Abstract
The present study verified that 1,8-dihydroxyanthraquinone (1), a common component in some industrial raw materials and dyes, could be converted into peniphenone (2), which possesses immunosuppressive activity and other medicinal potential, by Aleurodiscus mirabilis fermentation. The yield of peniphenone (2) after 7 days of fermentation was 11.05 ± 2.19%. To reveal the transformation mechanism, two secondary metabolites, emodin (3) and monodictyphenone (4), were isolated from the fermentation broth of A. mirabilis, implying that polyketide metabolic pathways from emodin (3) to monodictyphenone (4) might exist in A. mirabilis. 1,8-Dihydroxyanthraquinone (1) was suspected to be converted into peniphenone (2) via the same pathway since emodin (3) and 1,8-dihydroxyanthraquinone (1) share very similar skeletons. The P450 enzyme and Baeyer–Villiger oxidase in A. mirabilis were confirmed to catalyze this biotransformation on the basis of ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) analysis. This novel investigation could shed light on the mechanism and therefore development of peniphenone production from 1,8-dihydroxyanthraquinone by microbial fermentation.
Introduction
Biotransformation shows great advantages compared with chemical synthesis due to its regio- and stereoselectivity, environmentally friendly procedures, and mild reaction conditions.1,2 In general, biotransformations can replace a multistep synthesis with a single microbial transformation.3,4 Therefore, bioconversion could play a critical role in the synthesis of bioactive compounds.5 Our previous studies have shown that microbial autogenic metabolic processes and many transformations have been achieved by microorganisms.6−11
Peniphenone (2) is a benzophenone12,13 that was first isolated from the mangrove endophytic fungus Penicillium sp. ZJ-SY2 and has been reported to possess satisfactory immunosuppressive activity (the IC50 values of Con A-induced and LPS-induced were 8.1 and 9.3 μg/mL, respectively).14 Therefore, investigation into the large-scale production of peniphenone (2) by microbial fermentation is crucial in this field.
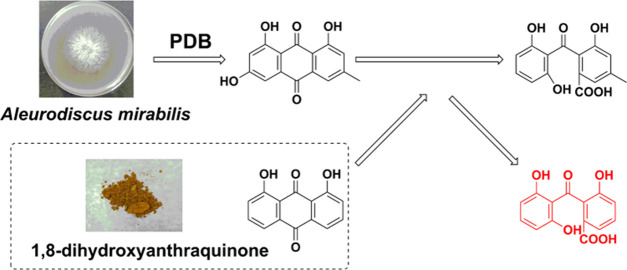
Aleurodiscus mirabilis is characterized by small disc-like or squamous fruiting bodies, large amyloid spores, and conspicuous sterile elements in the hymenium. Its active metabolites have been reported in the literature.15 However, there has been no report on the biotransformation of A. mirabilis. In the present study, the biotransformation of 1,8-dihydroxyanthraquinone (1) to peniphenone (2) was detected in A. mirabilis fermentation broth. 1,8-Dihydroxyanthraquinone (1) is a common component in some industrial raw materials and dyes.16 To investigate the process of this biotransformation, the secondary metabolites of A. mirabilis were isolated and analyzed. Two key compounds, emodin (3) and monodictyphenone (4), were isolated from the fungal fermentation broth without the addition of compound 1, which indicates that polyketide metabolism related to emodin may exist in A. mirabilis. As shown in Figure 1, the biological transformation pathway of 2 is probably identical to that of emodin because 1,8-dihydroxyanthraquinone (1) shares the same moiety with emodin (an intermediate product in the synthetic process of a fungal polyketide). Therefore, this biotransformation might be catalyzed by a P450 enzyme and a Baeyer–Villiger oxidase. The conversion efficiency was determined by high-performance liquid chromatography (HPLC). This is the first report to investigate the biotransformation of 1,8-dihydroxyanthraquinone and could serve as a reference for further studies on the internal metabolism of this compound.
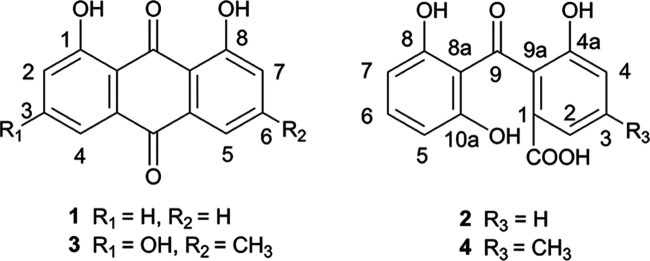
Figure 1.
Structures of compounds 1–4.
