Supplemental Digital Content is Available in the Text.
Key Words: heart failure, dapansutrile, NLRP3 inflammasome, IL-1β, inflammation
Abstract:
The NLRP3 inflammasome has been implicated in the development and progression of heart failure. The aim of this study was to determine the safety of an oral inhibitor of the NLRP3 inflammasome, dapansutrile (OLT1177), in patients with heart failure and reduced ejection fraction (HFrEF). This was a phase 1B, randomized, double-blind, dose escalation, single-center, repeat dose safety and pharmacodynamics study of dapansutrile in stable patients with HFrEF (New York Heart Association Class II–III). Subjects were randomized to treatment with dapansutrile for up to 14 days at a ratio of 4:1 into 1 of 3 sequential ascending dose cohorts (500, 1000, or 2000 mg) each including 10 patients. Subjects underwent clinical assessment, biomarker determination, transthoracic echocardiogram, and maximal cardiopulmonary exercise testing at baseline, day 14, and day 28 to ascertain changes in clinical status. Placebo cases (N = 2 per cohort) were used as a decoy to reduce bias and not for statistical comparisons. Thirty participants (20 men) were treated for 13 (12–14) days. No serious adverse events during the study were recorded. All clinical or laboratory parameters at day 14 compared with baseline suggested clinical stability without significant within-group differences in the dapansutrile-pooled group or the 3 dapansutrile cohorts. Improvements in left ventricular EF [from 31.5% (27.5–39) to 36.5% (27.5–45), P = 0.039] and in exercise time [from 570 (399.5–627) to 616 (446.5–688) seconds, P = 0.039] were seen in the dapansutrile 2000 mg cohort. Treatment with dapansutrile for 14 days was safe and well tolerated in patients with stable HFrEF.
INTRODUCTION
Heart failure (HF) is a clinical syndrome of impaired cardiac output and/or elevated filling pressures at rest or with exertion. Although HF is caused by an abnormality in cardiac structure and/or function, extracardiac factors such as systemic inflammation play a role in its development, progression, and outcomes.1,2
Anti-inflammatory drugs with broad action [eg, glucocorticoids and non-steroidal anti-inflammatory drugs (NSAIDs)] are commonly used to treat acute and chronic inflammatory diseases but are considered contraindicated in patients with HF because of the detrimental off-target effects leading to increased blood pressure (BP), water and sodium retention, and worsening clinical status.3–5 There is, therefore, a need for more targeted anti-inflammatory therapies to be explored in HF.
The NACHT, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) inflammasome is an intracellular macromolecular structure that senses danger and triggers an inflammatory response through the activation of caspase-1, finally leading to activation and release of interleukin (IL)-1β.6,7 IL-1β is known to be a soluble cardiodepressant factor, negatively modulating systolic and diastolic function.2 IL-1β induces a HF phenotype in the mouse and yet the effects are reversible, suggesting the modulation of IL-1β activity as a correctable factor in HF.8 Furthermore, IL-1 blockade has demonstrated beneficial effects in HF in terms of improved cardiorespiratory fitness, cardiac function, and quality of life.9–12 IL-18, another cytokine of the IL-1 family, also processed within the NLRP3 inflammasome, is abundant in the heart and has been shown to mediate IL-1–induced cardiac dysfunction in vivo.13 An upstream signaling blockade through NLRP3 inflammasome inhibition may therefore provide beneficial effects by inhibiting the production of both IL-1β and IL-18.13,14 To date, there have been no approved medications that directly inhibit the NLRP3 inflammasome (Box 1).
Box 1. NLRP3 inflammasome, a possible target to treat patients with heart failure.
The NLRP3 inflammasome is macromolecular machinery able to sense danger and trigger an inflammatory response through the activation of caspase-1, which is then responsible of the activation and release of IL-1β and IL-18. IL-1β is a known cardiodepressant factor, whereas IL-18 mediates the IL-1–induced cardiac dysfunction in vivo. As IL-1 blockade has been found beneficial in HF, an upstream signaling blockade through NLRP3 inflammasome inhibition seems promising to inhibit the production of both IL-1β and IL-18.
The compound OLT1177, dapansutrile (3-methanesulfonyl-propionitrile), is a small orally available molecule acting as a selective NLRP3 inflammasome inhibitor, which has shown preliminary efficacy in various experimental models in vitro and in vivo.14–16 In a phase 1 study in healthy subjects with oral OLT1177, the compound was safe and well tolerated.14 A subsequent study found OLT1177 to be well tolerated and reduced joint pain in patients with acute gouty arthritis17 (Box 2).
Box 2. Dapansutrile, a new selective NLRP3 inhibitor.
Dapansutrile (3-methanesulfonyl-propionitrile) is a small orally available molecule that selectively inhibits the NLRP3 inflammasome. Previous experimental models of infection, acute arthritis, and myocardial infarction showed the efficacy of this drug in reducing inflammation. A previous phase 1 study in healthy subjects showed that the compound was safe and well tolerated. Dapansutrile has been tested also in patients with acute gouty arthritis showing to be well tolerated and to reduce joint pain.
Before the study, the effects of dapansutrile (OLT1177) in subjects with HF are unknown; in this article, we investigated its safety and tolerability in subjects with stable symptomatic HF and reduced ejection fraction (HFrEF).
METHODS
Subjects
Male and female subjects aged 18 year or older were eligible if they had stable HFrEF, symptomatic for shortness of breath [New York Heart Association (NYHA) functional classification II–III], with a left ventricular ejection fraction (LVEF) ≤40%, peak aerobic exercise capacity [peak oxygen consumption (VO2)] ≤80% of age/gender predicted normative values in the cardiopulmonary exercise test (CPX) with a respiratory exchange ratio (RER) ≥1.00, and a high-sensitivity C-reactive protein (hsCRP) >2 mg/L. Exclusion criteria included pregnancy, active or recent (<14 days) infection, autoimmune disease, significant systemic illness or malignancy, concomitant use of systemic corticosteroids or other immunotherapies, abnormal BP or heart rate (HR) response, angina or electrocardiogram (ECG) changes occurring during the CPX. Additional details about inclusion and exclusion criteria can be found in the protocol in the Supplemental Digital Content 1 (see Supplementary material, http://links.lww.com/JCVP/A541).
The study was conducted in accordance with the Declaration of Helsinki (revised version 2000) and the International Conference on Harmonization Guidelines for Good Clinical Practice (revised version 2016) and approved by the local institutional review board. Each participant provided written informed consent before study entry. The study was registered on ClinicalTrials.gov (NCT03534297).
