Neuroendocrine, immune, and structural cells establish circadian circuits that optimize immune and physiological responses. In this review, Palomino-Segura and Hidalgo discuss the architecture of these circuits and speculate that immune cells can be a source of circadian signals in physiology.
Abstract
Immune responses are gated to protect the host against specific antigens and microbes, a task that is achieved through antigen- and pattern-specific receptors. Less appreciated is that in order to optimize responses and to avoid collateral damage to the host, immune responses must be additionally gated in intensity and time. An evolutionary solution to this challenge is provided by the circadian clock, an ancient time-keeping mechanism that anticipates environmental changes and represents a fundamental property of immunity. Immune responses, however, are not exclusive to immune cells and demand the coordinated action of nonhematopoietic cells interspersed within the architecture of tissues. Here, we review the circadian features of innate immunity as they encompass effector immune cells as well as structural cells that orchestrate their responses in space and time. We finally propose models in which the central clock, structural elements, and immune cells establish multidirectional circadian circuits that may shape the efficacy and strength of immune responses and other physiological processes.
Innate immunity and the circadian system
Every 24 h, organisms on Earth receive periodic signals from their environment, such as sunlight or availability of food. Virtually all lifeforms on our planet have evolved mechanisms to synchronize their physiology to these predictable cues (Gerhart-Hines and Lazar, 2015). These variations, known as circadian oscillations, govern most physiological activities (Scheiermann et al., 2018) and are the result of a collection of circadian clocks present in the central nervous system and in peripheral tissues (Dibner et al., 2010). In mammals, the master pacemaker of this network is the central clock located in the suprachiasmatic nucleus (SCN) of the brain, which integrates photic cues and synchronizes peripheral clocks through neural and humoral mediators (Dibner et al., 2010). The time-sensing clockwork is also cell autonomous, as it relies on the presence of a molecular clock expressed in virtually every cell (Reppert and Weaver, 2002), and is composed of a set of clock proteins that generate an autoregulatory transcriptional network with interlocked feedback loops. At the core of this central loop, the transcription factors Bmal1 and Clock form a heterodimer that binds E-box sequences and promotes the expression of its own repressors, whose degradation times establish the ∼24-h periodicity (Schibler, 2006).
Clock genes and related transcription factors have been described in all cells of the innate and adaptive immune systems (Silver et al., 2012a; Druzd et al., 2017; Adrover et al., 2019) and directly regulate many aspects of immunity, especially those related with frontline defense by innate immune cells (Scheiermann et al., 2012). However, as innate immune cells are growingly appreciated to accomplish homeostatic tasks (Aroca-Crevillén et al., 2020; Lavin and Merad, 2013), it is easy to foresee that, in addition to their own molecular clock, they need to integrate signals from a wide variety of sources. For example, circadian clocks in endothelial cells (ECs) control the expression of trafficking factors that influence the migration of leukocytes in the steady state or during inflammation (Scheiermann et al., 2012). Even more intriguing, studies have unearthed circadian connections between the brain and the intestine mediated through a subset of innate lymphoid cells (ILCs; Godinho-Silva et al., 2019b), thereby revealing a role for innate immune cells as mediators of brain-derived circadian signals in tissues. These circadian immune circuits appear ubiquitously (Fig. 1 and Table 1) and may underlie both housekeeping functions and disorders that display strong circadian patterns, including cardiovascular (Gupta and Shetty, 2005; Muller et al., 1985), metabolic (Oh et al., 2019), allergic (Paganelli et al., 2018), and even carcinogenic (Puram et al., 2016).
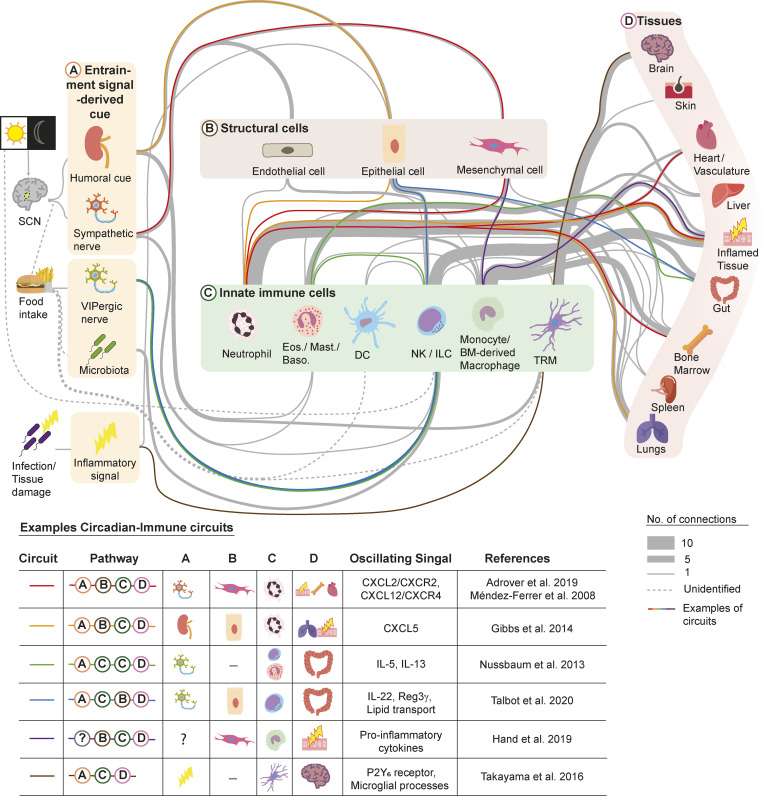
Figure 1.
Circadian immune circuits. Circadian regulation of innate immunity requires the coordinated action of at least three compartments: neurons of the SCN (and downstream endocrine organs), structural cells in peripheral tissues, and immune cells. The SCN integrates photic cues, which represent the main entrainment signals; however, the immune system can be also resynchronized by feeding or during infections. In this model, circadian information “flows” through these compartments, forming what we refer to as circadian immune circuits. We organize the distinct compartments of these circuits as those associated with the central clock or other entrainment cues (A), which deliver signals to structural (B) and/or innate immune cells (C) in peripheral organs. The circuits are organized such that structural and innate immune cells reciprocally communicate to orchestrate immune responses or specific physiological events (D). The upper diagram shows a nonexhaustive list of circadian immune circuits identified from the literature, of which some are highlighted by color lines and are referred to in the text and detailed in the lower table. All circuits shown here are detailed in Table 1. Eos., eosinophils; Baso., basophils; TRM, tissue-resident macrophages.
Table 1. List of reported circadian immune circuits.
| Circuit | Pathway | A | B | C | D | Oscillating signal | References |
|---|---|---|---|---|---|---|---|
| 1 | A-B-C-D | β-Adrenergic receptors | MCs | Neutrophils | Infected tissue, BM, cardiovasculature | CXCL2, CXCR2, CXCL12, CXCR4 | Méndez-Ferrer et al., 2008; Adrover et al., 2019 |
| 2 | A-B-C-D | Glucocorticoid receptors | EpCs | Neutrophils | Infected tissue, lungs | CXCL5 | Gibbs et al., 2014 |
| 3 | A-C-C-D | VIP neurons | — | ILC2s, eosinophils | Gut | IL-5, IL-13 | Nussbaum et al., 2013 |
| 4 | A-C-B-D | VIP neurons | EpCs | ILC3s | Gut | IL-22, Reg3γ, lipid transport | Talbot et al., 2020 |
| 5 | B-C-D | — | MCs | Monocytes | Inflamed tissue | Proinflammatory cytokines | Hand et al., 2019 |
| 6 | A-C-D | Infection | — | Microglia | Brain | P2Y6 receptor, microglial processes | Takayama et al., 2016 |
| 7 | A-B-C-D | β-Adrenergic receptors, cholinergic signals | MCs | Neutrophils | BM | CXCl12 | Méndez-Ferrer et al., 2008; García-García et al., 2019 |
| 8 | A-B-C-D | β-Adrenergic receptors | ECs | Neutrophils | Inflamed tissue, BM, cardiovasculature | Icam-1, Selectins, VCAM-1, CCL2 | Scheiermann et al., 2012 |
| 9 | C-C-D | — | — | Hematopoietic stem cell (HSC)–derived macrophages, neutrophils | Infected tissue | Proinflammatory cytokines | Kiessling et al., 2017 |
| 10 | C-C-B-D | — | MCs | Neutrophils, HSC-derived macrophages | BM | LXR, CXCL12 | Casanova-Acebes et al., 2013 |
| 11 | C-D | — | — | Neutrophils | Lungs | Lung transcriptome | Casanova-Acebes et al., 2018 |
| 12 | C-C-D | — | — | Neutrophils, HSC-derived macrophages | Gut, BM | IL-23, G-CSF | Casanova-Acebes et al., 2018 |
| 13 | C-C-D | — | — | HSC-derived macrophages, eosinophils | Lungs | IL-5, proinflammatory cytokines | Zasłona et al., 2017 |
| 14 | A-C-D | Glucocorticoids | — | Mast cells | Inflamed tissue | FcεRI expression | Nakamura et al., 2016 |
| 15 | A-C-C-D | Feeding metabolic cues | — | DC, lymphocytes | Infected tissue | IL-12 | Hopwood et al., 2018 |
| 16 | C-D | — | — | DC, lymphocytes | Spleen | TLR expression | Silver et al., 2012b, 2018 |
| 17 | A-C-D | β-Adrenergic receptors | — | NK cells | Spleen | TNF-α, granzyme B, perforin | Wahle et al., 2001; Logan et al., 2011 |
| 18 | A-B-C-D | β-Adrenergic receptors | ECs | NK cells | Infected tissue | CXCR4 | He et al., 2018 |
| 19 | A-C-D | Light-derived signals | — | ILC3s | Gut | Epithelial reactivity genes, microbiome composition, lipid epithelial transporters, CCR9 | Godinho-Silva et al., 2019b |
| 20 | A-C-D | Microbiota/light-derived signals | — | ILC3s | Gut | IL-17, IL-22, NFIL3, RORγT | Teng et al., 2019; Wang et al., 2019 |
| 21 | A-B-C-D | β-Adrenergic receptors | ECs | Monocytes | BM, inflamed tissue | Icam-1, Selectins, VCAM-1, CCL2 | Scheiermann et al., 2012 |
| 22 | C-D | — | — | Monocytes | BM, liver, lungs, infected/inflamed tissues, cardiovasculature | CXCR4, CCL2 | Chong et al., 2016; He et al., 2018; Nguyen et al., 2013; Huo et al., 2017; Winter et al., 2018; Schloss et al., 2017 |
| 23 | C-D | — | — | Microglia | Brain | Morphology, purinergic receptors | Hayashi, 2013 |
| 24 | C-D | — | — | Microglia | Brain | Cathepsin S | Hayashi et al., 2013 |
