Abstract
The activation of C. elegans spermatids to crawling spermatozoa is affected by a number of genes including spe-47. Here, we investigate a paralog to spe-47: spe-50, which has a highly conserved sequence and expression, but which is not functionally redundant to spe-47. Phylogenetic analysis indicates that the duplication event that produced the paralogs occurred prior to the radiation of the Caenorhabditis species included in the analysis, allowing a long period for the paralogs to diverge in function. Furthermore, we observed that knockout mutations in both genes, either alone or together, have little effect on sperm function. However, hermaphrodites harboring both knockout mutations combined with a third mutation in the him-8 gene are nearly self-sterile due to a sperm defect, even though they have numerous apparently normal sperm within their spermathecae. We suggest that the sperm in these triple mutants are defective in fusing with oocytes, and that the effect of the him-8 mutation is unclear but likely due to its direct or indirect effect on local chromatin structure and function.
Introduction
Sperm cells generally face a brief life of intense competition to realize their goal of fertilizing an oocyte. To have success, they must execute with extreme efficiency. They must activate at precisely the right moment, locomote with haste using chemotaxis to guide them to the fertilization site, and fuse with an oocyte as quickly as possible. All this is required of a cell stripped of its ability to express its genome, in most cases surviving only on the meager stores within its tiny volume. Given the unusual nature of sperm cells, it is not surprising that well in excess of 1,000 genes are specific to, or upregulated in, sperm development [1, 2].
Our studies are concerned with the activation of sperm from the nematode C. elegans. Spherical and immotile, C. elegans spermatids are so primed to activate that they require the activity of SPE-6 to remain in the spermatid stage [3]. Once a signal is received, the spermatids undergo rapid wholesale cellular reorganization that involves an influx of cations [4], a brief elevation in pH [5], the release of intracellular Ca2+ [6–8], induction of a MAPK cascade [9], and polymerization of major sperm protein (MSP) and fusion of the membranous organelles (MOs) with the plasma membrane [7]. As a result, a pseudopod is extended and motility is achieved through MSP mediated pseudopodal treadmilling [10].
As the first step in the life of a C. elegans sperm cell, activation (spermiogenesis) may be initiated via two redundant pathways. One pathway, utilized only in males, involves the extracellular signaling serine protease TRY-5, which is secreted with the seminal fluid [11] and activates the spermatid. TRY-5 interacts with the transporter protein SNF-10 to stimulate activation [12]. The second pathway present in both males and hermaphrodites proceeds through the SPE-8 group proteins, namely, SPE-8, SPE-12, SPE-19, SPE-27, SPE-29 [reviewed in 13], and the most recent addition, SPE-43 [14]. It is thought that these proteins are anchored to the plasma membrane and transduce the activation signal inward, perhaps through the non-receptor tyrosine kinase SPE-8, which appears to move inward, away from the plasma membrane, during activation [15].
Our focus has been on discovering the identities of a collection of mutations recovered from a suppressor screen of spe-27(it132ts) [3]. Mutant spe-27 hermaphrodites are sterile because their self-sperm do not activate. The suppressor mutations restore varying degrees of fertility due to the fact that they cause sperm to activate prematurely without the need for activation signal transduction. We have identified spe-27(it132ts) suppressor mutations in spe-4(hc196) [16], spe-46(hc197) [17], and spe-47(hc198) [18]. There is a paralog to spe-47 in the C. elegans genome with the sequence identifier Y48B6A.5. Here, we report that this paralog is a new sperm gene designated spe-50, but it is not functionally redundant to spe-47. However, knockouts of the two genes have an unusual genetic interaction with him-8 when combined in a triple mutant strain.
Methods
Worm strains and handling
All C. elegans strains were maintained on Escherichia coli OP50-seeded Nematode Growth Media (NGM) agar plates [19]. The Caenorhabditis Genetic Center kindly provided the following strains: N2, BA963: spe-27(it132ts) IV, BA966: spe-27(it132ts) unc-22(e66) IV, CB1489: him-8(e1489) IV, DR466: him-5(e1490) V, BA17: fem-1(hc17ts) IV, JK654: fem-3(q23ts) IV, EG5767: qqIr7 I; oxSi78 II; unc-119(ed3) III, and SP444 unc-4(e120) spe-7(mn252)/mnC1 [dpy-10(e128) unc-52(e444)] II. Strain IE4488 harboring the ttTi4488 Mos1 transposon insertion in Y48B6A.5 was received from the NEMAGENETAG consortium [20], and the transposon insertion was homozygosed to create strain ZQ117. Steven L’Hernault kindly provided BA771 spe-18(hc133)/mnC1 [dpy-10(e128) unc-52(e444)] II. Other strains were created by combining alleles. Brood size was measured by counting the progeny laid daily by hermaphrodites isolated in 35 mm petri dishes. In some cases, the effect of mating on hermaphrodite fertility was assessed, in which case individual hermaphrodites were maintained with four males each.
RT-PCR
To perform RT-PCR, RNA was extracted from mixed-age populations of worms. Large populations of each strain were collected and rinsed 4 times with M9 buffer. After freezing at -80°C, the worms were disrupted by sonication in TRI reagent, and the RNA was extracted using the Direct-zolTM RNA purification kit following the manufacturer’s protocol for DNase I digestion (Zymo Research). cDNA was synthesized with Maxima Reverse Transcriptase (Thermo Scientific™) and oligo(dT)18 primer (#SO131 Thermo Scientific™). cDNAs were adjusted to give the same concentration across samples prior to PCR amplification. A 536 bp region of spe-50 cDNA was amplified from exons 3 and 4 with primers that flank the ttTi4488 Mos1 insertion site (Forward primer: 5’-TTGACTTCTGTGCCTCCAGC -3’; Reverse primer: 5’-GGTTCAACAGATTCTTCCTCAAGTGG-3’). To determine if gene expression was upregulated in sperm, we multiplexed spe-50 specific primers with primers that amplify an 898 bp region of the transcript of act-2, the C. elegans ortholog of β-Actin (Forward primer: 5’-GTATGGGACAGAAAGACTCG-3’; Reverse primer: 5’-ATAGATCCTCCGATCCAGAC-3’). The primers spanned intronic regions to distinguish between products from genomic DNA and cDNA. To determine if the ttTi4488 Mos1 transposon insertion disrupted spe-50 transcription, we compared RT-PCR from a population of spe-50(ttTi4488) with that from an N2 population. To determine if the spe-50 transcript is upregulated in sperm, we compared RT-PCR products from populations of fem-3(q23ts), hermaphrodites of which make only sperm, with products from fem-1(hc13ts), hermaphrodites of which make only oocytes.
