Abstract
Plasmodium knowlesi, a simian malaria parasite, has been in the limelight since a large focus of human P. knowlesi infection was reported from Sarawak (Malaysian Borneo) in 2004. Although this infection is transmitted across Southeast Asia, the largest number of cases has been reported from Malaysia. The increasing number of knowlesi malaria cases has been attributed to the use of molecular tools for detection, but environmental changes including deforestation likely play a major role by increasing human exposure to vector mosquitoes, which coexist with the macaque host. In addition, with the reduction in human malaria transmission in Southeast Asia, it is possible that human populations are at a greater risk of P. knowlesi infection due to diminishing cross-species immunity. Furthermore, the possibility of increasing exposure of humans to other simian Plasmodium parasites such as Plasmodium cynomolgi and Plasmodium inui should not be ignored. We here review the current status of these parasites in humans, macaques, and mosquitoes to support necessary reorientation of malaria control and elimination in the affected areas.
Introduction
For many years, it has been accepted that only 4 species of Plasmodium namely Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale cause natural malaria infection in humans. However, this assumption was challenged when the first case of naturally acquired Plasmodium knowlesi infection was reported in an American army surveyor in the state of Pahang, Malaysia in 1965 [1].
The long-tailed macaque (Macaca fascicularis) was identified as a natural host of P. knowlesi, Plasmodium fieldi, Plasmodium cynomolgi, Plasmodium coatneyi, and Plasmodium inui, among others [2,3]. However, no mosquitoes were found to harbor sporozoites of these Plasmodium species in the area where the first case was reported. In contrast, Anopheles hackeri was incriminated as the vector of P. knowlesi in the state of Selangor and found to be attracted to nonhuman primates rather than humans, biting especially at canopy level [4]. Thus, it was postulated that human infection with simian malaria parasites is a rare event [5].
However, this scenario was greatly challenged when a large focus of human P. knowlesi infection was reported among the local population in Sarawak, Malaysia in 2004 [6]. It is believed that P. knowlesi infections in humans had been there much earlier but were only detected when molecular tools were adopted [7]. Under experimental conditions, both P. inui [8] and P. cynomolgi [9,10] can infect humans through mosquito bites, while natural P. cynomolgi infection had been reported recently [11–15]. On this background, we here review the current occurrence of P. knowlesi in human, mosquito, and macaque hosts, examining also the possible emergence of other simian Plasmodium species as zoonoses in Southeast Asia.
Methodology
A literature search was carried out using PubMed/Web of Science, Google Scholar, and other sites to find relevant materials related to simian malaria in humans, macaques, and vectors. The following search terms were used singly or in combination: simian malaria, primate malaria, zoonotic malaria, non-human primate, Anopheles, malaria vector, macaques, and monkeys. The most relevant publications related to the current topic were selected. All relevant early to present publications were included. Since the focus of the review article is on Southeast Asia, other simian Plasmodium species infecting humans in other parts of the world are not discussed in detail.
Epidemiology
Human infection by P. knowlesi had been reported from all Southeast Asia countries except Timor Leste [16] (Table 1).
Table 1. Knowlesi malaria cases in SEA based on the cumulative cases confirmed by PCR and/or sequencing and reported in peer-reviewed published articles.
| Country | Year | No. of P. knowlesi cases | References |
|---|---|---|---|
| Brunei | 2007–2017 | 73 | [17] |
| Cambodia | 2007–2010 | 2 | [18] |
| Indonesia | 2008–2015 | 418 | [19–24] |
| Laos | 2010–2016 | 10 | [25,26] |
| Malaysia | 2010–2018 | 18,687 | [27] |
| Myanmar | 2008–2013 | 49 | [28–30] |
| Philippines | 2006 | 5 | [31] |
| Singapore | 2007–2008 | 6 | [32,33] |
| Thailand | 2000–2018 | 44 | [29,34–40] |
| Vietnam | 2004–2010 | 38 | [26,41,42] |
PCR, polymerase chain reaction; SEA, Southeast Asia.
Knowlesi malaria in Malaysia
Malaysia, notably Sabah and Sarawak, reported the highest numbers of P. knowlesi cases in Southeast Asia [43] following the drastic reduction of malaria cases caused by the human malaria parasites. Knowlesi malaria now accounts for all local cases reported [44].
In Sabah, it was demonstrated that knowlesi malaria spread gradually from areas with no transmission of human malaria to other areas, as human malaria cases were reduced [45]. Most cases were found in the Southwest interior region, gradually spreading to the West Coast and then on to the northern area and finally to the East Coast where P. vivax was still present in substantial numbers [45]. In Sarawak, there were a total of 9,364 P. knowlesi cases from 1992 to 2014 [46]. From 73 cases in 1992, the number increased dramatically to 1,095 in 2014, far exceeding the incidence in Peninsular Malaysia [46]. Environmental changes especially associated with deforestation and land exploration bring human population in close contact with Anopheles mosquito vectors over time, which inevitably increases the risk [47,48].
Knowlesi malaria in other Southeast Asian countries
Besides Malaysia, large numbers of knowlesi malaria cases were recorded in neighboring countries, Brunei, Indonesia, and Thailand (Table 1). The low reported incidence of knowlesi malaria in other Southeast Asia countries might be due to misdiagnosis through microscopy and a scarcity of specific studies [49]. Thus, the knowlesi malaria cases reported from these countries may be just the tip of the iceberg. This underlines the importance of multinational collaboration in reducing or eliminating knowlesi malaria in future.
Knowlesi malaria among travelers
There are also increasing numbers of cases of knowlesi malaria imported from Southeast Asia to Europe, Asia, America, and Oceania (Fig 1) [50,51]. The countries outside Southeast Asia which had reported imported P. knowlesi malaria in international travelers are listed in S1 Table.
Fig 1. Plasmodium knowlesi exported from SEA to other areas across the world.
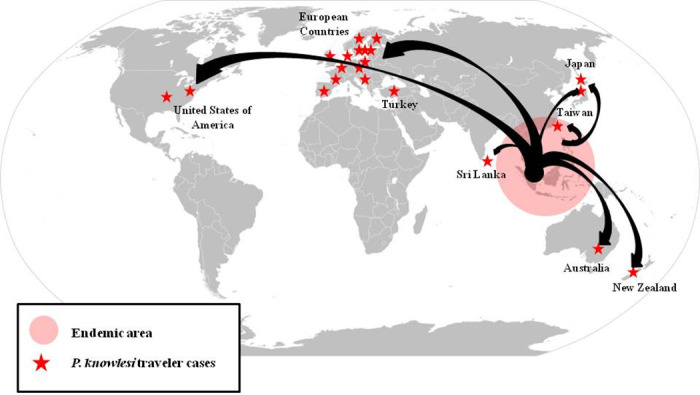
SEA, Southeast Asia.
