Abstract
Purpose.
This review provides a model for understanding polycystic ovary syndrome (PCOS) pathophysiology and updates the evidence on which it is based. Then it highlights complimentary molecular genetic and epigenetic advances in understanding PCOS etiology.
Recent findings.
Important studies into PCOS etiology built on the 2014 discovery of a novel regulatory protein variant that underlies the typical PCOS steroidogenic abnormalities: DENND1A.V2 (differentially expressed in normal and neoplastic development, isoform 1A, variant 2). Over 30 DENND1A variant genes have been found, the vast majority upstream of the coding sequence and potentially regulatory. These variants are individually uncommon but collectively plausibly cause 50% of PCOS. Anti-Müllerian hormone (AMH)/AMH receptor variants with decreased function possibly cause 6.7% of PCOS. DENNND1A was recently reported to belong to a signaling network that up-regulates luteinizing hormone (LH) receptor expression and insulin mitogenic signaling. Prenatal androgen administration has proven to be a potent epigenetic regulator that causes transgenerational epigenomic changes in a mouse PCOS model like those in human PCOS and PCOS daughters.
Summary.
In addition to finding how gene variants contribute to PCOS pathogenesis, better understanding of androgen epigenetic mechanisms of action in diverse tissues can be expected to expand our understanding of PCOS pathogenesis.
Keywords: Anti-Müllerian hormone (AMH), Differentially expressed in normal and neoplastic development (DENND), Epigenetics, Hyperandrogenism, Polycystic ovary syndrome (PCOS)
Introduction.
Polycystic ovary syndrome (PCOS) in adolescence is a heterogeneous disorder of androgen excess and anovulatory dysfunction that causes >90% of adolescent and adult hyperandrogenism (Table 1) (1–3). Hirsutism, acne, abnormal menstrual regularity, or obesity are common presenting features in the perimenarcheal stage (4, 5).
The pathogenesis of PCOS has long been controversial. However, evidence accrued over the past 30 years indicates that the immediate pathophysiologic abnormality underlying the vast majority of PCOS is functional ovarian hyperandrogenism and that the insulin-resistant hyperinsulinism found in half of PCOS aggravates it (1, 6, 7) (Figure 1). This review updates the evidence bearing on a pathophysiologic model and the molecular genetic and epigenetic advances in understanding PCOS etiology.
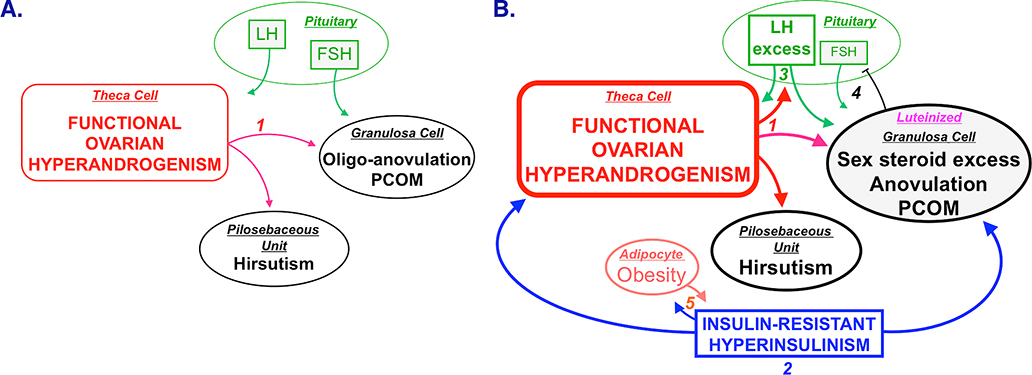
Figure 1.
Unified parsimonious model of PCOS pathophysiology. Panel A. Ovarian hyperandrogenism is demonstrable in nearly 90% of PCOS and can account for all the cardinal clinical features of the syndrome: hyperandrogenemia, oligo-anovulation, and polycystic ovaries. Mature pituitary LH secretion is necessary to sustain the ovarian androgen excess, but is not sufficient to cause it. Panel B. About half of patients with FOH have insulin-resistant hyperinsulinism (step 2). Insulin-resistant hyperinsulinism acts on theca cells to aggravate hyperandrogenism, synergizes with androgen to prematurely luteinize granulosa cells, and stimulates adipogenesis. The increased hyperandrogenemia provokes LH excess (step 3), which then acts on both theca and luteinized granulosa cells to worsen hyperandrogenism. LH also stimulates luteinized granulosa cells to secrete estradiol (step 4), which suppresses FSH secretion. These hyperinsulinism-initiated changes in granulosa cell function further hinder ovulation and exacerbate PCOM. Obesity causes insulin-resistance hyperinsulinism; the resultant hyprinsulinemia reciprocally stimulates fat formation (step 5) and further aggravates hyperandrogenism. Heaviness of lines and fonts represents severity.
