Abstract
Background
The course of coronavirus disease 2019 (COVID-19) seems to be aggravated by air pollution, and some industrial chemicals, such as the perfluorinated alkylate substances (PFASs), are immunotoxic and may contribute to an association with disease severity.
Methods
From Danish biobanks, we obtained plasma samples from 323 subjects aged 30–70 years with known SARS-CoV-2 infection. The PFAS concentrations measured at the background exposures included five PFASs known to be immunotoxic. Register data was obtained to classify disease status, other health information, and demographic variables. We used ordered logistic regression analyses to determine associations between PFAS concentrations and disease outcome.
Results
Plasma-PFAS concentrations were higher in males, in subjects with Western European background, and tended to increase with age, but were not associated with the presence of chronic disease. Of the study population, 108 (33%) had not been hospitalized, and of those hospitalized, 53 (16%) had been in intensive care or were deceased. Among the five PFASs considered, perfluorobutanoic acid (PFBA) showed an unadjusted odds ratio (OR) of 2.19 (95% confidence interval, CI, 1.39–3.46) for increasing severities of the disease. Among those hospitalized, the fully adjusted OR for getting into intensive care or expiring was 5.18 (1.29, 20.72) when based on plasma samples obtained at the time of diagnosis or up to one week before.
Conclusions
Measures of individual exposures to immunotoxic PFASs included short-chain PFBA known to accumulate in the lungs. Elevated plasma-PFBA concentrations were associated with an increased risk of a more severe course of COVID-19. Given the low background exposure levels in this study, the role of exposure to PFASs in COVID-19 needs to be ascertained in populations with elevated exposures.
Introduction
Elevated exposure to community pollution is associated with a worsened outcome of coronavirus disease 2019 (COVID-19) [1–4]. While replicated in different populations, this evidence relies solely on ecological study designs of air pollution without measures of individual exposures. Several environmental chemicals are known to suppress immune functions [5, 6] and worsen the course of infections [7]. Of particular relevance, the perfluorinated alkylate substances (PFASs) are persistent, globally disseminated chemicals known to be immunotoxic [8]. Thus, elevated blood-PFAS concentrations are associated with lower antibody responses to vaccinations in children [9] and in adults [10]. Also, infectious disease occurs more frequently in children with elevated exposure [11–13]. In support of the potential impact of these substances, a modeling study suggested that endocrine disruptors, including major PFASs, may interfere with proteins involved in critical pathways, such as IL-17, associated with severe clinical outcomes of the COVID-19 infection [14].
Substantial differences occur in the clinical course of the disease, and the reasons for this variability are only partially known [15, 16]. As a possible contributor, a deficient antibody response may be an important contributor to a more severe clinical course of the infection [17], as also suggested by the poorer prognosis in patients with bacterial co-infection [18]. The most serious clinical consequences are associated with male sex, older age, and the presence of co-morbidities, including obesity and diabetes [19–23]. In parallel, serum-PFAS concentrations are higher in men than in women and also tend to increase with age [8, 24]. Because elevated PFAS exposure has been linked to both obesity and diabetes [25, 26], these substances may potentially affect the progression of COVID-19 directly as well as indirectly.
Several PFASs can be reliably determined in human blood samples, where most of them show long biological half-lives of 2–3 years or more [27], thereby providing a measure of cumulated exposure. Still, blood concentrations may not accurately reflect the retention in specific organs, e.g., the short-chain perfluorobutanoic acid (PFBA), which accumulates in the lungs [28].
To assess if elevated background exposures to immunotoxic PFASs are associated with the clinical course of the infection, a study was undertaken in Denmark to determine individual plasma-PFAS concentrations in adults confirmed to be infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and examine the association with the severity of COVID-19 development.
Methods
Population
Plasma samples for PFAS analysis were obtained from medical biobanks that store excess material from diagnostic tests, viz., the Danish National Biobank at the Statens Serum Institut (SSI) and Odense University Hospital (OUH). Eligible subjects were identified from the Danish cohort of COVID-19 patients [29]. All cases were tested by quantitative polymerase-chain-reaction (PCR) and had a positive response for SARS-CoV-2 infection, as recorded in the Danish Microbiology Database (MiBa), a national database that contains both positive and negative results of the majority of microbiology testing done in Denmark [30].
