Abstract
The emerging biological understanding of metastatic cancer and proof-of-concept clinical trials suggest that debulking all gross disease holds great promise for improving patient outcomes. However, ablation of multiple targets with conventional external beam radiotherapy systems is burdensome, which limits investigation and utilization of complete metastatic ablation in the majority of patients with advanced disease. To overcome this logistical hurdle, technical innovation is necessary. Biology-guided radiotherapy (BgRT) is a new external beam radiotherapy delivery modality combining positron emission tomography-computed tomography (PET-CT) with a 6 MV linear accelerator. The key innovation is continuous response of the linear accelerator to outgoing tumor PET emissions with beamlets of radiotherapy at subsecond latency. This allows the deposited dose to track tumors in real time. Multiple new hardware and algorithmic advances further facilitate this low-latency feedback process. By transforming tumors into their own fiducials after intravenous injection of a radiotracer, BgRT has the potential to enable complete metastatic ablation in a manner efficient for a single patient and scalable to entire populations with metastatic disease. Future trends may further enhance the utility of BgRT in the clinic as this technology dovetails with other innovations in radiotherapy, including novel dose painting and fractionation schemes, radiomics, and new radiotracers.
The emerging role of local ablative therapy in later stage disease
There is a surprising dissonance in the contemporary approach to treating solid tumor cancers. In the setting of local and locally advanced solid tumors, evidence accumulated over decades has shown that the best outcomes arise from a combination of aggressive local debulking of gross disease along with adjuvant systemic therapies to address any remaining micrometastatic burden. And yet, in the setting of metastatic disease of any degree, this combination of local debulking with systemic therapy has gone under-investigated and, consequently, underutilized.
Instead, the general approach to patients with a site of distant metastasis has been to use drug therapy alone and to employ local therapies only as a palliative modality. Nonetheless, the concept of debulking grossly visible disease in Stage 4 cancers – which we label complete metastatic ablation (CMA) – has been of interest to oncologists since at least the 1990s when the Hellman-Weichselbaum metastatic spectrum theory was postulated.1 This theory held that there may be a stage in cancer progression where the cancer has spread beyond the tissue of origin but is not yet extensively metastatic and that some cancers in this phase may be rendered curable with the same combination of aggressive local treatment and drug therapy used in earlier cancer stages.
Twenty-five years later, our understanding of cancer biology is unequivocally more complex, but this expanded knowledge has not precluded the metastatic spectrum theory. Quite the contrary, our modern understanding suggests several mechanisms by which local ablation of all visible sites of disease may create synergies with systemic agents and improve survival. Specifically, we identify four mechanisms by which CMA can enhance systemic strategies.
Firstly, CMA can reduce the total cancer burden in the patient’s body, resulting in fewer viable cancer cells requiring eradication by systemic therapy. This mathematical argument applies equally whether the systemic therapy is directly cytotoxic or acts through a secondary agent such as an activated T cell.2
Secondly, locally ablative therapies – especially when deployed early – may reduce or eliminate the pool of subclones from which therapeutic resistance can evolve. This is especially relevant for cancers with driver mutations. For instance, in metastatic EGFR+ non-small cell lung cancer (NSCLC) treated with small molecule inhibitors, the rate at which resistant cancer subclones eventually emerge approaches 100%.3,4
Thirdly, in the context of immunotherapeutic drugs like checkpoint inhibitors, radiotherapy directed at multiple targets may have unique advantages. Ionizing radiation has been shown to induce pro-inflammatory signals via the cGAS-STING-IFN pathway,5–8 which has the potential to convert immune-sparse tumor microenvironments (“cold tumors”) into inflamed phenotypes that are more amenable to T cell trafficking. At a whole-patient level, CMA may improve the ratio of T cells to neoplastic cells and also release into circulation a diverse array of neoantigens from clonally distinct metastatic sites.
Finally, a less appreciated means by which local therapies can improve hard oncologic end points such as overall survival is their potential to forestall causes of death and morbidity attributable to local tumor effects in a subset of patients. Eliminating gross tumors near a sensitive structure may keep patients alive and fit so that the benefits of systemic therapy can fully accrue. Postponing the pace of cancer lethality this way may enhance systemic therapies whose effects are not immediate, such as biologics, injected radionuclides, and immune system potentiators.
The clinical evidence for complete metastatic ablation
Even more compelling than these mechanisms are the positive reports from multiple proof-of-concept studies that have formally tested the intervention of complete debulking in randomized controlled trials. Given the limitations of currently available technology, these investigations have focused only on the beginning of the metastatic spectrum, termed “oligometastatic disease.” This portion of the metastatic spectrum has been defined variably in clinical trials as 3 to 5 or fewer metastases, and more recently, an expert consensus definition of 5 or fewer metastatic lesions was put forward by radiation oncology professional societies.9
NSCLC has been the focus of many of the first Phase II proof-of-concept investigations of oligometastatic disease. Gomez et al investigated the addition of complete ablation of gross disease after induction systemic therapy in a randomized controlled trial of 49 NSCLC patients with fewer than 3 sites of metastases who had stable or responding disease. The addition of local consolidative therapy (radiation or surgery) in the experimental arm resulted in a statistically significant improvement in progression-free survival (PFS) (14.2 months vs 4.4 months, p = 0.022).10,11 Longer follow-up also demonstrated an improvement in overall survival (OS) (41.2 months vs 17.0 months, p = 0.017). A similarly designed randomized controlled trial by Iyengar et al, also investigated the addition of local consolidative stereotactic radiation to systemic therapy in “limited” NSCLC, defined as the primary plus up to five metastatic sites. The authors again observed a near tripling of the median PFS (9.7 months vs 3.5 months, p = 0.01).12
More recently, the SINDAS randomized controlled trial for oligometastatic EGFR+ NSCLC was reported.13 In this study, 133 patients were randomized to either TKI alone or SBRT to all sites of disease followed by TKI. The median PFS, the primary end point, was improved with complete debulking (20.2 months vs 12.5 months, p < 0.001) as was OS (25.5 months vs 17.4 months, p < 0.001). Importantly, adverse events were similar between the two groups despite the addition of SBRT in the experimental arm.
