Abstract
Excessive caloric intake in a form of high-fat diet (HFD) was long thought to be the major risk factor for development of obesity and its complications, such as fatty liver disease and insulin resistance. Recently, there has been a paradigm shift and more attention is attributed to the effects of sugar-sweetened beverages (SSBs) as one of the culprits of the obesity epidemic. In this review, we present the data invoking fructose intake with development of hepatic insulin resistance in human studies and discuss the pathways by which fructose impairs hepatic insulin action in experimental animal models. First, we described well-characterized pathways by which fructose metabolism indirectly leads to hepatic insulin resistance. These include unequivocal effects of fructose to promote de novo lipogenesis (DNL), impair fatty acid oxidation (FAO), induce endoplasmic reticulum (ER) stress and trigger hepatic inflammation. Additionally, we entertained the hypothesis that fructose can directly impede insulin signaling in the liver. This appears to be mediated by reduced insulin receptor and insulin receptor substrate 2 (IRS2) expression, increased protein-tyrosine phosphatase 1B (PTP1b) activity, whereas knockdown of ketohexokinase (KHK), the rate-limiting enzyme of fructose metabolism, increased insulin sensitivity. In summary, dietary fructose intake strongly promotes hepatic insulin resistance via complex interplay of several metabolic pathways, at least some of which are independent of increased weight gain and caloric intake. The current evidence shows that the fructose, but not glucose, component of dietary sugar drives metabolic complications and contradicts the notion that fructose is merely a source of palatable calories that leads to increased weight gain and insulin resistance.
Introduction
Obesity increases the risk of developing a myriad of negative health outcomes, including dyslipidemia, hypertension, insulin resistance, type 2 diabetes (T2D) and non-alcoholic fatty liver disease (NAFLD). NAFLD is a liver manifestation of obesity and is estimated to affect 1 billion individuals worldwide [1]. In the USA, NAFLD is the most common liver abnormality in children [2] and among the top three causes of liver transplant in adults [3]. Due to a strong link between obesity and hepatic fat deposition, it has been proposed that perhaps a more appropriate name for this entity is obesity associated fatty liver disease (OAFLD) or simply, fatty liver disease [4]. Hepatic insulin resistance is a key feature of NAFLD and is universally present when the gold standard test, euglycemic hyperinsulinemic clamp, is used to assess insulin action [5]. However, it remains undefined whether hepatic insulin resistance develops first and primes the liver for subsequent fat accumulation or if hepatic insulin resistance is simply a product of increased fat accumulation in the liver.
Excessive caloric intake, either from a high-fat diet (HFD) or sugar-sweetened beverages (SSBs), is associated with development of NAFLD and hepatic insulin resistance [6]. While historically HFDs were considered crucial to the development of fatty liver disease, experimental animal studies, epidemiological evidence and clinical trials in humans have documented the major role for added sugars, such as sucrose and high-fructose corn syrup, in the development of NAFLD and insulin resistance [7, 8, 9]. However, consensus is lacking whether the fructose or glucose components of table sugar (sucrose) and high-fructose corn syrup confers a greater risk or if sugar intake is simply a vehicle for increased caloric intake, which is the underlying driver of hepatic insulin resistance. Accumulating evidence indicates that fructose intake is strongly associated with development of hepatic insulin resistance, which in turn promotes development of NAFLD, even when total energy intake is matched by equal calories from glucose [8, 10, 11].
In this review, we will present the data invoking fructose intake with development of hepatic insulin resistance in human studies and discuss the pathways by which fructose impairs hepatic insulin action in experimental animal models. Initially, we will focus on well-characterized pathways by which fructose metabolism indirectly leads to hepatic insulin resistance. These include unequivocal effects of fructose to stimulate de novo lipogenesis (DNL), impair fatty acid oxidation (FAO), induce endoplasmic reticulum (ER) stress and initiate hepatic inflammation. Additionally, we will also entertain the hypothesis that fructose can directly impede insulin signaling in the liver. Overall, this review aims to answer whether fructose induces hepatic insulin resistance through indirect and direct pathways or if it is merely a source of palatable calories that leads to increased weight gain.
Human Studies Linking Fructose Intake to Hepatic Insulin Resistance
Initial evidence linking increased sugar intake to insulin resistance in humans dates back to studies conducted in the late 1970s. For example, high sucrose feeding for fourteen days in heathy adults resulted in a decreased response to insulin, with a 25% reduction in lowering of blood glucose levels following exogenous insulin administration [12]. In a subsequent study performed on a regular diet supplemented with 250 g of glucose or fructose (~ 3.5 g/kg) for seven days, fructose, but not glucose, supplementation was associated with decreased insulin sensitivity during an intravenous insulin tolerance test in fifteen healthy, normal-weight individuals [11]. Likewise, six days of hypercaloric fructose feeding (3 g/kg) in seven healthy men increased fasting blood glucose levels and blunted suppression of hepatic glucose production during a hyperinsulinemic-euglycemic clamp, as compared to their baseline levels when fed a control diet [13]. In agreement with these studies, seven days of hypercaloric fructose feeding (3.5 g/kg) increased hepatic glucose production as assessed by 2-step hyperinsulinemic-euglycemic clamp and lowered whole body insulin sensitivity, when compared to the standard diet-fed group. This crossover study was performed in sixteen healthy adult males who were offspring of patients with type 2 diabetes, a group at risk for development of metabolic disorders, and eight control subjects [14]. Hepatic glucose production, as measured by isotope dilution analysis using 6,6 2H2 glucose, was also increased in a study of thirty-five normal-weight men in which a healthy diet was supplemented with 4 g/kg of fructose for fourteen days [15]. In this study, fructose feeding also impaired insulin-induced suppression of adipose tissue lipolysis, compared to control diet-fed individuals, but total body insulin resistance did not develop. The last three studies suggest that hepatic insulin resistance is one of the earliest signs of impaired insulin action and, in some cases, hepatic insulin resistance is present even before whole body insulin resistance can be detected. However, not all studies demonstrated increased insulin resistance with short term hypercaloric fructose feeding. Johnston et al. [16] found no change in Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and insulin levels following hypercaloric fructose, as compared to glucose, supplementation for fourteen days in thirty-two overweight men. Of interest, in this study HOMA-IR was increased after two weeks of isocaloric fructose supplementation. Furthermore, there were strong trends toward higher BMI (p=0.07) and higher insulin levels (p=0.09) in the fructose, as compared to the glucose, group before initiation of the study [16].
These short-term hypercaloric studies in which fructose supplementation provides about 1000 additional calories (3–4 g/kg of fructose) have been taken with caution, since increased caloric intake alone is sufficient to induce insulin resistance. Consumption of lower amounts of fructose along with ad libitum diets, however, can also lead to insulin resistance. Stanhope et al. [8] reported that consumption of 25% of daily energy requirement (168 g/day) from fructose-sweetened beverages for ten weeks increased blood glucose, fasting insulin and insulin excursion following oral glucose tolerance test (OGTT). These increases were not observed in the glucose-supplemented group. Further, insulin sensitivity index as assessed by the deuterated glucose disposal was decreased by 17% in overweight/obese subjects consuming fructose [8]. Even a much lower amount of hypercaloric fructose (75 g/day) supplemented in drinks for twelve weeks increased insulin levels and HOMA-IR in obese men, compared to their baseline values before the intervention [17]. Similarly, in a study providing 80 g/day of fructose in sweetened beverages for three weeks, fructose increased baseline endogenous glucose production and lowered suppression of hepatic glucose production during euglycemic-hyperinsulinemic clamp study, compared to an equal amount of glucose [18]. Taken in aggregate, these studies indicate that fructose consumption that reflects habitual SSB consumption can lead to decreased hepatic insulin sensitivity over time.
Studies of acute and chronic fructose supplementation on ad libitum diets still leave the question of whether or not isocaloric fructose intake has an effect on hepatic insulin resistance. This question was addressed in a crossover experiment in which eight healthy men consumed weight-maintaining, isocaloric diets containing either high-fructose (25% energy requirement) or low-fructose diet (starch provided in place of fructose) for nine days. In this study, subjects exhibited lower suppression of hepatic glucose production during a euglycemic-hyperinsulinemic clamp when they consumed the high-fructose diet, compared to the low-fructose diet [19]. Hallfrisch et al. [20] also conducted a crossover study in which twelve men with hyperinsulinemia and twelve healthy age- and BMI-matched men were provided standardized equicaloric meals that contained either 0, 7.5, or 15% of the energy requirement as fructose in solid food for five weeks. All other components of the diet were matched so that the participants received 43% of the calories as total carbohydrate, 42% as fat and 15% as protein. Compared with the 0% fructose diet, the 15% fructose diet increased glucose and insulin responses to a 3-hour oral sucrose tolerance test [20]. As above, the studies that utilized euglycemic-hyperinsulinemic clamps to index both whole body and hepatic insulin resistance suggest that development of hepatic insulin resistance precedes development of whole body insulin resistance [18, 19].
