Abstract
The cinnamon-derived bioactive aroma compound cinnamaldehyde (CAL) has been identified as a promising antiobesity agent, inhibiting adipogenesis and decreasing lipid accumulation in vitro as well as in animal models. Here, we investigated the antiadipogenic effect of cinnamyl isobutyrate (CIB), another cinnamon-derived aroma compound, in comparison to CAL in 3T3-L1 adipocyte cells. In a concentration of 30 μM, CIB reduced triglyceride (TG) and phospholipid (PL) accumulation in 3T3-L1 pre-adipocytes by 21.4 ± 2.56 and 20.7 ± 2.05%, respectively. CAL (30 μM), in comparison, decreased TG accumulation by 37.5 ± 1.81% and PL accumulation by 28.7 ± 1.83%, revealing the aldehyde to be the more potent antiadipogenic compound. The CIB- and CAL-mediated inhibition of lipid accumulation was accompanied by downregulation of essential adipogenic transcription factors PPARγ, C/EBPα, and C/EBPβ on gene and protein levels, pointing to a compound-modulated effect on adipogenic signaling cascades. Coincubation experiments applying the TRPA-1 inhibitor AP-18 demonstrated TRPA1 dependency of the CAL, but not the CIB-induced antiadipogenic effect.
1. Introduction
Health care systems worldwide struggle with the challenges associated with a rising prevalence of obesity and its comorbidities.1 A sustained positive energy balance due to a caloric intake exceeding energy consumption ultimately leads to hyperplasia and/or hypertrophy of the adipose tissue.2 This pathophysiological overgrowth of adipose tissue increases the risk of developing noncommunicable diseases, calling for effective countermeasures.1 A potential approach to achieve an adipose tissue function that helps to maintain a healthy body weight and body composition is to target adipogenesis, the development of pre-adipocytes into mature adipocytes.3,4 Recent studies proposed antiadipogenic effects of naturally occurring bioactive aroma compounds, for example, present in red pepper5 or cinnamon spice.6 The antiobesity properties of cinnamon have been mainly allocated to its most abundant constituent in the essential oil of cinnamon bark, cinnamaldehyde (CAL), which has been hypothesized as a potential agent in preventing or treating overweight and obesity.4,7 It has been shown not only to exert anti-adipogenic effects in 3T3-L1 pre-adipocytes following a 4-day treatment with 10–40 μM CAL, but also to lower body weight gain, plasma lipids, and epididymal fat cell hypertrophy in mice after a 40 mg/kg CAL supplementation for 1 month compared to a high-fat diet control group.4 Moreover, CAL, in a concentration of 30 μM, has been shown to reduce fatty acid uptake in Caco-2 cells, pointing to an antiobesity effect as well.8 However, the molecular mechanisms regulating the CAL-mediated impact on adipocytes and lipid metabolism have not been entirely understood yet. Several possible modes of action, such as an impact on the adipogenic signaling cascade,4 on enzymes associated with the lipid metabolism9 as well as on thermogenesis have been described.7 Apart from CAL, also other cinnamon-derived aroma compounds such as cinnamyl alcohol and cinnamic acid, which exhibit structural similarities with CAL and constitute potential metabolites, have been reported to inhibit adipocyte differentiation in concentrations of 40–200 μM, accompanied by downregulation of CCAAT/enhancer binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) pathways.10,11 Moreover, for CAL, as a potent transient receptor potential channel A1 (TRPA1) agonist, a potential TRPA1 dependency in the CAL-induced effect on adipogenesis has been proposed, but not yet proven.9,12 Activation of TRPA1, however, is also associated with nociceptive reactions and sensation of pain.13 Considering its distinctive odor and spicy flavor qualities, the consumption of CAL is self-limited.14 A less well-investigated compound regarding potential antiobesity effects that is present in the essential oil of cinnamon bark is cinnamyl isobutyrate (CIB). Unlike its structural relative CAL, no strong flavor and pungent effects, but sweet and fruity flavor characteristics and a moderate strength of spicyness have been described for CIB.15
Because antiadipogenic effects for CIB have not been reported yet, we hypothesized such a potential role of the cinnamyl ester CIB in 3T3-L1 cells as a structural analog of CAL. To target this hypothesis, we investigated the impact of CIB in comparison to CAL on the adipogenesis of the well-defined model for adipocytes, 3T3-L1 cells, during differentiation and maturation.
The adipogenic pathways of 3T3-L1 cells from the initiation of differentiation into mature adipocytes is well investigated and constitutes an intricate operational sequence, determined by the integration of stimulating or repressing signaling factors via a cascade of transcription factors, ultimately driving the downstream expression of adipocyte specific genes.16 To that effect, complex interactions among various adipogenic transcription factors consecutively or synergistically play a decisive role in modulating the differentiation of adipocytes on a transcriptional level.16−19 Especially the PPARγ and members of the C/EBP family are considered key modulators in adipogenesis and lipid storage.18 However, many other transcription factors have been reported to have a regulatory effect in the different stages of the adipogenic network. Activation of the glucocorticoid receptor, cAMP response element—binding proteins (CREB) as well as ERK pathways are involved in the expression of C/EBPβ in the early stages of the adipogenic program.20−22 C/EBPβ in turn induces the expression of C/EBPα and PPARγ, which at the same time further stimulate the mutual expression of each and, as later adipogenic markers, regulate final differentiation processes, leading to the development of the mature adipocyte phenotype.16,23,24
The main objectives of the present study were (I) to compare the impact of the structural analogs CIB and CAL on the differentiation process of 3T3-L1 pre-adipocytes into mature adipocytes and (II) to assess potential underlying mechanisms of action. For this purpose, long-term lipid accumulation during differentiation, the short-term fatty acid uptake in mature adipocytes, the regulation of selected key transcription factors and markers of adipogenesis, and a potential involvement of TRPA1 were examined following treatment with CIB and CAL. Both compounds are flavoring substances (EFSA, Regulation EU 872/2012) and were tested in concentrations roughly following average use levels and as previously applied by Hoi et al. (2018).8
2. Results
2.1. Cell Viability
To rule out effects on cell viability after treatment with the test substances CIB, CAL, and AP-18 as well as combinations thereof, MTT assays were performed. No decrease in 3T3-L1 cell viability was determined after a 90 min treatment of fully matured adipocytes with CIB or CAL in concentrations of 0.3–300 μM compared to the untreated control cells. Additionally, no significant differences in cell viability were detected after treatment with 0.3 to 30 μM CIB or CAL with or without the addition of 2.5 μM AP-18 for 12 days compared to the control cells (data not shown). Higher concentrations of 300 μM tested for 12 days, however, significantly reduced cell viability.