Other notable prospective trials have looked at histologies beyond NSCLC. The SABR-COMET trial randomized patients with oligometastatic cancer arising from a basket of histologies to receive either standard-of-care systemic therapy and, if necessary, palliative radiotherapy or standard-of-care systemic therapy plus definitive metastasis-directed therapy to all sites of gross disease.14 Ultimately, 99 patients – including those with lung, prostate, breast, and colorectal tumors – with 1–5 metastatic sites of disease were randomized. Improvements in both OS (41 months vs 28 months, p = 0.09) and PFS (12 months vs 6 months, p = 0.001) were initially reported. Longer-term follow-up reported a 5-year OS of 42.3% in the ablative arm vs 17.7% in the non-ablative arm (p = 0.006).15 Two studies in prostate cancer, ORIOLE and STOMP, investigated SBRT to all sites of disease in oligorecurrent (1 to 3 metastases), hormone-sensitive disease.16,17 Interestingly, both studies investigated the intervention against observation to determine whether complete debulking had efficacy as a monotherapy. The primary end points, which were met in both studies, were based on clinically meaningful events: biochemical-recurrence free survival at 6 months in ORIOLE and androgen-deprivation therapy free survival in STOMP.
Finally, adding complete debulking with radiotherapy to a backbone of immunotherapy has been of keen interest. As described previously, the mechanisms of synergy between radiotherapy and immunotherapy are numerous and have the potential to be enhanced by multitarget as opposed to single-target ablation. Because immunotherapy is a more recent entrant to systemic therapy standard-of-care, prospective evidence for combinations with multi target radiotherapy are less mature. Notably, a single-arm study of patients with oligometastatic NSCLC (four or fewer lesions) who were treated with definitive local therapies to all sites followed by pembrolizumab found that patients had a median PFS of 19.1 months, compared to an expected historical control of 6.6 months (p = 0.005).18 Interestingly, PD-L1 expression was not correlated with better outcomes, suggesting that local therapies may potentiate pembrolizumab even among patients without high PD-L1 expression. Another single-arm study investigated the safety and clinical activity of combining pembrolizumab and multisite radiotherapy in a basket of heavily pretreated patients with metastatic solid tumors.19 They found that the combination was safe and observed a median OS of 9.6 months, which compared favorably with the expected survival outcomes for metastatic solid tumor patients refractory to multiple prior lines of systemic therapy.
Moving forward along the metastatic spectrum
The biological evidence-base and the results of multiple prospective clinical trials suggest there is real potential to enhance outcomes in metastatic solid tumors by combining systemic therapy with aggressive debulking of gross disease. In patients with few metastatic sites and low burden of disease, the aggregate evidence suggests that more durable remission and, in some cases, cure of disease is possible with this approach. However, this group represents a minority of patients with metastatic cancer. In fact, there is a heavy skew towards the lower end of the range (1–2 lesions) for allowed number of metastases among the enrolled subjects in the aforementioned trials. Therefore, a timely and intriguing question is whether we can translate the improvement in outcomes to patients on the higher end of the oligometastatic range and beyond in the polymetastatic range (i.e. 4 or more metastases).
In these patients, achieving a curative outcome may perhaps be less likely. At the same time, we may see extensions in DFS and OS that are on par with, or better than, those typically achieved with drug therapies. To effectively investigate the benefit of complete debulking in these patient populations, investigators need innovative approaches to overcome logistical limitations and efficiently ablate multiple sites of disease. This requirement remains a significant obstacle for current surgical and radiotherapeutic platforms. In the rest of this review, we will describe the strong potential of biology-guided radiotherapy to fill this unmet need.
Biology-guided radiotherapy
Anatomical vs biological imaging in radiotherapy
The history of employing imaging technologies in radiotherapy spans decades and is characterized by constant innovation.20–22 Image guidance has evolved with radiographic methods that provide anatomic information, such as 2D planar radiography, cone-beam CT, megavoltage CT, and, most recently, MRI. The evolution of combining anatomical imaging with radiotherapy has enabled progressively more precise delivery of ionizing radiation to targets delineated at finer and finer resolution. This has resulted in significant gains for patients because these technologies improve treatment planning, patient positioning and sparing of normal tissues, which result in reduced toxicities. Furthermore, these improvements in imaging have allowed for increased dose per fraction, clearing the way for more effective local ablation.
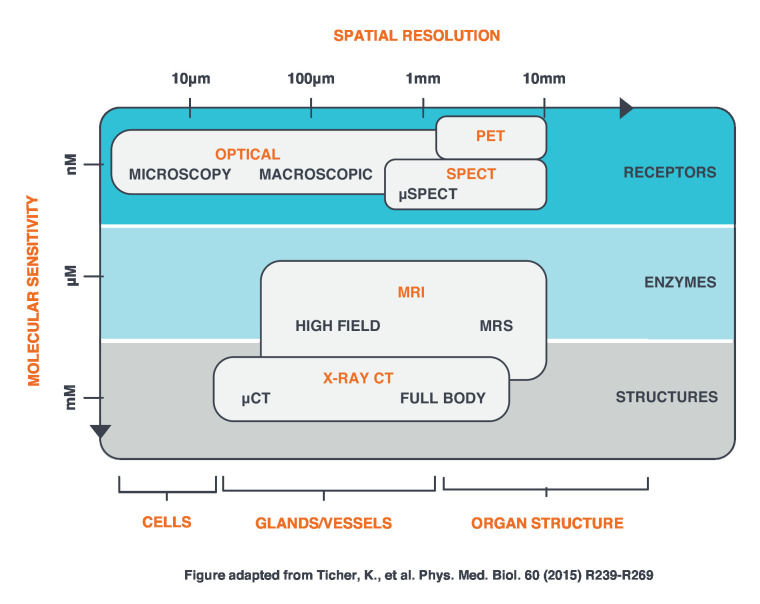
Despite the advantages of CT and MRI, molecular processes that take place at the nanometer scale are not well suited for visualization with anatomical imaging (Figure 1). As more attention is paid to categorizing tumors using phenotypic and genotypic features, there is renewed interest in incorporating imaging modalities that visualize biological and molecular aspects of the tissue targeted for treatment. Positron emission tomography (PET) is among the most powerful tools within this trend.