Source: Modified with permission from: Rosenfield RL and Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocrinol Rev 2016; 37: 467–520. Copyright ©2016 The Endocrine Society
PCOS Pathophysiology
Functional ovarian hyperandrogenism and functional adrenal hyperandrogenism (FOH and FAH)
FOH is central to PCOS pathogenesis because it is demonstrable in the vast majority (87%) of cases by ovarian androgenic function testing (1). Two-thirds of FOH is characterized by generalized ovarian steroid hyper-responsiveness to LH, as indicated by an off-label gonadotropin releasing-hormone agonist (GnRHag) or human chorionic gonadotropin (hCG) test, that is indexed by a disproportionate 17-hydroxyprogesterone rise. This typical pattern of response indicates that steroidogenesis is abnormally regulated (dysregiulated), particularly at the level of cytochrome P450c17 (CYP17) (Figure 2). Eighty-eight percent of these “functionally typical FOH” and the remaining third of FOH have an abnormal dexamethasone androgen-suppression test (DAST), the principle of which is that serum testosterone remaining after suppression of ACTH by dexamethasone is of ovarian origin. Those FOH cases that lack the typical 17-hydroxyprogesterone abnormality have “functionally atypical FOH”, which may indicate an atypical pathogenetic mechanism.
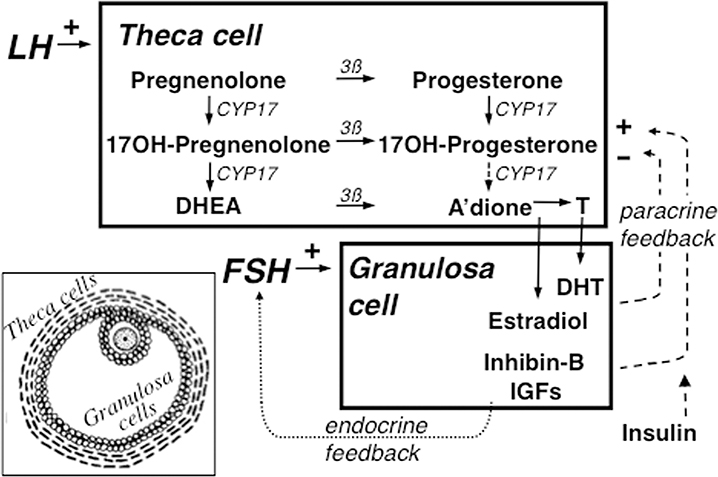
Figure 2.
Simplified version of the organization and regulation of the major steroid biosynthetic pathways in the small antral follicle of the ovary according to the 2-gonadotropin, 2-cell model of ovarian steroidogenesis. The main means of regulating theca cell androgen secretion is paracrine feedback by granulosa cell secretions on theca cell steroidogenesis to modulate LH action. This requires a fine balance of intraovarian down- and up-regulation processes in order to coordinate thecal androgen formation with granulosa cell estrogen formation. Down-regulation in part results from the paracrine inhibitory effects of granulosa cell sex steroids on CYP17. Down-regulation is counter-balanced by up-regulating paracrine factors produced by the granulosa cell, particularly inhibin-B and insulin-like growth factors (IGFs). Extra-ovarain factors like insulin and proinflammatory cytokines (not shown) also up-regulate steroidogenesis.. The two activities (17-hydroxylase and 17,20-lyase) of CYP17, the rate-limiting enzyme in androgen biosynthesis, are not appropriatedly regulated in PCOS. Inset shows diagram of small antral follicle: theca cells are stroma-like cells encircling the antral follicle and granulosa cells are epithelial-like cells lining the antrum, into which juts the ovum surrounded by the cumulus of specialized granulosa cells. Formation of androstenedione via 17-hydroxyprogesterone (dotted arrow) in the intact follicle is probably small, as is the amount of progesterone formed in granulosa cells (not shown). Stimulation indicated by + sign, inhibition by – sign. 3ß = 3ß-hydroxysteroid dehydrogenase, A’dione = androstenedione, DHEA = dehydroepiandrosterone, DHT=dihydrotestosterone, OH=hydroxy, T=testosterone.
Source: Modified with permission from: Rosenfield RL and Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocrinol Rev 2016; 37: 467–520. Copyright ©2016 The Endocrine Society.
The theca cell abnormality underlying FOH appears to be intrinsic: 1) FOH persists in response to gonadotropin stimulation after long-term suppression of endogenous gonadotropins (8), and 2) a steroidogenic defect like that detected by GnRHag/hCG testing, in which most steroidogenic enzymes are overexpressed, particularly CYP17, can be demonstrated in PCOS theca cells through multiple passages (9). 3) Recent genetic linkage studies discussed below showed that this is explicable by a variant in a previously unknown regulatory protein family, DENND (differentially expressed in normal and neoplastic development) (1, 10).
FOH appears to arise from intraovarian flaws in the down-regulation of androgen secretion in response to LH (Figure 2). Ovarian hyper-responsive to LH seems to result from subnormal homologous desensitization, which normally, when LH rises excessively a) down-regulates the expression of LH receptors, and b) down-regulates steroidogenesis, particularly at the level of CYP17.
FAH coexists with FOH in 27.5–46% of PCOS (1, 6, 11), as defined by hyper-responsiveness of 17-ketosteroids to ACTH without evidence of a steroidogenic block in cortisol secretion (Figure 3). Dehydroepiandrosterone (DHEA) is the major hyper-responsive 17-ketosteroid. Baseline serum DHEA-sulfate, an alternate marker of FAH, is elevated in slightly fewer cases and is moderately less specific for FAH, because DHEA sulfation is highly heritable. Adrenal 11-oxy-androgens may be elevated in PCOS (12–14). How these relate to the classic markers of FAH remains to be determined.
