Abstract
Aim
To investigate the association between regional cerebral oxygen saturation (rSO2) and neurological outcomes in extracorporeal cardiopulmonary resuscitation (ECPR) patients after out‐of‐hospital cardiac arrest (OHCA).
Methods
We used data from the Japan‐Prediction of Neurological Outcomes in Patients Post‐Cardiac Arrest Registry. This registry included consecutive comatose patients after OHCA who were transferred to 15 hospitals in Japan from 2011 to 2013. Our primary end‐point was a good neurological outcome (cerebral performance categories 1 or 2) at 90 days after OHCA.
Results
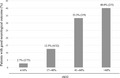
Among the enrolled patients, 121 (6.3%) received ECPR. Eleven (9.1%) had a good neurological outcome. Receiver operating characteristic curve analysis revealed the optimal cut‐off value as >16%. Good neurological outcomes were observed in 19.6% (9/46) and 2.7% (2/74) of patients with rSO2 >16% and rSO2 ≤16%, respectively.
Conclusion
The neurological outcome of ECPR patients differed according to their rSO2 values. When considering ECPR, the rSO2 value could be important in addition to other criteria. Further studies that focus on ECPR patients and serial rSO2 values are needed.
Keywords: Cardiac arrest, cardiopulmonary resuscitation, extracorporeal cardiopulmonary resuscitation, neurological outcome, regional cerebral oxygen saturation
Neurological outcomes of extracorporeal cardiopulmonary resuscitation (ECPR) patients differed according to regional cerebral oxygen saturation (rSO2) values. The optimal cut‐off rSO2 value was >16% among ECPR patients. Regional cerebral oxygen saturation could be considered if ECPR is indicated for out‐of‐hospital cardiac arrest patients.
Introduction
Despite recent improvements in advanced resuscitation therapy and post‐cardiac arrest care, out‐of‐hospital cardiac arrest (OHCA) remains a major public health problem.1 The survival rate after OHCA is low, and neurological deficits are common among survivors due to ischemic brain injury.2
Extracorporeal cardiopulmonary resuscitation (ECPR) is an effective technique to maintain organ perfusion while treating reversible causes of cardiac arrest and recovering spontaneous circulation. The 2015 American Heart Association guidelines recommended considering ECPR in select cardiac arrest patients if rapid induction is available.3 However, the indication criteria for ECPR vary, and there is no global consensus. Although the indication for ECPR should be carefully considered because of the invasiveness of ECPR and the high costs associated, quick judgment is needed to maximize ECPR efficacy. A previous systematic review showed that shockable rhythm and shorter CPR duration might be good neurological predictors in ECPR patients;4 however, the quality of evidence was low.
Recent studies reported that the value of regional cerebral oxygen saturation (rSO2) could be useful to assess cerebral oxygen perfusion during CPR5, 6 and predict neurological outcomes in cardiac arrest patients.7, 8, 9 These studies focused on whole cardiac arrest populations; however, the characteristics of patients with ECPR appear to be different from those without ECPR. Although it could be a prognostic factor for ECPR patients, few reports have assessed the rSO2 value of ECPR patients.10 Therefore, this study investigated the association between the rSO2 value and neurological outcome in patients treated with ECPR after OHCA.
Methods
Study design and data source
This was a secondary analysis of prospectively collected registry data of consecutive OHCA patients who were transferred to 15 tertiary emergency care hospitals in Japan between May 2011 and August 2013, based on the Japan‐Prediction of Neurological Outcomes in Patients Post‐Cardiac Arrest (J‐POP) Registry.7, 11 The database includes pre‐ and in‐hospital data collected from the emergency medical service (EMS) system and the medical records according to the Utstein style. The inclusion criteria of the J‐POP registry were unresponsive OHCA patients during and after resuscitation on hospital arrival. Exclusion criteria were trauma, accidental hypothermia, age <18 years, previous completion of the “Do Not Attempt Resuscitation” form, and a Glasgow Coma Scale (GCS) score of >8 on arrival at the hospital. The details of the protocol and main results of the J‐POP Registry have been published previously.7, 11, 12
According to the Emergency Life Guards Act, all EMS providers in Japan are obligated to perform CPR, and qualified EMS providers are authorized to intubate OHCA patients and administer i.v. adrenaline before hospital arrival.