Other simian malarias in humans
The first reported case of a natural transmission of P. cynomolgi was from Hulu Terengganu, the east coast of Peninsular Malaysia in 2011 [11]. Following that, natural infections of P. cynomolgi in humans became increasingly evident in Sarawak [13], northern Sabah [12], and western Cambodia [14]. A P. cynomolgi case was reported in a tourist from Denmark who had visited Peninsular Malaysia and Thailand in 2018 [15].
In addition to human Plasmodium species, more infections of P. knowlesi, P. cynomolgi, P. coatneyi, and P. inui were detected in Malaysian samples through a PCR approach, some of which comprised mixed infections of P. knowlesi + P. cynomolgi and P. knowlesi + P. coatneyi [52]. These were detected from hospital samples as well as from community surveys [52] and highlight the importance of using diagnostic tests specific for these species.
Macaque hosts
The main hosts of P. knowlesi are the long-tailed macaques (M. fascicularis), pig-tailed macaques (Macaca nemestrina), and banded leaf monkeys (Presbytis melalophos) [53]. The distribution and prevalence of simian Plasmodium in wild macaques in Southeast Asia are listed in Table 2. The compiled data from year 2004 until 2017 show that Malaysian Borneo has the highest prevalence of simian malaria in their wild macaques followed by Cambodia, Singapore, and Indonesia. In Malaysia, M. fascicularis has the highest prevalence of simian malaria. In Peninsular Malaysia, this species was positive for simian malaria in forested areas, but not in urban areas [54]. Overall, P. knowlesi and P. inui infections have similar rates in Peninsular Malaysia, followed by P. cynomolgi [54]. In contrast, in Sabah, P. inui infection is the most prevalent infection in long-tailed macaques, followed by P. knowlesi and P. cynomolgi with similar rate [48], while in Sarawak, P. knowlesi and P. inui have similarly high prevalence, followed by P. coatneyi and P. cynomolgi [55]. Most of the malaria–positive macaques in Peninsular Malaysia and Sarawak have mixed infections [55,56]. Besides, nonhuman primates in Southeast Asia harbor other simian Plasmodium parasites namely Plasmodium eylesi, P. fieldi, Plasmodium fragile, Plasmodium hylobati, Plasmodium jefferyi, Plasmodium pitheci, Plasmodium simiovale, Plasmodium silvaticum, and Plasmodium youngi [54]. Although these have not shown infection in humans, further studies are needed to determine if any of these species can cause human infections.
Table 2. Simian Plasmodium parasites in wild macaques in SEA.
| Country | Location | Years | Type of macaques studied | Number of positive /number of samples tested (percentage positive) | Percentage of multiple infection among positive samples (%) | P. knowlesi (%) | P. cynomolgi (%) | P. inui (%) | P. fieldi (%) | P. coatneyi (%) | Unknown Plasmodium sp. | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cambodia | Vanny | 2011 | M. fascicularis | 44/54 (81.5%) | 27.3 | - | 50.0 | 22.2 | 1.9 | 29.6 | - | [57] |
| Indonesia | Bintan Island | 2007 | M. fascicularis | 16/20 (80.0%) | 25.0 | - | 65.0 | 25.0 | 10.0 | 5.0 | - | [57] |
| Southern Sumatra | 2010 | M. fascicularis | 49/50 (98.0%) | 18.4 | - | 96.0 | 20.0 | - | - | - | [57] | |
| Sumatra | Not stated | M. fascicularis | 30/60 (50.0%) | NA | NA | NA | 48.3 | NA | NA | 3.3 | [58] | |
| West Java | Not stated | M. nemestrina | 4/4 (100.0%) | NA | NA | NA | NA | NA | NA | NA | [58] | |
| Laos | Guidong | 2013 | M. fascicularis | 30/44 (68.2%) | 3.3 | 2.3 | 63.6 | - | 2.3 | - | - | [57] |
| Malaysia | Hulu Selangor | 2014 | M. fascicularis | 35/70 (50.0%) | 74.3 | 30.0 | 25.7 | 32.9 | 1.4 | 22.9 | - | [56] |
| Peninsular Malaysia | 2015 | M. fascicularis | NA | NA | 34.3 | 27.9 | 33.2 | 27.6 | 16.6 | - | [59] | |
| Kapit, Sarawak | 2004–2008 | M. fascicularis | 80/82 (97.6%) | 92.5 | 86.6 | 63.4 | 84.1 | 4.9 | 76.8 | - | [55] | |
| Kapit, Sarawak | 2004–2008 | M. nemestrina | 21/26 (80.8%) | 66.7 | 50.0 | 34.6 | 76.9 | - | 34.6 | - | [55] | |
| Sepilok, Sabah | 2010–2011 | M. fascicularis | 26/26 (100%) | NA | 15.4 | 11.5 | 30.8 | 3.8 | 3.8 | 3.8 | [48] | |
| Sepilok, Sabah | 2010–2011 | M. nemestrina | 15/15 (100%) | NA | 13.3 | 6.7 | 60.0 | 20.0 | 6.7 | 6.7 | [48] | |
| Philippines | Montible subcolony and Iwahig Penal Colony, Palawan | 1971–1972 | Macaca irus | 11/20 (55.0%) | 27.3 | 0.0 | 9.1 | 9.1 | 0.0 | 0.0 | 54.5 | [60] |
| Batangas, Southwestern Luzon | 2012 | M. fascicularis | 3/28 (10.7%) | 33.3 | - | 10.7 | - | - | 3.6 | - | [57] | |
| Zamboanga, Western Mindanao | 2012 | M. fascicularis | 4/40 (10.0%) | - | - | 2.5 | 5.0 | - | 2.5 | - | [57] | |
| National Wildlife Rescue and Research Center, Manila | 2017 | Captive macaques | 0/30 (0%) | - | - | - | - | - | - | - | [61] | |
| Puerto Princesa Subterranean River National Park, Palawan | 2017 | M. fascicularis | 40/40 (100%) | 90.0 | 45.0 | 57.5 | 92.5 | 90.0 | 47.5 | - | [61] | |
| Palawan Wildlife Rescue and Conservation Center, Palawan | 2017 | M. fascicularis | 5/25 (20.0%) | 60.0 | - | - | 20.0 | 12.0 | - | - | [61] | |
| Singapore | Singapore | 2007 | M. fascicularis | 31/40 (77.5%) | 16.1 | - | 65.0 | 12.5 | 10.0 | - | - | [57] |
| Singapore | 2007–2011 | M. fascicularis | 66/92 (71.7%) | 42.4 | 48.9 | 43.5 | 1.1 | 12.0 | 2.2 | - | [62] | |
| Singapore | 2008–2011 | Peri-domestic macaque | 0/65 (0%) | - | - | - | - | - | - | - | [62] | |
| Thailand | Ranong Province | 2006 | M. fascicularis | 5/21 (23.8%) | 20.0 | - | - | 23.8 | - | 4.8 | - | [63] |
| Prachuab Khirikhan Province | 2006 | Peri-domestic macaque | 0/78 (0%) | - | - | - | - | - | - | - | [63] | |
| Southern Thailand | 2008–2009 | M. fascicularis | 12/195 (6.2%) | 16.6 | 0.5 | 0.5 | 3.6 | - | 1.5 | 0.5 | [64] | |
| Southern Thailand | 2008–2009 | M. nemestrina | 90/449 (20.0%) | 11.1 | 1.1 | 1.1 | 14.0 | 0.2 | 0.4 | 5.1 | [64] |
SEA, Southeast Asia.
