Abstract
microRNAs (miRNAs) modulate the expression of enzymes responsible for activation or detoxification of xenobiotics and toxicants. miRNAs are dysregulated in response to environmental exposure and have been implicated in toxicological events. Many in vivo and in vitro experimental approaches have been employed to delineate the mechanisms by which miRNAs regulate target genes; however, all these methods provide only indirect evidence for the interaction between miRNAs and their counterpart mRNA molecules. In this chapter, we describe a novel approach—a fluorescent-based RNA electrophoretic mobility shift assay (FREMSA) that is a sensitive and time-saving method, with a high specificity, to visualize the interactions among miRNAs, mRNAs, and proteins, as direct evidence of mRNA/miRNA complex formation.
Keywords: Fluorescent-based RNA electrophoretic mobility shift assay, miRNA target prediction, miRNA–RNA interaction
1. Introduction
To date, more than 100 algorithms [1, 2] have been developed to predict the gene targets of miRNAs, and multiple experimental approaches, such as reporter gene assays, correlation analyses between miRNAs and target genes, and evaluation of target gene expression based on exogenous miRNA mimics/inhibitors transfection, are used to validate the miRNA target prediction based on in silico analyses. However, researchers often are unable to verify experimentally target genes that are regulated by miRNAs, due to the poor accuracy of miRNA target prediction using in silico analysis [3, 4]. Furthermore, these experimental approaches only provide indirect evidence for the interaction between miRNAs and their counterpart mRNA molecules.
In this chapter, we provide a novel experimental approach, a fluorescent-based RNA electrophoretic mobility shift assay (FREMSA) that interrogates the interactions among miRNAs, mRNAs, and proteins to provide direct evidence that a miRNA molecule interacts directly with its cognate mRNA target. The FREMSA approach provides a powerful and practical tool to validate experimentally miRNA/RNA interactions [5–12].
2. Materials
2.1. Biological Samples
Cell lines used for cytoplasmic extracts preparation (see Note 1).
2.2. RNA Oligonucleotides
RNA oligonucleotide probes for miRNA species, 5′-labeled with the dye cy5.5™.
RNA oligonucleotide probes for mRNA fragments targeted by miRNAs, 5′-labeled with the dye IRDye 800.
Unlabeled specific miRNA oligonucleotides as “cold” probes for miRNA species.
Unlabeled nonspecific miRNA oligonucleotides as “cold” probes for a negative control miRNA (see Note 2).
All oligonucleotides can be obtained from Integrated DNA Technologies (Coraville, IA).
2.3. Apparatuses
An electrophoresis power supply (250 V, 200 mA), from any vendor.
A vertical electrophoresis apparatus for polyacrylamide gel, including the spacers, glass plates, combs, and a gel-casting stand, from any vendor.
A near-infrared fluorescence imaging system, e.g., an Odyssey CLx Infrared Imaging System from Li-COR (LI-COR Biosciences, Lincoln, NE).
A high-speed centrifuge (16,000 × g) from any vendor for cytoplasmic extracts preparation.
2.4. Chemical Solutions
All solutions used should be prepared using ultrapure water or obtained commercially and can be stored at room temperature unless stated otherwise.
- Native polyacrylamide gel
- N,N,N′,N′-Tetramethylethylenediamine (TEMED), tightly sealed and stored at 4 °C.
- 10% Ammonium persulfate (APS) solution, stored at 4 °C for at most 1 week.
- 40% Acrylamide/Bis solution, protected from light.
- Glycerol, molecular biology grade or better.
- 5× Tris-borate-EDTA buffer (TBE), 450 mM Tris, 450 mM boric acid, and 10 mM EDTA, pH 8.3.
- Running buffer
- 0.5× TBE Buffer, 1:10 dilution from 5× TBE buffer.
- miRNA–RNA binding reaction (see Note 3)
- 100 mM HEPES buffer (pH 7.3), stored frozen at −20 °C. 50% glycerol, 1:2 dilution from pure glycerol, stored at −20 °C.
