Abstract
Studies indicate that erythropoietin (EPO) has effect on lipid and energy metabolism; however, the impact of EPO on lipid oxidation in vivo has not been well documented. Here, we evaluate whether long-term erythropoiesis-stimulating agent (ESA) treatment affects the oxidation of plasma very low-density lipoprotein triglycerides (VLDL-TG) fatty acids (FA), plasma free fatty acids (FFA) and non-plasma (residual) FA in healthy, young, sedentary men. Infusion of [1-14C]VLDL-TG and [9,10-3H]palmitate was used in combination with indirect calorimetry to assess resting lipid fuel utilization and kinetics, and resting energy expenditure (REE) before and after 10 weeks of ESA exposure compared with placebo. REE increased significantly during ESA compared with placebo (P = 0.023, RM-ANOVA). Oxidation rates of VLDL-TG FA, FFA, and residual FA remained unchanged during ESA compared with placebo. The relative contribution of the lipid stores was greatest for FFA (47.1%) and the total lipid oxidation rate and was not significantly different between ESA and placebo-treated subjects. We conclude that long-term ESA treatment of healthy young men increases REE but does not alter the oxidation rates of plasma and non-plasma FA sources.
Keywords: erythropoietin, lipids, VLDL-TG, energy expenditure
Introduction
Human recombinant erythropoietin (rHuEpo) was introduced in 1989 for human use in patients with chronic renal failure, anemia and cancer, to increase hemoglobin mass and red blood cell volume, and to promote aerobic exercise capacity (1, 2). Since then, studies have shown that erythropoietin (EPO) has other protective functions, such as inhibition of inflammation and apoptosis, anti-oxidant effects, protection against ischemia-induced damage, ischemia-reperfusion, trauma, and stimulation of angiogenesis (3, 4). Some reports have also documented that EPO improves metabolic parameters in patients with diabetes, including fasting glucose levels, diet-induced weight gain, and insulin sensitivity (2, 5, 6, 7, 8, 9). Other studies indicate that EPO has effects on lipid and energy metabolism. However, the mechanisms behind these effects remain unclear (10, 11, 12, 13).
Treatment with rHuEpo has been reported to improve insulin sensitivity and dyslipidemia n patients with end-stage renal disease, independent of correction of anemia (14, 15, 16, 17, 18, 19, 20, 21). Moreover, a study from our group reported that acute erythropoiesis-stimulating agent (ESA) treatment increases resting energy expenditure (REE) (22) and plasma free fatty acids (FFA) concentrations albeit with non-significant changes in lipolysis (palmitate turnover) in healthy men (22). In addition, Caillaud et al. (4) found no effect on resting lipid oxidation, measured by indirect calorimetry, but improved lipid oxidation, during exercise (75% of VO2max) as well as improved VO2max following 4 weeks of EPO treatment in trained, lean men. However, the improvements in lipid oxidation and in VO2max were not significantly associated, which could suggest an independent role for EPO. Furthermore, studies from our group have consistently shown that plasma very low-density lipoprotein triglycerides (VLDL-TG) fatty acids (FA) are important substrates for lipid oxidation, contributing with 10%-20% of REE (23) and that the oxidation of all plasma (VLDL-TG FA and FFA) and non-plasma (residual) FA sources increase quantitatively during acute exercise in healthy men (12). Although EPO has shown stimulating effects on energy expenditure, studies regarding oxidation of lipid sources during prolonged EPO treatment are lacking.
To our knowledge, no studies have investigated the independent pharmacological impact of long-term EPO treatment on integrated lipid oxidation. We hypothesized, that, long-term EPO treatment increases the oxidation of VLDL-TG FA, plasma FFA and residual FA. We also hypothesized that the increased lipid oxidation is proportional to alterations in REE. To this end, we investigated the effects of 10 weeks of ESA exposure in healthy young sedentary subjects. Infusion of [1-14C]VLDL-TG and [9,10-3H]palmitate was used in combination with indirect calorimetry to assess resting lipid fuel utilization and kinetics.
Methods
Subjects
Seventeen healthy untrained men (age 18–36 years, BMI < 30 kg/m2) were recruited from an ongoing clinical study investigating the effects of physical training and ESA treatment (Darbepoetin alfa) alone and in combination. The data from the main study (11), as well as the effects on VLDL-TG kinetics in the training and control groups (12) have been published previously. All volunteers were normotensive, nonsmokers, used no medication, and had a normal blood and chemistry panel, lipid profile, fasting plasma glucose, HbA1c, as well as a hematocrit < 45%, a normal ECG and a VO2max < 50 mL/kg/min documented before participation. They were instructed to avoid strenuous physical activity, alcohol intake, or changes in the dietary intake 3 days before the study day. Written informed consent was obtained from all participants, the Central Jutland Regional Committee on Health Research Ethics in Denmark has approved the study (M-20110035) and the study was registered at www.clinicaltrials.gov (clinical trial number NCT01320449).