Immediately after hospital arrival, two disposal probes for a near‐infrared spectrometer (INVOS 5100C; Covidien, Boulder, CO, USA) were bilaterally positioned at the patient’s forehead. After several seconds of stable recordings, rSO2 was monitored for at least 1 min, and the lowest rSO2 value was used for the study. The lower limit of measurable rSO2 was 15%, and rSO2 values below this threshold were regarded as 15%. To evaluate the usefulness of rSO2 values in the decision to undertake ECPR, we analyzed patients who received ECPR after OHCA.
Treatment and outcomes
Advanced life support was provided according to the national guidelines for resuscitation. The study protocol basically predefined that ECPR would be induced in patients with initially documented ventricular fibrillation or pulseless ventricular tachycardia, if sustained return of spontaneous circulation (ROSC) for at least 20 min was not achieved. If sustained ROSC was achieved, targeted temperature management was induced with blood pressure over 90 mmHg and a GCS score of 3–8. The final decision to undertake all procedures was made by the attending physician.
The primary end‐point was a good neurological outcome at 90 days after cardiac arrest. Neurological outcomes were evaluated using cerebral performance categories (CPC);13 CPC 1 (good performance) and CPC 2 (moderate disability) were defined as “good neurological outcomes”, and CPC 3 (severe disability), CPC 4 (vegetative state), and CPC 5 (brain death or death) as “poor neurological outcomes” according to the Utstein‐style guidelines.14, 15 The CPC of an individual patient was determined by at least two physicians who were blinded to the patient’s rSO2 readings at hospital arrival. A detailed definition of the parameters has been previously published.7, 11, 12
Statistical analysis
Age and rSO2 values are expressed as median (interquartile range), and other continuous variables are presented as means (standard deviation). Categorical variables are described as numbers (percentages). Receiver operating characteristic (ROC) curve analysis was carried out, and the optimal rSO2 cut‐off value for good neurological outcomes in patients with ECPR was determined by the Youden index.16 We divided the patients with ECPR into two groups according to this cut‐off point and compared the neurological outcomes and patient backgrounds.
All analyses were undertaken with SAS version 9.4 for Windows (SAS Institute, Cary, NC, USA). The two‐sided significance level was set at P < 0.05 for each analysis.
Sensitivity analysis
We carried out a sensitivity analysis that was restricted to ECPR patients with initially detected shockable rhythm and time to hospital arrival within 45 min, considering the previously reported prognostic factors for neurological outcomes.4 We also compared the neurological outcomes at cut‐off values that we had previously used.17
Results
Of 3,086 consecutive OHCA patients, 1,921 adult patients whose rSO2 values had been recorded using near‐infrared spectroscopy were enrolled according to the protocol of the J‐POP Registry. Among those, 121 patients (6.3%) received ECPR (Fig. 1). Patient demographics are summarized in Table 1. A total of 11 patients (9.1%) had a good neurological outcome at 90 days after OHCA. As a result, 43% of patients with ECPR initially had detected non‐shockable rhythm such as pulseless electrical activity and asystole.
Fig. 1.
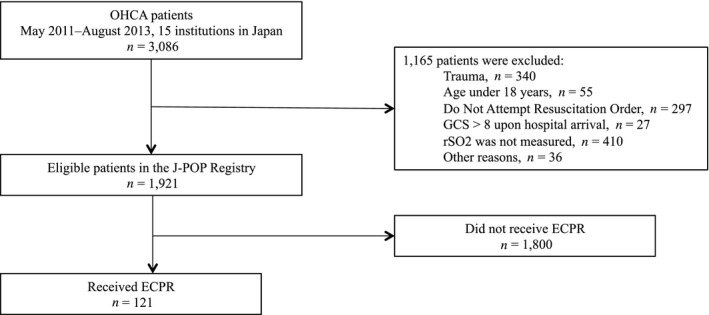
Study flow diagram. ECPR, extracorporeal cardiopulmonary resuscitation; GCS, Glasgow Coma Scale; J‐POP, Japan‐Prediction of Neurological Outcomes in Patients Post‐Cardiac Arrest; OHCA, out‐of‐hospital cardiac arrest; rSO2, regional cerebral oxygen saturation.