The study included non-pregnant subjects aged 30–70 years at the time of the positive test by early March 2020 through early May 2020, provided that the biobanks could provide a plasma sample of 0.15 mL. Although most blood samples were obtained soon after SARS-CoV-2 infection was identified, we also included subjects, mainly those not hospitalized, whose plasma in the SSI biobank had been obtained up to 28 months earlier, i.e., less than a half-life for major PFASs [27]. We calculated the time interval from blood sampling to the time of diagnosis, of relevance mainly for non-hospitalized subjects. In those hospitalized, we computed the interval from admission to the time of sampling the plasma used for PFAS analysis.
All samples were coded, and the Personal Identification Number for each subject was separately transferred to the Danish Health Data Authority (FSEID-00005000) to allow linkage to demographic and medical information from the Danish Civil Registration System (CRS) [31], the Danish National Register of Patients (DNRP) [32], and the National Health Insurance Service Register [33]. We used the following classification of disease status: no hospital admission and completed infection within 14 days of testing positive, hospitalization with COVID-19 up to, or above, 14 days, admission to intensive care unit, or death. Presence of chronic disease was based on the following diagnoses in the register data: diabetes type I and II (ICD10 codes E10-E11), malignant cancers (C00-C99), cerebrovascular and coronary disease (I00-I99), pulmonary disease (J00-J99), and obesity (E66-E68). Renal disease (N0-N2) was treated as a separate covariate due to the possible impact of kidney function on plasma-PFAS concentrations [34]. The linked data set was analyzed via secure server without access to information on the Personal Identification Numbers of the subjects involved. For confidentiality reasons, all tabular information had to be based on at least five subjects.
The protocol was approved by the Regional Committee on Health Research Ethics (S-20200064), which also allowed the project to proceed without seeking informed consent from the subjects identified for study participation. Additional approvals were obtained from the Danish Data Protection Agency as well as institutional and regional authorities for the transfer blood samples and linkage of subject information to the PFAS analyses, while protecting confidentiality.
Chemical analysis
The plasma samples were analyzed in successive series for PFAS concentrations, including PFBA, perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexane sulfonate (PFHxS), and perfluoronanoate (PFNA), which are known from previous studies to be associated with immunotoxicity in humans [8, 35, 36]. We also determined plasma concentrations of PFASs so far not linked to immunotoxicity, i.e., short-chain perfluorobutanesulfonate (PFBS), perfluoroheptanesulfonate (PFHpS), perfluorodecanoate (PFDA), and perfluoroundecanoate (PFUdA) (results shown in the Supporting information). We used online solid-phase extraction followed by liquid chromatography and triple quadropole mass spectrometry (LC–MS/MS) at the University of Southern Denmark [37]. Accuracy of the analysis was ensured by inclusion of quality control (QC) samples comprising proficiency test specimens from the HBM4EU program organized by Interlaboratory Comparison Investigations (ICI) and External Quality Assurance Schemes (EQUAS). All results of the QC samples were within the acceptance range. The between-batch CVs for the actual series ranged between 3% and 14% for all compounds. Both PFOS and PFOA were quantified in all blood samples, and all PFASs were detectable in at least 30% of the samples. Results below the limit of detection (LOD, 0.03 ng/ml) were replaced by LOD/2 before uploading to the secure server at the Danish Health Data Authority, where linkage to other information took place.
Statistical analysis
Correlations between PFASs were examined using Spearman’s correlation coefficient. The PFAS concentrations were compared between demographic groups (age in years, sex, national origin, place of inclusion), presence of comorbidities, and number of days between blood sampling and diagnosis, and differences were tested using Kruskal-Wallis and Wilcoxon rank-sum test. Furthermore, associations of COVID-19 severity with age were tested using Kruskal-Wallis test, and relations with each of the variables sex, national origin, presence of comorbidities, and number of days between blood sampling and diagnosis were tested using χ2 test. Associations between place of inclusion and COVID-19 severity could not be displayed and tested, as some cells contained less than five individuals.