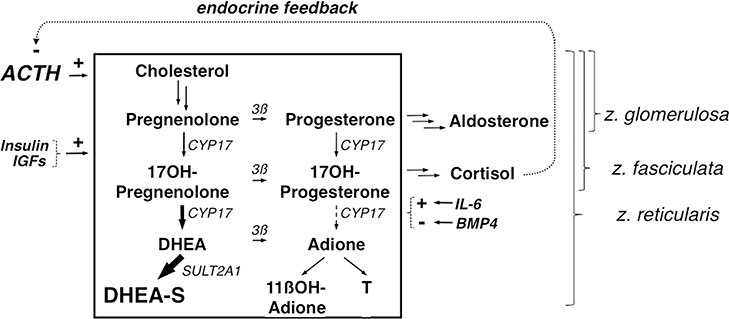
Figure 3.
Simplified outline of adrenal steroidogenesis emphasizing the zona reticularis. Adrenocortical steroidogenesis is stimulated primarily by ACTH, which is under negative feedback control by cortisol. Insulin, IGFs, interleukin-6 (IL-6) and bone morphogenetic protein type 4 (BMP4) modulate adrenal androgen secretion in response to ACTH. The unique secretory pattern of the zona reticularis, where adrenal androgens are formed, is due to uniquely low expression of 3ß-hydroxysteroid dehydrogenase (3ß), specific enhancement of the 17,20-lyase activity of CYP17, and unique expression of sulfotransferase activity (SULT2A). In FAH, adrenal androgens are formed in the zona reticularis disproportionately to cortisol formation in the zona fasciculata. The zona glomerulosa and zona fasciculata share core steroidogenic pathway steps with the zona reticularis, as indicated; individual arrows indicate steroidogenic steps in these zones.
FAH prevalence is similar in functionally typical and atypical FOH (11). Androgen hyper-responses to ACTH are accompanied by comparable androgen hyper-responsiveness to hCG (15). These findings suggest that FAH is usually a manifestation of the same disordered steroidogenesis that affects the ovary. That premature adrenarche may be the first manifestation of FAH is suggested by its 15–20% increased prevalence in PCOS and the resemblance of FAH to an exaggeration of adrenarche (1).
In the absence of intrinsic ovarian dysfunction, modest hyperandrogenemia of extra-ovarian origin (adrenal or peripheral sources) or severe insulin resistance are unusual causes of anovulation and PCOM (1, 6). However, in 5% of PCOS cases, FAH is the only detectable source of androgen excess (“functionally atypical PCOS due to FAH”). In 8% of PCOS no glandular source for the androgen excess has been detected: most such cases are obese (1). The “functionally atypical PCOS of obesity” seems to arise because excessive fat generates testosterone and obesity suppresses gonadotropin production.
Polycystic ovary morphology (PCOM) and oligo-anovulation
In PCOS an excess of small follicles forms, yet follicles prematurely luteinize, and few follicles reach the preovulatory follicle stage, accounting for oligo-anovulation and PCOM (1). Elevated serum anti-Müllerian hormone (AMH arises from the increased number of small follicles. FOH appears to be the usual cause of these effects; all are aggravated by hyperinsulinism. Progression of early follicle growth and development is normally regulated by AMH, which acts as a folliculogenesis gatekeeper (Figure 4) (1, 16). Some PCOS have been proposed to result from decreased AMH gatekeeper function (17).
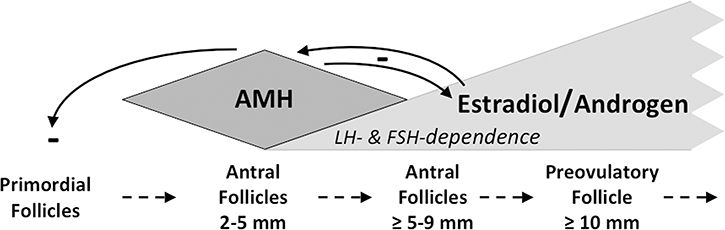
Figure 4.
Schematic depiction of AMH function as a folliculogenesis gatekeeper. AMH secreted by the granulosa cells of small growing follicles inhibits primordial follicle growth and development, which is gonadotropin-independent and in part stimulated by androgen (feedback loop not shown). AMH also inhibits CYP17, which restrains androgen and estrogen biosynthesis by larger antral follicles. As granulosa cells multiply in an increasingly gonadotropin-dependent manner and follicles grow, estradiol inhibits AMH secretion, confining it to follicles <9 mm. Increasing gonadotropin-dependence and waning AMH production by growing follicles permit emergence of the estrogen-predominant preovulatory follicle. Dashed arrows indicate key stages in follicular growth and development. Solid arrows with minus sign indicate inhibition by AMH and estradiol.