P. knowlesi has not been detected in wild macaques in Cambodia and Indonesia even though indigenous cases of knowlesi malaria in humans have been reported there [57,58]. Moreover, P. knowlesi is not the most prevalent malaria parasite found in macaques in each region/country in Southeast Asia except for Malaysian Borneo. The most prevalent simian malaria parasites in the other countries is either P. inui or P. cynomolgi. Both can reach more than 70% prevalence in some regions. Based on studies done thus far (Table 2), P. inui has the widest geographical distribution in Southeast Asia followed by P. cynomolgi. This is expected since these 2 parasites can infect a wide range of Old and New World monkeys (naturally or experimentally). Besides, P. inui is the widest-ranging parasite, likely due to its high chronicity in the hosts [65], followed by P. cynomolgi. It is also noteworthy that there is a generally high multispecies infection rate among the wild macaques. This leads to a bigger reservoir and higher risks of zoonotic transmission of P. inui and P. cynomolgi to humans.
Vectors
Distribution of P. knowlesi vectors
Most of the vector studies have been done in Malaysia [16] followed by Vietnam [42,66]. Studies in Malaysia first identified An. hackeri as the vector of P. knowlesi [4]. However, this species is so highly zoophilic that it has been considered impossible that it could infect humans [5]. After the recognition of the importance of P. knowlesi malaria in humans in Sarawak, vector studies were conducted in different ecological niches. In Kapit district, Anopheles latens was identified as the main natural vector of P. knowlesi for both macaques and humans [67], while in Sabah, Anopheles balabacensis was incriminated [68,69]. In both states, these 2 species, belonging to the Leucosphyrus group, are also vectors of human malaria [16].
In Pahang, Peninsular Malaysia, Anopheles cracens, a more anthropophilic member of the Leucosphyrus group was identified as the vector of P. knowlesi [70], while in Selangor, An. hackeri and Anopheles introlatus were found to be the vectors [4,71]. It seems that different geographical locations have a different predominant species of Leucosphyrus group. Within the same area, different ecological conditions play an important role. In Kapit, Sarawak An. latens and Anopheles watsonii were predominant in forested ecotypes, while in the farm areas, Anopheles donaldi and An. latens were the main species [67]. However, dissection and further examination of those mosquitoes revealed only An. latens to be positive with sporozoites and to be attracted to both humans and macaques. Thus, An. latens had been incriminated as the main vector for P. knowlesi in Kapit, Sarawak. The role of ecology was also observed by Wharton and Eyles, who incriminated An. hackeri as a vector for P. knowlesi at the coastal region of Selangor [4].
Other species of Anopheles like An. watsonii and An. donaldi found in sympatry with An. latens in Kapit were positive for oocysts with PCR–positive for the genus Plasmodium, but species identification by using primers for simian and human malaria parasites was not successful [67]. Anopheles kochi in Kuala Lipis, Pahang was found in considerable numbers in monkey baited traps but was not positive for oocyst or sporozoites by dissection [70]. A recent study by Hawkes and colleagues in Sabah found a non-Leucosphyrus group species, An. donaldi, to be positive for DNA of P. knowlesi, P. cynomolgi, and an unknown Plasmodium species [72]. Unfortunately, in this study, the whole mosquito was processed, so it is not known whether oocysts or sporozoites were present, and therefore, whether these mosquitoes were vectors.
In the forest and forest fringes of Khanh Phu commune, Vietnam, Anopheles dirus, the main vector of human malaria in this region [73], was identified as the primary vector of P. knowlesi [66]. It was the only Anopheles species found with sporozoites, and the highest number infected was detected in the forest compared to the forest fringes. P. knowlesi was the second most common Plasmodium species identified in the vectors using nested PCR assay followed by other simian plasmodia [66]. P. knowlesi was detected in the salivary glands of An. dirus as was the case in the previous study by Marchand and colleagues in the same locality [42].
In the other countries of the Mekong region (Cambodia, Laos, Myanmar, and Thailand), An. dirus is the main vector for human malaria besides Anopheles minimus [73]. Thus, based on results from Vietnam, it can be extrapolated that An. dirus could play a role in the transmission of knowlesi malaria in that region. In Palawan, Phillipines, Tsukamoto and colleagues demonstrated the presence of Plasmodium oocysts and sporozoites in An. balabacensis in an area where macaques were present. At that time, molecular tools were not available, so they carried out experiments to expose An. balabacensis, also a member of the Leucosphyrus group, to Plasmodium-positive macaques and showed that An. balabacensis could develop sporozoites [60]. In conclusion, it can be stated that, at present, only the Leucosphyrus group of Anopheles are confirmed vectors for P. knowlesi in Southeast Asia.
Bionomics of the vectors
With extensive deforestation and land exploration in Malaysia over the past decades, there have been changes in the bionomics of the mosquitoes. Deforestation has caused the migration of the long-tailed macaques from forested area to farms and semi-urban areas where they usually scavenge for food. This may have triggered mosquitoes to follow the host and adapt to forest fringes and farm areas [16]. Fruit orchards that were propagated in those areas were ideal sites for the Anopheles vectors [74].
Most of these vectors tend to bite humans early, between 1900 and 2100 hours, but this varies with the geographical locations and the mosquitoes species [16,67–71]. An. latens prefers to bite macaques at 3 to 6 m above ground. It was demonstrated that the periodicity of the gametocyte stage in the macaques’ host coincides with the biting times of the vector mosquito [75]. This raises the possibility that these vector mosquitoes become infected by biting macaques late at night and transmitting the infection to humans by biting them in the early part of the night, when they are not protected.
Other simian malaria parasites in vectors
Many studies have investigated the presence of other simian malaria parasites in salivary glands and oocysts from Anopheles mosquitoes using PCR. Based on the compiled data (Table 3), P. cynomolgi and P. inui were more prevalent in the vectors compared to P. knowlesi. These parasites were present as monoinfections and multiple infections. This observation is in parallel with the prevalence of simian plasmodia recovered from macaques [55,56,58]. The overlapping distribution of the vectors and the macaques in forested areas might explain the high prevalence of P. cynomolgi and P. inui in both the macaques and in the Anopheles mosquitoes. Anopheles mosquitoes identified as natural vectors for simian plasmodia in Southeast Asia are shown in Table 4. There are studies showing coinfections of these simian malaria parasites with human Plasmodium in mosquitoes [42,66,76] indicating possible simultaneous transmission.