2.2. Impact of CIB and CAL on Lipid Accumulation
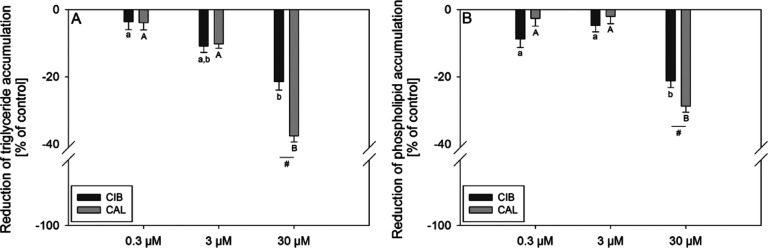
To assess and compare the impact of CIB and CAL on lipid accumulation, which is considered a marker for the extent of adipogenesis,16 3T3-L1 cells were treated with the test compounds during their differentiation and maturation in concentrations of 0.3–30 μM. First, the staining of lipids was carried out using the widely applied lysochrome diazo dye oil red O, which is considered a standard method for assessing lipid accumulation. The results demonstrated a decrease in lipid accumulation by 32.0 ± 3.10 and 17.2 ± 3.71% compared to the untreated control after treatment with 30 μM CAL and CIB, respectively (Figure 1). Moreover, CAL showed a significantly stronger decrease in lipid accumulation compared to CIB. Second, staining was also performed using the lipophilic stain nile red, which allows a further distinction between neutral and polar lipids. CAL and CIB, applied in a concentration of 30 μM, reduced triglyceride accumulation by 37.5 ± 1.81 and 21.4 ± 2.56%, respectively, compared to the untreated solvent control (Figure 2A). Additionally, both compounds also decreased phospholipid accumulation compared to the control after 12-day treatment in the same concentration by 28.7 ± 1.83% in the case of CAL and 21.2 ± 1.95% in the case of CIB (Figure 2B). In both cases, the decrease of lipid accumulation was stronger after 30 μM CAL compared to 30 μM CIB treatment (p ≤ 0.05). Moreover, calculations regarding the 30 μM CAL-mediated decrease in lipid accumulation applying a t-test revealed a stronger effect on the reduction of triglyceride compared to phospholipid accumulation (p = 0.001). In the case of 30 μM CIB treatment, there was no significant difference between the decrease in triglyceride and phospholipid accumulation.
Figure 1.
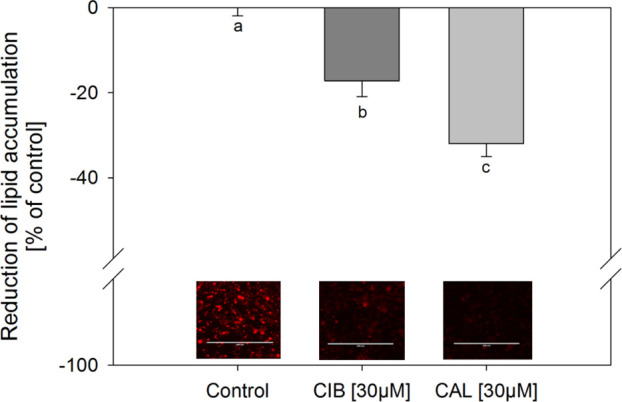
Reduction of lipid accumulation in % of control (0.1% ethanol; set to 0%) after addition of 0.3–30 μM CAL or CIB during differentiation and maturation of 3T3-L1 cells. Lipids in fully mature adipocytes were stained 12 d after initiation of differentiation with oil red O. Data are displayed as mean ± SEM. N = 6 (tr = 1–4). Significant differences are tested with one-way ANOVA followed by the Holm–Sidak post hoc test and marked with different letters (a = control).
Figure 2.
Reduction of lipid accumulation in % of control (0.1% ethanol; set to 0%) after addition of 0.3–30 μM CAL or CIB during differentiation and maturation of 3T3-L1 cells. Triglycerides (A) and phospholipids (B) in fully mature adipocytes were stained 12 d after initiation of differentiation with nile red. Data are displayed as mean ± SEM. N = 4–5 (tr = 3–6). Significant differences between control and treatments are tested with one-way ANOVA on ranks followed by Dunn’s method or one-way ANOVA followed by the Holm–Sidak post hoc test, and significant differences between treatments are tested with two-way ANOVA followed by the Holm–Sidak post hoc test. Significant differences between control and treatments are marked with different letters (a, A = control) and differences between treatments are marked with #p ≤ 0.05.
2.3. Impact of CIB and CAL on the Fatty Acid Uptake
To test the effect of the cinnamon compounds on short-term fatty acid uptake, fully mature adipocytes were pretreated with 0.3–300 μM CIB or CAL. As depicted in Table 1, both compounds did not change BODIPY-C12 uptake by the cells compared to the solvent control.
Table 1. BODIPY-C12 Fatty Acid Uptake after a 30 min Pretreatment with CAL and CIB in Concentrations of 0.3–300 μMa.
| CIB (%) | CAL (%) | |
|---|---|---|
| 0.3 μM | 105 ± 13.7 | 98.8 ± 8.72 |
| 3 μM | 102 ± 13.9 | 99.6 ± 4.90 |
| 30 μM | 97.0 ± 14.2 | 92.1 ± 4.71 |
| 300 μM | 98.5 ± 16.0 | 88.3 ± 7.82 |
Values are displayed as mean ± SD in percent compared to the control of 100 ± 14.1% (buffer with 0.1% ethanol). n = 4–7 (tr = 1–3).