Results and Discussion
Screening of Biotransformation and Transformed Product Yield
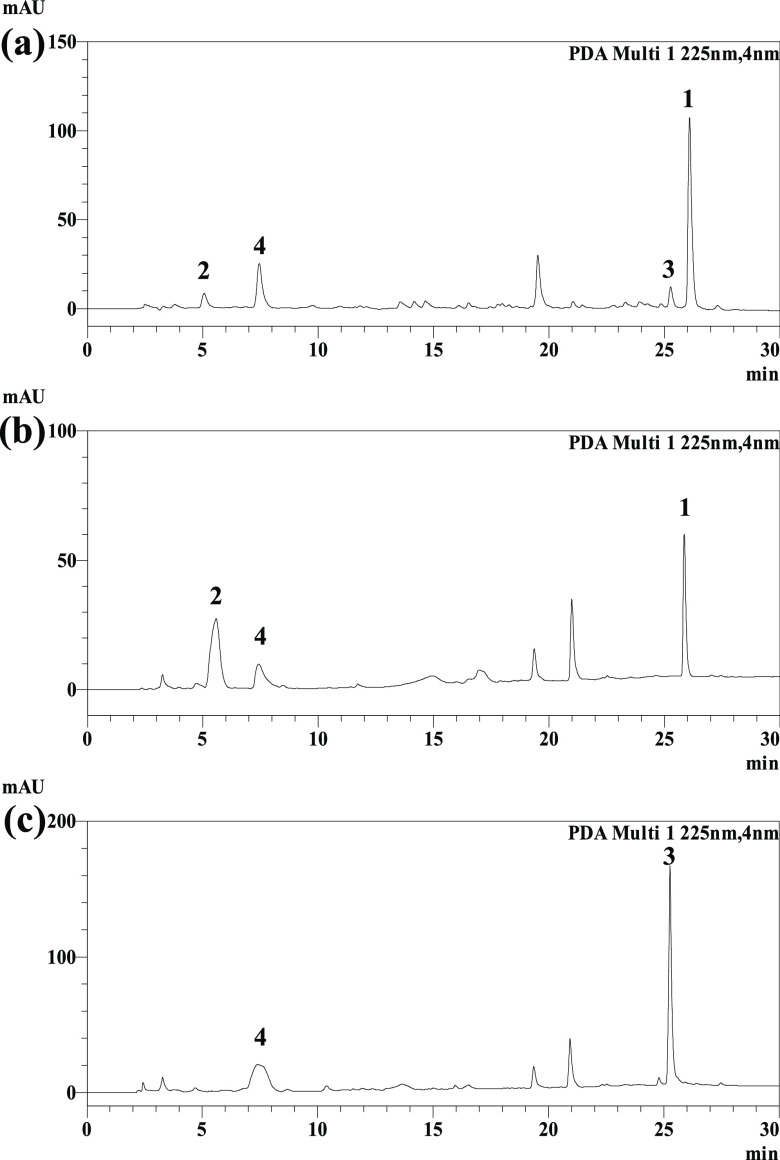
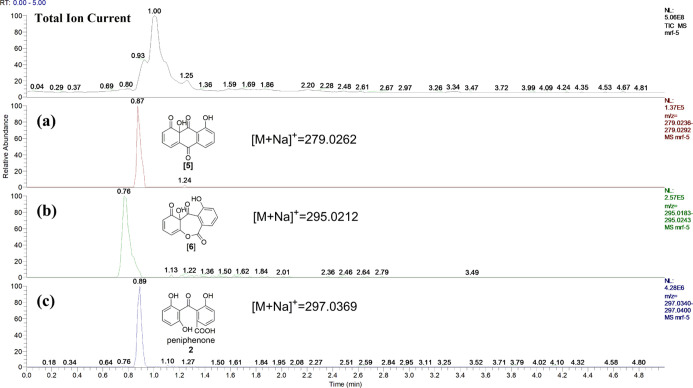
A. mirabilis was initially screened for its ability to catalyze biotransformation reactions using PDB with 1,8-dihydroxyanthraquinone as the substrate. HPLC experiments revealed that A. mirabilis fermentation could enhance the prominent biotransformation and reduce the metabolism of compound 3 (Figure 2). Fermentation of 1,8-dihydroxyanthraquinone by A. mirabilis for 7 days followed by separation of the transformation product yielded compound 2. The transformation process and transformed product yield under different culture conditions were also investigated (Figure S4). The yield of compound 2 was highest when the fermentation temperature was controlled at 28 °C and the pH value of the culture medium was 7. As the fermentation time increased, compound 2 gradually appeared, reaching its highest yield 7 days after substrate addition. Then, the yield remained the same, corresponding to a maximum yield of 11.15 ± 2.19%. The poor water solubility of 1,8-dihydroxyanthraquinone may be an obstacle for further yield improvement.17,18
Figure 2.
HPLC chromatograms of the extracts of 1,8-dihydroxyanthraquinone (1) by A. mirabilis fermentation for 3 days (a), 7 days (b), and the blank microbial sample fermented for 7 days (c).
Analysis of the Causes of Biotransformation
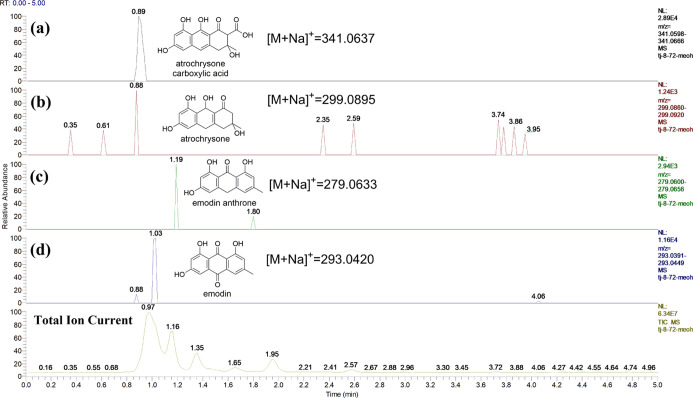
To better understand why the transformation occurs, the secondary metabolites of A. mirabilis are worthy of investigation. Compounds 3 and 4 were isolated from the extracts of A. mirabilis that had been fermented for 7 days. The biosynthetic pathway of compound 4 has been reported in Aspergillus nidulans,19 and it can provide guidance for the current bioconversion process because 4 has a similar molecular structure to the transformed product 2. Methylmalonyl-coenzyme A (Mal-CoA) was used as a starting material to produce atrochrysone carboxylic acid catalyzed by microbial polyketide synthases (PKSs). Atrochrysone carboxylic acid loses two CO2 molecules to emodin anthrone and is further oxidized to emodin (3); then, emodin (3) is catalyzed by microbial enzymes to form monodictyphenone (4). To confirm the presence of similar biological processes in A. mirabilis, the fermentation time was shortened to detect the intermediates. The broth of A. mirabilis was fermented for 3 days and analyzed by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS), and molecular signals from four key intermediates, atrochrysone carboxylic acid, atrochrysone, emodin anthrone, and emodin (3), were detected (Figure 3). The results showed that A. mirabilis has the same metabolic process as that of Aspergillus nidulans reported in the literature.19 The metabolic pathway of 4 implied that 1,8-dihydroxyanthraquinone (1) could be converted into peniphenone (2) via the same pathway since emodin (3) and 1,8-dihydroxyanthraquinone (1) share very similar moieties.
