Abstract
We aimed to compare the barrier function of the skin site with the color of hematoma induced by venipuncture and the area surrounding the skin site to help improve skin care for hospitalized elderly patients. There were 50 patients with a median age of 84 years who were included in the analysis. There was no significant difference between the hematoma site-induced venipuncture and the area surrounding the hematoma site in terms of transepidermal water loss and skin sebum level. The status of stratum corneum hydration and skin elasticity on the hematoma sites was significantly lower than that on nonhematoma sites. The median skin pH was significantly higher on hematoma sites than that on nonhematoma sites. The study variables did not reveal any significant correlation with the intensity of skin erythema. These findings showed that hematoma formation in the subcutaneous tissue affected the skin barrier function and that these sites need moisturizing skin care regardless of the intensity of skin erythema.
Keywords: venipuncture, hematoma formation, skin barrier function, elderly patients
Introduction
The skin protects the body from environmental factors, including physical (e.g., mechanical trauma, thermal injury, and radiation); chemical (e.g., destructive agents, surface active substances, xenobiotics, and allergens); and biological (e.g., bacteria and viruses) (Elias & Choi, 2005). The “skin barrier,” primarily composed of the epidermis, is the natural boundary that exists between an organism and its environment. The skin barrier’s functionality and appearance are maintained through the interaction of several surrogate markers, including transepidermal water loss, stratum corneum hydration, sebum content, and pH (Luebberding, Krueger, & Kerscher, 2013). An effective skin barrier is critical for maintaining skin function, and it also prevents tissue breakdown and the development of chronic wounds (Cowdell & Steventon, 2015). Skin damage and the compromised skin barrier that results, are common in clinical settings, often attributed to physical and mechanical irritation or chemical injury (Kezic & Nielsen, 2009).
Venipuncture for drug administration or blood extraction is the most common invasive procedure performed by healthcare providers (World Health Organization, 2010). Venipuncture creates skin friction and drying during disinfection and damages the veins, subcutaneous tissue, and skin by puncture. Even when a small volume of blood is withdrawn, venipuncture can produce complications, such as infection, nerve damage, arterial puncture, and hematoma formation. Among these, hematoma formation is the most common complication. According to some studies, the incidence rate is approximately 25% to 75% (Ascoli, Deguzman, & Rowlands, 2012; Dyson & Bogod, 1987; Godwin, Cuthbert, & Choyce, 1992; Laudenbach, Klaverkamp, & Hedman-Dennis, 2014; Legemaat et al., 2016). Some patients experience unpleasant symptoms at the site of venipuncture-induced hematoma (Fujita, Maeda, Ono, & Jyokyo, 2003), including itchiness and rough skin. These symptoms can occur due to dehydrated skin at the venipuncture site when compared with the surrounding tissue.
Investigations of the skin barrier’s function are leading to skin care for conditions such as xerosis (Danby et al., 2016), skin maceration (Ichikawa-Shigeta et al., 2014), and dermatoses in obese subjects (Guida et al., 2010).
We hypothesized that venipuncture implementation and hematoma formation in the subcutaneous tissue after venipuncture play a critical role in the skin barrier function. Does the skin barrier function after venipuncture differ between sites with hematoma and those surrounding a hematoma? Should we change skin care at the hematoma site as the intensity of skin erythema at the hematoma site changes? There exists no scientific consensus regarding which parameters accurately evaluate hematoma-related changes in the human skin barrier function. This study was performed to address these clinical questions for elderly patients. In Japan, we are experiencing the growth of the elderly patients population, who exhibit partial changes in skin physiology.
Aims
The goal of this basic study was to explore appropriate skin care for itchy and rough skin on venipuncture-induced hematoma formation based on skin barrier function. This study investigated post-venipuncture skin properties, such as transepidermal water loss (TEWL), stratum corneum hydration, sebum level, skin elasticity, and skin pH, of hematoma sites as compared with the surrounding sites. The aim of this study was to identify differences in the skin barrier function between venipuncture-induced hematoma sites, and the relevance of skin properties in relation to the intensity of skin erythema.
Methods
Design and Participants
This was an observational, cross-sectional study approved by the medical ethics committe of Noto General Hospital. Data of patients who were treated from December 2015 to February 2016 at three surgical and two internal medicine wards were prospectively collected. The inclusion criteria for study participation consisted of inpatients aged ≥65 years, no mental and physical problems in understanding the study and obtaining verbal and written consent, absence of other skin lesions on the investigated site, and the presence of hematoma after venipuncture for blood collection and catheter placement.
