Abstract
Objective:
To evaluate the use of transarterial chemoembolisation (TACE) combined with microwave ablation (MWA) to treat patients with hepatocellular carcinoma (HCC) and type Ⅱ–Ⅲ portal vein tumour thrombosis (PVTT) intolerant to targeted drug (TG) therapy.
Methods:
A total of 18 patients with HCC and type Ⅱ–Ⅲ PVTT intolerant to TG were enrolled between June 2015 and December 2019, who were treated with TACE + MWA (MWA group). 24 patients were treated with TACE + TG (TG group; control cohort). Time to progression and overall survival (OS) were analysed along with the incidence of adverse events.
Results:
The median follow-up time was 19.0 months (9.0–32.0 months). The median OS was 17.0 months (8.3–29.3 months; MWA group) and 13.5 months (5.5–22.5 months; TG group) and was not significantly different. The 1- and 2 year OS was also comparable (MWA group: 66.7%, 44.4% vs Target group: 41.7%, 29.2%). Time to progression showed no distinct differences (MWA group: 11.5 months; TG group: 9.0 months) between the two groups. Moreover, the incidence of major Grade 3–4 adverse events in the MWA group (5.6%) was similar to those in the TG group (8.3%).
Conclusion:
TACE + MWA and TACE + TG were comparable in their safety and efficacy in patients with HCC, type Ⅱ–Ⅲ PVTT, and intolerance to TG.
Advances in knowledge:
TACE + MWA can be used as a palliative treatment alternative for TACE + TG in patients with HCC, type Ⅱ–Ⅲ PVTT, and intolerance to TG.
Introduction
Approximately, 35–50% of patients with hepatocellular carcinoma (HCC) develop portal vein tumour thrombosis (PVTT). The main trunk is affected in 15–30% of these patients.1,2 PVTT is a very poor prognostic factor and presents limited treatment options for patients with HCC. If left untreated, the median survival of HCC patients ranges between 2.7 and 4 months.3 Classifications, such as the Barcelona-Clinic Liver Cancer (BCLC) staging system,4,5 include antiangiogenic targeted drugs (TG; e.g. sorafenib, lenvatinib, or regorafenib) as the standard treatment for patients with PVTT. However, TGs only enable short-term survival (median overall survival [OS]: 6.5–13.6 months).5,6 Thus, combining therapeutic strategies might enhance the efficacy of treatment in these patients. For example, combining transarterial chemoembolisation (TACE) and sorafenib shows complementary effects.7 The START trial, including Asian patients with unresectable HCC, reported excellent efficacy of combining TACE and sorafenib8 that resulted in improved OS and longer time to progression (TTP) of patients with PVTT as compared to treatment with TACE or sorafenib alone.9 Although most side-effects of TG therapy (e.g. hand-foot skin reaction, hypertension, fatigue, diarrhoea, etc) were tolerated well and manageable, the patients occasionally manifested with serious adverse events, such as cardiac complications (1–5%), arterial thromboembolic (4.9%), and bleeding (15–16.7%).10 Moreover, TG therapy was expensive for patients in developing countries that resulted in intolerance or refusal of treatment. Thus, percutaneous ablation therapies may be an alternative for this group of patients. Studies have shown that combining TACE and radiofrequency ablation (RFA) significantly improved survival in patients with PVTT (mean: 29.5 months).11 The technical features of microwave ablation (MWA) are similar to that of RFA and it had several advantages (e.g. improved convection profile, higher intratumoral temperatures, and faster ablation times) as compared to RFA.12 Combining TACE and MWA improves OS in patients with BCLC stage B HCC.13 However, there are limited studies on using this combination treatment in patients with PVTT, particularly those with type Ⅱ–Ⅲ PVTT and intolerance to TG.
In this study, we hypothesised that TACE + MWA improves the survival of HCC patients with type Ⅱ–Ⅲ PVTT and intolerance to TG. Therefore, we evaluated the safety and efficacy of TACE + MWA therapy.
