Abstract
This review performs a comprehensive assessment of the therapeutic potential of three native herbs of Crete (Thymbra capitata (L.) Cav., Salvia fruticosa Mill. and Origanum dictamnus L.), their phytochemical constituents, health benefits and issues relevant to their safety, within a translational context. Issues discussed comprise: 1) Ethnopharmacological uses of the three herbs, reviewed through an extensive search of the literature; 2) Systematic analysis of the major phytochemical constituents of each plant, and their medicinal properties; 3) To what extent could the existing medicinal properties be combined and produce an additive or synergistic effect; 4) Possible safety issues. We conclude with a specific example of the use of a combination of the essential oils of these plants as an effective anti-viral product and the experience gained in a case of a plant-based pharmaceutical development, by presenting the major steps and the continuum of the translational chain.
Keywords: traditional medicine, Southeastern Europe, Mediterranean, Near East, synergy, regulatory affairs, clinical trials, antiviral
Introduction
Plants are traditionally used, in different forms, for the treatment of diseases, since the Neolithic Era (Hardy, 2019). Different populations used native flora for the production of preparations, efficient in curing different diseases and conditions (Fabricant and Farnsworth, 2001). The Cretan area (KK, Crete and Karpathos floristic region) is such an example, as its evolutionary history preserved a very diverse flora, with more than 2000 indigenous species, including a high number of endemics (Dimopoulos et al., 2013). Cretan people abundantly use plants and greens in different aspects of their life. Cretan diet, a specific entity of the Mediterranean diet, uses a diversity of plants for culinary purposes (Renaud, 1995 and references herein), while a variety of plants are used as decoctions and infusions for recreational or medical purposes. We systematically analyzed the habits of a rural population in Crete and reported that use of different plant infusions, alone or in combination, resulted in a significant protection from common cold and influenza infections (Lionis et al., 1998). Based on this study, we have focused on three of the most efficient plants (Coridothymus capitatus, Salvia fruticosa and Origanum dictamnus) and developed a supplement, efficient at combatting upper respiratory tract infections (Duikler et al., 2015; Anastasaki et al., 2017).
In this review, we analyze in depth the health benefits of these plants, their most prominent active compounds and their combination. In addition, we review our experience with the development of a nutraceutical product, discussing potential bottlenecks encountered during the process.
The Plants
Taxonomy and Distribution
According to the Euro + Med plantbase, Coridothymus capitatus (L.) Reichenb. fil. is a dwarf-shrub. It is the only member of the monospecific genus Coridothymus. According to the Euro + Med plantbase (Accessed at June 2017), the name Coridothymus capitatus (L.) Reichenb. fil. is synonym of Thymbra capitata (L.) Cav., which is included in Kingdom–Plantae, Division–Tracheophyta, Subdivision–Spermatophytina, Class–Magnoliopsida, Superorder–Asteranae, Order–Lamiales, Family–Lamiaceae Lindl., Genus–Thymbra L.
Accepted name: Thymbra capitata (L.) Cav., Homotypic synonyms: Coridothymus capitatus (L.) Rchb. f., Origanum capitatum (L.) Kuntze, Satureja capitata L., Thymus capitatus (L.) Hoffmanns. & Link.
Salvia fruticosa Mill. is also a member of the Labiatae family. The taxon is included in Kingdom–Plantae, Division–Tracheophyta, Subdivision–Spermatophytina, Class–Magnoliopsida, Superorder–Asteranae, Order–Lamiales, Family–Lamiaceae Lindl., Genus–Salvia L.
Accepted name: Salvia fruticosa Mill, Heterotypic synonyms: Salvia baccifera Etl., Salvia clusii Jacq., Salvia cypria Unger & Kotschy, Salvia fruticosa subsp. cypria (Unger & Kotschy) Holmboe, Salvia fruticosa subsp. thomasii (Lacaita) Brullo and al., Salvia incarnata Etl., Salvia libanotica Boiss. & Gaill., Salvia lobryana Azn., Salvia marrubioides Vahl, Salvia ovata F. Dietr., Salvia sipylea Lam., Salvia subtriloba Schrank, Salvia sypilea Lam., Salvia thomasii Lacaita, Salvia triloba L. f., Salvia triloba subsp. calpeana (Dautez & Debeaux) P. Silva, Salvia triloba var. calpeana Dautez & Debeaux, Salvia triloba subsp. libanotica (Boiss. & Gaill.) Holmboe, Sclarea triloba(L. f.) Raf.
Origanum dictamnus L. is a local endemic of Crete, member of Labiatae family. The taxon is included in Kingdom–Plantae, Division–Tracheophyta, Subdivision–Spermatophytina, Class–Magnoliopsida, Superorder–Asteranae, Order–Lamiales, Family–Lamiaceae Lindl., Genus–Origanum L.
Accepted name: Origanum dictamnus L., Heterotypic synonyms: Amaracus dictamnus (L.) Benth., Majorana dictamnus (L.) Kostel., Heterotypic synonyms: Amaracus tomentosus Moench, Origanum dictamnifolium St.-Lag., Origanum saxatile Salisb.
Coridothymus capitatus is found throughout the Mediterranean region distributed in all Mediterranean countries with the exception of France. Salvia fruticosa is distributed in Italy, Sicily, and the eastern Balkans, including Cyprus, while Origanum dictamnus is local endemic of Crete (Fielding and Turland, 2005).
Methodology
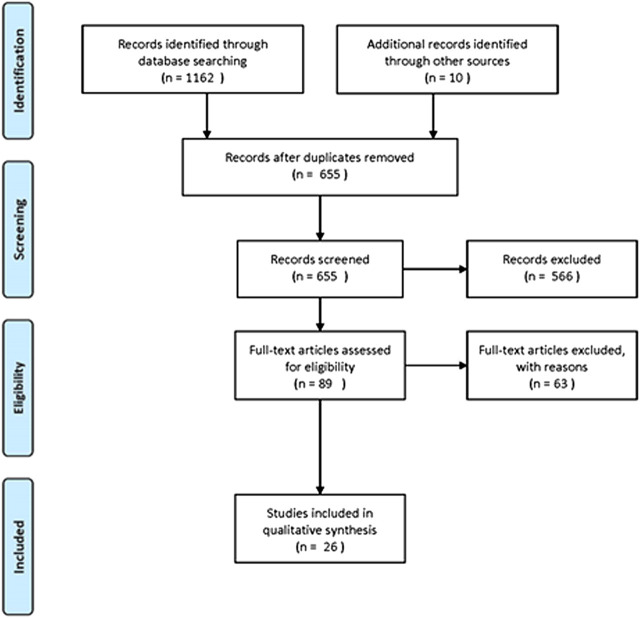
In the context of this review, PubMed, Scopus and Google Scholar were used for the retrieval of the relevant bibliography. The following inclusion criteria were used for the systematic review of the three plants: articles published in English, articles concerning countries of the Mediterranean basin and also including Jordan, Syria, Iran and Iraq, as well as studies with the following keywords in their text: Coridothymus capitatus, Salvia fruticosa and Origanum dictamnus. The PRISMA flow diagram, presenting the results of our search, is depicted in Figure 1. The primary search identified 1,162 articles, plus 10 articles from other sources. After the implementation of the eligibility criteria, 26 articles were finally incorporated. Any evaluation of studies concerning the plant quality was out of scope and has not been included in this review.
FIGURE 1.
PRISMA flow diagram for the ethnobotanical and ethnopharmacological data.
Concerning the synergistic interactions of the main constituents of the essential oil (see below) the review was carried out through a literature searching of the same databases using the following keywords in all fields: “synerg* AND (carvacrol OR eucalyptol OR 1,8-cineole OR beta-Caryophyllene).” The selection of manuscripts was based on the following criteria: articles published in English and articles where only documented synergy between pure substances was reported. The primary search identified 4,101 articles, with 1,538 from PubMed, 1,910 from Google Scholar and 653 from Scopus and after the implementation of the eligibility criteria, excluding the repetitions, 25 articles were finally included in the qualitative analysis.
Ethnopharmacological Uses of the Plants
Coridothymus capitatus, Origanum dictamnus and Salvia fruticosa are renown since antiquity for their pharmaceutical properties (Karousou et al., 1998). The first two are included in the Dioscorides book “De Materia Medica.” Salvia fruticosa was introduced in the Iberian Peninsula for cultivation by Greeks and Phoenicians and elements of these cultivations can be discovered today in several parts of the Iberian coast (Rivera et al., 1994). O. dictamnus is also mentioned from the Minoan era, through the centuries from several physicians and philosophers including Asklepios, Euripides, Aristotle, Hippocrates, Theophrastus, Virgil and Galen (Vrachnakis, 2003).
Coridothymus capitatus is known in local cultures with several common names among which Spanish oregano, thyme, headed thyme, conehead thyme, agrio thymari (αγριο θυμάρι), thymari (θυμάρι), tomilho de Creta and tomilho de Dioscórides, Salvia fruticosa is known with the names Greek sage and faskomilo (φασκόμηλο), while Origanum dictamnus is known as diktamo (δίκταμο), dittany of Crete and dictamo de Creta.
The ethnobotanical and ethnopharamacological studies concerning the medical use of Coridothymus capitatus, Salvia fruticosa and Origanum dictamnus, as well as from studies on local herbal markets in Eastern Mediterranean region, are presented in Tables 1–3 respectively, together with the plant part and their mode of preparation/use.
TABLE 2.