2.4. Impact of CIB and CAL on PPARγ, C/EBPα, C/EBPβ, FABP4, and FAS mRNA Levels
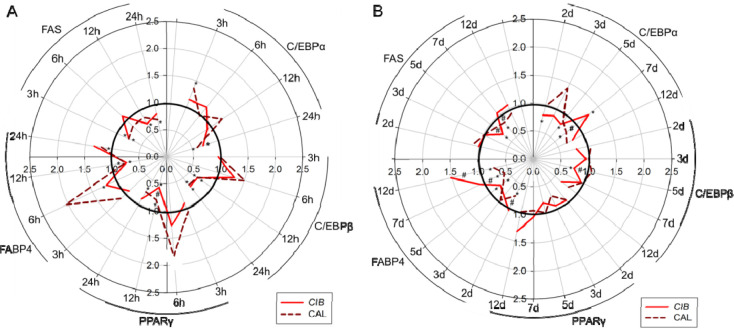
To further examine and compare the antiadipogenic effect of CAL and CIB on 3T3-L1 cells, the impact of both test compounds in a concentration of 30 μM was tested on the gene expression levels of selected transcription factors and markers associated with adipogenic pathways. As depicted in Figure 3A,B, the mRNA levels after CAL and CIB treatment over a period of 3h up to 12 days were studied in a time-dependent manner and revealed a regulation of the mRNA expression for all adipogenic transcription factors PPARγ, C/EBPα, and C/EBPβ as well as markers FABP4 and FAS over the course of the differentiation and maturation process. Compared to the solvent control, 30 μM CAL treatment revealed a regulation of the C/EBPα mRNA expression after 3 h, 24 h, and 7 days, whereas 30 μM CIB showed an effect on C/EBPα expression levels after 24 h, 2 d, 5 d, and 7 d treatment. C/EBPβ mRNA levels were downregulated after 12 and 24 h CAL treatment. Similarly, CIB treatment revealed C/EBPβ downregulation after 12 h, 24 h, and 5 days. Furthermore, PPARγ mRNA levels were regulated after 12 and 24 h CAL treatment as well as after 12 h CIB treatment. Gene expression levels of the adipogenic marker FAS were downregulated after 3 h, 24 h, and 7 d CAL treatment. CIB treatment led to FAS downregulation after 3 h, 12 h, and 5 days. Finally, FABP4 mRNA levels were altered after 12 h, 24 h, 2 d, 5 d, 7 d, and 12 d CAL treatment as well as after 6 h, 12 h, and 5 day CIB treatment. A stronger PPARy downregulation could be determined after 12 h CIB compared to CAL treatment. Additionally, CIB more strongly decreased FAS mRNA levels after 5 d treatment as well as C/EBPb mRNA levels after 5 d treatment compared to CAL. CAL showed a stronger effect on FABP4 downregulation after 2 d, 7 d, and 12 d treatment and a stronger FAS downregulation after 7 d treatment compared to CIB, as shown in Figure 3.
Figure 3.
Gene expression levels for C/EBPα, C/EBP, PPARy, FABP4, and FAS after treatment with 30 μM CAL and CIB (A) after 3 h, 6 h, 12 h, and 24 h and (B) after 2 d, 3 d, 5 d, 7 d, and 12 d. Data are shown as mean fold change compared to the controls (buffer with 0.1% ethanol) of 1.00 with SEMs of 0.00–0.06%; n = 3–4 (tr = 1–3). Significant differences are tested with one-way ANOVA followed by the Holm–Sidak post hoc test or Kruskal–Wallis one-way analysis of variance on ranks followed by Dunn’s Method or Tukey Test. Significant differences between treatments and controls were marked with *p ≤ 0.05, and significant differences between different treatments were marked with #p ≤ 0.05.
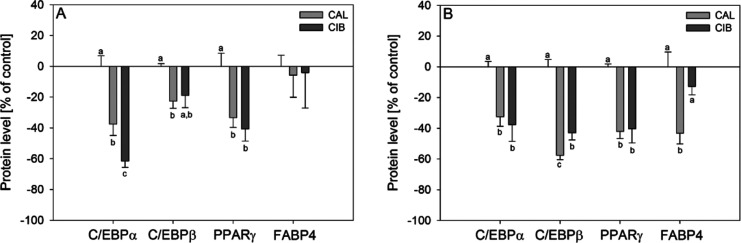
2.5. Impact of CIB and CAL on PPARγ, C/EBPα, C/EBPβ, FAS, and FABP4 Protein Levels
To additionally verify the CAL- and CIB-mediated impact on factors of the differentiation process, PPARγ, C/EBPα, C/EBPβ, and FABP4 protein levels were analyzed 24 h and 12 days after initiation of differentiation with or without compound treatment in a concentration of 30 μM by means of ELISA (Figure 4A,B). Treatment of 3T3-L1 cells with CAL for 24 h as well as 12 days decreased PPARγ (24 h: −33.3 ± 6.38%; 12 d: −42.1 ± 4.51%), C/EBPα (24 h: −37.5 ± 7.42%; 12 d: −32.6 ± 6.19%), and C/EBPβ (24 h: −22.6 ± 4.57%; 12 d: −57.6 ± 2.72%) levels compared to their untreated controls. Similarly, CIB treatment reduced PPARγ levels by 40.7 ± 7.69% and C/EBPα levels by 61.5 ± 4.13% after 24 h as well as PPARγ (−40.4 ± 9.15%), C/EBPα (−37.6 ± 10.9%), and C/EBPβ (−43.0 ± 4.61%) levels after 12 days. A CAL-induced lowered protein level could also be determined for FABP4 after 12-day treatment (−43.2 ± 6.97) compared to the control and CIB treatment, whereas CIB treatment over a 12-day differentiation period did not reduce the FABP4 expression.
Figure 4.
Protein levels for C/EBPα, C/EBPβ, PPARγ, and FABP4 after treatment with 30 μM CAL and CIB after (A) 24 h and (B) 12 d. Data are shown as mean ± SEM in % compared to the controls (buffer with 0.1% ethanol, set to 0%). n = 3–5 (tr = 1–2). Significant differences are tested with one-way ANOVA followed by the Holm–Sidak post hoc test and Kruskal–Wallis one-way analysis of variance on ranks followed by a Tukey test or Dunn's method and marked with different letters.
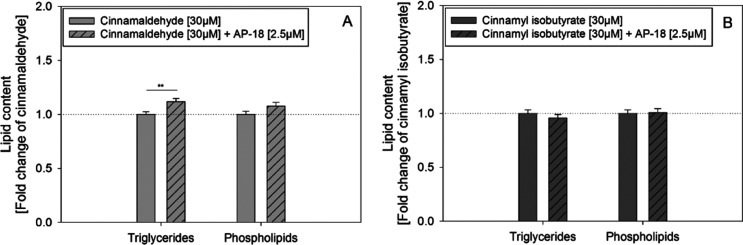
2.6. TRPA1 Involvement in CAL- and CIB-Mediated Decrease in Lipid Accumulation
CAL has been shown to be a potent activator of TRPA1 channels.25−27 In order to investigate if TRPA1 channels might play a role in the CAL- and CIB-induced inhibition of lipid accumulation during the adipogenesis, coincubation experiments using the TRPA1 inhibitor AP-18 were carried out. As presented in Figure 5, 12-day cotreatment with CAL (30 μM) and AP-18 (2.5 μM) reversed the 30 μM CAL-induced decrease in triglyceride accumulation (CAL: 1.00 ± 0.03 vs coincubation: 1.12 ± 0.03). No effect could be determined on the level of phospholipids. Lipid accumulation after 12-day coincubation with CIB (30 μM) and AP-18 (2.5 μM) also did not differ from the CIB-mediated decrease.