Table 3. Simian Plasmodium parasites in Anopheles Leucosphyrus group in SEA.
| Plasmodium species | Number of positive samples in An. balabacensis in Kudat and Pulau Banggi, Sabah, Malaysia [68] | Number of positive samples in An. balabacensis in Kudat and Ranau, Sabah, Malaysia [69,72,77] | Number of positive samples in An. latens in Kapit, Sarawak, Malaysia [78] | Number of positive samples in An. cracens in Kuala Lipis, Pahang, Malaysia [70] | Number of positive samples in An. dirus in Khanh Phu, Vietnam [66] | Number of positive samples in An. balabacensis in Palawan Island, Philippines [60] | |||
|---|---|---|---|---|---|---|---|---|---|
| Midgut | Salivary gland | Whole mosquito | Midgut | Salivary gland | Salivary gland | Salivary gland | Midgut | Salivary gland | |
| Pk | - | 1 | 1 | - | 5 | 3 | 4 (7*) | - | - |
| Pcy | 7 | 6 | 5 | - | - | - | 6 (3*) | - | - |
| Pin | 5 | 5 | 12 | - | 4 | - | 5 (1’) (3*) (1#) | - | - |
| Pfi | - | - | - | - | - | - | - | - | - |
| Pct | - | - | 3 | - | 1 | - | 7 | - | - |
| Pk + Pin | 2 | 2 | 1 | - | - | 1 | 2 (2*) | - | - |
| Pk + Pcy | 1 | 2 | 1 | - | - | - | 1* | - | - |
| Pcy + Pin | 8 | 4 | 2 | - | - | - | - | - | - |
| Pct + Pin | - | - | - | - | - | - | 1* | - | - |
| Pfi + Pcy | - | - | 1 | - | - | - | - | - | - |
| Pin + Pfi | - | - | - | - | 1 | - | - | - | - |
| Pin + Pct | - | - | - | - | 1 | - | - | - | - |
| Pk + Pcy + Pin | - | 4 | - | - | - | - | - | - | - |
| Pk + Pct + Pin | - | - | - | - | - | - | 1 | - | - |
| Pct + Pcy + Pin | - | - | - | - | - | - | 1* | - | - |
| Pk + Pct + Pcy + Pin | - | 1 | - | - | - | - | - | - | - |
| Not identified | 8 | 2 | - | 5 | - | - | - | 7 | 3 |
Pct, Plasmodium coatneyi; Pcy, Plasmodium cynomolgi; Pfi, Plasmodium fieldi; Pin, Plasmodium inui; Pk, Plasmodium knowlesi; SEA, Southeast Asia.
(‘) Mixed with P. falciparum;
(*) Mixed with P. vivax;
(#) Mixed with P. faciparum and P. vivax.
Table 4. Natural vectors of common simian Plasmodium in SEA and their distribution.
| Parasite | Natural vector | Place and method of incrimination | Distribution of the natural vector in SEA |
|---|---|---|---|
| P. coatneyi | An. balabacensis | Detection of parasite DNA in sporozoite-infected salivary gland. Sabah, Malaysian Borneo [68] | Brunei, Indonesia, Malaysian Borneo, and Philippines [79] |
| An. dirus | Detection of parasite DNA in sporozoite-infected salivary glands. Vietnam [66] | Cambodia, Lao PDR, Thailand, and Vietnam [73] | |
| An. hackeri | Inoculation of sporozoites into rhesus macaque. Peninsular Malaysia [80] | Malaysian Borneo, Peninsular Malaysia, Philippines, and Thailand [73,79] | |
| P. cynomolgi (including the variants cyclopis and ceylonensis) | An. balabacensis | Detection of parasite DNA in sporozoite-infected salivary gland. Sabah, Malaysian Borneo [68] | Brunei, Indonesia, Malaysian Borneo, and Philippines [79] |
| aAn. cracens (An. balabacensis balabacensis by Cheong et al. 1965) | Inoculation of sporozoites into rhesus macaque. Perlis, Peninsular Malaysia [81] | Indonesia, Peninsular Malaysia, and Thailand [79] | |
| An. dirus | Detection of parasite DNA in sporozoite-infected salivary glands. Vietnam [66] | Cambodia, Lao PDR, Thailand, and Vietnam [73] | |
| An. hackeri | Inoculation of sporozoites into rhesus macaque. Peninsular Malaysia [80] | Malaysian Borneo, Peninsular Malaysia, Philippines, and Thailand [73,79] | |
| aAn. introlatus (An. balabacensis introlatus by Eyles et al. 1963) | Inoculation of sporozoites into rhesus macaque. Peninsular Malaysia [82] | Indonesia, Peninsular Malaysia, and Thailand [73,79] | |
| P. fieldi | An. balabacensis (?) | Detection of parasite DNA in whole mosquito. Sabah, Malaysian Borneo [77] | Brunei, Indonesia, Malaysian Borneo, and Philippines [79] |
| An. hackeri | Inoculation of sporozoites into rhesus macaque [80] | Malaysian Borneo, Peninsular Malaysia, Philippines, and Thailand [73,79] | |
| aAn. introlatus (An. balabacensis introlatus by Warren and Wharton 1963) | Inoculation of sporozoites into rhesus macaque [80] | Indonesia, Peninsular Malaysia, and Thailand [73,79] | |
| P. inui (including the variant shortti) | An. balabacensis | Detection of parasite DNA in sporozoite-infected salivary gland. Sabah, Malaysian Borneo [68] | Brunei, Indonesia, Malaysian Borneo, and Philippines [79] |
| aAn. cracens (An. balabacensis balabacensis by Cheong et al. 1965) | Inoculation of sporozoites into rhesus macaque. Perlis, Peninsular Malaysia [81] | Indonesia, Peninsular Malaysia, and Thailand [79] | |
| An. dirus | Detection of parasite DNA in sporozoite-infected salivary glands. Vietnam [66] | Cambodia, Lao PDR, Thailand, and Vietnam [73] | |
| An. hackeri | Inoculation of sporozoites into rhesus macaque [80] | Malaysian Borneo, Peninsular Malaysia, Philippines, and Thailand [73,79] | |
| aAn. latens (An. leucosphyrus by Wharton et al. 1962) | Inoculation of sporozoites into rhesus macaque. Selangor, Peninsular Malaysia [83] | Indonesia, Malaysian Borneo, Peninsular Malaysia, and Thailand [79] | |
| P. knowlesi | An. balabacensis | Detection of parasite DNA in sporozoite-infected salivary gland. Sabah, Malaysian Borneo [68] | Brunei, Indonesia, Malaysian Borneo, and Philippines [79] |
| An. cracens | Detection of parasite DNA in sporozoite-infected salivary gland. Pahang, Peninsular Malaysia [70] | Indonesia, Peninsular Malaysia, and Thailand [79] | |
| An. dirus | Detection of parasite DNA in sporozoite-infected salivary gland. Vietnam [42] | Cambodia, Lao PDR, Thailand, and Vietnam [73] | |
| An. introlatus* | Detection of parasite DNA in the oocysts. Selangor, Peninsular Malaysia [71] | Indonesia, Peninsular Malaysia, and Thailand [73,79] | |
| An. hackeri | Inoculation of sporozoites into rhesus macaque. Selangor, Peninsular Malaysia [4] | Malaysian Borneo, Peninsular Malaysia, Philippines, and Thailand [73,79] | |
| An. latens | Detection of parasite DNA in sporozoite-infected salivary gland. Sarawak, Malaysian Borneo [67] | Indonesia, Malaysian Borneo, Peninsular Malaysia, and Thailand [79] |
a Species name revised based on Sallum et al. 2005 [79].