Study Protocol
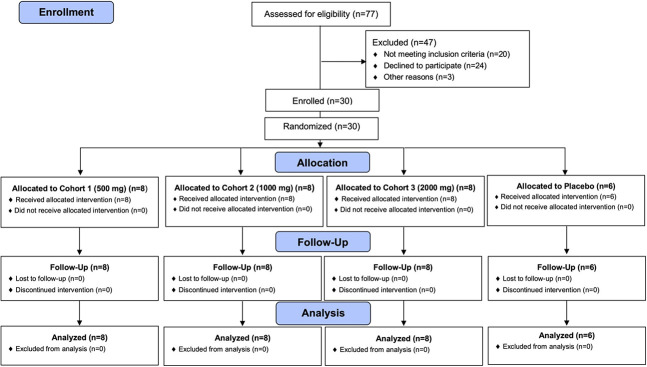
This phase 1B, single-center, randomized, double-blinded, placebo-controlled, dose escalation, repeat dose study was designed to evaluate the safety, tolerability, and pharmacodynamic profile of dapansutrile (OLT1177) in patients with stable, symptomatic HFrEF. Subjects were randomized to treatment with dapansutrile or placebo at a ratio of 4:1 into 1 of 3 sequential ascending dose cohorts [500 mg once daily—500 mg daily cohort (cohort 1); 500 mg twice daily—1000 mg daily cohort (cohort 2); and 500 mg 4 times daily—2000 mg daily cohort (cohort 3)], each of 10 patients (8 subjects under the investigational drug and 2 under placebo) (Figure 1). Additional information can be found in the protocol in the Supplemental Digital Content 1 (see Supplementary material, http://links.lww.com/JCVP/A541).
FIGURE 1.
CONSORT diagram of the flow of study participants.
Treatment and Concomitant Medications
The investigational products were provided as capsules containing either 100 mg of dapansutrile or placebo. Each treatment was constituted of 5 capsules. The first administration of the investigational product or placebo was performed at the clinical site on completion of all assessments and collection of baseline parameters. Subjects were monitored for at least one hour after treatment. Subjects then self-administered the investigational product at home for up to 14 consecutive days: 500 mg daily cohort (5 capsules once a day), 1000 mg daily cohort (5 capsules twice a day), and 2000 mg daily cohort (5 capsules 4 times a day). The percent of total intended dose has been calculated as the number of capsules taken divided by the intended number of capsules prescribed. Subjects reported the administration of investigational product using the supplied dosing diary. The site personnel had access to each subject's diary at the site visits and tracked subject's compliance with the investigational product on an ongoing basis. If a subject in cohort 2 or cohort 3 completed the study before their evening dose on day 14, the intended dose for their final dosing day was dependent on the number of doses taken before the last study visit.
Concomitant medications were recorded and reviewed at each study visit. Whenever possible, the investigators maintained subjects on stable doses of allowed concomitant medications. Prohibited concomitant medications included oral corticosteroids (within 2 weeks before enrolment) or other immunomodulating therapies (within a period of 5 half-lives before enrolment). Subjects using or intending to use prohibited concomitant medications were excluded from enrolment in the study.
Safety
Study visits occurred at screening, baseline (day 1, predose), day 4 ± 1, day 8 ± 2, day 12 ± 2, and day 28 ± 2. At each study visit, safety profile evaluation included physical examination, vital signs (HR, resting BP, temperature, and respiration rate), and safety laboratory measures. Blood samples for a complete blood count with differential, chemistry [albumin, total protein, aspartate aminotransferase (AST), bilirubin, lipase, blood urea nitrogen (BUN), creatinine, cystatin C, sodium, potassium, calcium, chloride, hsCRP, fasting plasma glucose (FPG), and glycated hemoglobin (HbA1c)], and cardiac biomarkers [N-terminal pro B-type natriuretic peptide (NT-proBNP)] were collected at screening, baseline (day 1, predose), day 4 ± 1, day 8 ± 2, day 12 ± 2, and day 28 ± 2 or at an early termination visit if occurred before the day 28 visit. Creatinine clearance was estimated using the Cockcroft–Gault equation.18 A 12-lead electrocardiogram (ECG) was collected at baseline (day 1, predose, and postdose), day 4 ± 1, and day 8 ± 2. We used a single-frequency bioelectrical impedance analyzer (Quantum IV, RJL System, Clinton Township, MI) to measure extracellular, intracellular, and total body water, fat-free mass, fat mass, and fat mass index during screening, at baseline (day 1, predose), day 12 ± 2, and day 28 ± 2.
Transthoracic Doppler echocardiography was performed before the CPX to measure cardiac size and function (e', E/e', left atrial volume index, LVEF, LV end-diastolic volume, LV end-systolic volume, and tricuspid annular plane systolic excursion [TAPSE]) according to the recommendations of the American Society of Echocardiography.19 The examination was performed by a qualified, trained professional, and the results were interpreted and reviewed by a cardiologist. Echocardiography was performed during screening, at baseline (day 1, predose), day 12 ± 2, and day 28 ± 2.
Cardiorespiratory fitness was measured using the CPX, providing an assessment of the integrative exercise responses involving the pulmonary, cardiovascular, and skeletal muscle systems. The American College of Cardiology/American Heart Association guidelines for exercise testing contraindications and test termination criteria were followed.20 A physician-supervised maximal aerobic exercise test was performed using a metabolic cart interfaced with a motorized treadmill. A conservative ramping protocol was used with incremental increases in workload of approximately 0.6 metabolic equivalents of task per minute. The CPX data were used to calculate VO2, carbon dioxide production (VCO2), RER (determined by the VCO2/VO2 ratio corresponding to the VO2), and minute ventilation/carbon dioxide production slope (VE/VCO2). The CPX procedure was performed at screening, baseline (day 1, predose), day 12 ± 2, and day 28 ± 2.
Safety profile evaluation included collection of adverse events (AEs) and serious AEs from randomization to day 42 ± 3. The AEs were graded by severity and relationship to the study drug. Detailed information about safety outcomes and procedures can be found in the protocol in the Supplemental Digital Content 1 (see Supplementary material, http://links.lww.com/JCVP/A541).
Pharmacokinetics
Blood samples for plasma pharmacokinetics (PK) analysis were collected into EDTA anticoagulation tubes, centrifuged, and drawn at baseline, day 4 ± 1, day 8 ± 2, day 12 ± 2, and day 28 ± 2. For the first 3 time points, the blood was drawn 60 ± 15 minutes after investigational drug administration.
Pharmacodynamics
Blood samples were collected for hsCRP at baseline, day 4 ± 1, day 8 ± 2, day 12 ± 2, and day 28 ± 2. For the first 3 time points, the blood was drawn 60 ± 15 minutes after investigational drug administration.
Quality of Life and Pain Assessment
Quality of life was assessed using 2 validated questionnaires: the Kansas City Cardiomyopathy Questionnaire (KCCQ)21 and the Duke Activity Status Index (DASI).22 The KCCQ is a 23-item, self-administered instrument that quantifies physical function, symptoms (frequency, severity, and recent change), social function, self-efficacy and knowledge, and quality of life. The DASI is a self-administered questionnaire measuring the subject's functional capacity to obtain a rough estimate of the subject's peak oxygen uptake.
A pain assessment was performed using the Brief Pain Index (BPI),23 a self-administered questionnaire evaluating the severity of pain and the impact of pain on daily functions. The quality of life and pain assessments were performed at baseline (day 1, predose), day 4 ± 1, day 8 ± 2, day 12 ± 2, and day 28 ± 2.
Statistical Analysis
Continuous variables are presented as median and interquartile range (IQR) for each cohort, whereas categorical variables are shown as frequencies and proportions. The Wilcoxon signed-rank test was performed to test for paired differences between baseline and subsequent visits for the subjects on dapansutrile capsules within each cohort separately and for the pooled dapansutrile-treated subjects. Placebo cases (N = 2 per cohort) were used as a decoy to reduce bias and not for statistical comparisons.