- 2 M KCl, stored at −20 °C.
- 1 M MgCl2, stored at −20 °C.
- 500 mM DTT (dithiothreitol), stored at −80 °C.
- tRNA, 10 mg/mL in 0.5× TBE buffer.
- Antibodies against Ago proteins, from any vendor.
- Loading buffer (6×)
- 15% Ficoll and 0.4% Orange G solution in water. One aliquot is stored at 4 °C for immediate use, and the remaining aliquots are stored at −20 °C (see Note 4).
3. Methods
3.1. Sample Preparation
3.1.1. In Silico Analyses
Various programs for miRNA target gene prediction, such as miR-Tar.human (http://mirtar.mbc.nctu.edu.tw/human/) [13] and PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html) [14], may be used to screen the potential genes targeted by a specific miRNA. RNAhybrid algorithm (http://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid) [15] is used to calculate the free energy of the binding affinity for miRNA/RNA duplexes. Our previous studies have indicated that a free energy threshold < −20 kcal/mol is sufficient to detect the formation of a miRNA/RNA complex using the FREMSA.
3.1.2. Oligonucleotides Synthetization
The RNA oligonucleotides corresponding to the mature miRNA species are synthesized commercially and 5′-labeled with cy5.5™ dye, while the cognate mRNA fragments corresponding to the miRNA targeting sequences are synthesized and 5′-labeled with IRDye 800 dye (see Note 5). Unlabeled oligonucleotides as “cold” probes, including specific miRNA sequences and cel-miR-67 sequences, used as negative controls or competitors, are synthesized.
3.1.3. Oligonucleotides Preparation
The dye-labeled miRNA and RNA oligonucleotides are dissolved in nuclease-free water to a final concentration of 200 nM, while unlabeled oligonucleotides are prepared at the concentration of 10 M (50×). All aliquots are stored at −20 °C; repeated freezing and thawing should be avoided.
3.1.4. Cytoplasmic Extracts
Many commercial products are available to extract the cytoplasmic and nuclear extracts. Proteins extracted with NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher, Waltham, MA) are suitable for FREMSA. Detailed procedures are listed in the manufacturer’s protocol. All aliquots were stored at −80 °C; repeated freezing and thawing should be avoided.
3.1.5. Native Polyacrylamide Gel
Mix 5.5 mL nuclease-free water, 1 mL of 5× TBE buffer, 1 mL of glycerol, 2.5 mL of 40% Acrylamide/Bis solution, 70 μL 10% APS, and 5 μL TEMED in a 50 mL conical flask, and gently cast one native polyacrylamide gel within a 7.25 cm × 10 cm × 1.5 mm gel cassette. Store the gel at 4 °C for no longer than 48 h.
3.2. Incubation and Interaction
On ice, thaw RNA oligonucleotides, cytoplasmic extracts, antibodies, and chemical solutions for the miRNA–RNA binding reaction.
A 20 μL reaction system is prepared in six PCR tubes (100 μL), as shown in Table 1 (see Note 6).
In each tube, mix 2 μL HEPES buffer, 2 μL 50% glycerol, 2 μL 2 M KCl, 2 μL 1 M MgCl2, and specific volumes of water for use as the basic binding buffer.
Add 2 μL of the corresponding RNA oligonucleotides (mRNA and miRNA probes), cytoplasmic extracts, and antibodies in each tube.
Incubate the reaction mixture at room temperature for 20 min.
Table 1.