The rigorous nature of these highly controlled, labor intensive studies precludes inclusion of a large number of patients. In contrast, epidemiological evidence provides information on large populations of subjects. In a study of 70,000 women followed for 18 years, those who reported consuming 2–3 SSBs per day had 31% higher risk of developing type 2 diabetes, compared to subjects who consumed less than one drink per month, even after controlling for BMI and physical activity [9]. Similarly, in a cross sectional study of nearly 2,000 healthy women, those in the highest quintile of self-reported energy-adjusted fructose intake had 13.9% higher plasma C-peptide concentrations, a marker of insulin secretion, compared to women in the lowest quintile of fructose intake [21]. Likewise, a meta-analysis of 17 prospective cohort studies found that one serving per day increment in SSB consumption was associated with an 18% higher risk of developing type 2 diabetes among the studies that did not adjust for adiposity and a 13% increased risk in the studies that adjusted for adiposity [22]. Another meta-analysis of 1005 participants observed that both hypercaloric and isocaloric fructose intake promotes the development of hepatic insulin resistance in nondiabetic adults, without affecting peripheral or muscle insulin sensitivity [23]. Together, these studies indicate that SSB, and fructose consumption in particular, is associated with an increased risk of developing insulin resistance, even when adjusted for caloric intake or BMI.
Indirect Effect of Fructose on Insulin Resistance
Acute and chronic intake of fructose-sweetened beverages in clinical trials and epidemiological evidence on consumption of SSBs, strongly suggest that excess fructose intake leads to hepatic and whole body insulin resistance (Figure 1). In certain studies where such determination was possible, hepatic insulin resistance was observed to develop before whole body insulin resistance, indicating that hepatic insulin resistance precedes or possibly initiates development of whole body insulin resistance. These effects of fructose on hepatic insulin resistance are generally thought to be secondary to an increase in hepatic DNL, a decrease in FAO, augmentation of ER stress and potentiation of inflammation.
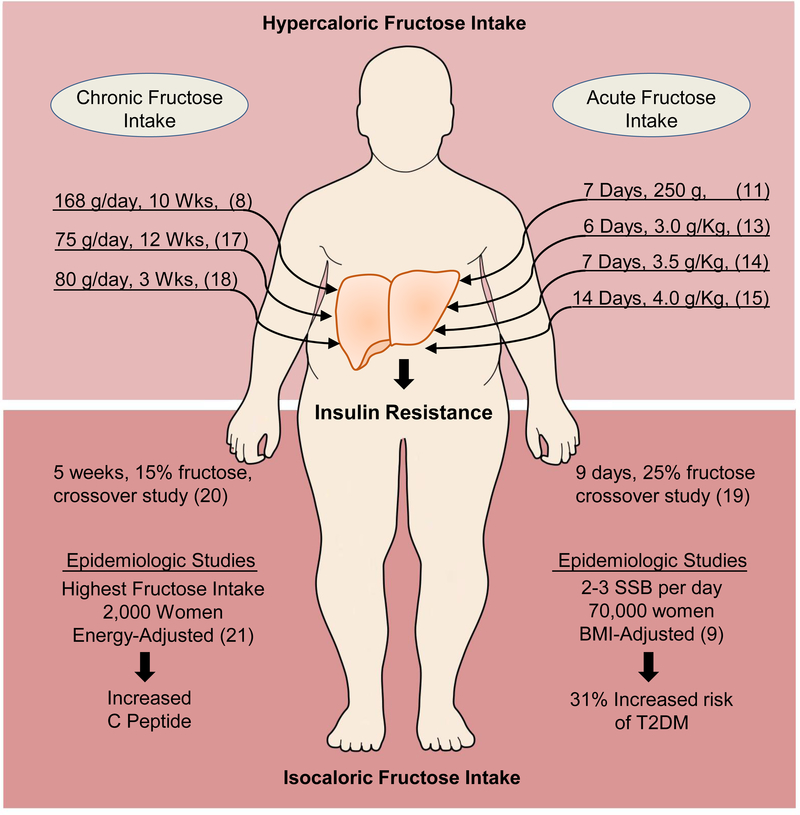
Figure 1.
Clinical Studies of Fructose and Hepatic Insulin Resistance
The top right side of the figure depicts short-term (~1–2 weeks) clinical studies in humans on a regular diet supplemented with additional 3–4g/kg of fructose. The top left side of the figure lists long-term (3–12 weeks) studies of fructose supplementation in human subjects on a regular diet, but with much lower amount of fructose. These short- and long-term studies document that hypercaloric fructose supplementation is associated with development of hepatic insulin resistance. The bottom panel depicts crossover studies of isocaloric short- (bottom right) and long-term (bottom left) consumption of dietary fructose. These studies demonstrate that weight maintaining diet supplemented with fructose is associated with hepatic insulin resistance. Lastly, epidemiologic evidence indicates that increased fructose intake, in sugar-sweetened beverages (SSB) on a population level, leads to outcomes suggestive of insulin resistance, even when adjusted for BMI or energy intake.
Fructose Increases Hepatic de novo Lipogenesis
Fructose is a highly lipogenic macronutrient, which stimulates hepatic DNL to a greater extent than it’s commonly compared counterpart glucose or even a HFD [6, 24, 25]. In our studies in chow and HFD-fed mice, 30% (W/V) fructose supplementation for 10 weeks induced a greater increase in enzymes regulating fatty acid synthesis, such as ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1), compared to mice receiving an equal amount of glucose [10]. Increased protein and mRNA levels of these DNL enzymes correlate with increased endogenous fatty acyl-CoA production, especially palmitoyl-CoA, in livers of fructose-, as compared to glucose-supplemented mice. A HFD itself did not robustly increase DNL enzymes and, interestingly, the effects of fructose were more robust on chow, than when paired with a HFD [10]. Human studies have also shown that carbohydrates support DNL more robustly than a HFD [26] and that fructose stimulates DNL more strongly than glucose [8, 27, 28] or starch [19].
On a molecular level, the effects of fructose to increase DNL are largely mediated via the two major lipogenic transcription factors, sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP). Insulin strongly increases DNL [29] and stimulates SREBP1c expression, post-translational processing and nuclear localization [30]. While fructose alone does not induce robust insulin secretion [31] and insulin is not required for acute fructose metabolism, fructose promotes insulin resistance and thus can chronically increase serum insulin levels. The effects of fructose on SREBP1c are not entirely dependent on insulin, as fructose can upregulate SREBP1c in the livers of insulin receptor knockout mice, which by design have no hepatic insulin signaling [32]. The effects of fructose on SREBP1c are also, at least in part, mediated via peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1b), as antisense oligonucleotide targeting PGC1b, reduces expression of SREBP-1c and its downstream lipogenic genes in livers of fructose-fed mice [33]. Further, fructose-induced expression of SREBP1c triggers a fast forward cycle, as its downstream target, SCD1, further increases SREBP1c [34].
Even though ChREBP transcriptional activity is mediated by glucose, fructose can also activate ChREBP [35] by inducing a second promoter in the ChREBP gene and an alternative splicing event to yield the ChREBP-β isoform [36]. Interestingly, ChREBP also promotes fructolysis by stimulating transcription of hepatic fructose transporter solute carrier family 2, member 5 (Glut5) [37] and the rate-limiting enzyme of fructose metabolism, ketohexokinase (KHK), also known as fructokinase [35, 37]. Similar to SREBP1c, ChREBP can increase expression of enzymes regulating DNL, including ACLY, ACC1, FASN and SCD1. Thus, ChREBP knockout decreases DNL and accumulation of fat in the liver [38, 39]. ChREBP also appears to protect liver from fructose-induced injury by attenuating ER stress and cholesterol synthesis [40]. The essential role of ChREBP in protection from fructose is best inferred from studies using ChREBP deficient mice, which stop eating, lose weight, and eventually die when fed a high-fructose diet [39]. Consistent with its lipogenic but protective roles, ChREBP overexpression results in hepatic steatosis, but hepatic steatosis in this context is dissociated from development of insulin resistance and metabolic complications [41]. The beneficial effects of ChREBP in fructose metabolism are consistent with its protective role in ethanol-induced liver injury [42].