Protocol
A detailed description of the original study design has been published previously (11). In brief, in the present sub-study, volunteers were allocated in a single-blinded, randomized, parallel design to either a sedentary and placebo treatment control (C) group (n = 9) or a sedentary and ESA (Darbepoietin–α, Aranesp; Amgen, Thousand Oaks, CA) treatment group (n = 8) for 10 weeks. Once a week, ESA was administered subcutaneously at a dose of 40 µg for the first 3 weeks and 20 µg for the remaining 7 weeks. Hematocrit was measured weekly throughout the study. The first three subjects, however, received treatment twice a week for the first 3 weeks, which led to a greater increase in the hematocrit than expected (50–54%). Hence, the number of injections was reduced to once a week. As per protocol, some subjects were intermittently switched to placebo in order to keep the hematocrit value below 55%. From 1 week before the treatment until the end of the study the subjects were supplemented with 100 mg iron orally/day (Ferrosulfat, Ferro Duretter; GlaxoSmithKline, Brentford, UK).
Metabolic study day
The participants arrived by taxi to the Clinical Research Unit (CRU) after an overnight fast (only mineral water was allowed) and were placed in bed under thermoneutral conditions for the rest of the study day. Catheters for infusion were placed in an antecubital vein and in a contralateral heated hand vein in order to obtain arterialized blood. Each study day consisted of a 4-h basal period (T = 0–240 min) and a 2-h hyperinsulinemic-euglycemic period (240–360 min). A primed–constant infusion of [1-14C]triolein labeled VLDL-TG (20% bolus, 80% constant) was administered during the whole study period to measure VLDL-TG kinetics. Blood samples for measurements of plasma VLDL-TG concentration and specific activity (SA) were collected together with 14CO2 in breath samples during the last 30 min of the basal period (T = 210, 225, and 240 min). A 2-h constant infusion of [9,10-3H]palmitate was given from T = 120–240 min to measure palmitate turnover and oxidation. Blood samples for measurements of plasma palmitate concentration and SA as well as plasma 3H2O concentration (dpm/mL) were collected at T = 120, 150, 160, 170, 180, 195, 210, 225, and 240 min. Indirect calorimetry was performed from T = 60–90 min. After completion of the study all catheters were removed, the volunteers had lunch, and when blood glucose had stabilized, they were dismissed.
VLDL-TG tracer preparation
The participants attended the CRU after a 12-h overnight fast 1 week before the metabolic study day. An 80 mL blood sample was obtained under sterile conditions from each volunteer to isolate VLDL-TG for ex vivo labeling as described earlier (24) with minor modifications. Plasma was immediately separated and then sonicated with 15 µCi of [1-14C]triolein (PerkinElmer) at 5°C for 2 h. The [1-14C]triolein–labeled plasma was transferred to sterile tubes and covered with a saline solution of d = 1.006 g/cm3 and ultracentrifuged (50.3 Ti rotor (37,000 g) or 5.4 Ti rotor (37,000 g), Beckman Instruments, Inc. (Palo Alto, CA, USA)) for 18 h and at 10°C. The supernatant containing the labeled VLDL fraction was removed with a Pasteur pipette, and the solution was then passed through a Millipore filter with a pore size of 0.20 µm and stored under sterile conditions at 5°C. A representative sample of the labeled [1-14C]VLDL-TG from all participants was cultured to ensure sterility before autologous infusion.
Plasma VLDL-TG concentration and SA
On the metabolic study day, VLDL-TG was isolated from approximately 3 mL plasma by ultracentrifugation. VLDL on the top layer was obtained by slicing the tube approximately 1 cm from the top with a tube slicer (Beckman Instruments, Inc.), and the exact volume was noted. TG content was analyzed on a 300 µL aliquot solution (Glycerol blanked assay; COBAS c111, Roche) and the VLDL-TG plasma concentration was calculated. The remaining VLDL-TG was transferred to a scintillation glass and [3H] and [14C] activity was measured by dual channel liquid scintillation counting to < 2% counting error.
Breath 14CO2SA
The activity of 14CO2 in expired air (IRIS-breath-bags; Wagner Analysen Technik, Bremen, Germany) was used to calculate [1-14C] VLDL-TG FA oxidation. The air was passed through a solution containing 0.5 mL hyamine hydroxide in 1 M methanol, 2 mL 96% ethanol, and one to two drops of phenolphthalein. A color change (pink to clear) occurred when exactly 0.5 mmol CO2 was trapped in the solution, where after [14C] activity was measured by liquid scintillation counting to <2% counting error.