Because COVID-19 severity was categorized, the association between the continuous plasma-PFAS concentrations and COVID-19 severity was tested in ordered logistic regression models. More than half the short-chain PFAS concentrations were below the LOD, and they were therefore treated as binary variables (below/above LOD). Potential confounding variables were identified based on a priori knowledge as summarized above and included age (continuous, years) sex, and national origin (Western European yes/no). Among those of Western European national origin, 94% were Danish, while most of the participants of non-Western European national origin were born in or of parents from Somalia (20% of the sample), Pakistan (13%), Iraq (12%), Morocco (11%), Eastern Europe (9%), and Turkey (9%). Kidney disease may affect PFAS elimination, and PFAS exposure could potentially increase the risk of certain other chronic diseases that may affect COVID-19 severity [8]. Kidney disease (yes/no) and other chronic disease (yes/no) were thus considered potential confounders to allow estimation of the direct, rather than the total effect of plasma-PFAS concentrations. Due to changes in PFAS exposures over time, the timing of blood sampling was included as covariate. Further, due to the short elimination half-life for short-chain PFASs [8], we carried out sensitivity analyses excluding plasma samples obtained more than one week before or after diagnosis. We also adjusted for the place of inclusion (OUH/SSI) but, under the circumstances of this study, detailed data on socioeconomic status (e.g., income, education or labor market affiliation) were unavailable for this study. Dichotomous analyses comparing severities of the disease were performed in logistic regression models.
The default assumption of dose-response linearity was tested by including PFAS squared along with PFAS in the regression models. No significant (p<0.05) deviation from linearity was found. The proportional odds assumption in the ordered logistic regression was tested by a likelihood-ratio test using the Stata omodel package. In a model adjusting for age, place of inclusion, and timing of blood sampling, the hypothesis of proportional odds was accepted (p>0.05) in all analyses. Odds ratios (ORs) between groups of COVID-19 severity were therefore calculated using logistic regression models.
Results
The predominant PFAS in plasma was PFOS, with an average concentration of 6.1 ng/mL (median, 4.7 ng/L), approximately equally distributed between the normal and branched isomers. Other PFASs quantified showed averages below 1 ng/mL. In a sensitivity analysis, one extreme PFHxS outlier at 12.9 ng/mL was omitted. The PFAS concentrations correlated well, with Spearman correlation coefficients generally above 0.5 (Table 1 and S1 Table), except for short-chained PFAS. PFOS on average contributed 69% of the total PFAS concentrations by weight and correlated particularly well with most other PFASs quantified.
Table 1. Spearman’s correlation coefficients for pairwise comparisons of detectable PFASs in plasma from 323 subjects included in the study.
| PFBA | PFHxS | PFOA | PFOS | |
| PFHxS | 0.0520 | |||
| PFOA | 0.0617 | 0.7072 | ||
| PFOS | 0.0591 | 0.8406 | 0.7248 | |
| PFNA | 0.0127 | 0.7133 | 0.7759 | 0.8406 |
In general, serum-PFAS concentrations were higher at older ages, in men, and among those of Western European origin. Although the presence of chronic disease did not seem to be associated with PFAS, the plasma concentrations appeared to be higher in the presence of kidney disease (Table 2 and S2 Table).