Figure 5.
Lipid content (triglycerides and phospholipids) after addition of 30 μM CAL (A) or CIB (B) during differentiation and maturation of 3T3-L1 cells alone (set to 1) and after cotreatment with TRPA1 inhibitor AP-18 [2.5 μM]. AP-18 was added 20 min prior to the test compounds. Values are presented as mean ± SEM compared to CAL or CIB alone (set to 1); n = 4–5 (tr = 3–8). Significant differences between treatments are tested with Student’s t-test and marked with **p ≤ 0.01.
3. Discussion
CAL, one of the major aroma compounds in cinnamon bark oil, has been shown to exert antiobesity properties by inhibiting body weight gain in mice after long-term supplementation in a concentration of 250 mg/kg body weight as well as adipogenesis and lipid accumulation in vitro after 4-day treatment with 10–40 μM CAL.4,7,9 Ongoing research indicates that CAL, however, might not be the only bioactive cinnamon-derived aroma compound associated with antiadipogenic activity.10,11 Moreover, its unique cinnamon flavor characteristics and nociceptive sensations might limit its application. Therefore, the less spicy CIB, a cinnamic ester and structurally related, naturally occurring cinnamon constituent, was examined for its antiobesity potential in the present study. We aimed to investigate the impact of CIB on the adipogenesis of 3T3-L1 pre-adipocytes as well as its potential effect size compared to CAL.
As hypothesized, the structurally related CIB also exhibited a reduced lipid accumulation after 12-day treatment with 30 μM of the test compound during the differentiation and maturation phase of 3T3-L1 cells, pointing to an antiadipogenic effect of CIB as well. However, in contrast to the CAL-mediated decrease in triglyceride accumulation by approximately 38%, which is comparable to the CAL-induced effect sizes reported in the literature,4,9 CIB decreased triglyceride accumulation by 21%. CAL and CIB treatment decreased not only the content of triglycerides as determined by nile red as well as oil red O staining, but also that of phospholipids, which has been found to increase during adipogenesis as well and has been suggested to be required for membrane biosynthesis.28 Again, CAL exhibited a more pronounced effect of approximately 7.5% compared to CIB. Interestingly, whereas CIB showed the same effect on triglyceride and phospholipid accumulation, in the case of CAL, a more pronounced effect on triglycerides compared to phospholipids was demonstrated. This result might point to an additional modulating impact of CAL on the lipid accumulation during the maturation phase of adipogenesis. Taken together, these results suggest that, although the cinnamyl ester CIB has antiadipogenic potential as well, the aldehyde CAL is more effective concerning the inhibiting impact on lipid accumulation. As bioactivities of naturally occurring compounds highly depend on their bioavailability and metabolization and numerous cinnamyl compounds have been shown to metabolize quickly to cinnamic acid and cinnamic acid derivatives in vivo, biotransformation of CIB and/or CAL in adipocytes needs to be investigated in future studies. Also, the stability of the test compounds has to be taken into account, making it difficult to specify exactly if the lipid accumulation-reducing effect of CIB is caused by the ester itself or a degradation product. In vivo, fast enzymatic hydrolyzation of aromatic esters has been reported, whereas CAL was also found in small doses in lipid tissue of animal models.29−37 Because of a possible hydrolyzation of the cinnamic ester into its respective components, it cannot be excluded that its derivatives cinnamic acid or cinnamyl alcohol might also be involved to a greater or lesser extent in the demonstrated decreased lipid accumulation in 3T3-L1 cells. Both have been reported to decrease triglyceride accumulation by approximately 20–25% when applied in similar concentrations as CIB.10,11 However, altogether, the net effect of CIB on lipid accumulation was still less than that of CAL.
Next, we examined whether a reduced short-term fatty acid uptake might also play a role in decreasing the lipid accumulation in mature adipocytes. Interestingly, however, no effect could be demonstrated for either test compound, further pointing to a stronger effect of CAL and CIB on the development of pre-adipocytes to adipocytes.11
For further verification of the CAL- and CIB-induced effect on markers of the differentiation process, protein levels of PPARγ, C/EBPα, and C/EBPβ as well as FABP4 were examined after selected time points. Protein levels were examined 24 h and 12 days after induction, selecting a time point in the early phase of adipogenesis and a time point after the differentiation process has been completed. Treatment with CAL led to reduced PPARγ, C/EBPα, and C/EBPβ levels after 24 h and 12 days, confirming the CAL-mediated downregulation of the transcription factors on the gene expression level. CIB treatment also led to reduced PPARγ and C/EBPα levels after 24 h and reduced PPARγ, C/EBPα, and C/EBPβ levels after 12 days. A stronger effect on C/EBPα levels could be shown after 24 h CIB treatment, whereas a stronger downregulation of C/EBPβ levels could be determined after 12 d CAL treatment. Altogether, these results suggest that CIB and CAL treatment, to a similar extent, affect key adipogenic transcription factors, which play a role especially in the earlier adipogenic phase. However, even though key transcription factors of adipogenesis, such as PPARγ, C/EBPα, and C/EBPβ were decreased after CIB treatment over a 12-day differentiation period, FABP4 protein levels were not reduced. In contrast, after 12-day CAL treatment, less FABP4 protein was detected in the fully matured cells. In accordance with the CAL-mediated bigger effect size in lipid accumulation detected by nile red and oil red O staining, these results further emphasize the stronger impact of CAL on diminishing the development to fully matured adipocytes and support the finding that CAL is the more potent antiadipogenic compound as compared to CIB.