An assessment of sample size or power analyses is not applicable for this Phase IB study because the goal of the study was to characterize any side effects after reasonable exposure to the drug. However, a sample size of 24 subjects receiving dapansutrile capsules (8 subjects for each of the 3 cohorts) would provide a power of 90% to exclude a relative difference >25% of any safety parameter, with an estimated SD of 30%. A P value <0.05 was considered as statistically significant. All statistical testing was performed for each dapansutrile-treated cohort separately (cohort 1, cohort 2, and cohort 3) and for all dapansutrile-treated subjects pooled from cohorts 1–3. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Clinical Characteristics
A total of 65 patients were screened and 30 randomized between June 2018 and October 2019 to receive dapansutrile 500 mg, 1000 mg, or 2000 mg (n = 8 each per cohort, total n = 24) or placebo (n = 2 each per cohort, total n = 6) (Figure 1—CONSORT diagram). All enrolled patients completed planned evaluations and were included in the safety analysis. Patients' demographics and baseline clinical characteristics are described in Table 1Table 2Table 3.
TABLE 1.
Subjects' Demographic, Medical History, and Medications at Baseline
| Dapansutrile 500 mg (n = 8) | Dapansutrile 1000 mg (n = 8) | Dapansutrile 2000 mg (n = 8) | All Dapansutrile Subjects (n = 24) | Pooled Placebo Subjects (n = 6) | |
| Age, yr | 57 (44–61) | 58 (51–62) | 60 (54–63) | 59 (50–62) | 58 (52–64) |
| Sex, n (%) | |||||
| Female | 3 (37.5) | 1 (12.5) | 3 (37.5) | 7 (29.2) | 3 (50.0) |
| Male | 5 (62.5) | 7 (87.5) | 5 (62.5) | 17 (70.8) | 3 (50.0) |
| Race, n (%) | |||||
| Black | 5 (62.5) | 5 (62.5) | 5 (62.5) | 15 (62.5) | 3 (50.0) |
| White | 3 (37.5) | 3 (37.5) | 3 (37.5) | 9 (37.5) | 3 (50.0) |
| Medical history, n (%) | |||||
| Diabetes mellitus | 5 (62.5) | 4 (50.0) | 4 (50.0) | 13 (54.2) | 5 (83.3) |
| Dyslipidemia | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Hypertension | 8 (100.0) | 7 (87.5) | 6 (75.0) | 21 (87.5) | 6 (100.0) |
| Previous MI | 5 (62.5) | 4 (50.0) | 1 (12.5) | 10 (41.7) | 2 (33.3) |
| Medications, n (%) | |||||
| Beta blockers | 7 (87.5) | 8 (100.0) | 8 (100.0) | 23 (95.8) | 5 (83.3) |
| RAAS blockers | 7 (87.5) | 7 (87.5) | 8 (100.0) | 22 (91.7) | 6 (100.0) |
| Aspirin | 6 (75.0) | 4 (50.0) | 3 (37.5) | 13 (54.2) | 5 (83.3) |
| Statins | 6 (75.0) | 5 (62.5) | 6 (75.0) | 17 (70.8) | 4 (66.7) |
| Aldosterone antagonists | 5 (62.5) | 5 (62.5) | 6 (75.0) | 16 (66.7) | 5 (83.3) |
| Loop diuretics | 7 (87.5) | 7 (87.5) | 7 (87.5) | 21 (87.5) | 6 (100.0) |
| Furosemide-equivalent PO dose | 40 (20–120) | 40 (20–60) | 80 (40–160) | 40 (40–80) | 60 (20–200) |
MI, myocardial infarction; PO, per os; RAAS, renin–angiotensin–aldosterone system.
TABLE 2.
Characteristics of the Cohort at Baseline
| Dapansutrile 500 mg (n = 8) | Dapansutrile 1000 mg (n = 8) | Dapansutrile 2000 mg (n = 8) | All Dapansutrile Subjects (n = 24) | Pooled Placebo Subjects (n = 6) | |
| Vitals | |||||
| SBP, mm Hg | 128 (122–131) | 123 (117–136) | 113 (105–125) | 122 (115–131) | 119 (116–134) |
| DBP, mm Hg | 76 (74–80) | 75 (69–81) | 71 (64–79) | 75 (70–81) | 74 (65–76) |
| HR, bpm | 74 (69–80) | 76 (71–83) | 70 (64–78) | 73 (69–80) | 85 (74–92) |
| Temperature, °C | 36.7 (36.4–36.8) | 36.9 (36.6–37.0) | 36.6 (36.5–36.9) | 36.8 (36.5–36.9) | 36.8 (36.7–36.8) |
| Respiratory rate, brpm | 18 (16–20) | 18 (17–18) | 18 (16–18) | 18 (16–18) | 20 (18–20) |
| Body composition | |||||
| BMI, kg/m2 | 33.7 (25.8–40.3) | 34.9 (31.8–39.6) | 33.5 (31.9–38.0) | 33.8 (30.8–38.1) | 37.2 (31.1–48.8) |
| Fat mass index, kg/m2 | 13.6 (7.8–15.2) | 12.5 (10.1–15.2) | 12.6 (11.5–17.9) | 12.9 (10.7–15.3) | 15.6 (9.6–20.9) |
| Extracellular water, L | 21.7 (14.7–27.6) | 23.4 (21.2–27.1) | 19.7 (17.7–21.6) | 21.3 (17.9–24.6) | 21.7 (19.7–25.2) |
| Intracellular water, L | 22.9 (20.3–32.2) | 29.7 (26.4.32.7) | 25.3 (18.3–28.7) | 27.3 (21.6–30.1) | 23.0 (20.1–27.5) |
| Echocardiography | |||||
| LVEF, % | 30 (26–36) | 31 (22–35) | 32 (28–39) | 31 (25–37) | 34 (30–36) |
| e', cm/s | 5.50 (4.25–6.80) | 6.93 (4.25–10.20) | 5.90 (5.13–7.65) | 5.90 (4.63–7.65) | 6.68 (4.50–8.90) |
| E/e' | 11.00 (8.73–19.50) | 12.05 (8.35–21.45) | 9.40 (7.60–13.90) | 10.55 (8.15–16.35) | 14.00 (11.50–16.20) |
| LAV, mL | 75 (67–103) | 102 (83–126) | 63 (55–95) | 79 (61–111) | 86 (71–99) |
| LVEDV, mL | 213 (164–241) | 207 (176–279) | 156 (121–200) | 200 (155–222) | 179 (117–229) |
| LVESV, mL | 150 (115–169) | 144 (117–215) | 113 (78–130) | 139 (97–157) | 116 (82–163) |
| TAPSE, cm | 2.15 (1.85–2.58) | 1.85 (1.35–2.15) | 2.51 (2.05–2.80) | 2.10 (1.80–2.57) | 1.48 (1.20–2.00) |
| CPX | |||||
| Exercise time, s | 569 (444–658) | 545.0 (450–660) | 570 (429–621) | 570 (444–649) | 365 (340–483) |
| Peak RER | 1.04 (1.02–1.06) | 1.13 (1.04–1.24) | 1.12 (1.03–1.22) | 1.06 (1.02–1.18) | 1.26 (1.11–1.40) |
| Peak VO2, mL/kg/min | 15.3 (13.6–17.3) | 14.5 (12.0–16.7) | 14.2 (12.1–17.3) | 15.0 (12.4–17.0) | 10.4 (9.9–15.7) |
| VE/VCO2 slope | 32.4 (31.5–39.3) | 28.2 (24.2–34.2) | 31.7 (30.3–33.3) | 31.8 (28.5–33.6) | 32.2 (30.1–37.8) |
BMI, body mass index; bpm, beats per minute; brpm, breaths per minute; DBP, diastolic blood pressure; LAV, left atrium volume; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SBP, systolic blood pressure.