CRISPR/Cas9-induced mutations
To induce specific mutations, we utilized the co-conversion strategy for CRISPR/Cas9 mediated gene edits [21]. Briefly, this strategy induces the dominant cn64 mutation in the dpy-10 gene in addition to the desired gene-specific edit. F1 worms heterozygous for cn64 roll while they crawl and are more likely to also harbor the desired edit than do non-rollers [21]. Our two specific edits were accomplished by different methods. To create a mutation that replicates the spe-47(hc198) amino acid substitution in spe-50, we utilized the expression vector pDD162, which has both a single guide RNA (sgRNA) backbone and the Cas9 gene for C. elegans expression [22] (obtained from Addgene). The spe-50 target sequence (5’-GATCTTGTTACAGTTCCAT-3’) was chosen using a CRISPR guide-finding feature in Geneious R11 (https://www.geneious.com) based upon a high predicted on-target activity [23] and a low likelihood of off-target activity. The targeting sequence was inserted into the sgRNA cassette of pDD162 using the Q5® Site-Directed Mutagenesis Kit (New England BioLabs, Inc.), resulting in identical plasmids (pTS11 and pTS12).
To make the specific spe-50 edit at the Cas9-induced double-stranded break, we designed an asymmetric ssDNA repair oligonucleotide having 34 bases upstream and 60 bases downstream of the beginning of the PAM site [24]. The oligo had a two-base pair (bp) substitution that changed the Asn at position 314 to Ile, a second silent substitution that created a TaqI restriction site, and a third silent substitution that disrupted the PAM site (Fig 1C). The dpy-10 co-conversion edit was accomplished with pDD162 derivative plasmids (pTS5 and pTS6) harboring the dpy-10 guide. The ssDNA repair oligo to induce the dpy-10(cn64) dominant mutation was as described in Arribere et al. [21]. The spe-50 and dpy-10 CRISPR/Cas9 plasmids and repair oligos were injected into the gonads of N2 hermaphrodites.
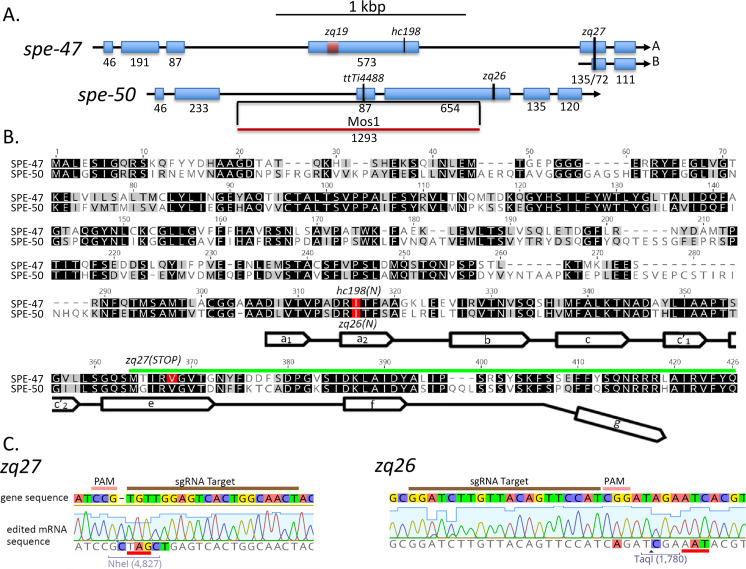
Fig 1. Comparison of spe-47 and spe-50 sequences.
(A) Exonic structure of spe-47 and its paralog spe-50. Shown are the locations of sequence variants for these genes. (B) Alignment of the SPE-47 and SPE-50 protein sequences. Darker background indicates greater similarity, and mutations are shown in red. Near the carboxy terminus a line with open arrows indicates the partial MSP domain, where the open arrows correspond to the seven β strands present in A. suum MSP-α. Note that the MSP domains are truncated at the carboxy terminus, missing the segment indicated by the downward bend in the MSP marker line. Also, the region encoded by the spe-47 isoform B is indicated by the green line. (C) Two mutations created in this study. The original gene sequence is shown above with the edited mRNA sequence below. The zq27 mutation created in spe-47 creates a stop codon (underlined in red) within an NheI restriction enzyme site for detection and a 1 bp insertion to shift the reading frame. The zq26 mutation in spe-50 induced an Asn to Ile mutation in the position corresponding the hc198 mutation in spe-47. Sequencing traces shown confirmation that the sequences were edited in the mutant strains.
A knockout mutation that affects both isoforms of spe-47 was accomplished with the Alt-R™ CRISPR/Cas9 components (Integrated DNA Technologies™) for in vitro assembled Cas9-crRNA-tracrRNA ribonucleoproteins (RNPs) following the protocol of Kohler et al. [25]. The spe-47 target sequence was chosen using the CRISPR guide-finding feature in Geneious R11 (5’-AGTTGCCAGTGACTCCAACA-3’). A specific spe-47 edit that affects both spliced isoforms was designed into an ssDNA repair oligo. The oligo consisted of 55 bp upstream and 58 bp downstream of the beginning of the PAM site. The oligo had an altered sequence that disrupted five of the six bp just upstream of the PAM site, induced an NheI site that created an in frame stop codon, and inserted one bp to shift the reading frame (Fig 1).