Methods and materials
Patient cohort
This retrospective study was approved by the Ethics Committee of Beijing Ditan Hospital. We enrolled patients with HCC and type Ⅱ–Ⅲ PVTT intolerant to TG (sorafenib or lenvatinib) and treated with TACE + MWA between June and December 2019. Similar patients who received TACE + TG constituted the control cohort. All patients provided written informed consent prior to treatment. HCC was diagnosed according to the guidelines for the diagnosis and treatment of primary liver cancer embodied by the BCLC classification.14 PVTT was diagnosed based on enhanced CT and type Ⅱ–Ⅲ PVTT was based on the classification proposed by Cheng et al15 (type Ⅱ PVTT: the thrombus occupied large branches of the portal vein; type Ⅲ PVTT: the thrombus involved the main trunk of the portal vein). All patients were informed of the advantages and disadvantages of TACE + MWA treatment and reluctant to accept other alternative treatments.
The inclusion criteria were as follows: (1) age 18–80 years; (2) Eastern Cooperative Oncology Group performance status score of 0–1; (3) liver function Child–Pugh class A or B; (4) maximum diameter of intrahepatic lesion ≤5 cm and ≤3 lesions; and (5) no history of anticancer treatment (e.g. liver transplantation, surgical resection, immunotherapy, 125I seed implantation, radiotherapy, chemotherapy, RF, cryoablation, or percutaneous ethanol injection).
The exclusion criteria were as follows: (1) superior mesenteric vein/hepatic vein tumour thrombosis; (2) severe liver malfunction (Child–Pugh score >9, serum total bilirubin level >3 mg dl−1, refractory ascites, and hepatic encephalopathy); (3) uncontrolled organ dysfunction syndrome (e.g. infection, dysfunction of heart, kidney, and brain, chronic obstructive pulmonary disease, and intrahepatic bile duct dilation); (4) untreatable coagulopathy (PLT <30 ×109/L, PT >30 s, PTA < 40%), and (5) presence of other malignancies or extrahepatic metastases.
Equipment
We used the KV2100 microwave tumour treatment device (Nanjing Kangyou Microwave Energy Sources Institute, China; frequency, 2450 MHz; needle type, internal water-cooling; electrode diameter, 15G; electrode length, 150 or 180 mm; power, 0–100 W; and distance from the aperture of the MW emission to the needle tip, 11 mm), Siemens AG CT scanner (Germany; tube voltage, 120 kV; tube current, 200 mA; slice thickness, 5 mm; and pitch, 1 mm), and Innova 3100-IQ digital subtraction angiography system (GE, USA) for TACE.
Treatment protocol
All patients were treated with TACE by experienced interventional radiologists. The protocol was as follows: hepatic artery angiography was performed using the Seldinger technique. Femoral arterial catheterisation (5.0 Fr, Terumo Corporation, Japan) was performed on the common or proper hepatic artery. Subsequently, a microcatheter (2.7 Fr, Terumo Corporation, Japan) was super-selectively inserted into the hepatic lobe or segmental artery branch and injected with a mixture of ethiodised poppyseed oil (5–10 ml, Jiangsu Hengrui Medicine Co., Ltd., China) and loplatin (40 mg, Hainan Chang'an International Pharmaceutical Co., Ltd., China). Finally, blank microspheres (100–300 µm, Merit Medical Systems, Inc., Biosphere medical SA, Paris, France.) were infused to embolise the artery until stasis flow was observed in the tumour vascularity. This procedure was repeated every 1–2 months according to the tolerance and treatment response of patients.
MWA was initiated 1–2 weeks after TACE. The procedure was performed under local anaesthesia and vital signs were monitored using electrocardiography. The patient was injected with pethidine hydrochloride and diazepam 30 min before the treatment. Procedures were performed using unenhanced CT scans by one of two doctors with 10 years of experience in HCC ablation.