TABLE 3.
Laboratory Findings of the Cohort at Baseline
| Dapansutrile 500 mg (n = 8) | Dapansutrile 1000 mg (n = 8) | Dapansutrile 2000 mg (n = 8) | All Dapansutrile Subjects (n = 24) | Pooled Placebo Subjects (n = 6) | |
| Chemistry | |||||
| ALT, U/L | 20 (13–36) | 15 (12–17) | 16 (14–23) | 16 (13–23) | 32 (22–36) |
| AST, U/L | 22 (18–31) | 18 (17–22) | 19 (18–22) | 19 (17–24) | 29 (25–42) |
| Bilirubin, mg/dL | 0.40 (0.35–0.70) | 0.70 (0.40–0.80) | 0.55 (0.45–0.65) | 0.50 (0.40–0.75) | 0.65 (0.60–0.70) |
| Lipase, U/L | 38 (23–53) | 38 (22–58) | 22 (19–32) | 29 (21–42) | 56 (13–59) |
| Total protein, g/dL | 7.15 (6.60–7.25) | 7.10 (7.00–7.30) | 7.35 (6.95–7.60) | 7.20 (6.85–7.40) | 7.20 (6.90–8.00) |
| Albumin, g/dL | 4.15 (4.00–4.50) | 4.20 (4.05–4.40) | 4.25 (4.20–4.40) | 4.20 (4.00–4.40) | 4.40 (3.50–4.50) |
| hsCRP, mg/L | 5.23 (2.62–7.36) | 3.46 (1.97–5.32) | 4.10 (2.29–20.75) | 4.10 (2.35–5.93) | 6.10 (2.87–17.12) |
| Creatinine, mg/dL | 1.16 (0.78–1.40) | 0.97 (0.87–1.12) | 0.94 (0.89–1.15) | 0.99 (0.86–1.19) | 1.03 (0.96–1.21) |
| Cystatin C, mg/L | 1.10 (0.89–1.74) | 1.08 (0.78–1.25) | 1.10 (0.94–1.34) | 1.08 (0.87–1.42) | 1.35 (0.85–1.64) |
| BUN, mg/dL | 14.5 (10.5–20.0) | 13.0 (13.0–16.0) | 15.5 (13.5–19.5) | 14.5 (13.0–17.0) | 19.0 (14.0–24.0) |
| Sodium (mmol/L) | 139 (138–139) | 138 (136–138) | 139 (136–140) | 138 (137–139) | 137 (134–138) |
| Potassium, mmol/L | 4.25 (3.80–4.50) | 4.15 (3.75–4.40) | 4.05 (3.80–4.35) | 4.15 (3.80–4.45) | 4.55 (4.30–4.80) |
| Chloride, mmol/L | 103 (102–107) | 104 (103–105) | 105 (98–106) | 104 (102–106) | 100 (97–102) |
| Calcium, mg/dL | 9.60 (9.40–9.80) | 9.40 (9.15–9.60) | 9.50 (9.15–9.60) | 9.50 (9.20–9.65) | 9.60 (9.30–10.00) |
| FPG, mg/dL | 131 (109–160) | 158 (91–192) | 128 (95–168) | 138 (98–173) | 125 (102–323) |
| HbA1c, % | 5.80 (5.25–7.25) | 7.60 (5.65–7.80) | 6.40 (6.00–8.40) | 6.40 (5.60–7.80) | 7.35 (5.80–9.70) |
| NT-proBNP, pg/mL | 631 (246–1478) | 641 (110–841) | 334 (263–958) | 520 (228–965) | 1549 (148–2207) |
| CBC | |||||
| WBC, 109/L | 7.15 (6.60–9.50) | 5.80 (5.10–8.60) | 6.35 (5.60–7.40) | 6.75 (5.45–8.60) | 6.95 (6.50–7.50) |
| Neutrophils, 109/L | 3.85 (3.50–6.05) | 3.60 (2.70–5.90) | 3.50 (3.20–4.70) | 3.60 (3.20–5.65) | 4.70 (3.50–5.40) |
| Lymphocytes, 109/L | 2.30 (1.85–2.80) | 1.60 (1.15–2.05) | 1.95 (1.75–2.20) | 1.95 (1.65–2.25) | 1.95 (1.20–2.30) |
| Monocytes, 109/L | 0.55 (0.40–0.75) | 0.55 (0.50–0.70) | 0.55 (0.35–0.60) | 0.55 (0.40–0.60) | 0.55 (0.50–0.70) |
| Basophils, 109/L | 0.00 (0.00–0.05) | 0.00 (0.00–0.05) | 0.00 (0.00–0.10) | 0.00 (0.00–0.10) | 0.05 (0.00–0.10) |
| Eosinophils, 109/L | 0.15 (0.10–0.30) | 0.10 (0.10–0.20) | 0.15 (0.10–0.20) | 0.10 (0.10–0.20) | 0.10 (0.10–0.20) |
| RBC, 1012/L | 4.76 (4.21–5.10) | 4.50 (4.06–5.14) | 4.48 (4.41–4.67) | 4.48 (4.24–5.05) | 4.32 (3.99–4.85) |
| Hematocrit, % | 41 (40–43) | 42 (38–43) | 39 (37–41) | 40 (38–43) | 40 (37–42) |
| Hemoglobin, g/dL | 13.60 (12.70–13.85) | 13.50 (12.75–14.35) | 12.70 (11.75–13.65) | 13.35 (12.55–13.85) | 13.10 (12.20–14.00) |
| Platelets, 109/L | 257 (197–283) | 232 (176–246) | 210 (197–230) | 224 (195–253) | 237 (202–291) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBC, complete blood count; RBC, red blood cells; WBC, white blood cells.
The median age was 59 (49–62) years for pooled dapansutrile and 58 (52–64) years for pooled placebo. In the dapansutrile-pooled group, 15 (62.5%) subjects were Black and 9 (37.5%) were White, whereas in the placebo-pooled group, 3 (50%) were Black American and 3 (50%) White. Men were 17 (71%) in the dapansutrile-pooled cohort and 3 (50%) in the placebo-pooled cohort.
Patients included in the study had a high prevalence of atherosclerotic risk factors and/or established coronary artery disease (Table 1).