Figure 3.
Molecular signals from four key intermediates, atrochrysone carboxylic acid (a), atrochrysone (b), emodin anthrone (c), and emodin (d), were detected by UPLC-MS.
Biotransformation Process of 1,8-Dihydroxyanthraquinone by A. mirabilis
The process of conversion from 3 to 4 is related to the expression of multiple monodictyphenone gene clusters, and VerA/aflN and aFlY/hypA play key roles in this biological process.19 The product of VerA/aflN is directly related to P450 mono-oxygenase in fungi,20,21 and aflY/hypA might encode a Baeyer–Villiger oxidase.22 Cytochrome P450 mono-oxygenases might catalyze epoxidation, typically by insertion of an oxygen atom from atmospheric dioxygen into a conjugated double bond.23 Baeyer–Villiger oxidases catalyze the oxidative cleavage of a carbon–carbon bond adjacent to a carbonyl, which converts ketones to esters and cyclic ketones to lactones.24 Referring to the conversion of 3 to 4, electron transfer starts from OH-3 and is concentrated in the A ring of emodin, but why the A ring migrates to the oxygen instead of the C ring still remains unknown.19 To determine whether the P450 enzyme system is involved in this biotransformation, and since 1,8-dihydroxyanthraquinone (1) lacks a key chemical group (OH-3) compared with emodin (3), special inhibition experiments were performed. 1-Aminobenzotriazole (ABT) and piperonyl butoxide (PBO) are P450 enzyme inhibitors that are widely used to estimate the inhibition and induction of reactions mediated by cytochromes P450.25−27 The rate of the biological reaction catalyzed by the P450 enzyme notably decreased after the inhibitors were added. As shown in Figure 4a,b, ABT and PBO exhibited a significant inhibitory effect on the conversion of 1,8-dihydroxyanthraquinone (1) into peniphenone (2) at a concentration of 40 μg/mL. Under such conditions, the production of peniphenone (2) was slower than that of the positive control. The experiments showed that P450 was involved in this biotransformation, and the electron transfer of this reaction could occur with OH-1 of compound 1.
Figure 4.
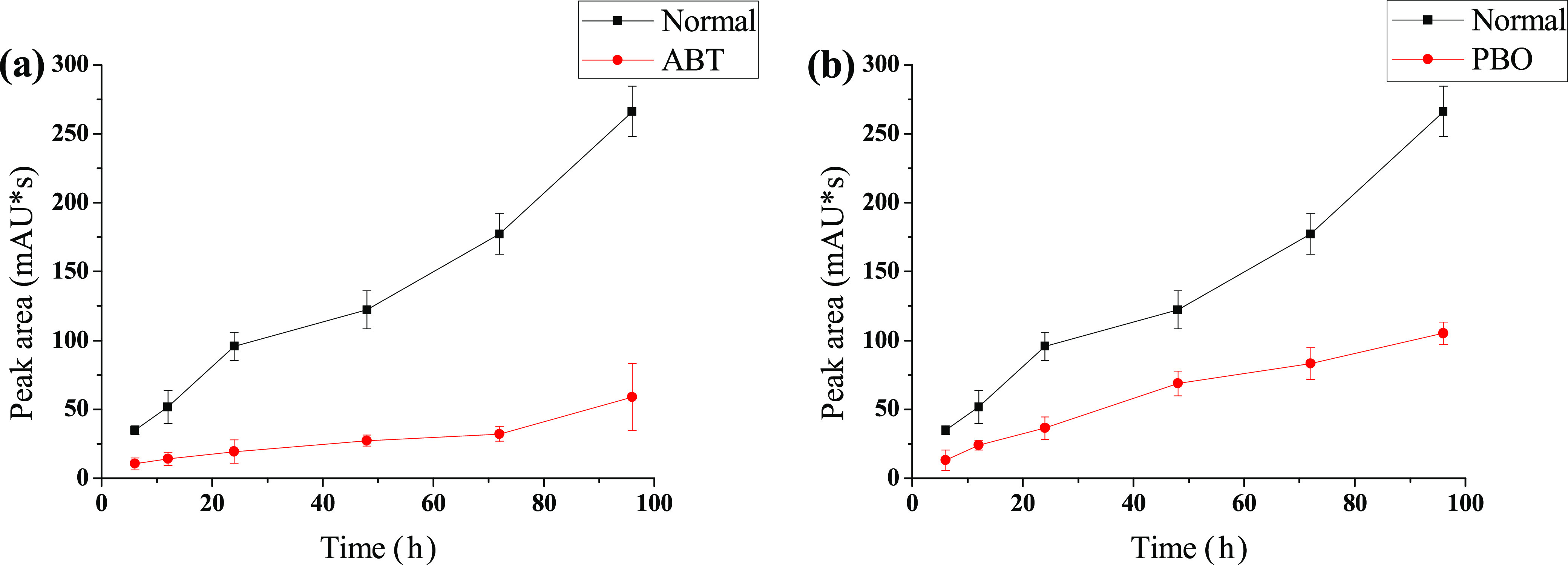
Effects of 1-aminobenzotriazole (ABT, 40 μg/mL) (a) and piperonyl butoxide (PBO, 40 μg/mL) (b) on the biotransformation of 1 to 2.