Figure 1.
Comparison of imaging modalities with respect to molecular sensitivity and spatial resolution (Adapted from Tichauer et al23). PET, Positron emission tomography; SPECT, single photon emission computed tomography.
PET is designed to visualize inherent biological processes rather than solely anatomy, which has implications for leveraging the innate characteristics of a tumor to direct treatment. It can also be used to investigate functional and molecular changes in the tumor in response to therapy that can be detected before anatomic changes.24–30 Furthermore, PET imaging is inherently compatible with a wide spectrum of positron-emitting tracers that can probe different biological states with exquisite sensitivity. The lack of an anatomic reference frame is a weakness of PET that has been addressed by combining PET with CT or MRI to enable precise correlations of molecular emissions with the surrounding anatomy.31 Although the concept of using biological information to inform radiotherapy has been an active area of interest,32–35 it has not been incorporated as an online guidance tool for radiotherapy procedures.
Biology-guided radiotherapy (BgRT), developed by RefleXion Medical (Hayward, CA), is the first fusion of PET/CT and radiotherapy delivery in a single system. It is an upcoming extension of the X1 which received FDA clearance in March 2020 for CT-based image-guided (i.e. non-BgRT) IMRT, SBRT and SRS applications. Importantly, BgRT simultaneously represents both a continuation of, and a departure from, conventional image guidance. Although BgRT fuses advanced imaging with radiotherapy, it does not fit within the typical paradigm of visualizing a fully formed image and then directing radiotherapy using that visualization. Rather, BgRT’s fundamental underpinning is that tumor PET emissions are detected and processed in real time so that the radiation dose is effectively tracked to lesion motion. Hence, this feedback loop between biological activity and the linear accelerator (LINAC) represents an evolution from classical image guidance. By enabling cross-talk between a tumor and the LINAC, BgRT transforms tumors into their own “biological fiducials,” with the aim of simplifying the process of radiotherapy delivery to multiple sites of disease throughout the body in the same treatment session.
BgRT hardware overview
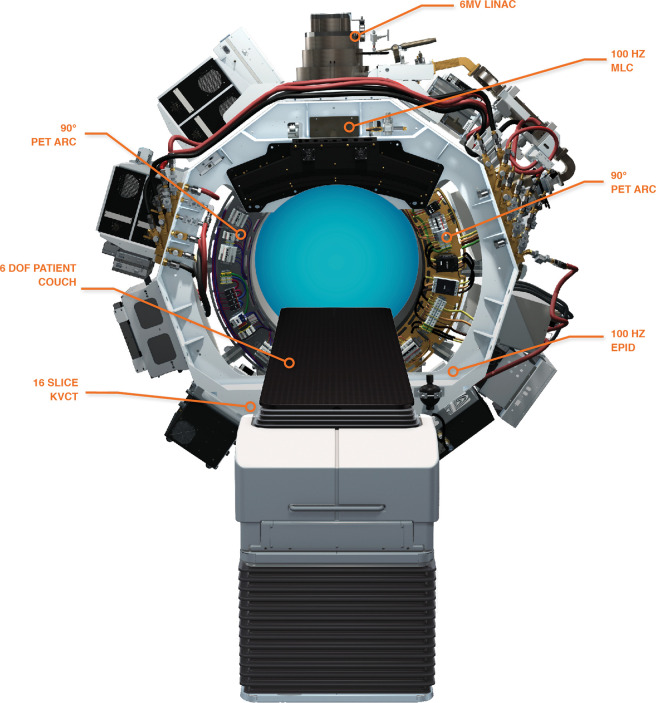
The X1 includes a 6 MV (flattening filter free) LINAC, a high-speed binary multi leaf collimator (MLC) with 64 leaves each at 6.25 mm width at isocenter to shape the beam, and dual 90° PET arcs for detecting biological signals. Leakage is minimized with a primary collimator as well as an MLC leaf size of 11 cm in the beam direction. The system also houses a 16-slice kilovoltage CT scanner for anatomical localization and a megavoltage detector for sensing of the portal radiation through the patient. These subsystems rotate together at 60 RPM on a ring-gantry platform with a 85 cm source-to-axis distance, delivering radiation from 100 discrete firing positions around the patient (Figure 2). The X1 also has an effective 6 degree-of-freedom (DOF) couch (the sixth DOF provided by the rotation of the gantry) that dwells at and translates through 2.1 mm incremental “beam stations” both in and out of bore directions during treatment. Varying the dwell time at each beam station provides variable pitch for the quasihelical nature of the delivery.
Figure 2.
The RefleXion™ X1 Biology-guided radiotherapy machine.
The high-speed rotation of the X1 system is required to form limited-time-sampled (LTS) PET images at a high frame rate; with PET detectors spanning symmetrically opposed 90° arcs, a 60 RPM rotation rate translates to a full tomographic sampling every 500 ms (half-rotation). This fast rotation allows the system to capture and process an LTS data set before the tumor has significantly changed position due to physiologic movements like respiration. The LTS PET images are derived from filtered-backprojection reconstruction. They are attenuation and randoms corrected, however they are not scatter corrected as all background outside of the pre-identified region of interest containing the “fiducial” is masked.
The other components of the X1 must also meet the demands for high speed and low latency. For example, fluences based on each LTS PET image are calculated in 100 ms windows and then delivered across 10 firing positions over a successive 100 ms window. To deliver fluence at this pace, the X1 has a high-speed binary MLC that transitions leaves at 100 Hz. Even with this unprecedented speed, a small degree of residual latency is unavoidable and is accounted for by adding a modest treatment margin to the tumor volume as described below.
BgRT workflow and algorithm
The BgRT workflow begins with a traditional simulation CT with the patient in the treatment position for contouring of the target(s) and organs-at-risk. An additional volume is contoured and denoted as a biology-tracking zone (BTZ) that is defined and registered relative to the simulation CT. The BTZ includes the anticipated full range of the target motion with an added margin accounting for residual BgRT-related uncertainties.36 The BTZ is not a prescription volume requiring uniform dose coverage. Rather, it acts as a bounding volume that eliminates interference from PET signals originating elsewhere from the tumor in organs such as the heart.
