87). A recent preliminary report of a secondary analysis of longitudinal sRAGE plasma levels in patients previously enrolled in the LIVE trial revealed an association between changes in plasma sRAGE over the first week after ARDS onset and 90-day survival. Similarly, a strategy of maximal alveolar recruitment (with higher PEEP and repeated recruitment maneuvers) was associated with increasing plasma sRAGE levels, suggesting increased injury to the lung alveolar epithelium due to this strategy in focal ARDS. Thus, plasma sRAGE may have potential value as a surrogate outcome for monitoring responses to ventilator settings in patients with ARDS (72,88). These approaches warrant further validation in prospective clinical studies, as they hold the promise for developing novel precision therapies that are effective in specific phenotypes (89).
The major limitations of biomarker-driven approaches to ARDS trials include the urgent need for prospective validation of most phenotypes described in secondary analyses of previous studies. The lack of a point-of-care assay for evaluating the candidate biomarkers at the bedside also limits the current application of biomarker-based enrichment strategies in “real time”. The Clinical Evaluation of a Point of Care Assay to Identify Phenotypes in the Acute Respiratory Distress Syndrome (PHIND) study is currently enrolling patients with ARDS for prospective identification of hyperinflammatory and hypoinflammatory phenotypes using a novel POC assay of serum IL-6 and sTNFr1 (ClinicalTrials.gov Identifier: NCT04009330). This POC assay has been recently used to identify phenotypes in patients with ARDS due to coronavirus disease 2019 (COVID-19) (58).
The hyperinflammatory and hypoinflammatory phenotypes of ARDS could be driven by genetic and/or environmental factors, and this deserves further investigation. This review has focused only on plasma protein biomarkers, as they have been most studied; however, measurements of biomarkers in the alveolar compartment (44,90), as well as the study of genetic variants, DNA methylation, transcriptomics (60,91,92), or metabolomics (93–96), may also be very important in meeting the challenge of precision ARDS medicine (49). In particular, examining the exhaled breath as a source of volatile organic compounds that can serve as ARDS biomarkers (97,98) or measuring biomarkers in the fluid collected from heat-and-moisture-exchange filters (as commonly used in mechanically ventilated patients) (99) represent promising, non-invasive methods for sampling the distal airspace in patients with ARDS. However, these methods and their potential value in ARDS management need to be further assessed.
Conclusion
Biomarker research has promise in elucidating the pathobiology of acute lung injury and repair, and protein biomarkers have been investigated for diagnosis and risk stratification in ARDS. Several biomarkers, such as proinflammatory cytokines and markers of lung epithelial and endothelial injury, can aid in establishing ARDS patient phenotypes and identifying potential biological treatment targets. Numerous challenges remain, but recent advances in both biomarker research and trial design open up opportunities for using biomarkers to facilitate more personalized approaches in future ARDS clinical trials.
Key points.
Biomarkers can provide major insights into the pathophysiologic mechanisms involved in ARDS, thereby aiding the diagnosis, risk stratification, and identification of candidate therapeutic targets.
The recent use of biomarkers has identified distinct phenotypes among patients with ARDS, with potential implications for assessment of prognosis and therapeutic responses in patients with ARDS or at risk of developing the syndrome.
Biomarkers should now be integrated as prognostic and predictive enrichment tools in future clinical trials to account for the heterogeneity of treatment effect observed in a number of negative ARDS clinical trials to date.
Financial support and sponsorship
This work was supported by grants from the Société Française d’Anesthésie et Réanimation (SFAR) French Ministry of Health (Bourse de Recherche 2018) (MJ) and by grants NIH HL103836, HL135849, HL126176 (LBW). The funders had no influence on the study design, conduct, and analysis or in the preparation of this article.
Footnotes
The authors declare that there is no conflict of interest regarding the publication of this paper.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012. June 20;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016. February 23;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 3.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017. August 10;377(6):562–72.. [DOI] [PubMed] [Google Scholar]
- 4.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019. March 14;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A recent review on ARDS, with major insights on pathophysiologic mechanisms and potential biomarkers.