Medicinal uses of Salvia fruticosa derived from ethnobotanical and ethnopharamacological studies.
| Area | Local name | Plant part | Medicinal use | Mode of preparation/use | Literature | |
|---|---|---|---|---|---|---|
| Israel (golan heights and west bank region) | Foliage | Stomach ache, intestinal gas and inflammation, diabetes and sexual weakness | An infusion of 50 g in 1 L is prepared and taken orally, 150 cc, 1–3 times/day until improvement occurs | Said et al. (2002) | ||
| Turkey (west Anatolia) | Almiya çalbasi = almiya yaği | Herbs | For flatulence and constipation for babies. For colds, cough, stomachache | Internally, volatile oil is applied on nipples before nursing to alleviate the flatulence and constipation of a baby. Infusion, as herbal tea | Honda et al. (1996) | |
| Greece | Faskomilo, elelisfakos | Erial parts | Hypotension, diabetes, laryngitis, pharyngitis, tonsillitis, constipation, diarrhea, spasmolytic, anemia, brain stimulant, calmative, depression, common cold, arthritis, hair loss, hair tonic, stomatitis, dysmenorrhoea, stimulant | Infusion, decoction, external application (footbath, message, washings) | Hanlidou et al. (2004) | |
| Israel | Sage | Leaf | Hemorrhages, intestinal diseases and pains | — | Lev & Amar (2000) | |
| Jordan | Sage | Leaf | Hemorrhages, intestinal diseases and pains | — | Lev & Amar (2002) | |
| Pelestinian area | White sage | — | Digestive system, prostate disorders, skin disorders | — | Ali-Shtayeh et al. (2000) | |
| Israel (northern Israel) | — | Leaves | Indigestion. Coughs and colds | Tisane. Taken as needed | Dafni et al. (1984) | |
| Jordan (northern Badia region) | Meirameieh | Foliage | Stomachache, flatulence, inflammation, diabetes and sexual weakness | Decoction | Alzweiri et al. (2011) | |
| Cyprus | Tree lobed sage | Leaf, tip of shoot | Febrile conditions (not specified), gastro-intestinal tract disorders (tenesmus), malaria fever, absent or delayed menstruation, repellent against arachnids, insects, snakes, respiratory tract diseases (catarrh and common cold, cough), supporting treatment in pleurisy and pneumonia, wounds | External (topical applications, baths), oral | Lardos (2006) | |
| Jordan (Showbak) | Sage, clary | Leaves stems | Sedative, for wounds healing | Syrup | Al-Qura’n (2009) | |
| Jordan | Meriamiah | — | Astrigent and antidandruff | Lotion | Al-Khalil (1995) | |
| Jordan (Mujib nature reserve | Meriamiah, Miramieh | Leaves | Spasm, common cold, intestinal gases | Infusion (50 g in l lof water) is made and taken orally 3 times a day. Infusion is also prepared with tea by adding 3–5 g to a cup of tea | Hudaib et al. (2008) | |
| Cyprus | Spatzia, faskomilo | — | Hypotension, diabetes, estrogen action, diarrhea, dyspepsia, spasmolytic, anti-aging, anti-perspirant, brain stimulant, depression, and nervous tonic, aphthae, gingivitis, dysmenorrhea, antiseptic, diuretic, tonic | Decoction, infusion/oral (potion), external (Compress, washings) | Karousou & Deirmentzoglou (2011) | |
| Jordan (Ailoun heights region) | Meriamia, Meirameieh | Leaves | Antispasmodic | Decoction | Aburjai et al. (2007) | |
| Palestinian area | White sage | Leaves | Anti inflammatory gargle, antiseptic, antitussive, antihaemorrhoids pain, antirheumatic, anti stomach, disturbances, astringent, carminative, hypotensive | — | Ali-Shtaueh et al. (1998) | |
| Israel (northern Israel) | — | Leaves | Indigestion goughs and colds | Tisane is taken as needed | Dafni et al. (1984) | |
| Turkey (Marmaris, Muğla) | Adaçayı, almakeyik, almageyik | Leaves | Stomachache, flatulence, cold, tonsillitis, laxative, antipyretic | Infusion, int | Gürdal and Kültür (2013) | |
| Palestine/west bank | — | Leaves | Diarrhea | — | Jaradat et al. (2016) | |
TABLE 1.
Medicinal uses of Coridothymus capitatus derived from ethnobotanical and ethnopharmacological studies.
| Area | Local name | Plant part | Medicinal use | Mode of preparation/use | Literature |
|---|---|---|---|---|---|
| Israel (Golan heights and west bank region) | Foliage | Heart diseases, paralysis, diabetes, track pain and inflammation and respiratory system | An infusion of one tsp foliage in a cup of water is made and taken 1/3 times/day until improvement occurs | Said et al. (2002) | |
| Turkey (west Anatolia) | Kekik | Herbs | For cough colds, stomachache | Internally, infusion, tea, 3 days on empty stomach | Honda et al. (1996) |
| Jordan (northern Badia region) | Zater faresy | Leaves | Heart and respiratory diseases, diabetes, inflammation and used as rubefacient | Infusion | Alzweiri et al. (2011) |
| Pelestinian area | Thyme | — | Respiratory and urinary digestive systems and inflammation | — | Ali-Shtayeh et al. (2000) |
| Israel | — | — | Cardiovascular system and digestive system | — | Palevirtch et al. (1986) |
| The levant countries | Headed thyme | — | Internal diseases, eye diseases, stomach intestine | — | Lev (2002) |
| Jordan | Conehead thyme | Branch | Digestive system, respiratory system | — | Lev and Amar (2002) |
| Cyprus | Conehead thyme | Aerial parts | Respiratory tract diseases (catarrh and common cold) | — | Lardos (2006) |
| Jordan (Showbak) | Thyme | Aerial parts | Antispasmodic | Syrup | Al-Qura’n (2009) |
| Jordan | — | Leaves | Pectoral, stomachic, and to treat urinary tract infections | Infusion | Al-Khalil (1995) |
| Israel (northern Israel) | — | Leaves | Heart disorders. Swelling and drops. Indigestion. Local paralysis | Tisane. Taken as needed decoction. Used as a drink for 40 days. Steam-bath. Treatment is given for a duration of a month | Dafni et al. (1984) |
| Spain (Barros area, Badajoz province) | — | Leaves | Disestive, tonic, carminative | — | Vázquez et al. (1997) |
| Cyprus | Throumpin, agrio thymari | Flowering herb, flower, leaf | Respiratory tract | Tea, chew | Lardos and Heinrich (2013) |
| Turkey | Ballı kekik,bal kekiği, zahter, beyazkekik | Erial parts, flowering branches, esssential oil,, fixed oil | Diabetes, analgesic, Pharyngitis, cold, flu, pleasureand medicinal tea | Drink oneteacup2–3 times a dayfor3–4 weeks/apply 2–3 timesadayfor1–2 Weeks in, Exo,Lo, Lpw,Spc | Sargin (2015) |
| Egypt (Sinai peninsula) | Zaatar, Hasha | Flowering tops, leaves | Infusion as analgesic, sedative, digestive diseases, spasms, vomiting, flatulence. Liver diseases (jaundice) | — | Eissa et al. (2014) |
TABLE 3.
Medicinal uses of Origanum dictamnus derived from ethnobotanical and ethnopharmacological studies.
| Area | Local name | Plant part | Medicinal use | Mode of preparation/use | References |
|---|---|---|---|---|---|
| Greece | Diktamos, erontas | Erial parts | Diabetes, liver disorders, spasmolytic, stomach ulcer, cholesterol, brain stimulant, headache, antiseptic, sanative, diuretic, dysmenorrhoea, antibacterial activities, aphrodisiac, stimulant | Infusion, external application (washings, compress) | Hanlidou et al. (2004) |
| Iran | Poneh kouhi | — | Dyspnea, bronchitis, allergy, depression, itch, dementia, abortifacient | Decoction | Naghibi et al. (2005) |
| Cyprus | Diktamo | Erial parts | Liver disorders, headache, neuralgia, cough, antiseptic, sanative, aphrodisiac, tonic | Infusion/oral (potion), external (compress) | Karousou and Deirmentzoglou (2011) |
| Europe | Dictamnus | — | Diabetes mellitus, obesity, disorders of lipoprotein metabolism and other lipidaemias, mood disorders, sexual dysfunction (not caused by organic disorder disease), epilepsy, acute nasopharyngitis (common cold), acute pharyngitis (unspecified), acute tonsillitis (unspecified), gingivitis and periodontal diseases, other specified disorders of teeth and supporting structures (toothache NOS), gastric ulcer, gastritis and duodenitis, diseases of liver, xerosis cutis, inflammatory polyarthropathies, pain in joint, acute nephritic syndrome, renal tubule-interstitial diseases, calculus of kidney and ureter, disorder of urinary system (unspecified), absent scanty and rare menstruation, pain and other conditions associated with female genital organs and menstrual cycle, dysmenorrhea unspecified, spontaneous abortion, long labor, cough, headache, convulsions (not elsewhere classified), multiple superficial injuries (unspecified), multiple open wounds (unspecified) | — | Martínez-Francés et al. (2015) |
Based on this information, medicinal uses, in an ethnobotanical context, suggest that the three species have a highly antimicrobial and anti-inflammatory action. In addition, they are active against various targets and diseases, including diseases of the respiratory, digestive and urinary systems.
Phytochemical Constituents
The essential oils of the three plants is produced from collected plant material, air dried in the dark, at room temperature (25°C) for 10 days. For analysis, after steam distillation, 1 ml of volatile oils were diluted with 2 ml of ether and filtered through anhydrous sodium sulfate to remove water traces and were stored at 4°C. Analysis was performed, as described previously (Duikler et al., 2015), by Gas Chromatography-Mass Spectroscopy (GC-MS, Shimadzu, QP 5050 A), with a MDN-5 column and a Quadrupole Mass Spectrometer as detector, after injection of 2 μL. The carrier gas was helium, the flow rate 0.9 ml/min. The sample was measured in a split mode procedure (1:35). For GS-MS detection an electron ionization system was used with ionization energy at 70 eV. The interested reader should refer to Duikler et al. (2015) for further analytical details.
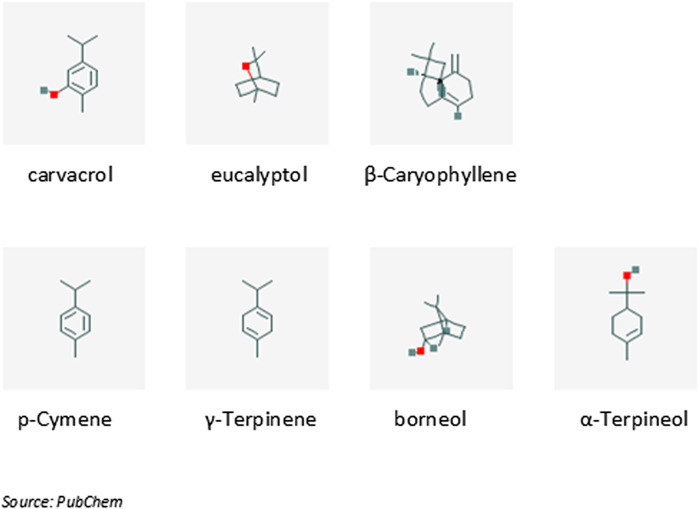
The chemical constituents of the essential oils of the three plants, as well as their combination (1.5% v/v of pure essential oils in olive oil carrier), used in the context of upper respiratory tract infections are presented in details as Supplemental Material in Duikler et al. (2015). According to this description (see also Figure 2) carvacrol (52.7%) is the main constituent of the mixture, followed by eucalyptol (12.77%) and β-caryophyllene (3.41%). The compounds p-cymene, γ-terpinene, borneol and α-terpineol participate with concentrations 1.32, 1.17, 1.68 and 1.06% respectively, while the rest 15 compounds participate with less than 1% (Figure 2).