On a mechanistic level, CAL has been proposed to exert its antiobesity effect via (i) inhibiting the differentiation of pre-adipocytes to mature adipocytes,4 (ii) modulating lipolysis and lipid biosynthesis of adipocytes,9 as well as (iii) activating thermogenesis and metabolic reprogramming.26,38 However, CAL is also known as a potent agonist of TRPA1 channels, constituting nonselective thermosensitive cation channels, that have been identified in a variety of neuronal and nonneuronal cell types.25−27 Multiple TRPA1-dependent actions for CAL have been reported over the last decades, such as immunomodulatory39 and vasodilatory40 actions as well as the secretion of hormones such as serotonin,27 ghrelin,12 and PYY.41 Additionally, the role of TRP channels in the physiological processes of adipogenesis has grown as a topic of extensive research.42 Activation of these multimodal receptors through physical and mechanical stimulation on the one hand and a wide range of endogenous and exogenous agents on the other hand is associated with altered intracellular Ca2+ concentrations and, therefore, it has the potential to regulate various cellular processes.27 With regard to the lipid metabolism, involvement of calcium signaling in the adipogenic process has been suggested. In 3T3-L1 cells, for instance, elevated intracellular Ca2+ levels ([Ca2+]i) have been reported to block early stages of the adipocyte differentiation process by inhibiting the post-confluent mitotic phase and modulating the expression of c-myc genes.43 It was also found, however, that, in later stages of the adipogenesis, elevated [Ca2+]i actually increased markers of differentiation in human adipocytes.44
We hypothesized a potential TRPA1 dependency in the CAL-mediated decrease in lipid accumulation, which was investigated by cotreatment of 3T3-L1 cells with CAL and the competitive TRPA1 inhibitor AP-18 for 12 days during the differentiation and maturation phase. The results showed an increased triglyceride accumulation compared to the effect of CAL alone, pointing to involvement of TRPA1 in the antiadipogenic effect of CAL. In contrast, no TRPA-1 involvement in the antiadipogenic effect of CIB could be determined, which might explain the smaller impact of CIB on lipid accumulation compared to CAL. Consistently, it was shown by Lieder et al. (2020) that TRPA1-mediated Ca2+ mobilization in transiently hTRPA1-transfected HEK293 cells is reduced after stimulation with cinnamon derivatives such as cinnamic acid, ferulic acid, or CIB compared to cinnamyl aldehyde.45 As mentioned above, it has been suggested that apart from directly inhibiting the differentiation process,4 CAL also modulates lipolysis and lipid biosynthesis in mature adipocytes.9 However, based on our data, it could not be distinguished whether the TRPA1 dependency in the CAL-mediated effect on the reduced lipid accumulation only plays a role in the early and intermediate differentiation phases or if a TRPA1-dependent effect of CAL is also involved in the subsequent terminal differentiation and maturation phase of the adipogenesis. As it was reported that the trigeminally active trans-pellitorine demonstrated a TRPA1-dependent antiadipogenic effect only in early to intermediate stages of adipogenesis, despite its continuing lipid accumulation reducing effect during maturation phase,25 CAL-mediated TRPA1 activation in the early differentiation might be hypothesized as well. Additionally, the time-dependent, biphasic regulatory effect of [Ca22+]i on adipogenesis44 could point to the fact that a CAL-mediated Ca2+ influx via TRPA1 might only be the case in early phases of the differentiation process. However, it cannot be excluded that CAL, as an exogenous inhibitory agent, regulates adipogenesis, its downstream cascade of transcription factors and lipid accumulation through different signaling pathways, especially because a modulating impact of CAL on lipolysis and lipid biosynthesis in adipocytes was also suggested.
In conclusion, analyzing and comparing the impact of the structural analogs CIB and CAL on adipogenesis in 3T3-L1 cells demonstrated the aldehyde to be the more potent antiadipogenic candidate as evidenced by a stronger inhibition in lipid accumulation and a stronger decrease in the expression of differentiation marker FABP4. This stronger effect size of CAL might be explained by its potential to activate TRPA1 channels, as TRPA1 dependency was found in the CAL-mediated decrease in triglyceride accumulation. The CIB- and CAL-induced decrease in lipid accumulation was further accompanied by a similar downregulation of the key adipogenic transcription factors PPARγ, C/EBPα, and C/EBPβ on a gene and protein level, indicating a compound-mediated effect on the signaling cascade of the adipogenic differentiation program.
4. Materials and Methods
4.1. Chemicals
All chemicals and reagents were purchased from Sigma-Aldrich (Vienna, Austria), unless stated otherwise. The murine fibroblast cell line 3T3-L1 was purchased from ATCC.
4.2. Cell Culture
3T3-L1 pre-adipoycte cells were cultured in Dulbecco’s modified eagle’s medium (DMEM) with the addition of 10% fetal bovine serum, 4% l-glutamine, and 1% penicillin/streptomycin at 37 °C and 5% CO2 in a humidified atmosphere. Cells were harvested and seeded after reaching a confluence of 70–80% and used between the passages 4 and 15. To induce the differentiation of pre-adipoyctes into mature adipocytes, cells were treated with differentiation medium containing growth medium with the addition of dexamethasone (1 μM), 3-isobutyl-1-methyl-xanthine (0.5 mM), and insulin (10 μg/mL) 2 days after reaching confluence (day 0), according to the protocol described by Riedel et al. (2012).46 After 2 days, the differentiation media were substituted with maturation medium comprising growth medium supplemented with 10 μg/mL insulin for additional 48 h. Cells were subsequently cultivated using normal growth medium for 5 more days and used for fatty acid uptake experiments on day 9.
Stock solutions of the test compounds CAL, CIB, and AP-18 were dissolved in ethanol or dimethyl sulfoxide (DMSO). Final ethanol and DMSO concentrations never exceeded 0.1% on the cells.