To induce the spe-47 knockout, we incubated equimolar solutions of our target-specific crRNAs (for both spe-47 and dpy-10) and standard tracrRNA (100 μM each) in IDT Nuclease-Free Duplex Buffer at 95°C for 5 minutes followed by 5 minutes at room temperature. The RNA duplex and Cas9-NLS were combined for a final concentration of 27 μM each and incubated at room temperature for 5 minutes to form the final ribonucleoprotein (RNP). We injected worm gonads with a mixture of 17.5 μM RNP and 6 μM ssDNA repair template for spe-47 along with 0.5 μM ssDNA repair template for dpy-10.
To recover the spe-50 edit, 38 F1 rolling cn64/+ worms were recovered and isolated. After laying eggs, the F1 worms placed in tubes; their DNA was then extracted and used as template in 5 μl PCR reactions with primers that flank the edit site (Forward primer: 5’-CATCAAGGGTGGACTTCTCG-3’; Reverse primer: 5’-AGCAGCAATGAGATGAGTGTCC-3’). To the completed PCR reactions, we added 5 μl containing restriction enzyme buffer and 5 units of TaqI. After an hour of incubation, the components were run on agarose gels to determine if the edit was induced. Of the 38 F1 rollers isolated, four appeared to have the edit via their restriction digested PCR products. Only one of them was pursued, and it contained the correct alteration of base pairs (Fig 1).
For spe-47, after injecting the constituted CRISPR/Cas9 RNPs, we found no F1 rollers. We combined three non-rolling F1s per petri dish in eight dishes and extracted their combined DNA for PCR/restriction analysis as previously described. One plate appeared to harbor a mutant. After isolating 24 offspring from this plate, we recovered a single worm that was homozygous for the edit (Fig 1). These mutations were designated spe-50(zq26) and spe-47(zq27).
Construction of a spe-50 translational reporter
We created an N-terminal mCherry translational reporter construct for spe-50 following the MosSCI technique [26, 27]. The mCherry sequence, amplified without its stop codon from plasmid pCFJ104 (Addgene), was placed directly downstream of 1,714 bp of the spe-50 promoter sequence and was followed by the spe-50 genomic sequence and 448 bp of the 3’ UTR. All worm sequences were amplified from N2 DNA, and all PCR was performed with Phusion High Fidelity DNA Polymerase (Thermo Scientific). The sequences were amplified with PCR primers engineered with regions of ~20 bp overlap, enabling us to join them together following the PCR fusion technique described by Hobert [28]. The final fusion was cloned into the multiple cloning site of the vector pCFJ352, which targets the ttTi4348 Mos1 insertion on Chromosome I for homologous recombination.
Microscopy, in vitro sperm activation, and microinjection transformation
Imaging was accomplished on a Nikon C2 confocal microscope also outfitted for Nomarski DIC and widefield epifluorescence. Widefield images were captured on a Nikon DS-Qi1 12 bit monochrome camera. Images were acquired and analyzed with Nikon NIS-Elements imaging software. All worms were dissected in SM1 buffer[29], and nuclear material was labeled with 30 ng/μl Hoechst 33342 in SM1 for live cells and with 20 ng/μl DAPI in PBS for fixed and permeabilized cells. Sperm were activated in vitro by exposure to SM1 containing 200 μg/ml Pronase. Imaging of reporter constructs was kept constant across experiments to reduce error (e.g. the laser power and gain were used for each fluorophore/fluorescent label). Compounds were microinjected of into the gonads of recipient young adult hermaphrodites using a Nikon Eclipse Ti inverted microscope outfitted for Nomarski DIC.
Phylogenetic analysis
In order to estimate the evolutionary relationships of the SPE-50 homologous proteins and detect gene duplication events, we conducted a phylogenetic analysis using 15 protein sequences from 7 species of Caenorhabditis, with the protein OVOC10046 of Onchocerca volvulus as the outgroup. The analysis was run in MrBayes 3.2.6 [30] with the GTR + I model and two runs of six chains for 10 million repetitions, with a sampling interval of 1,000 repetitions and burn-in of 25%.
Results
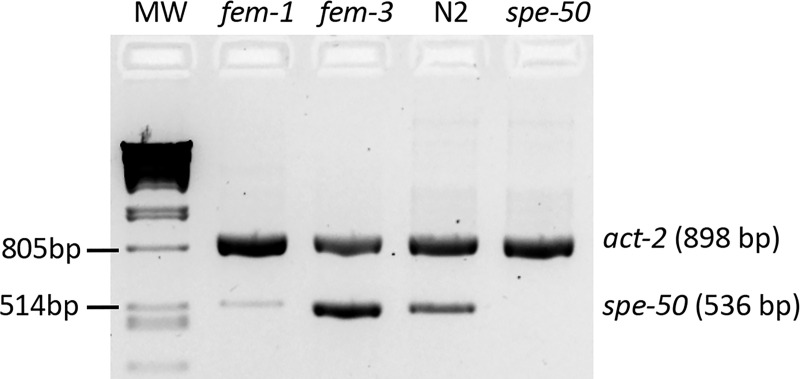
When we first discovered that spe-47 harbored the hc198 mutation that suppressed spe-27(it132ts) sterility by inducing premature spermatid activation [18], we became aware that there was a closely-related paralog present in the genome: Y48B6A.5 (Fig 1A and 1B). The SPE-47 and Y48B6A.5 proteins exhibit a high degree of sequence conservation, with both having an N-terminus of unknown function and a C-terminal MSP domain that lacks the final β strand (Fig 1B). To determine if Y48B6A.5 expression is upregulated in sperm, we performed differential RT-PCR. The Y48B6A.5 transcript is abundant in fem-3(q23) mutant hermaphrodites (Fig 2); these worms produce only spermatids but are otherwise somatically hermaphrodites. Alternatively, the transcript is nearly absent in fem-1(hc13ts) hermaphrodites that produce only oocytes. This pattern is characteristic of sperm genes.