Patients were instructed to be in the supine, lateral decubitus, or prone position depending on the designated route of puncture. HCC lesions were sequentially ablated. After the skin around the site of puncture was disinfected and numbed using local anaesthesia (lidocaine), a prepared guide pin (21G) was inserted and positioned to reach the edge of the lesion with the help of CT scans. Subsequently, the microwave electrode was inserted into the lesions in the direction of the guide pin, guide pin was removed, and microwave electrode was adjusted based on the CT scans. Microwave electrode placement was performed based on the expected ablation zone size described by the manufacturer, considering a sufficient (>5 mm) safety margin around the tumour. The microwave power was set to 50–60 W. Each lesion was ablated for 5–8 min; the area of ablation covering the lesion and its surroundings measured ≥5 mm. Figure 1 shows the protocol for ablation of PVTT lesions. The initial steps were the same as those for HCC lesions. The microwave electrode was inserted into the lesions parallel to PVTT maintaining 0.5–1.0 cm between the tip of the electrode to proximal end of the right or left branch of the PVTT; the procedure was performed in the far to near direction using 50–70 W power for 5–8 min. If a single treatment did not produce satisfactory results, further rounds of MWA were performed immediately until the ablation area covered the lesion. Routine ablation needle tracking was performed to prevent implantation metastasis and bleeding. A pressure dressing was placed to prevent haemorrhage immediately after the procedures and a post-operative CT scan was obtained to confirm lack of complications that required further management. After treatment, liver protection, anti-inflammatory, and analgesic therapies were prescribed.
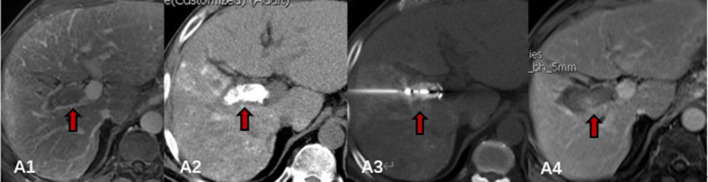
Figure 1.
MWA process of PVTT: A1, contrast-enhanced MRI before MWA, tumour thrombus was detected in right large branch of portal vein (thick arrow); A2, abdominal CT image after TACE, deposition of iodised oil within tumour thrombus was observed (thick arrow); A3, microwave electrode was inserted precisely into the lesions parallel to the direction of the PVTT under guidance of CT (thick arrow); A4, contrast-enhanced abdominal MRI after MWA, the ablated area completely covered the tumour thrombus (thick arrow). MWA, microwave ablation; PVTT, portal vein tumour thrombosis; TACE, transarterial chemoembolisation.
TG therapy was administered for 3 days after the first round of TACE. Sorafenib (Nexavar; Bayer, Leverkusen, Germany) was prescribed at a recommended dose of 400 mg bd (the dose could be reduced to 400 mg/day for sorafenib-related toxicities) and lenvatinib (Eisai Inc., Woodcliff Lake, NJ) was prescribed at 12 mg/day for patients with ≥60 kg or 8 mg/day for patients with <60 kg (dose interruptions followed by reduction for lenvatinib-related toxicities to 8 mg/day or 4 mg/day, respectively were permitted). A 3 day interruption was performed to efficiently control perioperative complications before and after TACE cycles.
Evaluating safety and efficacy
Patients were routinely followed up (every 3–6 months) immediately after treatment until death, study termination (December 2019), or till the patient withdrew consent. TACE and/or MWA was repeated using the same protocol upon detection of residual tumours or new lesions based on follow-up enhanced CT or MRI.
Efficacy was assessed based on the Modified Response Evaluation Criteria in Solid Tumour.16 The primary end points were OS (measured from the date of procedure until date of death or last follow-up) and TTP (measured from the date of procedure until date of tumour progression based on enhanced CT or MRI scans). Secondary end points comprised adverse events. Sorafenib or lenvatinib- and TACE-related adverse events were graded according to the Common Terminology Criteria for Adverse Events v. 4.017 and MWA-related adverse events were graded according to the updated standards established by the Society of Interventional Radiology.18
Statistical analysis
Statistical analyses were performed using SPSS19.0 (SPSS, IBM Company, USA). Continuous variables were represented as mean ± standard deviation and analysed using the Student’s t-test. Categorical variables were represented as frequencies and analysed using the χ2 test. OS and TTP were analysed using Kaplan–Meier plots and log-rank test. p < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 18 patients in the MWA group were used for data analysis; among these, 14 were intolerant to sorafenib presenting with persistent hand-foot skin reaction, hypertension, or diarrhoea and four were intolerant to lenvatinib presenting with severe hypertension, diarrhoea, or gastrointestinal bleeding. The control cohort comprised 24 patients (sorafenib: 21 patients; lenvatinib: 3 patients). The baseline characteristics of patients were similar between the groups, such as gender, age, hepatitis infection, Eastern Cooperative Oncology Group score, Child–Pugh grade, blood routine, coagulation, AFP, lesion number and size, PVTT type, and size (Table 1).