4.3. Cell Viability
The impact of the applied concentrations of the test compounds CAL (0.3–300 μM), CIB (0.3–300 μM), and AP-18 (2.5 μM) as well as combinations thereof on metabolic activity was examined using MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazoliumbromide) assays as described before.47
4.4. Nile Red Staining
Lipid accumulation was analyzed using nile red (9-diethylamino-5H-benzo [α] phenoxazine-5-one), a fluorescent lipophilic dye, which allows for distinction between neutral lipids and polar lipids through transition of emission from red to yellow based on the lipid hydrophobicity.48 For nile red staining, 3T3-L1 cells were seeded in 48-well plates at a density of 1.5 × 104. After initiating the differentiation as stated above, cells were cultured in maturation media for 10 days. Addition of the test compounds started at day 0. On day 12, cells were washed with 750 μL PBS, stained with nile red solution at a final concentration of 4 μg/mL, and incubated for 20 min at room temperature. Subsequently, fluorescence was measured at 485 nm excitation and 572 nm emission to determine triglyceride accumulation as well as 530 nm excitation and 635 nm emission for determination of the phospholipids using a Tecan plate reader (Tecan infinite M200, Tecan Austria). Lipid content after substance treatment was calculated as % to the untreated control cells. As a comparison, lipid staining was also performed using the oil red O staining protocol reported by Riedel et al. (2012).46
4.5. Fatty Acid Uptake
The uptake of free fatty acids in fully matured 3T3-L1 adipocytes was examined in 96-well plates applying the QBT fatty acid uptake kit (Molecular Devices Germany GmBH, Germany), which was used following manufacturers’ instructions. As described elaborately by Holik et al. (2016),49 cells were seeded and used for analysis on day 9 post-differentiation. After 30 min pretreatment of 3T3-L1 adipocytes with 0.3–300 μM CIB or CAL diluted in HBSS/HEPES, the BODIPY-C12 containing loading dye was added. BODIPY-C12 uptake was measured for 60 min with an excitation wavelength of 485 nm and emission wavelength of 515 nm. For quantification, the area under the curve (AUC) from the respective signal/time plots was determined using SigmaPlot and assessed relative to untreated control cells (100%).
4.6. Quantitative Real-Time Polymerase Chain Reaction
The gene expression of peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer binding protein α (C/EBPα) and β (C/EBPβ), fatty acid binding protein 4 (FABP4), and fatty acid synthase (FAS) was examined at different time points over 12 days, applying quantitative real-time polymerase chain reaction (PCR). RNA extraction using the MasterPure Complete DNA & RNA Purification Kit (Biozym) according to the manufacturer’s protocol was performed after 3, 6, 12, and 24 h as well as after 2, 3, 5, 7, and 12 days post-differentiation with or without 30 μM CIB or CAL treatment, which was added to the differentiation and maturation medium. Following a reverse transcription applying the high capacity cDNA Kit (Life Technology, Carlsbad, CA, USA), qRT-PCR analysis was carried out in triplicates on a StepOnePlus device by means of SYBR Green MasterMix (Life Technology, Carlsbad, CA, USA). The individual hypothetical starting mRNA levels were determined using LinRegPCR v.2012.2 and normalized to HPRT47 and ACTB50 as reference genes. Primers sequences are listed in Table 2.
Table 2. Sequence of Primers Used in qRT-PCR Experiments.
| target | forward primer | reverse primer |
|---|---|---|
| HPRT47 | GAGAGCGTTGGGCTTACCTC | ATCGCTAATCACGACGCTGG |
| ACTB50 | TCTTTGCAGCTCCTTCGTTG | CATTCCCACCATCACACCCT |
| PPARγ47 | GTGCCAGTTTCGATCCGTAGA | GGCCAGCATCGTGTAGATGA |
| C/EBPα47 | GCCCCGTGAGAAAAATGAAGG | ATCCCCAACACCTAAGTCCC |
| C/EBPβ51 | CGCCTTATAAACCTCCCGCT | TGGCCACTTCCATGGGTCTA |
| FABP447 | TTTGGTCACCATCCGGTCAG | TGATGCTCTTCACCTTCCTGTC |
| FAS52 | CACAGATGATGACAGGAGATGG | TCGGAGTGAGGCTGGGTTGAT |
4.7. PPARγ, C/EBPα, C/EBPβ, and FABP4 ELISA
Analysis of PPARγ, C/EBPα, C/EBPβ, and FABP4 protein expression was carried out 24 h as well as 12 days after initiation of differentiation with or without compound treatment (30 μM), applying specific ELISA kits (mouse PPARγ and C/EBPβ, Cloud-Clone Corp., USA; mouse C/EBPα and FABP4, ELISA Genie, United Kingdom). For sample preparation, 3T3-L1 cells were washed twice with ice-cold PBS and collected in lysis buffer (RIPA buffer) with the addition of 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium ortho-vanadate, and a protease inhibitor cocktail, as described by Rohm et al. (2015).47 After homogenization and subsequent agitation (30 min, 4 °C), the lysate was centrifuged for 19 min at 4 °C and 16,900g. The PPARγ, C/EBPα, C/EBPβ, and FABP4 protein content in the supernatant was determined by using the respective ELISA following the manufacturer's instructions and normalized to the protein content of each sample assessed by means of Bradford.
4.8. Statistical Analysis
Data from the in vitro experiments are presented as mean ± SD, unless indicated otherwise, or as fold change (treated over control: T/C) from at least three biological and two technical replicates. Outliers were identified and removed from statistical analysis according to the Nalimov outlier test. To test significant differences in treated versus untreated cells and in time course experiments, Student’s t-test, one-way ANOVA followed by the Holm–Sidak post hoc test or Kruskal–Wallis one-way analysis of variance on ranks followed by a Tukey test or Dunn’s Method were applied. Significant differences between different treatments and test concentrations were tested with two-way ANOVA followed by the Holm–Sidak post hoc test. To test a significant difference between the effect of CAL or CIB alone versus coincubation, Student’s t-test was performed. Statistical analysis was carried out using SigmaPlot 11.0.
Acknowledgments
The financial support by the Christian Doppler Research Association, the Austrian Federal Ministry for Digital and Economic Affairs, and the National Foundation for Research, Technology and Development is gratefully acknowledged.
Glossary
Abbreviations used
- CAL
cinnamaldehyde
- CIB
cinnamyl isobutyrate
- C/EBPα
CCAAT/enhancer binding protein α
- C/EBPβ
CCAAT/enhancer binding protein β
- PPARγ
peroxisome proliferator-activated receptor
- FABP4
fatty acid binding protein 4
- FAS
fatty acid synthase
- TRPA1
transient receptor potential channel A1
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05083.
MTT data (PDF)
Author Contributions
J.K.H., Barbara Lieder, J.P.L., J.H., and V.S. designed and established conditions for the experiments. J.K.H, Beatrix Liebisch, and C.C performed experiments and data analysis. The manuscript was written by J.K.H. and revised by Barbara Lieder, J.P.L., J.H., and V.S.
The authors declare the following competing financial interest(s): The authors J. Hans and J.P. Ley are employees at Symrise AG, Holzminden, Germany.