To verify the rationality of the transformation process, UPLC-MS was selected to detect the extracts of 1,8-dihydroxyanthraquinone (1) by A. mirabilis fermentation for 3 days, and two key intermediates, compounds 5 (P450 enzyme-catalyzed product) and 6 (Baeyer–Villiger oxidase-catalyzed product), were detected (Figure 5). The reason that this biological transformation is the most promising is because 1,8-dihydroxyanthraquinone (1) has a similar structure to that of emodin (3), which is involved in the metabolism of the fungus A. mirabilis. Reaction of 1,8-dihydroxyanthraquinone (1) was catalyzed by a P450 enzyme to form 5, and then it was subsequently catalyzed by Baeyer–Villiger oxidase to generate 6. Biotransformation product 2 was produced after hydrolysis, reduction of a ketone,19 and the dehydration of compound 6. The biological transformation process is speculated in Figure 6.
Figure 5.
Molecular signals from the P450 enzyme-catalyzed product (5), Baeyer–Villiger oxidase-catalyzed product (6), and peniphenone (2) were detected by UPLC-MS.
Figure 6.
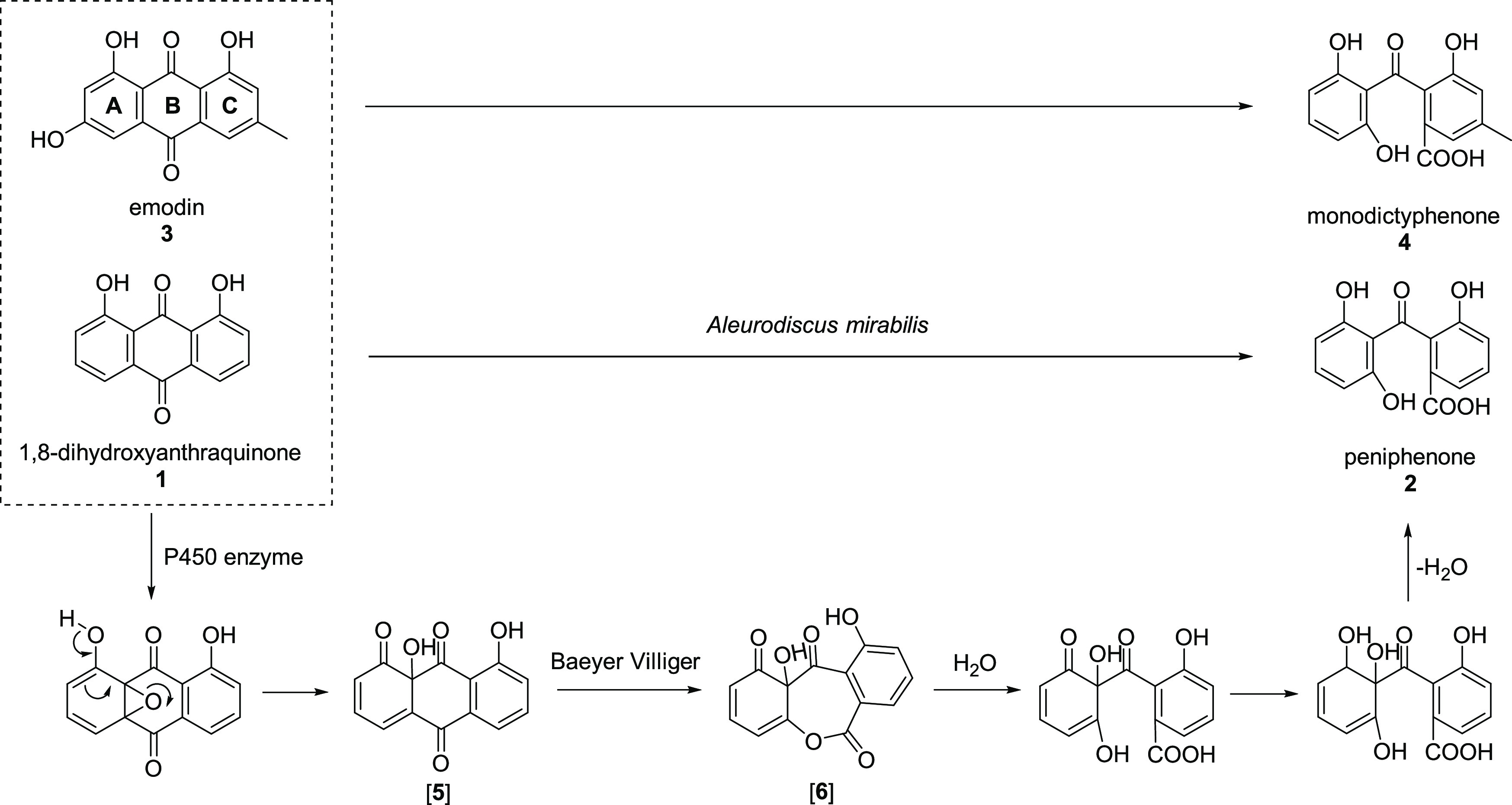
1,8-Dihydroxyanthraquinone (1) has a similar structure to that of emodin (3) and is catalyzed to peniphenone (2) by enzymes involved in the metabolism of the fungus A. mirabilis.
Conclusions
In summary, the present study confirmed that 1,8-dihydroxyanthraquinone could be converted into peniphenone (2) by A. mirabilis. The likely reason for the transformation is that 1,8-dihydroxyanthraquinone has a similar structure to emodin (an intermediate of fungal polyketide metabolism) and is catalyzed by a P450 enzyme and a Baeyer–Villiger oxidase. This is the first study to clarify the biotransformation of 1,8-dihydroxyanthraquinone, and this study could serve as a reference for further investigations on the internal metabolism of this compound.