FIGURE 2.
The main phytochemical constituents contained in the essential oil of plants discussed in this review. IUPAC names: 2-methyl-5-propan-2-ylphenol (carvacrol), 1,3,3-trimethyl-2-oxabicyclo [2.2.2] octane (eucalyptol), (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo [7.2.0] undec-4-ene (β-Caryophyllene), 1-methyl-4-propan-2-ylbenzene (p-Cymene), 1-methyl-4-propan-2-ylcyclohexa-1,4-diene (γ-Terpinene), (1S,2R,4R)-1,7,7-trimethylbicyclo [2.2.1] heptan-2-ol (borneol), 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol (α-Terpineol).
Carvacrol (5-isopropyl-2-methylphenol) is an aromatic monoterpene with molecular weight 150.2 g/mol. It is a constituent of many essential oils, especially those found in plants of the family Lamiaceae, where the Origanum vulgare subsp. hirtum (Link) (Kokkini and Vokou, 1989).
Eucalyptol (1,8-cineole) is a cyclic ether monoterpene (molecular weight 154.3 g/mol), which is found as a constituent of plant essential oils (its name is derived from its presence in essential oils of Eucalyptus globosus). It is also considered as a major monoterpene emitted by vegetation into the atmosphere (Guenther et al., 1994).
β-caryophyllene is a natural bicyclic sesquiterpene with molecular weight 204.4 g/mol that is a constituent of many essential oils, such as clove or rosemary oil (Bernardes et al., 2010).
Concerning the other compounds, p-cymene is a naturally occurring organic compound that is characterized as a hydrocarbon related to a monoterpene. Its molecular weight is 134.2 g/mol and it is a constituent of several essential oils, such as the oil of cumin (Allahghadri et al., 2010). γ-terpinene is a terpene with molecular weight 136.3 g/mol. It is a major component of essential oils made from Citrus fruits (Lücker et al., 2002). Borneol is a bicyclic monoterpene with molecular weight 154.3 g/mol containing exactly two rings which are fused to each other. α-Terpineol is a naturally occurring monoterpene alcohol, derived from several sources, including oil of pines. Terpineol, due to its pleasant odor (similar to lilac), is used as an ingredient in perfumes, cosmetics, and flavors (Kim and Chung, 2000).
Uses of Phytochemical Constituents
Apart from the ethnobotanical and ethnopharmacological uses of plants mentioned above, several uses of the aforementioned phytochemical constituents are also documented:
Carvacrol
Carvacrol is the basic constituent of many essential oils, mainly of oregano species (Labiatae) (Kokkini and Vokou, 1989; Kirimer et al., 1995; Baydar et al., 2004; Stefanaki et al., 2016); it is characterized by its strong antioxidant properties (similar to those of vitamin E and ascorbic acid) (Mastelic et al., 2008). It is also known for its pronounced antimicrobial and antibacterial action (Suntres et al., 2015). The antimicrobial activity of several essential oils has been related to their content of carvacrol (García-Beltrán and Esteban, 2016), while, in a number of studies, the antimicrobial and the antibacterial action of the pure compound has also been investigated (Ben Arfa et al., 2006).
The antibacterial action of carvacrol extends to a variety of Gram-positive and negative bacteria (Kim et al., 1995; Rattanachaikunsopon and Phumkhachorn, 2010a; Ravishankar et al., 2010; Rivas et al., 2010). The compound acts as a transmembrane monovalent cation (hydroxyl proton for a potassium cation) exchanger (Suntres et al., 32,015). Therefore, in addition to its hydrophobic characteristics, allowing its accumulation in the membrane, the presence of free hydroxyls is essential for its antibacterial and antimicrobial activity (Ben Arfa et al., 2006).
An anti-inflammatory action of carvacrol is also documented (Landa et al., 2009; Hotta et al., 2010) and relies to the decreased production of inflammatory mediators, including cytokines, prostaglandins, enzymes, nitric oxide (NOS) and reactive oxygen species (ROS) (da Silva Lima et al., 2013). The authors suggest that carvacrol’s anti-inflammatory effects specifically induced IL-10 release, leading, subsequently, to the reduction of IL-1β and prostanoids production. In addition, it was reported that the inhibition of prostaglandin synthesis (Wagner et al., 1986) by carvacrol, is the basis of its antinociceptive activity. Additional mechanisms of antinociceptive actions of carvacrol include its agonistic activity for Transient Receptor Potential Vanilloid 3 (TRPV3), an ionic channel implicated in hyperalgesia and possibly skin sensitization (Xu et al., 2006). In addition, carvacrol activates and promptly desensitizes the specific sensor of environmental irritants TRPA1 ion channel/receptor (Xu et al., 2006).
Eucalyptol (1,8-Cineole)
1,8-cineole (cineole), also known as eucalyptol, is the principal constituent of most Eucalyptus oil-preparations. It is employed in drug preparations, as a percutaneous enhancer and anti-inflammatory agent, decongestant and antitussive, while in aromatherapy it is used as a skin stimulant (Santos and Rao, 2000; Yuan et al., 2006).
Juergens et al. (2004), reported that 1,8-cineol inhibited Th1/Th2-associated cytokine production by human lymphocytes and monocytes. This anti-inflammatory action resulted in a subsequent inhibition of cytokine-induced airway mucus hypersecretion.
1,8 cineole is also known for its analgesic action (Guimarães et al., 2013). Santos and Rao (2000) suggested that the potent anti-inflammatory action of the compound was associated with an outstanding peripheral analgesic effect. They also suggested that 1,8- cineole-induced analgesic effects are not associated to neuronal toxicity, while Khalil et al. (2004) documented that 1,8-cineol, in a concentration-dependent manner, acted directly on sensory nerves and blocked nerve excitability (Lima-Accioly et al., 2006; Guimarães et al., 2013), through a direct activation of TRPM8 channels, which is a specific heat/cold receptor. TRPM8 activation in sensory nerves by 1,8-cineole, produced a specific analgesic effect, in cases of peripheral nerve injury (Proudfoot et al., 2006).
β-Caryophyllene
β-caryophyllene is a widespread plant volatile compound, which is a selective ligand of the CB2 cannabinoid receptor, specifically related to analgesia and inflammation, and devoted of psychotropic actions, mediated by CB1 (Gertsch et al., 2008; Klauke et al., 2014). Therefore, CB2 receptor-selective agonists, like β-caryophyllene, are potential analgesic drug candidates, in various types of pain (such as neuropathic pain) (Guindon and Hohmann, 2008; Kinsey et al., 2010).
p-Cymene
p-cymene is a precursor of carvacrol. It has an antimicrobial action per se, but it is less effective than carvacrol, when used alone (Kiskó and Roller, 2005), because p-cymene lacks a hydroxyl group, which is an important radical for the antimicrobial activity (Ultee et al., 1999; 2002). p-cymene also demonstrated an enhanced antinociceptive action in animal models of neurogenic and inflammatory pain (Guimarães et al., 2013).
γ-Terpinene
The antimicrobial activity of γ-terpinene is debatable, with positive (Sato et al., 2007) and negative (Sivropoulou et al., 1996) reports, against strains of Gram-positive or Gram-negative bacteria.
Borneol
Borneol is a known medicinal substance in Chinese and Indian traditional medicine. Borneol and its derivatives, possess antimicrobial (Corrêa et al., 2012), anti-inflammatory (Ehrnhöfer-Ressler et al., 2013) and antiviral activity. It also posesses a highly stimulatory action at GABAA receptors (Granger et al., 2005; Manayi et al., 2016). GABA is the predominant inhibitory transmitter in the mammalian central nervous system and stimulation of GABAA receptors produces anxiolysis, sedation, anesthesia and myorelaxation (Chebib and Johnston, 2000).
Borneol also expressed topical analgesic action. Wang et al. (2017), providing evidence for a topical analgesic efficacy in humans; TRPM8 was identified as its main molecular target. Moreover, borneol demonstrated anti-thrombotic effects, related to its anticoagulant properties (Li et al., 2008; Chen et al., 2015), possibly related to its potent modulation of the nitrite/nitrate reductase activity (Tang et al., 2009).
α-Terpineol
α-terpineol is characterized by a moderate antibacterial activity, with a broad antibacterial spectrum. It is worth mentioning that a-terpineol showed antibacterial activities against penicillin-resistant bacterial strains (Kotan et al., 2007).
α-terpineol exerts central and peripheral antinociceptive activity (Quintans-Júnior et al., 2011). It also inhibits NF-κB and subsequently down-regulats the expression of proinflammatory IL-1β and IL-6 cytokines (Held et al., 2007; Hassan et al., 2010), mainly explaining the compound’s antinociceptive and anti-inflammatory properties (Oliveira et al., 2012).
α-terpineol also exhibited a gastroprotective action in ethanol-induced gastric ulcers (Souza et al., 2011) and beneficial effects in the cardiovascular system (Ribeiro et al., 2010). The proposed mechanism of action included endothelial NO-related vasorelaxation and activation of the NO–cGMP pathway.
Registered Medical Applications
A series of clinical studies (most of them double-bind placebo-controlled trials, Table 4) evaluated the efficacy of 1,8-cineol (eucalyptol), in several medical issues. 1,8-cineol (eucalyptol) is a licensed medicinal product in Germany, since many years, in intestine-soluble capsules, for the treatment of acute and chronic bronchitis, sinusitis, respiratory infections and rheumatoid-like joint diseases (Juergens et al., 2003; Guimarães et al., 2013).
TABLE 4.