BgRT simulation also includes a BgRT Imaging-Only Session, which is an additional input to the treatment planning process. This involves administering a radiotracer with the same dose that would be used for each fraction (anticipated tracer dose will be similar to that used for diagnostic procedures), and then positioning the patient on the X1 to collect PET and CT imaging data necessary for creating the treatment plan. The X1 planning system dose calculation is based on a collapsed cone convolution superposition algorithm.
In traditional treatment planning, the desired dose to the target and constraints to normal tissue are met by optimizing for and generating a set of machine-deliverable radiotherapy fluences delivered from many pre-determined angles around the patient. In contrast, BgRT requires reacting to stochastically received LTS PET images with partial fluences, which means that those partial fluences cannot be calculated in advance. As such, the treatment planning process instead optimizes for fluence indirectly by calculating a transfer function, termed the firing matrix, that best translates a given PET image to the desired fluences. The BgRT Imaging-Only Session is therefore useful in approximating what the LTS PET images and thus partial fluences will be during treatment.
Although the firing matrix is calculated using a full PET image obtained through filtered-backprojection at the BgRT Imaging-Only Session (to generate a complete fluence), it can also be applied to LTS PET images to generate partial fluences. This follows from the fact that the firing matrix is confined by the treatment planner to take the form of a linear, shift-invariant operator that can be applied to LTS PET images to derive a partial fluence. In turn, just as the sequential LTS PET images sum to a full PET image, the partial fluences will sum to the complete intended fluence. Figure 3 is a visual representation of this principle of linear superposition.
Figure 3.
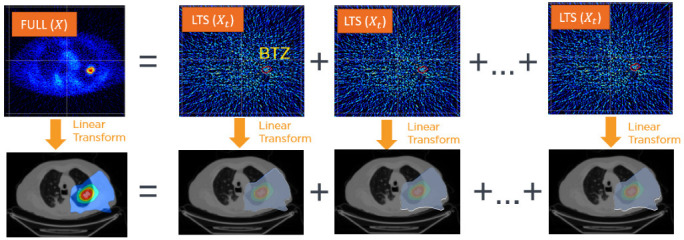
Just as the LTS PET images, Xt, sum to the full PET image X, the derived partial fluences sum to the complete intended fluence. LTS, limited-time-sampled; PET, positron emmision tomography. BTZ, biology-tracking zone.
Importantly, a tumor’s PET profile and, by extension, its constituent LTS PET images can vary between radiotracer administrations that may occur over a 1–2 week span due to a variety of biological, physiological, and logistical factors. Therefore, BgRT treatment planning generates a continuum of possible radiotherapy dose distributions that reflect possible variations in tumor position and tumor contrast, visually represented in a bounded dose–volume histrogram (bDVH). Specifically, different initial positions of the tumor within the BTZ as well as variations of contrast up to 25% are modeled. This distinct feature of BgRT robustly accounts for and models the likelihood that the tumor’s behavior and appearance at each treatment fraction may change. During treatment planning, the physician approves the comprehensive set of dosimetric scenarios represented by the bDVH that can occur during BgRT delivery. Finally, as detailed in the next section, the delivery workflow incorporates a verification step (PET pre-scan) prior to delivery that checks whether observed PET activity on the day of treatment falls within the continuum of modeled PET variations. Of note, preliminary evidence suggests that such variation is expected to be modest, at least in the case of FDG; the target PET signal does not appear to rise significantly due to inflammation or diminish due to cancer cell death during the time-frame of ablative radiotherapy delivery.37
Investigational studies of BgRT treatment plans have shown compelling dosimetry in comparison with conventional image-guided RT plans. These have included studies of lung and breast cancer using FDG-based BgRT38,39 and prostate cancer with PSMA-based BgRT.40 Of interest, the unique features of the X1 hardware – including kVCT localization, 60 RPM rotation and variable dwell couch translation – also enable robust treatment planning in non-BgRT modes such as IMRT, SBRT, and SRS.41–44
BgRT delivery
When treatment delivery is ready to begin, the patient is set up in the appropriate treatment position and aligned with on-board kVCT imaging. Next, a PET pre-scan is conducted to verify the fidelity of the biological signal as compared to that observed at planning. This check includes specific confirmation that the expected radiotherapy distribution, based upon the tumor’s current-day PET profile, lies within the variation range pre-specified by the bDVH that was calculated and approved in the final treatment plan.
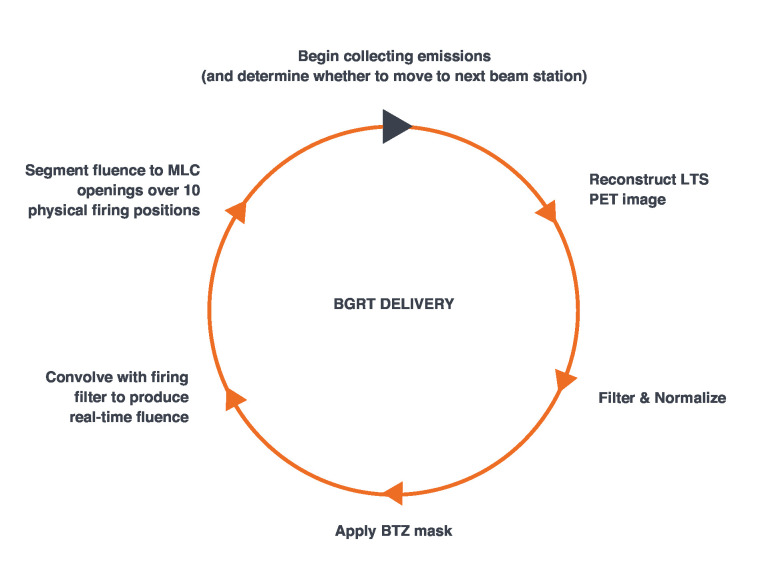
Once the PET pre-scan checks are completed, BgRT delivery can begin. During delivery, LTS PET images are continuously reconstructed at 10 frames/s. This involves filtering, normalizing, and masking each LTS frame every 100 ms so that an enhanced contrast image of the BTZ region can be generated. The pre-calculated firing matrix from the treatment planning process is then applied to the processed image to arrive at a partial fluence for each of the subsequent 10 firing positions over the 100 ms window, segmented as beamlets of dose though corresponding binary MLC leaf openings (Figure 4). Every 1 second rotation therefore results in the processing of 10 LTS frames, and the delivery of partial fluences across 100 firing positions. Notably, the LINAC and PET detectors both operate continuously; even as the LINAC is firing, the PET subsystem actively gathers emission data for the next LTS.