Materials and Methods
Chemicals
1,8-Dihydroxyanthraquinone (compound 1, CAS: 117-10-2) was purchased from J&K Scientific Ltd. (Beijing, China). Methanol (Hipure Chem, China) was of HPLC grade and purchased from Guangdong Xilong Chemical Reagent Co., Ltd. (Guangdong, China). 1-Aminobenzotriazole (ABT, 98%) and piperonyl butoxide (PBO, 90%) were obtained from J&K Scientific Ltd. (Beijing, China). Water (resistivity ≥18.25 MΩ/cm) was purified using a water purification system (Chengdu, China). MeOD (CAS: 881-98-3, 99.8 atom % D, containing 0.03% tetramethylsilane (TMS)) and dimethyl sulfoxide (DMSO)-d6 (CAS: 2206-27-1, 99.8 atom % D, containing 0.03% TMS) were purchased from Energy-Chemical (Shanghai, China). All other reagents were analytically pure and obtained from Shengbi Co., Ltd. (Yunnan, China).
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer (400.00 MHz, 1H; 100.00 MHz, 13C). Chemical shifts are given in ppm (δ) downfield from the TMS internal standard.
HPLC and UPLC-MS
HPLC analysis was performed using a Shimadzu LC-20 Series equipped with a Shimadzu LC-20AR dual pump, an SPD-M20A photodiode array (PDA) detector, and an Agilent ZORBAX XDB-C18 column (4.6 × 250 mm2 5-micron). MeOH–0.1% HOAc was used as the mobile phase under gradient elution mode (0–5 min, 45:55, v/v; 5–10 min, 55:45, v/v; 10–15 min, 60:40, v/v; 15–20 min, 75:25, v/v; 20–35 min, 100:0 v/v; 1.0 mL/min; 225 nm). The injection volume was set to 10 μL for each injection, and analytical experiments were carried out at 25 °C.
High-resolution UPLC-MS on an Ultimate 3000-LTQ Orbitrap XL was performed using the following eluents: (A) 0.1% aqueous formic acid and (B) MeOH under isocratic elution mode. The applied gradient was 95% solvent B held for 5 min at a flow rate of 0.3 mL/min. The chromatographic column was a Hypersil GOLD C18 column (2.1 × 100 mm2 1.9-micron). Full-scan mass spectra were measured from m/z 50–1200, the spray voltage was 3.5 kV, and the capillary temperature was 300 °C.
Microorganisms and Culture Medium
A. mirabilis was isolated from fresh Glyceria elata and frozen at −80 °C in the lab at the Yunnan Institute of Microbiology, Yunnan Province, China. The fungus was maintained in potato dextrose agar (PDA, 1 L of water, 200 g of potato, 20 g of dextrose, and 15 g of agar) slants and stored at 4 °C for mutation. The seed culture medium and fermentation media were potato dextrose broth (PDB, 1 L of water, 200 g of fresh potato, and 20 g of dextrose). All media were sterilized in an autoclave at 121 °C and 1.06 kg/cm2 for 30 min and cooled prior to use.
Fermentation of 1,8-Dihydroxyanthraquinone
A. mirabilis was inoculated into the PDA slant culture medium and incubated at 28 °C for 7 days. Then, the fungal spores on the mature slants were picked out with the tip of a needle and put into 150 mL Erlenmeyer flasks containing 50 mL of PDB at 180 rpm and 28 °C for 3 days to prepare a spore suspension for the seed culture medium. Then, 500 mL Erlenmeyer flasks each containing 200 mL of PDB were individually inoculated with 20 μL of mature seed culture to prepare a spore suspension followed by incubation at 28 °C on a rotary shaker at 180 rpm for 72 h. Then, 1 mL of substrate (1,8-dihydroxyanthraquinone ethanol solution, 10 mg/mL) was injected, and the fermentation broth was incubated at 180 rpm and 28 °C for 7 days. The injection operation was carried out on a super clean bench. Blank control samples were cultured in the same manner, and the substrate was replaced by ethanol. Then, the fermented broth was extracted with an ultrasonic cleaner three times with ethyl acetate (EtOAc, 200 mL, 30 min each time), and the solvent was removed under vacuum. The extracts were dissolved in MeOH and prepared as a 20 mg/mL solution. Then, the extracts were filtered through a 0.22 μm filter for HPLC sample analysis.
Biotransformation Process Investigation by UPLC-MS
A. mirabilis was grown in a shaking culture at 28 °C for 72 h in 500 mL Erlenmeyer flasks that contained 200 mL of sterile PDB. Then, 1 mL of substrate (1,8-dihydroxyanthraquinone ethanol solution, 10 mg/mL) was injected. The injection operation was carried out on a super clean bench. The fermentation broth was incubated at 180 rpm and 28 °C for 3 days. The blank control samples were cultured in the same manner, and the substrate was replaced by ethanol. The fungal fermentation broth was filtered through a 0.22 μm filter for UPLC-MS sample analysis.
Purification of the Metabolite
Compounds 2–4 were isolated with a Shimadzu LC-20 Series HPLC equipped with a Waters Spherisorb S10 ODS2 semi-prep column (20 × 250 mm2). The extracts of 1,8-dihydroxyanthraquinone that had been fermented for 7 days were subjected to preparative HPLC and eluted with MeOH–H2O (0–25 min, 35:65, v/v; 16.0 mL/min; 225 nm) to yield 2 (8.3 mg); the extracts of the blank control that had been fermented for 7 days were subjected to preparative HPLC and eluted with MeOH–H2O (0–15 min, 15:85; 15–20 min, 30:70; 20–25 min, 45:55, v/v; 16.0 mL/min; 225 nm) to yield 3 (2.7 mg) and 4 (6.2 mg).