Two clinical research nurses checked the implementation of venipuncture for blood collection or catheter placement using the data from medical charts. Patients were excluded if they were unstable or if emergency intravenous access was required.
One day prior to commencing the study, a clinical research associate contacted the eligible patients meeting the inclusion criteria for this study, explained the study details, and obtained verbal and written informed consent. All participants were informed not to take shower or bed bath and use cream on the investigated site at least eight hours before measurement. Verbal consent was granted prior to commencing the study procedures. A clinical research associate measured and recorded body temperature, pulse rate, and blood pressure before the investigation of skin properties. A clinical research associate reviewed the medical chart of each participant to obtain clinical data within 1 week before and after the study measurement, including age, gender, primary illness, body mass index, recent hemoglobin level, total protein, and platelet count.
Measures
Environmental measurements
A clinical research associate measured and recorded the ambient temperature and illumination intensity within each patient’s bedroom throughout the study. During the study, the illumination intensity in each patient’s bedroom was >750 lux as specified by the Japanese Industrial Standards Committee (Z-9110) for Clinical Examination and Injection as the standard hospital illumination. A digital meter (Model 51001; Yokogawa Meters & Instruments Corporation, Tokyo, Japan) was used to measure the illumination intensity.
Investigated sites
For each patient, two clinical nurses selected two sites around the antebrachial area, including the cubital fossa for observation and measurement. The palest area of the hematoma that clinical nurses were able to distinguish was designated as the “hematoma site,” and the area surrounding the hematomas site where the clinical nurses were unable to distinguish changes in skin color was subjectively designated as the “nonhematoma site.” We also investigated the skin color at the “hematoma site” and the “nonhematoma site” to ensure the reliability and objectivity of the colorimetric instrumentation. Using a tri-stimulus colorimetric instrument (NF 333; Nippon Denshoku Industries Co. Ltd., Tokyo, Japan), skin color was measured to distinguish between hematoma and nonhematoma sites and was quantified according to the most commonly used Commission International de l’Eclairage L*a*b* values (Weatherall & Coombs, 1992). The luminance (L*) value measured brightness ranging from total black (low value) to total white (high value). The skin color a* value expressed color from green (−) to red (+), whereas the b* value expressed color from blue (−) to yellow (+). The b* value and melanin index correlated almost linearly with the amount of epidermal melanin, whereas the a* value correlated almost linearly with the amount of hemoglobin (Takiwaki, 1998), where erythema of the skin is indicated by a* (Fullerton et al., 1996).
Transepidermal water loss
TEWL is a sensitive biophysical measure of the epidermal water barrier function (Darlenski, Sassning, Tsakov, & Fluhr, 2009; Fluhr, Feingold, & Elias, 2006; Hassing, Nater, & Bleumink, 1982; Pinnagoda, Tupker, Agner, & Serup, 1990). When the epidermis is injured, TEWL increases (Fluhr et al., 2006; Matoltsy, Schragger, & Matoltsy, 1962; Shahidullah, Raffle, Rimmer, & Frain-Bell, 1969; van der Valk, Nater, & Bleumink, 1985). In addition, chapped skin exhibits a higher rate of TEWL when compared with that exhibited by healthy skin (Smit et al., 1990). The TEWL measure has been validated in humans and rodents using in vivo and ex vivo models (Fluhr et al., 2006). We measured TEWL by evaporimetry (Tewameter® TM 300; Courage & Khazaka).
Stratum corneum hydration level
The water content of the stratum corneum affects barrier permeability and is measured as the total impedance applied to the skin or alternatively as electrical conductance or capacitance (Gabard, Clarys, & Barel, 2006; Verdier-Sévrain & Bonte, 2007). The results are displayed in arbitrary units (CM units), indicating very dry skin (<30 CM units), dry skin (30–40 CM units), or well-hydrated skin (Heinrich et al., 2003). The more water contained in the epidermis, the higher its electrical capacity (Barel & Clarys, 1997). The failure of the stratum corneum to retain water induces dryness and impairs the epidermal barrier function (Tupker, Pinnagoda, Coenraads, & Nater, 1990; Verdier-Sévrain & Bonte, 2007).