Figure 4.
Steps in BgRT delivery which occur every 100 ms. BgRT, biology-guided radiotherapy; BTZ, biology-tracking zone; LTS, limited-time-sampled; PET, positron emmision tomography.
Finally, in contrast to other systems, the couch does not continuously translate but dwells at a series of positions denoted as beam stations that are 2.1 mm apart. In this fashion, multiple rotations of the LINAC can occur for each beam station to deliver the desired dose for that particular slice.
The vision for BgRT in the metastatic setting is to improve throughput and efficiency by allowing the clinician to treat multiple lesions without the usual bevy of intervening actions required between each target, which may include changes to patient position, immobilization devices, and employed motion management technologies. Therefore, the ambition of BgRT is to create a unified process for tracking and ablating all tumors that is anchored to a single radiotracer injection. These logistical gains fortify the potential for investigating CMA in scalable fashion among both oligometastatic and polymetastatic patient populations. From a practical workflow perspective, as the X1 device is capable of delivering CT-guided IMRT, SBRT and SRS treatments, in addition to BgRT, it is anticipated that a typical radiotherapy department will be able to maintain a high machine utilization and patient throughput.
Future possibilities with BgRT
Dose and fractionation in the era of immunotherapy
Today, most applications of radiotherapy serve a single goal: to fully ablate or sterilize a volume of tumor tissue. However, as described in the first section, the scientific community has turned its attention to the effects of radiotherapy on the tumor microenvironment45 and its interactions with the immune system.46 In this setting, it is unknown whether fully ablative versus sub ablative doses, or single-fraction vs hypofractionated radiotherapy best maximizes the potential synergy between radiotherapy and immunotherapy. As such, investigators are actively exploring innovative radiotherapy schedules in the setting of immunotherapy that are vastly different than standard ablative dose-fractionation schemes.7,47,48
BgRT is expected to enhance these efforts by empowering clinical scientists to efficiently and scalably investigate different approaches in larger populations of patients with metastatic cancer, including those beyond the oligometastatic range. As BgRT aims to enable multisite planning and treatment in these patients, of particular interest will be the opportunity to use BgRT to administer different doses and/or fractionation schemes to different lesions in the same patient and during the same treatment session, in order to measure disparities in response.
Biology-modulated radiotherapy
Cancer is a very heterogenous disease and fully capturing this heterogeneity with a single sensor such as anatomical imaging is fundamentally limiting. Rather, adequate spatiotemporal resolution of the biological richness of the tumor requires the ability to sense diverse biological signals. In turn, these biological signals need to be multiplexed in such a way that they can be understood and acted on efficiently throughout the entire course of radiotherapy.
Several research groups have considered giving a spatially non-uniform dose to some regions of the tumor based on biological images, e.g. hypoxic radioresistance regions. This is sometimes called biological ‘‘dose painting”.49–51 Some investigators have taken this a step further whereby individual voxels are targeted with different radiotherapy doses based on their brightness.52–56
Although these techniques have traditionally been explored through offline treatment planning (i.e. registering diagnostic PET images to simulation CT images), BgRT has the potential to integrate dose painting into delivery itself with possible advantages of avoiding registration errors and adapting to changes over a treatment course.
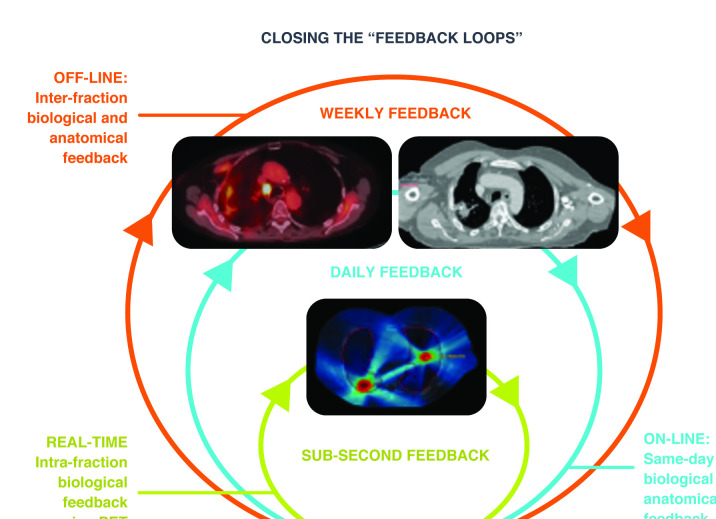
One can envision an application of modifying dose at each fraction during an ongoing course of treatment based upon dynamic changes in the PET signal, in essence extending the concept of radiotherapy adaptation from a paradigm of offline planning to an online approach (Figure 5), which one might term “biology-modulated radiotherapy”. Efficient on-table replanning that reproduces the role of the Imaging-Only session just prior to delivery would likely be an important component of pursuing this advanced application. Regardless of its label, this utilization of patient-specific dose per fraction and heterogeneous dose painting delivered in a convenient workflow is a compelling approach for enhancing the efficacy of radiotherapy by personalizing treatment for each tumor.32–35
Figure 5.
Closing the feedback loops using temporal monitoring of biological signals.
Radiomics
Molecular biomarkers have shown promise as indicators of changes in tumor bulk, tumor heterogeneity, and tumor pace, but they are limited to aggregate changes rather than changes in specific lesions.57–62 Alternative imaging-based biomarkers are being explored that can concisely convey the rich information content of 3D volumetric data sets. The extraction of features historically too subtle for experts to detect are now emerging as a source of predictive biomarkers. While the underlying mechanisms have not been consistently elucidated, early results have correlated radiomic features with molecular biomarkers derived from clinical laboratory data.63–68
In the context of BgRT, PET/CT images of the tumor are a byproduct of each treatment fraction. Applying radiomics principles to the interrogation of these images creates the possibility of discovering predictive or prognostic signatures predicated on subtle changes in PET/CT signals observed during the course of radiotherapy.69–71 Although PET sensitivity for the X1 is significantly lower than a diagnostic PET device due to a smaller solid-angle coverage geometry, nonetheless, pending clinical validation, an exciting application would be to utilize both PET and CT signatures to adjust the final radiotherapy dose based on radiomic data from the initial fractions of therapy or to inform systemic therapy decisions after the course of radiotherapy is complete.