| 25 | C-D | Not glucocorticoids | — | Microglia | Brain | IL-1β, TNF-α, IL-6 | Fonken et al., 2015 |
| 26 | A-C-D | Glucocorticoids | — | Microglia | Brain | Phagocytosis | Choudhury et al., 2020 |
| 27 | C-D | — | — | KCs | Liver | TLR4 signals | Wang et al., 2018 |
| 28 | A-C-D | Feeding metabolic cues | — | KCs | Liver | TNF-α | Guerrero-Vargas et al., 2015 |
| 29 | C-D | — | — | LCs | Skin | IRF7 | Greenberg et al., 2020 |
| 30 | A-C-C-D | Melatonin | — | LCs, lymphoid T cells | Infected tissue | Migration to lymph nodes | Prendergast et al., 2013 |
| 31 | A-B-D | Glucocorticoids | EpCs | — | Lungs | cell molecular clock | Gibbs et al., 2009 |
| 32 | A-B-D | LPS treatment | EpCs | — | Lungs | Proinflammatory cytokines, REV-ERBα degradation | Pariollaud et al., 2018 |
| 33 | A-C-B-D | Microbiota | EpCs | ILC3s | Gut | NFIL3 | Wang et al., 2017 |
Here, we summarize evidence highlighting the prevalence and importance of circadian immune circuits, with particular consideration to neural and structural elements in tissues that coordinate with immune cells to orchestrate multiple aspects of circadian physiology and immunity.
Circadian rhythms in innate immune cells
Almost every hematopoietic and immune cell is subjected to circadian influence by more than one mechanism. In some instances, a cell-intrinsic clock drives circadian behaviors; however, these are often the result of the coordinated action of several cell types, including those that form part of the tissue architecture. In this section, we summarize the major circadian features of innate immune cells and present them in the broader context of the tissue so as to illustrate the nature and abundance of immune circadian circuits (Fig. 1).
Granulocytes
Neutrophils are characterized by a short lifespan, a feature that may explain their strong circadian patterns (Aroca-Crevillén et al., 2020). Both their numbers in blood and migratory behavior are orchestrated, at least in part, by a cell-autonomous clock (Adrover et al., 2019) in combination with cues originating from tissue-resident or structural cells (He et al., 2018). For example, the circadian trafficking of neutrophils to and from the bone marrow (BM) likely depends on the oscillatory expression of the chemokine CXCL12 (Ella et al., 2016; De Filippo and Rankin, 2018) and its cognate receptor, CXCR4 (Casanova-Acebes et al., 2013), a feature shared with other hematopoietic cells (Lucas et al., 2008; Méndez-Ferrer et al., 2008). Expression of Cxcl12 is regulated in a circadian manner by direct sympathetic innervation of the marrow and appears to be under direct control of the central clock. This axis critically relies on β3-adrenergic receptor signaling in mesenchymal cells (MCs) that form the hematopoietic niche (Méndez-Ferrer et al., 2010), resulting in blunted CXCL12 production at daytime in mice (Fig. 1, red circuit; Méndez-Ferrer et al., 2008). Concomitant with this axis, inhibitory cholinergic signals from the parasympathetic nervous system damp the sympathetic tone at night (García-García et al., 2019), altogether establishing a neural mesenchymal circuit that controls neutrophil dynamics in the BM. In addition to MCs, neutrophil dynamics can be controlled by other structural cells, at least during inflammation. In the context of bacterial infections in the lung, for example, expression of Cxcl5 regulated by the circadian machinery and by glucocorticoids on epithelial cells (EpCs) controls neutrophil recruitment and the magnitude of the response (Fig. 1, orange circuit; Gibbs et al., 2014).
During their brief circulating time (6–10 h in mice), neutrophils undergo oscillations in phenotype and function, a phenomenon referred to as aging (Adrover et al., 2016). These changes are controlled in a cell-intrinsic circadian manner though Bmal1 and CXCR2 and are subjected to light entrainment (Adrover et al., 2019). CXCR4 acts as an internal inhibitor of circadian aging by interfering with CXCR2 signaling in a process that correlates with oscillatory levels of CXCL12 in plasma, suggesting that cell-autonomous and systemic signals contribute to neutrophil aging. This in turn regulates the migratory and toxic properties of circulating neutrophils (Adrover et al., 2019, 2020) and impacts the outcome of infectious, ischemic, and inflammatory responses (Adrover et al., 2019; Zhang et al., 2015). Finally, the finding that neutrophils infiltrate healthy tissues in a circadian manner (Casanova-Acebes et al., 2018) suggests that they could instruct circadian programs in tissues. This intriguing possibility is supported by the finding that neutrophils infiltrating the BM control the circadian activity of hematopoietic niches (Casanova-Acebes et al., 2013), as well as the transcriptional activity in the lungs (Casanova-Acebes et al., 2018), ultimately modulating the migration of hematopoietic stem or metastatic cells, respectively.
The number of eosinophils follow circadian oscillations in blood (Acland and Gould, 1956), and in the intestine, their migration is controlled by an extrinsic circuit involving hormonal cues and type 2 ILCs (ILC2s; Nussbaum et al., 2013). Specifically, food intake generates rhythmic expression of vasoactive intestinal peptide (VIP), which entrains the circadian production of IL-5 and IL-13 by ILC2s and drives the accumulation of eosinophils within tissues (Fig. 1, green circuit; Nussbaum et al., 2013). Together with feeding, independent cues involving adrenal hormones have been long known to entrain fluctuations of eosinophils in blood (Brown and Dougherty, 1956). Thus, a circadian immune–metabolic axis can control immune fluxes and shape specific aspects of tissue homeostasis or disease. For example, the circadian dynamics of eosinophils in blood (Haus and Smolensky, 1999) and lungs (Panzer et al., 2003), the number of circulating low-density eosinophils (Calhoun et al., 1992), and variations of eosinophil-derived GM-CSF (Esnault et al., 2007) associate with the diurnal onset and clinical manifestations of asthma. Similarly, allergy onset follows circadian patterns in which eosinophils are major cellular effectors and correlates with rhythmic expression of the eosinophil cationic protein (Baumann et al., 2013). In these type 2 as well as in other inflammatory responses, the molecular clock in myeloid cells generally functions to blunt the magnitude of the response (Zasłona et al., 2017).
Mast cells are also major effectors of anaphylactic reactions (Baumann et al., 2015). Expression of tryptase and FcεRI chain and release of prestored histamine, leukotrienes, and proinflammatory cytokines by mast cells are all under circadian regulation and allow temporal gating of mast cell activation and effector functions (Baumann et al., 2013, 2015). Despite the clear circadian patterns of allergy, however, the role of internal versus external clocks in controlling mast cell activation remain poorly defined. Studies in adrenalectomized mice determined that degranulation after an IgE challenge was extrinsically dependent on glucocorticoids, yet the process was shown to additionally require a functional internal clock (Nakamura et al., 2016).
Monocytes, monocyte-derived macrophages, and dendritic cells (DCs)
Monocytes exhibit circadian oscillations in blood counts (He et al., 2018) and in recruitment to tissues, in part mediated by external cues delivered by the sympathetic nerves to ECs (Scheiermann et al., 2012). The release of inflammatory monocytes from the BM is, in contrast, controlled by CCL2-producing MCs (Shi et al., 2011), a cell type that is under strong circadian control (Méndez-Ferrer et al., 2008), suggesting that circadian monocyte release may be regulated through these cells. In the context of inflammation, the molecular clock in local synoviocytes (a type of fibroblast) blunts monocyte recruitment and the magnitude of inflammation in arthritis (Fig. 1, violet circuit; Hand et al., 2019). Complementing these extrinsic cues, the intrinsic clock of monocytes controls expression of genes important for their migration. For example, oscillations in CXCR4 regulate their egress from BM (Chong et al., 2016) and homing into the murine liver and lung (Chong et al., 2016; He et al., 2018). Inflammatory, but not patrolling, monocytes show circadian expression of Ccl2, which amplifies their migration to infected tissues. Bmal1 deletion results in exaggerated expression of Ccl2 and predisposes to septic shock and chronic inflammatory disease (Nguyen et al., 2013). Thus, intrinsic regulation of inflammatory cytokines or their receptors by the circadian clock appears to be generally protective, as also shown in the context of atherosclerosis (Huo et al., 2017; Winter et al., 2018) and myocardial infarction (Schloss et al., 2017).