Fig 2. RT-PCR results for the Y48B6A.5 (spe-50) transcript.
Primers specific for the Y48B6A.5 transcript amplified a robust product in fem-3(q23ts) hermaphrodites that produce only sperm, but such a product was nearly absent amplifying from fem-1(hc13ts) hermaphrodites that produce only oocytes. The Y48B6A.5 transcript was also present in the N2 strain but not in the Y48B6A.5 mutant that has the ttTi4488 Mos1 transposon insertion in Exon 3. Had the transcript with the Mos1 transposon been amplified, it would have been 1,829 bp in length, and the extension time was designed to allow a product that large to be amplified. The PCR reactions also had primers for act-2, the C. elegans β-actin gene. The act-2 product demonstrates that there was equivalent mRNA present in the samples. MW is the molecular weight marker: Phage lambda DNA digested with PstI.
There are two sperm genes mapped to the region of Y48B6A.5: spe-7 [31] and spe-18 (Steven L’Hernault, personal communication). In order to determine if Y48B6A.5 is actually one of the two nearby genes, we conducted complementation tests using the strain ZQ117 with the ttTi4488 Mos1 transposon insertion in Y48B6A.5. This insertion disrupts Y48B6A.5 and results in the absence of a transcript (Fig 2). Hermaphrodites homozygous for mutations in spe-7(mn252) and spe-18(hc133) are sterile due to primary spermatocytes that arrest in Meiosis I [31; Steven L'Hernault, personal communication]. It should be noted that the ttTi4488 strain has near normal fertility (see phenotypic descriptions below), so this test would only identify Y48B6A.5 as an allele of the other two genes if the mutated alleles of those genes were not strict loss of function but perhaps gain-of-function alleles. Males from the ttTi4488 bearing strain were crossed with sterile unc-4 spe-7 mutant hermaphrodites or with sterile spe-18 mutant hermaphrodites. The F1 hermaphrodites were isolated at 25°C and their progeny counted. The F1 hermaphrodites had wild-type fertility: for spe-7, F1 fertility = 198 progeny (n = 10, SEM = 14.4), and for spe-18, F1 fertility = 190 progeny (n = 12, SEM = 9.3). Thus, the ttTi4488 strain complemented both spe-7 and spe-18, because it carried wild-type alleles of both. Y48B6A.5 is a new sperm gene and was given the designation spe-50 (Steven L’Hernault, personal communication).
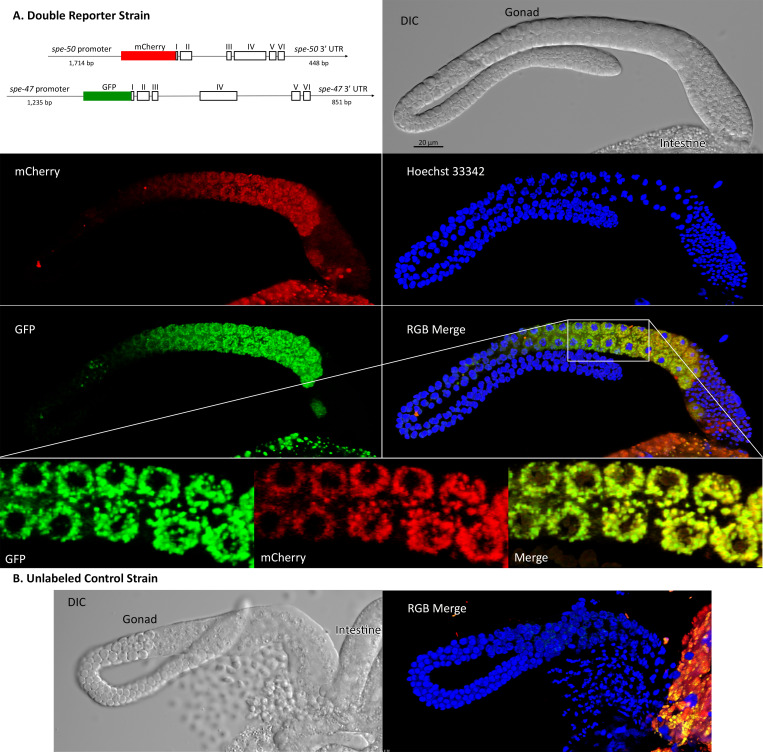
To examine SPE-50 protein localization, we created an N-terminal translational reporter with mCherry via the mosSCI protocol [26, 27]. The mosSCI process inserts the reporter into specific chromosomal locations, allowing us to combine the spe-50::mCherry reporter with a spe-47::GFP reporter we created earlier [18] in a double reporter strain. Imaging of male gonads showed that SPE-50::mCherry colocalizes almost completely with SPE-47::GFP (Fig 3A). Both appear as small puncta surrounding nuclei that are entering the pachytene stage. The puncta enlarge and expand to fill the cells as they mature into primary spermatocytes. Both also then disappear as the secondary spermatocytes form with the spermatids being completely devoid of the reporters. No such fluorescence was found in males lacking the reporters (Fig 3B).
Fig 3. Localization of SPE-50::mCherry and SPE-47::GFP translational reporters in male gonads.
The fluorescent images are 3D reconstructions of a stack of images. (A) The double reporter strain constructs and imaging in blue (nuclei), green (spe-47::GFP), and red (spe-50::mCherry). A region of the gonad in the merge image shown by the box is enlarged to give better detail of the localization. In this enlargement, only the middle of the 3D reconstruction is shown to give better understanding of colocalization. (B) Imaging from the unlabeled wild-type strain for comparison. In both the reporter images and the wild-type control, there are remnants of the intestine present. The intestine is highly autofluorescent in green and red.