Binding reactions for miRNA–RNA interaction
| Component | Lane 1 | Lane 2 | Lane 3 | Lane 4 | Lane 5 | Lane 6 |
|---|---|---|---|---|---|---|
| HEPES buffer | 2 | 2 | 2 | 2 | 2 | 2 |
| KCl | 2 | 2 | 2 | 2 | 2 | 2 |
| MgCl2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 50% glycerol | 2 | 2 | 2 | 2 | 2 | 2 |
| Dye-labeled miRNA | 2 | – | 2 | 2 | 2 | 2 |
| Dye-labeled mRNA | – | 2 | 2 | 2 | 2 | 2 |
| Unlabeled miRNA | – | – | – | 2 | – | – |
| Unlabeled negative control | – | – | – | – | 2 | – |
| tRNA | – | – | – | – | – | 1 |
| Cytoplasmic extracts | – | – | – | – | – | Xa |
| Antibody | – | – | – | – | – | Xa |
| Water | 10 | 10 | 8 | 6 | 6 | Xa |
Adjusted based on the protein concentration of cytoplasmic extracts and antibody
3.3. Separation of Probes by Gel Electrophoresis
Set the voltage at 100 V to pre-electrophorese the native polyacrylamide gel for 30 min at 4 °C, in the vertical electrophoresis apparatus filled with 0.5× TBE.
Flush wells gently.
Add 4 μL of loading buffer to each binding reaction system.
Use pipette to gently mix the loading samples. Do not vortex.
Load mixtures into the wells of the polyacrylamide gel.
Set the voltage at 100 V to electrophorese the samples for 2–3 h at 4 °C, until the orange G dye has migrated to the bottom of the gel.
3.4. Visualization and Detection
Wash the glass containing the gel with water. Do not strip the gel from the glass plates.
Place the whole glass plates onto an Odyssey CLx Infrared Imaging System to detect the fluorescent signals.
Scan the RNA oligonucleotides labeled with cy5.5™ and IRDye 800 dye by checking the box next to the desired channel(s) (700, 800, or both) in Image Studio™ software (LI-COR Biosciences), and quantify the complexes including miRNAs, mRNAs, and/or proteins following the directions in the software manual.
3.5. Explanation of Results
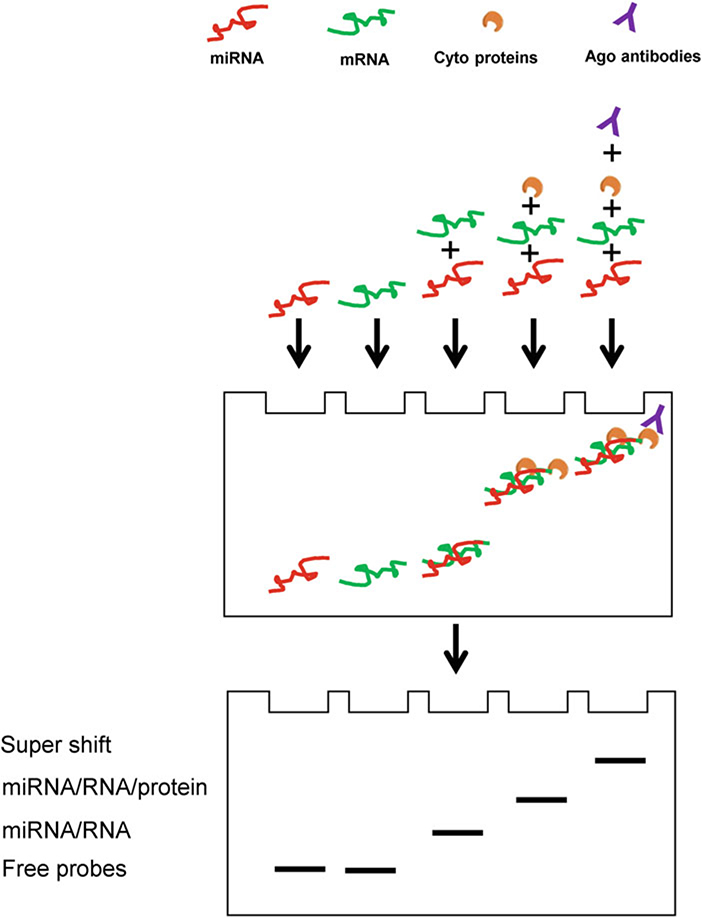
Probes representing miRNAs, mRNAs, and miRNA/mRNA complexes can be visualized by band shifts with different wavelengths of fluorescence dyes. Two-color fluorescence channels, with specific wavelengths of 700 nm and 800 nm, are used to detect the oligonucleotide probes for a miRNA (red) and its cognate mRNA (green), respectively. As shown in Fig. 1, free (unbound) RNA oligonucleotides (mRNA and miRNA probes), miRNA/RNA complex, miRNA/RNA/protein complex, and miRNA/RNA/protein/antibody complex have different mobilities and exhibit distinct shift patterns, under ideal experimental conditions. Each band can be recorded by the fluorescence scanner.