Monocyte-derived macrophages have been mostly studied in vitro from peritoneal exudates or in vivo in the peritoneum and spleen, where BM-derived macrophages dominate in adulthood (Guilliams et al., 2018). These macrophages display circadian gating in the response against pathogens that is controlled by a Bmal1- and Rev–Erbα–dependent clock (Curtis et al., 2015; Gibbs et al., 2012). For instance, they display rhythmic expression of genes encoding pattern recognition receptors (Keller et al., 2009; Silver et al., 2018, 2012b) and cytokines (Curtis et al., 2015; Gibbs et al., 2012; Kiessling et al., 2017) or involved in production of reactive oxygen species (Early et al., 2018). Their phagocytic activity is also regulated in a circadian manner, thereby ensuring efficient elimination of pathogens (Kitchen et al., 2020; Oliva-Ramírez et al., 2014) and preservation of tissue homeostasis (A-Gonzalez et al., 2017). The rhythmic changes in phagocytosis appears to be mediated, at least in part, through regulation of the cytoskeletal regulator RhoA by an internal Bmal1-dependent clock (Kitchen et al., 2020). Interestingly, inflammatory macrophages express receptors for melatonin (Maestroni et al., 2002), a circadian neurohormone that modulates phagocytosis (Pires-Lapa et al., 2013). Additionally, circadian peaks in mitochondrial dynamics and activity are needed for, and precede, phagocytic activity in macrophages (Wang et al., 2017; Oliva-Ramírez et al., 2014). Interestingly, Bmal1 is induced upon inflammation and promotes mitochondrial reprograming, which in turn elicits protective inflammatory responses in the context of infection or cancer (Alexander et al., 2020). Thus, molecular coordination of circadian, metabolic, and immune programs regulates both the timing and type of response in macrophages (Xu et al., 2014; Sato et al., 2014).
Because DCs link innate and adaptive immunity, studies have typically explored DC rhythmicity by observing downstream adaptive responses. For example, morning exposure to helminths generates a more protective response, and ablation of DC-specific Bmal1 biases the T helper type 1 and 2 response and compromises pathogen clearance (Hopwood et al., 2018). In this case, DC responses are modulated by an intrinsic clock and externally by feeding-derived cues, ultimately regulating the production of T helper 1 cell–type cytokines (Hopwood et al., 2018). DC abundance in the lymph nodes and spleen, a critical parameter to initiate adaptive responses, also features circadian dynamics (Druzd et al., 2017; Silver et al., 2018). It is noteworthy that the peak numbers of DC and T cells align in the lymph nodes, suggesting mechanisms of immune synchronization that optimize adaptive immune responses (Fortier et al., 2011). Likewise, circadian regulation of pattern recognition receptor expression in DCs may have evolved to overlap with higher exposure to pathogens (Silver et al., 2012b, 2018).
Embryo-derived macrophages
Here, we present an overview of representative subsets of tissue-resident macrophages of embryonic origin and emphasize that the circadian biology for many of these highly specialized cells remains unknown.
Microglia, the resident macrophages of the brain, perform highly dynamic tasks that support neural homeostasis; for example, by “pruning” synaptic terminal and producing neurotrophic factors (Wu et al., 2015; Kierdorf and Prinz, 2017), both of which are under circadian influence. Studies focused on microglial morphology revealed circadian patterns in branching through expression of a purinergic receptor (P2Y12R) that was controlled by core clock genes (Hayashi, 2013). This process is relevant to support oscillatory patterns in synaptic strength and spine density and to prevent neuropsychiatric disorders (Hayashi et al., 2014). Intriguingly, this pattern could be inverted upon exposure to bacterial infection (Takayama et al., 2016), thus uncovering multiple regulatory inputs in microglia (Fig. 1, brown circuit). Expression of cathepsin S, another regulator of synaptic strengthening in the cortex and memory formation (Hayashi et al., 2014), is also under control of the intrinsic microglial clock (Hayashi et al., 2013). External regulators, such as glucocorticoids and noradrenaline, in turn, tune the expression of opsonins and phagocytic receptors, thereby enabling the removal of weak synapses by microglia during the resting phase (Choudhury et al., 2020). Of particular interest for our discussion are the links between circadian oscillations in microglia and neuroinflammatory and cognitive disorders, such as Alzheimer’s or Parkinson’s disease (Liu et al., 2020; Ni et al., 2019). These patients manifest conspicuous loss of circadian patterns, which in mice is associated with exacerbated inflammatory profiles in microglia that lack core circadian genes. Indeed, an internal clock, rather than extrinsic hormonal cues, appears to control the oscillatory response of microglia to sterile challenges (Fonken et al., 2015). Microglia are therefore both regulators of neuronal synapses and targets of neurohormonal signals, implying important influence of both intrinsic and extrinsic circadian inputs.
Recent proteomic analyses in the murine liver found a strong correlation between the circadian changes in the proteome of Kupffer cells (KCs) and liver physiology (Wang et al., 2018). For example, pathway interactions for KCs and whole-liver proteins were predominantly immune at daytime and metabolic at nighttime. A challenge now is to understand how KCs coordinate with the rest of the tissue to synchronize functional pathways and to define its relevance for organ physiology. KCs isolated from rats subjected to shifts in food intake showed increased production of TNF-α after endotoxin stimulation, again highlighting links of circadian oscillations with metabolism and a general role in limiting inflammation (Guerrero-Vargas et al., 2015). The extent to which the cell-autonomous clock modulates other critical functions of KCs in the liver, such as iron recycling, remains unknown.
Although the skin displays prominent circadian rhythms (Plikus et al., 2015; Sherratt et al., 2019) and is heavily altered in arrhythmic mice lacking Bmal1 (Welz et al., 2019), little is known about the actual circadian biology of Langerhans cells (LCs), the epidermal macrophages. Global circadian transcriptomic analysis of the skin, however, identified abundant immune-related genes (Geyfman et al., 2012) that suggested circadian regulation of LCs. Consistently, LCs undergo diurnal changes in the subcellular distribution of IFN-sensitive genes in the context of psoriasis, and the absence of Bmal1 blunted oscillations and exacerbated the IFN-driven response (Greenberg et al., 2020). Interestingly, the antigen-presenting capacity and migration of LCs are extrinsically regulated by the circadian hormone melatonin (Doebel et al., 2017), resulting in defective antigen-specific hypersensitivity reactions in the skin of arrhythmic hamsters (Prendergast et al., 2013). Thus, similar principles of intrinsic and extrinsic circadian regulation, including blunted immune activation by the intrinsic clock, may apply to LCs. Nonetheless, much remains to be learned in order to rigorously substantiate these concepts in LCs, as well as in multiple other populations of resident macrophages.
ILCs
ILCs are an expanding group of immune cells of the lymphoid lineage that do not express antigen-specific receptors and have functions typically assigned to the innate immune arm, including response to infections, homeostasis, and inflammation. They have different origins, distributions, and functions and are therefore classified in three main groups.
The cytotoxic activity of natural killer (NK), a type 1 ILC, is under the control of both intrinsic, Per2-dependent clock regulation (Arjona and Sarkar, 2006; Liu et al., 2006) and extrinsic neurohormonal signals dependent on the central clock (Liu et al., 2006; Logan et al., 2011). For example, NK cells in the spleen express β-adrenergic receptors (Wahle et al., 2001) that regulate daily variations in TNF-α, granzyme B, and perforin (Logan et al., 2011) but not IFN-γ production, suggesting that additional nonneural cues are necessary to entrain effector functions in NK cells. Disruption of diurnal cycles of rats has major effects in the rhythmic production of inflammatory mediators and cytotoxic activity of NK cells and enhances tumor growth (Logan et al., 2012). Contrasting with the direct regulatory function of circadian cues, the migratory patterns of NK cells into tissues is regulated indirectly by sympathetic nervous system (SNS)–dependent signals that control the molecular clock in ECs (He et al., 2018). An additional, intriguing source of regulation for NK cells is during their lineage specification, through the circadian clock gene Nfil3, which in turn is regulated by feeding (Yang et al., 2015). Thus, multiple sources of circadian control dictate different aspects of NK biology.
In contrast to NK cells, type 2 ILCs reside in tissues and respond to parasites and allergens (Vivier et al., 2018). In the small intestine, ILC2s are stimulated by caloric intake through the circadian synchronizer VIP to produce IL-5 and IL-13, thereby regulating eosinophil maintenance in the tissue (Fig. 1, green circuit; Nussbaum et al., 2013). Despite the stark circadian nature of ILC2s, clock-dependent gene expression has not yet been demonstrated, and their influence on the circadian control of other immune cells in relevant tissues, such as alveolar macrophages (Guilliams et al., 2020), remains unexplored. Another particularly interesting aspect of ILCs is the tight connection with local nerves, which in the case of ILC2s have been shown to regulate their activation and effector functions against helminths through neuromedin U (Cardoso et al., 2017), raising the possibility of circadian regulation through this neural–ILC2 circuit.