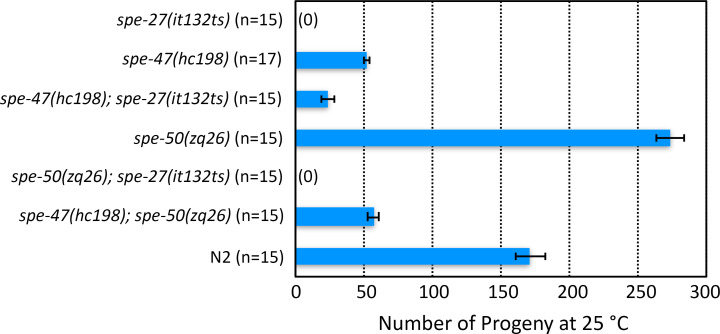
The colocalization of the two reporters in space and time suggested that these proteins are involved in the same cellular processes. We tested this hypothesis by examining mutations in both genes. The spe-47 gene was discovered in a suppressor screen of spe-27(it132ts). The spe-27 mutation causes hermaphrodite sterility because the self-spermatids are unresponsive to the signal to activate [32]. The spe-47(hc198) mutation recovered from the screen causes some spermatids to activate precociously without the need for an activation signal, overcoming the sterility of spe-27(it132ts) at its restrictive temperature of 25°C (Fig 4) [18]. If SPE-50 is functionally redundant to SPE-47, then a mutation similar to hc198 in spe-50 should also result in precocious spermatid activation and suppression of spe-27(it132ts) at 25°C. Fig 1B shows the location of the amino acid changed by spe-47(hc198): an isoleucine to asparagine substitution in the a2 ß-strand of the MSP domain. Using CRISPER/Cas9, we induced the same amino acid change in spe-50 by creating the zq26 mutation. In addition to altering the two base pairs that cause the amino acid change, we made two other silent substitutions: one that created a TaqI restriction site to allow detection of the alteration, and the other that altered the PAM site to eliminate further Cas9 activity (Fig 1C). Interestingly, the spe-50(zq26) mutation did not suppress spe-27(it132ts) sterility (Fig 4). In fact, there was no fertility deficit associated with the spe-50(zq26) mutation, while spe-47(hc198) causes a significant reduction in fertility due to problems with sperm function (Fig 4) [18]. When both spe-47(hc198) and spe-50(zq26) were combined in the same strain, the fertility was nearly identical to that of spe-47(hc198) alone (Fig 4). Thus, in terms of function, the two genes are not identical.
Fig 4. Suppression of spe-27(it132ts) sterility at 25°C.
spe-27(it132ts) mutants are sterile at 25°C, but they regain some fertility if they are also homozygous for the spe-47(hc198) mutation. On its own, the hc198 mutation results in a loss of fertility compared to wild type (N2). Conversely, the spe-50(zq26) mutation, which encodes the equivalent amino acid change as hc198, is entirely fertile on its own and does not suppress spe-27(it132ts) sterility. A strain with both hc198 and zq26 has approximately the same fertility as hc198 on its own. Thus, the spe-50(zq26) mutation has no apparent effect on fertility. Error bars represent one Standard Error of the Mean (SEM).
We also examined knockout alleles of the two genes (Fig 1). Even though the spe-50(ttTi4488) transposon insertion disrupts spe-50, it had essentially no effect on fertility at 25°C (Fig 5). In our previous study of spe-47, we created a knockout allele, spe-47(zq19) (Fig 1A), which caused only a slight reduction in fertility [18]. In the interim, a second isoform of the spe-47 transcript, which is unaffected by the spe-47(zq19) mutation, was identified. To ensure that we disabled both isoforms, we inserted a stop codon and frame-shift in the fifth exon common to both isoforms to create a new knockout allele: spe-47(zq27) (Fig 1A and 1C). This new mutation also had little effect on fertility at 25°C (Fig 5). Combining both spe-50(ttTi4488) and spe-47(zq27) mutations in the same strain of worms had only a modest effect on fertility, reducing it to just over 100 self-progeny per worm at 25°C (Fig 5). Thus, these genes are not essential to spermatogenesis, but they do seem to have an interaction that reduces fertility when both gene products are absent, suggesting that the two genes may have some redundancy.
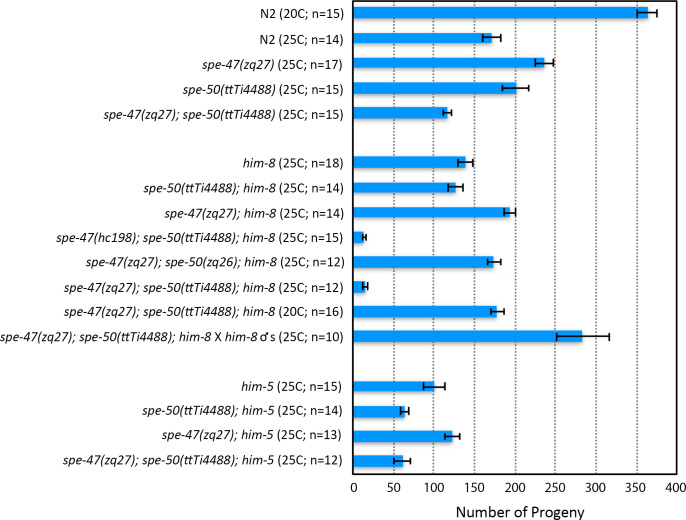
Fig 5. Fertility associated with spe-47 and spe-50 knockout mutations and him mutant backgrounds.