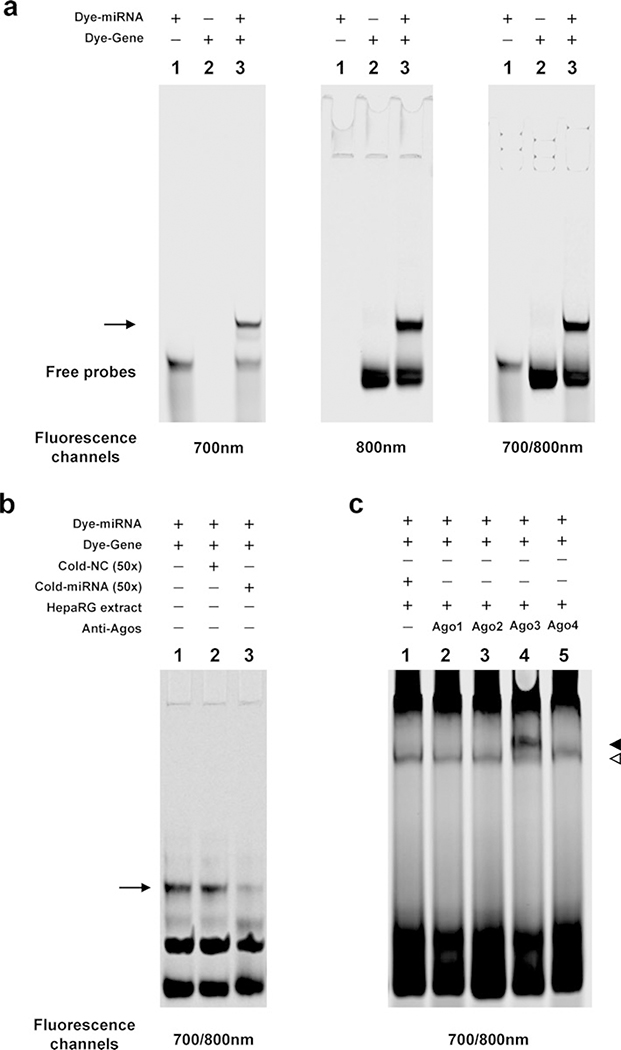
The specificity of the interaction between a miRNA and its cognate mRNA can be analyzed using cold probe competition assays, and antibodies can be used to show the involvement of proteins in the interaction process. As shown in Fig. 2a, the miRNA or/and mRNA oligonucleotides exhibit significantly different band mobilities (shifts), which were recorded by the fluorescence scanner at the 700 nm channel, 800 nm channel, and/or both channels. In Fig. 2b, the miRNA/RNA complex is eliminated by an excess of unlabeled hsa-miR-128–3p oligonucleotides (cold probe), but not the nonspecific competitors, indicating the miRNA/RNA complex was formed via a sequence-specific manner. In Fig. 2c, a complex of miRNA/mRNA/proteins is shown by a band shift, indicating that cytoplasmic extracts from HepaRG cells were able to bind to the miRNA/RNA complex formed by the miRNA/RNA interaction (lane 1). Antibodies against Ago3 created supershifted bands when incubated with protein/RNA complexes (lane 4).