Supplementary Material
References
- Ginsberg H. N.; MacCallum P. R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. CardioMetab. Syndr. 2009, 4, 113–119. 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K.; Kusminski C. M.; Scherer P. E. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011, 121, 2094–2101. 10.1172/jci45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E.; Kim C. Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. 10.3390/molecules24061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.; Yuan H. D.; Kim D. Y.; Quan H. Y.; Chung S. H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 2011, 59, 3666–3673. 10.1021/jf104814t. [DOI] [PubMed] [Google Scholar]
- Kim H.-J.; You M.-K.; Wang Z.; Kim H.-A. Red Pepper Seed Inhibits Differentiation of 3T3-L1 Cells during the Early Phase of Adipogenesis via the Activation of AMPK. Am. J. Chin. Med. 2018, 46, 107–118. 10.1142/s0192415x18500064. [DOI] [PubMed] [Google Scholar]
- Lopes B. P.; Gaique T. G.; Souza L. L.; Paula G. S. M.; Kluck G. E. G.; Atella G. C.; Gomes A. C. C.; Simas N. K.; Kuster R. M.; Ortiga-Carvalho T. M.; Pazos-Moura C. C.; Oliveira K. J. Cinnamon extract improves the body composition and attenuates lipogenic processes in the liver and adipose tissue of rats. Food Funct. 2015, 6, 3257–3265. 10.1039/c5fo00569h. [DOI] [PubMed] [Google Scholar]
- Zuo J.; Zhao D.; Yu N.; Fang X.; Mu Q.; Ma Y.; Mo F.; Wu R.; Ma R.; Wang L.; Zhu R.; Liu H.; Zhang D.; Gao S. Cinnamaldehyde Ameliorates Diet-Induced Obesity in Mice by Inducing Browning of White Adipose Tissue. Cell. Physiol. Biochem. 2017, 42, 1514–1525. 10.1159/000479268. [DOI] [PubMed] [Google Scholar]
- Hoi J. K.; Lieder B.; Pignitter M.; Hans J.; Ley J. P.; Lietard J.; Hoelz K.; Somoza M.; Somoza V. Identification of Cinnamaldehyde as Most Effective Fatty Acid Uptake Reducing Cinnamon-Derived Compound in Differentiated Caco-2 Cells Compared to Its Structural Analogues Cinnamyl Alcohol, Cinnamic Acid, and Cinnamyl Isobutyrate. J. Agric. Food Chem. 2019, 67, 11638–11649. 10.1021/acs.jafc.9b04274. [DOI] [PubMed] [Google Scholar]
- Khare P.; Jagtap S.; Jain Y.; Baboota R. K.; Mangal P.; Boparai R. K.; Bhutani K. K.; Sharma S. S.; Premkumar L. S.; Kondepudi K. K.; Chopra K.; Bishnoi M. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. BioFactors 2016, 42, 201–211. 10.1002/biof.1265. [DOI] [PubMed] [Google Scholar]
- Hwang D. I.; Won K.-J.; Kim D.-Y.; Kim B.; Lee H. M. Cinnamyl Alcohol, the Bioactive Component of Chestnut Flower Absolute, Inhibits Adipocyte Differentiation in 3T3-L1 Cells by Downregulating Adipogenic Transcription Factors. Am. J. Chin. Med. 2017, 45, 833–846. 10.1142/s0192415x17500446. [DOI] [PubMed] [Google Scholar]
- Kang N. H.; Mukherjee S.; Yun J. W. Trans-Cinnamic Acid Stimulates White Fat Browning and Activates Brown Adipocytes. Nutrients 2019, 11, 577. 10.3390/nu11030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho S.; Michlig S.; de Senarclens-Bezençon C.; Meylan J.; Meystre J.; Pezzoli M.; Markram H.; le Coutre J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M.; Story G. M.; Hwang S. W.; Viswanath V.; Eid S. R.; Petrus M. J.; Earley T. J.; Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Burdock G. A. (2010). Fenaroli's Handbook of Flavor Ingredients, CRC Press, Boca Raton, FL. [Google Scholar]
- Additives, Joint FAO/WHO Expert Committee on Food . Compendium of Food Additive Specifications; FAO. Addendum 8.: Rome, Geneva, 2000; Vol. 55th session.
- Siersbaek R.; Mandrup S. Transcriptional networks controlling adipocyte differentiation. Cold Spring Harbor Symp. Quant. Biol. 2011, 76, 247–255. 10.1101/sqb.2011.76.010512. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Rosen E. D.; Brun R.; Hauser S.; Adelmant G.; Troy A. E.; McKeon C.; Darlington G. J.; Spiegelman B. M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Farmer S. R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen M. S.; Siersbaek R.; Boergesen M.; Nielsen R.; Mandrup S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 2014, 34, 939–954. 10.1128/mcb.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z.; Umek R. M.; McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Reusch J. E. B.; Colton L. A.; Klemm D. J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 2000, 20, 1008–1020. 10.1128/mcb.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.-Q.; Gronborg M.; Huang H.; Kim J.-W.; Otto T. C.; Pandey A.; Lane M. D. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 9766–9771. 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm J. K.; Park B. H.; Farmer S. R. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 2001, 276, 18464–18471. 10.1074/jbc.m100797200. [DOI] [PubMed] [Google Scholar]
- Zuo Y.; Qiang L.; Farmer S. R. Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J. Biol. Chem. 2006, 281, 7960–7967. 10.1074/jbc.m510682200. [DOI] [PubMed] [Google Scholar]
- Lieder B.; Zaunschirm M.; Holik A. K.; Ley J. P.; Hans J.; Krammer G. E.; Somoza V. The Alkamide trans-Pellitorine Targets PPARgamma via TRPV1 and TRPA1 to Reduce Lipid Accumulation in Developing 3T3-L1 Adipocytes. Front. Pharmacol. 2017, 8, 316. 10.3389/fphar.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes E.; Fernandes M.; Keeble J. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K.; Kawabata-Shoda E.; Doihara H.; Kojima R.; Okada H.; Mochizuki S.; Sano Y.; Inamura K.; Matsushime H.; Koizumi T.; Yokoyama T.; Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 3408–3413. 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi R.; Wakil S. J. Increased synthesis and accumulation of phospholipids during differentiation of 3T3-L1 cells into adipocytes. J. Biol. Chem. 1983, 258, 3559–3564. [PubMed] [Google Scholar]