In the top set of bars, the two knockout mutations are compared to N2 both alone and combined in the same strain. Neither knockout alone caused sterility at 25°C, nor did a double knockout. In the middle set of bars, the various knockout and spe-27 suppressor mutations are combined with him-8(e1489). The spe-47(hc198) suppressor mutation in a spe-50 knockout and him-8 mutant background resulted in a drastic reduction of fertility at 25°C. This fertility reduction did not occur when the spe-50(zq26) suppressor-like mutation was combined with the spe-47(zq27) knockout in a him-8 mutant background at 25°C. The spe-47(zq27); spe-50(ttTi4488) double knockout in a him-8 mutant background again exhibited the drastic reduction of fertility at 25°C. This reduction was due to a defect in sperm, because mating the strain to him-8 males restored full fertility at 25°C. Further, the defect is temperature sensitive, as the spe-47(zq27); spe-50(ttTi4488) double knockout in a him-8 mutant background regained fertility when reared at 20°C. In the bottom set of bars, combining the knockout mutations with him-5 did not have a drastic reduction in fertility. Error bars represent one SEM.
In order to obtain male worms for easy observation of sperm, we combined our alleles with alleles of him-5 and him-8, both of which cause non-disjunction of the X Chromosome resulting in the production of male progeny from unmated hermaphrodites [33, 34]. We were surprised to find that combining the him-8(e1489) mutation with the two knockout mutations caused a substantial reduction of fertility at 25°C compared with all other allele combinations (Fig 5). Combining either knockout alone with him-8(e1489) did not reduce fertility more than what we found in the him-8(e1489) strain alone at 25°C. It was only when both knockouts were present in the him-8 background that fertility was reduced to approximately 15 progeny per hermaphrodite at 25°C (Fig 5). This fertility deficit was temperature sensitive: the double spe-47(zq27); spe-50(ttTi4488) knockout in a him-8 mutant background regained fertility when reared at 20°C. Further, the defect is due to self-sperm dysfunction, as mating these hermaphrodites to him-8(e1489) males increased their fertility greatly (Fig 5). Interestingly, combining the spe-27-suppressor mutation spe-47(hc198) with spe-50(ttTi4488) in a him-8(e1489) background lead to a similar drop in fertility at 25°C. The same was not true when we combined the spe-47(zq27) knockout mutation with the spe-27-suppressor-like mutation spe-50(zq26) in a him-8(e1489) strain: the fertility was much higher at 25°C (Fig 5). There was no similar fertility deficit associated with him-5. The triple spe-47(zq27); spe-50(ttTi4488); him-5(e1490) mutant hermaphrodites laid in excess of 50 offspring (Fig 5), indicating that the interaction with him-8 is not due just to X Chromosome non-disjunction problems common to both him-5 and him-8 mutants.
If the two knockout mutations in a him-8(e1489) background (the triple mutant) have defective sperm, then we might expect to see some defects in the sperm themselves. Of 145 sperm dissected from seven triple mutant males, all appeared as normal spermatids (Table 1). This is similar to 162 sperm we dissected from five him-8(e1489) virgin males: all were spermatids. Because him-8 has a role in X-Chromosome pairing and synapsis [34], we also looked for gross abnormalities in sperm nuclei. The vast majority of sperm from triple mutants had normal nuclei, similar to what we found for sperm from him-8(e1489) single mutants (Table 1). Alternatively, spermatids from triple mutants could have defective activation, so we exposed spermatids to the in vitro activator Pronase [5, 35]. Sperm from triple mutants kept at 25°C activated at a slightly but significantly reduced rate (88.5%) compared with sperm from him-8(e1489) mutants (96.3%) (Table 1), although this does not seem a large enough effect to explain the fertility deficit in this strain. Finally, we looked at the sperm remaining in hermaphrodites one day after being transferred as L4s from 20°C to 25°C. The triple mutants had fewer sperm remaining in each gonad arm (mean = 31.7, SEM = 5.9, n = 21 gonad arms) than did him-8 mutants (mean = 73.5, SEM = 6.9, n = 14 gonad arms), a significant difference (t = 4.88, P<0.001). Again, this does not explain the small number of fertilized eggs produced by the triple mutants, because the number of sperm cells remaining per gonad arm is greater than the number of progeny produced by the triple mutants. In most instances, the sperm in the triple mutants were in or very near the spermatheca, while in others some sperm were well away from the spermatheca, being scattered in the uterus, as if they were unable to remain localized in their target organ. Thus, overall there were more sperm remaining within triple mutants than the number of fertilized eggs they produce, suggesting that these sperm are unable to fertilize oocytes.
Table 1. Sperm activation and nuclear anatomy.
| Male dissected in | Sperm morphology | Nuclear anatomy | |||
|---|---|---|---|---|---|
| SM1 buffer | Spermatids | Spermatozoa | Normal | Tiny | Double |
| Triple mutant | 145 | 0 | 141 | 2 | 2 |
| him-8 | 162 | 0 | 159 | 1 | 2 |
| SM1 + Pronase* | |||||
| Triple mutant | 15 | 116 | |||
| him-8 | 6 | 154 | |||
Sperm phenotypes were examined in the triple mutant, spe-47(zq27); spe-50(ttTi4488); him-8(e1489) with him-8(e1489) as the control when both were reared at 25°C. In SM1 buffer alone, dissected virgin males release only inactive spermatids. In 200 μg/ml Pronase in SM1, spermatids activate to crawling spermatozoa. The gross anatomy of sperm nuclei was examined in the worms dissected in SM1, which also contained nuclear label Hoechst 33342 at 20μg/ml.
*A G-test of independence was performed on the data from Pronase activation: P = 0.011; G = 6.456.