(?) The parasite DNA was detected in the whole body (not in saliva or sporozoite form, so it is still questionable).
* Vector had been incriminated based on epidemiological grounds.
Lao PDR, Lao People’s Democratic Republic; SEA, Southeast Asia.
Challenges and future direction: What’s next?
Increasing case numbers of knowlesi malaria
With the reduction in human malaria cases, in Southeast Asia, more and more cases of simian malaria, especially due to P. knowlesi, are detected in humans as seen in Malaysia and Sumatra in Indonesia [23]. One factor could be simply increased awareness and the availability of molecular diagnosis. Besides, increasing exposure of humans to mosquitoes that transmit simian malaria may also be a factor. This is compounded by human encroachment into areas where deforestation and environmental changes are taking place.
However, this hardly explains the continued steep increases in Malaysia over the last 25 years. With reduction of human malaria, control measures may be relaxed, increasing the risk of simian malaria. In addition, diminishing cross-species protective immunity may play a role. P. knowlesi and P. vivax share important antigenic properties [84–86]. In vitro, specific antibodies against P. vivax antigens from animals and malaria patients inhibit erythrocyte invasion by P. knowlesi [86,87]. Data from neurosyphilis malariotherapy demonstrated that patients previously infected with P. vivax had lower susceptibility to P. knowlesi [88].
In addition, are we beginning to see human-to-human transmission occurring? At least in some areas, in Malaysia and Vietnam, the main vectors of simian malaria are also the main vectors of human malaria. While monkey-to-human transmission currently remains the main route of transmission for knowlesi malaria [89], human-to-human transmission could well occur, and may become more likely as prevalence continues to increase.
Possibilities of other simian malaria infecting humans
As noted above, cases of other simian malaria parasites infecting humans have been detected with increasing frequency over the last 10 years. With reports of P. cynomolgi in humans [11–15], the results of the study demonstrate the importance of being proactive to prevent outbreaks. Previous studies have indicated that antisera to P. falciparum, P. malariae and P. ovale from humans can cross-react with the P. cynomolgi antigen [90–92]. Therefore, if P. knowlesi emerged in humans due to the waning cross-immunity [45], this could occur also for other simian malarias.
As P. inui and P. cynomolgi are the most prevalent simian malaria parasites in the macaque hosts and vectors in Southeast Asia, humans are exposed to these parasites by bites of infectious mosquitoes [66]. The vectors of simian malaria are now known to bite both macaques and humans [67,68,70] unlike in the 1960s when An. hackeri was the only verified vector of simian malaria [4]. As human infections with P. inui and P. cynomolgi result in milder infections which do not require hospitalization compared to infection caused by human malaria or P. knowlesi [93], these infections could go unnoticed as asymptomatic infections or be misdiagnosed. Under these circumstances, those parasites could adapt to humans in the years to come. Indeed, some studies have shown that host switching is common in the evolution of malaria parasites [94,95]. Thus, we believe that the surveillance and investigation of naturally acquired infections with other simian plasmodia should be given high priority.
Control of simian malarias
While human malaria control strategies mainly rely on insecticide-treated nets or indoor residual spraying and chemotherapy, the control of simian malaria poses a serious challenge, as neither the nonhuman primate reservoir nor the mainly forest-dwelling vectors are much affected by the human-focused interventions. A study in Sabah, Malaysia [96] indicated that mosquito nets have less protective effect against P. knowlesi infection mainly due to the bionomics of the local vector, An. balabacensis. New vector control tools like repellents and insecticide treated clothing need to be investigated. Besides, more robust vector studies should be conducted to enable the design of new tools for vector control. Given the importance of environmental change for the epidemiology of these infections, Forest, Agricultural, and Public Health Departments should work together to identify and investigate possible infections thoroughly and seek to curb transmission [74].
Certification of malaria-free status is granted by WHO if the transmission of human malaria in an entire country has been interrupted for at least 3 consecutive years [97]. For Southeast Asian countries aiming to obtain WHO certification of malaria-free status, it would be helpful if WHO could state whether P. knowlesi is to be considered a human malaria parasite in the context of certification. More generally, we call on WHO to spearhead the recognition of simian malarias in humans as a threat, for example, (but not only) by including simian malaria in the annual World Malaria Reports. This could spur increased surveillance and research to the benefit of the populations at risk and the ultimate goal of malaria eradication.
Case management of simian malarias
A rapid diagnostic test with high specificity and sensitivity is yet to be developed for P. knowlesi. In moving forward, it is also crucial to initiate the development of diagnostic kits for both P. inui and P. cynomolgi. In a recent case reported in Kelantan, Malaysia [98], a patient infected with P. cynomolgi was misdiagnosed as P. vivax through microscopic examinations. When later reexamined molecularly, it was found that the patient was infected with P. cynomolgi, not P. vivax. Furthermore, the knowledge of clinicians about simian malarias is insufficient [98]. It is not generally known, for example, that P. cynomolgi produce hypnozoites [99] and thus requires primaquine treatment.
Conclusions
Many countries in Southeast Asia are rapidly reducing indigenous human malaria transmission. However, the ongoing increase in P. knowlesi cases poses a major challenge to malaria control. Besides P. knowlesi, priority should be given to surveillance and control of other simian Plasmodium species, especially P. inui and P. cynomolgi, which have the potential to infect human. Simian malaria could emerge and spread as a major public health problem, as the 4 classical human malarias are reduced and as environmental change brings human into increased contact with simian malaria. The early recognition and containment of transmission of these simian malaria among humans should be given high priority.
Key Learning Points
The reduction in human malaria cases may have exposed human populations in some areas of Southeast Asia to a greater risk of being infected by Plasmodium knowlesi due to diminishing cross-species immunity.
Increased number of imported cases of simian malaria in countries outside Southeast Asia shows that simian malaria is now an international concern.
Plasmodium cynomolgi and Plasmodium inui are more prevalent in Anopheles vectors and macaques hosts in Southeast Asia than P. knowlesi, raising the possibility of increased natural transmission of these simian malaria parasites to human in future.
The possible emergence of other simian parasites (besides P. knowlesi) necessitates rapid diagnostic tests, appropriate treatment regimes, and new control strategies for malaria caused by these parasites.
Top Five Papers
Grignard L, Shah S, Chua TH, William T, Drakeley CJ, Fornace KM. Natural human infections with Plasmodium cynomolgi and other malaria species in an elimination setting in Sabah, Malaysia. J Infect Dis. 2019;220(12):1946–9.
Herdiana H, Irnawati I, Coutrier FN, Munthe A, Mardiati M, Yuniarti T, et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar J. 2018;17(1):186.