- 5.Jabaudon M, Blondonnet R, Audard J, et al. Recent directions in personalised acute respiratory distress syndrome medicine. Anaesth Crit Care Pain Med. 2018. June;37(3):251–8. [DOI] [PubMed] [Google Scholar]
- 6.Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019. February;25(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review highlights the recent identification of distinct phenotypes among patients with ARDS and how it can improve our understanding of the pathophysiology of acute lung injury and open novel clinical perspectives for ARDS research.
- 7.Binnie A, Tsang JLY, dos Santos CC. Biomarkers in acute respiratory distress syndrome. Curr Opin Crit Care. 2014. February;20(1):47–55. [DOI] [PubMed] [Google Scholar]
- 8.Blondonnet R, Constantin J-M, Sapin V, Jabaudon M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis Markers. 2016. February 11;2016:3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Zee P, Rietdijk W, Somhorst P, et al. A systematic review of biomarkers multivariately associated with acute respiratory distress syndrome development and mortality. Crit Care. 2020. May 24;24(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang P, Esper AM, Martin GS. The Future of ARDS Biomarkers: Where Are the Gaps in Implementation of Precision Medicine? In: Vincent J-L, editor. Annual Update in Intensive Care and Emergency Medicine 2020. Cham: Springer International Publishing; 2020. p. 91–100. [Google Scholar]
- 11.Walter JM, Wilson J, Ware LB. Biomarkers in acute respiratory distress syndrome: from pathobiology to improving patient care. Expert Rev Respir Med. 2014. October;8(5):573–86. [DOI] [PubMed] [Google Scholar]
- 12.Meyer NJ, Calfee CS. Novel translational approaches to the search for precision therapies for acute respiratory distress syndrome. Lancet Respir Med. 2017. June;5(6):512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu AC, Kiley JP, Noel PJ, et al. Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am J Respir Crit Care Med. 2018. December 15;198(12):e116–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spadaro S, Park M, Turrini C, et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm. 2019. January 15;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarma A, Calfee CS, Ware LB. Biomarkers and Precision Medicine: State of the Art. Crit Care Clin 2020. January;36(1):155–65. [DOI] [PubMed] [Google Scholar]
- 16.Sprung CL, Rackow EC, Fein IA, et al. The spectrum of pulmonary edema: differentiation of cardiogenic, intermediate, and noncardiogenic forms of pulmonary edema. Am Rev Respir Dis. 1981. December;124(6):718–22. [DOI] [PubMed] [Google Scholar]
- 17.Fein A, Grossman RF, Jones JG, et al. The value of edema fluid protein measurement in patients with pulmonary edema. Am J Med. 1979. July;67(1):32–8. [DOI] [PubMed] [Google Scholar]
- 18.Parsons PE, Matthay MA, Ware LB, Eisner MD, National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials Network. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005. March;288(3):L426–31. [DOI] [PubMed] [Google Scholar]
- 19.Meduri GU, Kohler G, Headley S, et al. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995. November;108(5):1303–14. [DOI] [PubMed] [Google Scholar]
- 20.Idell S, Kueppers F, Lippmann M, et al. Angiotensin converting enzyme in bronchoalveolar lavage in ARDS. Chest. 1987. January;91(1):52–6. [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990. August;86(2):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly SC, Haslett C, Dransfield I, et al. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994. July 23;344(8917):215–9. [DOI] [PubMed] [Google Scholar]
- 23.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001. May;163(6):1376–83. [DOI] [PubMed] [Google Scholar]
- 24.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med. 2014. June 1;189(11):1301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003. November;58(11):983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware LB, Koyama T, Zhao Z, Jet et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013. October 24;17(5):R253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006. May 1;173(9):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011. March;39(3):480–8. [DOI] [PubMed] [Google Scholar]; * This small single-center study was the first to confirm that plasma sRAGE was elevated in patients with ARDS, whatever their septic status, and correlated with lung injury severity.