PET tracers beyond FDG
FDG is the anticipated workhorse radiotracer for BgRT. However, non-FDG tracers are being investigated for specific clinical indications. For example, FDG may not be suitable for BgRT of brain tumors if the FDG background signal is too high. Also, some tumor types such as prostate cancer may not have adequate FDG uptake. Therefore, new tracers, such as PSMA-targeted tracers for prostate cancer72 or CA9-targeted tracers for renal cancers,73 may replace FDG in these indications.
One shortcoming of FDG in the context of BgRT is its chemical inflexibility: the FDG molecule cannot be modified to accommodate long-lived PET isotopes, such as 64Cu or 89Zr. Novel tracer designs may improve the flexibility in matching the pharmacokinetic and isotope half-life to the specific clinical indication. As one example, PET tracers targeting fibroblast activation protein (FAP) have been created with a host of different isotopes, which might allow investigations of BgRT that combine the versatile injection and uptake schedules of different isotopes with the pan-cancer detection ability of FAP.74
Lastly, disease-agnostic tracers such as those that highlight hypoxic regions (e.g. FMISO, FAZA) or areas of cellular proliferation (e.g. FLT) may enhance dose delivery tailored to tumor biology.
Conclusion
Cancer is a biological process, and the prospect of using BgRT to target it with PET tracers as probes may lead to new applications for disease management. As the field of radiotherapy advances to incorporate both molecular and anatomic information, the paradigm of closing the feedback loop during treatment becomes attainable. Moreover, as evidence for the effectiveness of EBRT in metastatic disease continues to grow, BgRT may become an important tool for efficiently ablating multiple sites of disease to potentiate systemic therapy.
Footnotes
Acknowledgments: The authors would like to acknowledge input on both technical and clinical matters including James W Welsh, MD, Ralph Weichselbaum, MD, George R Simon, MD, David Mankoff, MD, PhD, Sanjiv Sam Gambhir, MD, PhD, and Norbert J Pelc, ScD. Authors also acknowledge SBIR funding from the National Cancer Institute (R44CA228753). The authors would like to acknowledge the members of the Research and Development team and other contributors at RefleXion Medical who have worked tirelessly to develop the machine, algorithm and workflows that are described in this article. Finally, the authors dedicate this work to the late Dr Sanjiv “Sam” Gambhir who was a critical supporter of RefleXion from the very beginning and who continues to inspire us all to make a positive impact for patient care.
Funding: All authors are employees and stockholders of RefleXion Medical Inc., a company that is commercializing biology-guided radiotherapy. All acknowledged individuals are advisors and stockholders of RefleXion. Authors acknowledge funding support from the National Cancer Institute (National Institutes of Health) Grant Nos. 1R43CA153466, 2R44CA153466 and R44CA228753.
Contributor Information
Shervin M Shirvani, Email: sean@reflexion.com.
Calvin J Huntzinger, Email: cal@reflexion.com.
Thorsten Melcher, Email: thorsten@reflexion.com.
Peter D Olcott, Email: polcott@reflexion.com.
Yevgen Voronenko, Email: yevgen@reflexion.com.
Judy Bartlett-Roberto, Email: judy@reflexion.com.
Samuel Mazin, Email: sam@reflexion.com.
REFERENCES
- 1.Hellman S. Weichselbaum Rr. Oligometastases. J Clin Oncol 1995; 13: 8–10. [DOI] [PubMed] [Google Scholar]
- 2.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-Cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545: 60–5. doi: 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart EL, Tan SZ, Liu G, Tsao M-S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl Lung Cancer Res 2015; 4: 67–81. doi: 10.3978/j.issn.2218-6751.2014.11.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res 2011; 17: 5530–7. doi: 10.1158/1078-0432.CCR-10-2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014; 2: 831–8. doi: 10.1158/2326-6066.CIR-14-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks ED, Schoenhals JE, Tang C, Micevic G, Gomez DR, Chang JY, et al. Stereotactic ablative radiation therapy combined with immunotherapy for solid tumors. Cancer J 2016; 22: 257–66. doi: 10.1097/PPO.0000000000000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10: 718–26. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85: 293–5. doi: 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol 2020; 148: 157–66. doi: 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 10.Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016; 17: 1672–82. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol 2019; 37: 1558–65. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol 2018; 4: e173501. doi: 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Zeng M. First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small cell lung cancer: interim results of a randomized phase III, open-label clinical trial (SINDAS) (NCT02893332). J Clin Oncol 2020; 38(15_suppl): 9508. doi: 10.1200/JCO.2020.38.15_suppl.9508 [DOI] [Google Scholar]
- 14.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. The Lancet 2019; 393: 2051–8. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 15.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 2020; 38: JCO.20. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2018; 36: 446–53. doi: 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 17.Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol 2020; 6: 650–9. doi: 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauml JM, Mick R, Ciunci C, Aggarwal C, Davis C, Evans T, et al. Pembrolizumab after completion of locally ablative therapy for oligometastatic Non–Small cell lung cancer. JAMA Oncol 2019; 5: 1283. doi: 10.1001/jamaoncol.2019.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. JCO 2018; 36: 1611–8. doi: 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans PM. Anatomical imaging for radiotherapy. Phys Med Biol 2008; 53: R151–91. doi: 10.1088/0031-9155/53/12/R01 [DOI] [PubMed] [Google Scholar]