Skin sebum level
The protective hydrolipid film on the skin surface is a major component of the superficial layer. Sebum lipids contribute to nonspecific protective mechanisms of the skin barrier (Darlenski et al., 2009). We used sebumetry (Sebumeter® SM 815; Courage & Khazaka) to quantify sebum production. This photometry system measures the translucency of a special tape that becomes transparent after contacting sebum on the skin surface (Luebberding et al., 2013).
Skin elasticity
Maintaining an effective skin barrier is critical for maintaining skin function and preventing tissue breakdown and chronic wound development (Cowdell & Steventon, 2015). Aged skin exhibits higher violability in response to mechanical exposure and skin disease (Lemperle, Holmes, Cohen, & Lemperle, 2001; Li et al., 2006; Makrantonaki & Zouboulis, 2007). Suction chamber devices were commonly used to noninvasively determine the mechanical properties of the skin (Ahn, Kim, Lee, Moon, & Chang, 2007; Cua, Wilhelm, & Maibach, 1990; Ryu, Joo, Kim, Park, & Youn, 2008). To evaluate skin elasticity parameters, a Cutometer® MPA (Courage & Khazaka Electronic GmbH, Cologne, Germany) with a probe and a 2-mm aperture size was used. The mechanical properties of aging skin are evaluated using the parameter ratio of elastic recovery to distensibility (Ur/Uf; R7), as well as the gross elasticity (Ua/Uf; R2) (Krueger, Luebberding, Oltmer, Streker, & Kerscher, 2011).
Skin surface pH
The acidic milieu of the skin surface plays a central role in maintaining the homeostasis of epidermal permeability, restoring a disrupted skin barrier, and defending the skin using nonspecific antimicrobial methods (Fluhr et al., 2001; Hachem et al., 2003; Schmid-Wendtner & Korting, 2006). The importance of the skin’s “acid mantel” is demonstrated by a number of diseases (e.g., diaper dermatitis) (Schmid-Wendtner & Korting, 2006) and aging skin (Blaak, Wohlfart, & Schürer, 2011). We measured the pH of the skin surface with the Skin-pH-Meter® PH 905(Courage & Khazaka). The specially designed probe consists of a flat-topped glass electrode for full skin contact, which is then connected to a voltmeter. The system measures energy changes due to hydrogen cation activity surrounding the very thin layer of hydrated gel at the top of the probe (Luebberding, Krueger, & Kerscher, 2014).
Outcomes
The primary finding of this study was the difference noted in the skin barrier function between venipuncture-induced hematoma and nonhematoma sites. The secondary outcomes were the relationship between parameters that were significantly different between the two investigated sites and the intensity of skin erythema.
Statistical Analysis
Each variable was represented using the median (interquartile range, IQR). The Wilcoxon signed-rank test was used to compare between hematoma and nonhematoma sites. The statistical significance level was defined as a p value <.05. The variables found to be significant in the univariate analysis were compared with the skin color a* intensity ratio using a Spearman’s correlation coefficient test. The skin color a* intensity ratio was calculated using the following equation
where a is the skin color a* at the hematoma site and b is the skin color a* at the nonhematoma site. The statistical relationship was assessed on the basis of the correlation coefficient as follows: r > .7 was high, r = .4–.7 was moderate, and r < .4 was low. The statistical analysis was conducted using JMP® software, version 9.0 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 52 patients were eligible for study participation; however, two withdrew consent before the study. Table 1 shows the baseline characteristics of the study environment and the 50 participants. The median patient age was 84 years (IQR, 77–89 years), and the median BMI was 22.2 kg/m2 (IQR, 18.0–24.9 kg/m2). Comorbidities included 24.0% (12/50) musculoskeletal, 20.0% (10/50) pulmonary, and 20.0% (10/50) gastrointestinal (Table 1). The hematoma investigation was performed after 4–14 days of venipuncture.
Table 1.
Baseline Characteristics of the Clinical Environment and Participants.
| Environment | |
| Room temperature, ℃ | 23.8 (23.0−25.0) |
| Humidity, % | 29.0 (25.0−34.5) |
| Participants, n = 50 | |
| Age, years | 84 (77–89) |
| Body mass index, kg/m2 | 22.2 (18.0–24.9) |
| Body temperature, ℃ | 36.7 (36.4–36.9) |
| Pulse rate, beats per minute | 78 (71–87) |
| Maximum blood pressure, mmHg | 123 (108–136) |
| Minimum blood pressure, mmHg | 67 (59–76) |
| Total protein, g/dL | 6.0 (5.5–6.6) |
| Hemoglobin, g/dL | 10.3 (8.8–11.5) |
| Platelet, ×104/µL | 23.7 (18.6–31.3) |
| Sex, n (%) | |
| Men | 14 (28.0) |
| Underlying disease, n (%) | |
| Musculoskeletal | 12 (24.0) |
| Pulmonary | 10 (20.0) |
| Gastrointestinal | 10 (20.0) |
| Cancer | 9 (18.0) |
| Circulatory | 6 (12.0) |
| Cerebrovascular | 3 (6.0) |
Note. Data are presented as median (interquartile range) unless otherwise indicated.