In addition to stimulating lipogenic transcription factors, tracer studies show that fructose carbons can be immediately utilized as substrate in lipogenesis, whereas carbons labeled in glucose molecule are not observed to enter lipids, at least during a short four-hour observation period [31]. The difference in carbon appearance can be explained by increased flux through the fructolysis pathway. Glyceraldehyde-3 phosphate (GA3P) and dihydroxyacetone phosphate (DHAP) are common intermediates of both glycolysis and fructolysis pathways, downstream of which glucose and fructose metabolism is indistinguishable. However, prior to formation of these intermediates fructose is metabolized by KHK and aldolase, both of which are not regulated by insulin or end products of fructolysis. On the other hand, phosphofructokinase, an upstream enzyme in glycolysis pathway, is inhibited by both ATP and citrate [43], which are the products of this pathway. Thus, while both fructose and glucose carbons converge onto a common pathway, fructolysis is not subjected to feedback inhibition and allows for unrestrained flux through the pathway.
Whereas fructose strongly induces hepatic lipogenesis, it is hepatic lipid composition that correlates with progression of liver disease and development of metabolic complications, rather than the total amount of liver lipids [44]. Fructose has been associated with increased hepatic synthesis of free fatty acids (FFAs), diacylglycerol, ceramides and acyl-carnitines, which are known mediators of insulin resistance. In healthy subjects, fructose stimulates hepatic fatty acid synthesis at least twice as much as glucose [28], and many studies document that increased hepatic FFA synthesis strongly contributes to development of hepatic insulin resistance [45, 46, 47, 48, 49]. On the other hand, increased oxidation of FFA via upregulation of PPARα is sufficient to reverse hepatic steatosis and insulin resistance in mice fed high-fructose diet [50]. FFAs also serve as building blocks for synthesis of more complex lipid species, which in turn have been associated with development of fructose-induced insulin resistance. Diacylglycerols (DAG), formed when two fatty acids are covalently bound to a glycerol backbone, are increased with fructose consumption [33, 51, 52, 53]. On a molecular level, DAG activates PKC epsilon, a potential mediator of hepatic insulin resistance [54]. Fructose intake can increase condensation of endogenously produced fatty acids, such as palmitoyl-CoA, with an amino acid serine to commence de novo synthesis of ceramides. Ceramides are known to decrease Akt phosphorylation and induce hepatic insulin resistance [55, 56]. Acylcarnitines are produced from fatty acyl-CoAs by the action of carnitine palmitoyltransferase 1 (CTP1α) and are destined for mitochondrial oxidation. We observed increased acylcarnitine profile in HFD-fed and fructose-supplemented mice [57]. Increased acylcarnitine profile is strongly associated with insulin resistance, but this is thought to be a sign of decreased FAO, rather than a direct pathologic effect of acylcarnitines. However, in at least one study, infusion of acylcarnitines was shown to be sufficient to induce insulin resistance in muscle [58]. Taken together, the above studies strongly support the concept that increased DNL is one pathway by which fructose intake leads to hepatic insulin resistance.
Fructose Decreases FAO by Inducing Mitochondrial Dysfunction
In addition to promoting DNL, fructose decreases FAO in human [17, 19, 59], animal [60, 61, 62] and in vitro studies [57], and this can contribute to the development of hepatic steatosis and insulin resistance. Traditionally, decreased FAO has been thought to be secondary to increased DNL. This is because malonyl-CoA, an intermediate in lipogenesis, inhibits CPT1α, the rate limiting enzyme of FAO. Furthermore, chronic fructose intake leads to hyperinsulinemia, which also decreases FAO. Similar to the potent effects of insulin, fructose decreased FAO in perfused rat liver and their effects were additive [63], suggesting that fructose impairs FAO, independent of hyperinsulinemia that commonly occurs with chronic fructose intake. Furthermore, fructose inhibits FAO even in isolated liver mitochondria, an effect that is independent of DNL, since lipogenesis does not occur in mitochondria [64]. The decrease in mitochondrial beta-oxidation is, at least in part, mediated by fructose-induced impairment of peroxisome proliferator activated receptor alpha (PPARα) signaling, leading to decreased expression of its target genes, such as CPT1α, long chain acyl-CoA dehydrogenase (ACADL) and very long chain acyl-CoA dehydrogenase (ACADVL) [65]. This may be mediated by fructose-induced hypermethylation of PPARα and CTP1α promoter regions, resulting in decreased mRNA levels of these genes [66]. These effects are also, in part, mediated via fructose-induced upregulation of ChREBP as previously discussed, since ChREBP is known to negatively regulate PPARα expression in the liver and other tissues [67, 68]. Our recent work in mice supports a direct role of fructose to decrease expression of FAO genes, as knockdown of KHK leads to increased CPT1α mRNA and protein levels [57].
Fructose metabolism depletes hepatic ATP levels. This is thought to be secondary to the rapid conversion of fructose to fructose-1 phosphate, leading to rapid generation of AMP and its conversion to uric acid [69]. Indeed, acute fructose metabolism is 10 times faster than that of glucose [70] and leads to decreased ATP levels, while concomitantly increasing inorganic phosphate and ADP [71]. However, depletion of hepatic ATP could also be in part due to a decrease in mitochondrial ATP synthesis, as the degree and time course of fructose-induced ATP depletion in isolated hepatocytes is similar to the effects of oligomycin, an inhibitor of mitochondrial ATP synthesis [72]. Human subjects with high habitual fructose consumption also have chronically lower hepatic ATP levels, and these subjects exhibit further decrease in ATP during times of acute fructose metabolism [73], providing additional evidence in favor of impaired mitochondrial ATP synthesis. Indeed, we reported that fructose pretreated hepatocytes generate less ATP, as compared to glucose pretreated ones, and this is associated with decreased mitochondrial beta-oxidation [57].
As discussed above, when fructose is metabolized by ketohexokinase, there is a transient decrease in intracellular ATP that activates the nucleotide degradation pathway and subsequent uric acid formation [74] [75]. A central role of uric acid in mediating fructose effects is inferred from studies demonstrating that it further stimulates KHK expression in a fast-forward loop, which accelerates fructose metabolism [35]. Furthermore, uric acid can also play a part in increasing lipogenesis and decreasing beta oxidation. Uric acid blocks enoyl-CoA hydratase and decreases AMPK activation, thus contributing to fructose-induced decrease in FAO [76]. It also can stimulate lipogenesis by inducing NADPH oxidase, which associates with the mitochondria, resulting in oxidative stress that reduces aconitase-2, leading to generation of citrate, a lipogenic precursor [60, 75]. In addition to being produced by fructose metabolism, uric acid may stimulate endogenous fructose production by activating aldose reductase in the polyol pathway, which drives development of non-alcoholic fatty liver disease [77].
Fructose-induced increase in uric acid production is one of the mechanisms by which fructose leads to increased reactive oxygen species (ROS) levels [78, 79]. While fructose induces ROS generation, fructose-fed mice are more sensitive to ROS, as fructose feeding decreases expression of several ROS defense genes. Furthermore, mice with diminished antioxidant capacity, due to hepatocyte specific deficiency in eIF2a phosphorylation, have more severe hepatocyte cell death and increased liver fibrosis when exposed to dietary fructose [80]. On the other hand, a decrease in ROS, achieved by resveratrol treatment, is sufficient to reverse fructose-induced insulin resistance [81], further implicating ROS in the pathogenesis of fructose-induced metabolic syndrome. A fructose-induced increase in ROS is not only a sign of mitochondrial dysfunction, but it can also result in increased mitochondrial DNA (mtDNA) damage, thus itself causing mitochondrial dysfunction. Indeed, in one study, fructose supplementation decreased hepatic mtDNA copy number and increased mtDNA damage, as evident by increased plasma 8-hydroxy-2’-deoxyguanosine levels [82].
In our studies of sugar metabolism, fructose, but not glucose, supplementation of mice on a HFD induced mitochondrial dysfunction, characterized by decreased FAO, decreased hepatic ATP levels and increased hepatic ROS [57]. Interestingly, we also found that fructose affects mitochondrial fusion/fission, so that mice supplemented with fructose on a HFD had the highest mitochondrial number, but the lowest mitochondrial size, as compared to the mice supplemented with regular or glucose-sweetened water on a HFD. This was accompanied by increased levels of mitochondrial fission protein FIS1 and decreased levels of fusion protein OPA1 in mice fed HFD plus fructose. We also show that the effects on mitochondrial morphology were dependent on fructose metabolism, as knockdown of KHK normalized mitochondrial number and decreased the proportion of smallest mitochondria. Our mitochondrial proteome analysis revealed that a HFD profoundly decreased levels of many mitochondrial proteins, and addition of fructose further decreased global mitochondrial proteome, but addition of glucose normalized some parts of proteome towards the levels found in chow-fed mice. Thus, fructose metabolism has profound effects, not only on mitochondrial ATP and FAO, but also on mitochondrial number, structure and protein abundance.