Palmitate turnover and oxidation
A 2-h constant infusion of [9,10-3H] palmitate (0.3 µCi/min; Department of Clinical Physiology and Nuclear Medicine, Aarhus University Hospital, Denmark) was employed to measure systemic palmitate turnover and oxidation. Plasma palmitate concentration and SA were measured by HPLC using [2H31] palmitate as an internal standard. Steady-state SA was verified for each individual. Palmitate turnover (µmol/min) was calculated as [9,10-3H] palmitate infusion rate (dpm/min) divided by the steady-state palmitate SA (dpm/µmol). The slope of the increase in 3H2O in total body water vs time to calculate 3H2O production rate (T = 120–240 min) was used to measure oxidation of plasma palmitate. The rate of 3H2O production (dpm/mL/min) was divided by the average plasma [3H]palmitate SA during the same period to calculate plasma palmitate oxidation rates. The plasma 3H2O concentration and body water were determined as described previously (12, 25, 26).
Body composition
At the end of both metabolic study days, Dual-energy X-ray absorptiometry (QDR-2000, Hologic Marlborough, MA, USA) was performed to measure total body fat mass (FM), fat percentage, and lean body mass (LBM).
Indirect calorimetry
Indirect calorimetry (Deltatrac monitor, Datex Instrumentarium, Helsinki, Finland) was used to measure REE and respiratory exchange ratio (RER). Net lipid and glucose oxidation were calculated after correction for protein oxidation (27). Urine was collected during the basal period and used to calculate protein oxidation from urea excretion.
Calculations
VLDL-TG secretion rate (µmol/min) = VLDL-TG infusion rate/VLDL-TG SA
VLDL-TG clearance rate (mL/min) = VLDL-TG secretion rate/(CVLDL-TG)
Fractional VLDL-TG oxidation (% of the infused tracer) = (14CO2SA × VCO2)/(k × Ar × F), where k is the volume of CO2 at 20°C and 1 atm. pressure (22.4 L/mol), Ar is the fractional acetate carbon recovery factor in breath CO2 (0.56 at rest) (28), and F is the tracer infusion rate.
VLDL-TG oxidation rate (µmol/min) = Fractional VLDL-TG oxidation × VLDL-TG secretion rate
VLDL-TG FA energy production (kcal/day) = VLDL-TG oxidation rate × 3 (3 FA’s per TG molecule) × 282 g/mol × 9.1 kcal/g × 1440 min/day, where 282 g/mol is the molecular weight of oleic acid, 9.1 kcal/g is the caloric density.
Plasma FFA oxidation rate (kcal/day) = (palmitate oxidation (µmol/min) × 1440 min × 256.42 g/mol × 9.1 kcal/g)/ (0.29 × 1,000,000), where 0.29 is the average ratio of palmitate in TG long chain FA and 256.42 g/mol is the molecular weight of palmitate.
Residual FA oxidation rate was calculated as: Total lipid oxidation rate – (VLDL-TG oxidation rate + plasma FFA oxidation rate). The relative contributions of plasma FFA, VLDL-TG FA and Residual FA oxidation rates were calculated as the percentage of the total lipid oxidation.
Statistical analyses
Data were analyzed with SPSS 13.0 and Sigmaplot. Data following a normal distribution are presented as mean ± s.e.m. Data not following a normal distribution are presented as median (range). Data were tested for normality by QQ-plots, plots of residuals, and equal variance test. Student’s t-test or Mann–Whitney’s two sample test for parametric and non-parametric data were used to examine differences at baseline. Difference between the effects of ESA and placebo group regarding kinetic parameters were examined using a linear mixed model analysis with total FFA oxidation, VLDL-TG oxidation and residual lipid oxidation as the dependent variables, treatment and time as factors, and LBM as a covariate. Differences between substrates and body composition were examined using two-way ANOVA for repeated measurements (RM-ANOVA); if the RM-ANOVA was significant post hoc analysis was performed using Bonferroni’s test. Correlation analysis were performed using Pearson’s r.
Results
Patient characteristics are shown in Table 1 LBM was not significantly different between the two groups, but decreased significantly during the intervention (ANOVA, time effect P = 0.04).
Table 1.
Subject characteristics.
| C – before (n = 9) | C – after | ESA – before (n = 8) | ESA – after | Interaction P value | |
|---|---|---|---|---|---|
| Age | 26.1 ± 1.6 | 23 ± 1 | |||
| BMI (kg/m2) | 24.0 ± 1.0 | 24.0 ± 1.0 | 23.4 ± 0.6 | 23.5 ± 0.6 | 0.931 |
| LBM (kg) | 61 ± 2.4 | 59.9 ± 2.1 | 65.4 ± 2.7 | 64.6 ± 2.7 | 0.897a |
| Fat mass (kg) | 18.2 ± 1.9 | 19.2 ± 1.6 | 16.9 ± 2.3 | 17.9 ± 2.5 | 0.953 |
| Fat % | 21.3 ± 1.5 | 21.9 ± 1.4 | 18.0 ± 1.7 | 18.6 ± 2.0 | 0.715 |
| REE (kcal/24 h) | 1705 ± 48 | 1698 ± 48 | 1757 ± 50 | 1893 ± 50 | 0.023a,b |
Student’s t-test was used for age (NS). For all other parameters RM ANOVA was used. Data are mean ± s.e.m.
aP < 0.05 (time effect, RM-ANOVA); bP = 0.010 (C vs ESA after treatment, RM-ANOVA).