LH excess
LH elevation at baseline and in response to GnRH occurs in about half of adolescent PCOS (1). It ordinarily results from androgen-induced resistance to the negative feedback effect of estrogen-progestin (1, 18). Androgen resistance to estrogen-progestin suppression of LH was less frequently demonstrable in hyperandrogenic adolescents than in adult PCOS (19). This difference may be due in part to milder hyperandrogenemia or higher sensitivity to estrogen negative feedback of the immature pubertal neuroendocrine axis.
FOH is a gonadotropin–dependent disorder, because the expression of thecal steroidogenic enzymes is dependent on LH stimulation (6). Thus, any treatment that suppresses gonadotropin secretion suppresses ovarian androgen secretion.
Insulin-resistant hyperinsulinism
About half of PCOS women have an abnormal degree of insulin resistance for their adiposity (1). Intrinsic post-receptor defects in insulin metabolic signaling account for this (20). This insulin resistance is specific for the glucose-metabolic effect and some other effects of insulin in a tissue-specific manner. Many non-classic actions of insulin are spared because they remain sensitive to the compensatory hyperinsulinemia that maintains euglycemia. This hyperinsulinism counters normal homologous desensitization, up-regulating thecal LH receptors and CYP17 activities (Figure 1–2), aggravating FOH. Hyperinsulinism also synergizes with androgen and FSH to luteinize granulosa cells at a premature stage: this further disrupts orderly follicle maturation, aggravating the severity of anovulation and PCOM. Hyperinsulinism also stimulates adiposity, and when severe its mitogenic actions cause pseudoacromegaly. A similar paradox of hyperinsulinism with insulin resistance exists in acquired obesity (21).
Both insulin resistance and defective insulin secretion are heritable in PCOS, thereby increasing risk for type 2 diabetes mellius (22). Pro-and anti-inflammatory cytokines and lipopolysaccharide that arise in circulating adipose or intestinal monocytes also directly modulate insulin resistance and steroidogenesis (1, 23–26). Glucose or saturated fat ingestion aggravate insulin resistance and FOH by triggering increased serum levels of several of these proinflammatory factors, often moreso in PCOS than in obesity (25, 26).
Obesity
Body fat is excessive in PCOS, even in many lean cases (1). In part, this is because insulin excess stimulates adipogenesis and abdominal lipogenesis and inhibits lipolysis, leading to adipocyte hypertrophy (1, 27). Testosterone opposes insulin effects on subcutaneous fat stores (1, 27, 28), while inducing insulin resistance in these cells and suppressing levels of the insulin-sensitizing adipokine adiponectin (27). Paradoxically, however, chronic mild hyperandrogenemia induced in female primates fed a high-fat diet promotes omental adipose accumulation (29). This paradox may explain the role of androgen in determining the size of the metabolically adverse visceral fat depot of “android obesity”.
Obesity is mainly important in PCOS pathogenesis because it causes insulin resistance, which in turn aggravates FOH (1). Obesity induces insulin resistance through some of the same nutrient-mediated proinflammatory pathways as PCOS (25, 26).
Brown fat metabolic activity is reduced in PCOS (30, 31). It is inversely associated with central fat and androgen levels.
Summary: a parsimonious model of PCOS pathophysiology
The essential features of PCOS are consistent with a model in which the core pathophysiologic abnormality is FOH (Figure 1A) (1). FOH actions on granulosa cells cause oligo-anovulation and PCOM, actions on pilosebaceous units cause hirsutism or acne (Figure 1A, step 1).
Insulin-resistant hyperinsulinism occurs in about half of PCOS (Figure 1B, step 2). This aggravates FOH effects on theca and granulosa cells, increasing hyperandrogenism severity and premature luteinization of follicles. Increased hyperandrogenemia interferes with estrogen-progestin negative feedback on gonadotropes to cause LH excess (Figure 1B, step 3). LH excess further stimulates the prematurely luteinized follicles, further aggravating FOH and stimulating excess estrogen secretion. Excess estrogen secretion enhances suppression of FSH by PCOS’ inhibin excess (not shown, (8)) (Figure 1B, step 4). Obesity aggravates the insulin-resistant state, and the compensatory hyperinsulinism in turn promotes adiposity (Figure 1B, step 5)..
The figure does not depict other associated defects, such as the FAH that often accompanies FOH and the contribution of excess adiposity to peripheral androgen production and gonadotropin suppression.
This model does not exclude the possibility that the intrinsic defects that underpin the ovarian steroidogenic dysfunction of typical FOH also directly dysregulate granulosa cell folliculogenesis and adipose development as well. Atypical FOH may be particularly heterogeneous in origin, with small subsets caused, for example, by primary folliculogenesis (17) or insulin resistance abnormalities (32).
Etiology of PCOS
PCOS is a complex trait that results from the interaction of multiple heritable and environmental factors. At its simplest, it is explicable by a “two-hit” hypothesis (Figure 5), i.e., PCOS results from a congenitally programmed predisposing factor that becomes clinically manifest upon exposure to a postnatal provocative factor. The congenital factors causing abnormal theca cell androgenic function may have genetic or environmentally acquired causes. The major postnatal factor is insulin-resistant hyperinsulinism, which may also either have a congenital or acquired basis. Genetic and epigenetic studies within the last several years have led to important advances in understanding the particulars.
Figure 5.