Fig. 1.
Schemes of the FREMSA technique. Idealized results showing the significantly different mobility shifts for free RNA oligonucleotides (miRNA and mRNA probes), miRNA/RNA complex, miRNA/RNA/protein complex, and miRNA/RNA/protein/antibody complex, respectively, due to their diverse molecular weights
Fig. 2.
Typical results of the FREMSA technique. (a) Dye-labeled oligonucleotide miRNA probe (lane 1), its target mRNA probe (lane 2), and the miRNA/mRNA complex (lane 3). (b) Competition assays in FREMSA. Lanes 2 and 3 show the competition effects by excessive unlabeled miRNA oligonucleotides (lane 3), but not by nonspecific competitor (lane 2). The arrow indicates the miRNA/mRNA complex. (c) miRNA interacting with the cognate target mRNA in vitro through the involvement of Ago 3 protein. The hollow triangle indicates miRNA/mRNA/protein complex; the solid triangle represents supershift complex
3.6. Advantages and Further Consideration
Compared to the classic RNA EMSA technique, FREMSA is a simpler and time-saving process. A researcher needs only 2–3 h to complete an assay because neither electrophoretic transfer of binding reactions nor a chemiluminescence procedure is needed.
FREMSA is highly sensitive; only a very minimum amount of experimental materials is needed, due to the use of fluorescence dyes.
FREMSA has high specificity since “cold” probes and nonspecific competitors can be used to exclude the nonspecific interactions. Combined with a free energy threshold (<20 kcal/mol) from in silico analysis, FREMSA exhibits a high success rate in detecting mRNAs targeted by miRNAs.
A low concentration (such as 3%) of native polyacrylamide gel can be used to detect mRNA/miRNA/protein complexes; thus, macromolecules with high molecular weights, such as proteins, can be separated by the system.
The dyes are stable and the gels can be stored in the glass plates for months, so multiple gels can be re-scanned for comparison in the future studies.
The method can be coupled with proteomic technologies to identify proteins involved in the interaction between miRNA and mRNA. The gel band representing the mRNA/miRNA/protein complex can be excised from the gel and analyzed by mass spectrometry to determine the proteins involved in the miRNA/mRNA complex. For example, FUS, a protein component of heterogeneous nuclear ribonucleoprotein complex, was found to interact with the hsa-miR-370–3p/CYP2D6-mRNA duplex [12].
Acknowledgment
The information in these materials is not a formal dissemination of the U.S. Food and Drug Administration.
Footnotes
To detect the role of miRNAs in liver toxicity, human primary hepatocytes or HepaRG cell lines are recommended to extract the cytoplasmic proteins for FREMSA.
Cel-miR-67, which shows minimal sequence identity with miRNAs in humans, mice, and rats, is a good negative control used in competition assays in FREMSA.
LightShift RNA EMSA Optimization and Control Kit (Thermo Fisher) contains most of components for miRNA/RNA binding reaction.
Do not use bromophenol blue dye in loading buffer. This could seriously impair the fluorescent signals from RNA oligonucleotide probes.
The 2′-O-methylation is recommended to modify the RNA oligonucleotides corresponding to the miRNA target sequences, to enhance the stability of oligonucleotides.
2 μg of cytoplasmic extracts and 1 μg of nonspecific tRNA are recommended to be added to form RNA/protein complexes.