Post hoc testing could not identify a significant decrease in any of the two groups, but no significant interaction of the intervention was found. As reported previously (11), hemoglobin, hematocrit, plasma FFA concentrations, and REE increased significantly in the ESA treated group compared with placebo, whereas BMI, plasma insulin and lipids, insulin sensitivity (M-value), palmitate turnover, total fat mass, and % body fat remained unchanged in the two groups.
VLDL-TG kinetics
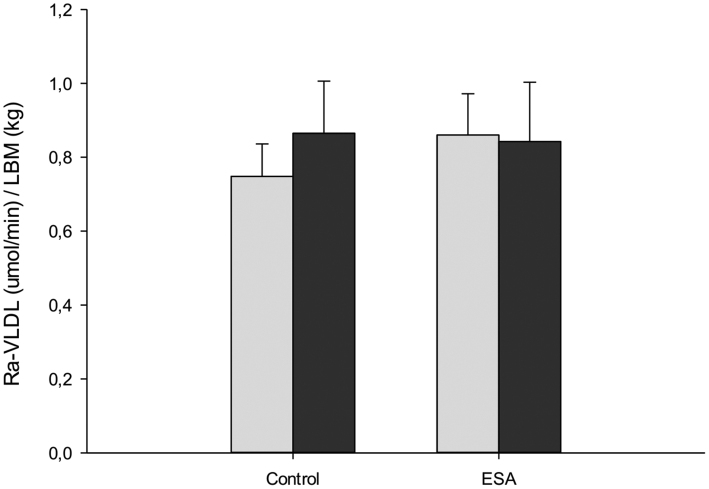
VLDL-TG oxidation and secretion rates did not change significantly during the intervention (Figs 1A and 2). Similar results were obtained when the analyses were performed without using LBM as covariate in the statistical testing. Fractional VLDL-TG oxidation (%) (i.e. the percent of circulating VLDL-TG FAs that are oxidized) was greater in the ESA compared with the placebo group, but no significant interaction between groups was observed (C-before: 47.9 ± 1.8; C-after: 53.5 ± 4.4; ESA-before: 44.6 ± 1.6; ESA-after: 53.5 ± 4.1 (RM-ANOVA, P = 0.024 for group difference). VLDL-TG clearance rate remained unaltered (data not shown).
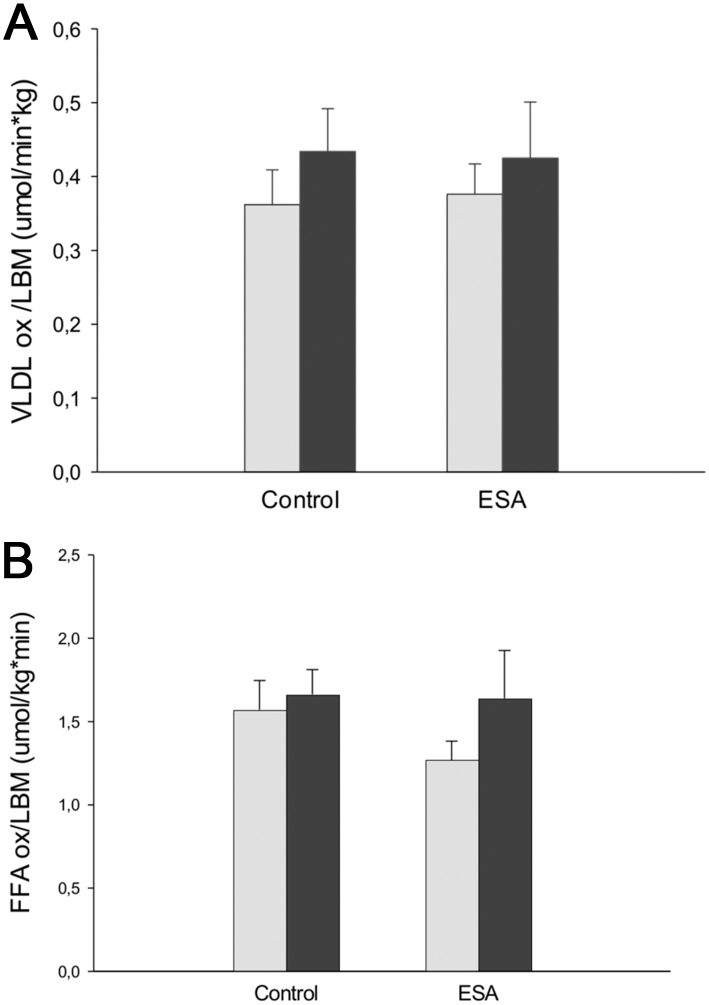
Figure 1.