Table 2 represents a comparison of the skin color L* and a* values between the hematoma and nonhematoma sites. When compared with the nonhematoma sites, the hematoma sites had significantly less stratum corneum hydration (39.9 [IQR: 34.0–45.4] vs. 43.3 [IQR: 39.1–50.1], p < .001); lower R7 (0.22 [IQR: 0.17–0.31] vs. 0.27 [IQR: 0.21–0.39], p < .001) and R2 (0.54 [IQR: 0.51–0.59] vs. 0.62 [IQR: 0.57–0.66], p < .001); and higher skin pH (5.52 [IQR: 5.24–5.98] vs. 5.28 [IQR: 4.98–5.65]; p < .001).
Table 2.
Comparison Between Hematoma and Nonhematoma Sites After Venipuncture.
| Variable | Hematoma sites (n = 50) | Nonhematoma sites (n = 50) | p valuea |
|---|---|---|---|
| Skin color L* | 51.72 (48.22–53.04) | 59.09 (57.43–61.24) | <.001 |
| Skin color a* | 4.28 (2.73–6.22) | 2.05 (1.06–3.15) | <.001 |
| Skin color b* | 11.45 (8.11–13.94) | 10.56 (8.60–12.34) | .891 |
| Transepidermal water loss | 8.38 (6.79–10.74) | 7.71 (5.98–9.77) | .075 |
| Stratum corneum hydration (AU) | 39.9 (34.0–45.4) | 43.3 (39.1–50.1) | <.001 |
| Sebum level | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| Cutometer R7 | 0.22 (0.20–0.24) | 0.25 (0.23–0.28) | .011 |
| Cutometer R2 | 0.54 (0.44–0.66) | 0.62 (0.50–0.75) | <.001 |
| Skin pH | 5.52 (5.24–5.98) | 5.28 (4.98–5.65) | <.001 |
Note. Values are presented as median (interquartile range).
aWilcoxon signed-rank test.
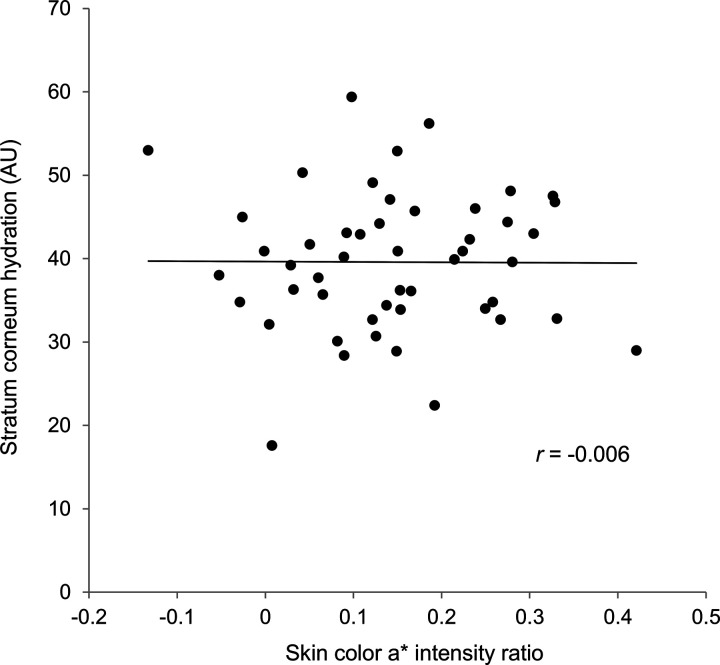
There were no significant differences between the two sites regarding skin color b*, TEWL, and sebum levels. Figure 1 shows the correlation coefficient between the skin color a* intensity ratio and mean skin hydration. The skin color a* intensity ratio had very low correlations (r < .4) with stratum corneum hydration and skin elasticity; hence, these were not statistically significant. Furthermore, the Cutometer R0 and R2 values had very low correlations (r = .008 and .019, respectively).