Recent years have seen a surge of interest in ILC3, a subset of ILCs that critically regulate the intestinal barrier by integrating immune and neural cues (Godinho-Silva et al., 2019a). The regulatory functions of these cells in mucosal integrity appear to be intimately linked with circadian oscillations at different levels. First, a cell-intrinsic REV–ERBα–NFIL3 axis regulates the master transcription factor RORγ and instructs ILC3 specification (Wang et al., 2019). Second, ablation of Bmal1 leads to reduced cell numbers in the gut due to defective expression of migratory receptors, causing massive alterations that are specific to the gut, including impaired epithelial reactivity, dysregulated microbiome, susceptibility to bowel infection, and disrupted lipid metabolism (Godinho-Silva et al., 2019b). Finally, ILC3 oscillations are under the influence of light and prandial cues. Specifically, a neuroimmune circuit involving production of VIP by enteric neurons after feeding represses Il22 expression by ILC3, in turn favoring lipid absorption through EpCs (Fig. 1, blue circuit; Talbot et al., 2020).
γδ T cells localize mostly in mucosal areas (in mice) and are specialized in containing pathogen invasion (Palomino-Segura et al., 2020). While their circadian biology remains poorly characterized, their numbers in human blood oscillate during the day (Mazzoccoli et al., 2011), possibly regulated by humoral cues triggered by the autonomous nervous system (Suzuki et al., 1997). More recently, the circadian gene clock has been implicated in the direct modulation of Il23r expression on γδ T cells and linked with susceptibility to psoriasis in mice (Ando et al., 2015).
Circadian features of structural immune cells
In addition to immune leukocytes, structural cell lineages within tissues can act as orchestrators of the immune response. Indeed, ECs, EpCs, and MCs embedded within tissues sense danger, provide spatial and temporal guidance for immune cell recruitment (Krausgruber et al., 2020), can store so-called immune memory (Ordovas-Montanes et al., 2020), and are integral elements of the circadian immune circuits discussed herein.
ECs line the inside of blood vessels are therefore key regulators of leukocyte migration to tissues. ECs display marked oscillations in clock genes as well as adhesion receptors and chemokines, which is in line with the circadian adhesion of leukocytes to, and migration through, inflamed vessels (Scheiermann et al., 2012). A puzzling finding was that the oscillatory expression of adhesive and chemotactic genes is out of phase for arteries and veins, despite synchronized expression of their clock genes. Elegant surgical and genetic models revealed a role for NG2-positive mural cells around arteries in sensing SNS signals and instructing circadian adhesion of leukocytes to arteries and adjacent veins (de Juan et al., 2019). Additionally, factors released by myeloid leukocytes such as CCL2 can introduce circadian changes in the adhesive properties of large and small vessels (Winter et al., 2018). Contrasting with this, the EC-intrinsic circadian clock only impacts circadian leukocyte adhesion in venous, but not arterial, vessels (de Juan et al., 2019), a property that may vary across tissues (Kalucka et al., 2020). Finally, surprising new findings suggest that light can entrain metabolic rewiring and elicit cardioprotective responses through Per2 in ECs (Oyama et al., 2019). These immune- and SNS-driven features in vessels are likely to underlie inflammatory and thrombotic processes in atherosclerosis and other cardiovascular disorders (Winter et al., 2018; de Juan et al., 2019).
MCs include fibroblasts and various types of perivascular cells present in every tissue that control multiple aspects of tissue physiology, including immune and hematopoietic regulation (Stark et al., 2013; Méndez-Ferrer et al., 2010). As discussed above, a prominent role for MCs in the control of circadian leukocyte dynamics has been best described in the BM (Shi et al., 2011; Méndez-Ferrer et al., 2008). Local SNS innervation and delivery of catecholamines appears to be a common mechanism that regulates circadian immune trafficking; indeed, expression of adrenergic receptors in MCs relays signals locally by modulating expression of chemotactic or adhesive factors such as CXCL12 in the marrow (Méndez-Ferrer et al., 2008). Interestingly, the activity of MCs and their ability to influence hematopoietic trafficking in the BM is additionally regulated by innate immune cells, including medullary-resident macrophages and neutrophils (Casanova-Acebes et al., 2013), revealing reciprocal regulation between MCs and innate immune cells. While the contribution of the intrinsic clock in MCs in immune homeostasis has not been broadly explored, a role for Bmal1 in synoviocytes was shown to keep inflammation in check during arthritis (Fig. 1, violet circuit; Hand et al., 2019).
EpCs feature functional clocks in most tissues, although the most relevant immune-circadian analyses have been performed in the intestine (Sládek et al., 2007) and pulmonary airways (Gibbs et al., 2009), both of which manifest marked circadian patterns in basal function and disease manifestation. In the intestine, circadian patterns of EpC function, such lipid transport, are directly regulated by IL-22 secreted by ILC3s and indirectly by feeding and the microbiota (Fig. 1, blue circuit; Talbot et al., 2020). In the lungs, expression of clock genes is largely restricted to club cells, a type of bronchiolar EpC, and is strongly responsive to glucocorticoids (Gibbs et al., 2009). Elegant studies demonstrated that rhythmic expression of the inflammatory gene Cxcl5 is under direct control of glucocorticoid receptors and Bmal1 in club cells and was responsible for the time-of-day variation in the severity of bacterial infection in the lungs (Gibbs et al., 2014). As shown for other cell types, the intrinsic clock in EpCs appears to be protective by blunting expression of inflammatory gene products, both in basal and inflammatory settings (Pariollaud et al., 2018). Interestingly, inflammatory cytokines induce degradation of the Rev–Erbα and exacerbate pulmonary inflammation (Pariollaud et al., 2018), altogether evidencing the existence of multiple regulatory layers and the general protective function of the epithelial clock during immune responses.
Modeling circadian immune circuits
The classical view of circadian biology in mammals posits a hierarchical organization where peripheral clocks in cells and tissues are “enslaved” by the master pacemaker in the SCN (Fig. 2 A; Dibner et al., 2010). In our discussion above, we present examples supporting this prevailing dogma of a hierarchical control of circadian patterns in immune function (Nakamura et al., 2016; Choudhury et al., 2020). We highlight, however, that regulation of immune cells by the central clock is often indirect, i.e., relayed by intermediary cells. These are typically part of the so-called structural system and are increasingly recognized as integral to immune responses (Krausgruber et al., 2020), as also highlighted above. A central theme that arises is that networks composed of multiple signals and cellular intermediaries exist that coordinate immune responses in time and that leukocytes are typically final effector components of the network (see examples in Fig. 1). These networks, which we refer to here as circadian immune circuits, remain only superficially characterized and are important to comprehend key features of innate immunity and tissue physiology.
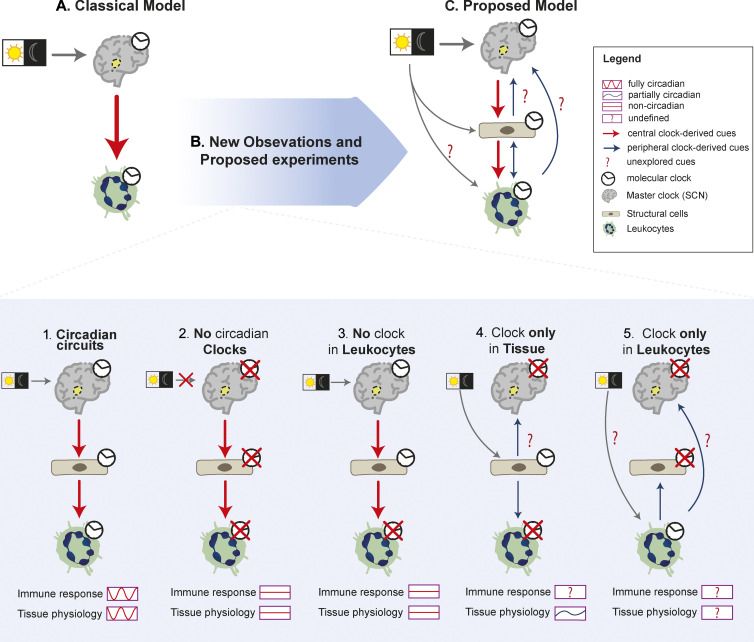
Figure 2.
Modeling the architecture of circadian immune circuits. (A) Depiction of the classical model of circadian immune circuits whereby circadian clocks in innate immune subsets are under direct control of the master clock. (B) The model is refined by recent observations that consider at least three elements in the circuit (SCN and structural and immune cells). The presence or depletion of the internal clock in each component of the circuit leads to specific circadian patterns in immune response or tissue physiology. Rescue of a functional clock in specific components in an otherwise arrhythmic mouse (models 4 and 5) will be needed to understand how the circuits are actually organized. (C) Integrating current and future analyses proposed in the text and shown in B will enable building a more accurate model of how these circuits work, including defining whether peripheral clocks sense diurnal changes and deliver circadian cues to the other components of the circuit.