Pharmacokinetics
A dose-dependent increase in dapansutrile levels was seen across the 3 cohorts, with no detectable levels in the placebo cohort (Figure 2). Levels were measured 1 hour after the initial 500 mg dose resulting in similar levels across the 3 cohorts [10,200 (6000–15,000) ng/mL, 8300 (7400–10,500) ng/mL, and 9200 (5400–10,100) ng/mL]. Measurements of the dapansutrile levels were repeated after 12 ± 2 days of treatment, and a dose-dependent increase in dapansutrile levels was found [up to 23,300 (19,000–39,100) ng/mL, 30,200 (27,500–39,700) ng/mL, and 80,700 (50,700–94,200) ng/mL for the 500, 1000, and 2000 mg cohorts, respectively] (Figure 2).
FIGURE 2.
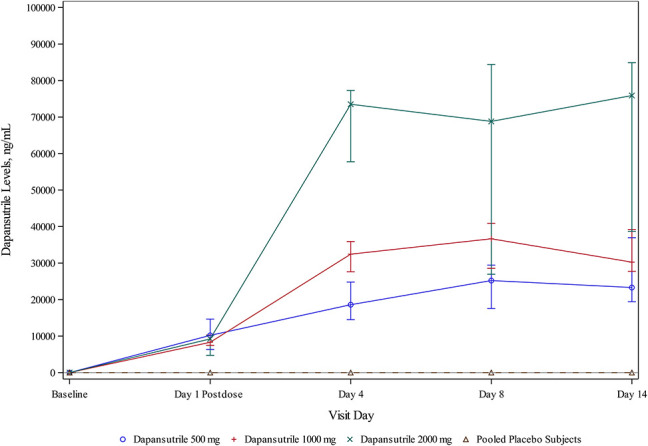
Dapansutrile levels during the study period. A dose-dependent increase in the dapansutrile level was observed across the 3 cohorts, whereas no detectable levels were recorded in the placebo cohort.
Safety and Tolerability Profile
A total of 40 AEs were reported in the overall population. Eighteen of 30 subjects (60%) experienced at least one AE, although none experienced a serious AE or death. A list of AEs is presented in Table 4.
TABLE 4.
Adverse Events
| Dapansutrile 500 mg (n = 8) | Dapansutrile 1000 mg (n = 8) | Dapansutrile 2000 mg (n = 8) | All Dapansutrile Subjects (n = 24) | Pooled Placebo Subjects (n = 6) | |
| Subjects with at least 1 AE, n (%) | 4 (50.0) | 4 (50.0) | 5 (62.5) | 13 (54.2) | 5 (83.3) |
| Total AEs, n | 9 | 6 | 11 | 26 | 14 |
| Cardiac disorders, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Atrial fibrillation, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 0 (0) |
| Cardiac failure, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| GI disorders, n (%) | 1 (12.5) | 1 (12.5) | 4 (50.0) | 6 (25.0) | 3 (50.0) |
| Abdominal discomfort, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Abdominal pain, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Diarrhea, n (%) | 0 (0) | 0 (0) | 4 (50.0) | 4 (16.7) | 0 (0) |
| Flatulence, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| Nausea, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| General disorders and administration site conditions, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Edema, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Infections and infestations, n (%) | 1 (12.5) | 1 (12.5) | 0 (0) | 2 (8.3) | 2 (33.3) |
| Nasopharyngitis, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| URTI, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 1 (16.7) |
| UTI, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Investigations, n (%) | 3 (37.5) | 0 (0) | 0 (0) | 3 (12.5) | 1 (16.7) |
| BP increase, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| Lipase increased, n (%) | 3 (37.5) | 0 (0) | 0 (0) | 3 (12.5) | 0 (0) |
| Metabolism and nutrition disorders, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 1 (16.7) |
| Hyperglycemia, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| Hypokalemia, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
| Musculoskeletal and connective tissue disorders, n (%) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 3 (12.5) | 1 (16.7) |
| Arthralgia, n (%) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 3 (12.5) | 0 (0) |
| Muscle spasms, n (%) | 0 (0) | 1 (12.5) | 0 (0) | 1 (4.2) | 0 (0) |
| Neuropathic arthropathy, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| Nervous system disorders, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 2 (33.3) |
| Dizziness, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
| Headache, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (33.3) |
| Renal and urinary disorders, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Acute kidney injury, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 1 (16.7) |
| Respiratory, thoracic, and mediastinal disorders, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Cough, n (%) | 1 (12.5) | 0 (0) | 0 (0) | 1 (4.2) | 0 (0) |
| Skin and SCT disorders, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 1 (16.7) |
| Angioedema, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
| Decubitus ulcer, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) |
| Vascular disorders, n (%) | 0 (0) | 0 (0) | 3 (37.5) | 3 (12.5) | 0 (0) |
| Hypertension, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
| Hypotension, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
| Orthostatic hypertension, n (%) | 0 (0) | 0 (0) | 1 (12.5) | 1 (4.2) | 0 (0) |
GI, gastrointestinal; SCT, subcutaneous tissue; URTI, upper respiratory tract infection; UTI, urinary tract infection.
Four of 8 (50%) subjects in the dapansutrile 500 mg daily cohort experienced 9 total AEs. Of these 9 AEs, the most frequent was 4 episodes of increase in lipase levels (45% of AEs), which occurred in 3 of the 8 (38%) patients. Other AEs were single occurrences of abdominal pain, urinary tract infection, arthralgia, acute kidney injury, and cough.
In the dapansutrile 1000 mg daily cohort, 4 of the 8 (50%) individuals reported 6 total AEs. These AEs were single occurrences of atrial fibrillation, abdominal discomfort, edema, upper respiratory tract infection, arthralgia, and muscle spasms.
Five of the 8 (63%) subjects in the dapansutrile 2000 mg daily cohort reported a total of 11 AEs. These AEs included 4 episodes of diarrhea and 3 vascular disorders (1 each of hypertension, hypotension, and orthostatic hypertension). The 4 remaining AEs included single occurrences of hypokalemia, arthralgia, dizziness, and angioedema. The severity of these events ranged from mild to moderate.
Five of the 6 (83%) subjects receiving placebo experienced 14 total AEs. This included 2 episodes of headache and single occurrences of cardiac failure, abdominal discomfort, flatulence, nausea, edema, nasopharyngitis, upper respiratory tract infection, hypertension, hyperglycemia, neuropathic arthropathy, acute kidney injury, and decubitus ulcer.
No clinically significant change in systolic BP was seen from baseline to day 12 ± 2 in the dapansutrile-pooled group or in the 3 dose-escalating dapansutrile cohorts (see Supplementary Table 1 and Supplementary Figure 1, Supplemental Digital Contents 2 and 3, http://links.lww.com/JCVP/A534 and http://links.lww.com/JCVP/A526). The systolic BP was significantly higher at day 12 ± 2 versus baseline in the placebo-pooled cohort [9.5 (5–18) mm Hg, P = 0.031]. No clinically significant change in diastolic BP was seen from baseline to day 12 ± 2 in the dapansutrile-pooled group or placebo-pooled group. A reduction in diastolic BP was seen in the dapansutrile 500 mg daily [−6.5 (−12.0 to −2.5) mm Hg, P = 0.008], but not in the 1000 mg or 2000 mg daily cohorts (see Supplementary Table 1 and Supplementary Figure 1, Supplemental Digital Contents 2 and 3, http://links.lww.com/JCVP/A534 and http://links.lww.com/JCVP/A526). No clinically significant change in HR was seen from baseline to day 12 ± 2 in the dapansutrile-pooled group or placebo-pooled group. A significant reduction in HR was noted in the dapansutrile 500 mg daily cohort (−4 [−6 to −1/min] pulse/min, P = 0.008), but not in the 1000 mg daily, 2000 mg daily, or in the dapansutrile-pooled cohort (see Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JCVP/A534).