Clinical studies concerning the evaluation of efficacy of the main constituents of essential oil preparation from Thymbra capitata (L.) Cav., Salvia fruticosa Mill. and Origanum dictamnus L., in several medical conditions.
| Compound | Symptome | Clinical trial | References |
|---|---|---|---|
| 1,8-cineol (eucalyptol) | Anti-inflammatory activity in bronchial asthma | Double-bind placebo-controlled trial | Juergens et al. (2003) |
| Cineole | Acute nonpurulent rhinosinusitis | Double-blind, randomized, placebo-controlled trial | Kehrl et al. (2004) |
| Cineole | Acute non-purulent rhinosinusitis | Prospective, randomised, double-blinded controlled study | Tesche et al. (2008) |
| Cineole (eucalyptole) | Chronic obstructive pulmonary disease | Placebo-controlled double-blind trial | Worth et al. (2009) |
| Cineole | Asthma | Placebo-controlled, double-blind trial | Worth and Dethlefsen (2012) |
| Cineole | Acute bronchitis | Placebo-controlled double-blind trial | Fischer and Dethlefsen (2013) |
| 1,8-cineol (in eucalyptus oil) | Pain and inflammatory responses after total knee replacement | Randomized clinical trial | Jun et al. (2013) |
| 1,8-Cineole | Preoperative anxiety | Randomized clinical trial | Kim et al. (2014) |
Borneol and p-cymene have also been subjects of clinical studies for induced analgesia and treatment of the Fish Tapeworm disease respectively (Vartiainen, 1950; Wang et al., 2017).
Synergy of Phytochemical Constituents
According to a classical definition, synergy occurs when the combined effect of two or more substances is greater than the sum of the individual agents (Breitinger, 2012). When the registered effect is an add up of individual actions, the action is reported as additive. Recently, the definition of synergy has been clarified from two points of view, the pharmacodynamic (enhanced therapeutic actions on the same target) and pharmacokinetic (no direct interaction but a multi-target behavior) (Yang et al., 2014; Zhou et al., 2016). The main reason of employing combinations of active substances with synergistic interactions is to reduce the administered amount of each compound and to increase the biological activity of a preparation/mixture against a specific target. In addition, this strategy diminishes the chance of pharmaceutical resistance of the pathogenic organism (Araújo et al., 2016).
Synergistic interactions were known for specific substances long time ago. For example, Barbaste et al. (2002) proposed a “cascade” reaction from lipophilic to hydrophilic antioxidants, enhancing their biological effects, while Vardar-Ünlü et al. (2003) suggested that the crude essential oil of Thymus pectinatus var pectinatus was more effective as antioxidant (DPPH assay) than its main active components (thymol and carvacrol). Testing the antioxidant activity of thymol, carvacrol and p-cymene, Milos and Makota (2012) showed synergistic effects in any combination of any two of the above compounds. Moreover, p-cymene, the precursor of carvacrol was found to enhance the bactericidal activity of carvacrol when used in combination (Kiskó and Roller, 2005; Rattanachaikunsopon and Phumkhachorn, 2010b). Synergistic effects of p-cymene have also been reported in relation to thymol and γ-terpinene (Dauqan and Abdullah, 2017).
Synergistic interactions of carvacrol, eucalyptol (1,8-cineole) and β-caryophyllene are presented in Table 5. Synergy was also reported between carvacrol and other minor constituents, such as, p-cymene, carvacrol and thymol. Eucalyptol also appeared to have synergistic effects with other constituent, including camphor, terpinene-4-ol and caryophyllene oxide. Additionally, synergy was also reported between minor constituents, such as between camphor and p-cymene.
TABLE 5.
Synergistic interactions of carvacrol, eucalyptol and β-Caryophyllene. Synergistic interactions of minor constituents of the essential oil reported in the same articles are also reported.
| Substances | Biological activity | Interaction | References |
|---|---|---|---|
| Thymol/carvacrol | Antigenotoxicity | Synergy | Quintero Ruiz et al. (2017) |
| Carvacrol/p-cymene | Antigenotoxicity | Synergy | Quintero Ruiz et al. (2017) |
| Thymol/p-cymene | Antigenotoxicity | Synergy | Quintero Ruiz et al. (2017) |
| Carvacrol/thymol | Against pathogenic bacteria | Synergy | Kissels et al. (2017) |
| Carvacrol/thymol | Against rhipicephalus microplus, R. sanguineus (Acari:Ixodidae) | Synergy | Araújo et al. (2016) |
| Carvacrol/thymol | Cytotoxicity | Synergy | Coccimiglio et al. (2016) |
| Carvacrol/1,8-cineole | Inhibition of staphylococcus aureus | Synergy | Honório et al. (2015) |
| Thymol/carvacrol | Antibacterial – against Brachyspira hyodysenteriae | Synergy | Maele et al. (2016) |
| Thymol/carvacrol | Against dermacentor nitens (Acari: Ixodidae) | Synergy | Novato et al. (2015) |
| Carvacrol/20 substances | Against culex quinquefasciatus (mosquito) | Synergy | Pavela (2015) |
| Borneol/20 substances | Against culex quinquefasciatus (mosquito) | Synergy | Pavela (2015) |
| Camphor/20 substances | Against culex quinquefasciatus (mosquito) | Synergy | Pavela (2015) |
| Thymol/carvacrol | Against cisplatin – induced nephrotoxicity | Synergy | EL-Sayed et al. (2015) |
| Carvacrol/thymol | Against culex pipiens pallens (Diptera: Culicidae) | Synergy | Ma et al. (2014) |
| Carvacrol/thymol | Against enterococcus faecalis | Synergy | Gutiérrez-Fernández et al. (2013) |
| Carvacrol/thymol | Antimicrobial | Synergy | Guarda et al. (2011) |
| Carvacrol/p-cymene | Antimicrobial | Synergy | Custódio et al. (2011) |
| Thymol/p-cymene | Antimicrobial | Synergy | Custódio et al. (2011) |
| Thymol/carvacrol | Against E. coli | Synergy | Pei et al. (2009) |
| Carvacrol/cymene | Against listeria monocytogenes | Synergy | Periago et al. (2004) |
| Carvacrol/p-cymene | Antibacterial | Synergy | Burt (2004) |
| Thymol/carvacrol | Antioxidant | Synergy | Vardar-Unlü et al. (2003) |
| Carvacrol/cymene | Antimicrobial | Synergy | Ultee et al. (2000) |
| Thymol/carvacrol | Antibacterial | Synergy | Didry et al. (1994) |
| 1,8-cineole/camphor | Insecticide | Synergy | Tak and Isman (2017) |
| Carvacrol/1,8-cineol | Against staphylococcus aureus | Synergy | Honório et al. (2015) |
| 1,8 cineol/terpinene-4-ol | Against botrytis cinerea | Synergy | Yu et al. (2015) |
| 1,8 cineol/camphor | Against trichoplusia ni | Synergy | Tak et al. (2016) |
| 1,8-cineol/carvacrol | Antibacteria | Synergy | De Sousa et al. (2012) |
| 1,8-cineol/caryophyllene oxide | Anticholinesterase | Synergy | Savelev et al. (2003) |
Safety Issues
Carvacrol, eucalyptol and β-caryophyllene, p-cymene, γ-terpinene, borneol and α-terpineol have been approved by the Food and Drug Administration (FDA) for food use (Code for Federal Reguation: 21CFR172.515). Moreover, the European Commission Implementing Regulation (EU No 872/2012) of October 1, 2012, based on the evaluations of EFSA, included carvacrol, eucalyptol and β-caryophyllene, in the Union’s List of Flavorings and Source Materials (Table 6). Their use is therefore permitted, in accordance with good agricultural and manufacturing practices and without given specific restrictions. This EU list takes also into consideration the reports of the Chemical Abstracts Service (CAS), the Joint FAO/WHO Expert Committee on Food additives (JECFA) and the Council of Europe. The rest of the constituents included in the essential oil’s composition of the mixture of Coridothymus capitatus, Salvia fruticosa and Origanum dictamnus (Duikler et al., 2015), are also included in the same list, concerning Food supplements among others, as defined in Directive 2002/46/EC of the European Parliament and the Council, excluding food supplements for infants and young children.
TABLE 6.
Chemical constituents of herb essential oil preparations, which are included in the Union List of Flavourings and Source Materials (Commission Implementing Regulation (EU) No 872/2012 of October 1, 2012) based on EFSA and/or JECFA evaluations.
| FL-no | Substance | CAS-number | JECFA-number | CoE-number | Restrictions of use | References |
|---|---|---|---|---|---|---|
| 04.031 | Carvacrol | 499–75-2 | 710 | 2055 | - | EFSA |
| 03.001 | 1,8-cineol | 470–82-6 | 1,234 | 182 | - | EFSA |
| 01.007 | β-caryophyllene | 87–44-5 | 1,324 | 2,118 | - | EFSA |
| 01.002 | p-cymene | 99–87-6 | 1,325 | 620 | - | EFSA |
| 01.020 | γ-terpinene | 99–85-4 | 1,340 | 11,025 | - | EFSA |
| 02.016 | Borneol | 507–70-0 | 1,385 | 64 | - | EFSA |
| 02.014 | α-terpineol | 98–55-5 | 366 | 62 | - | JECFA |
| 01.008 | β-myrcene | 123–35-3 | 1,327 | 2,197 | — | EFSA |
| 02.085 | cis-sabinene hydrate | 546–79-2 | 441 | 10,309 | — | JECFA |
| 02.085 | trans-sabinene hydrate | 546–79-2 | 441 | 10,309 | — | JECFA |
| 02.013 | Linalool | 78–70-6 | 356 | 61 | — | JECFA |
| 07.215 | Camphor | 464–49-3 | 1,395 | 140 | There is maximum dose per day | EFSA |
| 02.230 | δ-terpineol | 8,000–41-7 | — | — | — | EFSA |
| 02.072 | Terpinen-4-ol | 562–74-3 | 439 | 2,229 | — | JECFA |
| 04.006 | Thymol | 89–83-8 | 709 | 174 | — | EFSA |
| — | δ-terpinyl acetate | — | — | — | — | — |
| 16.043 | Caryophyllene oxide | 1,139–30-6 | 1,575 | 10,500 | — | EFSA |
| 08.014 | n-hexadecanoic acid | 57–10-3 | 115 | 14 | — | JECFA |
However, in addition to these beneficiary compounds, the extract of the three aromatic plants includes also 0.74 and 0.52% of cis- and trans-thujone respectively (Duijker et al., 2015). Alpha and beta thujone are, according to the Regulation EC No 1334/2008 of the European Parliament and of the Council of December 15, 2008, among the substances which shall not be added as such, to food or food supplements. Maximum concentrations of thujone, naturally present in flavorings and food ingredients with flavoring properties, have been introduced. According to Regulation EC No 1334/2008, “The maximum levels shall not apply where a compound food contains no added flavourings and the only food ingredients with flavoring properties which have been added are fresh, dried or frozen herbs and spices. After consultation with the Member States and the Authority, based on data made available by the Member States and on the newest scientific information, and taking into account the use of herbs and spices and natural flavoring preparations, the Commission, if appropriate, proposes amendments to this derogation.” EMA/HMPC (2010) reported that exposures in the range between 3 and 7 mg/day do not pose special concerns. For higher concentrations, a case-by-case benefit/risk assessment is necessary (Lachenmeier and Uebelacker, 2010; Pelkonen et al., 2013; Sotiropoulou et al., 2016). Finally, Dettling et al. (2004) showed that a single dose of 0.28 mg/kg in men (20 mg/70 kg) and of 0.24 mg/kg (17 mg/70 kg) in women provided “borderline relevance” of adverse effects, mainly related to perturbations in driving, operating machinery, etc.