Spectroscopic Data
Peniphenone (2)
White amorphous powder; high-resolution electrospray ionization mass spectrometry (HRESI-MS) (m/z 297.0370 [M + Na]+, C14H10O6); 1H NMR (MeOD, 400 MHz) δH: 7.45 (1H, d, J = 7.7 Hz, H-2), 7.23 (1H, t, J = 8.0 Hz, H-3), 6.97 (1H, d, J = 8.0 Hz, H-4), 6.25 (1H, d, J = 8.2 Hz, H-5), 7.19 (1H, t, J = 5.6 Hz, H-6), and 6.25 (1H, d, J = 8.2 Hz, H-7); 13C NMR (MeOD, 100 MHz) δC: 134.6 (C-1), 121.9 (C-2), 129.6 (C-3), 120.6 (C-4), 154.6 (C-4a), 108.1 (C-5), 137.2 (C-6), 108.1 (C-7), 163.4 (C-8), 113.0 (C-8a), 203.6 (C-9), 130.8 (C-9a), 163.4 (C-10a), and 169.8 (−COOH).
Emodin (3)
Yellow amorphous powder; HRESI-MS (m/z 293.0428 [M + Na]+, C15H10O5); 1H NMR (DMSO-d6, 400 MHz) δH: 12.02 (1H, s, 1-OH), 11.95 (1H, s, 8-OH), 7.05 (1H, s, H-2), 7.40 (1H, s, H-4), 7.09 (1H, s, H-5), 6.54 (1H, s, H-7), and 2.37 (3H, s, 6-CH3); 13C NMR (DMSO-d6, 100 MHz) δC: 164.5 (C-1), 108.8 (C-2), 148.2 (C-3), 107.9 (C-4), 120.5 (C-5), 161.4 (C-6), 124.1 (C-7), 165.6 (C-8), 189.6 (C-9), 181.3 (C-10), 132.7 (C-11), 113.3 (C-12), 108.9 (C-13), 135.0 (C-14), and 21.6 (−CH3).
Monodictyphenone (4)
Amorphous powder; HRESI-MS (m/z 287.0559 [M – H]−, C15H12O6); 1H NMR (MeOD, 400 MHz) δH: 7.19 (1H, s, H-2), 6.72 (1H, s, H-4), 6.19 (1H, d, J = 8.1 Hz, H-5), 7.08 (1H, t, J = 8.1 Hz, H-6), 6.17 (1H, d, J = 8.1 Hz, H-7), and 2.23 (3H, s, H-11); 13C NMR (MeOD, 100 MHz) δC: 129.6 (C-1), 122.4 (C-2), 140.1 (C-3), 120.9 (C-4), 154.5 (C-4a), 108.0 (C-5), 137.1 (C-6), 108.2 (C-7), 162.9 (C-8), 113.4 (C-8a), 202.5 (C-9), 131.5 (C-9a), 163.2 (C-10a), 21.2 (-CH3), and 167.7 (−COOH).
Determination of the Reaction Yield by HPLC Analysis
The quantification of compound 2 was analyzed with the standard curve method by HPLC. Different concentrations of compound 2 in MeOH were injected into the HPLC instrument, and a regression equation correlating the concentration and peak area was constructed. The contents of compound 2 were determined according to the regressive equation; then, the yield of compound 2 could be calculated. The PDB medium was autoclaved and inoculated under the same conditions after it had been incubated for 72 h, and 1 mL of substrate (1,8-dihydroxyanthraquinone ethanol solution, 10 mg/mL) was injected. The injection operation was carried out on a super clean bench. The mixtures were incubated at 180 rpm and 28 °C for 1, 3, 5, 7, 9, 11, 13, or 15 days. Then, the fermented broth was extracted with an ultrasonic cleaner three times with EtOAc (200 mL, 30 min each time), and the solvent was removed under vacuum. Extracts were analyzed by HPLC.
Effects of Fermentation Temperature
Effects of fermentation temperature were evaluated using the fermentation method described in the Determination of the Reaction Yield by HPLC Analysis section. The fermentation broth was incubated at 180 rpm for 7 days. The fermentation temperature was set to 20 °C, 28 °C, or 37 °C. Extracts were analyzed by HPLC. Each sample was repeated in triplicate.
Effects of pH Value of Fermentation Broth
Effects of pH value of fermentation broth were evaluated using the fermentation method described in the Determination of the Reaction Yield by HPLC Analysis section. The pH value of the fermentation broth was buffered to 5, 6, 7, 8, or 9 by phosphate buffer. Then 1 mL of substrate (1,8-dihydroxyanthraquinone ethanol solution, 10 mg/mL) was injected. The mixtures were incubated at 180 rpm and 28 °C for 7 days. Extracts were analyzed by HPLC. Each sample was repeated in triplicate.
Effects of the Inhibitors ABT and PBO
The 500 mL Erlenmeyer flasks each containing 200 mL of PDB were individually inoculated with 20 μL of mature seed culture to prepare a spore suspension and then incubated at 28 °C on a rotary shaker at 180 rpm for 24 h. Then, 1 mL of a solution of ABT and PBO in ethanol (8.0 mg/mL) was added to the spore suspension (40 μg/mL, final concentration). After an additional 48 h of culture, 1 mL of substrate (1,8-dihydroxyanthraquinone ethanol solution, 20 mg/mL) was added. Spore suspensions without inhibitors were used as positive control. All injection operations were carried out on a super clean bench. The concentration of compound 2 was determined by HPLC analysis after 6, 12, 24, 48, 72, and 96 h. Each sample was repeated in triplicate.
Acknowledgments
The authors extend their sincere thanks to all of the Functional Molecules Analysis and Biotransformation Key Laboratory members. Thanks to Advanced Analysis and Measurement Center of Yunnan University for providing sample testing service.
Glossary
Abbreviations
- UPLC
ultra-performance liquid chromatography
- MS
mass spectrometry
- PKS
polyketone synthases
- HPLC
high-performance liquid chromatography
- NMR
nuclear magnetic resonance
- ABT
1-aminobenzotriazole
- PBO
piperonyl butoxide
- Mal-CoA
methylmalonyl-coenzyme A
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05216.