(A) VLDL oxidation/LBM, (B) total FFA oxidation/LBM, and (C) residual lipid oxidation/LBM before and after 10 weeks intervention for C and ESA groups, respectively. Gray bars: before treatment, black bars: after treatment. Values are mean ± s.e.m.; All comparisons NS, RM-ANOVA.
Figure 2.
VLDL-TG secretion rate before and after 10 weeks intervention for C and ESA groups, respectively. Gray bars: before treatment, black bars: after treatment. Values are mean ± s.e.m.; All comparisons NS, RM-ANOVA.
Plasma FFA oxidation rate
Plasma FFA oxidation rate was not significantly different between the groups during the intervention (Fig. 1B). Similarly, no significant difference was observed when the analyses were performed without using LBM as covariate in the statistical testing.
Residual FA oxidation rate
No significant difference was found in residual FA oxidation rate between the groups during the intervention (Fig. 1C).
REE and substrate oxidation rates
The contribution of the different FA sources to total lipid oxidation is shown in Table 2. Total lipid oxidation and residual FA was significantly greater in the ESA group compared with the placebo group, but no significant interaction with treatment was observed. In relative terms (% of total lipid oxidation), residual FA oxidation was significantly greater and FFA oxidation significantly lower in the ESA group compared with the placebo group, but, again, no significant interaction with treatment was found.
Table 2.
Distribution of total lipid oxidation between plasma FFA, VLDL-TG FA, the residual lipid oxidation.
| C-before (n = 9) | C-after | ESA-before (n = 8) | ESA-after | Interaction P value | |
|---|---|---|---|---|---|
| TL ox/LBM (kcal/24 h kg) | 10.0 ± 1.3 | 9.7 ± 1.3 | 10.3 ± 1.3 | 11.6 ± 1.3 | 0.426a |
| VLDL-TG ox/LBM (kcal/24 h kg) | 1.3 ± 0.2 | 1.6 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.2 | 0.710 |
| Total FFA ox/LBM (kcal/24 h kg) | 5.3 ± 0.6 | 5.6 ± 0.6 | 4.3 ± 0.7 | 5.5 ± 0.7 | 0.443 |
| Residual lipid ox/LBM (kcal/24 h kg) | 3.4 ± 1.1 | 2.6 ± 1.1 | 4.6 ± 1.2 | 4.5 ± 1.2 | 0.717a |
| VLDL-TG ox (% of TLO) | 16.4 ± 3.9 | 20.4 ± 3.9 | 15.3 ± 4.1 | 14.5 ± 4.1 | 0.519 |
| FFA ox (% of TLO) | 59.2 ± 7.7 | 65.6 ± 7.7 | 44.0 ± 8.2 | 47.1 ± 8.2 | 0.815a |
| Residual FA ox (% of TLO) | 24.3 ± 10.4 | 14.0 ± 10.4 | 40.6 ± 11.0 | 38.5 ± 11.0 | 0.696a |
Linear mixed model.
aP <0.05 (group effect). Data are mean ± s.e.m. (RM ANOVA).
Ox, oxidation; TLO, total lipid.
As aforementioned, REE increased significantly during ESA compared with placebo (Table 1). When LBM was entered as a covariate in the analyses the increase in REE was no longer significant (P = 0.09). There were no significant correlations between changes in REE and the individual lipid oxidation rates.
Glucose, protein and lipid oxidation rates as measured by indirect calorimetry were not significantly different between the two groups and did not change significantly during the intervention.
Discussion
The present study is the first to assess the pharmacological effect of long-term erythropoiesis stimulation on plasma and non-plasma FA oxidation rates. We found no significant effects of 10 weeks ESA vs placebo exposure on VLDL-TG FA oxidation or secretion rates or in plasma FFA oxidation rates. Consequently, as total lipid oxidation was not altered, the residual FA oxidation was also unaffected by ESA treatment.
This present study is unique in that it investigates the combined effects on VLDL-TG FA and FFA oxidation, as well as whole-body substrate oxidation rates using validated methods (11, 12, 27). Even though ESA treatment was associated with an increase in REE we did not observe any significant changes in the oxidation rates of plasma and non-plasma FA sources. Moreover, we found no significant correlations between changes in REE and FA oxidation rates. We, therefore, conclude that ESA treatment of healthy, sedentary men has no major impact on oxidation of individual lipid fuel sources. Previous studies have reported greater (22) or unchanged (29) REE following ESA treatment of healthy individuals. In the present study we were unable to demonstrate significant changes in the overall oxidation of glucose, lipids or proteins despite the significant increase in REE during ESA treatment. Since body weight and body composition were not altered during the treatment period, we, therefore, cannot rule out that our ESA treated participants increased their food intake during the treatment period compared with those in the placebo group, thereby remaining weight stable. The mechanism whereby ESA facilitates a potential increase in REE is so far not understood in detail. We previously reported uncoupling protein 2 (UCP2) mRNA to be significantly greater during long-term ESA compared with placebo, which could account for some of the calorigenic effect of ESA (11). Still, increased UCP2 activity relies on preceding substrate oxidation. Hence, our inability to demonstrate significant differences in individual substrate oxidation rates precludes any speculation regarding which mechanisms might be involved in a potential ESA driven change in REE.