How are these circuits organized? To address this fundamental question, we resort to multiple experimental models in which the molecular clock of different components of the circuit are disabled (Fig. 2 B). For example, analysis of mice with global clock deficiency demonstrate the importance of circadian regulation in the response to pathogens (Sundar et al., 2015; Majumdar et al., 2017) and organismal physiology (Fig. 2, B.1 and B.2; Kondratova and Kondratov, 2012; Koronowski et al., 2019). However, because these arrhythmic models target all cells, they cannot be used to tease out the contribution of each separate component of the immune circuits. In contrast, studies in animals in which the molecular clock is targeted in a specific element of the circuit (immune or structural) evidenced that deleting specific clocks in cells along the circuits still causes profound changes in the magnitude and timing of antimicrobial and inflammatory responses, as well as (certain) homeostatic properties of tissues (Fig. 2 B.3; Godinho-Silva et al., 2019b; Gibbs et al., 2014; Nguyen et al., 2013; Kitchen et al., 2020; Adrover et al., 2019). Important insights into these circuits can be also gained by direct analyses of the central clock. Indeed, while a functional clock in the SCN is sufficient to generate circadian rhythms in organismal behavior and in tissues (Sujino et al., 2003), studies in mice with a genetically ablated central clock demonstrated the existence of peripheral, SCN-independent clocks that could be entrained by light or feeding regimens (Izumo et al., 2014; Husse et al., 2014). In line with these surprising findings, elegant genetic models allowing cell- or tissue-specific rescue of a functional clock in otherwise arrhythmic mice have recently demonstrated that peripheral clocks are sufficient to preserve circadian oscillations in many genes and in organ physiology, autonomously from the SCN (Fig. 2 B.4; Welz et al., 2019; Koronowski et al., 2019). Thus, although much progress has been made in understanding general circadian circuits, we are still far from understanding the fundamental organization of circadian immune circuits. It is unclear, for example, whether and to what extent these circuits are autonomous from the SCN, because no experiments have addressed if clocks in structural or immune cells are by themselves sufficient to support circadian immune responses.
While still hypothetical, the potential for circadian clocks in structural and innate immune cells to sense and generate their own circadian cues can be speculated based on their distinct transcriptional profiles. For instance, a search in public databases (Immgen) reveals that subsets of ILCs, macrophages, neutrophils, mast cells, and EpCs express genes encoding several opsin genes, a family of G protein–coupled receptors that detect light and could render these cells independent of photic cues delivered through the SCN (Fig. 2, B.4 and B.5), as already shown for specific tissues (Buhr et al., 2019; Zhang et al., 2020). Further, because several subsets of innate immune cells, including macrophages, monocytes, and neutrophils, are capable of synthesizing and secreting catecholamines and glucocorticoids (Flierl et al., 2008; Acharya et al., 2020), it is conceivable that they generate neurohormonal signals similar to those used by the central clock to deliver circadian cues to their surrounding tissue. Rescue of functional clocks in immune and structural cells using newly generated mouse models (Welz et al., 2019) should allow exploring the autonomy, directionality, and relevance of the circadian immune circuits proposed herein (Fig. 2 C).
Concluding remarks
We aimed here to present an immunologist’s view of how innate immunity and circadian oscillations interact in physiology rather than providing a comprehensive description of circadian immunity, which has been the subject of recent reviews (Scheiermann et al., 2018; Man et al., 2016). We provide an overview of exciting recent findings illustrating that circadian oscillations in peripheral tissues may operate independently of the central clock, that dedicated sensors of light and possibly other environmental cues exist in cells, that multiple cell types coordinate circadian responses, and that innate immune cells can be a source of circadian signals. Integrating these ideas may allow us to better understand how immunity and physiology intersect; for example, the observation that tissue-infiltrating neutrophils entrain circadian oscillations in the lung and the BM (Casanova-Acebes et al., 2018) or that ablation of the molecular clock in these cells blunts diurnal changes in immune responses (Adrover et al., 2019) suggests a remarkable degree of “circadian autonomy” of innate immunity and its ability to entrain organ physiology. Further, the well-established role of microglia in housekeeping neural connectivity in the brain (Hayashi et al., 2014) and the ability of inflammatory mediators to act directly on the master clock (Kwak et al., 2008) suggest potential roles of immune cells in supporting normal SCN activity, in turn suggesting immune influence in organismal rhythms. We expect that by proposing exploration of multidirectional interactions between circadian oscillations and innate immune cells, including structural cells as essential intermediaries of the circadian immune circuits, we will obtain a better understanding of how these two systems (immune and circadian) orchestrate normal physiology. The proposed studies may additionally enable identification of immune defects as the source of dysregulated circadian oscillations and loss of fitness in tissues in a wide array of pathologies, including those associated with aging.
Acknowledgments
We thank I. Ballesteros for critical reading of and suggestions on the manuscript.
M. Palomino-Segura is supported by a Federation of European Biochemical Societies long-term fellowship. A. Hidalgo is supported by the Ministerio de Ciencia e Innovacion (RTI2018-095497-B-I00), Fundación La Caixa (HR17_00527), and the Leducq Foundation Transatlantic Network of Excellence (TNE-18CVD04). The Centro Nacional de Investigaciones Cardiovasculares Carlos III is supported by the Ministerio de Ciencia e Innovacion and the Pro-CNIC Foundation and is a Severo Ochoa Center of Excellence (Ministerio de Ciencia e Innovacion award SEV-2015-0505).
Author contributions: M. Palomino-Segura and A. Hidalgo conceived, wrote, and edited the text and designed the figures.
References
- A-Gonzalez, N., Quintana J.A., García-Silva S., Mazariegos M., González de la Aleja A., Nicolás-Ávila J.A., Walter W., Adrover J.M., Crainiciuc G., Kuchroo V.K., et al. . 2017. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 214:1281–1296. 10.1084/jem.20161375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya, N., Madi A., Zhang H., Klapholz M., Escobar G., Dulberg S., Christian E., Ferreira M., Dixon K.O., Fell G., et al. . 2020. Endogenous Glucocorticoid Signaling Regulates CD8+ T Cell Differentiation and Development of Dysfunction in the Tumor Microenvironment. Immunity. 53:658–671.e6. 10.1016/j.immuni.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland, J.D., and Gould A.H.. 1956. Normal variation in the count of circulating eosinophils in man. J. Physiol. 133:456–466. 10.1113/jphysiol.1956.sp005600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrover, J.M., Nicolás-Ávila J.A., and Hidalgo A.. 2016. Aging: A Temporal Dimension for Neutrophils. Trends Immunol. 37:334–345. 10.1016/j.it.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Adrover, J.M., Del Fresno C., Crainiciuc G., Cuartero M.I., Casanova-Acebes M., Weiss L.A., Huerga-Encabo H., Silvestre-Roig C., Rossaint J., Cossío I., et al. . 2019. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity. 50:390–402.e10. 10.1016/j.immuni.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Adrover, J.M., Aroca-Crevillén A., Crainiciuc G., Ostos F., Rojas-Vega Y., Rubio-Ponce A., Cilloniz C., Bonzón-Kulichenko E., Calvo E., Rico D., et al. . 2020. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 21:135–144. 10.1038/s41590-019-0571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, R.K., Liou Y.-H., Knudsen N.H., Starost K.A., Xu C., Hyde A.L., Liu S., Jacobi D., Liao N.-S., and Lee C.-H.. 2020. Bmal1 integrates mitochondrial metabolism and macrophage activation. eLife. 9:e54090 10.7554/eLife.54090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, N., Nakamura Y., Aoki R., Ishimaru K., Ogawa H., Okumura K., Shibata S., Shimada S., and Nakao A.. 2015. Circadian gene clock regulates psoriasis-like skin inflammation in mice. J. Invest. Dermatol. 135:3001–3008. 10.1038/jid.2015.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona, A., and Sarkar D.K.. 2006. The circadian gene mPer2 regulates the daily rhythm of IFN-γ. J. Interferon Cytokine Res. 26:645–649. 10.1089/jir.2006.26.645 [DOI] [PubMed] [Google Scholar]
- Aroca-Crevillén, A., Adrover J.M., and Hidalgo A.. 2020. Circadian Features of Neutrophil Biology. Front. Immunol. 11:576 10.3389/fimmu.2020.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, A., Gönnenwein S., Bischoff S.C., Sherman H., Chapnik N., Froy O., and Lorentz A.. 2013. The circadian clock is functional in eosinophils and mast cells. Immunology. 140:465–474. 10.1111/imm.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, A., Feilhauer K., Bischoff S.C., Froy O., and Lorentz A.. 2015. IgE-dependent activation of human mast cells and fMLP-mediated activation of human eosinophils is controlled by the circadian clock. Mol. Immunol. 64:76–81. 10.1016/j.molimm.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Brown, H.E., and Dougherty T.F.. 1956. The diurnal variation of blood leucocytes in normal and adrenalectomized mice. Endocrinology. 58:365–375. 10.1210/endo-58-3-365 [DOI] [PubMed] [Google Scholar]