Table 1.
Baseline data of patients and tumours in the two groups
| Variable | MWA group | TG group | p |
|---|---|---|---|
| Total (n) | 18 | 24 | 0.42 |
| Gender | |||
| Male (n) | 15 | 18 | 0.68 |
| Female (n) | 3 | 6 | 0.54 |
| Age (year) | 58.0 (55.5–64.0) | 60.4 (53.2–68.6) | 0.76 |
| Hepatitis type | |||
| HBV (n) | 17 | 23 | 0.46 |
| HCV (n) | 1 | 1 | 1 |
| ECOG | |||
| 0 (n) | 16 | 20 | 0.60 |
| 1 (n) | 2 | 4 | 0.66 |
| Child-Pugh grade | |||
| A (n) | 17 | 22 | 0.64 |
| B (n) | 1 | 2 | 0.87 |
| Blood routine | |||
| WBC (109/L) | 4.0 (2.9–5.1) | 3.7 (2.3–5.3) | 0.59 |
| PLT (109/L) | 83.0 (58.0–98.5) | 76.2 (51.5–91.8) | 0.78 |
| HB (g/L) | 128.0 (120.5–140.5) | 121.5 (112.2–138.8) | 0.66 |
| Coagulation function | |||
| PT (s) | 12.9 (12.0–14.1) | 13.2 (12.6–14.7) | 0.68 |
| PTA (%) | 86.0 (73.5–90.5) | 84.2 (68.7–88.4) | 0.73 |
| AFP (ng/ml) | 27.1 (3.4–1850.6) | 33.8 (5.4–2349.2) | 0.42 |
| Tumour number | |||
| 1 (n) | 10 | 9 | 0.85 |
| ≥2 (n) | 8 | 15 | 0.58 |
| Tumour max diameter (cm) | 2.0 (1.8–4.4) | 3.1 (2.5–4.8) | 0.36 |
| PVTT type | |||
| Ⅱ (n) | 14 | 15 | 0.82 |
| Ⅲ (n) | 4 | 9 | 0.64 |
| PVTT max diameter (cm) | 3.6 (3.2–5.1) | 3.9 (3.4–6.2) | 0.68 |
MWA, microwave ablation; PVTT, portal vein tumour thrombosis.
There was no significant difference in all variables in the two groups.
Analysis of efficacy
The median treatment time for TACE was 3.0 (1.5–5.0) in the MWA group and 3.5 (2–5.8) in the TG group; this difference was not statistically significant. Median treatment power and time for PVTT was 55 W (50–70 W) and 6 min (6–8 min); the median time for MWA was 1.3 (1.0–2.0).
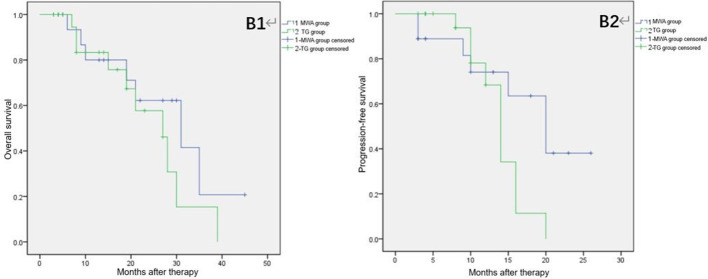
Median follow-up duration was 19.0 months (9.0–32.0 months) in both patient groups. Median OS and TTP were 17.0 months (8.3–29.3 months) and 11.5 months (3.8–20.0 months), respectively for patients treated with MWA and 13.5 months (5.5–22.5 months) and 9.0 months (4.0–13.5 months), respectively in patients administered TG. The 1- and 2 year rates of survival in MWA patients were 66.7 and 44.4%, respectively and 41.7 and 29.2%, respectively in the TG group. Patients treated with TACE + MWA showed higher OS, TTP, and 1- or 2 year rates of survival as compared to those in patients treated with TACE + TG; however, these differences were not statistically significant. Table 2 shows the data for clinical efficacy. Figure 2 represents the cumulative survival and progression-free survival curves and Figures 3 and 4 show the MRI images at diagnosis and follow-up of patients.
Table 2.