Few studies, including the present one (22), have examined the influence of rHuEpo treatment on the circulating lipid profile and the results are controversial (5, 30, 31, 32, 33). Bucciante et al. demonstrated that rHuEpo in some patients increased cholesterol and triglyceride and reduced HDL cholesterol, and suggested that these results might be attributed to increase in food intake (5). In comparison, administration of rHuEpo in hemodialysis patients has been reported to have no effect on lipid and lipoprotein patterns neither acutely (5) nor at doses varying from 1.5 to 500 units per kilogram of body weight for a period of 14–29 weeks (34). Importantly, other effects of ESA may secondarily affect lipid metabolism. One trial (35) described normalization of several hypothalamic–pituitary hormonal systems after the use of rHuEpo, which in turn may improve the lipid profile.
There are limitations to the study. First, the limited number of participants recruited, although the statistical power was high enough to show significant results for the dependent variables. Second, the results might have been different, if the population investigated were either females, obese, had increased basal VLDL-TG secretion, augmented intrahepatic content or were insulin resistant. Other studies have described sex-difference in lipid metabolism including both FFA and VLDL (36, 37). Third, consumption of food was not recorded in the current study. Fourth, palmitate was chosen as a representative FA (38). However, previous studies have pointed toward different uptake of individual FFA’s in different tissue beds (38). Therefore, small differences between palmitate and total FFA turnover and oxidation cannot be completely ruled out.
In conclusion, ESA treatment of healthy young men does not alters neither total nor proportional lipid fuel oxidation or metabolism despite a concomitant increase in REE. The mechanisms behind this paradox is unknown, but could be a complex interplay between unaccounted for changes in energy expenditure and/or physical fitness in combination with increased food intake. Further studies are needed in order to investigate the mechanistic effects of EPO in healthy subjects.
Declaration of interest
Jens Otto Jorgensen is a Senior Editor for Endocrine Connections. J O J was not involved in the review or editorial process for this paper, on which he is listed as an author.
Funding
The World Anti-Doping Agency, Independent Research Fund Denmark, Denmark, and Clinical Institute, Aarhus University, Denmark, kindly supported this study.
Acknowledgements
The authors thank Lone Kvist, Susanne Sørensen, Elsebeth Hornemann, Hanne F Petersen, Kirsten Nyborg Rasmussen, and Eva Schriver for excellent technical assistance.
References
- 1.Fisher JW.Erythropoietin: physiology and pharmacology update. Experimental Biology and Medicine 2003. 228 1–14. ( 10.1177/153537020322800101) [DOI] [PubMed] [Google Scholar]
- 2.Hojman P, Taudorf S, Lundby C, Pedersen BK.Erythropoietin augments the cytokine response to acute endotoxin-induced inflammation in humans. Cytokine 2009. 45 154–15. ( 10.1016/j.cyto.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 3.Lundby C, Olsen NV.Effects of recombinant human erythropoietin in normal humans. Journal of Physiology 2011. 589 1265–12. ( 10.1113/jphysiol.2010.195917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caillaud C, Connes P, Ben Saad H, Mercier J.Erythropoietin enhances whole body lipid oxidation during prolonged exercise in humans. Journal of Physiology and Biochemistry 2015. 71 9–16. ( 10.1007/s13105-014-0374-8) [DOI] [PubMed] [Google Scholar]
- 5.Allegra V, Martimbianco L, Vasile A.Lipid and apolipoprotein patterns during erythropoietin therapy: roles of erythropoietin, route of administration, and diet. Nephrology, Dialysis, Transplantation 1997. 12 924–9. ( 10.1093/ndt/12.5.924) [DOI] [PubMed] [Google Scholar]
- 6.Hojman P, Brolin C, Gissel H, Brandt C, Zerahn B, Pedersen BK, Gehl J.Erythropoietin over-expression protects against diet-induced obesity in mice through increased fat oxidation in muscles. PLoS ONE 2009. 4 e5894. ( 10.1371/journal.pone.0005894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinberg M, Hojman P, Pedersen BK, Kessing LV, Miskowiak KW.Effects of erythropoietin on body composition and fat-glucose metabolism in patients with affective disorders. Acta Neuropsychiatrica 2018. 30 342–349. ( 10.1017/neu.2018.16) [DOI] [PubMed] [Google Scholar]