Figure 1.
A correlation between skin color a* intensity ratio and stratum corneum hydration at hematoma sites.
Discussion
To date, the skin barrier function of hematoma sites has not been investigated. Many patients do not share skin changes with their families, friends, and healthcare providers in Japan. Most cases of hematoma formation can be attributed to venipuncture induced by healthcare providers; therefore, appropriate skin care is needed in accordance with the barrier function at these sites. One of the most important functions of the skin is to protect against water loss and prevent the penetration of foreign molecules into the body (Roskos & Guy, 1989). This study yielded two important observations: (a) hematoma sites after venipuncture were more dehydrated, less elastic, and had a higher skin pH compared to nonhematoma sites; and (b) the intensity of skin erythema was not significantly correlated with skin hydration and elasticity. These observations suggest that the skin barrier function in hematoma sites after venipuncture was different from that in nonhematoma sites and that the application of moisturizing agents to hematoma sites is needed, regardless of the intensity of skin erythema. Healthcare providers may induce most cases of hematoma formation sites with the specific skin care in accordance with this study results (e.g., the use of emollients and other products). Currently, many patients have refused using skin cream at the hematoma formation sites due to an unarticulated anxiety regarding the appearance of both the hematoma and pinhole from the venipuncture.
This study showed that stratum corneum hydration and skin elasticity differed between the hematoma and nonhematoma sites. Similar studies have focused on dry skin caused by aging and the anatomical position (Darlenski et al., 2009). Heinrich et al. (2003) classified dry skin using stratum corneum hydration in arbitrary CM units as very dry (<30 CM units), dry (30–40 CM units), and well-hydrated (>40 CM units). The sites of hematoma formation in our patients corresponded to dry skin, whereas the nonhematoma sites indicated well-hydrated skin. The average values for a well-hydrated stratum corneum on the forearm of elderly people were reported as 43.30 (Luebberding et al., 2014), 48.09 (Luebberding et al., 2013), and 46.1, if with skin tears (Koyano et al., 2016), whereas that for dry skin was reported as approximately 35.0 (Danby et al., 2016). Our results were consistent with these values. One study demonstrated a clinically significant increase (+2.09 ± 0.95 units) in the level of skin hydration after the application of a test emollient for 28 days (Danby et al., 2016). Previous research has suggested that the application of cream on the skin with a hematoma significantly increased hydration by 5.0 to 6.5 AU (Blaak et al., 2011; Woods, Brown, Baig-Lewis, & Simpson, 2011). We expect that the use of a moisturizing cream on a hematoma skin site improves the hydration of the stratum corneum to a level similar to that of a nonhematoma site. Therefore, we considered the difference in stratum corneum hydration to be clinically significant between the two sites. In this study, TEWL in the hematoma sites tended to be greater when compared with that in the nonhematoma sites. When the epidermis is injured, TEWL increases (Fluhr et al., 2006), and chapped skin would exhibit a higher TEWL rate than that exhibited by healthy skin (Smit et al., 1990). Thus, we presume that the decreased level of stratum corneum hydration might have increased TEWL in the hematoma sites.
This study also demonstrated that the Cutometer R0 value of the hematoma formation sites was lower than that of the nonhematoma formation sites. The status of stratum corneum hydration and skin elasticity changes with aging (Krueger et al., 2011). These changes may be halted by the application of natural moisturizing factors, such as oil or water nanoemulsions containing ceramide 3B, and natural stratum corneum lipids (Yilmaz & Borchert, 2006). Our results implied a decrease in water retention at the hematoma formation sites after venipuncture. Although the pathophysiology of skin dehydration after venipuncture is unclear, we suppose that the application of isopropyl alcohol for skin disinfection and the use of tape (Konya et al., 2010) may have contributed to a decrease in the moisture retention function at these sites.
Although there was no significant correlation between the intensity of skin erythema and skin hydration and elasticity in this study population of elderly patients, we have experienced cases in which erythematous hematoma skin sites after venipuncture became more dehydrated over time. We consider the intensity of skin erythema to have affected the skin barrier function and to be a useful measure of skin care. Based on our results, if hematoma formation is caused by venipuncture, skin care with emollients and the maintenance of the humectant function should be routine practice regardless of the intensity of skin erythema. Skin care products intended to remain on the skin for softening and smoothing the skin (emollient function) increase the stratum water content (humectant function) while also working toward protecting the skin (occlusive and protective functions) (Kottner & Surber, 2016).