PCOS etiology is a complex trait conceptualized as consisting of a congenital predisposing condition (“first hit”) and an activating postnatal condition (“second hit”). Examples are shown of conditions conferring PCOS risk according to clinical studies.
Heritable Factors Underlying PCOS
PCOS has long been known to have a hereditary component; family studies suggested pseudo-autosomal dominant inheritance with variable penetrance (1). Studies in identical twin sisters indicate PCOS heritability to be over 70%. Approximately half of PCOS sisters are hyperandrogenic or have PCOM, and half of these are also amenorrheic and thus have PCOS. Roughly one-quarter of PCOS mothers have PCOS. Type 2 diabetes mellitus and insulin resistance prevalence are increased in fathers and other primary relatives (22, 33). The longest prospective study of PCOS daughters found significantly increasing PCOS manifestations, central obesity, and blood pressure during the post-menarcheal years: by 18–20 years of age (n=21), 15 met adult hyperandrogenic PCOS criteria, 11 having hyperandrogenic anovulation (34, 35).
The search for the underlying genetic defects by genome wide association screening (GWAS) led to the 2014 discovery of a novel regulatory protein variant that seemingly explains the typical PCOS secretory abnormalities: DENND1A.V2 (DENND isoform 1A, variant 2) (1, 10). V2 is an extra-potent splice variant of DENND1A, a guanine nucleotide exchange factor that is localized to cytoplasmic pits adjacent to the cell membrane. DENND1A.V2 is up-regulated in PCOS theca and zona reticularis cells and mediates the PCOS steroidogenic abnormality in culture.
GWAS recently showed that half of PCOS families had ≥1 of 32 rare DENND1A variants (36). Thirty were noncoding. Most of these were predicted to alter RNA-binding protein binding motifs likely to alter V2 expression (e.g., alternate splicing), others to alter transcription factor binding. The data are consistent with the concept that causal DENND1A variants are individually uncommon, but collectively occur in key genes regulating core pathophysiologic pathways.
Recently DENND1A.V2 expression was found to be inversely correlated with that of a small noncoding mRNA (miRNA) miR-130b-3p, which is involved in post-translational regulation of gene expression (37). This miRNA also interacts with 5 of the 22 other gene candidates identified as associated with PCOS by GWAS (38): among these are the LH receptor and RAB5B (RAS-related protein 5B), a GTPase involved in vesicular trafficking. Pathway and network analyses indicate that miR-130b-3p inhibits DENND1A and RAB5B up-regulation of theca cell LH receptor expression at the cell surface; miR-130b-3p also interacts with insulin signaling through the MAP kinase pathway. Forskolin stimulation caused DENND1A.V2 and RAB5B translocation to the nucleus, suggesting they also directly stimulate steroidogenesis (39).
Genes identified to date explain less than 10% of heritability (40, 41). Several of the other 20-or-so loci/genes identified to date by GWAS and meta-analysis (38), several candidates are not clearly mechanistically related to the essential reproductive abnormality (37). Some may link to metabolic risk, while others, like the LH/CG or FSH receptor linkages, may link to epiphenomena (40).
Anti-Müllerian hormone (AMH) and AMH receptor (AMHR) coding variants with decreased signaling have been identified by next-generation sequencing in 6.7% PCOS patients, who have elevated serum AMH (17). Poor restraint by variant AMH of its gatekeeper functions of inhibiting foliculogenesis and steroidogenesis (Figure 4) possibly accounts for this PCOS. This study suggests that some PCOS cases may be caused by a primary folliculogenesis defect rather than a primary theca cell defect.
Intrauterine Environmental Factors Underlying PCOSs
Congenital virilization is a common cause of secondary PCOS (1, 42) and the most common method of producing experimental models of PCOS. In rhesus monkeys, prenatal testosterone administration reproduces the entire reproductive and metabolic PCOS spectrum, including obesity, insulin resistance, defective insulin secretion, and diabetes mellitus (1, 41, 43). Several methods, from administering dihydrotestosterone to pharmacologic dosing with AMH to inhibit placental aromatase, have been used to congenitally virilize a variety of species. However, congenital virilization is unlikely to account for ordinary PCOS because maternal-fetal testosterone passage is hindered by high placental aromatase activity and fetal ovarian follicle development does not begin until mid-gestation (1).
Disturbed fetal nutrition, particularly fetal undernutrition, predisposes to metabolic syndrome and related cardiovascular disease in adulthood (“developmental metabolic programming”). The proposal that low birth weight is likewise a risk factor for PCOS has been supported in some populations, not in others (1). In some studies, high birth weight has been associated with PCOS. However, most PCOS cases in most populations occur in individuals with normal birth weight (1, 44).
Such intrauterine perturbations cause adult disease via epigenetic programming (43, 45, 46). Epigenomic alterations in PCOS are indicated in PCOS granulosa cells by >100 differentially methylated sites affecting a wide variety of functions (47) and androgen receptor splice variants (48, 49); abnormal methylation of ovarian aromatase, AMH/AMHR, and genes involved in insulin/IGF signaling (50); and miRNA expression abnormalities in PCOS theca cells (37) and adipose tissue (1). Furthermore, DNA is differentially methylated in PCOS and PCOS daughters versus controls (43).