Lee KS, Divis PCSS, Zakaria SK, Matusop A, Julin RA, Conway DJ, et al. Plasmodium knowlesi: Reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011;7(4):e1002015.
Moyes CL, Henry AJ, Golding N, Huang Z, Singh B, Baird JK, et al. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Negl Trop Dis. 2014;8(3):e2780.
Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman W-Y, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLOS Negl Trop Dis. 2015;9(10):e0004135.
Supporting information
(DOC)
Acknowledgments
The authors would like to thank Dr. Allan Schapira, formerly of WHO Geneva, for his constructive comments on this manuscript.
Funding Statement
This study was funded by Ministry of Education, Malaysia, Long-term Research Grant Scheme (LRGS 2018-1) awarded to IV. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149(3686):865. [DOI] [PubMed] [Google Scholar]
- 2.Coatney G, Collins WE, Warren W, Contacos PG. The primate malarias. Washington: US Government Printing Office; 1971. [Google Scholar]
- 3.Eyles DE, Dunn FL, Warren M, Guinn E. Plasmodium coatneyi from the Philippines. J Parasitol. 1963;49(6):1038. [PubMed] [Google Scholar]
- 4.Wharton RH, Eyles DE. Anopheles hackeri, a vector of Plasmodium knowlesi in Malaya. Science. 1961;134(3474):279–80. [DOI] [PubMed] [Google Scholar]
- 5.Warren M, Fredericks HK, Coatney GR, Cheong WH. Cycles of jungle malaria in West Malaysia. Am J Trop Med Hyg. 1970;19(3):383–93. [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SSG, Cox-Singh J, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363(9414):1017–24. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Daneshvar C. Plasmodium knowlesi malaria in Malaysia. Med J Malaysia. 2010;65(3):224–30. [PubMed] [Google Scholar]
- 8.Coatney GR, Chin W, Contacos PG, King HK. Plasmodium inui, a quartan-type malaria parasite of old world monkeys transmissible to man. J Parasitol. 1966;52(4):660. [PubMed] [Google Scholar]
- 9.Schmidt LH, Greenland R, Genther CS. The transmission of Plasmodium cynomolgi to man. Am J Trop Med Hyg. 1961;10(5):679–88. [DOI] [PubMed] [Google Scholar]
- 10.Eyles DE, Coatney GR, Getz ME. Vivax-type malaria parasite of macaques transmissible to man. Science. 1960;131(3416):1812–3. [DOI] [PubMed] [Google Scholar]
- 11.Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grignard L, Shah S, Chua TH, William T, Drakeley CJ, Fornace KM. Natural human infections with Plasmodium cynomolgi and other malaria species in an elimination setting in Sabah, Malaysia. J Infect Dis. 2019;220(12):1946–9. 10.1093/infdis/jiz397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh B, Kadir KAA, Hu THH, Raja TNN, Mohamad DSS, Lin LWW, et al. Naturally acquired human infections with the simian malaria parasite, Plasmodium cynomolgi, in Sarawak, Malaysian Borneo. Int J Infect Dis. 2018;73:68. [Google Scholar]
- 14.Imwong M, Madmanee W, Suwannasin K, Kunasol C, Peto TJ, Tripura R, et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi. J Infect Dis. 2019;219(5):695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmeyer GN, Stensvold CR, Fabricius T, Marmolin ES, Hoegh SV, Nielsen HV, et al. Plasmodium cynomolgi as cause of malaria in tourist to Southeast Asia. 2018. Emerg Infect Dis. 2019;25(10):1936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vythilingam I, Wong ML, WanYussof WS. Current status of Plasmodium knowlesi vectors: A public health concern? Parasitology. 2018;145(1):32–40. [DOI] [PubMed] [Google Scholar]
- 17.Koh GJN, Ismail PK, Koh D . Occupationally acquired Plasmodium knowlesi malaria in Brunei Darussalam. Saf Health Work. 2019;10(1):122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khim N, Siv S, Kim S, Mueller T, Fleischmann E, Singh B, et al. Plasmodium knowlesi infection in humans, Cambodia, 2007–2010. Emerg Infect Dis. 2011;17(10):1900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulistyaningsih E, Fitri LE, Löscher T, Berens-Riha N. Diagnostic difficulties with Plasmodium knowlesi infection in humans. Emerg Infect Dis. 2010;16(6):1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figtree M, Lee R, Bain L, Kennedy T, Mackertich S, Urban M, et al. Plasmodium knowlesi in human, Indonesian Borneo. Emerg Infect Dis. 2010;16(4):672–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herdiana H, Irnawati I, Coutrier FN, Munthe A, Mardiati M, Yuniarti T, et al. Two clusters of Plasmodium knowlesi cases in a malaria elimination area, Sabang Municipality, Aceh, Indonesia. Malar J. 2018;17(1):186 10.1186/s12936-018-2334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setiadi W, Sudoyo H, Trimarsanto H, Sihite BA, Saragih RJ, Juliawaty R, et al. A zoonotic human infection with simian malaria, Plasmodium knowlesi, in Central Kalimantan, Indonesia. Malar J. 2016;15(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herdiana H, Cotter C, Coutrier FN, Zarlinda I, Zelman BW, Tirta YK, et al. Malaria risk factor assessment using active and passive surveillance data from Aceh Besar, Indonesia, a low endemic, malaria elimination setting with Plasmodium knowlesi, Plasmodium vivax, and Plasmodium falciparum. Malar J. 2016;15(1):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubis IND, Wijaya H, Lubis M, Lubis CP, Divis PCS, Beshir KB, et al. Contribution of Plasmodium knowlesi to multispecies human malaria infections in North Sumatera, Indonesia. J Infect Dis. 2017;215(7):1148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwagami M, Nakatsu M, Khattignavong P, Soundala P, Lorphachan L, Keomalaphet S, et al. First case of human infection with Plasmodium knowlesi in Laos. PLoS Negl Trop Dis. 2018;12(3):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pongvongsa T, Culleton R, Ha H, Thanh L, Phongmany P, Marchand RP, et al. Human infection with Plasmodium knowlesi on the Laos-Vietnam border. Trop Med Health. 2018;46(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministry of Health Malaysia (MOH). Knowlesi Malaria: Malaysia’s Experience in Vector Control [Internet]. 2019 [cited 2019 Nov 18]. https://endmalaria.org/sites/default/files/5_ChristinaRundi.pdf.