Table 2. Median plasma-PFAS concentrations (25th, 75th percentiles) in ng/mL by population characteristics.
| PFAS (ng/mL) median (25th,75th percentile) | ||||||
|---|---|---|---|---|---|---|
| Population characteristics | n (%) | PFBA | PFHXS | PFOA | PFOS | PFNA |
| Total | 323 (100) | <LOD (<LOD, 0.04) | 0.48 (0.28, 0.71) | 0.77 (0.43, 1.18) | 4.86 (2.85, 8.29) | 0.38 (0.23, 0.59) |
| Age (years) | ||||||
| 30–39 | 37 (11) | <LOD (<LOD, 0.03) | 0.32 (0.19, 0.46) | 0.59 (0.43, 0.86) | 3.30 (1.89, 5.27) | 0.29 (0.21, 0.43) |
| 40–49 | 64 (20) | <LOD (<LOD, 0.03) | 0.35 (0.15, 0.57) | 0.58 (0.35, 0.89) | 3.11 (2.24, 5.06) | 0.27 (0.19, 0.39) |
| 50–59 | 106 (33) | <LOD (<LOD, <LOD) | 0.50 (0.31, 0.75) | 0.83 (0.43, 1.18) | 5.41 (2.79, 8.84) | 0.40 (0.24, 0.61) |
| 60–70 | 116 (36) | <LOD (<LOD, 0.05) | 0.56 (0.39, 0.89) | 0.97 (0.56, 1.51) | 6.11 (3.83, 9.60) | 0.48 (0.30, 0.70) |
| p-value a | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Sex | ||||||
| Male | 174 (54) | <LOD (<LOD, 0.04) | 0.59 (0.40, 0.87) | 0.81 (0.51, 1.26) | 5.96 (3.65, 10.17) | 0.40 (0.25, 0.61) |
| Female | 149 (46) | <LOD (<LOD, 0.04) | 0.35 (0.17, 0.52) | 0.70 (0.40, 1.04) | 3.43 (2.06, 5.66) | 0.36 (0.22, 0.56) |
| p-value b | 0.713 | <0.001 | 0.011 | <0.001 | 0.131 | |
| Kidney disease | ||||||
| yes | 34 (11) | <LOD (<LOD, 0.06) | 0.55 (0.34, 0.77) | 0.91 (0.54, 1.46) | 5.60 (3.08, 8.38) | 0.50 (0.24, 0.67) |
| no | 289 (89) | <LOD (<LOD, 0.03) | 0.47 (0.28, 0.71) | 0.76 (0.43, 1.15) | 4.76 (2.82, 8.10) | 0.36 (0.23, 0.57) |
| p-value b | 0.040 | 0.466 | 0.065 | 0.489 | 0.141 | |
| Other chronic disease | ||||||
| Yes | 220(68) | <LOD (<LOD, 0.04) | 0.47 (0.28, 0.68) | 0.71 (0.42, 1.15) | 4.70 (2.87, 7.99) | 0.38 (0.23, 0.57) |
| No | 103 (32) | <LOD (<LOD, 0.03) | 0.51 (0.28, 0.76) | 0.87 (0.47, 1.23) | 5.35 (2.72, 8.41) | 0.41 (0.23, 0.65) |
| p-value b | 0.075 | 0.314 | 0.124 | 0.850 | 0.407 | |
| National origin | ||||||
| Western Europe | 224 (69) | <LOD (<LOD, 0.04) | 0.52 (0.35, 0.76) | 0.91 (0.60, 1.29) | 5.61 (3.40, 9.18) | 0.43 (0.29, 0.64) |
| Other | 99 (31) | <LOD (<LOD, 0.04) | 0.34 (0.16, 0.57) | 0.44 (0.31, 0.80) | 2.86 (1.61, 5.13) | 0.23 (0.16, 0.36) |
| p-value b | 0.552 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Place of inclusion | ||||||
| Odense | 48 (15) | <LOD (<LOD, 0.06) | 0.45 (0.32, 0.69) | 0.67 (0.42, 0.95) | 4.67 (3.29, 8.09) | 0.36 (0.24, 0.45) |
| Copenhagen | 275 (85) | <LOD (<LOD, 0.03) | 0.48 (0.28, 0.72) | 0.79 (0.44, 1.20) | 4.89 (2.72, 8.31) | 0.39 (0.23, 0.62) |
| p-value b | 0.003 | 0.967 | 0.203 | 0.697 | 0.299 | |
| Timing of blood sampling | ||||||
| After diagnosis—1 week before | 193 (60) | <LOD (<LOD, 0.04) | 0.48 (0.30, 0.71) | 0.70 (0.40, 1.11) | 4.63 (2.83, 7.65) | 0.34 (0.23, 0.56) |
| >1 week—1 year before | 46 (14) | <LOD (<LOD, 0.03) | 0.45 (0.21, 0.66) | 0.82 (0.38, 1.35) | 4.81 (2.36, 8.62) | 0.38 (0.20, 0.65) |
| > 1year before diagnosis | 84 (26) | <LOD (<LOD, 0.03) | 0.50 (0.30, 0.72) | 0.87 (0.57, 1.22) | 5.48 (3.10, 10.28) | 0.45 (0.28, 0.65) |
| p-value a | 0.185 | 0.756 | 0.085 | 0.209 | 0.053 | |
a Variables with more than two categories tested using Kruskal-Wallis rank test.
b Binary variables tested using Wilcoxon rank-sum test.
In the study population, males, older subjects, and those with chronic disease, were more frequently represented among subjects with severe COVID-19, while there was no difference in regard to national origin for disease severity (Table 3). The PFAS-associations with disease severity were similar in Western Europeans and subjects with other backgrounds (P > 0.2 for population differences).