A Case Study of the Use of Plant Extracts as a Pharmaceutical Product: Lessons Learned
The idea of using herb extracts for the treatment of upper respiratory infections was raised several years ago, when epidemiological observations revealed that people who consumed certain herbs infusions had low rates of respiratory infections and rarely suffered from common colds or influenza infections. The efficacy of the selected herbs was attributed to their antioxidant properties (Lionis et al., 1998). After years of in vitro and in vivo research, an essential oil combination was developed, based on the extracts of the three plants discussed in the present review (Coridοthymus capitatus, Salvia fruticosa and Origanum dictamnus) in extra-virgin olive oil (Duijker et al., 2015); we showed that this combination exerted a synergistic effect against viral upper respiratory tract infections, including influenza (Anastasaki et al., 2017). Moreover, an in vitro study revealed that this combination exhibited a remarkable direct antiviral activity against influenza A/H1N1 viral strains, influenza B and human rhinovirus 14 (HRV14), related to a defective trafficking of influenza A Nucleoprotein (NP) (Tseliou et al., 2019). In this respect, the “one drug, one target, one disease” approach (Zhou et al., 2016) was “violated.” Indeed, the combinatorial use of herbal preparations resulted in an synergistic effect, beyond the reported properties of each plant. Hence, here we address the case of the development and commercialization of a product, containing this essential oils’ combination.
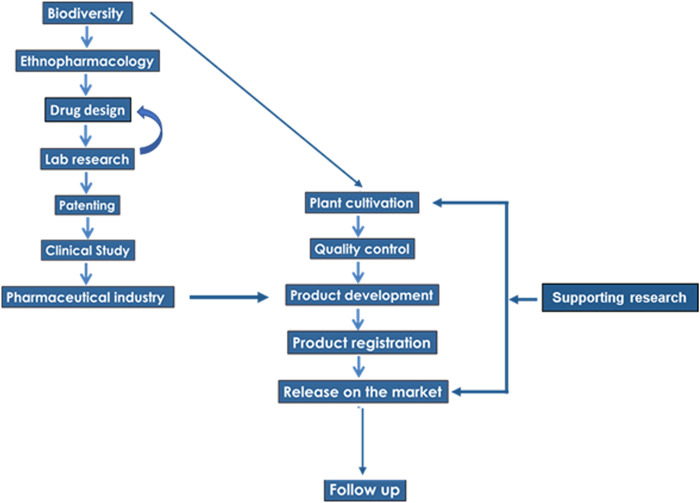
The scheme of the translational chain, from the step of biodiversity to the development of a commercial product, is presented in Figure 3. The first edge of the chain, leading from a plant extract to a final product, relies on the choice of plants, as well as the choice of secondary metabolites, whose biological activity is expected to ensure the desired health benefit (drug design). For example, the preparation containing the essential oils of Thymbra capitata (L.) Cav., Salvia fruticosa Mill. and Origanum dictamnus L. avoided plant extracts rich in alkaloids, as preliminary (Lionis et al., 1988) and clinical (Duijker et al., 2015) or laboratory evidence (Tseliou et al., 2019) documented a potent health benefit of the preparation.
FIGURE 3.
The flow-chart of a translational chain development.
Another part of the continuum is related to the implementation of a clinical study, which is one of the main obstacles in the translational process. In the presented case, it was valuable not only for the demonstration of an antiviral action, but also for the evaluation of the amelioration of the symptoms of the disease, due to supplementary actions of the substances involved in the combination of essential oils.
The supply of raw material is an important part of the translational chain. It is well known that several restrictions rule the natural collections (harvests) and trade of herbs and spices, especially within the framework of the EU environmental policy, as well as within the framework of the United Nations Convention on Biological Diversity. For example, 232 medicinal and aromatic plant species are listed on Appendix II of CITES, which regulates the international trade in endangered species. Worldwide, the increased needs for medicinal plants, in combination with the strengthening of the regulatory framework for their collection and trade, have caused supply constraints of medicinal plants of high ethnopharmacological interest, which introduced a serious constrain in the development and commercialization of plant-derived products (Amirkia and Heinrich, 2014). In the presented case, the plants are cultivated under controlled and monitored conditions, while local farmers have an important income from this agricultural activity. This activity constitutes a viable developmental axis for the local communities.
Recently, a new tool based on Ecological Niche Modeling has been developed, in order to support local farmers in the decision-making process, concerning the suitability of the area where their land is located, for cultivation of medicinal taxa of high ethnopharmacological interest (Bariotakis et al., 2019). A web-based, easy-to-use application was created in the framework of precision agriculture, where the predicted suitability scores for each area of interest can be made accessible to anyone, by the use of its GPS coordinates. So, in our case, the raw material is produced from organic farming, with Global GAP and precision agriculture.
Finally, it should be clear that the continuum in plant-based drug development, is not terminated at the step of release on the market. A follow-up of the final product, after its market release and additional supporting research is necessary. In the example case presented here, a follow up was made through a pragmatic prospective observational study (Anastasaki et al., 2017). In our opinion, this “supporting research” step is necessary as, in many cases, there are further research queries which need clarification even after the pharmaceutical evidence of an essential oil combination. In the example case, the effectiveness of the combination of essential oils in humans was documented (Duijker et al., 2015), but the mode of biological action was not understood. Limited in vitro data was available with ongoing research (Tseliou et al., 2019), providing a possible mechanism, at a cellular level.
In summary, two main lessons emerged from the development of a new pharmaceutical product: The first is resumed in the words “mind the gap,” a well-known phrase from the London underground, as it reveals the necessity of the bridging between successional steps, or successional links of the translational chain. The second concerns the time lag in bridging some successional steps. Indeed, in practice, many subsequent successive steps are fulfilled before others, imposing several loops in the development of the final preparation, which should be treated and resolved accordingly.
However, in spite of the successful use of the three plants, further research is required, in order to decipher additional multi-target action(s), in view of supplementary beneficial effects, which need to be investigated/screened not only in vitro but also in preclinical and clinical studies in the context of Evidence Based Medicine (Stavrou et al., 2013). The results of this screening are expected to clarify whether the ethnopharmacological gap, between reported traditional uses in ethnobotanical studies and the tested properties, is due to a noise of data collection in ethnobotanical practice or reflects underline biological activities, which should be incorporated in the formal therapeutic practice. Moreover, there are no preclinical or clinical evidence about the exposure of sensitive groups (i.e., pregnant women, children, etc.).
A logical subsequent step might be the development a drug, instead of a dietary supplement. A number of items are required for this shift, especially: 1) the repetition of phase I/II trial, with a greater number of participants, together with a detailed pharmacokinetic study of the major active compounds; 2) the performance of a phased III trial. Phase III studies, undertaken in large numbers of patients, often in multiple centers, assess real outcomes in a variety of patients, approximating the global population of patients, who will receive the drug. Their aim is to compare new treatments with existing ones and to demonstrate long-term safety and tolerance (Hobbs and McCarthy, 2009).
Finally, taking into consideration reported variability of the plants’ chemical fingerprint, at least in two of the three native Cretan herbs, which have been used for the development of the active extract (see Karousou et al., 2000; Karousou et al., 2005), it becomes clear that the biological variability in nature does not conform to the requirements for stability in the composition required by market regulations. Thus, further study is suggested concerning the interaction of environmental, chemical, genetic and epigenetic factors, for the quality assurance process. Last but not least, further studies on the cultivation and storage of the above species are required for the standardization and quality control measures, along the whole supply chain.
Author Contributions
EC, SP, CL contributed conception and design of the study, SP and EC wrote the first draft of the manuscript, CL, GS, MK and MB wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
SP, CL, and EC are inventors of a patent (WO2010GB01836 20,100,929) on the use of the three examined plants for combating upper respiratory tract infections.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Funding
This work was partially supported by a grant from OLVOS Pharmaceuticals. During the peer review of this manuscript, an additional study of our group was published (Kalyvianaki K, Malamos P, Mastrodimou N, Manoura-Zonou I, Vamvoukaki R, Notas G, Malliaraki N, Moustou E, Tzardi M, Pirintsos S, Lionis C, Sourvinos G, Castanas E and Kampa M. Toxicity evaluation of an essential oil mixture from the Cretan herbs Thyme, greek sage and cretan ddittany, npj Science of Food (2020) 4:20; doi:10.1038/s41538-020-00080-1, suggesting the absence of accute or sub-chronic toxicity of the three herb preparation.
Acknowledgments
We are grateful to Michael Heinrich for his valuable comments on an earlier version of this manuscript.