- 28.Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, et al. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg Infect Dis. 2010;16(9):1476–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sermwittayawong N, Singh B, Nishibuchi M, Sawangjaroen N, Vuddhakul V. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar J. 2012;11(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghinai I, Cook J, Tun T, Hla W, Myat H, Htet T, et al. Malaria epidemiology in central Myanmar: Identification of a multi-species asymptomatic reservoir of infection. Malar J. 2017;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, et al. Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14(5):811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng OT, Eng EO, Cheng CL, Piao JL, Lee CN, Pei SW, et al. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14(5):814–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeslyn WPS, Huat TC, Vernon L, Irene LMZ, Sung LK, Jarrod LP, et al. Molecular epidemiological investigation of Plasmodium knowlesi in humans and macaques in Singapore. Vector Borne Zoonotic Dis. 2011;11(2):131–5. 10.1089/vbz.2010.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10(12):2211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, et al. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J Infect Dis. 2009;199(8):1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jongwutiwes S, Buppan P, Kosuvin R, Seethamchai S, Pattanawong U, Sirichaisinthop J, et al. Plasmodium knowlesi malaria in humans and macaques, Thailand. Emerg Infect Dis. 2011;17(10):1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Traipattanakul J, Changpradub D, Trakulhun K, Phiboonbanakit D, Mungthin M. A first case of Plasmodium knowlesi malaria in Phramongkutklao Hospital. J Infect Dis Antimicrob Agents. 2014;31(2):91–100. [Google Scholar]
- 38.Nakaviroj S, Kobasa T, Teeranaipong P, Putaporntip C, Jongwutiwes S. An autochthonous case of severe Plasmodium knowlesi malaria in Thailand. Am J Trop Med Hyg. 2015;92(3):569–72. 10.4269/ajtmh.14-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chantaramongkol J, Buathong R. A fatal malaria caused by Plasmodium knowlesi infection in a healthy man, Betong, Yala, Thailand April, 2016. Int J Infect Dis. 2016;53:124. [Google Scholar]
- 40.Ngernna S, Rachaphaew N, Thammapalo S, Prikchoo P, Kaewnah O, Manopwisedjaroen K, et al. Case report: Case series of human Plasmodium knowlesi infection on the Southern Border of Thailand. Am J Trop Med Hyg. 2019;101(6):1397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.den Eede P, Van HN, Van OC, Vythilingam I, Duc TN, Hung LX, et al. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar J. 2009;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S, Population S. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, Southern Vietnam. Emerg Infect Dis. 2011;17(7):1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaw MT, Lin Z. Human Plasmodium knowlesi infections in South-East Asian countries. J Microbiol Immunol Infect. 2019;52(5):679–84. [DOI] [PubMed] [Google Scholar]
- 44.Ministry of Health Malaysia (MOH). Press statement Zoonotic malaria and the prevention program in Malaysia [Internet]. 2018 [cited 2019 Nov 18]. http://www.moh.gov.my/index.php/database_stores/attach_download/337/1087.
- 45.William T, Rahman HA, Jelip J, Ibrahim MY, Menon J, Grigg MJ, et al. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax malaria in Sabah, Malaysia. PLoS Negl Trop Dis. 2013;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ooi CH, Bujang MA. Tg Abu Bakar Sidik TMI, Ngui R, Lim YAL. Over two decades of Plasmodium knowlesi infections in Sarawak: Trend and forecast. Acta Trop. 2017;176:83–90. [DOI] [PubMed] [Google Scholar]
- 47.Fornace KM, Alexander N, Abidin TR, Brock PM, Chua TH, Vythilingam I, et al. Local human movement patterns and land use impact exposure to zoonotic malaria in Malaysian Borneo. Elife. 2019;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muehlenbein MP, Pacheco MA, Taylor JE, Prall SP, Ambu L, Nathan S, et al. Accelerated diversification of non-human primate malarias in Southeast Asia: Adaptive radiation or geographic speciation? Mol Biol Evol. 2015;32(2):422–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyes CL, Henry AJ, Golding N, Huang Z, Singh B, Baird JK, et al. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Negl Trop Dis. 2014;8(3):e2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller M, Schlagenhauf P. Plasmodium knowlesi in travellers, update 2014. Int J Infect Dis. 2014;22:55–64. [DOI] [PubMed] [Google Scholar]
- 51.Cramer JP. Plasmodium knowlesi malaria: Overview focussing on travel-associated infections. Curr Infect Dis Rep. 2015;17(3). [DOI] [PubMed] [Google Scholar]
- 52.Yap NJ. Molecular investigation of human and simian Plasmodium species among human archived blood samples in Malaysia. PhD Thesis. University of Malaya; 2019.
- 53.Eyles DE, Sandosham AA, Guinn E, Wharton RH, Warren M, Fong YL. Plasmodium coatneyi, a new species of primate malaria from Malaya. Am J Trop Med Hyg. 1962;11(5):597–604. [Google Scholar]
- 54.Lee KS, Vythilingam I. Plasmodium knowlesi: Emergent human malaria in Southeast Asia In: Parasites and their vectors. Springer; Vienna; 2013. p. 5–31. [Google Scholar]
- 55.Lee KS, Divis PCSS, Zakaria SK, Matusop A, Julin RA, Conway DJ, et al. Plasmodium knowlesi: Reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 2011;7(4):e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akter R, Vythilingam I, Khaw LT, Qvist R, Lim YAL, Sitam FT, et al. Simian malaria in wild macaques: First report from Hulu Selangor district, Selangor. Malaysia. Malar J. 2015;14(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Kadir KA, Quintanilla-Zariñan LF, Villano J, Houghton P, Du H, et al. Distribution and prevalence of malaria parasites among long-tailed macaques (Macaca fascicularis) in regional populations across Southeast Asia. Malar J. 2016;15(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siregar JE, Faust CL, Murdiyarso LS, Rosmanah L, Saepuloh U, Dobson AP, et al. Non-invasive surveillance for Plasmodium in reservoir macaque species. Malar J. 2015;14(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khajeaian P. Identification and determination of genetic diversity in simian malaria parasites among wild long-tailed monkey populations from various regions of Peninsular Malaysia. Masters Thesis. Universiti Putra Malaysia; 2015.
- 60.Tsukamoto M, Miyata A, Miyagi I. Surveys on simian malaria parasites and their vector in Palawan Island, the Philippines. Trop Med. 1978;20(1):39–50. [Google Scholar]
- 61.Gamalo LE, Dimalibot J, Kadir KA, Singh B, Paller VG. Plasmodium knowlesi and other malaria parasites in long-tailed macaques from the Philippines. Malar J. 2019;18(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li MZ. Identification and molecular characterisation of simian malaria parasites in wild monkeys of Singapore. MSc Thesis. National University of Singapore; 2011.