Table 3. COVID-19 severity by population characteristics.
| COVID-19 severity | ||||
|---|---|---|---|---|
| Population characteristics | No. of subjects | No hospitalization | Hospitalization | Intensive care unit and/or deceased |
| Total No. of subjects (%) | 323 (100) | 108 (33) | 162 (50) | 53 (16) |
| Age (years) median (25th,75th percentile) | 55 (46, 62) | 49 (41, 57) | 57 (51, 63) | 62 (53, 67) |
| P value a | <0.001 | |||
| Sex | ||||
| Male, n (%) | 174 (100) | 44 (25) | 94 (54) | 36 (21) |
| Female, n (%) | 149 (100) | 64 (43) | 68 (46) | 17 (11) |
| P value b | 0.002 | |||
| Kidney disease | ||||
| Yes, n (%) | 34 (11) | 7 (21) | 13 (38) | 14 (41) |
| No, n (%) | 289 (89) | 101 (35) | 149 (52) | 39 (13) |
| P value b | <0.001 | |||
| Other chronic disease | ||||
| Yes, n (%) | 220 (100) | 54 (25) | 119 (54) | 47 (21) |
| No, n (%) | 103 (100) | 54 (52) | 43 (42) | 6 (6) |
| P value b | <0.001 | |||
| National origin | ||||
| Western Europe, n (%) | 224 (100) | 76 (34) | 113 (50) | 35 (16) |
| Other, n (%) | 99 (100) | 32 (32) | 49 (49) | 18 (18) |
| P value b | 0.844 | |||
| Days between blood sampling and diagnosis | ||||
| median (25th,75th percentile) | 0 (-1, 393) | 335 (22.5, 639.5) | 0 (-1, 0) | 0 (-2, 1) |
| P value a | <0.001 | |||
a Associations tested using Kruskal-Wallis rank test.
b Associations tested using Pearson’s chi-squared test.
A more severe disease outcome was associated with higher plasma-PFBA concentrations, also after adjustment for all covariates (Table 4 and S4 Table). None of the other PFASs showed a similar tendency. If leaving out presence of chronic disease as a non-significant predictor, the adjusted OR for PFBA was 1.77 (95% CI, 1.09, 2.87). More importantly, when excluding samples collected earlier than one week before the time of diagnosis (148 samples), or more than one week later (5 samples), stronger ORs emerged for PFBA (Table 4). Counter to the a priori hypothesis, some PFASs, including PFHxS, seemed associated with a lower risk, but this tendency was weakened when relying on plasma samples collected in close connection to the diagnosis of corona infection (Table 4 and S3 Table).
Table 4. Ordered logistic regression OR of increased Covid-19 severity for an increase by 1 ng/mL in plasma-PFAS concentrations.
| PFAS | No. of subjects | OR (95% CI) | No. of subjects | OR (95% CI) | |
|---|---|---|---|---|---|
| Crude | Adjusted for main covariatesa | Exposure at time of diagnosisa,b | |||
| PFBA (>LOD/<LOD) | 104/219 | 2.19 (1.39, 3.46) | 1.57 (0.96, 2.58) | 61/109 | 2.10 (1.02, 4.33) |
| PFHxS (ng/mL) | 323 | 0.85 (0.63, 1.15) | 0.52 (0.29, 0.91) | 170 | 0.52 (0.24, 1.14) |
| PFHxS c (ng/mL) | 322 | 1.00 (0.62, 1.61) | 0.52 (0.29, 0.93) | 169 | 0.53 (0.22, 1.27) |
| PFOA (ng/mL) | 323 | 0.99 (0.72, 1.36) | 0.83 (0.57, 1.20) | 170 | 0.62 (0.36, 1.08) |
| PFOS (ng/mL) | 323 | 1.00 (0.96, 1.04) | 0.97 (0.92, 1.02) | 170 | 0.98 (0.89, 1.07) |
| PFNA (ng/mL) | 323 | 1.18 (0.67, 2.09) | 1.04 (0.54, 2.02) | 170 | 0.73 (0.25, 2.11) |
a Adjusted for age, sex, kidney disease, other chronic disease, national origin, place of testing, and days between blood sampling and diagnosis.
b Excluding individuals who had blood sampled more than one week before or after diagnosis.
c PFHxS >10 ng/mL excluded.