Limitations
This study had several limitations. First, we only investigated elderly patients participants from an Asian population. It is important to note that variations in ethnicity, the presence of skin disorders (e.g., xerosis), and the presence of venous diseases or circulatory problems may affect the skin barrier function. Therefore, future research should involve a study population that will represent the general population. Second, the cross-sectional design and retrospective review of medical records might have added bias. The timing of hematoma formation after venipuncture was not measured precisely. Therefore, prospective studies are required to address and account for these limitations. Third, the investigated sites may reflect an unknown medical condition (e.g., the leakage of medical fluid from the veins) despite the clinical nurses’ observations and patients’ medical charts.
Conclusion
The skin barrier function of hematoma sites induced by venipuncture differed from the nonhematoma formation sites. In particular, the sites where hematomas formed were more dehydrated and less elastic than the surrounding tissue. These skin properties did not correlate with the intensity of skin erythema after venipuncture. Further prospective trials on the skin barrier function with follow-up until the resolution of hematoma are required to validate our results.
Acknowledgments
The authors greatly appreciate the contribution of Noto General Hospital, Kanazawa Medical University Hospital, and Saiseikai Kanazawa Hospital in collecting data for this study. Special appreciation goes to all the hospitalized elderly patients who gave consent to take part in this research.
Authors' Note
Masaru Matsumoto is now affiliated with Department of Imaging Nursing Science, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was partially supported by a grant-in-aid for Exploratory Research from the Japan Society for the Promotion of Science, 2015–2016 (no. 15K15803, Keiko Kimori).
References
- Ahn S., Kim S., Lee H., Moon S., Chang I. (2007) Correlation between a Cutometer and quantitative evaluation using Moire topography in age-related skin elasticity. Skin Research and Technology 13: 280–284. [DOI] [PubMed] [Google Scholar]
- Ascoli G. B., Deguzman P. B., Rowlands A. (2012) Peripheral intravenous catheter complication rates between those indwelling >96 hours to those indwelling 72-96 hours: A retrospective correlational study. International Journal of Nursing 1: 7–12. [Google Scholar]
- Barel A. O., Clarys P. (1997) In vitro calibration of the capacitance method (Corneometer CM 825) and conductance method (Skicon-200) for the evaluation of the hydration state of the skin. Skin Research and Technology 3: 107–113. [DOI] [PubMed] [Google Scholar]
- Blaak J., Wohlfart R., Schürer N. Y. (2011) Treatment of aged skin with a pH 4 skin care product normalizes increased skin surface pH and improves barrier function: Results of a pilot study. Journal of Cosmetics, Dermatological Sciences and Applications 1: 50–58. [Google Scholar]
- Cowdell F., Steventon K. (2015) Skin cleansing practices for older people: A systematic review. International Journal of Older People Nursing 10: 3–13. [DOI] [PubMed] [Google Scholar]
- Cua A. B., Wilhelm K. P., Maibach H. (1990) Elastic properties of human skin: Relation to age, sex, and anatomical region. Archives of Dermatological Research 282: 283–288. [DOI] [PubMed] [Google Scholar]
- Danby S. G., Brown K., Higgs-Bayliss T., Chittock J., Albenali L., Cork M. J. (2016) The effect of an emollient containing urea, ceramide NP, and lactate on skin barrier structure and function in older people with dry skin. Skin Pharmacology Physiology 29: 135–147. [DOI] [PubMed] [Google Scholar]
- Darlenski R., Sassning S., Tsankov N., Fluhr J. W. (2009) Non-invasive in vivo methods for investigation of the skin barrier physical properties. European Journal of Pharmaceutics and Biopharmaceutics 72: 295–303. [DOI] [PubMed] [Google Scholar]
- Dyson A., Bogod D. (1987) Minimising bruising in the antecubital fossa after venipuncture. British Medical Journal (Clinical Research Edition) 294: 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P. M., Choi E. H. (2005) Interactions among stratum corneum defensive functions. Experimental Dermatology 14: 719–726. [DOI] [PubMed] [Google Scholar]