- Adams T. B.; Cohen S. M.; Doull J.; Feron V. J.; Goodman J. I.; Marnett L. J.; Munro I. C.; Portoghese P. S.; Smith R. L.; Waddell W. J.; Wagner B. M. The FEMA GRAS assessment of cinnamyl derivatives used as flavor ingredients. Food Chem. Toxicol. 2004, 42, 157–185. 10.1016/j.fct.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Sapienza P. P.; Ikeda G. J.; Warr P. I.; Plummer S. L.; Dailey R. E.; Lin C. S. Tissue distribution and excretion of 14C-labelled cinnamic aldehyde following single and multiple oral administration in male Fischer 344 rats. Food Chem. Toxicol. 1993, 31, 253–261. 10.1016/0278-6915(93)90075-a. [DOI] [PubMed] [Google Scholar]
- Hsu C.-L.; Yen G.-C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. 10.1021/jf071695r. [DOI] [PubMed] [Google Scholar]
- Mitterberger M. C.; Lechner S.; Mattesich M.; Kaiser A.; Probst D.; Wenger N.; Pierer G.; Zwerschke W. DLK1(PREF1) is a negative regulator of adipogenesis in CD105(+)/CD90(+)/CD34(+)/CD31(-)/FABP4(-) adipose-derived stromal cells from subcutaneous abdominal fat pats of adult women. Stem Cell Res. 2012, 9, 35–48. 10.1016/j.scr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Bijland S.; Mancini S. J.; Salt I. P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. 10.1042/cs20120536. [DOI] [PubMed] [Google Scholar]
- Yin W.; Mu J.; Birnbaum M. J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. 10.1074/jbc.m308484200. [DOI] [PubMed] [Google Scholar]
- Chen S. C.; Brooks R.; Houskeeper J.; Bremner S. K.; Dunlop J.; Viollet B.; Logan P. J.; Salt I. P.; Ahmed S. F.; Yarwood S. J. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol. Cell. Endocrinol. 2017, 440, 57–68. 10.1016/j.mce.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M.; Hotamisligil G. S. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulin J.; Berget I.; Lien S.; Sundvold H. Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comp. Biochem. Physiol B Comp. Biochem. Mol. Biol. 2008, 151, 147–152. 10.1016/j.cbpb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Emont M. P.; Jun H.; Qiao X.; Liao J.; Kim D.-i.; Wu J. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metab., Clin. Exp. 2017, 77, 58–64. 10.1016/j.metabol.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes S. J. F.; Sousa F. I. A. B.; Pereira D. M. S.; Ferro T. A. F.; Pereira I. C. P.; Silva B. L. R.; Pinheiro A. J. M. C. R.; Mouchrek A. Q. S.; Monteiro-Neto V.; Costa S. K. P.; Nascimento J. L. M.; Grisotto M. A. G.; da Costa R.; Fernandes E. S. Cinnamaldehyde modulates LPS-induced systemic inflammatory response syndrome through TRPA1-dependent and independent mechanisms. Int. Immunopharmacol. 2016, 34, 60–70. 10.1016/j.intimp.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Aubdool A. A.; Kodji X.; Abdul-Kader N.; Heads R.; Fernandes E. S.; Bevan S.; Brain S. D. TRPA1 activation leads to neurogenic vasodilatation: involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br. J. Pharmacol. 2016, 173, 2419–2433. 10.1111/bph.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J.; Son H. J.; Song S. H.; Jung M.; Kim Y.; Rhyu M.-R. The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One 2013, 8, e71603 10.1371/journal.pone.0071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z.; Luo Z.; Ma S.; Liu D. TRP channels and their implications in metabolic diseases. Pfluegers Arch 2011, 461, 211–223. 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M.; Takova T. Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Differentiation 1996, 60, 151–158. 10.1046/j.1432-0436.1996.6030151.x. [DOI] [PubMed] [Google Scholar]
- Shi H.; Halvorsen Y.-D.; Ellis P. N.; Wilkison W. O.; Zemel M. B. Role of intracellular calcium in human adipocyte differentiation. Physiol. Genom. 2000, 3, 75–82. 10.1152/physiolgenomics.2000.3.2.75. [DOI] [PubMed] [Google Scholar]
- Lieder B.; Hoi J.; Burian N.; Hans J.; Holik A.-K.; Beltran Marquez L. R.; Ley J. P.; Hatt H.; Somoza V. Structure-Dependent Effects of Cinnamaldehyde Derivatives on TRPA1-Induced Serotonin Release in Human Intestinal Cell Models. J. Agric. Food Chem. 2020, 68, 3924–3932. 10.1021/acs.jafc.9b08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel A.; Pignitter M.; Hochkogler C. M.; Rohm B.; Walker J.; Bytof G.; Lantz I.; Somoza V. Caffeine dose-dependently induces thermogenesis but restores ATP in HepG2 cells in culture. Food Funct. 2012, 3, 955–964. 10.1039/c2fo30053b. [DOI] [PubMed] [Google Scholar]
- Rohm B.; Holik A. K.; Kretschy N.; Somoza M. M.; Ley J. P.; Widder S.; Krammer G. E.; Marko D.; Somoza V. Nonivamide enhances miRNA let-7d expression and decreases adipogenesis PPARgamma expression in 3T3-L1 cells. J. Cell. Biochem. 2015, 116, 1153–1163. 10.1002/jcb.25052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P.; Mayer E. P.; Fowler S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holik A. K.; Lieder B.; Kretschy N.; Somoza M. M.; Held S.; Somoza V. N(ϵ) -Carboxymethyllysine Increases the Expression of miR-103/143 and Enhances Lipid Accumulation in 3T3-L1 Cells. J. Cell. Biochem. 2016, 117, 2413–2422. 10.1002/jcb.25576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieder B.; Schweiger K.; Somoza V., The TRP channel agonists nonivamide and cinnamaldehyde augment cold-induced mitochondrial biogenesis in white adipocytes. In Flavour Science, Proceedings of the XV Weurman Flavour Research Symposium; Barbara Siegmund E. L., Ed.; Verlag der Technischen Universität Graz: Graz, Austria, 2018. [Google Scholar]
- Chen K.; Zhou J.-D.; Zhang F.; Zhang F.; Zhang R.-R.; Zhan M.-S.; Tang X.-Y.; Deng B.; Lei M.-G.; Xiong Y.-Z. Transcription factor C/EBPbeta promotes the transcription of the porcine GPR120 gene. J. Mol. Endocrinol. 2016, 56, 91–100. 10.1530/jme-15-0200. [DOI] [PubMed] [Google Scholar]
- Hewitt K. N.; Pratis K.; Jones M. E. E.; Simpson E. R. Estrogen replacement reverses the hepatic steatosis phenotype in the male arromatase knockout mouse. Endocrinology 2004, 145, 1842–1848. 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.