- 63.Seethamchai S, Putaporntip C, Jongwutiwes S, Cui L, Malaivijitnond S. Malaria and Hepatocystis species in wild macaques, Southern Thailand. Am J Trop Med Hyg. 2008;78(4):646–53. [PubMed] [Google Scholar]
- 64.Putaporntip C, Jongwutiwes S, Thongaree S, Seethamchai S, Grynberg P, Hughes AL. Ecology of malaria parasites infecting Southeast Asian macaques: Evidence from cytochrome b sequences. Mol Ecol. 2010;19(16):3466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eyles DE. The species of simian malaria: Taxonomy, morphology, life cycle, and geographical distribution of the monkey species. J Parasitol. 1963;49(6):866. [PubMed] [Google Scholar]
- 66.Maeno Y, Quang NT, Culleton R, Kawai S, Masuda G, Nakazawa S, et al. Humans frequently exposed to a range of non-human primate malaria parasite species through the bites of Anopheles dirus mosquitoes in South-central Vietnam. Parasit Vectors. 2015;8(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar J. 2008;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman W-Y, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis. 2015;9(10):e0004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chua TH, Manin BO, Vythilingam I, Fornace K, Drakeley CJ. Effect of different habitat types on abundance and biting times of Anopheles balabacensis Baisas (Diptera: Culicidae) in Kudat district of Sabah. Malaysia Parasit Vectors. 2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiram AI, Vythilingam I, Noorazian YM, Yusof YM, Azahari AH, Fong MY. Entomologic investigation of Plasmodium knowlesi vectors in Kuala Lipis, Pahang, Malaysia. Malar J. 2012;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vythilingam I, Lim YAL, Venugopalan B, Ngui R, Leong CS, Wong ML, et al. Plasmodium knowlesi malaria an emerging public health problem in Hulu Selangor, Selangor, Malaysia (2009–2013): Epidemiologic and entomologic analysis. Parasit Vectors. 2014;7(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawkes FM, Manin BO, Cooper A, Daim S, Homathevi R, Jelip J, et al. Vector compositions change across forested to deforested ecotones in emerging areas of zoonotic malaria transmission in Malaysia. Sci Rep. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hii J, Rueda LM. Malaria vectors in the Greater Mekong Subregion: Overview of malaria vectors and remaining challenges. Southeast Asian J Trop Med Public Health. 2013;44:73–165. [PubMed] [Google Scholar]
- 74.Overgaard HJ, Ekbom B, Suwonkerd W, Takagi M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landsc Ecol. 2003;18(6):605–19. [Google Scholar]
- 75.Anderios F, NoorRain A, Vythilingam I. In vivo study of human Plasmodium knowlesi in Macaca fascicularis. Exp Parasitol. 2010;124(2):181–9. [DOI] [PubMed] [Google Scholar]
- 76.Nakazawa S, Marchand RP, Quang NT, Culleton R, Manh ND, Maeno Y. Anopheles dirus co-infection with human and monkey malaria parasites in Vietnam. Int J Parasitol. 2009;39(14):1533–7. [DOI] [PubMed] [Google Scholar]
- 77.Chua TH, Manin BO, Daim S, Vythilingam I, Drakeley C. Phylogenetic analysis of simian Plasmodium spp. infecting Anopheles balabacensis Baisas in Sabah, Malaysia. PLoS Negl Trop Dis. 2017;11(10):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan CH. Identification of vectors of Plasmodium knowlesi and other malaria parasites, and studies on their bionomics in Kapit, Sarawak, Malaysia. Master Thesis. Universiti Malaysia Sarawak; 2008.
- 79.Sallum MAM, Peyton EL, Harrison BA, Wilkerson RC. Revision of the Leucosphyrus group of Anopheles (Cellia) (Diptera, Culicidae). Rev Bras Entomol. 2005;49(SUPPL. 1):1–152. [Google Scholar]
- 80.Wharton RH, Eyles DE, Warren M, Cheong WH. Studies to determine the vectors of monkey malaria in Malaya. Ann Trop Med Parasitol. 1964;58(1):56–77. [DOI] [PubMed] [Google Scholar]
- 81.Cheong WH, Warren M, Omar AH, Mahadevan S. Anopheles balabacensis balabacensis identified as vector of simian malaria in Malaysia. Science. 1965;150(3701):1314–5. [DOI] [PubMed] [Google Scholar]
- 82.Eyles DE, Warren M, Guinn E, Lumpur K, Wharton RH, Ramachandran CP. Identification of Anopheles balabacensis introlatus as a vector of monkey malaria in Malaya. Bull World Health Organ. 1963. [PMC free article] [PubMed] [Google Scholar]
- 83.Wharton RH, Eyles DE, Warren M, Moorhouse DE. Anopheles leucosphyrus identified as a vector of monkey malaria in Malaya. Science. 1962;137(3532):758. [DOI] [PubMed] [Google Scholar]
- 84.Miller L, Mason S, Dvorak J, McGinniss M, Rothman I. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189(4202):561–3. [DOI] [PubMed] [Google Scholar]
- 85.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks: The Duffy-blood-group genotype, FyFy. N Engl J Med. 1976;295(6):302–4. [DOI] [PubMed] [Google Scholar]
- 86.Muh F, Ahmed MA, Han JH, Nyunt MH, Lee SK, Lau YL, et al. Cross-species analysis of apical asparagine-rich protein of Plasmodium vivax and Plasmodium knowlesi. Sci Rep. 2018;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muh F, Kim N, Nyunt MH, Firdaus ER, Han JH, Hoque MR, et al. Cross-species reactivity of antibodies against Plasmodium vivax blood-stage antigens to Plasmodium knowlesi. PLoS Negl Trop Dis. 2020;14(6):e0008323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Rooyen CE, Pile GR. Observations on infection by Plasmodium knowlesi (ape malaria) in the treatment of general paralysis of the insane. Br Med J. 1935;2(3901):662–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amir A, Cheong FW, de Silva JR, Liew JWK, Lau YL. Plasmodium knowlesi malaria: Current research perspectives. Infect Drug Resist. 2018;11:1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Collins WE, Jeffery GM, Guinn E, Skinner JC. Fluorescent antibody studies in human malaria. IV. Cross-reactions between human and simian malaria. Am J Trop Med Hyg. 1966;15(1):11–5. [DOI] [PubMed] [Google Scholar]
- 91.Meuwissen JHET. Antibody responses of patients with natural malaria to human and simian Plasmodium antigens measured by the flurescent antibody test. Trop Geogr Med. 1968;20:137–40. [PubMed] [Google Scholar]
- 92.Kuvin SF, Voller A. Malarial antibody titres of west Africans in Britain. Br Med J. 1963;2(5355):477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coatney GR. The simian malarias: Zoonoses, anthroponoses, or both? Am J Trop Med Hyg. 1971;20(6):795–803. [DOI] [PubMed] [Google Scholar]
- 94.Hayakawa T, Culleton R, Otani H, Horii T, Tanabe K. Big bang in the evolution of extant malaria parasites. Mol Biol Evol. 2008;25(10):2233–9. [DOI] [PubMed] [Google Scholar]
- 95.Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol Phylogenet Evol. 2008;47(1):261–73. [DOI] [PubMed] [Google Scholar]
- 96.Grigg MJ, Cox J, William T, Jelip J, Fornace KM, Brock PM, et al. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: A case-control study. Lancet Planet Health. 2017;1(3):e97–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.World Health Organization. A framework for malaria elimination. World Health Organization; 2017. [Google Scholar]
- 98.Mohd Nor F, Azeana R, Aziz A, Azimullah M, Zakaria A, Adura S, et al. P. vivax or P. cynomolgi?: Public health challenges in detection and control measures. Int J Publ Health Sci. 2020;6(6):2289–7577. [Google Scholar]
- 99.Cogswell FB. The hypnozoite and relapse in primate malaria. Clin Microbiol Rev. 1992;5(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)