Fructose and ER stress
Increased metabolic demands on the endoplasmic reticulum, a major site of protein folding and lipid synthesis, leads to ER stress and unfolded protein response (UPR), which is strongly associated with development of insulin resistance. Fructose increases all three pathways of UPR, including activating transcription factor 6 (ATF6), inositol-requiring enzyme alpha (IREa) and PKR-like kinase (PERK) [50, 83, 84]. However, the IREa branch of ER stress response, which leads to activation of XBP1s, appears to be increased first, only one day after fructose feeding [85]. A seminal study by Lee et al. [86] established the importance of XBP1s in mediating hepatic lipogenesis induced by a 60% fructose diet. Subsequent studies showed that XBP1s is required for the ER stress-mediated development of insulin resistance induced by fructose, as fructose-fed mice with conditional knockout of XPB1s had improved insulin sensitivity and decreased lipogenesis, characterized by reduced hepatic DAG content and reduced PKCɛ activity [52]. Reduction of ER stress via treatment with ER chaperones was also sufficient to prevent hepatic lipid accumulation via inhibition of fructose-induced increase in SREBP-1c and its downstream targets [87, 88].
The function of the ER is intimately connected with that of the mitochondria, as these organelles physically interact with 20% of the mitochondrial surface being in direct contact with the ER [89]. ER stress can thus directly induce mitochondrial dysfunction through excess calcium flux, leading to increased mitochondrial fragmentation and decreased membrane potential [90]. Unresolved ER stress resulting in mitochondrial dysfunction, increases cell death via cytochrome c release and autophagy. Intriguingly, in spite of inducing ER stress, fructose decreases hepatic autophagy, as a result of activation of a mammalian target of rapamycin [91]. In agreement with this, we showed that knockdown of KHK in mice on a HFD and a HFD supplemented with fructose restores autophagy/mitophagy to normal levels found in HFD-fed and glucose-supplemented mice [57]. Regulation of autophagy may be a critical step in ER stress-mediated insulin resistance, since restoration of autophagy decreases ER stress and leads to improved insulin signaling in fructose-fed mice [91]. In summary, ER stress is a pivotal part of fructose-associated metabolic effects, as it directly increases lipogenesis through XBP1s, leads to decreased FAO via accelerating mitochondrial dysfunction and induces liver inflammation.
Liver Inflammation and Fructose
Dietary intake of high-fructose corn syrup sweetened drinks has been reported to result in increased liver inflammation [92]. Dysregulated immune response is an integral part in the pathogenesis of hepatic insulin resistance, which is thought to be, in large part, mediated by increased serine phosphorylation of insulin receptor and insulin receptor substrates (IRS) [93]. However, it remains to be defined whether fructose effects are dependent on inflammation triggered by liver stromal cell or direct effects on immune cells in the liver.
Initial evidence indicates that fructose strongly upregulates an inflammatory cascade via c-jun NH(2)-terminal kinase (JNK) activity in liver parenchyma, even after a single sucrose-containing meal. In this study, intraperitoneal fructose injection after only 6 hours also increased hepatic JNK activity and induced hepatic insulin resistance [94]. These effects can occur specifically in hepatocytes, since fructose supplementation in isolated primary rat hepatocytes is sufficient to activate JNK activity and decrease insulin signaling [95]. Normalization of the inflammatory response, on the other hand, is sufficient to reverse fructose-induced JNK activation, lipid dysregulation and hyperinsulinemia [96]. Mechanistically, fructose-induced effects on hepatic inflammation are, at least in part, mediated via induction of ER stress, as it can directly lead to JNK activation via IREa branch of ER stress response. It is not clear, however, whether fructose-induced JNK activation and subsequent insulin resistance are entirely independent of hepatic lipid metabolism [52]. Adequate disposal of lipids in adipose tissue, achieved by pioglitazone administration, can decrease hepatic inflammatory response and liver insulin resistance induced by fructose [97]. Conversely, inability of adipose tissue to adequately store lipids, such as in lipodystrophy, is highly associated with development of hepatic inflammation and insulin resistance [98]. Aberrant lipid oxidation may be a source of ROS, which has been implicated in the initiation of fructose-induced hepatic inflammation. In support of this claim, antioxidant administration has been shown to reverse increased inflammation in cultured hepatocytes treated with 5 mM of fructose [99]. Thus, fructose can induce liver inflammation by activating JNK specifically in hepatocytes, leading to hepatic insulin resistance.
Recent reports suggest that fructose can also induce liver inflammation by acting directly on inflammatory cells. For example, fructose induces dendritic cell inflammatory capacity, with specific increases in production of interleukin 6 (IL-6) and IL-1β [100]. These proinflammatory cytokines are known to modulate T helper cell polarization and promote skewing of naive CD4+ T cells into Th17 subset [101, 102]. In fact, Th17 cells play a central role in the pathogenesis of diet-induced liver inflammation as mice lacking either IL-17A or IL-17RA, needed for Th17 signaling, are protected from either a high-fat or high-fat, high-sugar diet-driven progression of fatty liver disease [103]. In addition, the link between ROS and Th17 cell polarization has been demonstrated in context of liver inflammation [104]. Further, peroxisomal beta-oxidation, which impacts ER stress and mitochondrial function, also regulates inflammation and Th17 cell polarization in NAFLD [105]. Detailed contribution of the IL-17 axis in immunopathogenesis of fatty liver disease has been reviewed elsewhere [106]. Lastly, a recently described model of fatty liver and cardiovascular disease, which more accurately reflects human pathology, shows increased uric acid and ROS production, and is linked with increased IL-6, IL-1b and IL-17A production [107, 108, 109]. In summary, fructose can induce inflammation in hepatocytes through activation of JNK and/or aberrant lipid metabolism. Some of these effects may also occur in inflammatory cells, which further drives production of proinflammatory cytokines and liver inflammation that underpins hepatic insulin resistance.
Direct Effect of Fructose on Insulin Signaling
Above we have described unequivocal effects of fructose to induce hepatic insulin resistance via its effects on several pathways such us hepatic lipogenesis, mitochondrial dysfunction, ER stress and liver inflammation (Figure 2). But fructose, its metabolites or the enzymes involved in fructose metabolism may also directly contribute to insulin resistance. In the next section we will explore this hypothesis by discussing the effects of fructose on the insulin signaling pathway, that are independent of its effects to promote obesity, and its role as a source of additional calories in obesogenic diets.
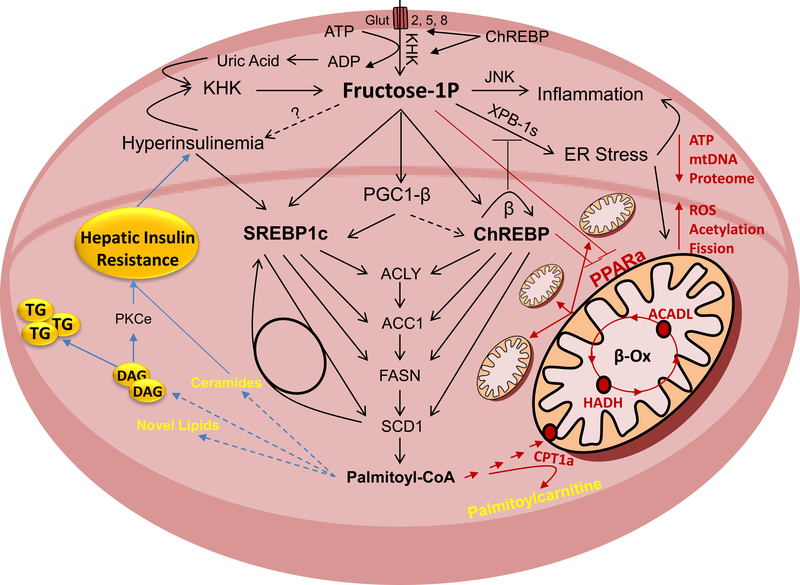
Figure 2.