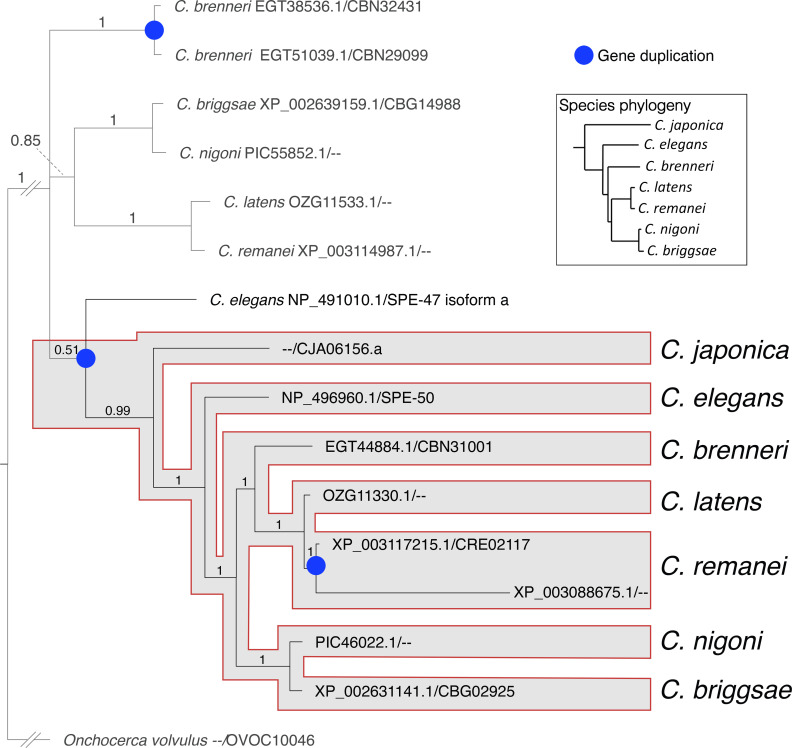
We examined the phylogeny of spe-50 and spe-47 by comparing their protein products to those from closely related species. The evolutionary analysis in Fig 6 shows that the SPE-50 protein is clearly more closely related to orthologous proteins in the genus than to its paralog-encoded SPE-47. Indeed, the hypothetical duplication of the ancestral coding sequence must have occurred prior to the radiation of the species examined. Further duplication events have created two paralogs from spe-50 in C. remanei and two paralogs from the ancestral coding sequence in C. brenneri.
Fig 6. Evolutionary relationship of the SPE-50 homologous proteins.
The proteins were identified from a BLASTP search of the NCBI non-redundant protein sequence database. For each protein, the accession number is listed before the slash, and the WormBase identifier after the slash. The duplication event that resulted in creation of the two paralogs occurred prior to the radiation of the species included in the analysis. The phylogeny of the species within Caenorhabditis follows Stevens et al. [36].
Discussion
The paralogs spe-47 and spe-50 have a high degree of protein sequence conservation, and they retain a very similar exonic structure (Fig 1). Further, the gene products are expressed in a nearly identical fashion within the spermatogenic tissue. Such similarity would suggest a similar function. While knockout mutations in either gene had no effect on fertility, a double knockout of both genes resulted in slightly reduced fertility, suggesting some common function. However, spe-50 does not share the spe-47 premature sperm activation phenotype found in spe-47(hc198) mutants, and the spe-50(zq26) mutation, which replicates the homologous amino acid substitution associated with spe-47(hc198), did not suppress spe-27(it132ts) sterility. Indeed, spe-50(zq26) had no phenotype, while spe-47(hc198) exhibits a distinct loss of fertility. How do such similar paralogs vary so greatly in function? Hypothetically, genes have multiple selection pressures acting on their function, and these pressures may act in opposition, constraining sequence evolution [37]. After a gene duplication event, the paralogs are thought to undergo functional divergence to satisfy different selective pressures with opposing effects on sequence evolution. Thus, true functionally redundant paralogs are very rare [37]. The duplication event that gave rise to spe-47 and spe-50 occurred early in the radiation of the genus (Fig 6), so the two paralogs have had ample time to evolve in response to different selection pressures.
However, the spe-47and spe-50 genes do show a phenotype when the knockout mutations are combined in a triple mutant strain with him-8(e1489). Fertility in the triple mutant strain is dramatically reduced due to a sperm defect that is most likely due to problems associated with sperm-oocyte fusion. Although we did not test an independent allele of him-8, if him-8 is indeed interacting with spe-47 and spe-50, it is unclear how this interaction arises. HIM-8 is a C2H2 zinc-finger protein that binds to the pairing center of the X-chromosome and initiates the pairing and synapsis of the X chromosome homologs [34]; him-8 mutants show high levels of X-chromosome nondisjunction leading to increased rates of male production. Because we did not find a sperm defect in triple spe-47; spe-50; him-5 knockout mutants, the him-8 triple mutant defect is not apparently associated with X chromosome non-disjunction, which is elevated in him-5(e1490) mutants [33, 38]. However, we cannot say whether or not chromosome pairing in general is involved with the him-8 triple mutant defect.
Another possible source of the defect seen in the spe-47; spe-50; him-8 triple mutants involves chromatin remodeling. In its role in X chromosome pairing, HIM-8 protein binds to specific short sequences concentrated in the chromosomal pairing centers, but these binding sequences are present at other sites on the X-chromosome and on the autosomes [39]. Further, HIM-8 protein has been shown to bind more diffusely at other sites on the X chromosome and the autosomes [40], likely as a result of the scattered binding sequences. Further, mutations in him-8 can suppress the defects associated with hypomorphic mutations in egl-13, pop-1, sptf-3, and lin-39, each encoding a transcription factor [41]. The him-8 mutations suppress only those transcription factor mutations that affect the DNA binding domains, prompting the hypothesis that HIM-8 also has a chromatin remodeling function that affects gene expression [41]. The spe-47 and spe-50 coding sequences do not have DNA binding domains, so the interaction between them and him-8 cannot be the same as what is observed for the four transcription factor encoding genes.