- 29.Jabaudon M, Blondonnet R, Roszyk L, et al. Soluble Receptor for Advanced Glycation End-Products Predicts Impaired Alveolar Fluid Clearance in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2015. July 15;192(2):191–9. [DOI] [PubMed] [Google Scholar]
- 30.Jones TK, Feng R, Kerchberger VE, et al. Plasma sRAGE Acts as a Genetically Regulated Causal Intermediate in Sepsis-associated Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020. January 1;201(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fremont RD, Koyama T, Calfee CS, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010. May;68(5):1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terpstra ML, Aman J, van Nieuw Amerongen GP, Groeneveld ABJ. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis*. Crit Care Med. 2014. March;42(3):691–700. [DOI] [PubMed] [Google Scholar]
- 33.Jabaudon M, Berthelin P, Pranal T, et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep. 2018. February 8;8(1):2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J, Pabon M, Choi AMK, et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: validation in US and Korean cohorts. BMC Pulm Med. 2017. December 15;17(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008. October;63(10):903–9. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013. April 1;187(7):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011. February 15;183(4):462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004. October 1;170(7):766–72. [DOI] [PubMed] [Google Scholar]
- 39.Calfee CS, Eisner MD, Parsons PE, et al. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009. February;35(2):248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007. August;35(8):1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008. December;63(12):1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jabaudon M, Blondonnet R, Pereira B, et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med. 2018. September;44(9):1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This meta-analysis of individual data from 746 patients with ARDS previously enrolled in 8 studies found that higher baseline plasma sRAGE was associated with higher 90-day mortality, independently of tidal volume and driving pressure.
- 43.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015. June 1;147(6):1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This secondary analysis of 953 patients from two previous cohorts was the first to report distinct lung epithelial and endothelial biomarker profiles between direct and indirect ARDS.
- 44.García-Laorden MI, Lorente JA, Flores C, et al. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017. July;5(14):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovach MA, Stringer KA, Bunting R, et al. Microarray analysis identifies IL-1 receptor type 2 as a novel candidate biomarker in patients with acute respiratory distress syndrome. Respir Res. 2015. February 21;16(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng J-S, Chan M-C, Hsu J-Y, et al. Procalcitonin is a valuable prognostic marker in ARDS caused by community-acquired pneumonia. Respirology. 2008. June;13(4):505–9. [DOI] [PubMed] [Google Scholar]
- 47.Ware LB, Koyama T, Billheimer DD, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010. February;137(2):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first study to investigate the value of combined biomarkers to predict ARDS development and outcome.
- 48.Zhao Z, Wickersham N, Kangelaris KN, May AK, Bernard GR, Matthay MA, et al. External validation of a biomarker and clinical prediction model for hospital mortality in acute respiratory distress syndrome. Intensive Care Med. 2017. August;43(8):1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beitler JR, Goligher EC, Schmidt M, et al. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med. 2016. May;42(5):756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prescott HC, Calfee CS, Thompson BT, et al. Toward Smarter Lumping and Smarter Splitting: Rethinking Strategies for Sepsis and Acute Respiratory Distress Syndrome Clinical Trial Design. Am J Respir Crit Care Med. 2016. July 15;194(2):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]; * An important perspective paper on heterogeneity in critical care syndromes such as ARDS and sepsis and how future trial design strategies could help moving towards more personalized approaches.
- 51.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000. May 4;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 52.Brower RG, Lanken PN, MacIntyre N, Met al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004. July 22;351(4):327–36. [DOI] [PubMed] [Google Scholar]
- 53.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014. August;2(8):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The first use of latent class analysis to identify the hyperinflammatory and hypoinflammatory ARDS phenotypes, with distinct outcomes and prognostic response to the level of PEEP, through secondary analysis of the ARMA and ALVEOLI trials.
- 54.Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med. 2017. February 1;195(3):331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018. September;6(9):691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018. November;44(11):1859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinha P, Delucchi KL, McAuley DF, et al. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med. 2020. March;8(3):247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Recent report of a simplified 3-variable model (IL-8, bicarbonate, and protein C) developed and validated to further ease the identification of inflammatory phenotypes in future studies.