Fructose Activated Pathways That Lead to Insulin Resistance
Fructose is metabolized in hepatocytes by ketohexokinase (KHK). This leads to decreased adenosine triphosphate (ATP) levels and increased uric acid production. Uric acid further stimulates KHK expression in a feed forward loop. Fructose strongly increases hepatic de novo lipogenesis (DNL). This is mediated through sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP) transcription factors that are at least in part regulated through peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1b). They upregulate enzymes involved in free fatty acid (FFA) synthesis, such as ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), fatty acid synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1). Accumulation of FFA in hepatocyte leads to insulin resistance, either directly or through buildup of more complex lipids and intermediates of lipid oxidation. In addition to DNL, fructose decreases mitochondrial fatty acid oxidation (FAO). This leads to a further decrease in ATP, decreased mitochondrial size and attenuated mitochondrial proteome, whereas mitochondrial fission, protein acetylation and reactive oxygen species (ROS) are increased. Decreased FAO is signaled through upregulation of ChREBP and a decrease in peroxisome proliferator activated receptor alpha (PPARα). Mitochondrial dysfunction and decreased FAO have been strongly linked with the development of hepatic insulin resistance. Fructose also strongly induces ER stress and hepatic inflammation, both of which can lead to hepatic insulin resistance.
Fructose Decreases Hepatic Insulin Receptor and IRS2
Some reports indicate that fructose feeding is associated with a decrease in proximal insulin signaling. This can be either a consequence of decreased protein levels of insulin signaling molecules, including a decrease in insulin receptor and insulin receptor substrates 1 and 2 (IRS1/IRS2), or impaired signal transduction of these molecules. Indeed, it has been shown that fructose-fed rats have lower insulin receptor levels in the liver and muscle [110]. This was noted after only two weeks of feeding diet containing 66% fructose, an intervention that was not long enough to result in changes in body weight, fasting plasma glucose, and plasma insulin levels. A decrease in protein levels of insulin receptor was associated with a decrease in insulin receptor mRNA, indicating that less protein was transcribed. This resulted in decreased binding of radiolabeled insulin, which can be explained by decreased receptor numbers in fructose-fed rats, as binding affinity of insulin to insulin receptor was not affected [110].
Similarly, two weeks of fructose-sweetened water (10% W/V) supplementation in female rats resulted in decreased hepatic IRS2, but not IRS1 protein levels [111], without having an effect on total body weight. A decrease in IRS2 is found in the setting of NAFLD [112], where insulin is unable to suppress hepatic glucose production, but is sufficient to stimulate hepatic lipogenesis via IRS1, a term called selective insulin resistance [113]. A decrease in IRS2 was associated with decreased insulin sensitivity and impaired glucose tolerance in these rats, but hepatic insulin receptor levels were not reported in this study [111]. Subsequent studies showed that decreased hepatic IRS2 protein and mRNA levels in fructose-supplemented rats (10% W/V) are likely mediated by increased activity of mammalian target of rapamycin 1 and inactivation of the hepatic transcription factor forkhead box O1 (FoxO1) [114]. An extended dietary intervention over two months was also associated with decreased hepatic IRS2 protein levels and impaired glucose tolerance in fructose-, but not glucose-, supplemented rats. Again, this was observed without a change in total caloric intake and body weight gain between fructose- and glucose-supplemented groups, whereas both groups gained more weight than chow-fed group [115]. Together, these studies suggest that fructose supplementation leads to decreased protein and mRNA levels of insulin receptor and IRS2, which contributes to hepatic insulin resistance. Furthermore, these effects are evident before weight gain occurs from increased fructose intake and are also indepenent of total calories, as equivalent caloric intake from glucose does not lead to these changes.
Fructose Upregulates PTP1b
Protein tyrosine phosphatase non-receptor type 1 (PTP1b) negatively regulates insulin signal transduction by removing tyrosine phosphorylation on insulin receptor and insulin receptor substrates. Thus, a decrease in PTP1b improves insulin sensitivity in diabetic mice [116, 117] and several PTP1b inhibitors are being developed for treatment of insulin resistance [118]. Adeli’s [119] group reported increased PTP1b protein and enzymatic activity in isolated hepatocytes from fructose-fed hamsters. This was associated with reduced tyrosine phosphorylation of the insulin receptor and IRS1/IRS2, indicative of reduced proximal insulin signaling [119]. They also reported decreased phosphatidylinositol 3-kinase activity and decreased insulin-stimulated phosphorylation of Akt on Ser473 and Thr308. Similar to findings in hamster, fructose feeding in rats also decreases hepatic tyrosine-phosphorylation of insulin receptor and IRS1 to about 70% of control levels after insulin stimulation [120, 121]. Increased hepatic PTP1b levels and activity in fructose-fed rats could be reversed by curcumin, an intervention that also results in increased insulin receptor, IRS1/IRS2 and Akt phosphorylation [122]. The effects of fructose to increase PTP1b may not be completely independent of hepatic lipogenesis, as fructose-induced elevation of PTP1b leads to increased mRNA, as well as promoter activity of SREBP1 and its downstream target FASN. This is mediated via induction of protein phosphatase 2A (PP2A), as its inhibition results in normalization of SREBP1 levels [123]. The effects of fructose to decrease tyrosine phosphorylation of insulin signaling molecules via induction of PTP1b activity provide further evidence that fructose feeding can directly decrease early steps of insulin signal transduction.
Knockdown of Fructose Metabolism Increases Hepatic Insulin Sensitivity
The direct effect of fructose to induce hepatic insulin resistance can also be inferred from studies utilizing silencing of fructose metabolizing enzyme, KHK. We showed that siRNA targeting KHK improves glucose tolerance in HFD-fed mice supplemented with fructose in drinking water, but also in HFD-fed mice provided with regular water. This was associated with an increase in insulin stimulated Akt phosphorylation (Ser473) in livers of these mice, as well as improved NAFLD activity score and hepatic triglyceride content [10]. We could not consistently document increase in insulin receptor following KHK knockdown and had not yet assessed total IRS1/IRS2 levels. Ishimoto et al. [124] also published that global KHK knockout mice are protected from steatohepatitis and hyperinsulinemia induced by high-fat, high-sucrose diet, but assessment of hepatic insulin resistance was not reported in this manuscript. Others, have also published that KHK knockout mice are protected from diet-induced metabolic dysregulation, but again hepatic insulin sensitivity was not directly tested in these mice [125].
The above discussed studies pave the way for fructose to exert a direct role in inducing hepatic insulin resistance in addition to its indirect effects (Figure 3). This hypothesis needs to be adequately addressed in future studies. If confirmed, it would be reasonable to suggest that reducing excess fructose intake takes priority over reducing total caloric intake as a first step on the road to improve hepatic insulin sensitivity.
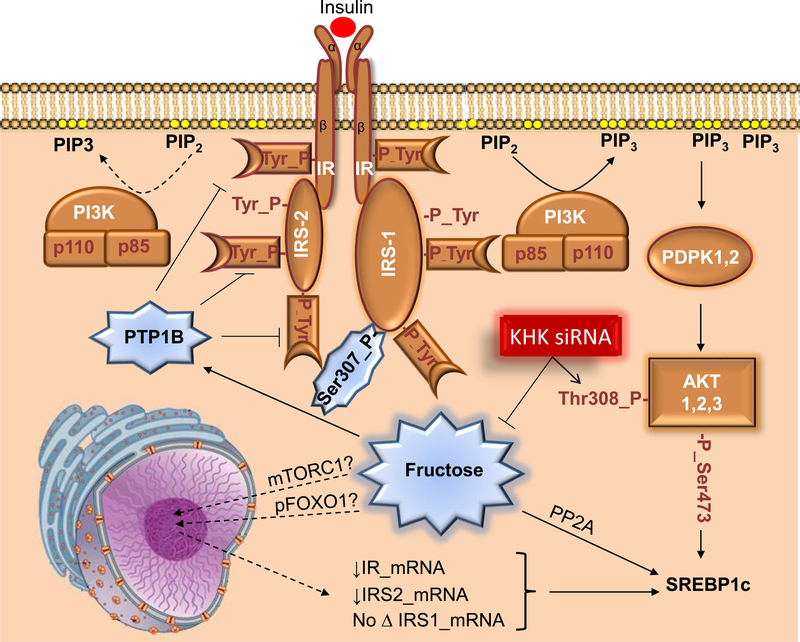
Figure 3.