Figure S1: morphology of Aleurodiscus mirabilis; Figure S2: standard working curve for compound 2; Figure S3: UV-spectrum for compound 2 by PDA detector; Figure S4: yields of compound 2 from different fermentation temperatures, pH, and time; Figure S5: 1H NMR spectrum (400 MHz, MeOD) of compound 2; Figure S6: 13C NMR spectrum (100 MHz, MeOD) of compound 2; Figure S7: H–H COSY spectrum of compound 2; Figure S8: HMBC spectrum of compound 2; Figure S9: HSQC spectrum of compound 2; Figure S10: 1H NMR spectrum (400 MHz, DMSO-d6) of compound 3; Figure S11: 13C NMR spectrum (100 MHz, DMSO-d6) of compound 3; Figure S12: 1H NMR spectrum (400 MHz, MeOD) of compound 4; Figure S13: 13C NMR spectrum (100 MHz, MeOD) of compound 4; Figure S14: H–H COSY spectrum of compound 4; Figure S15: HMBC spectrum of compound 4; Figure S16: HSQC spectrum of compound 4; Figure S17: ESI-MS of compound 2; Figure S18: ESI-MS of compound 3; Figure S19: ESI-MS of compound 4; Figure S20: ESI-MS of compound 5; and Figure S21: ESI-MS of compound 6. (PDF)
Author Contributions
All authors have made a substantial contribution to this work: conceptualization, R.M. and Y.S.; methodology, R.M.; software, R.M.; validation, Y.S., S.Z., and J.H.; formal analysis, L.C.; investigation, L.Z.; resources, Z.D.; data curation, Y.S.; writing—original draft preparation, R.M.; writing—review and editing, R.M.; visualization, Y.S.; supervision, S.Z. and J.G.; project administration, L.C.; funding acquisition, L.C. and Z.D. All authors have read and agreed to the published version of the manuscript.
This research was funded by the Natural Science Foundation of China, Grant Numbers 81960760 and 81660719; The Program for Changjiang Scholars and Innovative Research Team in University, Grant Number IRT_17R94; A Project of Yunling Scholars of Yunnan Province; and A Program for Excellent Young Talents in Yunnan University.
The authors declare no competing financial interest.
Supplementary Material
References
- Cheng Y.; Zhang H.; Qu L.; He Y.; Routledge M. N.; Gong Y.; Qiao B. Identification of rhein as the metabolite responsible for toxicity of rhubarb anthraquinones. Food Chem. 2020, 331, 127363 10.1016/j.foodchem.2020.127363. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Lv B.; Feng X.; Li C. Perspective on biotransformation and De Novo Biosynthesis of licorice constituents. J. Agric. Food Chem. 2017, 65, 11147–11156. 10.1021/acs.jafc.7b04470. [DOI] [PubMed] [Google Scholar]
- Savinova O. S.; Solyev P. N.; Vasina D. V.; Tyazhelova T. V.; Fedorova T. V.; Savinova T. S. Biotransformation of progesterone by Aspergillus nidulans VKPM F-1069 (wild type). Steroids 2019, 149, 108421 10.1016/j.steroids.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Yildirim K.; Saran H.; Dolu O. F.; Kuru A. Biotransformation of some steroids by Mucor hiemalis MRC 70325. J. Chem. Res. 2013, 37, 566–569. 10.3184/174751913X13745069090242. [DOI] [Google Scholar]
- Kozłowska E.; Matera A.; Sycz J.; Kancelista A.; Kostrzewa-Suslow E.; Janeczko T. New 6,19-oxidoandrostan derivatives obtained by biotransformation in environmental filamentous fungi cultures. Microb. Cell Fact. 2020, 19, 37 10.1186/s12934-020-01303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei R. F.; Shi Y. X.; Duan W. H.; Ding H.; Zhang X. R.; Cai L.; Ding Z. T. Biotransformation of α-terpineol by Alternaria alternata. RSC Adv. 2020, 10, 6491–6496. 10.1039/C9RA08042B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L.; Dong J. W.; Zhao L. X.; Zhou H.; Xing Y.; Li Y.; Li Z. J.; Duan W. H.; Li X. J.; Ding Z. T. An improved water-soluble/stereospecific biotransformation of aporphine alkaloids in Stephania epigaea to 4R-hydroxyaporphine alkaloids by Clonostachys rogersoniana. Process Biochem. 2016, 51, 933–940. 10.1016/j.procbio.2016.04.016. [DOI] [Google Scholar]
- Dong J.; Zhao L.; Cai L.; Fang H.; Chen X.; Ding Z. Antioxidant activities and phenolics of fermented Bletilla formosana with eight plant pathogen fungi. J. Biosci. Bioeng. 2014, 118, 396–399. 10.1016/j.jbiosc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Dong J. W.; Cai L.; Xiong J.; Chen X. H.; Wang W. Y.; Shen N.; Liu B. L.; Ding Z. T. Improving the antioxidant and antibacterial activities of fermented Bletilla striata with Fusarium avenaceum and Fusarium oxysporum. Process Biochem. 2015, 50, 8–13. 10.1016/j.procbio.2014.09.008. [DOI] [Google Scholar]
- Li Y.; Cai L.; Dong J. W.; Xing Y.; Duan W. H.; Zhou H.; Ding Z. T. Innovative Approach to the accumulation of rubrosterone by fermentation of Asparagus filicinus with Fusarium oxysporum. J. Agric. Food Chem. 2015, 63, 6596–6602. 10.1021/acs.jafc.5b02570. [DOI] [PubMed] [Google Scholar]