- 58.Sinha P, Calfee CS, Cherian S, et al. Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med. 2020. August 27; S2213–2600(20)30366–0. doi: 10.1016/S2213-2600(20)30366-0 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bos LDJ, Scicluna BP, Ong DSY, et al. Understanding Heterogeneity in Biologic Phenotypes of Acute Respiratory Distress Syndrome by Leukocyte Expression Profiles. Am J Respir Crit Care Med. 2019. July 1;200(1):42–50. [DOI] [PubMed] [Google Scholar]; ** The first study to report distinct underlying biological mechanisms between the hypoinflammatory and the hyperinflammatory phenotypes, with differential gene expression profiles in peripheral blood leukocytes with regards to pathways such as oxidative phosphorylation and mitochondrial dysfunction.
- 60.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding Heterogeneity in Biologic Phenotypes of Acute Respiratory Distress Syndrome by Leukocyte Expression Profiles. Am J Respir Crit Care Med. 2019. July 1;200(1):42–50. [DOI] [PubMed] [Google Scholar]
- 61.Kitsios GD, Yang L, Manatakis DV, et al. Host-Response Subphenotypes Offer Prognostic Enrichment in Patients With or at Risk for Acute Respiratory Distress Syndrome. Crit Care Med. 2019. December;47(12):1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first study to report the presence of inflammatory phenotypes among ICU patients without ARDS.
- 62.Serpa Neto A, Bos LD, Campos PPZA, et al. Association between pre-operative biological phenotypes and postoperative pulmonary complications: An unbiased cluster analysis. Eur J Anaesthesiol. 2018. September;35(9):702–9. [DOI] [PubMed] [Google Scholar]; * The first study to report the presence of inflammatory phenotypes among surgical patients at risk of developing postoperative pulmonary complications.
- 63.Iwashyna TJ, Burke JF, Sussman JB, et al. Implications of Heterogeneity of Treatment Effect for Reporting and Analysis of Randomized Trials in Critical Care. Am J Respir Crit Care Med. 2015. November 1;192(9):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seymour CW, Gomez H, Chang C-CH, et al. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017. October 18;21(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.FDA. Draft guidance: enrichment strategies for clinical trials to support approval of human drugs and biological products. Available at: https://wwwfdagov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm332181pdf. 2012.
- 66.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013. June 6;368(23):2159–68. [DOI] [PubMed] [Google Scholar]
- 67.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006. June 15;354(24):2564–75. [DOI] [PubMed] [Google Scholar]
- 68.McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin in the Acute Respiratory Distress Syndrome. N Engl J Med. 2014. October 30;371(18):1695–703. [DOI] [PubMed] [Google Scholar]
- 69.Puybasset L, Cluzel P, Gusman P, et al. Regional distribution of gas and tissue in acute respiratory distress syndrome. I. Consequences for lung morphology. Intensive Care Med. 2000. July 1;26(7):857–69. [DOI] [PubMed] [Google Scholar]
- 70.Mrozek S, Jabaudon M, Jaber S, et al. Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. Chest. 2016. November;150(5):998–1007. [DOI] [PubMed] [Google Scholar]; * This is the first observational study to report elevated levels of plasma sRAGE in patients with a nonfocal radiographic phenotype of ARDS, suggesting increased lung epithelial injury in this subgroup.
- 71.Jabaudon M, Blondonnet R, Lutz J, et al. Net alveolar fluid clearance is associated with lung morphology phenotypes in acute respiratory distress syndrome. Anaesth Crit Care Pain Med. 2016. April;35(2):81–6. [DOI] [PubMed] [Google Scholar]
- 72.Jabaudon M, Pereira B, Laroche E, et al. Changes in Plasma Soluble RAGE Are Associated with Survival in Patients with ARDS: Secondary Analysis of a Trial of Lung Imaging for Ventilator Setting. In: A104 Lungs, Bugs, and the Diaphragm: Translational Studies in the ICU. American Thoracic Society International Conference 2020. A2628–A2628 Available at: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2628. [Google Scholar]
- 73.Constantin J-M, Jabaudon M, Lefrant J-Y, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019. October;7(10):870–80. [DOI] [PubMed] [Google Scholar]; ** This French multicenter RCT was the first to use pre-randomization phenotyping in 400 patients with moderate-severe ARDS to assess the impact of a personalized ventilation strategy tailored to radiographic phenotypes of focal versus nonfocal ARDS, compared to a conventional, low-tidal volume ventilation strategy.