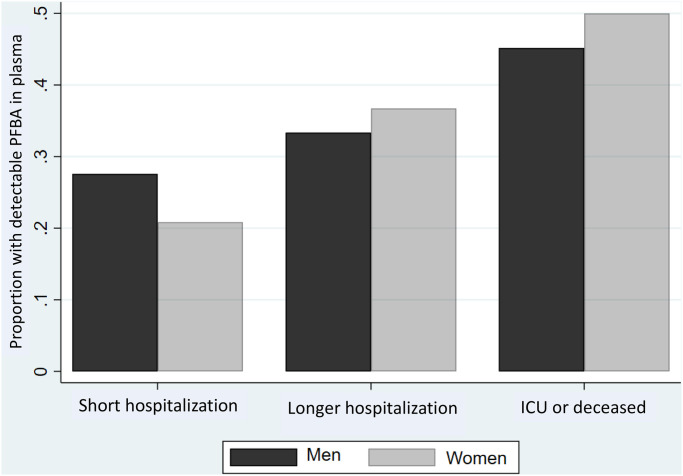
In dichotomous analyses comparing severities of the disease (S4 Table), detectable PFBA in plasma also showed a clear association with a more severe clinical course of the disease, most pronounced for odds between hospitalization and admission to intensive care unit/death, especially when based on plasma samples obtained at the time of diagnosis or up to one week before where the adjusted OR was 5.18 (1.29, 20.72). No such tendency was seen for the other PFASs detected (S4 Table). The association between PFBA and disease severity was similar for men and women (Fig 1).
Fig 1. Proportion of plasma samples with detectable PFBA concentrations at different disease severities.
Results are shown for 44 men and 64 women with up to two weeks of hospitalization, 94 men and 68 women with longer hospitalization, and 36 men and 17 women admitted to the intensive care unit (ICU) or deceased (P = 0.003).
Discussion
The present study aimed at determining the potential aggravation of COVID-19 associated with elevated exposures to PFASs. Several of these substances are known immunotoxicants in laboratory animals [35] and in humans [8, 9]. In addition to immunotoxicity, major PFASs can potentially interfere with major pathways that are predictive of a serious clinical outcome of the infection [14]. An association of PFAS exposure with disease severity therefore appears biologically plausible.
Among the PFASs, presence of detectable PFBA in plasma showed the strongest positive association with the severity of the disease. This finding may at first seem surprising, as this PFAS has a short elimination half-life in the blood and is often considered of less importance to health [27]. However, in tissue samples from autopsies, PFBA is the only PFAS that is substantially accumulated in the lungs [28]. Given the persistence of the PFASs in general, the unique retention of PFBA in lung tissue may offer a clue to interpreting the findings in this study.
Some odds ratios for PFBA were weakened after adjustment for covariates. However, adjustment for all covariates may result in over-adjustment bias. Thus, older age and male sex are known to be strong predictors of higher blood-PFAS concentrations, and simple adjustment for these factors could potentially result in a bias toward the null. As PFAS exposure has been linked to important comorbidities, such as diabetes and obesity [25, 26], both of which may exacerbate the virus infection, adjustment for chronic disease may also not be justified. Leaving it out slightly strengthened the PFBA association with the disease severity. The strongest associations for PFBA, but not for other PFASs, appeared when focusing on the most representative blood samples obtained close to the time of diagnosis.
An additional consideration is that the present study relates to low background exposure levels, in comparison with PFAS concentrations to findings in, e.g., U.S. adults [38]. Given the wide occurrence of highly contaminated drinking water in other countries [39], the present study results should not be interpreted as evidence that most PFASs do not contribute to a worsened clinical course of COVID-19.
The results for PFBA in this study appear to parallel the findings in regard to other environmental toxicants, viz., air pollutants [1–4] and suggest a need to ascertain the impact of relevant occupational or environmental exposures on COVID-19 severity. Of note, the evidence on air pollution relies solely on ecological study designs without measures of individual levels of exposure, while the present study benefitted from measurements of plasma-PFAS concentrations of all study subjects.
In regard to limitations, the study population may not be representative of corona-positive subjects, as inclusion in the study depended solely on the existence of plasma from diagnostic blood samples at the participating hospitals. Thus, subjects with chronic disease or more severe COVID-19 likely had more frequent hospital visits or longer admissions and thereby a greater chance of having plasma available for inclusion in this study. With a corona-related fatality rate of Danish blood donors below 70 years of age at 89 per 100,000 infections [40], the presence of 17 deaths in the present material (i.e., against 0.3 deaths expected) confirms that the blood samples represent a highly selected population. Still, a total of 108 subjects were known to have been infected, though not hospitalized. In many cases, their plasma had been stored on previous occasions, and the PFAS concentrations may reflect slightly higher exposures in the recent past [8], which could possibly explain the apparent protective effects of some PFASs, although adjustment for the time interval since sample collection was included in the analyses. However, the strongest associations for PFBA, but not for other PFASs, were seen when excluding samples not obtained in close temporal connection with the infection.