Effects of Fructose on Insulin Signaling Pathway
Insulin binds to the insulin receptor (IR), which leads to autophosphorylation of IR and triggers the insulin signaling cascade. Insulin receptor substrates (IRS) dock to IR to further propagate insulin signaling by interacting with phosphoinositol 3 kinase. PI3K phosphorylates phosphoinositol diphosphate to phosphoinositol triphosphate (PIP3), which activates 3-phosphoinositide-dependent protein kinase 1 (PDPK1) that subsequently phosphorylates Akt, one of the critical nodes in the insulin-signaling network. Fructose intake has been associated with a decrease in IR and IRS2 expression, with no change in IRS1 levels, leading to decreased Akt phosphorylation. Additionally, fructose may increase protein-tyrosine phosphatase 1b (PTP1b) activity, which results in dephosphorylation of IR and IRS2, further leading to decreased Akt phosphorylation. This process may not be entirely independent of lipogenesis as fructose through PTP1b induces protein phosphatase 2A (PP2A), which increases SREBP1. Whereas dietary fructose decreases insulin signaling, knockdown of ketohexokinase (KHK) reduces fructose metabolism and increases phosphorylation of Akt.
Summary and Future Perspective
Dietary fructose intake is strongly associated with development of hepatic insulin resistance [124], and this effect appears to be independent of total caloric intake [126] or body weight gain [115]. Furthermore, while both glucose and fructose can induce features of metabolic syndrome, it is the internal conversion of glucose to fructose in the liver that is responsible for increased lipogenesis and insulin resistance [127]. Fructose effects are largely dependent on increased hepatic lipogenesis, manifesting as NAFLD. However, not all hepatic fat accumulation leads to insulin resistance, as deposition of triglycerides, observed with glucose supplementation, does not result in hepatic insulin resistance [10]. This is further highlighted in studies of mice with overexpression of Dgat2, an enzyme that mediates the final step in TG synthesis, where increased hepatic triglyceride accumulation does not result in hepatic insulin resistance [128]. In addition to lipogenesis, fructose intake decreases mitochondrial FAO, induces ER stress and potentiates liver inflammation, which are all pathways that play a marked role in the development of hepatic insulin resistance. Interestingly, dietary fructose may also have direct effects on the insulin signaling pathway, so that insulin resistance develops first in the liver, which is one of the primary sites of fructose metabolism.
This review is exclusively focused on the pathways in the liver by which fructose leads to insulin resistance. Future work is needed to investigate the effects of dietary fructose on insulin resistance via its other reported effects, such as to promote accumulation of visceral adipose tissue, alter gut microbiome, induce inflammatory response in adipose and muscle tissue, impact exercise physiology, increase production of uric acid in the kidney and central effects leading to hyperphagia and leptin resistance. Furthermore, improved laboratory techniques, such as RNA sequencing to identify microRNAs and long non-coding RNAs [129], proteomic analysis of post-translational modification of mitochondrial proteins [57] and new metabolomic platforms to identify fructose-specific lipids, may uncover new pathways that could mediate detrimental effects of fructose of insulin resistance.
In summary, dietary fructose intake strongly promotes hepatic insulin resistance via complex interplay of several metabolic pathways, at least some of which are independent of increased weight gain and total caloric intake. Further studies are needed to more completely understand the underlying mechanisms and to identify opportunities for intervention where modified fructose metabolism may be used for treatment of insulin resistance and fatty liver disease. The current evidence contradicts the notion that fructose is merely a source of palatable calories that leads to increased weight gain.
Acknowledgements:
The authors would like to thank George Fuchs, MD., for critically reading the manuscript and Tomas Dolan for help with illustrating figures. This work was supported in part by NIH grants R01 DK031036 and R01 DK033201 to C.R.K.; R01 DK099222 and American Diabetes Association (ADA) 1–18-IBS-100 to S.D. and P30 DK40561 and NASPGHAN Foundation Young Investigator Award to S.S.
References:
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013. November;10(11):686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006. October;118(4):1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015. March;148(3):547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Softic S, Kahn CR. Fatty liver disease: is it nonalcoholic fatty liver disease or obesity-associated fatty liver disease? Eur J Gastroenterol Hepatol. 2019. January;31(1):143. doi: 10.1097/MEG.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001. August;50(8):1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 6.Softic S, Cohen DE, Kahn CR. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci. 2016. May;61(5):1282–93. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018. May;68(5):1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009. May;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazzano LA, Li TY, Joshipura KJ, et al. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008. July;31(7):1311–7. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Softic S, Gupta MK, Wang GX, et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J Clin Invest. 2017. November 1;127(11):4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr. 1980. February;33(2):273–8. doi: 10.1093/ajcn/33.2.273. [DOI] [PubMed] [Google Scholar]
- 12.OPaNSS Beck-Nielsen H. Effects of diet on the cellular insulin binding and the insulin sensitivity in young normals. Diabetolgia. 1978;15:289. [DOI] [PubMed] [Google Scholar]
- 13.Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005. July;54(7):1907–13. doi: 10.2337/diabetes.54.7.1907. [DOI] [PubMed] [Google Scholar]
- 14.Le KA, Ith M, Kreis R, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009. June;89(6):1760–5. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 15.Lecoultre V, Carrel G, Egli L, et al. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men [Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. Am J Clin Nutr. 2014. February;99(2):268–75. doi: 10.3945/ajcn.113.069526. [DOI] [PubMed] [Google Scholar]
- 16.Johnston RD, Stephenson MC, Crossland H, et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013. November;145(5):1016–1025 e2. doi: 10.1053/j.gastro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Taskinen MR, Soderlund S, Bogl LH, et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J Intern Med. 2017. August;282(2):187–201. doi: 10.1111/joim.12632. [DOI] [PubMed] [Google Scholar]
- 18.Aeberli I, Hochuli M, Gerber PA, et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care. 2013. January;36(1):150–6. doi: 10.2337/dc12-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz JM, Noworolski SM, Wen MJ, et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J Clin Endocrinol Metab. 2015. June;100(6):2434–42. doi: 10.1210/jc.2014-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallfrisch J, Ellwood KC, Michaelis OEt, et al. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr. 1983. September;113(9):1819–26. doi: 10.1093/jn/113.9.1819. [DOI] [PubMed] [Google Scholar]
- 21.Wu T, Giovannucci E, Pischon T, et al. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Am J Clin Nutr. 2004. October;80(4):1043–9. [DOI] [PubMed] [Google Scholar]
- 22.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sports Med. 2016. April;50(8):496–504. doi: 10.1136/bjsports-2016-h3576rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ter Horst KW, Schene MR, Holman R, et al. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: a systematic review and meta-analysis of diet-intervention trials. Am J Clin Nutr. 2016. December;104(6):1562–1576. doi: 10.3945/ajcn.116.137786. [DOI] [PubMed] [Google Scholar]
- 24.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011. February;22(2):60–5. doi: S1043–2760(10)00171–2 [pii] 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy AR, Pissios P, Otu H, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007. June;292(6):E1724–39. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr. 1996;16:523–57. doi: 10.1146/annurev.nu.16.070196.002515. [DOI] [PubMed] [Google Scholar]
- 27.Lecoultre V, Egli L, Carrel G, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring). 2013. April;21(4):782–5. doi: 10.1002/oby.20377. [DOI] [PubMed] [Google Scholar]