Table 1.
Characteristics and neurological outcomes of patients who received extracorporeal cardiopulmonary resuscitation
| Characteristic | Total (n = 121) |
Patients with rSO2 ≤16% (n = 75) |
Patients with rSO2 >16% (n = 46) |
|---|---|---|---|
| Age, years; median (IQR) | 60 (47, 70) | 59 (44, 67) | 64 (48, 72) |
| Male sex, n (%) | 93 (76.9) | 55 (73.3) | 38 (82.6) |
| rSO2 on arrival at the hospital, median (IQR) | 15 (15, 24) | 15 (15, 15) | 35 (21, 42) |
| Locations of cardiac arrest, n (%) | |||
| Home | 53 (43.8) | 32 (42.7) | 21 (45.7) |
| Nursing home/assisted living | 2 (1.7) | 1 (1.3) | 1 (2.2) |
| Public building | 17 (14.1) | 10 (13.3) | 7 (15.2) |
| Street | 16 (13.2) | 8 (10.7) | 8 (17.4) |
| Others | 33 (27.3) | 24 (32.0) | 9 (19.6) |
| Type of bystander‐witness status, n (%) | |||
| No witness | 32 (26.5) | 21 (28.0) | 11 (23.9) |
| Family member | 42 (34.7) | 27 (36.0) | 15 (32.6) |
| EMS | 12 (9.9) | 8 (10.7) | 4 (8.7) |
| Other | 35 (28.9) | 19 (25.3) | 16 (34.8) |
| Bystander‐initiated CPR, n (%) | 56 (46.3) | 36 (48.0) | 20 (43.5) |
| Presumed cardiac origin, n (%) | 95 (78.5) | 58 (77.3) | 37 (80.4) |
| Initially documented rhythms at the scene of cardiac arrest, n (%) | |||
| VF/pulseless VT | 66 (54.6) | 36 (48.0) | 30 (65.2) |
| PEA | 32 (26.5) | 22 (29.3) | 10 (21.7) |
| Asystole | 20 (16.5) | 17 (22.7) | 3 (6.5) |
| Prehospital procedures, n (%) | |||
| Advanced airway devices | 59 (48.8) | 43 (57.3) | 16 (34.8) |
| Intravenous adrenaline administration | 34 (28.1) | 23 (30.7) | 11 (23.9) |
| Defibrillation | 71 (58.7) | 39 (52.0) | 32 (69.6) |
| Emergency call to hospital arrival, min; mean (SD) | 33 (12.3) | 33 (9.8) | 34 (15.7) |
| Rhythms at rSO2 measurement, n (%) | |||
| VF/pulseless VT | 39 (32.2) | 21 (28.0) | 18 (39.1) |
| PEA | 30 (24.8) | 18 (24.0) | 12 (26.1) |
| Asystole | 43 (35.5) | 35 (46.7) | 8 (17.4) |
| Others (pulses detectable at hospital arrival) | 9 (7.4) | 1 (1.3) | 8 (17.4) |
| Procedures after hospital arrival, n (%) | |||
| Therapeutic hypothermia | 78 (64.5) | 46 (61.3) | 32 (69.6) |
| Coronary angiography | 68 (56.2) | 38 (50.6) | 30 (65.2) |
| Primary percutaneous coronary intervention | 32 (26.4) | 16 (21.3) | 16 (34.8) |
| Neurological outcomes at 90 days after OHCA | |||
| CPC 1, good performance | 10 (8.3) | 2 (2.7) | 8 (17.4) |
| CPC 2, moderate disability | 1 (0.8) | 0 (0.0) | 1 (2.2) |
| CPC 3, severe disability | 2 (1.7) | 1 (1.3) | 1 (2.2) |
| CPC 4, vegetative state | 6 (5.0) | 3 (4.0) | 3 (6.5) |
| CPC 5, death | 102 (84.3) | 68 (91.9) | 33 (71.7) |
Lower limit of measurable regional cerebral oxygen saturation (rSO2) was 15%.
