Within a month after the first case was reported on February 2020 in Brazil, all countries in Latin America had reported cases of the novel coronavirus disease 2019 (COVID-19); Brazil, Argentina, and Colombia were the most affected countries, with 9,467,320 cases among them reported by early December.1 Increased poverty, limited water access, poor sanitation, and distrust in governments are important factors that have affected the transmission and have facilitated COVID-19 outbreaks in Latin America.2
Acute kidney injury (AKI) is common among critically ill patients with COVID-19, affecting approximately 20% to 40% of patients admitted to intensive care units; unfortunately, the overall burden of AKI in COVID-19 might be underestimated in Latin America. Available information on epidemiology and risk factors for AKI in the region is generally scarce, and this situation has not improved during the COVID-19 pandemic. A recent study from 15 countries in Latin America, the “Estudio Epidemiológico Longitudinal de Injuria Renal Aguda” (EPILAT-IRA), provided data on 905 patients with AKI; the median age was 64 (range, 50–74) years, and 61% were male. AKI was community acquired in 62% of patients, with dehydration, shock, and nephrotoxic drugs being the most common causes. Renal replacement therapy (RRT) was performed in 29% of cases, and the incidence of all-cause in-hospital mortality was 26.5%.3 Preliminary and unpublished data from the Latin American Society of Nephrology and Hypertension AKI Committee registry of AKI in COVID-19 patients, which has enrolled 393 subjects so far, showed that >50% of patients were female, the median age was 60 (interquartile range, 48–71) years, 36% of patients developed proteinuria, and 17% presented with hematuria. Most of the AKI was hospital acquired (66%), and 225 of the 393 patients (57.2%) had indications for starting RRT. In 27 of the 225 patients (12%), RRT was not initiated; unfortunately, the reasons why RRT was not initiated were not documented. Most of the patients (72.7%) were admitted to the intensive care unit. The main 3 causes of AKI were cytokine storm (38.5%), hypovolemia (22.9%), and nephrotoxicity (22.9%). Only 30% recovered renal function after AKI.
The infrastructure, human resources, and equipment available to treat AKI in Latin America are variable; most patients who require RRT are treated in units that are located in urban teaching hospitals. Lombardi et al. showed that, in Latin America, intermittent hemodialysis (HD) was universally performed in 246 dialysis units from the 14 countries that participated in the survey, whereas 40% performed prolonged intermittent RRT (PIRRT), 30% performed peritoneal dialysis (PD), and only 23%, provided continuous RRT (CRRT), which is mainly available in large units.4 Preliminary data from the IRA– Latin American Society of Nephrology and Hypertension registry of AKI in COVID-19 patients show that HD was used in 48.8% of cases, PIRRT was used in 25.1% of cases, CRRT was used in 18.3% of cases, and PD was used in 2.3% of cases; in the remaining of 5.3% of cases, different types of RRT were used (i.e., patients experiencing hemodynamic instability were placed on CRRT for 48 to 72 hours, and once their condition improved, they were placed on HD).
The use of extracorporeal organ support therapies for the treatment of severe pneumonia is not new in our region. In 2009, Mexico faced the beginning of the swine influenza A (H1N1) epidemic; thousands of patients experienced severe pneumonia, and many died from pneumonia complications. Of the 14 patients with serum creatinine available at admission, 6 (42.8%) developed AKI, and all of them required RRT; 5 of the patients were placed on PD, and 1 was placed on HD.5
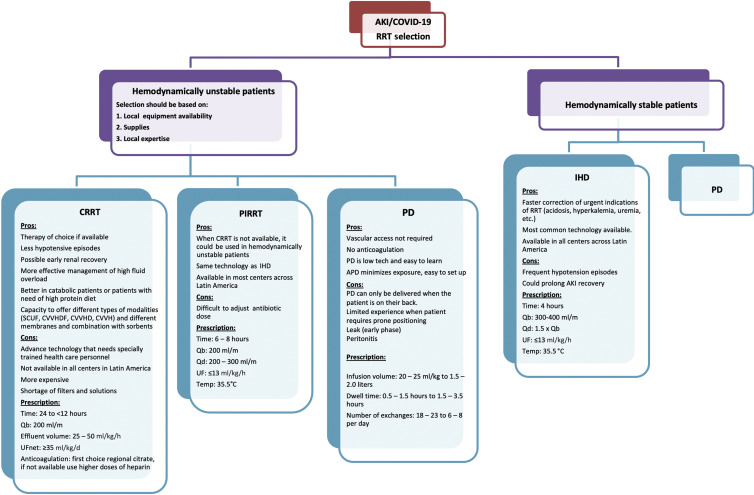
During the COVID-19 pandemic, the proportion of patients needing RRT increased to 5.6% to 23% with severe infection.6 Nephrologists in Latin America need to consider all the possible RRT options available in each center for treating severe AKI (Figure 1 ).