Testosterone is a potent epigenetic programmer (35, 45, 51, 52). Prenatal androgen exposure, but not obesity, was found to cause differential, transgenerational expression of several genes in the oocytes of mice and also in PCOS (35). One was up-regulated in mice, PCOS adipose, and PCOS daughter serum: Tia1 cytotoxic granule-associated RNA binding protein-like 1 (Tial1). This encodes a member of a family of RNA-binding proteins that regulate RNA stability, localization and post-transcriptional regulation, suggesting a potential role in androgen excess similar to that of miRNA.
Postnatal Heritable and Environmental Factors Activating PCOS
Ordinary obesity appears to be the most common precipitant of PCOS symptoms during adolescence (1). Heritable factors underlying insulin resistance and type 2 diabetes mellitus (22) seem to predispose to the development of childhood obesity and PCOS.
Moderately severe insulin-resistant hyperinsulinism underlies two unusual syndromes of intractable obesity in mid-childhood that herald PCOS in adolescence: pseudo-Cushing syndrome and pseudoacromegaly (53). The cause of these is unknown, but probably is inborn (53). Rare genetic disorders of extreme insulin resistance (eg, insulin receptor mutations and generalized lipodystrophy) are all associated with PCOS.
Virilization of sexually mature women, whether arising from treatment of female-to-male transsexuals or undertreated virilizing congenital adrenal hyperplasia, causes reversible FOH and PCOM (42). This can lead to a confusing clinical picture in girls with FOH secondary to virilizing congenital hyperplasia.
Research Horizons
Two of the fundamental pathophysiological mysteries about PCOS are what the common denominator may be that links hyperandrogenism to the common but inconstant features of insulin resistance and obesity.
A direct effect of hyperandrogenism on insulin resistance in adult humans is unlikely: reducing PCOS androgen action or levels by anti-androgen or gonadotropin-suppression treatment has improved insulin sensitivity in only a minority of studies (20, 54). In monkeys testosterone programing of PCOS likely has an epigenetic basis. Although fetal testosterone excess itself is unlikely to program naturally-occurring human PCOS (1), other molecules can potentially mimic/mediate testosterone’s epigenetic actions. For example, testosterone administration to the perinatal rodent reduces activity of sexually dimorphic brain methyltransferases, thereby releasing masculinizing genes from epigenetic repression (52, 55). This (52) and other (56) androgen effects are mediated by post-receptor signaling through prostaglandins.
Regarding hyperandrogenism and obesity, steroidogenic and adipose cells share a common transcription factor, Kruppel-like factor 15, that both increases testosterone biosynthesis and stimulates adipogenesis in response to insulin, while promoting hepatic steatosis and muscle mass (57, 58). This suggests that many of the diverse organ system abnormalities of PCOS may be linked to dysregulation of shared transcription factors. An alternative hypothesis is that a PCOS subset is caused by insulin resistance such as that resulting from fundamentally excessive fat storage (32). Mechanistic understanding is meager about androgens’ epigenetic role in obesity (41).
Conclusion
In addition to finding how gene variants contribute to PCOS pathogenesis, better understanding of androgen epigenetic mechanisms of action in diverse tissues can be expected to expand our understanding of PCOS pathogenesis.
Key Points:
The vast majority of PCOS is explicable by functional ovarian hyperandrogenism (FOH). It is aggravated by insulin-resistant hyperinsulinism in about half of cases.
FOH is usually characterized by a typical pattern of steroid hyper-responsiveness to luteinizing hormone in which there is disproportionate 17-hydroxyprogesterone response.
This steroidogenic pattern is characteristic of cultured PCOS theca cells, and seems explicable by overexpression of a DENND (differentially expressed in normal and neoplastic development) variant protein that modeling suggests to up-regulate LH receptors. Variants in this gene are found in half of PCOS; their causal role remains to be proven by gene function studies.
Functionally atypical FOH, detectable by dexamethasone androgen-suppression testing, but lacking 17-hydroxyprogesterone hyper-responsiveness to LH, may have other causes, such as the defective anti-Müllerian hormone (AMH) or AMH receptor (AMHR) variants recently found in 6.7% of PCOS.
Epigenetic programming seems to be an important contributor to PCOS etiology. Prenatal androgen administration is a potent inducer of epigenetic changes that induces models of PCOS in several species. It was recently shown to cause transgenerational epigenomic changes in a mouse PCOS model that are like those in human PCOS and PCOS daughters.
Acknowledgments
Financial support: The author’s research was supported in part by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement [U54–041859] as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and RR-00055 and UL1RR024999 from the National Center For Research Resources.
Footnotes
Conflicts of interest: none.