CPC, cerebral performance category; CPR, cardiopulmonary resuscitation; EMS, emergency medical service; IQR, interquartile range; OHCA, out‐of‐hospital cardiac arrest; PEA, pulseless electrical activity; SD, standard deviation; VF, ventricular fibrillation; VT, ventricular tachycardia.
The ROC analysis indicated an optimal rSO2 cut‐off value of >16% for predicting a good neurological outcome (area under the ROC curve 0.76, 95% confidence interval [CI], 0.61–0.91; sensitivity 0.82, 95% CI, 0.52‐0.95; specificity 0.66, 95% CI, 0.57–0.75; Fig. 2). We stratified patients into two groups with rSO2 >16% and rSO2 ≤16%. The patient characteristics and neurological outcomes of these two groups are shown in Table 1. A good neurological outcome was observed in 19.6% (9/46) and 2.7% (2/75) of patients with rSO2 >16% and rSO2 ≤16%, respectively (P = 0.003).
Fig. 2.
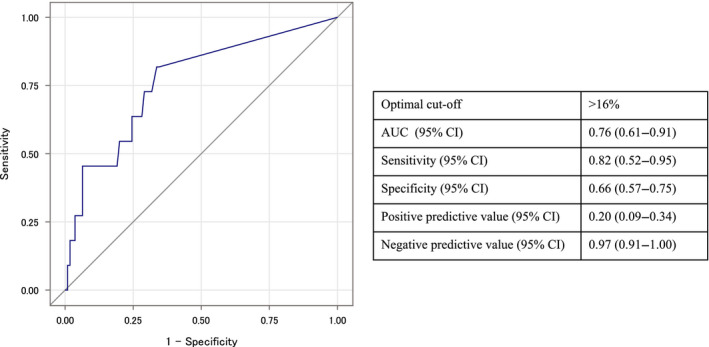
Receiver operating characteristic analysis of the regional cerebral oxygen saturation values in patients with extracorporeal cardiopulmonary resuscitation. AUC, area under the curve; CI, confidence interval.
In the sensitivity analysis restricted to ECPR patients with initial shockable rhythm and time to hospital arrival within 45 min, patients with rSO2 values >16% showed significantly better neurological outcomes than those with rSO2 ≤16% (26.9% versus 3.5%, respectively; P = 0.021). Using additional cut‐off values, we categorized ECPR patients into four groups: rSO2 ≤16%, rSO2 17‐40%, rSO2 41‐60%, and rSO2 >60%. The proportion of good neurological outcome was 2.7%, 12.5%, 33.3%, and 40.0%, respectively (Fig. 3).
Fig. 3.
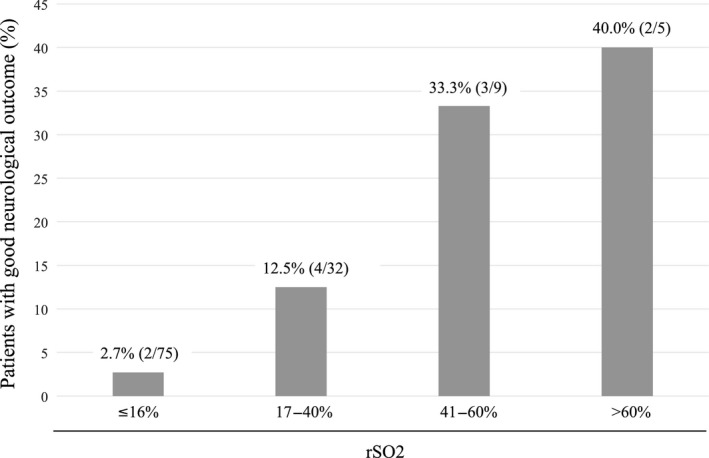
Proportion of patients with good neurological outcome following extracorporeal cardiopulmonary resuscitation, according to each regional cerebral oxygen saturation (rSO2) value.