- 21.Nestle U, Weber W, Hentschel M, Grosu A-L. Biological imaging in radiation therapy: role of positron emission tomography. Phys Med Biol 2009; 54: R1–25. doi: 10.1088/0031-9155/54/1/R01 [DOI] [PubMed] [Google Scholar]
- 22.Verellen D, De Ridder M, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol 2008; 86: 4–13. doi: 10.1016/j.radonc.2007.11.023 [DOI] [PubMed] [Google Scholar]
- 23.Tichauer KM, Wang Y, Pogue BW, Liu JTC. Quantitative in vivo cell-surface receptor imaging in oncology: kinetic modeling and paired-agent principles from nuclear medicine and optical imaging. Phys Med Biol 2015; 60: R239–69. doi: 10.1088/0031-9155/60/14/R239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinahan PE, Mankoff DA, Linden HM. The value of establishing the quantitative accuracy of PET/CT imaging. J Nucl Med 2015; 56: 1133–4. doi: 10.2967/jnumed.115.159178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffray DA, Chung C, Coolens C, Foltz W, Keller H, Menard C, et al. Quantitative imaging in radiation oncology: an emerging science and clinical service. Semin Radiat Oncol 2015; 25: 292–304. doi: 10.1016/j.semradonc.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 26.Zaidi H, Karakatsanis N. Towards enhanced PET quantification in clinical oncology. Br J Radiol 2018; 91: 20170508. doi: 10.1259/bjr.20170508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Press RH, Shu H-KG, Shim H, Mountz JM, Kurland BF, Wahl RL, et al. The use of quantitative imaging in radiation oncology: a quantitative imaging network (Qin) perspective. Int J Radiat Oncol Biol Phys 2018; 102: 1219–35. doi: 10.1016/j.ijrobp.2018.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Heide UA, Thorwarth D. Quantitative imaging for radiation oncology. Int J Radiat Oncol Biol Phys 2018; 102: 683–6. doi: 10.1016/j.ijrobp.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 29.Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006. doi: 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackie TR, Jackson EF, Giger M. Opportunities and challenges to utilization of quantitative imaging: report of the AAPM practical big data workshop. Med Phys 2018; 45: e820–8. doi: 10.1002/mp.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000; 41: 1369–79. [PubMed] [Google Scholar]
- 32.Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000; 47: 551–60. doi: 10.1016/S0360-3016(00)00467-3 [DOI] [PubMed] [Google Scholar]
- 33.Lambin P, Petit SF, Aerts HJWL, van Elmpt WJC, Oberije CJG, Starmans MHW, et al. The ESTRO Breur lecture 2009. from population to voxel-based radiotherapy: exploiting intra-tumour and intra-organ heterogeneity for advanced treatment of non-small cell lung cancer. Radiother Oncol 2010; 96: 145–52. doi: 10.1016/j.radonc.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Toma-Dasu I, Dasu A. Towards multidimensional radiotherapy: key challenges for treatment individualisation. Comput Math Methods Med 2015; 2015: 934380. doi: 10.1155/2015/934380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambin P, Zindler J, Vanneste BGL, De Voorde LV, Eekers D, Compter I, et al. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev 2017; 109: 131–53. doi: 10.1016/j.addr.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 36.Da Silva A, Mazin S. Treatment planning and delivery overview of biology-guided Radiotherapy [Internet]. 2019. Available from: https://reflexion.com/wp-content/uploads/2019/10/BgRT_WhitePaper_Final.pdf.
- 37.Tian S, Sethi I, Yang X, Da Silva A, Switchenko J, Owonikoko TK, et al. Characterization of inter-fraction 18-FDG PET variability during lung SBRT: results of a prospective pilot study. Int J Radiat Oncol Biol Phys 2019; 105: E536. doi: 10.1016/j.ijrobp.2019.06.2449 [DOI] [Google Scholar]
- 38.Fan Q, Nanduri A, Yang J, Yamamoto T, Loo B, Graves E, et al. Toward a planning scheme for emission guided radiation therapy (EGRT): FDG based tumor tracking in a metastatic breast cancer patient. Med Phys 2013; 40: 081708. doi: 10.1118/1.4812427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang J, Da Silva A, Han C, Neylon J, Amini A, Sampath S, et al. Biology-guided radiotherapy for lung SBRT reduces planning target volume and organs at risk doses. Int J Radiat Oncol Biol Phys 2019; 105: S254. doi: 10.1016/j.ijrobp.2019.06.2468 [DOI] [Google Scholar]
- 40.Hrinivich WT, Phillips R, Da Silva AJ, Radwan N, Gorin MA, Rowe SP, et al. Online prostate-specific membrane antigen and positron emission tomography-guided radiation therapy for oligometastatic prostate cancer. Adv Radiat Oncol 2020; 5: 260–8. doi: 10.1016/j.adro.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang J, Liu A, Han C, Da Silva A, Zhang S, Wong JYC. A dosimetric study to assess the feasibility of prototype treatment planning software for a new biology-guided radiation therapy system. Int J Radiat Oncol Biol Phys 2018; 102: e477. doi: 10.1016/j.ijrobp.2018.07.1363 [DOI] [Google Scholar]
- 42.Han C, Liu A, Liang J, Da Silva A, Zhang S, Wong JYC. Dosimetric evaluation of treatment plans for a biology-guided radiation therapy system in treatment of nasopharyngeal cancer. Int J Radiat Oncol Biol Phys 2018; 102: e527. doi: 10.1016/j.ijrobp.2018.07.1482 [DOI] [Google Scholar]
- 43.Han C, Liang J, Neylon J, Liu A, Da Silva A, Dandapani SV, et al. Dosimetric evaluation of intracranial stereotactic radiosurgery treatment plans for a prototype Biology-Guided radiotherapy system. Int J Radiat Oncol Biol Phys 2019; 105: E763–4. doi: 10.1016/j.ijrobp.2019.06.799 [DOI] [Google Scholar]
- 44.Partouche J, Chmura SJ, Luke JJ, Da Silva A, Aydogan B. Evaluation of a prototype treatment planning system (TPS) for biology-guided radiation therapy (BgRT) in the context of stereotactic body radiation therapy (SBRT) for oligo-metastases. Int J Radiat Oncol Biol Phys 2018; 102: e514–5. doi: 10.1016/j.ijrobp.2018.07.1454 [DOI] [Google Scholar]