References
- 1.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine reviews. 2016;37(5):467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pena AS, Witchel SF, Hoeger KM, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol 2020; S1083–3188(20)30254–0. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfield RL, Ehrmann DA, Littlejohn E. Adolescent polycystic ovary syndrome due to functional ovarian hyperandrogenism persists into adulthood. The Journal of clinical endocrinology and metabolism. 2015;100(4):1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfield RL. The diagnosis of polycystic ovary syndrome in adolescents. Pediatrics. 2015;136(6):1154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocrine reviews. 1995;16(3):322–53. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfield RL. Polycystic ovary syndrome in adolescents. In: Rose BD, editor. UpToDate. Waltham, MA: 2018. p. http://www.uptodate.com/index. [Google Scholar]
- 8.Hirshfeld-Cytron J, Barnes RB, Ehrmann DA, Caruso A, Mortensen MM, Rosenfield RL. Characterization of functionally typical and atypical types of polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2009;94(5):1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson VL, Legro RS, Strauss JF 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Molecular endocrinology. 1999;13(6):946–57. [DOI] [PubMed] [Google Scholar]
- 10.McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(15):E1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Human reproduction. 2011;26(11):3138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2017;102(3):840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida T, Matsuzaki T, Miyado M, Saito K, Iwasa T, Matsubara Y, et al. 11-oxygenated C19 steroids as circulating androgens in women with polycystic ovary syndrome. Endocrine journal. 2018;65(10):979–90. [DOI] [PubMed] [Google Scholar]
- 14.Mody A, Lodish MB, Auchus RJ, Huddleston H. 11-Oxygenated C19 steroids in polycystic ovarian syndrome. Proc Annual Meeting of The Endocrine Society, March 20, 2020. 2020;P 77:Abstract 5119. [Google Scholar]
- 15.Maas KH, Chuan S, Harrison E, Cook-Andersen H, Duleba AJ, Chang RJ. Androgen responses to adrenocorticotropic hormone infusion among individual women with polycystic ovary syndrome. Fertility and sterility. 2016;106(5):1252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Human reproduction update. 2014;20(3):370–85 (Erratum in: Hum Reprod Update. 2014 Sep-Oct;20(5):804). [DOI] [PubMed] [Google Scholar]
- 17.Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional genetic variation in the anti-Mullerian hormone pathway in women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2019;104(7):2855–74.•• Functional mutations in the AMH signaling pathway were demonstrated: some disrupt the coding region of the AMH gene, others disrupt noncoding regions that reduce AMH or AMHR expression; it remains to be determined whether these actions are dissociated from the ipremature ovarian failure characteristic of the AMH-null state.
- 18.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. The Journal of clinical endocrinology and metabolism. 2000;85(11):4047–52. [DOI] [PubMed] [Google Scholar]
- 19.Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, et al. Modulation of GnRH pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls - Implications for regulation of pubertal maturation. The Journal of clinical endocrinology and metabolism. 2009;94(7):2360–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbould A Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24(7):520–32. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, et al. Obesity induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63(4):1270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yilmaz B, Vellanki P, Ata B, Yildiz BO. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertility and sterility. 2018;110(3):523–33 e14. [DOI] [PubMed] [Google Scholar]
- 23.Fox CW, Zhang L, Sohni A, Doblado M, Wilkinson MF, Chang RJ, et al. Inflammatory Stimuli Trigger Increased Androgen Production and Shifts in Gene Expression in Theca-Interstitial Cells. Endocrinology. 2019;160(12):2946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–33.•• This paper reports evidence that the microbiome plays a role in PCOS pathogenesis: transplantation of fecal microbiota from women with PCOS, in whom Bacteroides vulgaris is elevated, or B. vulgatus-colonized mice into recipient mice, resulted in a PCOS-like state with, altered bile acid metabolism, and reduced secretion of the anti-inflammatory cytokine interleukin-22.
- 25.Gonzalez F, Considine RV, Abdelhadi OA, Acton AJ. Saturated fat ingestion promotes lipopolysaccharide-mediated inflammation and insulin resistance in polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2019;104(3):934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez F, Considine RV, Abdelhadi OA, Acton AJ. Inlammation triggered by saturated fat ingestion is linked to insulin resistance and hyperandrogenism in PCOS. The Journal of clinical endocrinology and metabolism. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiffer L, Kempegowda P, Arlt W, O’Reilly MW. The sexually dimorphic role of androgens in human metabolic disease. European journal of endocrinology / European Federation of Endocrine Societies. 2017;177(3):R125–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arner P Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87(1):39–43. [DOI] [PubMed] [Google Scholar]
- 29.Varlamov O, Bishop CV, Handu M, Takahashi D, Srinivasan S, White A, et al. Combined androgen excess and Western-style diet accelerates adipose tissue dysfunction in young adult, female nonhuman primates. Human reproduction. 2017;32(9):1892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira FR, Mamede M, Bizzi MF, Ana Luiza LR, Ferreira CN, Gomes KB, et al. Brown adipose tissue activity is reduced in women with polycystic ovary syndrome. European journal of endocrinology / European Federation of Endocrine Societies. 2019;181(5):473–80. [DOI] [PubMed] [Google Scholar]
- 31.Shorakae S, Jona E, de Courten B, Lambert GW, Lambert EA, Phillips SE, et al. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clinical endocrinology. 2019;90(3):425–32. [DOI] [PubMed] [Google Scholar]
- 32.Ibanez L, Diaz M, Sebastiani G, Sanchez-Infantes D, Salvador C, Lopez-Bermejo A, et al. Treatment of androgen excess in adolescent girls: ethinylestradiol-cyproteroneacetate versus low-dose pioglitazone-flutamide-metformin. The Journal of clinical endocrinology and metabolism. 2011;96(11):3361–6. [DOI] [PubMed] [Google Scholar]
- 33.Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2006;91(4):1275–83. [DOI] [PubMed] [Google Scholar]
- 34.Crisosto N, Ladron de Guevara A, Echiburu B, Maliqueo M, Cavada G, Codner E, et al. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertility and sterility. 2019;111(2):381–8. [DOI] [PubMed] [Google Scholar]
- 35.Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–904.•• This paper is doubly important: 1) The clinical studies show that 71% of the 21 PCOS daughters in the Chilean series who were followed longitudinally into adulthood met adult criteria for PCOS, with 11/21 having hyperandrogenism and menstrual irregularity; 2) The molecular genetic studies show that prenatal androgen administration to mice leads to some of the same transmissible epigenetic changes in the mouse genome that are found found in PCOS and PCOS daughters.