Discussion
Our results showed that neurological outcomes of ECPR patients differ according to their rSO2 values on hospital arrival. This result was maintained when limited to patients with initial shockable rhythm and time to hospital arrival within 45 min. However, the results of this study should be carefully interpreted because our data only included the initial rSO2 values, and serial change from the baseline might also provide important information to predict patient outcomes.6
A prior systematic review on predictors of neurological outcome for adult patients with ECPR showed that initial shockable rhythm and CPR duration were independent predictors;4 however, the rSO2 value was not analyzed. Recent reviews concluded that rSO2 values during CPR could predict neurological outcome,18 and potentially facilitate clinical decision‐making.19 Our study also indicated that there was a significant difference in neurological outcomes between patients with rSO2 >16% and rSO2 ≤16%. The difference was maintained among patients with initial shockable rhythm and time to hospital arrival within 45 min. This suggests that the rSO2 value could be important in predicting the neurological outcome in ECPR patients in addition to previously reported parameters.
Because predicting the neurological outcome in ECPR patients is difficult, it is challenging for emergency physicians to establish the indication for ECPR. Although patient characteristics, such as type of bystander‐witness status, origin of cardiac arrest, and time to hospital arrival, did not differ between the groups in our study, the neurological outcome was significantly different depending on the rSO2 value. Those parameters are usually included in ECPR indication criteria;20, 21, 22 however, they may be insufficient to decide for or against ECPR use. The rSO2 value on hospital arrival could comprehensively reflect the reversibility of brain function impairments at the time, which might be influenced by the quality of prehospital CPR in addition to previously established parameters. To define an indication for ECPR in OHCA patients, we should consider not only these parameters but also the rSO2 value.
Our determined cut‐off for the rSO2 value was much lower than previously described in OHCA patients. Most studies reported that rSO2 values in patients with a good neurological outcome were above 30%,19, 23, 24, 25 which might suggest terminating resuscitation could be considered for patients whose rSO2 values were constantly below 30% during resuscitation.26 However, most patients included in those studies did not receive ECPR. After ECPR induction, vital organs can receive almost as much perfusion as in ROSC, and ECPR can recover more severe patients with much lower rSO2 values.
Our study has several limitations. First, because of the small number of patients, we could not undertake a multivariate analysis. We carried out a sensitivity analysis restricted to patients with initial shockable rhythm and time to hospital arrival within 45 min; the rSO2 value was still associated with neurological outcome. Second, our registry data included only the initial rSO2 value. Recently, Takegawa et al. reported that the trend pattern of the rSO2 value during CPR was important for patient outcome prediction.6 We agree that only the initial rSO2 value might be insufficient to assess the prognosis of ECPR patients. However, few studies have assessed the rSO2 value among ECPR patients, and it is uncertain which rSO2 value (mean, initial, highest, and serial change from the baseline) should be used for ECPR indication. Further studies focused on ECPR patients and assessing several types of rSO2 values are needed. Third, the time from OHCA to ECPR initiation was not available. Because all included hospitals were tertiary emergency care hospitals in Japan, we assumed that ECPR could be rapidly implemented. We assessed the time from emergency call to hospital arrival instead; however, this might be insufficient. Fourth, the lower limit of measurable rSO2 was 15% in the device used in this study, and there were no in vivo data to confirm the accuracy of lower rSO2 values. Several previous studies have used this device for cardiac arrest patients whose rSO2 values were very low;27, 28 however, lower values could provide more information, especially for ECPR patients. Further studies using devices that can accurately measure lower values are required. Finally, in other countries, where the EMS system and the severity of OHCA patients could differ from those in Japan, the results of this study might not be applicable.
Conclusion
In patients receiving ECPR, the neurological outcome differed according to their rSO2 values. It could be important to consider the rSO2 value in addition to other criteria when deciding the indication for ECPR. Further studies focusing on ECPR patients and serial rSO2 values are needed to identify the significance of rSO2 monitoring as an indication criterion for ECPR.