No statistically significant changes from baseline to day 12 ± 2 were found for temperature and respiratory rate (see Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JCVP/A534) and body weight (see Supplementary Figure 2, Supplemental Digital Content 4, http://links.lww.com/JCVP/A527) in the dapansutrile-pooled group, the individual dapansutrile cohorts, or the placebo-pooled group.
No clinically significant changes in body composition were found in the placebo-pooled group. A small but statistically significant decrease in the fat mass index was found in the dapansutrile-pooled group from baseline to day 12 ± 2 [−0.4 (−0.9 to 0.1) kg/m2, P = 0.042] and in the dapansutrile 1000 mg daily cohort [−0.6 (−1.2 to −0.1) kg/m2, P = 0.031], but not in the 500 mg daily or the 2000 mg daily cohorts (see Supplementary Table 2 and Supplementary Figure 3, Supplemental Digital Contents 5 and 6, http://links.lww.com/JCVP/A535 and http://links.lww.com/JCVP/A528). No changes were observed for the percentage of variation in intracellular and extracellular water distribution (see Supplementary Table 2 and Supplementary Figure 4, Supplemental Digital Contents 5 and 7, http://links.lww.com/JCVP/A535 and http://links.lww.com/JCVP/A529) from baseline to day 12 ± 2 neither for the dapansutrile-pooled nor for the single cohorts (see Supplementary Table 2, Supplemental Digital Content 5, http://links.lww.com/JCVP/A535).
Chemistry and Hematology
No clinically significant changes in chemistry and hematology laboratory parameters [albumin, total protein, alanine aminotransferase (ALT), AST, bilirubin, lipase, BUN, creatinine, cystatin C, sodium, potassium, calcium, chloride, hematocrit, hemoglobin, red blood cell and white blood cell count with differential, and HbA1c] between baseline and day 12 ± 2 were seen in the dapansutrile-pooled cohort, in the dapansutrile individual dose cohorts, or in the placebo cohort (see Supplementary Tables 3, 4, and Supplementary Figure 5, Supplemental Digital Contents 8, 9, and 10, http://links.lww.com/JCVP/A536, http://links.lww.com/JCVP/A537 and http://links.lww.com/JCVP/A530). A small but statistically significant reduction in ALT was seen in the dapansutrile-pooled group [−1.5 (−4.5 to 0.5) U/L, P = 0.012] and in the 2000 mg daily cohort from baseline to day 12 ± 2 [−2.0 (−4.0 to −0.5) U/L, P = 0.047], but not in the 500 mg daily or the 1000 mg daily cohorts (see Supplementary Table 3, Supplemental Digital Content 8, http://links.lww.com/JCVP/A536). Dapansutrile was associated with a small but statistically significant reduction in hematocrit [−1.1 (−2.8 to 0.4)%, P = 0.010], hemoglobin [−0.3 (−0.8 to 0) g/dL, P = 0.007], red blood cells [−0.1 (−0.3 to 0) ×109/L, P = 0.015], white blood cells [−0.6 (−1.0 to 0.2) ×109/L, P = 0.041], and monocytes [0 (−0.1 to 0) ×109/L, P = 0.042] in the pooled cohort, without any significant difference when the 3 cohorts were analyzed separately (see Supplementary Table 4, Supplemental Digital Content 9, http://links.lww.com/JCVP/A537).
In the dapansutrile-pooled cohort, a statistically significant decrease in FPG was observed from baseline to day 12 ± 2 [−10.5 (−40 to 7) mg/dL, P = 0.021]; however, the change in FPG was not found to be statistically significant in the 3 dapansutrile cohorts when analyzed separately (see Supplementary Table 3 and Supplementary Figure 6, Supplemental Digital Contents 8 and 11, http://links.lww.com/JCVP/A536 and http://links.lww.com/JCVP/A531). In the pooled group of patients with type 2 diabetes mellitus who received dapansutrile (n = 13, 54%), a statistically significant decrease of 32.5 mg/dL in the median FPG was observed after the 12 ± 2-day treatment period [−32.5 (−47 to 11) mg/dL, P = 0.029] (see Supplementary Table 5 and Supplementary Figure 7, Supplemental Digital Contents 12 and 13, http://links.lww.com/JCVP/A538 and http://links.lww.com/JCVP/A532).
Inflammatory and Cardiac Biomarkers
There were no significant effects of dapansutrile on hsCRP and NT-proBNP (see Supplementary Table 3, Supplemental Digital Content 8, http://links.lww.com/JCVP/A536).
Echocardiography
The LVEF significantly increased from 31% (25%–37%) at baseline to 33% (26%–38%) at day 12 ± 2 (P = 0.046) in the dapansutrile-pooled cohort. This was because of an improvement in LVEF in the dapansutrile 2000 mg daily cohort [from 32% (28–39) to 36% (28–45), P = 0.039], with no significant changes in the 500 mg daily cohort, 1000 mg daily cohort, or in the placebo-pooled group (Figure 3 and see Supplementary Table 6, Supplemental Digital Content 14, http://links.lww.com/JCVP/A539). No clinically significant changes in LV volumes, e', E/e', left atrial volume, and TAPSE were seen in the dapansutrile-pooled cohort, the 3 individual dapansutrile cohorts, or in the placebo-pooled group (see Supplementary Table 6, Supplemental Digital Content 14, http://links.lww.com/JCVP/A539).
FIGURE 3.
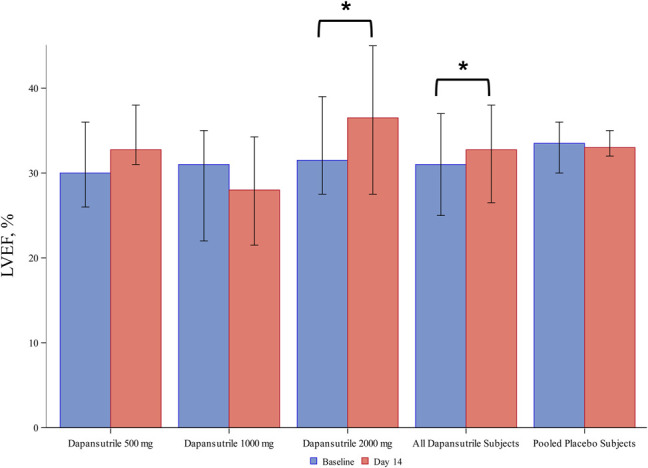
LVEF modifications across the study period. LVEF significantly increased from baseline at day 12 ± 2 in the dapansutrile-pooled cohort. This was mainly because of an improvement in LVEF in the dapansutrile 2000 mg daily cohort, whereas no significant changes in the 500 mg daily cohort, 1000 mg daily cohort, or in the placebo-pooled group were observed. *P < 0.05 for Wilcoxon signed-rank.