- Fluhr J. W., Feingold K. R., Elias P. M. (2006) Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivo models. Experimental Dermatology 15: 483–492. [DOI] [PubMed] [Google Scholar]
- Fluhr J. W., Kao J., Jain M., Ahn S. K., Feingold K. R., Elias P. M. (2001) Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. The Journal of Investigative Dermatology 117: 44–51. [DOI] [PubMed] [Google Scholar]
- Fujita H., Maeda Y., Ono T., Jyokyo R. (2003) Clinical features with various troubles against venipuncture in the blood drawing room. Journal of Medical Technology 47: 1571–1573. [Google Scholar]
- Fullerton A., Fischer T., Lahti A., Wilhelm K., Takiwaki H., Serup J. (1996) Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 15: 1–10. [DOI] [PubMed] [Google Scholar]
- Gabard B., Clarys P., Barel A. O. (2006) Comparison of commercial electrical measurement instruments for assessing the hydration state of the stratum corneum. In: Serup J., Jemec G. B. E., Grove G. L. (eds) Handbook of noninvasive methods and the skin, 2nd ed Boca Raton, FL: Taylor & Francis, pp. 351–358. [Google Scholar]
- Godwin P. G., Cuthbert A. C., Choyce A. (1992) Reducing bruising after venipuncture. Quality in Health Care 1: 245–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida B., Nino M., Perrino N. R., Laccetti R., Trio R., Labella S., Balato N. (2010) The impact of obesity on skin disease and epidermal permeability barrier status. Journal of the European Academy of Dermatology and Venereology 24: 191–195. [DOI] [PubMed] [Google Scholar]
- Hachem J. P., Crumrine D., Fluhr J., Brown B. E., Feingold K. R., Elias P. M. (2003) pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. The Journal of Investigative Dermatology 121: 345–353. [DOI] [PubMed] [Google Scholar]
- Hassing J. H., Nater J. P., Bleumink E. (1982) Irritancy of low concentrations of soap and synthetic detergents as measured by skin water loss. Dermatologica 164: 314–321. [DOI] [PubMed] [Google Scholar]
- Heinrich U., Koop U., Leneveu-Duchemin M. C., Osterrieder K., Bielfeldt S., Chkarnat C., Rohr M. (2003) Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). International Journal of Cosmetic Science 25: 45–53. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Shigeta Y., Sugama J., Sanada H., Nakatani T., Konya C., Nakagami G., Mugita Y. (2014) Physiological and appearance characteristics of skin maceration in elderly women with incontinence. Journal of Wound Care 23: 18–30. [DOI] [PubMed] [Google Scholar]
- Kezic S., Nielsen J. B. (2009) Absorption of chemicals through compromised skin. International Archives of Occupational and Environmental Health 82: 677–688. [DOI] [PubMed] [Google Scholar]
- Konya C., Sanada H., Sugama J., Okuwa M., Kamatani Y., Nakagami G., Sakaki K. (2010) Skin injuries caused by medical adhesive tape in older people and associated factors. Journal of Clinical Nursing 19: 1236–1242. [DOI] [PubMed] [Google Scholar]
- Koyano Y., Nakagami G., Iizaka S., Minematsu T., Noguchi H., Tamai N., Murayama R. (2016) Exploring the prevalence of skin tears and skin properties related to skin tears in elderly patients at a long-term medical facility in Japan. International Wound Journal 13: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottner, J., & Surber, C. (2016). Skin care in nursing: A critical discussion of nursing practice and reseach. International Journal of Nursing Studies, 61, 20–28. [DOI] [PubMed]
- Krueger N., Luebberding S., Oltmer M., Streker M., Kerscher M. (2011) Age-related changes in skin mechanical properties: A quantitative evaluation of 120 female subjects. Skin Research and Technology 17: 141–148. [DOI] [PubMed] [Google Scholar]
- Laudenbach N., Klaverkamp L., Hedman-Dennis S. (2014) Peripheral i.v. stabilization and the rate of complications in children: an exploratory study. Journal of Pediatric Nursing 29: 348–353. [DOI] [PubMed] [Google Scholar]
- Legemaat M., Carr P. J., van Rens R. M., van Dijk M., Poslawsky I. E., van den Hoogen A. (2016) Peripheral intravenous cannulation: Complication rates in the neonatal population: A multicenter observational study. Journal of Vascular Access 17: 360. [DOI] [PubMed] [Google Scholar]