- 8.Katz O, Stuible M, Golishevski N, Lifshitz L, Tremblay ML, Gassmann M, Mittelman M, Neumann D.Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. Journal of Endocrinology 2010. 205 87–95. ( 10.1677/JOE-09-0425) [DOI] [PubMed] [Google Scholar]
- 9.Maiese K.Erythropoietin and diabetes mellitus. World Journal of Diabetes 2015. 6 1259–12. ( 10.4239/wjd.v6.i14.1259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodo K, Sugimoto S, Nakajima H, Mori J, Itoh I, Fukuhara S, Shigehara K, Nishikawa T, Kosaka K, Hosoi H.Erythropoietin (EPO) ameliorates obesity and glucose homeostasis by promoting thermogenesis and endocrine function of classical brown adipose tissue (BAT) in diet-induced obese mice. PLoS ONE 2017. 12 e0173661. ( 10.1371/journal.pone.0173661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen B, Nellemann B, Larsen MS, Thams L, Sieljacks P, Vestergaard PF, Bibby BM, Vissing K, Jørgensen HS, Pedersen SB.et al Whole body metabolic effects of prolonged endurance training in combination with erythropoietin treatment in humans: a randomized placebo controlled trial. American Journal of Physiology: Endocrinology and Metabolism 2013. 305 E879–E8. ( 10.1152/ajpendo.00269.2013) [DOI] [PubMed] [Google Scholar]
- 12.Nellemann B, Christensen B, Vissing K, Thams L, Sieljacks P, Larsen MS, Jørgensen JOL, Nielsen S.Ten weeks of aerobic training does not result in persistent changes in VLDL triglyceride turnover or oxidation in healthy men. European Journal of Endocrinology 2014. 171 603–6. ( 10.1530/EJE-14-0333) [DOI] [PubMed] [Google Scholar]
- 13.Guadalupe-Grau A, Plenge U, Helbo S, Kristensen M, Andersen PR, Fago A, Belhage B, Dela F, Helge JW.Effects of an 8-weeks erythropoietin treatment on mitochondrial and whole body fat oxidation capacity during exercise in healthy males. Journal of Sports Sciences 2015. 33 570–57. ( 10.1080/02640414.2014.951872) [DOI] [PubMed] [Google Scholar]
- 14.Borissova AM, Djambazova A, Todorov K, Dakovska L, Tankova T, Kirilov G.Effect of erythropoietin on the metabolic state and peripheral insulin sensitivity in diabetic patients on haemodialysis. Nephrology, Dialysis, Transplantation 1993. 8 93. ( 10.1093/oxfordjournals.ndt.a092282) [DOI] [PubMed] [Google Scholar]
- 15.Khedr E, El-Sharkawy M, Abdulwahab S, Eldin EN, Ali M, Youssif A, Ahmed B.Effect of recombinant human erythropoietin on insulin resistance in hemodialysis patients. Hemodialysis International: International Symposium on Home Hemodialysis 2009. 13 340–34. ( 10.1111/j.1542-4758.2009.00367.x) [DOI] [PubMed] [Google Scholar]
- 16.Mak RH.Correction of anemia by erythropoietin reverses insulin resistance and hyperinsulinemia in uremia. American Journal of Physiology 1996. 270 F839–F8. ( 10.1152/ajprenal.1996.270.5.F839) [DOI] [PubMed] [Google Scholar]
- 17.Mak RH.Metabolic effects of erythropoietin in patients on peritoneal dialysis. Pediatric Nephrology 1998. 12 660–66. ( 10.1007/s004670050524) [DOI] [PubMed] [Google Scholar]
- 18.Mak RH.Effect of recombinant human erythropoietin on insulin, amino acid, and lipid metabolism in uremia. Journal of Pediatrics 1996. 129 97–104. ( 10.1016/s0022-3476(9670195-6) [DOI] [PubMed] [Google Scholar]
- 19.Spaia S, Pangalos M, Askepidis N, Pazarloglou M, Mavropoulou E, Theodoridis S, Dimitrakopoulos K, Milionis A, Vayonas G.Effect of short-term rHuEPO treatment on insulin resistance in haemodialysis patients. Nephron 2000. 84 320–32. ( 10.1159/000045606) [DOI] [PubMed] [Google Scholar]
- 20.Tuzcu A, Bahceci M, Yilmaz E, Bahceci S, Tuzcu S.The comparison of insulin sensitivity in non-diabetic hemodialysis patients treated with and without recombinant human erythropoietin. Hormone and Metabolic Research 2004. 36 716–7. ( 10.1055/s-2004-826021) [DOI] [PubMed] [Google Scholar]
- 21.Allegra V, Mengozzi G, Martimbianco L, Vasile A.Early and late effects of erythropoietin on glucose metabolism in maintenance hemodialysis patients. American Journal of Nephrology 1996. 16 304–30. ( 10.1159/000169014) [DOI] [PubMed] [Google Scholar]