- 45.Monjazeb AM, Schalper KA, Villarroel-Espindola F, Nguyen A, Shiao SL, Young K. Effects of radiation on the tumor microenvironment. Semin Radiat Oncol 2020; 30: 145–57. doi: 10.1016/j.semradonc.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guha C. Trials and tribulations of Radio-Immuno-Oncology. Semin Radiat Oncol 2020; 30: 108–12. doi: 10.1016/j.semradonc.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 47.Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer 2019; 7: 237. doi: 10.1186/s40425-019-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savage T, Pandey S, Guha C. Postablation modulation after single high-dose radiation therapy improves tumor control via enhanced immunomodulation. Clin Cancer Res 2020; 26: 910–21. doi: 10.1158/1078-0432.CCR-18-3518 [DOI] [PubMed] [Google Scholar]
- 49.Brahme A, Argren AK. Optimal dose distribution for eradication of heterogeneous tumors. Acta Oncol 1987; 26: 377–85. doi: 10.3109/02841868709104364 [DOI] [PubMed] [Google Scholar]
- 50.Källman P, Ågren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992; 62: 249–62. doi: 10.1080/09553009214552071 [DOI] [PubMed] [Google Scholar]
- 51.Bentzen SM, Gregoire V. Molecular Imaging–Based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol 2011; 21: 101–10. doi: 10.1016/j.semradonc.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bentzen SM. Theragnostic imaging for radiation oncology: dose-painting by numbers. Lancet Oncol 2005; 6: 112–7. doi: 10.1016/S1470-2045(05)01737-7 [DOI] [PubMed] [Google Scholar]
- 53.Thorwarth D, Eschmann S-M, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys 2007; 68: 291–300. doi: 10.1016/j.ijrobp.2006.11.061 [DOI] [PubMed] [Google Scholar]
- 54.Grönlund E, Johansson S, Montelius A, Ahnesjö A. Dose painting by numbers based on retrospectively determined recurrence probabilities. Radiother Oncol 2017; 122: 236–41. doi: 10.1016/j.radonc.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 55.Bowen SR, Flynn RT, Bentzen SM, Jeraj R. On the sensitivity of IMRT dose optimization to the mathematical form of a biological imaging-based prescription function. Phys Med Biol 2009; 54: 1483–501. doi: 10.1088/0031-9155/54/6/007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan D, Chen S, Krauss DJ, Chen PY, Chinnaiyan P, Wilson GD. Tumor voxel dose-response matrix and dose prescription function derived using 18F-FDG PET/CT images for adaptive dose painting by number. Int J Radiat Oncol Biol Phys 2019; 104: 207–18. doi: 10.1016/j.ijrobp.2019.01.077 [DOI] [PubMed] [Google Scholar]
- 57.Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 2005; 5: 845–56. doi: 10.1038/nrc1739 [DOI] [PubMed] [Google Scholar]
- 58.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017; 14: 531–48. doi: 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- 59.Heitzer E, Speicher MR. Digital circulating tumor cell analyses for prostate cancer precision oncology. Cancer Discov 2018; 8: 269–71. doi: 10.1158/2159-8290.CD-18-0075 [DOI] [PubMed] [Google Scholar]
- 60.Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer 2020; 1: 176–83. doi: 10.1038/s43018-019-0011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol 2019; 16: 341–55. doi: 10.1038/s41571-019-0173-9 [DOI] [PubMed] [Google Scholar]
- 62.Cescon DW, Bratman SV, Chan SM, Siu LL. Circulating tumor DNA and liquid biopsy in oncology. Nat Cancer 2020; 1: 276–90. doi: 10.1038/s43018-020-0043-5 [DOI] [PubMed] [Google Scholar]
- 63.Rogers W, Thulasi Seetha S, Refaee TAG, Lieverse RIY, Granzier RWY, Ibrahim A, et al. Radiomics: from qualitative to quantitative imaging. Br J Radiol 2020; 93: 20190948. doi: 10.1259/bjr.20190948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nyflot MJ, Yang F, Byrd D, Bowen SR, Sandison GA, Kinahan PE. Quantitative radiomics: impact of stochastic effects on textural feature analysis implies the need for standards. J Med Imaging 2015; 2: 041002. doi: 10.1117/1.JMI.2.4.041002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aerts HJWL. The potential of radiomic-based phenotyping in precision medicine. JAMA Oncol 2016; 2: 1636–42. doi: 10.1001/jamaoncol.2016.2631 [DOI] [PubMed] [Google Scholar]
- 67.Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–62. doi: 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 68.McNitt-Gray M, Napel S, Jaggi A, Mattonen SA, Hadjiiski L, Muzi M, et al. Standardization in quantitative imaging: a multicenter comparison of radiomic features from different software packages on digital reference objects and patient data sets. Tomogr Ann Arbor Mich 2020; 6: 118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dissaux G, Visvikis D, Da-Ano R, Pradier O, Chajon E, Barillot I, et al. Pretreatment 18F-FDG PET/CT radiomics predict local recurrence in patients treated with stereotactic body radiotherapy for early-stage non-small cell lung cancer: a multicentric study. J Nucl Med 2020; 61: 814–20. doi: 10.2967/jnumed.119.228106 [DOI] [PubMed] [Google Scholar]
- 70.Mu W, Tunali I, Gray JE, Qi J, Schabath MB, Gillies RJ. Radiomics of 18F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur J Nucl Med Mol Imaging 2020; 47: 1168–82. doi: 10.1007/s00259-019-04625-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolsztynski E, O’Sullivan F, Keyes E, O’Sullivan J, Eary JF. Positron emission tomography-based assessment of metabolic gradient and other prognostic features in sarcoma. J Med Imaging 2018; 5: 1. doi: 10.1117/1.JMI.5.2.024502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siva S, Udovicich C, Tran B, Zargar H, Murphy DG, Hofman MS. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol 2020; 17: 107–18. doi: 10.1038/s41585-019-0272-5 [DOI] [PubMed] [Google Scholar]
- 73.Verhoeff SR, van Es SC, Boon E, van Helden E, Angus L, Elias SG, et al. Lesion detection by [89Zr]Zr-DFO-girentuximab and [18F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur J Nucl Med Mol Imaging 2019; 46: 1931–9. doi: 10.1007/s00259-019-04358-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindner T, Loktev A, Giesel F, Kratochwil C, Altmann A, Haberkorn U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI radiopharm chem 2019; 4: 16. doi: 10.1186/s41181-019-0069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]