- 28.Parks EJ, Skokan LE, Timlin MT, et al. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr. 2008. June;138(6):1039–46. doi: 10.1093/jn/138.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Softic S, Kirby M, Berger NG, et al. Insulin concentration modulates hepatic lipid accumulation in mice in part via transcriptional regulation of fatty acid transport proteins. PLoS One. 2012;7(6):e38952. doi: 10.1371/journal.pone.0038952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002. May;109(9):1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007. June;85(6):1511–20. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 32.Haas JT, Miao J, Chanda D, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012. June 6;15(6):873–84. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai Y, Yonemitsu S, Erion DM, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009. March;9(3):252–64. doi: S1550–4131(09)00036–9 [pii] 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki M, Dobrzyn A, Man WC, et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. J Biol Chem. 2004. June 11;279(24):25164–71. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 35.Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One. 2012;7(10):e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MS, Krawczyk SA, Doridot L, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2016. November 01;126(11):4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006. September 29;281(39):28721–30. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 38.Dentin R, Benhamed F, Hainault I, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006. August;55(8):2159–70. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 39.Iizuka K, Bruick RK, Liang G, et al. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004. May 11;101(19):7281–6. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Tong X, VanDommelen K, et al. Lipogenic transcription factor ChREBP mediates fructose-induced metabolic adaptations to prevent hepatotoxicity. J Clin Invest. 2017. June 30;127(7):2855–2867. doi: 10.1172/JCI89934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benhamed F, Denechaud PD, Lemoine M, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012. June;122(6):2176–94. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marmier S, Dentin R, Daujat-Chavanieu M, et al. Novel role for carbohydrate responsive element binding protein in the control of ethanol metabolism and susceptibility to binge drinking. Hepatology. 2015. October;62(4):1086–100. doi: 10.1002/hep.27778. [DOI] [PubMed] [Google Scholar]
- 43.Tornheim K, Lowenstein JM. Control of phosphofructokinase from rat skeletal muscle. Effects of fructose diphosphate, AMP, ATP, and citrate. J Biol Chem. 1976. December 10;251(23):7322–8. [PubMed] [Google Scholar]
- 44.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007. October;46(4):1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 45.Kovacs P, Stumvoll M. Fatty acids and insulin resistance in muscle and liver. Best Pract Res Clin Endocrinol Metab. 2005. December;19(4):625–35. doi: 10.1016/j.beem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005. December;54(12):3458–65. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 47.Lewis GF, Carpentier A, Adeli K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002. April;23(2):201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 48.Perry RJ, Camporez JG, Kursawe R, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015. February 12;160(4):745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevilacqua S, Bonadonna R, Buzzigoli G, et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987. May;36(5):502–6. [DOI] [PubMed] [Google Scholar]
- 50.Chan SM, Sun RQ, Zeng XY, et al. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress [Research Support, Non-U.S. Gov’t]. Diabetes. 2013. June;62(6):2095–105. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012. March;23(3):203–8. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Jurczak MJ, Lee AH, Jornayvaz FR, et al. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012. January 20;287(4):2558–67. doi: 10.1074/jbc.M111.316760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kopf T, Schaefer HL, Troetzmueller M, et al. Influence of fenofibrate treatment on triacylglycerides, diacylglycerides and fatty acids in fructose fed rats. PLoS One. 2014;9(9):e106849. doi: 10.1371/journal.pone.0106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010. June 26;375(9733):2267–77. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vila L, Roglans N, Alegret M, et al. Suppressor of cytokine signaling-3 (SOCS-3) and a deficit of serine/threonine (Ser/Thr) phosphoproteins involved in leptin transduction mediate the effect of fructose on rat liver lipid metabolism [Research Support, Non-U.S. Gov’t]. Hepatology. 2008. November;48(5):1506–16. doi: 10.1002/hep.22523. [DOI] [PubMed] [Google Scholar]
- 56.Crescenzo R, Mazzoli A, Di Luccia B, et al. Dietary fructose causes defective insulin signalling and ceramide accumulation in the liver that can be reversed by gut microbiota modulation. Food Nutr Res. 2017;61(1):1331657. doi: 10.1080/16546628.2017.1331657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Softic S, Meyer JG, Wang GX, et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. 2019. October 1;30(4):735–753 e4. doi: 10.1016/j.cmet.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Zhang Y, Chen N, et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007. June;117(6):1679–89. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox CL, Stanhope KL, Schwarz JM, et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011. December;96(12):E2034–8. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanaspa MA, Cicerchi C, Garcia G, et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7(11):e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rawat AK, Menahan LA. Antiketogenic action of fructose, glyceraldehyde, and sorbitol in the rat in vivo. Diabetes. 1975. October;24(10):926–32. doi: 10.2337/diab.24.10.926. [DOI] [PubMed] [Google Scholar]
- 62.Ter Horst KW, Serlie MJ. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients. 2017. September 6;9(9). doi: 10.3390/nu9090981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Topping DL, Mayes PA. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972. January;126(2):295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prager GN, Ontko JA. Direct effects of fructose metabolism on fatty acid oxidation in a recombined rat liver mitochondria-hish speed supernatant system. Biochim Biophys Acta. 1976. March 26;424(3):386–95. [DOI] [PubMed] [Google Scholar]
- 65.Su Q, Baker C, Christian P, et al. Hepatic mitochondrial and ER stress induced by defective PPARalpha signaling in the pathogenesis of hepatic steatosis [Research Support, Non-U.S. Gov’t]. Am J Physiol Endocrinol Metab. 2014. June 1;306(11):E1264–73. doi: 10.1152/ajpendo.00438.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohashi K, Munetsuna E, Yamada H, et al. High fructose consumption induces DNA methylation at PPARalpha and CPT1A promoter regions in the rat liver. Biochem Biophys Res Commun. 2015. December 4–11;468(1–2):185–9. doi: 10.1016/j.bbrc.2015.10.134. [DOI] [PubMed] [Google Scholar]
- 67.Iizuka K, Wu W, Horikawa Y, et al. Feedback looping between ChREBP and PPARalpha in the regulation of lipid metabolism in brown adipose tissues [Research Support, Non-U.S. Gov’t]. Endocr J. 2013;60(10):1145–53. [DOI] [PubMed] [Google Scholar]
- 68.Boergesen M, Poulsen L, Schmidt SF, et al. ChREBP mediates glucose repression of peroxisome proliferator-activated receptor alpha expression in pancreatic beta-cells [Research Support, Non-U.S. Gov’t]. J Biol Chem. 2011. April 15;286(15):13214–25. doi: 10.1074/jbc.M110.215467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006. March;290(3):F625–31. [DOI] [PubMed] [Google Scholar]
- 70.Asipu A, Hayward BE, O’Reilly J, et al. Properties of normal and mutant recombinant human ketohexokinases and implications for the pathogenesis of essential fructosuria. Diabetes. 2003. September;52(9):2426–32. [DOI] [PubMed] [Google Scholar]
- 71.Boesiger P, Buchli R, Meier D, et al. Changes of liver metabolite concentrations in adults with disorders of fructose metabolism after intravenous fructose by 31P magnetic resonance spectroscopy. Pediatr Res. 1994. October;36(4):436–40. [DOI] [PubMed] [Google Scholar]
- 72.Cannon JR, Harvison PJ, Rush GF. The effects of fructose on adenosine triphosphate depletion following mitochondrial dysfunction and lethal cell injury in isolated rat hepatocytes. Toxicology and applied pharmacology. 1991. May;108(3):407–16. [DOI] [PubMed] [Google Scholar]
- 73.Abdelmalek MF, Lazo M, Horska A, et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes [Research Support, N.I.H., Extramural]. Hepatology. 2012. September;56(3):952–60. doi: 10.1002/hep.25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maenpaa PH, Raivio KO, Kekomaki MP. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968. September 20;161(847):1253–4. [DOI] [PubMed] [Google Scholar]
- 75.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012. November 23;287(48):40732–44. doi: M112.399899 [pii] 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cicerchi C, Li N, Kratzer J, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014. August;28(8):3339–50. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez-Lozada LG, Andres-Hernando A, Garcia-Arroyo FE, et al. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J Biol Chem. 2019. March 15;294(11):4272–4281. doi: 10.1074/jbc.RA118.006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ives A, Nomura J, Martinon F, et al. Xanthine oxidoreductase regulates macrophage IL1beta secretion upon NLRP3 inflammasome activation. Nat Commun. 2015. March 24;6:6555. doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan X, Xu C, Lin Y, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016. April;64(4):925–32. doi: 10.1016/j.jhep.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Choi WG, Han J, Kim JH, et al. eIF2alpha phosphorylation is required to prevent hepatocyte death and liver fibrosis in mice challenged with a high fructose diet. Nutrition & metabolism. 2017;14:48. doi: 10.1186/s12986-017-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bagul PK, Middela H, Matapally S, et al. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats [Research Support, Non-U.S. Gov’t]. Pharmacol Res. 2012. September;66(3):260–8. doi: 10.1016/j.phrs.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Cioffi F, Senese R, Lasala P, et al. Fructose-Rich Diet Affects Mitochondrial DNA Damage and Repair in Rats. Nutrients. 2017. March 24;9(4). doi: 10.3390/nu9040323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren LP, Chan SM, Zeng XY, et al. Differing endoplasmic reticulum stress response to excess lipogenesis versus lipid oversupply in relation to hepatic steatosis and insulin resistance [Research Support, Non-U.S. Gov’t]. PLoS ONE. 2012;7(2):e30816. doi: 10.1371/journal.pone.0030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhuvaneswari S, Yogalakshmi B, Sreeja S, et al. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-kappaB-mediated inflammation in high fructose and high fat diet-fed mice [Research Support, Non-U.S. Gov’t]. Cell stress & chaperones. 