The study population included mostly older subjects who were more frequently male, and a large proportion of foreign-born subjects and second-generation immigrants (Table 3), thereby possibly deviating from the background population of corona-infected patients in Denmark. Still, the results do not suggest major biases affecting PFAS exposure and its association with COVID-19 outcomes.
Among immigrants, adverse associations appeared slightly stronger, also after adjustments, in accordance with national origin, perhaps as related to demographic or social factors, resulting in a greater likelihood also to PFAS-associated aggravation of the infection. Difference in age, sex, or comorbidities did not explain this tendency, but is in agreement with previous findings of ethnic differences in vulnerability [41]. However, national origin may be a surrogate marker for other factors, such as exposure at work or exposure within crowded households, as immigrant origin tends to be associated with certain occupations including front-line workers and living in areas with higher population density [42]. Still, in agreement with higher PFAS exposure being associated with higher socioeconomic position [43], we found that the association between PFBA exposure and disease severity was independent of national origin.
Conclusions
Increased plasma-PFBA concentrations were associated with a greater severity of COVID-19 prognosis, and this tendency remained after adjustment for sex, age, comorbidities, national origin, sampling location and time. Although occurring in fairly low concentrations in plasma, PFBA is known to accumulate in the lungs. Thus, as immunotoxic substances, the PFASs may well contribute to the severity of COVID-19. The present findings on a short-chain PFAS at background exposures suggest a need to ascertain if elevated exposures to environmental immunotoxicants may worsen the outcome of the SARS-CoV-2 infection.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The Danish Departments of Clinical Microbiology, the Danish Microbiology Database and the section for Data Integration and Analysis at Statens Serum Institut collated the national COVID-19 data. Plasma samples were identified and provided by Statens Serum Institut and the Department of Clinical Biochemistry and Pharmacology at Odense University Hospital.
Data Availability
Data cannot be shared publicly because of the need to protect personal data. Anonymous data are available from the secure server at the Danish Health Data Authority, pending necessary approvals from the Authority (instructions at www.sundhedsdata.dk) and the Regional Committee on Health Research Ethics for researchers who meet the criteria for access to confidential data. Aggregated data underlying the results presented in the study are available from the corresponding author, provided that no data are based on less than 5 individuals.
Funding Statement
Novo Nordisk Foundation (NNF20SA0062871) (LB, KM). National Institute for Environmental Health Sciences (ES027706) (PG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut 2020;264:114732 10.1016/j.envpol.2020.114732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ 2020;727:138704 10.1016/j.scitotenv.2020.138704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir MF, Ma BJ, Bilal, et al. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Californian context. Environ Res 2020;187:109652 10.1016/j.envres.2020.109652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci Adv 2020;6 10.1126/sciadv.abd4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rooney AA, Luebke RW, Selgrade MK, Germolec DR. Immunotoxicology and its application in risk assessment. Exp Suppl 2012;101:251–87. 10.1007/978-3-7643-8340-4_9 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization W. Guidance for Immunotoxicity Risk Assessment for Chemicals: IPCS Harmonization Project. Geneva, Switzerland: 2012. [Google Scholar]
- 7.DeWitt JC, Germolec DR, Luebke RW, Johnson VJ. Associating Changes in the Immune System with Clinical Diseases for Interpretation in Risk Assessment. Curr Protoc Toxicol 2016;67:18.1–1-.1.22. 10.1002/0471140856.tx1801s67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Food Safety Authority. Risk to human health related to the presence of perfluoroalkyl substances in food Panel on Contaminants in the Food Chain. Parma: EFSA: 2020:e06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandjean P, Andersen EW, Budtz-Jorgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 2012;307:391–7. 10.1001/jama.2011.2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kielsen K, Shamim Z, Ryder LP, et al. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol 2016;13:270–3. 10.3109/1547691X.2015.1067259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalsager L, Christensen N, Husby S, et al. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1–4 years among 359 children in the Odense Child Cohort. Environ Int 2016;96:58–64. 10.1016/j.envint.2016.08.026 [DOI] [PubMed] [Google Scholar]
- 12.Granum B, Haug LS, Namork E, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 2013;10:373–9. 10.3109/1547691X.2012.755580 [DOI] [PubMed] [Google Scholar]