Figure 1.
Proposed approach for the provision of renal replacement therapy (RRT) and modality selection in Latin America during coronavirus disease 2019 (COVID-19) pandemic. Overview of a stepwise approach for providing RRT to patients with COVID-19; in hemodynamically unstable patients, continuous renal replacement therapy (CRRT) is the therapy of choice if available, and prolonged intermittent renal replacement therapy (PIRRT) and peritoneal dialysis (PD) can be used if CRRT is not available. In hemodynamically stable patients, intermittent hemodialysis (IHD) and PD can be used. Selection should be based on local equipment availability, supplies, and local expertise. APD, automated peritoneal dialysis; AKI, acute kidney injury; CVVH, continuous veno-venous hemofiltration; CVVHD, continuous veno-venous hemodialysis; CVVHDF, continuous veno-venous hemodiafiltration; Qb, blood flow rate; Qd, dialysis fluid flow rate; SCUF, slow continuous ultrafiltration; UF, ultrafiltration; UFnet, net ultrafiltration.
Because some patients with COVID-19 pneumonia have lower lung compliance, higher right to left shunt, higher lung recruitability, and symptoms that mimic acute respiratory distress syndrome, and because hemodynamic instability and fluid overload are frequent in COVID-19 patients, CRRT should be the first option of treatment if available.7 However, CRRT capacity was overwhelmed during this pandemic, with shortage of filters and solutions in some countries of the region, such as Mexico, Colombia, and Bolivia. In those scenarios, CRRT machines were used to administer long intermittent treatments (e.g., 10 hours instead of continuous [24 hours] with higher effluent flow rates [40–50 ml/kg per hour]). This strategy, which is commonly used in many centers across Latin America, will allow the CRRT machine to more quickly become available earlier for the care of another patient after cleaning the terminal. In the case where no CRRT devices are available, conventional HD machines could be used to provide prolonged intermittent RRT, which has been shown to have similar hemodynamic effects, and provides similar metabolic control as CRRT.8
PD is an important alternative to consider in the region.9 The experience of the use of PD for COVID-19 patients requiring RRT in Latin America was preliminary reported by Dr. Bazarra Durand from Clínica Ricardo Palma, in Peru, and by Dr. Ponce from Botucatu School of Medicine, in Brazil, with data presented in a webinar entitled “COVID-19–Associated AKI Treatment Protocol and Latin America Experiences.” They reported using automated cycler high-volume PD in 5 critically ill patients with low fill volumes (20 ml/kg), rapid exchanges (dwell times from 35–60 minutes), and 18 to 20 exchanges over 16 to 24 hours. PD catheter insertion was performed bedside by nephrologists with local anesthesia. They did not report any mechanical or infectious complications related to PD; unfortunately, 4 of the 5 patients died (80%).
Nonetheless, there are specific issues of concern regarding PD in AKI from COVID-19.
When acute respiratory distress syndrome occurs, PD should not be used as the first modality for providing RRT, except when other modalities of RRT are not available. When patients need to be prone, PD with a lower infusion volume (20–25 ml/kg) can be used; this low dwell volume will usually not cause mechanical or hemodynamic disturbances. A Brazilian review study reported that ventilated AKI patients with 2 L of PD dwell exchange presented with slightly increased intra-abdominal pressure on the first dwell. However, this did not interfere with patients’ ventilation and oxygenation.10
The use of automated cycler PD reduces the contact with patients, PD fluid exchange occurs automatically, and the exchange of used PD bags with fresh bags is only performed once daily with automated cycler PD. This minimization of contact with the patients reduces not only the risk of contagion, but also the need for personal protective equipment usage, especially in the times when resources are limited, and there is thus far no evidence of virus spreading from PD effluent. However, in a case report of a patient with small-bowel volvulus who required surgery, severe acute respiratory syndrome coronavirus 2 was found in reactive peritoneal fluid at higher concentrations than in the respiratory tract.11
PD is simple and efficient and requires less equipment and infrastructure than other RRTs and reduces the exposure of health care staff, which is a critical issue amidst the ongoing pandemic.