- Buhr, E.D., Vemaraju S., Diaz N., Lang R.A., and Van Gelder R.N.. 2019. Neuropsin (OPN5) Mediates Local Light-Dependent Induction of Circadian Clock Genes and Circadian Photoentrainment in Exposed Murine Skin. Curr. Biol. 29:3478–3487.e4. 10.1016/j.cub.2019.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, W.J., Bates M.E., Schrader L., Sedgwick J.B., and Busse W.W.. 1992. Characteristics of peripheral blood eosinophils in patients with nocturnal asthma. Am. Rev. Respir. Dis. 145:577–581. 10.1164/ajrccm/145.3.577 [DOI] [PubMed] [Google Scholar]
- Cardoso, V., Chesné J., Ribeiro H., García-Cassani B., Carvalho T., Bouchery T., Shah K., Barbosa-Morais N.L., Harris N., and Veiga-Fernandes H.. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 549:277–281. 10.1038/nature23469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes, M., Pitaval C., Weiss L.A., Nombela-Arrieta C., Chèvre R., A-González N., Kunisaki Y., Zhang D., van Rooijen N., Silberstein L.E., et al. . 2013. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 153:1025–1035. 10.1016/j.cell.2013.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova-Acebes, M., Nicolás-Ávila J.A., Li J.L., García-Silva S., Balachander A., Rubio-Ponce A., Weiss L.A., Adrover J.M., Burrows K., A-González N., et al. . 2018. Neutrophils instruct homeostatic and pathological states in naive tissues. J. Exp. Med. 215:2778–2795. 10.1084/jem.20181468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, S.Z., Evrard M., Devi S., Chen J., Lim J.Y., See P., Zhang Y., Adrover J.M., Lee B., Tan L., et al. . 2016. CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses. J. Exp. Med. 213:2293–2314. 10.1084/jem.20160800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury, M.E., Miyanishi K., Takeda H., Islam A., Matsuoka N., Kubo M., Matsumoto S., Kunieda T., Nomoto M., Yano H., and Tanaka J.. 2020. Phagocytic elimination of synapses by microglia during sleep. Glia. 68:44–59. 10.1002/glia.23698 [DOI] [PubMed] [Google Scholar]
- Curtis, A.M., Fagundes C.T., Yang G., Palsson-McDermott E.M., Wochal P., McGettrick A.F., Foley N.H., Early J.O., Chen L., Zhang H., et al. . 2015. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA. 112:7231–7236. 10.1073/pnas.1501327112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo, K., and Rankin S.M.. 2018. CXCR4, the master regulator of neutrophil trafficking in homeostasis and disease. Eur. J. Clin. Invest. 48(Suppl 2):e12949 10.1111/eci.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Juan, A., Ince L.M., Pick R., Chen C.S., Molica F., Zuchtriegel G., Wang C., Zhang D., Druzd D., Hessenauer M.E.T., et al. . 2019. Artery-associated sympathetic innervation drives rhythmic vascular inflammation of arteries and veins. Circulation. 140:1100–1114. 10.1161/CIRCULATIONAHA.119.040232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner, C., Schibler U., and Albrecht U.. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72:517–549. 10.1146/annurev-physiol-021909-135821 [DOI] [PubMed] [Google Scholar]
- Doebel, T., Voisin B., and Nagao K.. 2017. Langerhans Cells - The Macrophage in Dendritic Cell Clothing. Trends Immunol. 38:817–828. 10.1016/j.it.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Druzd, D., Matveeva O., Ince L., Harrison U., He W., Schmal C., Herzel H., Tsang A.H., Kawakami N., Leliavski A., et al. . 2017. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity. 46:120–132. 10.1016/j.immuni.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early, J.O., Menon D., Wyse C.A., Cervantes-Silva M.P., Zaslona Z., Carroll R.G., Palsson-McDermott E.M., Angiari S., Ryan D.G., Corcoran S.E., et al. . 2018. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA. 115:E8460–E8468. 10.1073/pnas.1800431115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella, K., Csépányi-Kömi R., and Káldi K.. 2016. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 57:209–221. 10.1016/j.bbi.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Esnault, S., Fang Y., Kelly E.A.B., Sedgwick J.B., Fine J., Malter J.S., and Jarjour N.N.. 2007. Circadian changes in granulocyte-macrophage colony-stimulating factor message in circulating eosinophils. Ann. Allergy Asthma Immunol. 98:75–82. 10.1016/S1081-1206(10)60863-0 [DOI] [PubMed] [Google Scholar]
- Flierl, M.A., Rittirsch D., Huber-Lang M., Sarma J.V., and Ward P.A.. 2008. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol. Med. 14:195–204. 10.2119/2007-00105.Flierl [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken, L.K., Frank M.G., Kitt M.M., Barrientos R.M., Watkins L.R., and Maier S.F.. 2015. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav. Immun. 45:171–179. 10.1016/j.bbi.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier, E.E., Rooney J., Dardente H., Hardy M.-P., Labrecque N., and Cermakian N.. 2011. Circadian variation of the response of T cells to antigen. J. Immunol. 187:6291–6300. 10.4049/jimmunol.1004030 [DOI] [PubMed] [Google Scholar]
- García-García, A., Korn C., García-Fernández M., Domingues O., Villadiego J., Martín-Pérez D., Isern J., Bejarano-García J.A., Zimmer J., Pérez-Simón J.A., et al. . 2019. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood. 133:224–236. 10.1182/blood-2018-08-867648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines, Z., and Lazar M.A.. 2015. Circadian metabolism in the light of evolution. Endocr. Rev. 36:289–304. 10.1210/er.2015-1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyfman, M., Kumar V., Liu Q., Ruiz R., Gordon W., Espitia F., Cam E., Millar S.E., Smyth P., Ihler A., et al. . 2012. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA. 109:11758–11763. 10.1073/pnas.1209592109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, J.E., Beesley S., Plumb J., Singh D., Farrow S., Ray D.W., and Loudon A.S.I.. 2009. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology. 150:268–276. 10.1210/en.2008-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, J.E., Blaikley J., Beesley S., Matthews L., Simpson K.D., Boyce S.H., Farrow S.N., Else K.J., Singh D., Ray D.W., and Loudon A.S.I.. 2012. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA. 109:582–587. 10.1073/pnas.1106750109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, J., Ince L., Matthews L., Mei J., Bell T., Yang N., Saer B., Begley N., Poolman T., Pariollaud M., et al. . 2014. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 20:919–926. 10.1038/nm.3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho-Silva, C., Cardoso F., and Veiga-Fernandes H.. 2019a Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 37:19–46. 10.1146/annurev-immunol-042718-041812 [DOI] [PubMed] [Google Scholar]
- Godinho-Silva, C., Domingues R.G., Rendas M., Raposo B., Ribeiro H., da Silva J.A., Vieira A., Costa R.M., Barbosa-Morais N.L., Carvalho T., and Veiga-Fernandes H.. 2019b Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature. 574:254–258. 10.1038/s41586-019-1579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, E.N., Marshall M.E., Jin S., Venkatesh S., Dragan M., Tsoi L.C., Gudjonsson J.E., Nie Q., Takahashi J.S., and Andersen B.. 2020. Circadian control of interferon-sensitive gene expression in murine skin. Proc. Natl. Acad. Sci. USA. 117:5761–5771. 10.1073/pnas.1915773117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Vargas, N.N., Guzmán-Ruiz M., Fuentes R., García J., Salgado-Delgado R., Basualdo M.C., Escobar C., Markus R.P., and Buijs R.M.. 2015. Shift Work in Rats Results in Increased Inflammatory Response after Lipopolysaccharide Administration: A Role for Food Consumption. J. Biol. Rhythms. 30:318–330. 10.1177/0748730415586482 [DOI] [PubMed] [Google Scholar]
- Guilliams, M., Mildner A., and Yona S.. 2018. Developmental and Functional Heterogeneity of Monocytes. Immunity. 49:595–613. 10.1016/j.immuni.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Guilliams, M., Thierry G.R., Bonnardel J., and Bajenoff M.. 2020. Establishment and Maintenance of the Macrophage Niche. Immunity. 52:434–451. 10.1016/j.immuni.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Gupta, A., and Shetty H.. 2005. Circadian variation in stroke - a prospective hospital-based study. Int. J. Clin. Pract. 59:1272–1275. 10.1111/j.1368-5031.2005.00678.x [DOI] [PubMed] [Google Scholar]
- Hand, L.E., Dickson S.H., Freemont A.J., Ray D.W., and Gibbs J.E.. 2019. The circadian regulator Bmal1 in joint mesenchymal cells regulates both joint development and inflammatory arthritis. Arthritis Res. Ther. 21:5 10.1186/s13075-018-1770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus, E., and Smolensky M.H.. 1999. Biologic rhythms in the immune system. Chronobiol. Int. 16:581–622. 10.3109/07420529908998730 [DOI] [PubMed] [Google Scholar]
- Hayashi, Y. 2013. Diurnal Spatial Rearrangement of Microglial Processes through the Rhythmic Expression of P2Y12 Receptors. J. Neurol. Disord. 01:1–7. 10.4172/2329-6895.1000120 [DOI] [Google Scholar]
- Hayashi, Y., Koyanagi S., Kusunose N., Okada R., Wu Z., Tozaki-Saitoh H., Ukai K., Kohsaka S., Inoue K., Ohdo S., and Nakanishi H.. 2013. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Sci. Rep. 3:2744 10.1038/srep02744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, Y., Wu Z., and Nakanishi H.. 2014. A possible link between microglial process dysfunction and neuropsychiatric disorders. J Neurol Disord Stroke. 2:1–5. [Google Scholar]