References
- Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. (2007). Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 110, 294–304. 10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Al-Khalil S. (1995). A survey of plants used in Jordanian traditional medicine. Int. J. Pharmacogn. 33, 317–323. 10.3109/13880209509065385. [DOI] [Google Scholar]
- Al-Qura’n S. (2009). Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 123, 45–50. [DOI] [PubMed] [Google Scholar]
- Ali-Shtayeh M. S., Yaghmour R. M.-R., Faidi Y. R., Salem K., Al-Nuri M. A. (1998). Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 60, 265–271. 10.1016/s0378-8741(97)00153-0. [DOI] [PubMed] [Google Scholar]
- Ali-Shtayeh M. S., Yaniv Z., Mahajna J. (2000). Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J. Ethnopharmacol. 73, 221–232. 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- Allahghadri T., Rasooli I., Owlia P., Nadooshan M. J., Ghazanfari T., Taghizadeh M., et al. (2010). Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J. Food Sci. 75, H54–H61. 10.1111/j.1750-3841.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- Altmann R. E., Campbell E. L., Johnston G. A. R. (2005). (+)- and (−)-borneol: efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem. Pharmacol. 69, 1101–1111. 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Alzweiri M., Sarhan A. A., Mansi K., Hudaib M., Aburjai T. (2011). Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 137, 27–35. 10.1016/j.jep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Amirkia V., Heinrich M. (2014). Alkaloids as drug leads — a predictive structural and biodiversity-based analysis. Phytochem. Lett. 10, xlviii–liii. 10.1016/j.phytol.2014.06.015 [DOI] [Google Scholar]
- Anastasaki M., Bertsias A., Pirintsos S. A., Castanas E., Lionis C. (2017). Post-market outcome of an extract of traditional Cretan herbs on upper respiratory tract infections: a pragmatic, prospective observational study. BMC Compl. Alternative Med. 17, 466 10.1186/s12906-017-1978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade e Silva H. G. (2012). Drug synergy-mechanisms and methods of analysis, toxicity and drug testing. Rijeka: InTech, The German University of Cairo. [Google Scholar]
- Araújo L. X., Novato T. P. L., Zeringota V., Maturano R., Melo D., Da Silva B. C., et al. (2016). Synergism of thymol, carvacrol and eugenol in larvae of the cattle tick, Rhipicephalus microplus, and brown dog tick, Rhipicephalus sanguineus. Med. Vet. Entomol. 30, 377–382. 10.1111/mve.12181. [DOI] [PubMed] [Google Scholar]
- Ay G., Bertsias A., Symvoulakis E. K., Moschandreas J., Malliaraki N., Derdas S. P., et al. (2015). Reporting effectiveness of an extract of three traditional Cretan herbs on upper respiratory tract infection: results from a double-blind randomized controlled trial. J. Ethnopharmacol. 163, 157–166. 10.1016/j.jep.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariotakis M., Georgescu L., Laina D., Oikonomou I., Ntagounakis G., Koufaki M.-I., et al. (2019). From wild harvest towards precision agriculture: use of ecological Niche modelling to direct potential cultivation of wild medicinal plants in Crete. Sci. Total Environ. 694, 133681 10.1016/j.scitotenv.2019.133681. [DOI] [PubMed] [Google Scholar]
- Ben Arfa A., Combes S., Preziosi-Belloy L., Gontard N., Chalier P. (2006). Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 43, 149–154. 10.1111/j.1472-765x.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- Bernardes W. A., Lucarini R., Tozatti M. G., Bocalon Flauzino L. G., Souza M. G. M., Turatti I. C. C., et al. (2010). Antibacterial activity of the essential oil from Rosmarinus officinalis and its major components against oral pathogens. Z. Naturforsch. C Biosci. 65, 588–593. 10.1515/znc-2010-9-1009. [DOI] [PubMed] [Google Scholar]
- Burt S. (2004). Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 94, 223–253. 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Carazza J., Ribeiro M. V., Silva F., Rodrigues Machado S. M., Sousa M. (2011). The essential oils component p-cymene induces proton leak through Fo-ATP synthase and uncoupling of mitochondrial respiration. JEP (J. Environ. Psychol.) 3, 69–76. 10.2147/jep.s16387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebib M., Johnston G. A. R. (2000). GABA-activated ligand gated ion channels: medicinal chemistry and molecular biology. J. Med. Chem. 43, 1427–1447. 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- Chen C., Yang F.-Q., Zhang Q., Wang F.-Q., Hu Y.-J., Xia Z.-N. (2015). Natural products for antithrombosis, Evid. base Compl. Alternative Med., 2015, 1, 10.1155/2015/876426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccimiglio J., Alipour M., Jiang Z. H., Gottardo C., Suntres Z. (2016). Antioxidant, antibacterial, and cytotoxic activities of the ethanolic Origanum vulgare extract and its major constituents. Oxid Med Cell Longev 2016 10.1155/2016/1404505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa P. R. C., Miranda R. R. S., Duarte L. P., Silva G. D. F., Filho S. A. V., Okuma A. A., et al. (2012). Antimicrobial activity of synthetic bornyl benzoates againstTrypanosoma cruzi. Pathog. Glob. Health 106, 107–112. 10.1179/2047773212y.0000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemon M., Verge S., Dumas M., Soulet S., Nay B., Arnaudinaud V., et al. (2002). Dietary antioxidants, peroxidation and cardiovascular risks. J. Nutr. Health Aging 6, 209–223. [PubMed] [Google Scholar]
- Dafni A., Yaniv Z., Palevitch D. (1984). Ethnobotanical survey of medicinal plants in northern Israel. J. Ethnopharmacol. 10, 295–310. 10.1016/0378-8741(84)90017-5. [DOI] [PubMed] [Google Scholar]
- Dauqan E. M., Abdullah A. (2017). Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. Biotechnol. 5, 017–022. [Google Scholar]
- de Sousa J. P., de Azerêdo G. A., de Araújo Torres R., da Silva Vasconcelos M. A., da Conceição M. L., de Souza E. L. (2012). Synergies of carvacrol and 1,8-cineole to inhibit bacteria associated with minimally processed vegetables. Int. J. Food Microbiol. 154, 145–151. 10.1016/j.ijfoodmicro.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Dettling A., Grass H., Schuff A., Skopp G., Strohbeck-Kuehner P., Haffner H. T. (2004). Absinthe: attention performance and mood under the influence of thujone. J. Stud. Alcohol 65, 573–581. 10.15288/jsa.2004.65.573. [DOI] [PubMed] [Google Scholar]
- Didry N., Dubreuil L., Pinkas M. (1994). Activity of thymol, carvacrol, cinnamaldehyde and eugenol on oral bacteria. Pharm. Acta Helv. 69, 25–28. 10.1016/0031-6865(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Dimopoulos P., Raus T., Bergmeier E., Constantinidis T., Iatrou G., Kokkini S., et al. (2013). “An annotated checklist. – Berlin Englera 31,” in Vascular plants of Greece: botanischer Garten und Botanisches Museum Berlin-Dahlem. Athens: Hellenic Botanical Society. [Google Scholar]
- Eissa T. A. F., Palomino O. M., Carretero M. E., Gómez-Serranillos M. P. (2014). Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 151, 317–332. 10.1016/j.jep.2013.10.041. [DOI] [PubMed] [Google Scholar]
- El-Sayed E. M., Abd-Allah A. R., Mansour A. M., El-Arabey A. A. (2015). Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 29, 165–172. 10.1002/jbt.21681. [DOI] [PubMed] [Google Scholar]
- EMA/HMPC (2010). Public statement on the use of herbal medicinal products containing thujone. EMA/HMPC/732886/2010 . [Google Scholar]
- Euro+Med (2019). Euro+Med PlantBase - the information resource for Euro-Mediterranean plant diversity. Available at: http://ww2.bgbm.org/EuroPlusMed/Published on the Internet (Accessed June 2017).
- Fabricant D. S., Farnsworth N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 109 (Suppl. 1), 69–75. 10.2307/3434847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding J., Turland N. (2005). Flowers of Crete. Editor: B. Mathiew (London, England: Kev Royal Botanic Gardens). [Google Scholar]
- Fischer J., Dethlefsen U. (2013). Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough 9, 25 10.1186/1745-9974-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Beltrán J. M., Esteban M. A. (2016). Properties and applications of plants of Origanum sp. Genus. SM Journal of Biology 2, 1006. [Google Scholar]
- Gertsch J., Leonti M., Raduner S., Racz I., Chen J.-Z., Xie X.-Q., et al. (2008). Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A. 105, 9099–9104. 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda A., Rubilar J. F., Miltz J., Galotto M. J. (2011). The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 146, 144–150. 10.1016/j.ijfoodmicro.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Guenther A., Zimmerman P., Wildermuth M. (1994). Natural volatile organic compound emission rate estimates for U.S. woodland landscapes. Atmos. Environ. 28, 1197–1210. 10.1016/1352-2310(94)90297-6. [DOI] [Google Scholar]
- Guimarães A. G., Quintans J. S. S., Quintans-Júnior L. J. (2013). Monoterpenes with analgesic activity-A systematic review. Phytother Res. 27, 1–15. 10.1002/ptr.4686. [DOI] [PubMed] [Google Scholar]
- Guindon J., Hohmann A. G. (2008). Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 153, 319–334. 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürdal B., Kültür Ş. (2013). An ethnobotanical study of medicinal plants in Marmaris (Muğla, Turkey). J. Ethnopharmacol. 146, 113–126. 10.1016/j.jep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Fernández J., García-Armesto M. R., Álvarez-Alonso R., Del Valle P., de Arriaga D., Rúa J. (2013). Antimicrobial activity of binary combinations of natural and synthetic phenolic antioxidants against Enterococcus faecalis . J. Dairy Sci. 96, 4912–4920. 10.3168/jds.2013-6643. [DOI] [PubMed] [Google Scholar]
- Hanlidou E., Karousou R., Kleftoyanni V., Kokkini S. (2004). The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 91, 281–299. 10.1016/j.jep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Hardy K. (2019). Paleomedicine and the use of plant secondary compounds in the Paleolithic and Early Neolithic. Evol. Anthropol. 28, 60–71. 10.1002/evan.21763. [DOI] [PubMed] [Google Scholar]
- Hassan S. B., Gali-Muhtasib H., Göransson H., Larsson R. (2010). Alpha terpineol: a potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 30, 1911–1919. [PubMed] [Google Scholar]
- Held S., Schieberle P., Somoza V. (2007). Characterization of α-terpineol as an anti-inflammatory component of orange juice by in Vitro studies using oral buccal cells. J. Agric. Food Chem. 55, 8040–8046. 10.1021/jf071691m. [DOI] [PubMed] [Google Scholar]