Cardiorespiratory Fitness
During the CPX, no significant change in exercise time was seen in the dapansutrile-pooled group; however, an increase in the exercise time [from 570 (399.5–627.0) to 616 (446.5–688.0) seconds, P = 0.039] was seen in the dapansutrile 2000 mg daily cohort and not in the 500 mg daily, 1000 mg daily, or the placebo-pooled group (Figure 4 and see Supplementary Table 7, Supplemental Digital Content 15, http://links.lww.com/JCVP/A540). No significant changes in peak RER, VO2, and VE/VCO2 were seen in the dapansutrile-pooled cohort nor the 3 individual dapansutrile cohorts or placebo-pooled cohort (see Supplementary Table 7 and Supplementary Figure 8, Supplemental Digital Contents 15 and 16, http://links.lww.com/JCVP/A540 and http://links.lww.com/JCVP/A533) (Box 3).
FIGURE 4.
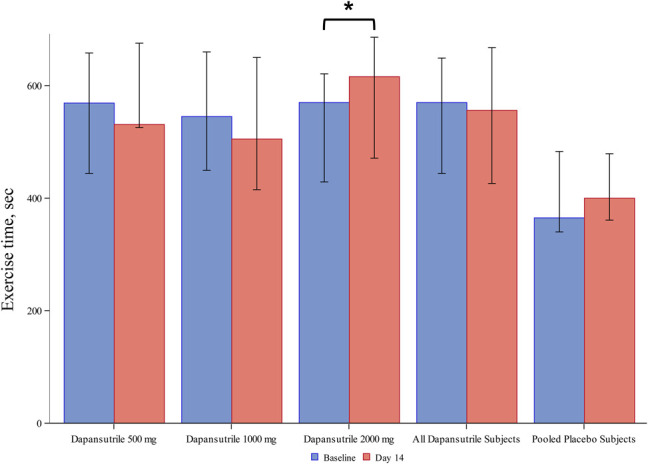
Exercise time changes across the study period. A significant increase in the exercise time was found in the dapansutrile 2000 mg daily cohort, but not in the dapansutrile-pooled, the 500 mg daily, the 1000 mg daily, or the placebo-pooled groups. *P < 0.05 for Wilcoxon signed-rank.
Box 3. Dapansutrile is safe and well tolerated in patients with stable HFrEF.
The 3 tested doses of dapansutrile (500 mg daily, 1000 mg daily, and 2000 mg daily) did not show any clinically significant differences in safety parameters between baseline and day 14. Improvements in LVEF and exercise time were seen in the dapansutrile 2000 mg cohort. No serious AEs during the study were recorded. These data together suggest that dapansutrile is a safe and well-tolerated drug in patients with stable HFrEF.
Quality of Life
No significant changes were observed for the KCCQ and DASI scores from baseline to day 12 ± 2 in the dapansutrile-pooled cohort or the 3 individual cohorts or the placebo cohort. There were also no significant changes found in the BPI pain score in the dapansutrile-pooled cohort, the 3 individual cohorts, or the placebo cohort.
DISCUSSION
Inhibition of systemic inflammation has been explored as a therapeutic target in HF for decades.2,24–27 However, the use of nontargeted anti-inflammatory drugs such as glucocorticoids and NSAIDs is fraught by significant off-target effects leading to adverse cardiovascular events (such as hypertension and sodium and water retention) that prohibit their use in patients with HF.3–5
In this article, we show that dapansutrile, an oral NLRP3 inflammasome inhibitor, is safe and well tolerated in patients with stable HFrEF. Dapansutrile had no clinically relevant, untoward effects on BP and HR nor showed any signs of impaired renal function or water or sodium retention. This was seen in the context of adequate exposure to the drug, based on the PK analysis and on a previous safety study with dapansutrile in healthy adult humans for 8 days.14 Marchetti et al found that dapansutrile reached a mean maximum concentration of 41,400 ng/mL at day 8 for the 1000-mg dose,14 which is similar to what found in this study at day 8 (37,962 ng/mL), followed by a further increase until day 14. Also in a preclinical study in an experimental acute myocardial infarction in the mouse, dapansutrile showed a dose-dependent reduction in infarct size within a serum concentration range of 10–1000 ng/mL.16
The lack of adverse effects attributable to dapansutrile in this HF population is particularly relevant because it may differentiate NLRP3 inhibition from other anti-inflammatory drug classes, such as NSAIDs (eg, diclofenac or ibuprofen) or glucocorticoids. Dapansutrile has already been shown to be effective in a phase 2 clinical trial of patients with acute gouty arthritis.17 Patients with gout are very often older with chronic cardiometabolic diseases or risk factors and have contraindications to treatment with NSAIDs or glucocorticoids due to an increased risk of hypertension, edema, and hyperglycemia. The favorable profile of dapansutrile makes it an ideal candidate for an anti-inflammatory drug in patients with or at risk for HF.
A product of the NLRP3 inflammasome, IL-1β is recognized as a soluble cardiodepressant factor that detrimentally influences both systolic and diastolic functions.2 Animal studies have shown that IL-1β-induces a phenotype of cardiac dysfunction that is reversible,8 thus modulation of IL-1β activity is considered a modifiable factor in HF. Accordingly, preliminary studies of IL-1 blockade suggest beneficial effects in HF in terms of improved cardiorespiratory fitness, cardiac function, and quality of life.2 In the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), treatment with canakinumab—a fully human monoclonal antibody targeting IL-1β—in patients with previous acute myocardial infarction reduced recurrent ischemic events and hospitalizations for HF.28,29 In a substudy of the CANTOS trial including patients with pre-existing HFrEF (NYHA class II-III), canakinumab improved cardiorespiratory fitness and LVEF.30 Phase 2 studies with an IL-1 receptor antagonist, anakinra, showed improved cardiac function and/or cardiorespiratory fitness in patients with HF and a reduced incidence of new onset HF in patients with ST-segment elevation MI.9–11,31–36
The current phase 1B study was not powered to detect significant changes in functional parameters and any findings can be viewed as hypothesis-generating alone. Although promising and in line with the results of IL-1 blockers in patients with HFrEF, the favorable effects of dapansutrile 2000 mg daily on LVEF and exercise time were not compared directly with placebo nor adjusted for multiple comparisons and will require testing in adequately designed and powered phase 2/3 trials. Importantly, 18 of the 30 (60%) patients had diabetes, consistent with the strong link between diabetes and HF.37 A beneficial effect was found in these patients with a statistically significant decrease in blood glucose that was highest in the 2000-mg dose group. Conversely, the lack of hsCRP reduction may simply reflect the lack of statistical power or that treatment for 12 ± 2 days with dapansutrile 2000 mg/d may incompletely suppress the IL-1/IL-6 signaling leading to liver synthesis of CRP in patients with chronic HFrEF. It is possible that a higher dose or a longer duration of treatment would lead to a reduction of CRP. The lack of effect of dapansutrile on CRP levels is unlikely to depend on baseline CRP levels that were rather similar to those observed in the CANTOS trial28 and also in other trials with an IL-1 blocker—anakinra—conducted by our group.11,31,35,38 It is also possible that despite the NLRP3 inflammasome inhibition, a residual IL-6 signaling in HFrEF may persist because of NLRP3-independent pathways. These aspects will require being investigated in future studies.