Follow-up survey of patientsin the two groups
| Variable | MWA group | TG group | p |
|---|---|---|---|
| OS (months) | 17.0 (8.3–29.3) | 13.5 (5.5–22.5) | 0.212 |
| 1 year survival rate (%) | 66.7 | 41.7 | 0.16 |
| 2 year survival rate (%) | 44.4 | 29.2 | 0.20 |
| TTP (months) | 11.5 (3.8–20.0) | 9.0 (4.0–13.5) | 0.32 |
MWA, microwave ablation; OS, overall survival; TG, targeted drug; TTP, time to progression.
Figure 2.
Overall survival and progression-free survival of patients in the two groups: B1, overall survival; B2, progression-free survival.
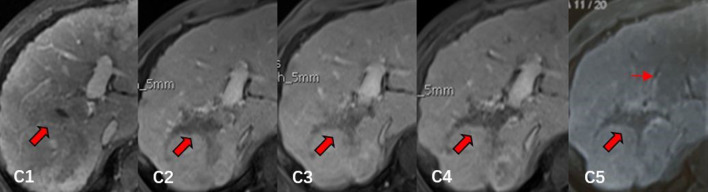
Figure 3.
Contrast-enhanced MRI in a 54-year-old male who had HCC with PVTT: C1, contrast-enhanced MRI before MWA, PVTT was detected in right main branch (thick arrow); C2, 6 month follow-up MRI after MWA, most of the PVTT was ablated and the extent of PVTT was gradually reduced (thick arrow); C3-C4, 12 month follow-up and 18 month follow-up MRI after MWA, the extent of PVTT continued to reduce and remained stable (thick arrow); C5, 24 month follow-up MRI after MWA, the extent of right main branch PVTT remained stable (thick arrow), but the tumour thrombus in left main branch of portal vein was nascent (thin arrow). HCC, hepatocellular carcinoma; MWA, microwave ablation; PVTT, portal vein tumour thrombosis.
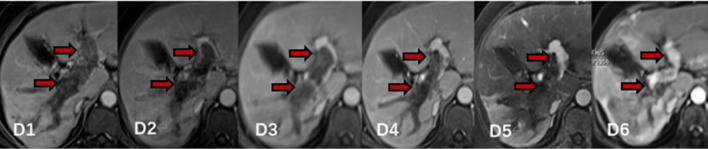
Figure 4.
Contrast-enhanced MRI from a 64-year-old female who had HCC with PVTT: D1, contrast-enhanced MRI before MWA, PVTT was detected in left and right main branch (thick arrow); D2, 3 month follow-up MRI after MWA, most of the PVTT was ablated and obstructed left branch of portal vein was partly recanalised (thick arrow); D3-D5, 6-month, 9-month and 12-month follow-up MRI after MWA, the extent of PVTT continued to reduce and remained stable, obstructed left and right branch of portal vein was opened further (thick arrow). D6, 15-month follow-up MRI after MWA, the extent of PVTT significantly reduced and obstructed left and right branch of portal vein were almost completely recanalised (thick arrow). HCC, hepatocellular carcinoma; MWA, microwave ablation; PVTT, portal vein tumour thrombosis.
Safety analysis
Side-effects associated with TACE included ascites, liver dysfunction, fatigue, fever, nausea, and vomiting. Majority of these were Grade 1–2 symptoms (MWA: 83.3% of patients vs TG: 87.5% of patients) according to Common Terminology Criteria for Adverse Events v. 4.0 and did not require treatment. The primary side-effects observed in patients administered TG were hand-foot skin reaction, hypertension, fatigue, and diarrhoea. These comprised 91.7% of Grade 1–2 symptoms; only two patients (8.3%) suffered from serious hypertension and diarrhoea and required treatment. The most common MWA-related adverse events were pain, fever, nausea, and vomiting that were observed intratreatment and 1–3 days after treatment; according to the SIR grading system, 88.9% were classified as Grade A or B and resolved spontaneously without treatment. Delayed bleeding was detected in one patient (5.6%), 2 days after the procedure and required prompt intervention with angiographic embolisation. There was no significant difference between the adverse events found in patients of the two groups (Table 3).
Table 3.