- Hobbs M., McCarthy M. W. (2009). “Clinical trials,” in Oxford American handbook of clinical pharmacy. Editors McCarthy M. W., Kockler D. R. (New York: Oxford University Press; ), 115–126. [Google Scholar]
- Honda G., Yeşilada E., Tabata M., Sezik E., Fujita T., Takeda Y., et al. (1996). Traditional medicine in Turkey VI. Folk medicine in west Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydin provinces. J. Ethnopharmacol. 53, 75–87. 10.1016/s0378-8741(96)01426-2. [DOI] [PubMed] [Google Scholar]
- Hotta M., Nakata R., Katsukawa M., Hori K., Takahashi S., Inoue H. (2010). Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J. Lipid Res. 51, 132–139. 10.1194/jlr.m900255-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudaib M., Mohammad M., Bustanji Y., Tayyem R., Yousef M., Abuirjeie M., et al. (2008). Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 120, 63–71. 10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Jaradat N. A., Ayesh O. I., Anderson C. (2016). Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 182, 57–66. 10.1016/j.jep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Juergens U. R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. (2003). Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir. Med. 97, 250–256. 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- Juergens U. R., Engelen T., Racké K., Stöber M., Gillissen A., Vetter H. (2004). Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm. Pharmacol. Therapeut. 17, 281–287. 10.1016/j.pupt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Jun Y. S., Kang P., Min S. S., Lee J.-M., Kim H.-K., Seol G. H. (2013). Effect of Eucalyptus oil inhalation on pain and inflammatory responses after total knee replacement: a randomized clinical trial, Evid. base Compl. Alternative Med., 2013, 1, 10.1155/2013/502727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karousou R., Deirmentzoglou S. (2011). The herbal market of Cyprus: traditional links and cultural exchanges. J. Ethnopharmacol. 133, 191–203. 10.1016/j.jep.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Karousou R., Hanlidou E., Kokkini S. (2000). “The Sage plants of Greece: distribution and infraspecific variation,” inThe Sage. Editor Kintzios S. E. (Singapore: Harwood Academic Publishers; ), 27–46. [Google Scholar]
- Karousou R., Koureas D. N., Kokkini S. (2005). Essential oil composition is related to the natural habitats: Coridothymus capitatus and Satureja thymbra in NATURA 2000 sites of Crete. Phytochemistry 66, 2668–2673. 10.1016/j.phytochem.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Karousou R., Vokou D., Kokkini S. (1998). Variation ofSalvia fruticosaEssential oils on the island of Crete (Greece). Plant Biol. 111, 250–254. 10.1111/j.1438-8677.1998.tb00705.x. [DOI] [Google Scholar]
- Kehrl W., Sonnemann U., Dethlefsen U. (2004). Therapy for acute nonpurulent rhinosinusitis with cineole: results of a double-blind, randomized, placebo-controlled trial. Laryngoscope 114, 738–742. 10.1097/00005537-200404000-00027. [DOI] [PubMed] [Google Scholar]
- Kim J. M., Marshall M., Cornell J. A., Iii J. F. P., III, Wei C. I. (1995). Antibacterial activity of carvacrol, citral, and geraniol against Salmonella typhimurium in culture medium and on fish cubes. J. Food Sci. 60, 1364–1368. 10.1111/j.1365-2621.1995.tb04592.x. [DOI] [Google Scholar]
- Kim K.-Y., Chung H.-J. (2000). Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 48, 1269–1272. 10.1021/jf9900229. [DOI] [PubMed] [Google Scholar]
- Kim K. Y., Seo H. J., Min S. S., Park M., Seol G. H. (2014). A randomized clinical trial. The effect of 1.8-cineole inhalation on preoperative anxiety. Evidence-Based Complementary Altern. Med. 2014 10.1155/2014/820126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey S. G., Long J. Z., Cravatt B. F., Lichtman A. H. (2010). Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J. Pain 11, 1420–1428. 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirimer N., Başer K. H. C., Tümen G. (1995). Carvacrol-rich plants in Turkey. Chem. Nat. Compd. 31, 37–41. 10.1007/bf01167568. [DOI] [Google Scholar]
- Kiskó G., Roller S. (2005). Carvacrol and p-cymene inactivate Escherichia coli O157: H7 in apple juice. BMC Microbiol. 5, 36 10.1186/1471-2180-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissels W., Wu X., Santos R. R. (2017). Short communication: interaction of the isomers carvacrol and thymol with the antibiotics doxycycline and tilmicosin: in vitro effects against pathogenic bacteria commonly found in the respiratory tract of calves. J. Dairy Sci. 100, 970–974. 10.3168/jds.2016-11536. [DOI] [PubMed] [Google Scholar]
- Klauke A.-L., Racz I., Pradier B., Markert A., Zimmer A. M., Gertsch J., et al. (2014). The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol 24, 608–620. 10.1016/j.euroneuro.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Kokkini S., Vokou D. (1989). Carvacrol-rich plants in Greece. Flavour Fragrance J. 4, 1–7. 10.1002/ffj.2730040102. [DOI] [Google Scholar]
- Kotan R., Kordali S., Cakir A. (2007). Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Z. Naturforsch. C Biosci. 62, 507–513. 10.1515/znc-2007-7-808. [DOI] [PubMed] [Google Scholar]
- Lachenmeier D. W., Uebelacker M. (2010). Risk assessment of thujone in foods and medicines containing sage and wormwood – evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 58, 437–443. 10.1016/j.yrtph.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Landa P., Kokoska L., Pribylova M., Vanek T., Marsik P. (2009). In vitro anti-inflammatory activity of carvacrol: inhibitory effect on COX-2 catalyzed prostaglandin E2 biosynthesisb. Arch Pharm. Res. (Seoul) 32, 75–78. 10.1007/s12272-009-1120-6. [DOI] [PubMed] [Google Scholar]
- Lardos A., Heinrich M. (2013). Continuity and change in medicinal plant use: the example of monasteries on Cyprus and historical iatrosophia texts. J. Ethnopharmacol. 150, 202–214. 10.1016/j.jep.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Lardos A. (2006). The botanical materia medica of the Iatrosophikon-A collection of prescriptions from a monastery in Cyprus. J. Ethnopharmacol. 104, 387–406. 10.1016/j.jep.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Lev E., Amar Z. (2000). Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 72, 191–205. 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Lev E., Amar Z. (2002). Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J. Ethnopharmacol. 82, 131–145. 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Lev E. (2002). Reconstructed materia medica of the Medieval and Ottoman al-Sham. J. Ethnopharmacol. 80, 167–179. 10.1016/s0378-8741(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Li Y.-H., Sun X.-P., Zhang Y.-Q., Wang N.-S. (2008). The antithrombotic effect of borneol related to its anticoagulant property. Am. J. Chin. Med. 36, 719–727. 10.1142/s0192415x08006181. [DOI] [PubMed] [Google Scholar]
- Lima M. d. S., Quintans-Júnior L. J., de Santana W. A., Martins Kaneto C., Pereira Soares M. B., Villarreal C. F. (2013). Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur. J. Pharmacol. 699, 112–117. 10.1016/j.ejphar.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Lima‐Accioly P. M., Lavor‐Porto P. R., Cavalcante F. S., Magalhães P. J. C., Lahlou S., Morais S. M. et al. (2006). Essential oil of Croton nepetaefolius and its main constituent, 1,8‐cineole, block excitability of rat sciatic nerve in vitro. Clin. Exp. Pharmacol. Physiol. 33, 1158--1163. 10.1111/j.1440-1681.2006.04494.x [DOI] [PubMed] [Google Scholar]
- Lionis C., Faresjö Å., Skoula M., Kapsokefalou M., Faresjö T. (1998). Antioxidant effects of herbs in Crete. Lancet 352, 1987–1988. 10.1016/s0140-6736(05)61333-5. [DOI] [PubMed] [Google Scholar]
- Lücker J., El Tamer M. K., Schwab W., Verstappen F. W. A., van der Plas L. H. W., Bouwmeester H. J., et al. (2002). Monoterpene biosynthesis in lemon (Citrus limon). FEBS J. 269, 3160–3171. 10.1046/j.1432-1033.2002.02985.x. [DOI] [PubMed] [Google Scholar]
- Ma W.-B., Feng J.-T., Jiang Z.-L., Wu H., Ma Z.-Q., Zhang X. (2014). Fumigant activity of eleven essential oil compounds and their selected binary mixtures against Culex pipiens pallens (Diptera: Culicidae). Parasitol. Res. 113, 3631–3637. 10.1007/s00436-014-4028-0. [DOI] [PubMed] [Google Scholar]
- Maele L. V., Heyndrickx M., De Pauw N., Verlinden M., Haesebrouck F., Martel A., et al. (2016). In vitro susceptibility of Brachyspira hyodysenteriae to organic acids and essential oil components. J. Vet. Med. Sci. 78, 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manayi A., Nabavi S. M., Daglia M., Jafari S. (2016). Natural terpenoids as a promising source for modulation of GABAergic system and treatment of neurological diseases. Pharmacol. Rep. 68, 671–679. 10.1016/j.pharep.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Martínez-Francés V., Rivera D., Heinrich M., Obón C., Ríos S. (2015). An ethnopharmacological and historical analysis of “Dictamnus”, a European traditional herbal medicine. J. Ethnopharmacol. 175, 390–406. 10.1016/j.jep.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Mastelic J., Jerkovic I., Blazevic I., Poljak-Blaži M., Borović S., Ivančić-Baće I., et al. (2008). Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 56, 3989–3996. [DOI] [PubMed] [Google Scholar]
- Milos M., Makota D. (2012). Investigation of antioxidant synergisms and antagonisms among thymol, carvacrol, thymoquinone and p-cymene in a model system using the Briggs-Rauscher oscillating reaction. Food Chem. 131, 296–299. 10.1016/j.foodchem.2011.08.042. [DOI] [Google Scholar]
- Naghibi F., Mosaddegh M., Mohammadi Motamed M., Ghorbani A. (2005). Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iran. J. Pharm. Res. (IJPR) 4, 63–79. [Google Scholar]
- Novato T. P. L., Araújo L. X., de Monteiro C. M. O., Maturano R., Senra T. d. O. S., da Silva Matos R., et al. (2015). Evaluation of the combined effect of thymol, carvacrol and ( E )-cinnamaldehyde on Amblyomma sculptum (Acari: ixodidae) and Dermacentor nitens (Acari: ixodidae) larvae. Vet. Parasitol. 212, 331–335. 10.1016/j.vetpar.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Oliveira M. G., Marques R. B., Santana M. F., Santos A. B., Brito F. A., Barreto E. O. et al. (2012). α‐Terpineol reduces mechanical hypernociception and inflammatory response. Basic & Clin. Pharm. & Toxicol. 111, 120--125. 10.1111/j.1742-7843.2012.00875.x [DOI] [PubMed] [Google Scholar]