- 74.Hendrickson CM, Calfee CS. A new frontier in ARDS trials: phenotyping before randomisation. Lancet Respir Med. 2019. October;7(10):830–1. [DOI] [PubMed] [Google Scholar]
- 75.Forel J-M, Guervilly C, Hraiech S, et al. Type III procollagen is a reliable marker of ARDS-associated lung fibroproliferation. Intensive Care Med. 2015. January 1;41(1):1–11. [DOI] [PubMed] [Google Scholar]
- 76.Hamon A, Scemama U, Bourenne J, et al. Chest CT scan and alveolar procollagen III to predict lung fibroproliferation in acute respiratory distress syndrome. Ann Intensive Care. 2019. March 27;9(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janz DR, Bastarache JA, Rice TW, et al. Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: the Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis trial. Crit Care Med. 2015. March;43(3):534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015. February 17;313(7):677–86. [DOI] [PubMed] [Google Scholar]
- 79.Reilly JP, Calfee CS, Christie JD. Acute Respiratory Distress Syndrome Phenotypes. Semin Respir Crit Care Med. 2019. February;40(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011. September 1;184(5):561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006. February 1;173(3):281–7. [DOI] [PubMed] [Google Scholar]
- 82.Gao Smith F, Perkins GD, Gates S, et al. Effect of intravenous β−2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012. January 21;379(9812):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perkins GD, Gates S, Park D, et al. The beta agonist lung injury trial prevention. A randomized controlled trial. Am J Respir Crit Care Med. 2014. March 15;189(6):674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blondonnet R, Audard J, Belville C, et al. RAGE inhibition reduces acute lung injury in mice. Sci Rep. 2017. August 3;7(1):7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Audard J, Godet T, Blondonnet R, et al. Inhibition of the Receptor for Advanced Glycation End-Products in Acute Respiratory Distress Syndrome: A Randomised Laboratory Trial in Piglets. Sci Rep. 2019. June 25;9(1):9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001. March;69(3):89–95. [DOI] [PubMed] [Google Scholar]
- 87.Determann RM, Royakkers AANM, Haitsma JJ, et al. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med. 2010. February 16;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jabaudon M, Hamroun N, Roszyk L, et al. Effects of a recruitment maneuver on plasma levels of soluble RAGE in patients with diffuse acute respiratory distress syndrome: a prospective randomized crossover study. Intensive Care Med. 2015. March 20;41(5):846–55. [DOI] [PubMed] [Google Scholar]
- 89.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017. June;5(6):524–534. [DOI] [PubMed] [Google Scholar]
- 90.Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002. November;161(5):1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kangelaris KN, Prakash A, Liu KD, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015. June 1;308(11):L1102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morrell ED, Radella F 2nd, Manicone AM, et al. Peripheral and Alveolar Cell Transcriptional Programs Are Distinct in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2018. February 15;197(4):528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stringer KA, Serkova NJ, Karnovsky A, et al. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma 1H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Lung Cell Mol Physiol. 2011. January;300(1):L4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinha N, Viswan A, Singh C, et al. Metabolomics based predictive biomarker model of ARDS: A systemic measure of clinical hypoxemia. PLoS One. 2017. November 2;12(11):e0187545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Metwaly S, Cote A, Donnelly SJ, et al. Evolution of ARDS biomarkers: Will metabolomics be the answer? Am J Physiol Lung Cell Mol Physiol. 2018. October 1;315(4):L526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Metwaly SM, Winston BW. Systems Biology ARDS Research with a Focus on Metabolomics. Metabolites. 2020. May 19;10(5):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bos LDJ, Weda H, Wang Y, et al. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur Respir J. 2014. July;44(1):188–97. [DOI] [PubMed] [Google Scholar]
- 98.Bos LDJ, Schultz MJ, Sterk PJ. Exhaled breath profiling for diagnosing acute respiratory distress syndrome. BMC Pulm Med. 2014. April 26;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McNeil JB, Shaver CM, Kerchberger VE, et al. Novel Method for Noninvasive Sampling of the Distal Airspace in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2018. April 15;197(8):1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