The shortage of resources that is facing physicians is one of the most difficult dilemmas in our region as it can affect decision of who should and who should not receive lifesaving treatments. Selection criteria must be explicit and transparent, produced collectively by the staff, and accessible. The “first come, first served” criteria are in fact selection criteria because once the available places have been filled, the subsequent patients will not have access to them, without the distinction of priorities of any kind. For this reason, guidelines and recommendations have been proposed for a decision-making process.12 Age (relevant to the probability of survival and the expected years of additional living), comorbidities, functional state, and the related long-term prognosis are the most frequently adopted criteria.
Finally, those patients excluded or withdrawn from active treatment have the right to receive the standard of care at the end of life, and the staff is ethically obliged to provide it; in these cases, maximal medical management is attempted (i.e., appropriate doses of loop diuretics for fluid overload, use of potassium binders, and use of sodium bicarbonate). The decision-making process is a 3-step process based on introducing choice, deliberating among options, and helping patients analyze preferences and make decisions; palliative care and transfer from the intensive care unit to an isolated room, if possible, should be provided as the last action of the staff.
In summary, all types of RRT are available in Latin America for treating COVID-19 patients with AKI. We have adapted therapies, such as CRRT, to provide adequate treatment with limited resources; when available HD or CRRT machines are scarce, PD can be used. A proposed approach for the early identification of AKI and for providing RRT for patients with COVID-19 is shown in Table 1 .
Table 1.
Proposed approach for early identification of AKI and for providing RRTs in patients with COVID-19
|
|
|
|
|
|
|
AKI, acute kidney injury; APD, automated peritoneal dialysis; CAPD; continuous ambulatory peritoneal dialysis; COVID-19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; IHD, intermittent hemodialysis; KDIGO, Kidney Disease: Improving Global Outcomes; PIRRT, prolonged intermittent renal replacement therapy; RRT, renal replacement therapy.
Disclosure
All the authors declared no competing interests.
References
- 1.Burki T. COVID-19 in Latin America. Lancet Infect Dis. 2020;20:547–548. doi: 10.1016/S1473-3099(20)30303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller M.J., Loaiza J.R., Takyar A., Gilman R.H. COVID-19 in Latin America: novel transmission dynamics for a global pandemic? PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lombardi R., Ferreiro A., Claure-Del Granado R., et al. EPILAT-IRA study: a contribution to the understanding of the epidemiology of acute kidney injury in Latin America. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombardi R., Rosa-Diez G., Ferreiro A., et al. Acute kidney injury in Latin America: a view on renal replacement therapy resources. Nephrol Dial Transplant. 2014;29:1369–1376. doi: 10.1093/ndt/gfu078. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S., et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 6.Adapa S., Aeddula N.R., Konala V.M., et al. COVID-19 and renal failure: challenges in the delivery of renal replacement therapy. J Clin Med Res. 2020;12:276–285. doi: 10.14740/jocmr4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geara A.S., Neyra J.A. COVID-19: nephrology preparedness checklist. Clin Nephrol. 2020;94:14–17. doi: 10.5414/CN110225. [DOI] [PubMed] [Google Scholar]
- 8.Kitchlu A., Adhikari N., Burns K.E., et al. Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study. BMC Nephrol. 2015;16:127. doi: 10.1186/s12882-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16:308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida C.P., Ponce D., de Marchi A.C., Balbi A.L. Effect of peritoneal dialysis on respiratory mechanics in acute kidney injury patients. Perit Dial Int. 2014;34:544–549. doi: 10.3747/pdi.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coccolini F., Tartaglia D., Puglisi A., et al. SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann Surg. 2020;272:e240–e242. doi: 10.1097/SLA.0000000000004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergano M., Bertolini G., Giannini A., et al. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: the Italian perspective during the COVID-19 epidemic. Crit Care. 2020;24:165. doi: 10.1186/s13054-020-02891-w. [DOI] [PMC free article] [PubMed] [Google Scholar]