- Palevitch D., Yaniv Z., Dafni A., Simon J. E. (1986). “Medicinal plants of Israel: an ethnobotanical survey,” in Herbs, spices and medicinal plants. Editor Craker L. A. (Phoenix, AZ: Oryx Press; ), 281–345. [Google Scholar]
- Pavela R. (2015). Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 114, 3835–3853. 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- Pei R. S., Zhou F., Ji B. P., Xu J. (2009). Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 74, 379–383. 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Abass K., Wiesner J. (2013). Thujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regul. Toxicol. Pharmacol. 65, 100–107. 10.1016/j.yrtph.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Periago P. M., Delgado B., Fernández P. S., Palop A. (2004). Use of carvacrol and cymene to control growth and viability of Listeria monocytogenes cells and predictions of survivors using frequency distribution functions. J. Food Protect. 67, 1408–1416. 10.4315/0362-028x-67.7.1408. [DOI] [PubMed] [Google Scholar]
- Proudfoot C. J., Garry E. M., Cottrell D. F., Rosie R., Anderson H., Robertson D. C., et al. (2006). Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 16, 1591–1605. 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Quintans‐Júnior L. J., Oliveira M. G. B., Santana M. F., Santana M. T., Guimarães A. G., Siqueira J. S. et al. (2011) α‐Terpineol reduces nociceptive behavior in mice. Pharm. Biol. 49, 583--586. 10.3109/13880209.2010.529616 [DOI] [PubMed] [Google Scholar]
- Rattanachaikunsopon P., Phumkhachorn P. (2010b). Assessment of synergistic efficacy of carvacrol and cymene against Edwardsiella tarda in vitro and in Tilapia (Oreochromis niloticus). Afr. J. Microbiol. Res. 4, 420–425. [Google Scholar]
- Rattanachaikunsopon P., Phumkhachorn P. (2010a). Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. J. Biosci. Bioeng. 110, 614–619. 10.1016/j.jbiosc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Ravishankar S., Zhu L., Reyna-Granados J., Law B., Joens L., Friedman M. (2010). Carvacrol and cinnamaldehyde inactivate antibiotic-resistant Salmonella enterica in buffer and on celery and oysters. J. Food Protect. 73, 234–240. 10.4315/0362-028x-73.2.234. [DOI] [PubMed] [Google Scholar]
- Renaud S. (1995). Le régime santé. Paris, France: Editions: Odile Jacob. [Google Scholar]
- Ribeiro T. P., Porto D. L., Menezes C. P., Antunes A. A., Silva D. F., De Sousa D. P., et al. (2010). Unravelling the cardiovascular effects induced by alpha-terpineol: a role for the nitric oxide-cGMP pathway. Clin. Exp. Pharmacol. Physiol. 37, 811–816. 10.1111/j.1440-1681.2010.05383.x. [DOI] [PubMed] [Google Scholar]
- Rivera D., Obon C., Cano F. (1994). The botany, history and traditional uses of three-lobed sage (Salvia fruticosa miller) (Labiatae). Econ. Bot. 48, 190–195. 10.1007/bf02908216. [DOI] [Google Scholar]
- Said O., Khalil K., Fulder S., Azaizeh H. (2002). Ethnopharmacological survey of medicinal herbs in Israel, the Golan heights and the west bank region. J. Ethnopharmacol. 83, 251–265. 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- Santos F. A., Rao V. S. N. (2000). Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 14, 240–244. . [DOI] [PubMed] [Google Scholar]
- Sargin S. A. (2015). Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey. J. Ethnopharmacol. 173, 105–126. [DOI] [PubMed] [Google Scholar]
- Sato K., Krist S., Buchbauer G. (2007). Antimicrobial effect of vapours of geraniol, (R)-(-)-linalool, terpineol,γ-terpinene and 1,8-cineole on airborne microbes using an airwasher. Flavour Fragrance J. 22, 435–437. 10.1002/ffj.1818. [DOI] [Google Scholar]
- Savelev S., Okello E., Perry N. S. L., Wilkins R. M., Perry E. K. (2003). Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 75, 661–668. 10.1016/s0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Sivropoulou A., Papanikolaou E., Nikolaou C., Kokkini S., Lanaras T., Arsenakis M. (1996). Antimicrobial and cytotoxic activities ofOriganumEssential oils. J. Agric. Food Chem. 44, 1202–1205. 10.1021/jf950540t. [DOI] [Google Scholar]
- Song H., Schacher C., Dethlefsen U. (2009). Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: a placebo-controlled double-blind trial. Respir. Res. 10, 69 10.1186/1465-9921-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou Ν.-S., Kokkini M., P.Megremi S.-F., Daferera D., Skotti E., Kimbaris A., et al. (2016). Determination of Α- and Β-thujone in wormwood and sage infusions of Greek flora and estimation of their average toxicity. Curr. Res. Nutr. Food Sci. 4, 152–160. 10.12944/crnfsj.4.special-issue-october.21. [DOI] [Google Scholar]
- Souma H., Sağdiç O., Özkan G., Karadoğan T. (2004). Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Contr. 15, 169–172. 10.1016/s0956-7135(03)00028-8. [DOI] [Google Scholar]
- Stavrou A., Challoumas D., Dimitrakakis G. (2013). Archibald Cochrane (1909-1988): the father of evidence-based medicine. Interact. Cardiovasc. Thorac. Surg. 18, 121–124. 10.1093/icvts/ivt451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanaki A., Cook C. M., Lanaras T., Kokkini S. (2016). The Oregano plants of Chios Island (Greece): essential oils of Origanum onites L. growing wild in different habitats. Ind. Crop. Prod. 82, 107–113. 10.1016/j.indcrop.2015.11.086. [DOI] [Google Scholar]
- Suntres Z. E., Coccimiglio J., Alipour M. (2015). The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nutr. 55, 304–318. 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- Tak J.-H., Jovel E., Isman M. B. (2016). Comparative and synergistic activity of Rosmarinus officinalis L. essential oil constituents against the larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni (Lepidoptera: noctuidae). Pest Manag. Sci. 72, 474–480. 10.1002/ps.4010. [DOI] [PubMed] [Google Scholar]
- Tak J. H., Isman M. B. (2017). Penetration-enhancement underlies synergy of plant essential oil terpenoids as insecticides in the cabbage looper, Trichoplusia ni. Sci. Rep. 7, 42432 10.1038/srep42432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi V. G., Bezerra J., Souza G. T., Carvalho R. J., Gomes-Neto N. J., Figueiredo R. C., et al. (2015). Inhibition of Staphylococcus aureus cocktail using the synergies of oregano and rosemary essential oils or carvacrol and 1, 8-cineole. Front. Microbiol. 6, 1223 10.3389/fmicb.2015.01223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Garg H., Geng Y.-J., Bryan N. S. (2009). Nitric oxide bioactivity of traditional Chinese medicines used for cardiovascular indications. Free Radic. Biol. Med. 47, 835–840. 10.1016/j.freeradbiomed.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Tarantilis R. H. L., Cardoso M. S. P., Menezes C. T., Silva J. P., De Sousa D. P., Batista J. S. (2011) .Gastroprotective activity of α-terpineol in two experimental models of gastric ulcer in rats. Daru: J. Faculty Phar. 19, 277. [PMC free article] [PubMed] [Google Scholar]
- Tesche S., Metternich F., Sonnemann U., Engelke J.-C., Dethlefsen U. (2008). The value of herbal medicines in the treatment of acute non-purulent rhinosinusitis. Eur. Arch. Oto-Rhino-Laryngol. 265, 1355 10.1007/s00405-008-0683-z. [DOI] [PubMed] [Google Scholar]
- Tseliou M., Pirintsos S. A., Lionis C., Castanas E., Sourvinos G. (2019). Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. f., Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 17-18, 100288 10.1016/j.hermed.2019.100288. [DOI] [Google Scholar]
- Ultee A., Bennik M. H. J., Moezelaar R. (2002). The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus . AEM 68, 1561–1568. 10.1128/aem.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A., Kets E. P. W., Smid E. J. (1999). Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus . Appl. Environ. Microbiol. 65, 4606–4610. 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultee A., Slump R. A., Steging G., Smid E. J. (2000). Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Protect. 63, 620–624. 10.4315/0362-028x-63.5.620. [DOI] [PubMed] [Google Scholar]
- Vardar-Ünlü G., Candan F., Sökmen A., Daferera D., Polissiou M., Sökmen M., et al. (2003). Antimicrobial and Antioxidant Activity of the Essential Oil and Methanol Extracts ofThymus pectinatusFisch. et Mey. Var.pectinatus(Lamiaceae). J. Agric. Food Chem. 51, 63–67. 10.1021/jf025753e. [DOI] [PubMed] [Google Scholar]
- Vartiainen O. (1950). The anthelmintic effects of thymol and p-cymene; a pharmacological and clinical study, with special consideration of the fish tapeworm disease. Ann. Med. Intern. Fenn. 39, 1--87. [PubMed] [Google Scholar]
- Vázquez F. M., Suarez M. A., Pérez A. (1997). Medicinal plants used in the Barros area, Badajoz province (Spain). J. Ethnopharmacol. 55, 81–85. 10.1016/s0378-8741(96)01491-2. [DOI] [PubMed] [Google Scholar]
- Vrachnakis T. (2003). Trichomes of Origanum dictamnus L. (Labiatae). Phyton 43, 109–133. [Google Scholar]
- Wagner H., Wierer R. (1986). In vitro-Hemmung der Prostaglandin-Biosynthese durch etherische Öle und phenolische Verbindungen. Planta Med. 52, 184–187. 10.1055/s-2007-969117. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang D., Hu J., Jia Q., Xu W., Su D., et al. (2017). A clinical and mechanistic study of topical borneol‐induced analgesia. EMBO Mol. Med. 9, 802–815. 10.15252/emmm.201607300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth H., Dethlefsen U. (2012). Patients with asthma benefit from concomitant therapy with cineole: a placebo-controlled, double-blind trial. J. Asthma 49, 849–853. 10.3109/02770903.2012.717657. [DOI] [PubMed] [Google Scholar]
- Xu H., Delling M., Jun J. C., Clapham D. E. (2006). Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 9, 628–635. 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhang Z., Li S., Ye X., Li X., He K. (2014). Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia 92, 133–147. 10.1016/j.fitote.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Yu D., Wang J., Shao X., Xu F., Wang H. (2015). Antifungal modes of action of tea tree oil and its two characteristic components againstBotrytis cinerea. J. Appl. Microbiol. 119, 1253–1262. 10.1111/jam.12939. [DOI] [PubMed] [Google Scholar]
- Yuan G., Wahlqvist M., He G., Yang M., Li D. (2006). Natural products and anti-inflammatory activity. Asia Pac. J. Clin. Nutr. 15, 143. [PubMed] [Google Scholar]
- Zhou X., Seto S. W., Chang D., Kiat H., Razmovski-Naumovski V., Chan K., et al. (2016). Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front. Pharmacol. 7, 201 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]