- He, W., Holtkamp S., Hergenhan S.M., Kraus K., de Juan A., Weber J., Bradfield P., Grenier J.M.P., Pelletier J., Druzd D., et al. . 2018. Circadian Expression of Migratory Factors Establishes Lineage-Specific Signatures that Guide the Homing of Leukocyte Subsets to Tissues. Immunity. 49:1175–1190.e7. 10.1016/j.immuni.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood, T.W., Hall S., Begley N., Forman R., Brown S., Vonslow R., Saer B., Little M.C., Murphy E.A., Hurst R.J., et al. . 2018. The circadian regulator BMAL1 programmes responses to parasitic worm infection via a dendritic cell clock. Sci. Rep. 8:3782 10.1038/s41598-018-22021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, M., Huang Y., Qu D., Zhang H., Wong W.T., Chawla A., Huang Y., and Tian X.Y.. 2017. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 31:1097–1106. 10.1096/fj.201601030R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husse, J., Leliavski A., Tsang A.H., Oster H., and Eichele G.. 2014. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 28:4950–4960. 10.1096/fj.14-256594 [DOI] [PubMed] [Google Scholar]
- Izumo, M., Pejchal M., Schook A.C., Lange R.P., Walisser J.A., Sato T.R., Wang X., Bradfield C.A., and Takahashi J.S.. 2014. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife. 3:e04617 10.7554/eLife.04617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalucka, J., de Rooij L.P.M.H., Goveia J., Rohlenova K., Dumas S.J., Meta E., Conchinha N.V., Taverna F., Teuwen L.A., Veys K., et al. . 2020. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell. 180:764–779.e20. 10.1016/j.cell.2020.01.015 [DOI] [PubMed] [Google Scholar]
- Keller, M., Mazuch J., Abraham U., Eom G.D., Herzog E.D., Volk H.D., Kramer A., and Maier B.. 2009. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA. 106:21407–21412. 10.1073/pnas.0906361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf, K., and Prinz M.. 2017. Microglia in steady state. J. Clin. Invest. 127:3201–3209. 10.1172/JCI90602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling, S., Dubeau-Laramée G., Ohm H., Labrecque N., Olivier M., and Cermakian N.. 2017. The circadian clock in immune cells controls the magnitude of Leishmania parasite infection. Sci. Rep. 7:10892 10.1038/s41598-017-11297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen, G.B., Cunningham P.S., Poolman T.M., Iqbal M., Maidstone R., Baxter M., Bagnall J., Begley N., Saer B., Hussell T., et al. . 2020. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA. 117:1543–1551. 10.1073/pnas.1915932117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova, A.A., and Kondratov R.V.. 2012. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13:325–335. 10.1038/nrn3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronowski, K.B., Kinouchi K., Welz P.S., Smith J.G., Zinna V.M., Shi J., Samad M., Chen S., Magnan C.N., Kinchen J.M., et al. . 2019. Defining the Independence of the Liver Circadian Clock. Cell. 177:1448–1462.e14. 10.1016/j.cell.2019.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausgruber, T., Fortelny N., Fife-Gernedl V., Senekowitsch M., Schuster L.C., Lercher A., Nemc A., Schmidl C., Rendeiro A.F., Bergthaler A., and Bock C.. 2020. Structural cells are key regulators of organ-specific immune responses. Nature. 583:296–302. 10.1038/s41586-020-2424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, Y., Lundkvist G.B., Brask J., Davidson A., Menaker M., Kristensson K., and Block G.D.. 2008. Interferon-gamma alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J. Biol. Rhythms. 23:150–159. 10.1177/0748730407313355 [DOI] [PubMed] [Google Scholar]
- Lavin, Y., and Merad M.. 2013. Macrophages: gatekeepers of tissue integrity. Cancer Immunol. Res. 1:201–209. 10.1158/2326-6066.CIR-13-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Malkani G., Shi X., Meyer M., Cunningham-Runddles S., Ma X., and Sun Z.S.. 2006. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 74:4750–4756. 10.1128/IAI.00287-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W.W., Wei S.Z., Huang G.D., Liu L.B., Gu C., Shen Y., Wang X.H., Xia S.T., Xie A.M., Hu L.F., et al. . 2020. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 34:6570–6581. 10.1096/fj.201901565RR [DOI] [PubMed] [Google Scholar]
- Logan, R.W., Arjona A., and Sarkar D.K.. 2011. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav. Immun. 25:101–109. 10.1016/j.bbi.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, R.W., Zhang C., Murugan S., O’Connell S., Levitt D., Rosenwasser A.M., and Sarkar D.K.. 2012. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J. Immunol. 188:2583–2591. 10.4049/jimmunol.1102715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, D., Battista M., Shi P.A., Isola L., and Frenette P.S.. 2008. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 3:364–366. 10.1016/j.stem.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni, G.J.M., Sulli A., Pizzorni C., Villaggio B., and Cutolo M.. 2002. Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann. N. Y. Acad. Sci. 966:271–275. 10.1111/j.1749-6632.2002.tb04226.x [DOI] [PubMed] [Google Scholar]
- Majumdar, T., Dhar J., Patel S., Kondratov R., and Barik S.. 2017. Circadian transcription factor BMAL1 regulates innate immunity against select RNA viruses. Innate Immun. 23:147–154. 10.1177/1753425916681075 [DOI] [PubMed] [Google Scholar]
- Man, K., Loudon A., and Chawla A.. 2016. Immunity around the clock. Science. 354:999–1003. 10.1126/science.aah4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli, G., Sothern R.B., De Cata A., Giuliani F., Fontana A., Copetti M., Pellegrini F., and Tarquini R.. 2011. A timetable of 24-hour patterns for human lymphocyte subpopulations. J. Biol. Regul. Homeost. Agents. 25:387–395. [PubMed] [Google Scholar]
- Méndez-Ferrer, S., Lucas D., Battista M., and Frenette P.S.. 2008. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 452:442–447. 10.1038/nature06685 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer, S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., and Frenette P.S.. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466:829–834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, J.E., Stone P.H., Turi Z.G., Rutherford J.D., Czeisler C.A., Parker C., Poole W.K., Passamani E., Roberts R., Robertson T., et al. . 1985. Circadian variation in the frequency of onset of acute myocardial infarction. N. Engl. J. Med. 313:1315–1322. 10.1056/NEJM198511213132103 [DOI] [PubMed] [Google Scholar]
- Nakamura, Y., Nakano N., Ishimaru K., Ando N., Katoh R., Suzuki-Inoue K., Koyanagki S., Ogawa H., Okumura K., Shibata S., and Nakao A.. 2016. Inhibition of IgE-mediated allergic reactions by pharmacologically targeting the circadian clock. J. Allergy Clin. Immunol. 137:1226–1235. 10.1016/j.jaci.2015.08.052 [DOI] [PubMed] [Google Scholar]
- Nguyen, K.D., Fentress S.J., Qiu Y., Yun K., Cox J.S., and Chawla A.. 2013. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 341:1483–1488. 10.1126/science.1240636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, J., Wu Z., Meng J., Saito T., Saido T.C., Qing H., and Nakanishi H.. 2019. An impaired intrinsic microglial clock system induces neuroinflammatory alterations in the early stage of amyloid precursor protein knock-in mouse brain. J. Neuroinflammation. 16:173 10.1186/s12974-019-1562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum, J.C., Van Dyken S.J., von Moltke J., Cheng L.E., Mohapatra A., Molofsky A.B., Thornton E.E., Krummel M.F., Chawla A., Liang H.-E., and Locksley R.M.. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 502:245–248. 10.1038/nature12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, G., Koncevičius K., Ebrahimi S., Carlucci M., Groot D.E., Nair A., Zhang A., Kriščiūnas A., Oh E.S., Labrie V., et al. . 2019. Circadian oscillations of cytosine modification in humans contribute to epigenetic variability, aging, and complex disease. Genome Biol. 20:2 10.1186/s13059-018-1608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva-Ramírez, J., Moreno-Altamirano M.M.B., Pineda-Olvera B., Cauich-Sánchez P., and Sánchez-García F.J.. 2014. Crosstalk between circadian rhythmicity, mitochondrial dynamics and macrophage bactericidal activity. Immunology. 143:490–497. 10.1111/imm.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas-Montanes, J., Beyaz S., Rakoff-Nahoum S., and Shalek A.K.. 2020. Distribution and storage of inflammatory memory in barrier tissues. Nat. Rev. Immunol. 20:308–320. 10.1038/s41577-019-0263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama, Y., Bartman C.M., Bonney S., Lee J.S., Walker L.A., Han J., Borchers C.H., Buttrick P.M., Aherne C.M., Clendenen N., et al. . 2019. Intense Light-Mediated Circadian Cardioprotection via Transcriptional Reprogramming of the Endothelium. Cell Rep. 28:1471–1484.e11. 10.1016/j.celrep.2019.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli, R., Petrarca C., and Di Gioacchino M.. 2018. Biological clocks: their relevance to immune-allergic diseases. Clin. Mol. Allergy. 16:1–8. 10.1186/s12948-018-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomino-Segura, M., Latino I., Farsakoglu Y., and Gonzalez S.F.. 2020. Early production of IL-17A by γδ T cells in the trachea promotes viral clearance during influenza infection in mice. Eur. J. Immunol. 50:97–109. 10.1002/eji.201948157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzer, S.E., Dodge A.M., Kelly E.A.B., and Jarjour N.N.. 2003. Circadian variation of sputum inflammatory cells in mild asthma. J. Allergy Clin. Immunol. 111:308–312. 10.1067/mai.2003.65 [DOI] [PubMed] [Google Scholar]