Adverse events related to treatment in the two groups
| Variable | MWA group | TG group | p |
|---|---|---|---|
| TACE related adverse events | |||
| Grade 1–2 (%) | 83.3 | 87.5 | 0.628 |
| Grade 3–4 (%) | 0 | 0 | 1 |
| TG related adverse events | |||
| Grade 1–2 (%) | / | 91.7 | / |
| Grade 3–4 (%) | / | 8.3 | / |
| MWA related adverse events | |||
| Grade A-B (%) | 88.9 | / | / |
| Grade C (%) | 5.6 | / | / |
| Total | |||
| Grade 1–2 and Grade A-B (%) | 94.4% | 95.8 | 0.853 |
| Grade 3–4 and Grade C (%) | 5.6 | 8.3 | 0.652 |
MWA, microwave ablation; TACE, transarterial chemoembolisation; TG, targeted drug.
Discussion
There are no efficient therapeutic strategies currently available for PVTT. Sorafenib has been the primary treatment in Europe and America. Southeast Asian countries focuses on using comprehensive treatment. Studies have shown that combining TACE and sorafenib imparted survival benefits as compared to that using only TACE in patients with PVTT or unresectable HCC19,20; median survival was 13 months for first-order patients and 15 months in second- or low-order PVTT19 and was consistent with our findings.
The widespread use of sorafenib as the primary TG for advanced HCC leads to intolerance and serious adverse events in some patients, such as cardiac events (1–5%), arterial thromboembolic (4.9%), bleeding (15–16.7%).10 Moreover, TG is very expensive. Alternatively, patients with HCC and PVTT benefit from combining TACE with thermal ablation. TACE + RFA enhances patient survival (median OS: 29.5 months [16.6–42.4 months]) in HCC patients with type I–III PVTT.11 Compared to RFA, MWA has a better convection profile, reaches higher intratumoral temperatures, and faster ablation times.12 Similar to our data, TACE + MWA benefits patients with HCC and type I–III (median OS: 13.5 months; 1- and 3 year OS: 48 and 23%, respectively).21
This study focused on patients with type Ⅱ–Ⅲ PVTT and intolerance to TG. Although OS and TTP were not significantly different between the two groups, median TTP and OS seemed longer in MWA patients as compared to that in the TG-administered patients. TACE + MWA may improve patient survival via multiple mechanisms. First, TACE significantly reduces arterial flow in tumours as well as in the PVTT, thereby delaying its progression.11 Second, MWA removes the obstruction from the portal vein, thereby improving blood supply to the liver parenchyma, while ablation destroys tumours and most of the accompanying PVTT.22 Finally, repeated TACE helps kill and inhibit residual tumours and reduces post-treatment recurrence and metastasis.23 Therefore, based on the published literature, we speculated that MWA eliminates the tumour and TACE induces local ischaemia, thereby enabling TACE + MWA to significantly improve the survival of patients with type Ⅱ–Ⅲ PVTT and intolerance to TG.
To the best of our knowledge, this is the first report on the use of TACE + MWA in treating patients with type Ⅱ–Ⅲ PVTT and intolerance to TG. This regimen was simple, involved minimal injury, and was comparable to the efficacy of TACE + TG.
However, this study has some limitations. First, we had a small patient cohort and lacked long-term follow-up data. Second, it was a single-centre retrospective study that may affect the generalisation of data. Third, the study did not include patients with huge (diameter >5 cm) or diffuse HCC. Finally, PVTT was confirmed only by imaging and not histopathologicAL examination.
Conclusion
In conclusion, the combined TACE + MWA regimen was safe and effective for patients with HCC and type Ⅱ–Ⅲ PVTT. We observed comparable OS and TTP as that obtained in patients having undergone TACE + TG. Thus, TACE + MWA could be a palliative treatment alternative for patients with intolerance to TG.
Footnotes
Acknowledgment: This article was supported by research fund program of Beijing Ditan Hospital (DTDR201806)
The authors Wen Peng Zhao and Honglu Li contributed equally to the work.
Contributor Information
Wen Peng Zhao, Email: zwp215@163.com.
Honglu Li, Email: lhl999_120@sina.com.
Jiang Guo, Email: guojiang2002105@aliyun.com.
Liang Cai, Email: cailiangmc@163.com.
Youjia Duan, Email: yutian110@163.com.
Xiaopu Hou, Email: hxp0525@163.com.
Hongliu Du, Email: duhongliu@163.com.