- 22.Christensen B, Vendelbo MH, Krusenstjerna-Hafstrom T, Madsen M, Pedersen SB, Jessen N, Møller N, Jørgensen JOL.Erythropoietin administration acutely stimulates resting energy expenditure in healthy young men. Journal of Applied Physiology 2012. 112 1114–11. ( 10.1152/japplphysiol.01391.2011) [DOI] [PubMed] [Google Scholar]
- 23.Sondergaard E, Nellemann B, Sorensen LP, Christensen B, Gormsen LC, Nielsen S.Lean body mass, not FFA, predicts VLDL-TG secretion rate in healthy men. Obesity 2015. 23 1379–13. ( 10.1002/oby.21108) [DOI] [PubMed] [Google Scholar]
- 24.Gormsen LC, Jensen MD, Nielsen S.Measuring VLDL-triglyceride turnover in humans using ex vivo-prepared VLDL tracer. Journal of Lipid Research 2006. 47 99–106. ( 10.1194/jlr.M500205-JLR200) [DOI] [PubMed] [Google Scholar]
- 25.Guo Z, Burguera B, Jensen MD.Kinetics of intramuscular triglyceride fatty acids in exercising humans. Journal of Applied Physiology 2000. 89 2057–20. ( 10.1152/jappl.2000.89.5.2057) [DOI] [PubMed] [Google Scholar]
- 26.Koutsari C, Ali AH, Mundi MS, Jensen MD.Measuring plasma fatty acid oxidation with intravenous bolus injection of 3H- and 14C-fatty acid. Journal of Lipid Research 2013. 54 254–2. ( 10.1194/jlr.P031153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondergaard E, Rahbek I, Sorensen LP, Christiansen JS, Gormsen LC, Jensen MD, Nielsen S.Effects of exercise on VLDL-triglyceride oxidation and turnover. American Journal of Physiology: Endocrinology and Metabolism 2011. 300 E939–E9. ( 10.1152/ajpendo.00031.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR.A new correction factor for use in tracer estimations of plasma fatty acid oxidation. American Journal of Physiology 1995. 269 E649–E6. ( 10.1152/ajpendo.1995.269.4.E649) [DOI] [PubMed] [Google Scholar]
- 29.Lundby C, Robach P, Boushel R, Thomsen JJ, Rasmussen P, Koskolou M, Calbet JAL.Does recombinant human Epo increase exercise capacity by means other than augmenting oxygen transport? Journal of Applied Physiology 2008. 105 581–58. ( 10.1152/japplphysiol.90484.2008) [DOI] [PubMed] [Google Scholar]
- 30.Mat O, Stolear JC, Georges B.Blood lipid profile in hemodialysis patients treated with human erythropoietin. Nephron 1992. 60 236–23. ( 10.1159/000186747) [DOI] [PubMed] [Google Scholar]
- 31.Prata MM, Sousa FT, Barbas JM, Rodrigues MC.Blood lipids in haemodialysis patients treated with erythropoietin. Nephrology, Dialysis, Transplantation 1990. 5 474. ( 10.1093/ndt/5.6.474) [DOI] [PubMed] [Google Scholar]
- 32.Viron B, Donsimoni R, Michel C, al Khayat R, Mignon F.Effect of recombinant human erythropoietin on nutritional status and plasma lipids in uremic patients. Nephron 1992. 60 249. ( 10.1159/000186755) [DOI] [PubMed] [Google Scholar]
- 33.Pollock CA, Wyndham R, Collett PV, Elder G, Field MJ, Kalowski S, Lawrence JR, Waugh DA, George CR.Effects of erythropoietin therapy on the lipid profile in end-stage renal failure. Kidney International 1994. 45 897–902. ( 10.1038/ki.1994.118) [DOI] [PubMed] [Google Scholar]
- 34.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW.Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. New England Journal of Medicine 1987. 316 73–7. ( 10.1056/NEJM198701083160203) [DOI] [PubMed] [Google Scholar]
- 35.Schaefer RM, Kokot F, Kuerner B, Zech M, Heidland A.Normalization of serum prolactin levels in hemodialysis patients on recombinant human erythropoietin. International Journal of Artificial Organs 1989. 12 445–44. [PubMed] [Google Scholar]
- 36.Mittendorfer B, Patterson BW, Klein S.Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. American Journal of Clinical Nutrition 2003. 77 573–57. ( 10.1093/ajcn/77.3.573) [DOI] [PubMed] [Google Scholar]
- 37.Gormsen LC, Jensen MD, Schmitz O, Moller N, Christiansen JS, Nielsen S.Energy expenditure, insulin, and VLDL-triglyceride production in humans. Journal of Lipid Research 2006. 47 2325–23. ( 10.1194/jlr.M600175-JLR200) [DOI] [PubMed] [Google Scholar]
- 38.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD.Energy expenditure, sex, and endogenous fuel availability in humans. Journal of Clinical Investigation 2003. 111 981–98. ( 10.1172/JCI16253) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a