Disclosure
Approval of the research protocol: The study protocol was approved by the Ethics Committee of the Kyoto University Graduate School and Faculty of Medicine (No. R1666; 6 September 2018), which also waived the requirement for informed consent.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: T. Seki received personal fees from Pfizer outside the submitted work. K. Nishiyama has conducted an investigator‐sponsored study (Covidien, Japan) entitled “Pre‐hospital rSO2 Study” (“Pre‐hospital Resuscitation for Sustaining Cerebral Oxidation: Observational Cohort Study”). K. Kawakami received honoraria from Shin Nippon Biomedical Laboratories, research funds from Olympus, Sumitomo Dainippon Pharma, Bayer Yakuhin, Stella Pharma, Novartis Pharma, CMIC, Amgen Astellas BioPharma, Suntory Beverage & Food, and Medical Platform, advisor fees from JMDC, and holds stocks in Real World Data. There are no patent products under development or marketed products relevant to those companies to declare.
Acknowledgements
We thank Editage for English language editing.
Funding Information
This study was supported by the Japan Society for the Promotion of Science Grant‐in‐Aid for Scientific Research (KAKENHI, grant nos. JP24390400, JP26462753, and JP17K11603).
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE et al Heart disease and stroke statistics‐2017 update: A report from the American Heart Association. Circulation 2017; 135: e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drennan IR, Lin S, Sidalak DE et al Survival rates in out‐of‐hospital cardiac arrest patients transported without prehospital return of spontaneous circulation: An observational cohort study. Resuscitation 2014; 85: 1488–93. [DOI] [PubMed] [Google Scholar]
- 3. Link MS, Berkow LC, Kudenchuk PJ et al Part 7: Adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132: S444–64. [DOI] [PubMed] [Google Scholar]
- 4. Wang J, Ma Q, Zhang H et al Predictors of survival and neurologic outcome for adults with extracorporeal cardiopulmonary resuscitation: A systemic review and meta‐analysis. Medicine (Baltimore) 2018; 97: e13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogawa Y, Shiozaki T, Hirose T et al Load‐distributing‐band cardiopulmonary resuscitation for out‐of‐hospital cardiac arrest increases regional cerebral oxygenation: a single‐center prospective pilot study. Scand. J. Trauma. Resusc. Emerg. Med. 2015; 23: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takegawa R, Shiozaki T, Ogawa Y et al Usefulness of cerebral rSO2 monitoring during CPR to predict the probability of return of spontaneous circulation. Resuscitation 2019; 139: 201–7. [DOI] [PubMed] [Google Scholar]
- 7. Nishiyama K, Ito N, Orita T et al Regional cerebral oxygen saturation monitoring for predicting interventional outcomes in patients following out‐of‐hospital cardiac arrest of presumed cardiac cause: A prospective, observational, multicentre study. Resuscitation 2015; 96: 135–41. [DOI] [PubMed] [Google Scholar]
- 8. Parnia S, Yang J, Nguyen R et al Cerebral oximetry during cardiac arrest: A multicenter study of neurologic outcomes and survival. Crit. Care. Med. 2016; 44: 1663–74. [DOI] [PubMed] [Google Scholar]
- 9. Storm C, Leithner C, Krannich A et al Regional cerebral oxygen saturation after cardiac arrest in 60 patients–A prospective outcome study. Resuscitation 2014; 85: 1037–41. [DOI] [PubMed] [Google Scholar]
- 10. Ehara N, Hirose T, Shiozaki T et al The relationship between cerebral regional oxygen saturation during extracorporeal cardiopulmonary resuscitation and the neurological outcome in a retrospective analysis of 16 cases. J. Intensive Care 2017; 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ito N, Nishiyama K, Callaway CW et al Noninvasive regional cerebral oxygen saturation for neurological prognostication of patients with out‐of‐hospital cardiac arrest: A prospective multicenter observational study. Resuscitation 2014; 85: 778–84. [DOI] [PubMed] [Google Scholar]