- 13.Goudarzi H, Miyashita C, Okada E, et al. Prenatal exposure to perfluoroalkyl acids and prevalence of infectious diseases up to 4years of age. Environ Int 2017;104:132–8. 10.1016/j.envint.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Coumoul X, Grandjean P, Barouki R, Audouze K. Endocrine disrupting chemicals and COVID-19 relationships: a computational systems biology approach. Environ Int 2020;(in press):106232 10.1016/j.envint.2020.106232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W, Liang H, Ou L, et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med 2020;180:1081–9. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atyeo C, Fischinger S, Zohar T, et al. Distinct Early Serological Signatures Track with SARS-CoV-2 Survival. Immunity 2020;53:524–32 e4. 10.1016/j.immuni.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncalves Mendes Neto A, Lo KB, Wattoo A, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol 2020;(in press). 10.1002/jmv.26441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, He Y, Yang H, et al. Development and validation a nomogram for predicting the risk of severe COVID-19: A multi-center study in Sichuan, China. PLoS One 2020;15:e0233328 10.1371/journal.pone.0233328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Zhang B, Li P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: a systematic review and meta-analysis. medRxiv 2020:2020.03.17.20037572. [Google Scholar]
- 22.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020;395:1763–70. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartof SY, Qian L, Hong V, et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann Intern Med 2020;173:773–81. 10.7326/M20-3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu CH, Riker CD, Lu SE, Fan ZT. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016–2018. Int J Hyg Environ Health 2020;223:34–44. 10.1016/j.ijheh.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U. S. Women. Environ Health Perspect 2018;126:037001 10.1289/EHP2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Dhana K, Furtado JD, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med 2018;15:e1002502 10.1371/journal.pmed.1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ATSDR. Draft toxicological profile for perfluoroalkyls. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2018. [PubMed] [Google Scholar]
- 28.Perez F, Nadal M, Navarro-Ortega A, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 2013;59:354–62. 10.1016/j.envint.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 29.Reilev M, Kristensen KB, Pottegard A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol 2020. 10.1093/ije/dyaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voldstedlund M, Haarh M, Molbak K, MiBa Board of R. The Danish Microbiology Database (MiBa) 2010 to 2013. Euro Surveill 2014;19 10.2807/1560-7917.es2014.19.1.20667 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 32.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen JS, de Fine Olivarius N, Krasnik A. The Danish National Health Service Register. Scand J Public Health 2011;39:34–7. 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 34.Jain RB, Ducatman A. Perfluoroalkyl substances follow inverted U-shaped distributions across various stages of glomerular function: Implications for future research. Environ Res 2019;169:476–82. 10.1016/j.envres.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 35.DeWitt JC, Blossom SJ, Schaider LA. Exposure to perfluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol 2019;29:148–56. 10.1038/s41370-018-0097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng XW, Li QQ, Chu C, et al. Alternatives of perfluoroalkyl acids and hepatitis B virus surface antibody in adults: Isomers of C8 Health Project in China. Environ Pollut 2020;259:113857 10.1016/j.envpol.2019.113857 [DOI] [PubMed] [Google Scholar]
- 37.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A 2009;1216:385–93. 10.1016/j.chroma.2008.10.113 [DOI] [PubMed] [Google Scholar]
- 38.CDC. National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. Atlanta, GA: Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 39.Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ Sci Technol Lett 2016;3:344–50. 10.1021/acs.estlett.6b00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erikstrup C, Hother CE, Pedersen OBV, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis 2020:in press. 10.1093/cid/ciaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lassale C, Gaye B, Hamer M, Gale CR, Batty GD. Ethnic disparities in hospitalisation for COVID-19 in England: The role of socioeconomic factors, mental health, and inflammatory and pro-inflammatory factors in a community-based cohort study. Brain Behav Immun 2020;88:44–9. 10.1016/j.bbi.2020.05.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montazeri P, Thomsen C, Casas M, et al. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health 2019;222:864–72. 10.1016/j.ijheh.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]