CONCLUSIONS
Treatment with dapansutrile, an oral NLRP3 inflammasome inhibitor, was well tolerated and safe over a 14-day treatment period in patients with stable HFrEF. These findings provide preliminary safety evidence for NLRP3 inhibition in patients with HFrEF who are expected to poorly tolerate side effects of NSAIDs or glucocorticoids.
Footnotes
Supported by Olatec Therapeutics (New York, NY).
B. W. Van Tassell has served as a consultant for Novartis and Serpin Pharma. A. Abbate has served as a consultant for AstraZeneca, Janssen, Kiniksa Pharmaceuticals Ltd, Merck, Novartis, Olatec, and Serpin Pharma. A. Bonaventura and A. Vecchié received a travel grant from Kiniksa Pharmaceuticals Ltd to attend the 2019 American Heart Association Scientific Sessions. S. Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association. The remaining authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
G. F. Wohlford and B. W. Van Tassell equally contributed to this manuscript.
Contributor Information
George F. Wohlford, Email: wohlfordgf@vcu.edu.
Benjamin W. Van Tassell, Email: bvantassell@vcu.edu.
Hayley E. Billingsley, Email: Hayley.Billingsley@vcuhealth.org.
Dinesh Kadariya, Email: dinesh.kadariya@vcuhealth.org.
Justin M. Canada, Email: Justin.M.Canada@vcuhealth.org.
Salvatore Carbone, Email: scarbone@vcu.edu.
Virginia L. Mihalick, Email: Virginia.Mihalick@vcuhealth.org.
Aldo Bonaventura, Email: aldo.bonaventura@vcuhealth.org.
Alessandra Vecchié, Email: alessandra.vecchie@vcuhealth.org.
Juan Guido Chiabrando, Email: juan.chiabrando@hospitalitaliano.org.ar.
Edoardo Bressi, Email: edo.bressi@gmail.com.
Georgia Thomas, Email: Georgia.Thomas@vcuhealth.org.
Ai-Chen Ho, Email: AiChen.Ho@vcuhealth.org.
Amr A. Marawan, Email: Amr.Marawan@vcuhealth.org.
Megan Dell, Email: megansdell@gmail.com.
Cory R. Trankle, Email: cory.trankle@vcuhealth.org.
Jeremy Turlington, Email: jeremy.turlington@vcuhealth.org.
Roshanak Markley, Email: roshanak.markley@vcuhealth.org.
REFERENCES
- 1.Murphy SP, Kakkar R, McCarthy CP, et al. Inflammation in heart failure: JACC state-of-the-art Review. J Am Coll Cardiol. 2020;75:1324–1340. [DOI] [PubMed] [Google Scholar]
- 2.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–2069. [DOI] [PubMed] [Google Scholar]
- 3.Page RL, II, O'Bryant CL, Cheng D, et al. American heart association clinical P, heart F, transplantation committees of the council on clinical C, council on cardiovascular S, anesthesia, council on C, stroke N, council on quality of C, outcomes R. Drugs that may cause or exacerbate heart failure: a scientific statement from the American heart association. Circulation. 2016;134:e32–69. [DOI] [PubMed] [Google Scholar]
- 4.Pepine CJ, Gurbel PA. Cardiovascular safety of NSAIDs: additional insights after PRECISION and point of view. Clin Cardiol. 2017;40:1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16:505–511. [PubMed] [Google Scholar]
- 6.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–214. [DOI] [PubMed] [Google Scholar]
- 7.Mauro AG, Bonaventura A, Mezzaroma E, et al. NLRP3 inflammasome in acute myocardial infarction. J Cardiovasc Pharmacol. 2019;74:175–187. [DOI] [PubMed] [Google Scholar]
- 8.Van Tassell BW, Seropian IM, Toldo S, et al. Interleukin-1beta induces a reversible cardiomyopathy in the mouse. Inflamm Res. 2013;62:637–640. [DOI] [PubMed] [Google Scholar]
- 9.Van Tassell BW, Arena R, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105:1371–1377 e1. [DOI] [PubMed] [Google Scholar]
- 11.Van Tassell BW, Canada J, Carbone S, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure anakinra response trial). Circ Heart Fail. 2017;10:e004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckley LF, Carbone S, Trankle CR, et al. Effect of interleukin-1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol. 2018;72:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toldo S, Mauro AG, Cutter Z, et al. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553–H68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchetti C, Swartzwelter B, Gamboni F, et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc Natl Acad Sci U S A. 2018;115:E1530–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti C, Swartzwelter B, Koenders MI, et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res Ther. 2018;20:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toldo S, Mauro AG, Cutter Z, et al. The NLRP3 inflammasome inhibitor, OLT1177 (dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J Cardiovasc Pharmacol. 2019;73:215–222. [DOI] [PubMed] [Google Scholar]
- 17.Klück V, Jansen TLTA, Janssen M, et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020;2:e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of, cardiovascular imaging. Eur Heart J Cardiovasc Imag. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher GF, Ades PA, Kligfield P, et al. American heart association exercise CR, prevention committee of the council on clinical Cardiology CoNPA, metabolism CoC, stroke N, council on E, prevention. Exercise standards for testing and training: a scientific statement from the American heart association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 22.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 23.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. [DOI] [PubMed] [Google Scholar]
- 24.Shirazi LF, Bissett J, Romeo F, et al. Role of inflammation in heart failure. Curr Atheroscler Rep. 2017;19:27. [DOI] [PubMed] [Google Scholar]
- 25.Wintrich J, Kindermann I, Ukena C, et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol. 2020;109:1079–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montecucco F, Liberale L, Bonaventura A, et al. The role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19:11. [DOI] [PubMed] [Google Scholar]
- 27.Ayoub KF, Pothineni NVK, Rutland J, et al. Immunity, inflammation, and oxidative stress in heart failure: emerging molecular targets. Cardiovasc Drugs Ther. 2017;31:593–608. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 29.Everett BM, Cornel JH, Lainscak M, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 30.Trankle CR, Canada JM, Cei L, et al. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol. 2018;122:1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbate A, Trankle CR, Buckley LF, et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9:e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trankle CR, Canada JM, Kadariya D, et al. IL-1 blockade reduces inflammation in pulmonary arterial hypertension and right ventricular failure: a single-arm, open-label, phase IB/II pilot study. Am J Respir Crit Care Med. 2019;199:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115:288–292. [DOI] [PubMed] [Google Scholar]
- 34.Van Tassell BW, Abouzaki NA, Oddi Erdle C, et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Tassell BW, Trankle CR, Canada JM, et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11:e005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mechanick JI, Farkouh ME, Newman JD, et al. Cardiometabolic-based chronic disease, adiposity and dysglycemia drivers: JACC state-of-the-art Review. J Am Coll Cardiol. 2020;75:525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canada JM, Van Tassell BW, Christopher S, et al. Clinical predictors of response to anakinra in patients with heart failure. Int J Cardiol. 2014;173:537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]