- Lemperle G., Holmes R. E., Cohen S. R., Lemperle S. M. (2001) A classification of facial wrinkles. Plastic and Reconstructive Surgery 108: 1735–1750. discussion 1751–1752. [DOI] [PubMed] [Google Scholar]
- Li L., Mac-Mary S., Marsaut D., Sainthillier J. M., Nouveau S., Gharbi T., Humbert P. (2006) Age-related changes in skin topography and microcirculation. Archives of Dermatological Research 297: 412–416. [DOI] [PubMed] [Google Scholar]
- Luebberding S., Krueger N., Kerscher M. (2013) Age-related changes in skin barrier function—Quantitative evaluation of 150 female subjects. International Journal of Cosmetic Science 35: 183–190. [DOI] [PubMed] [Google Scholar]
- Luebberding S., Krueger N., Kerscher M. (2014) Age-related changes in male skin: quantitative evaluation of one hundred and fifty male subjects. Skin Pharmacology and Physiology 27: 9–17. [DOI] [PubMed] [Google Scholar]
- Makrantonaki E., Zouboulis C. C. (2007) Molecular mechanisms of skin aging: state of the art. Annals of the New York Academy of Science 1119: 40–50. [DOI] [PubMed] [Google Scholar]
- Matoltsy A. G., Schragger A., Matoltsy M. N. (1962) Observations on regeneration of the skin barrier. The Journal of the Investigative Dermatology 38: 251–253. [DOI] [PubMed] [Google Scholar]
- Pinnagoda J., Tupker R. A., Agner T., Serup J. (1990) Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 22: 164–178. [DOI] [PubMed] [Google Scholar]
- Roskos K. V., Guy R. H. (1989) Assessment of skin barrier function using transepidermal water loss: Effect of age. Pharmaceutical Research 6: 949–953. [DOI] [PubMed] [Google Scholar]
- Ryu H. S., Joo Y. H., Kim S. O., Park K. C., Youn S. W. (2008) Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Research Technology 14: 354–358. [DOI] [PubMed] [Google Scholar]
- Schmid-Wendtner M. H., Korting H. C. (2006) The pH of the skin surface and its impact on the barrier function. Skin Pharmacology and Physiology 19: 296–302. [DOI] [PubMed] [Google Scholar]
- Shahidullah M., Raffle E. J., Rimmer A. R., Frain-Bell W. (1969) Transepidermal water loss in patients with dermatitis. The British Journal of Dermatology 81: 722–730. [DOI] [PubMed] [Google Scholar]
- Smit H. A., Pinnagoda J., Tupker R. A., Burema J., Coenraads P. J., Nater J. P. (1990) Variability in transepidermal water loss of the skin: Evaluation of a method to assess susceptibility to contact dermatitis in epidemiological studies. International Archives of Occupational and Environmental Health 62: 509–512. [DOI] [PubMed] [Google Scholar]
- Takiwaki H. (1998) Measurement of skin color: Practical application and theoretical considerations. Journal of Medical Investigation 44: 121–126. [PubMed] [Google Scholar]
- Tupker R. A., Pinnagoda J., Coenraads P. J., Nater J. P. (1990) Susceptibility to irritants: role of barrier function, skin dryness and history of atopic dermatitis. The British Journal of Dermatology 123: 199–205. [DOI] [PubMed] [Google Scholar]
- van der Valk P. G., Nater J. P., Bleumink E. (1985) The influence of low concentrations of irritants on skin barrier function as determined by water vapour loss. Dermatosen in Beruf und Umwelt 33: 89–91. [PubMed] [Google Scholar]
- Verdier-Sévrain S., Bonté F. (2007) Skin hydration: A review on its molecular mechanisms. Journal of Cosmetic Dermatology 6: 75–82. [DOI] [PubMed] [Google Scholar]
- Weatherall I. L., Coombs B. D. (1992) Skin color measurements in terms of CIELAB color space values. Journal of Investigative Dermatology 99: 468–473. [DOI] [PubMed] [Google Scholar]
- Woods M. T., Brown P. A., Baig-Lewis S. F., Simpson E. L. (2011) Effects of a novel formulation of fluocinonide 0.1% cream on skin barrier function in atopic dermatitis. Journal of Drugs in Dermatology 10: 171. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2010). WHO guidelines on drawing blood: Best practices in phlebotomy. Retrieved from http://www.euro.who.int/__data/assets/pdf_file/0005/268790/WHO-guidelines-on-drawing-blood-best-practices-in-phlebotomy-Eng.pdf?ua-1. [PubMed]
- Yilmaz E., Borchert H. H. (2006) Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—An in vivo study. International Journal of Pharmaceutics 307: 232–238. [DOI] [PubMed] [Google Scholar]