References
- 1.Fan X, Kurgan L (2015) Comprehensive overview and assessment of computational prediction of microRNA targets in animals. Brief Bioinform 16(5):780–794. 10.1093/bib/bbu044 [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Zhang Z (2015) Computational biology in microRNA. Wiley Interdiscip Rev RNA 6 (4):435–452. 10.1002/wrna.1286 [DOI] [PubMed] [Google Scholar]
- 3.Pinzon N, Li B, Martinez L, Sergeeva A, Presumey J, Apparailly F, Seitz H (2017) microRNA target prediction programs predict many false positives. Genome Res 27 (2):234–245. 10.1101/gr.205146.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M, Lieberman J, Lal A (2010) Desperately seeking microRNA targets. Nat Struct Mol Biol 17(10):1169–1174. 10.1038/nsmb.1921 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zeng L, Wang Y, Tolleson WH, Knox B, Chen S, Ren Z, Guo L, Mei N, Qian F, Huang K, Liu D, Tong W, Yu D, Ning B (2017) The expression, induction and pharmacological activity of CYP1A2 are post-transcriptionally regulated by microRNA hsa--miR-132–5p. Biochem Pharmacol 145:178–191. 10.1016/j.bcp.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, Yu D, Tolleson WH, Knox B, Wang Y, Chen S, Ren Z, Deng H, Guo Y, Ning B (2016) MicroRNA hsa-miR-25–3p suppresses the expression and drug induction of CYP2B6 in human hepatocytes. Biochem Pharmacol 113:88–96. 10.1016/j.bcp.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezquita-Pla J (2018) Gordon H Dixon’s trace in my personal career and the quantic jump experienced in regulatory information. Syst Biol Reprod Med 64(6):448–468. 10.1080/19396368.2018.1503752 [DOI] [PubMed] [Google Scholar]
- 8.Yu D, Green B, Marrone A, Guo Y, Kadlubar S, Lin D, Fuscoe J, Pogribny I, Ning B (2015) Suppression of CYP2C9 by microRNA has--miR-128–3p in human liver cells and association with hepatocellular carcinoma. Sci Rep 5:8534 10.1038/srep08534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu D, Green B, Tolleson WH, Jin Y, Mei N, Guo Y, Deng H, Pogribny I, Ning B (2015) MicroRNA hsa-miR-29a-3p modulates CYP2C19 in human liver cells. Biochem Pharmacol. 10.1016/j.bcp.2015.08.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Tolleson WH, Knox B, Jin Y, Guo L, Guo Y, Kadlubar SA, Ning B (2015) Modulation of ALDH5A1 and SLC22A7 by microRNA hsa-miR-29a-3p in human liver cells. Biochem Pharmacol. 10.1016/j.bcp.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Wu L, Gill P, Tolleson WH, Chen S, Sun J, Knox B, Jin Y, Xiao W, Hong H, Wang Y, Ren Z, Guo L, Mei N, Guo Y, Yang X, Shi L, Chen Y, Zeng L, Dreval K, Tryndyak V, Pogribny I, Fang H, Shi T, McCullough S, Bhattacharyya S, Schnackenberg L, Mattes W, Beger RD, James L, Tong W, Ning B (2018) Multiple microRNAs function as self-protective modules in acetaminophen-induced hepatotoxicity in humans. Arch Toxicol 92(2):845–858. 10.1007/s00204-017-2090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng L, Chen Y, Wang Y, Yu LR, Knox B, Chen J, Shi T, Chen S, Ren Z, Guo L, Wu Y, Liu D, Huang K, Tong W, Yu D, Ning B (2017) MicroRNA hsa-miR-370–3p suppresses the expression and induction of CYP2D6 by facilitating mRNA degradation. Biochem Pharmacol 140:139–149. 10.1016/j.bcp.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu JB, Chiu CM, Hsu SD, Huang WY, Chien CH, Lee TY, Huang HD (2011) miRTar: an integrated system for identifying miRNA-target interactions in human. BMC Bioinf 12:300 10.1186/1471-2105-12-300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry VJ, Bandrowski AE, Pepin AS, Gonzalez BJ, Desfeux A (2014) OMICtools: an informative directory for multi-omic data analysis. Database (Oxford). 10.1093/database/bau069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger J, Rehmsmeier M (2006) RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 34(Web Server): W451–W454. 10.1093/nar/gkl243 [DOI] [PMC free article] [PubMed] [Google Scholar]