- Pariollaud, M., Gibbs J.E., Hopwood T.W., Brown S., Begley N., Vonslow R., Poolman T., Guo B., Saer B., Jones D.H., et al. . 2018. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J. Clin. Invest. 128:2281–2296. 10.1172/JCI93910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-Lapa, M.A., Tamura E.K., Salustiano E.M.A., and Markus R.P.. 2013. Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. J. Pineal Res. 55:240–246. 10.1111/jpi.12066 [DOI] [PubMed] [Google Scholar]
- Plikus, M.V., Van Spyk E.N., Pham K., Geyfman M., Kumar V., Takahashi J.S., and Andersen B.. 2015. The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythms. 30:163–182. 10.1177/0748730414563537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast, B.J., Cable E.J., Patel P.N., Pyter L.M., Onishi K.G., Stevenson T.J., Ruby N.F., and Bradley S.P.. 2013. Impaired leukocyte trafficking and skin inflammatory responses in hamsters lacking a functional circadian system. Brain Behav. Immun. 32:94–104. 10.1016/j.bbi.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram, R.V., Kowalczyk M.S., de Boer C.G., Schneider R.K., Miller P.G., McConkey M., Tothova Z., Tejero H., Heckl D., Järås M., et al. . 2016. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 165:303–316. 10.1016/j.cell.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert, S.M., and Weaver D.R.. 2002. Coordination of circadian timing in mammals. Nature. 418:935–941. 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- Sato, S., Sakurai T., Ogasawara J., Takahashi M., Izawa T., Imaizumi K., Taniguchi N., Ohno H., and Kizaki T.. 2014. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 192:407–417. 10.4049/jimmunol.1301982 [DOI] [PubMed] [Google Scholar]
- Scheiermann, C., Kunisaki Y., Lucas D., Chow A., Jang J.E., Zhang D., Hashimoto D., Merad M., and Frenette P.S.. 2012. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 37:290–301. 10.1016/j.immuni.2012.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann, C., Gibbs J., Ince L., and Loudon A.. 2018. Clocking in to immunity. Nat. Rev. Immunol. 18:423–437. 10.1038/s41577-018-0008-4 [DOI] [PubMed] [Google Scholar]
- Schibler, U. 2006. Circadian time keeping: the daily ups and downs of genes, cells, and organisms. Prog. Brain Res. 153:271–282. 10.1016/S0079-6123(06)53016-X [DOI] [PubMed] [Google Scholar]
- Schloss, M.J., Hilby M., Nitz K., Guillamat Prats R., Ferraro B., Leoni G., Soehnlein O., Kessler T., He W., Luckow B., et al. . 2017. Ly6Chigh Monocytes Oscillate in the Heart During Homeostasis and After Myocardial Infarction-Brief Report. Arterioscler. Thromb. Vasc. Biol. 37:1640–1645. 10.1161/ATVBAHA.117.309259 [DOI] [PubMed] [Google Scholar]
- Sherratt, M.J., Hopkinson L., Naven M., Hibbert S.A., Ozols M., Eckersley A., Newton V.L., Bell M., and Meng Q.J.. 2019. Circadian rhythms in skin and other elastic tissues. Matrix Biol. 84:97–110. 10.1016/j.matbio.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Shi, C., Jia T., Mendez-Ferrer S., Hohl T.M., Serbina N.V., Lipuma L., Leiner I., Li M.O., Frenette P.S., and Pamer E.G.. 2011. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 34:590–601. 10.1016/j.immuni.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, A.C., Arjona A., Hughes M.E., Nitabach M.N., and Fikrig E.. 2012a Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav. Immun. 26:407–413. 10.1016/j.bbi.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, A.C., Arjona A., Walker W.E., and Fikrig E.. 2012b The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 36:251–261. 10.1016/j.immuni.2011.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver, A.C., Buckley S.M., Hughes M.E., Hastings A.K., Nitabach M.N., and Fikrig E.. 2018. Daily oscillations in expression and responsiveness of Toll-like receptors in splenic immune cells. Heliyon. 4:e00579 10.1016/j.heliyon.2018.e00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sládek, M., Rybová M., Jindráková Z., Zemanová Z., Polidarová L., Mrnka L., O’Neill J., Pácha J., and Sumová A.. 2007. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology. 133:1240–1249. 10.1053/j.gastro.2007.05.053 [DOI] [PubMed] [Google Scholar]
- Stark, K., Eckart A., Haidari S., Tirniceriu A., Lorenz M., von Brühl M.L., Gärtner F., Khandoga A.G., Legate K.R., Pless R., et al. . 2013. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat. Immunol. 14:41–51. 10.1038/ni.2477 [DOI] [PubMed] [Google Scholar]
- Sujino, M., Masumoto K.H., Yamaguchi S., van der Horst G.T.J., Okamura H., and Inouye S.T.. 2003. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr. Biol. 13:664–668. 10.1016/S0960-9822(03)00222-7 [DOI] [PubMed] [Google Scholar]
- Sundar, I.K., Ahmad T., Yao H., Hwang J.W., Gerloff J., Lawrence B.P., Sellix M.T., and Rahman I.. 2015. Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci. Rep. 5:9927 10.1038/srep09927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S., Toyabe S., Moroda T., Tada T., Tsukahara A., Iiai T., Minagawa M., Maruyama S., Hatakeyama K., Endoh K., and Abo T.. 1997. Circadian rhythm of leucocytes and lymphocytes subsets and its possible correlation with the function of the autonomic nervous system. Clin. Exp. Immunol. 110:500–508. 10.1046/j.1365-2249.1997.4411460.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, F., Hayashi Y., Wu Z., Liu Y., and Nakanishi H.. 2016. Diurnal dynamic behavior of microglia in response to infected bacteria through the UDP-P2Y6 receptor system. Sci. Rep. 6:30006 10.1038/srep30006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot, J., Hahn P., Kroehling L., Nguyen H., Li D., and Littman D.R.. 2020. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature. 579:575–580. 10.1038/s41586-020-2039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, F., Goc J., Zhou L., Chu C., Shah M.A., Eberl G., and Sonnenberg G.F.. 2019. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci. Immunol. 4(40):eaax1215 10.1126/sciimmunol.aax1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier, E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., et al. . 2018. Innate Lymphoid Cells: 10 Years On. Cell. 174:1054–1066. 10.1016/j.cell.2018.07.017 [DOI] [PubMed] [Google Scholar]
- Wahle, M., Stachetzki U., Krause A., Pierer M., Häntzschel H., and Baerwald C.G.O.. 2001. Regulation of beta2-adrenergic receptors on CD4 and CD8 positive lymphocytes by cytokines in vitro. Cytokine. 16:205–209. 10.1006/cyto.2001.0965 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Subramanian M., Yurdagul A. Jr., Barbosa-Lorenzi V.C., Cai B., de Juan-Sanz J., Ryan T.A., Nomura M., Maxfield F.R., and Tabas I.. 2017. Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell. 171:331–345.e22. 10.1016/j.cell.2017.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Song L., Liu M., Ge R., Zhou Q., Liu W., Li R., Qie J., Zhen B., Wang Y., et al. . 2018. A proteomics landscape of circadian clock in mouse liver. Nat. Commun. 9:1553 10.1038/s41467-018-03898-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q., Robinette M.L., Billon C., Collins P.L., Bando J.K., Fachi J.L., Sécca C., Porter S.I., Saini A., Gilfillan S., et al. . 2019. Circadian rhythm-dependent and circadian rhythm-independent impacts of the molecular clock on type 3 innate lymphoid cells. Sci. Immunol. 4:eaay7501 10.1126/sciimmunol.aay7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz, P.S., Zinna V.M., Symeonidi A., Koronowski K.B., Kinouchi K., Smith J.G., Guillén I.M., Castellanos A., Furrow S., Aragón F., et al. . 2019. BMAL1-Driven Tissue Clocks Respond Independently to Light to Maintain Homeostasis. Cell. 177:1436–1447.e12. 10.1016/j.cell.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, C., Silvestre-Roig C., Ortega-Gomez A., Lemnitzer P., Poelman H., Schumski A., Winter J., Drechsler M., de Jong R., Immler R., et al. . 2018. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 28:175–182.e5. 10.1016/j.cmet.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Wu, Y., Dissing-Olesen L., MacVicar B.A., and Stevens B.. 2015. Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol. 36:605–613. 10.1016/j.it.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H., Li H., Woo S.L., Kim S.M., Shende V.R., Neuendorff N., Guo X., Guo T., Qi T., Pei Y., et al. . 2014. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. J. Biol. Chem. 289:16374–16388. 10.1074/jbc.M113.539601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M., Li D., Chang Z., Yang Z., Tian Z., and Dong Z.. 2015. PDK1 orchestrates early NK cell development through induction of E4BP4 expression and maintenance of IL-15 responsiveness. J. Exp. Med. 212:253–265. 10.1084/jem.20141703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasłona, Z., Case S., Early J.O., Lalor S.J., McLoughlin R.M., Curtis A.M., and O’Neill L.A.J.. 2017. The circadian protein BMAL1 in myeloid cells is a negative regulator of allergic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 312:L855–L860. 10.1152/ajplung.00072.2017 [DOI] [PubMed] [Google Scholar]
- Zhang, D., Chen G., Manwani D., Mortha A., Xu C., Faith J.J., Burk R.D., Kunisaki Y., Jang J.E., Scheiermann C., et al. . 2015. Neutrophil ageing is regulated by the microbiome. Nature. 525:528–532. 10.1038/nature15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K.X., D’Souza S., Upton B.A., Kernodle S., Vemaraju S., Nayak G., Gaitonde K.D., Holt A.L., Linne C.D., Smith A.N., et al. . 2020. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature. 585:420–425. 10.1038/s41586-020-2683-0 [DOI] [PMC free article] [PubMed] [Google Scholar]