Xihong Shao, Email: shaoxihong5052@sina.com.
Zhenying Diao, Email: dzy-cindy@sohu.com.
Changqing Li, Email: changqing0402@163.com.
REFERENCES
- 1.Manzano-Robleda MDC, Barranco-Fragoso B, Uribe M, Méndez-Sánchez N. Portal vein thrombosis: what is new? Ann Hepatol 2015; 14: 20–7. doi: 10.1016/S1665-2681(19)30797-5 [DOI] [PubMed] [Google Scholar]
- 2.Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg 2001; 233: 379–84. doi: 10.1097/00000658-200103000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MdelC, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999; 29: 62–7. doi: 10.1002/hep.510290145 [DOI] [PubMed] [Google Scholar]
- 4.Llovet J, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19: 329–38. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 5. European Associationfor the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;. [DOI] [PubMed] [Google Scholar]
- 6.Liu P-H, Huo T-I, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis 2018; 38: 242–51. doi: 10.1055/s-0038-1666805 [DOI] [PubMed] [Google Scholar]
- 7.Han K, Kim JH, Ko G-Y, Gwon DI, Sung K-B. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol 2016; 22: 407–16. doi: 10.3748/wjg.v22.i1.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao Y, Chung Y-H, Han G, Yoon J-H, Yang J, Wang J, et al. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the start trial. Int J Cancer 2015; 136: 1458–67. doi: 10.1002/ijc.29126 [DOI] [PubMed] [Google Scholar]
- 9.Kim G-A, Shim JH, Yoon SM, Jung J, Kim JH, Ryu M-H, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Journal of Vascular and Interventional Radiology 2015; 26: 320–9. doi: 10.1016/j.jvir.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Gao Z-H, Qu X-J. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol 2015; 116: 216–21. doi: 10.1111/bcpt.12365 [DOI] [PubMed] [Google Scholar]
- 11.Zheng J-S, Long J, Sun B, Lu N-N, Fang D, Zhao L-Y, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival of patients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication for ablation? Clin Radiol 2014; 69: e253–63. doi: 10.1016/j.crad.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 12. Francesco Izzo Vincenza Granata, Roberto Grassi, Roberta Fusco, Raffaele Palaia, Paolo Delrio, et al. radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist 2019; 24: e990–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Shen L, Zhao L, Guan Z, Chen Q, Li W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn Interv Radiol 2018; 24: 219–24. doi: 10.5152/dir.2018.17528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999; 19: 329–38. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 15.Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology 2007; 54: 499–502. [PubMed] [Google Scholar]
- 16.Lencioni R, Llovet JM, Modified R. mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Protocol development. Cancer therapy evaluation program.. Available from: https://ctep.cancer.gov/protocolDevelopment/ electronicapplications/ctc.htm [accessed March 21, 2017].
- 18.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology 2014; 132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology 2014; 272: 284–93. doi: 10.1148/radiol.14131946 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Ikeda M, Torimura N, Tanabe N, Aikata H, Ueshima K, et al. Randomised, multicentre prospective trial of TransarterialChemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: tactics trial. Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long J, Zheng J-sheng, Sun B, Lu N. Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int 2016; 10: 175–84. doi: 10.1007/s12072-015-9673-6 [DOI] [PubMed] [Google Scholar]
- 22.Giorgio A, Di Sarno A, de Stefano G, Farella N, Scognamiglio U, de Stefano M, NunziaFarella US, Stefano Mde, et al. Hepatocellular carcinoma with cirrhosis: are patients with neoplastic main portal vein invasion eligible for percutaneous radiofrequency ablation of both the nodule and the portal venous tumor thrombus? American Journal of Roentgenology 2009; 193: 948–54. doi: 10.2214/AJR.08.2087 [DOI] [PubMed] [Google Scholar]
- 23.Kattipatanapong T, Nishiofuku H, Tanaka T, Sato T, Masada T, Tatsumoto S, et al. Improved local tumor control and survival rates by obtaining a 3D-Safety margin in superselective transarterial chemoembolization for small hepatocellular carcinoma. Cardiovasc Intervent Radiol 2020; 43: 423–33. doi: 10.1007/s00270-019-02365-9 [DOI] [PubMed] [Google Scholar]