- 12. Nishiyama K, Ito N, Orita T et al Characteristics of regional cerebral oxygen saturation levels in patients with out‐of‐hospital cardiac arrest with or without return of spontaneous circulation: A prospective observational multicentre study. Resuscitation 2015; 96: 16–22. [DOI] [PubMed] [Google Scholar]
- 13. Resuscitation Brain. Clinical Trial I Study Group. A randomized clinical study of cardiopulmonary‐cerebral resuscitation: Design, methods, and patient characteristics. Am. J. Emerg. Med. 1986; 4: 72–86. [PubMed] [Google Scholar]
- 14. Jacobs I, Nadkarni V, Bahr J et al Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa). Resuscitation 2004; 63: 233–49. [DOI] [PubMed] [Google Scholar]
- 15. Perkins GD, Jacobs IG, Nadkarni VM et al Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out‐of‐Hospital Cardiac Arrest: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care. Perioperative and Resuscitation. Circulation 2015; 132: 1286–300. [DOI] [PubMed] [Google Scholar]
- 16. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–5. [DOI] [PubMed] [Google Scholar]
- 17. Nakatani Y, Nakayama T, Nishiyama K et al Effect of target temperature management at 32–34 degrees C in cardiac arrest patients considering assessment by regional cerebral oxygen saturation: A multicenter retrospective cohort study. Resuscitation 2018; 126: 185–90. [DOI] [PubMed] [Google Scholar]
- 18. Genbrugge C, Dens J, Meex I et al Regional cerebral oximetry during cardiopulmonary resuscitation: Useful or useless? J. Emerg. Med. 2016; 50: 198–207. [DOI] [PubMed] [Google Scholar]
- 19. Schnaubelt S, Sulzgruber P, Menger J et al Regional cerebral oxygen saturation during cardiopulmonary resuscitation as a predictor of return of spontaneous circulation and favourable neurological outcome ‐ A review of the current literature. Resuscitation 2018; 125: 39–47. [DOI] [PubMed] [Google Scholar]
- 20. Kagawa E, Inoue I, Kawagoe T et al Assessment of outcomes and differences between in‐ and out‐of‐hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 2010; 81: 968–73. [DOI] [PubMed] [Google Scholar]
- 21. Maekawa K, Tanno K, Hase M et al Extracorporeal cardiopulmonary resuscitation for patients with out‐of‐hospital cardiac arrest of cardiac origin: a propensity‐matched study and predictor analysis. Crit. Care. Med. 2013; 41: 1186–96. [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto T, Morimura N, Nagao K et al Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out‐of‐hospital cardiac arrest: A prospective observational study. Resuscitation 2014; 85: 762–8. [DOI] [PubMed] [Google Scholar]
- 23. Singer AJ, Ahn A, Inigo‐Santiago LA et al Cerebral oximetry levels during CPR are associated with return of spontaneous circulation following cardiac arrest: an observational study. Emerg. Med. J. 2015; 32: 353–6. [DOI] [PubMed] [Google Scholar]
- 24. Schewe JC, Thudium MO, Kappler J et al Monitoring of cerebral oxygen saturation during resuscitation in out‐of‐hospital cardiac arrest: a feasibility study in a physician staffed emergency medical system. Scand. J. Trauma. Resusc. Emerg. Med. 2014; 22: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hirose T, Shiozaki T, Nomura J et al Pre‐hospital portable monitoring of cerebral regional oxygen saturation (rSO2) in seven patients with out‐of‐hospital cardiac arrest. BMC Res. Notes. 2016; 9: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cournoyer A, Iseppon M, Chauny JM et al Near‐infrared spectroscopy monitoring during cardiac arrest: A systematic review and meta‐analysis. Acad. Emerg. Med. 2016; 23: 851–62. [DOI] [PubMed] [Google Scholar]
- 27. Newman DH, Callaway CW, Greenwald IB et al Cerebral oximetry in out‐of‐hospital cardiac arrest: standard CPR rarely provides detectable hemoglobin‐oxygen saturation to the frontal cortex. Resuscitation 2004; 63: 189–94. [DOI] [PubMed] [Google Scholar]
- 28. Parnia S, Nasir A, Shah C et al A feasibility study evaluating the role of cerebral oximetry in predicting return of spontaneous circulation in cardiac arrest. Resuscitation 2012; 83: 982–5. [DOI] [PubMed] [Google Scholar]