- Wang J. P.; Shu Y.; Hu J. T.; Liu R.; Cai X. Y.; Sun C. T.; Gan D.; Zhou D. J.; Mei R. F.; Ding H.; Zhang X. R.; Cai L.; Ding Z. T. Roquefornine A, a sesterterpenoid with a 5/6/5/5/6-fused ring system from the fungus Penicillium roqueforti YJ-14. Org. Chem. Front. 2020, 7, 1463–1468. 10.1039/D0QO00301H. [DOI] [Google Scholar]
- Bashiri S.; Abdollahzadeh J.; Di Lecce R.; Alioto D.; Gorecki M.; Pescitelli G.; Masi M.; Evidente A. Rabenchromenone and Rabenzophenone, Phytotoxic tetrasubstituted chromenone and hexasubstituted benzophenone constituents produced by the Oak-Decline-Associated fungus Fimetariella rabenhorstii. J. Nat. Prod. 2020, 83, 447–452. 10.1021/acs.jnatprod.9b01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Park C.; Oh E.; Sung Y.; Lee J.; Park K. H.; Kang H. Benzophenone compounds, from a Marine-Derived strain of the fungus Pestalotiopsis neglecta, Inhibit proliferation of pancreatic cancer cells by targeting the MEK/ERK pathway. J. Nat. Prod. 2019, 82, 3357–3365. 10.1021/acs.jnatprod.9b00646. [DOI] [PubMed] [Google Scholar]
- Liu H.; Chen S.; Liu W.; Liu Y.; Huang X.; She Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY(2). Mar. Drugs 2016, 14, 217–224. 10.3390/md14120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer U.; Anke T.; Sheldrick W. S.; Scherer A.; Steglich W. Antibiotics from basidiomycetes. XXXI. Aleurodiscal: an antifungal sesterterpenoid from Aleurodiscus mirabilis (Berk. & Curt.) Hhn. J. Antibiot. 1989, 42, 875 10.7164/antibiotics.42.875. [DOI] [PubMed] [Google Scholar]
- Kraus G. A.; Chen L. Synthesis of 1,5- and 1,8-Dihydroxyanthraquinones from a common intermediate. A direct synthesis of racemic 7-deoxyaklavinone. J. Org. Chem. 1992, 56, 5098–5100. 10.1002/chin.199203172. [DOI] [Google Scholar]
- Huang J.; Gong W.; Chen Z.; Huang J.; Chen Q.; Huang H.; Zhao C. Emodin self-emulsifying platform ameliorates the expression of FN, ICAM-1 and TGF-β1 in AGEs-induced glomerular mesangial cells by promoting absorption. Eur. J. Pharm. Sci. 2017, 99, 128–136. 10.1016/j.ejps.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Marques M. P. C.; Carvalho F.; de Carvalho C. C. C. R.; Cabral J. M. S.; Fernandes P. Steroid bioconversion: towards green processes. Food Bioprod. Process. 2010, 88, 12–20. 10.1016/j.fbp.2010.01.009. [DOI] [Google Scholar]
- Chiang Y. M.; Szewczyk E.; Davidson A. D.; Entwistle R.; Keller N. P.; Wang C. C.; Oakley B. R. Characterization of the Aspergillus nidulans monodictyphenone gene cluster. Appl. Environ. Microbiol. 2010, 76, 2067–2074. 10.1128/AEM.02187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry K. M.; Townsend C. A. Synthesis and fate of O-carboxybenzophenones in the biosynthesis of aflatoxin. J. Am. Chem. Soc. 2005, 127, 3300–3309. 10.1021/ja045520z. [DOI] [PubMed] [Google Scholar]
- Chang P. K.; Hua S. S.; Sarreal S. B.; Li R. W. Suppression of aflatoxin biosynthesis in Aspergillus flavus by 2-phenylethanol is associated with stimulated growth and decreased degradation of Branched-Chain amino acids. Toxins 2015, 7, 3887 10.3390/toxins7103887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. C.; Montalbano B.; Boue S. M.; Bhatnagar D. An aflatoxin biosynthesis cluster gene encodes a novel oxidase required for conversion of versicolorin a to sterigmatocystin. Appl. Environ. Microbiol. 2005, 71, 8963–8965. 10.1128/AEM.71.12.8963-8965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. Cytochrome P450 oxidations in the generation of reactive electrophiles: Epoxidation and related reactions. Arch. Biochem. Biophys. 2003, 409, 59–71. 10.1016/S0003-9861(02)00415-0. [DOI] [PubMed] [Google Scholar]
- Tanner A.; Hopper D. J. Conversion of 4-hydroxyacetophenone into 4-phenyl acetate by a flavin adenine Dinucleotide-Containing Baeyer-Villiger-Type monooxygenase. J. Bacteriol. 2000, 182, 6565–6569. 10.1128/JB.182.23.6565-6569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder C. D.; Renaud N. A.; Hutzler J. M. Is 1-aminobenzotriazole an appropriate in vitro tool as a nonspecific cytochrome P450 inactivator?. Drug Metab. Dispos. 2009, 37, 10–13. 10.1124/dmd.108.024075. [DOI] [PubMed] [Google Scholar]
- Kotze A. C. Peroxide-supported in-vitro cytochrome P450 activities in Haemonchus contortus. Int. J. Parasitol. 1999, 29, 389–396. 10.1016/S0020-7519(98)00224-0. [DOI] [PubMed] [Google Scholar]
- Rasool A.; Joußen N.; Lorenz S.; Ellinger R.; Schneider B.; Khan S. A.; Ashfaq M.; Heckel D. G. An independent occurrence of the chimeric P450 enzyme CYP337B3 of Helicoverpa armigera confers cypermethrin resistance in Pakistan. Insect Biochem. Mol. Biol. 2014, 53, 54–65. 10.1016/j.ibmb.2014.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.