2014. March;19(2):183–91. doi: 10.1007/s12192-013-0443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun RQ, Wang H, Zeng XY, et al. IRE1 impairs insulin signaling transduction of fructose-fed mice via JNK independent of excess lipid [Research Support, Non-U.S. Gov’t]. Biochim Biophys Acta. 2015. January;1852(1):156–65. doi: 10.1016/j.bbadis.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 86.Lee AH, Scapa EF, Cohen DE, et al. Regulation of hepatic lipogenesis by the transcription factor XBP1 [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Science. 2008. June 13;320(5882):1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang C, Chen X, Zhu RM, et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice [Research Support, Non-U.S. Gov’t]. Toxicol Lett. 2012. August 3;212(3):229–40. doi: 10.1016/j.toxlet.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Ge CX, Yu R, Xu MX, et al. Betaine prevented fructose-induced NAFLD by regulating LXRalpha/PPARalpha pathway and alleviating ER stress in rats [Research Support, Non-U.S. Gov’t]. Eur J Pharmacol. 2016. January 5;770:154–64. doi: 10.1016/j.ejphar.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 89.Kornmann B, Currie E, Collins SR, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Science. 2009. July 24;325(5939):477–81. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Breckenridge DG, Stojanovic M, Marcellus RC, et al. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol [Research Support, Non-U.S. Gov’t]. J Cell Biol. 2003. March 31;160(7):1115–27. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H, Sun RQ, Zeng XY, et al. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice [Research Support, Non-U.S. Gov’t]. Endocrinology. 2015. January;156(1):169–81. doi: 10.1210/en.2014-1454. [DOI] [PubMed] [Google Scholar]
- 92.Kohli R, Kirby M, Xanthakos SA, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010. September;52(3):934–44. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014. January 1;6(1). doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei Y, Pagliassotti MJ. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: implications for hepatic insulin resistance [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Am J Physiol Endocrinol Metab. 2004. November;287(5):E926–33. doi: 10.1152/ajpendo.00185.2004. [DOI] [PubMed] [Google Scholar]
- 95.Wei Y, Wang D, Pagliassotti MJ. Fructose selectively modulates c-jun N-terminal kinase activity and insulin signaling in rat primary hepatocytes. J Nutr. 2005. July;135(7):1642–6. doi: 10.1093/jn/135.7.1642. [DOI] [PubMed] [Google Scholar]
- 96.Kelley GL, Allan G, Azhar S. High dietary fructose induces a hepatic stress response resulting in cholesterol and lipid dysregulation. Endocrinology. 2004. February;145(2):548–55. [DOI] [PubMed] [Google Scholar]
- 97.Collino M, Aragno M, Castiglia S, et al. Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation [Research Support, Non-U.S. Gov’t]. Br J Pharmacol. 2010. August;160(8):1892–902. doi: 10.1111/j.1476-5381.2010.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Softic S, Boucher J, Solheim MH, et al. Lipodystrophy Due to Adipose Tissue-Specific Insulin Receptor Knockout Results in Progressive NAFLD. Diabetes. 2016. August;65(8):2187–200. doi: 10.2337/db16-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, Zhang JH, Chen XY, et al. Reactive oxygen species-induced TXNIP drives fructose-mediated hepatic inflammation and lipid accumulation through NLRP3 inflammasome activation. Antioxid Redox Signal. 2015. April 1;22(10):848–70. doi: 10.1089/ars.2014.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaiswal N, Agrawal S, Agrawal A. High fructose-induced metabolic changes enhance inflammation in human dendritic cells. Clin Exp Immunol. 2019. August;197(2):237–249. doi: 10.1111/cei.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010. October 21;467(7318):967–71. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011. June;12(6):568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harley IT, Stankiewicz TE, Giles DA, et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014. May;59(5):1830–9. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mukherjee R, Moreno-Fernandez ME, Giles DA, et al. Nicotinamide adenine dinucleotide phosphate (reduced) oxidase 2 modulates inflammatory vigor during nonalcoholic fatty liver disease progression in mice. Hepatol Commun. 2018. May;2(5):546–560. doi: 10.1002/hep4.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moreno-Fernandez ME, Giles DA, Stankiewicz TE, et al. Peroxisomal beta-oxidation regulates whole body metabolism, inflammatory vigor, and pathogenesis of nonalcoholic fatty liver disease. JCI Insight. 2018. March 22;3(6). doi: 10.1172/jci.insight.93626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Giles DA, Moreno-Fernandez ME, Divanovic S. IL-17 Axis Driven Inflammation in Non-Alcoholic Fatty Liver Disease Progression. Curr Drug Targets. 2015;16(12):1315–23. doi: 10.2174/1389450116666150531153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giles DA, Moreno-Fernandez ME, Stankiewicz TE, et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat Med. 2017. July;23(7):829–838. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giles DA, Ramkhelawon B, Donelan EM, et al. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab. 2016. November;5(11):1121–1130. doi: 10.1016/j.molmet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian XY, Ganeshan K, Hong C, et al. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016. January 12;23(1):165–78. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Catena C, Giacchetti G, Novello M, et al. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am J Hypertens. 2003. November;16(11 Pt 1):973–8. doi: 10.1016/s0895-7061(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 111.Vila L, Roglans N, Perna V, et al. Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose. J Nutr Biochem. 2011. August;22(8):741–51. doi: 10.1016/j.jnutbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 112.Honma M, Sawada S, Ueno Y, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes (Lond). 2018. September;42(9):1544–1555. doi: 10.1038/s41366-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shimomura I, Matsuda M, Hammer RE, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000. July;6(1):77–86. [PubMed] [Google Scholar]
- 114.Rebollo A, Roglans N, Baena M, et al. Liquid fructose down-regulates liver insulin receptor substrate 2 and gluconeogenic enzymes by modifying nutrient sensing factors in rats. J Nutr Biochem. 2014. February;25(2):250–8. doi: 10.1016/j.jnutbio.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 115.Baena M, Sanguesa G, Davalos A, et al. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci Rep. 2016. May 19;6:26149. doi: 10.1038/srep26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gum RJ, Gaede LL, Koterski SL, et al. Reduction of protein tyrosine phosphatase 1B increases insulin-dependent signaling in ob/ob mice. Diabetes. 2003. January;52(1):21–8. doi: 10.2337/diabetes.52.1.21. [DOI] [PubMed] [Google Scholar]
- 117.Zinker BA, Rondinone CM, Trevillyan JM, et al. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci U S A. 2002. August 20;99(17):11357–62. doi: 10.1073/pnas.142298199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tamrakar AK, Maurya CK, Rai AK. PTP1B inhibitors for type 2 diabetes treatment: a patent review (2011 – 2014). Expert Opin Ther Pat. 2014. October;24(10):1101–15. doi: 10.1517/13543776.2014.947268. [DOI] [PubMed] [Google Scholar]
- 119.Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, et al. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002. January 4;277(1):793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 120.Bezerra RM, Ueno M, Silva MS, et al. A high fructose diet affects the early steps of insulin action in muscle and liver of rats. J Nutr. 2000. June;130(6):1531–5. doi: 10.1093/jn/130.6.1531. [DOI] [PubMed] [Google Scholar]
- 121.Ueno M, Bezerra RM, Silva MS, et al. A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats. Braz J Med Biol Res. 2000. December;33(12):1421–7. doi: . [DOI] [PubMed] [Google Scholar]
- 122.Li JM, Li YC, Kong LD, et al. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology. 2010. May;51(5):1555–66. doi: 10.1002/hep.23524. [DOI] [PubMed] [Google Scholar]
- 123.Shimizu S, Ugi S, Maegawa H, et al. Protein-tyrosine phosphatase 1B as new activator for hepatic lipogenesis via sterol regulatory element-binding protein-1 gene expression. J Biol Chem. 2003. October 31;278(44):43095–101. doi: 10.1074/jbc.M306880200. [DOI] [PubMed] [Google Scholar]
- 124.Ishimoto T, Lanaspa MA, Rivard CJ, et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology. 2013. November;58(5):1632–43. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miller CO, Yang X, Lu K, et al. Ketohexokinase knockout mice, a model for essential fructosuria, exhibit altered fructose metabolism and are protected from diet-induced metabolic defects. Am J Physiol Endocrinol Metab. 2018. September 1;315(3):E386–E393. doi: 10.1152/ajpendo.00027.2018. [DOI] [PubMed] [Google Scholar]
- 126.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011. September;60(9):1259–70. doi: S0026–0495(11)00020–5 [pii] 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lanaspa MA, Ishimoto T, Li N, et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun. 2013;4:2434. doi: 10.1038/ncomms3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monetti M, Levin MC, Watt MJ, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007. July;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 129.Sud N, Zhang H, Pan K, et al. Aberrant expression of microRNA induced by high-fructose diet: implications in the pathogenesis of hyperlipidemia and hepatic insulin resistance. J Nutr Biochem. 2017. May;43:125–131. doi: 10.1016/j.jnutbio.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]