- 36.Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2019.•• Half of PCOS families were found to have a variety of rare DENND1A variants: nearly 95% were non-coding variants, most of which altered the affinities for transcription factors or RNA binding proteins, thus plausibly driving DENND1A.V2 expression via post-transcriptional regulation.
- 37.McAllister JM, Han AX, Modi BP, Teves ME, Mavodza GR, Anderson ZL, et al. miRNA profiling reveals miRNA-130b-3p mediates DENND1A variant 2 expression and androgen biosynthesis. Endocrinology. 2019;160(8):1964–81.•• This report suggests that, in addition to the genetic factor in DENND1AV2 transmission, post-transcriptional regulatory mechanisms mediated by a specific microRNA underlie androgen excess in PCOS. The analyses provide further evidence that DENND1A stimulates gonadotropin signalimg by altering LH receptor expression.
- 38.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni R, Teves ME, Han AX, McAllister JM, Strauss JF, 3rd. Colocalization of polycystic ovary syndrome candidate gene products in theca cells suggests novel signaling pathways. J Endocr Soc. 2019;3(12):2204–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crespo RP, Bachega T, Mendonca BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction. 2020;159(1):R1–R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, et al. Ovarian hyperandrogenism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. The Journal of clinical endocrinology and metabolism. 1994;79(5):1328–33. [DOI] [PubMed] [Google Scholar]
- 43.Abbott DH, Rogers J, Dumesic DA, Levine JE. Naturally occurring and experimentally induced Rhesus macaque models for polycystic ovary syndrome: translational gateways to clinical application. Med Sci (Basel). 2019;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paschou SA, Ioannidis D, Vassilatou E, Mizamtsidi M, Panagou M, Lilis D, et al. Birth weight and polycystic ovary syndrome in adult life: is there a causal link? PloS one. 2015;10(3):e0122050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha N, Roy S, Huang B, Wang J, Padmanabhan V, Sen A. Developmental Programming: Prenatal Testosterone-induced Epigenetic Modulation and its Effect on Gene Expression in Sheep Ovary. Biology of reproduction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(44):17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Makrinou E, Drong AW, Christopoulos G, Lerner A, Chapa-Chorda I, Karaderi T, et al. Genome-wide methylation profiling in granulosa lutein cells of women with polycystic ovary syndrome (PCOS). Molecular and cellular endocrinology. 2020;500:110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Pan J, Liu Y, Meng Q, Lv P, Qu F, et al. Alternative splicing of the androgen receptor in polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(15):4743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walters KA, Handelsman DJ. Androgen receptor splice variants and polycystic ovary syndrome: cause or effect? Asian J Androl. 2016;18(3):442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu YY, Sun CX, Liu YK, Li Y, Wang L, Zhang W. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertility and sterility. 2015;104(1):145–53 e6. [DOI] [PubMed] [Google Scholar]
- 51.Bramble MS, Roach L, Lipson A, Vashist N, Eskin A, Ngun T, et al. Sex-specific effects of testosterone on the sexually dimorphic transcriptome and epigenome of embryonic neural stem/progenitor cells. Sci Rep. 2016;6:36916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy MM, Wright CL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry. 2017;81(5):402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Littlejohn EE, Weiss RE, Deplewski D, Edidin DV, Rosenfield RL. Intractable early childhood obesity as the initial sign of insulin resistant hyperinsulinism and precursor of polycystic ovary syndrome. Journal of pediatric endocrinology & metabolism : JPEM. 2007;20(1):41–51. [DOI] [PubMed] [Google Scholar]
- 54.Ibañez L, Potau N, Marcos MV, de Zegher F. Treatment of hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism in nonobese, adolescent girls: effect of flutamide. The Journal of clinical endocrinology and metabolism. 2000;85(9):3251–5. [DOI] [PubMed] [Google Scholar]
- 55.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18(5):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta C, Goldman A. The arachidonic acid cascade is involved in the masculinizing action of testosterone on embryonic external genitalia in mice. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du X, Rosenfield RL, Qin K. KLF15 is a transcriptional regulator of the human 17ß-hydroxysteroid dehydrogenase type 5 gene. A potential link between regulation of testosterone production and fat stores in women. The Journal of clinical endocrinology and metabolism. 2009;94(7):2594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Reilly MW, Kempegowda P, Walsh M, Taylor AE, Manolopoulos KN, Allwood JW, et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2017;102(9):3327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]