If the interaction of him-8 with spe-47 and spe-50 is due to the chromatin remodeling role for HIM-8 protein, then it might be that the him-8 mutation is altering the expression of other genes, one or more of which has a more direct interaction with spe-47 and spe-50. We could not identify a sperm defect for the spe-47; spe-50; him-8 triple mutants other than there were more sperm in the reproductive tract than fertilized eggs produced, suggesting a defect in fusion with the oocytes. SPE-47 localizes to the fibrous body-membranous organelle complexes [FB-MOs; 18], and by its colocalization with SPE-47, so does SPE-50. These complexes are involved in many aspects of sperm development, from acting as vehicles for MSP transport during the meiotic divisions to the remodeling of the spermatid during its transformation to an active spermatozoon. Many gene products are involved in FB-MOs: at least nine different genes have mutant phenotypes that affect FB-MO morphogenesis or function [10]. Here, our results suggest that, in combination with altered gene expression from a HIM-8 deficit, the FB-MO-associated SPE-47 and SPE-50 proteins are important to the ability of sperm to fuse with passing oocytes, even though the two proteins disappear before the spermatids form.
Supporting information
The image used in Fig 2 is on the right. The set of bands to the left had a larger volume of RT-PCR products loaded into the wells than the set of bands that were included in Fig 2.
(TIF)
Acknowledgments
We are grateful to Steven L'Hernault for suggesting the complementation tests in identifying the spe-50 gene and in providing the spe-50 designation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by U.S. National Institutes of Health award 5SC3GM087212 to CWL. Some strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
- 1.Ma X, Zhu Y, Li C, Xue P, Zhao Y, Chen S, et al. Characterisation of Caenorhabditis elegans sperm transcriptome and proteome. BMC Genomics. 2014;15:168 10.1186/1471-2164-15-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131(2):311–23. 10.1242/dev.00914 [DOI] [PubMed] [Google Scholar]
- 3.Muhlrad PJ, Ward S. Spermiogenesis initiation in Caenorhabditis elegans Involves a casein kinase 1 encoded by the spe-6 gene. Genetics. 2002;161(1):143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson GA, Ward S. Vesicle fusion, pseudopod extension and amoeboid motility are induced in nematode spermatids by the ionophore monensin. Cell. 1980;19(2):457–64. 10.1016/0092-8674(80)90520-6 [DOI] [PubMed] [Google Scholar]
- 5.Ward S, Hogan E, Nelson GA. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev Biol. 1983;98(1):70–9. 10.1016/0012-1606(83)90336-6 [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay J, Lee J, Lee J, Lee JI, Yu JR, Jee C, et al. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol Biol Cell. 2002;13(9):3281–93. 10.1091/mbc.e02-01-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.L'Hernault SW. Spermatogenesis In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Cold Spring Harbor, NY USA: Cold Spring Harbor Laboratory Press; 1997. p. 271–94. [PubMed] [Google Scholar]
- 8.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119(Pt 12):2552–62. 10.1242/jcs.02980 [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Wang B, He R, Zhao Y, Miao L. Calcium signaling and the MAPK cascade are required for sperm activation in Caenorhabditis elegans. Biochimica et biophysica acta. 2014;1843(2):299–308. 10.1016/j.bbamcr.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 10.L'Hernault SW. Spermatogenesis. WormBook. 2006:1–14. Epub 2007/12/01. 10.1895/wormbook.1.85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JR, Stanfield GM. TRY-5 is a sperm-activating protease in Caenorhabditis elegans seminal fluid. PLoS Genet. 2011;7(11):e1002375 10.1371/journal.pgen.1002375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenker KE, Hansen AA, Chong CA, Jud MC, Duffy BA, Norton JP, et al. SLC6 family transporter SNF-10 is required for protease-mediated activation of sperm motility in C. elegans. Dev Biol. 2014;393(1):171–82. 10.1016/j.ydbio.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis RE, Stanfield GM. The regulation of spermatogenesis and sperm function in nematodes. Semin Cell Dev Biol. 2014;29:17–30. Epub 2014/04/11. 10.1016/j.semcdb.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauchunas AR, Mendez E, Ni JZ, Druzhinina M, Mulia A, Parry J, et al. spe-43 is required for sperm activation in C. elegans. Dev Biol. 2018;436(2):75–83. 10.1016/j.ydbio.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhlrad PJ, Clark JN, Nasri U, Sullivan NG, LaMunyon CW. SPE-8, a protein-tyrosine kinase, localizes to the spermatid cell membrane through interaction with other members of the SPE-8 group spermatid activation signaling pathway in C. elegans. BMC genetics. 2014;15:83 10.1186/1471-2156-15-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosney R, Liau WS, LaMunyon CW. A novel function for the presenilin family member spe-4: inhibition of spermatid activation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:44 10.1186/1471-213X-8-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liau WS, Nasri U, Elmatari D, Rothman J, LaMunyon CW. Premature sperm activation and defective spermatogenesis caused by loss of spe-46 function in Caenorhabditis elegans. PloS one. 2013;8(3). 10.1371/journal.pone.0057266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMunyon CW, Nasri U, Sullivan NG, Shaw MA, Prajapati G, Christensen M, et al. A new player in the spermiogenesis pathway of Caenorhabditis elegans. Genetics. 2015;201(3):1103–16. 10.1534/genetics.115.181172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. Epub 1974/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallin E, Gallagher J, Granger L, Martin E, Belougne J, Maurizio J, et al. A genome-wide collection of Mos1 transposon insertion mutants for the C. elegans research community. PLOS ONE. 2012;7(2):e30482 10.1371/journal.pone.0030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arribere JA, Bell RT, Fu BXH, Artiles KL, Hartman PS, Fire AZ. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics. 2014;198(3):837–46. 10.1534/genetics.114.169730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickinson DJ, Ward JD, Reiner DJ, Goldstein B. Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nature Methods. 2013;10(10):1028–34. Epub 09/01. 10.1038/nmeth.2641 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology. 2013;31:827–32. 10.1038/nbt.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang X, Potter J, Kumar S, Ravinder N, Chesnut JD. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. Journal of Biotechnology. 2017;241:136–46. 10.1016/j.jbiotec.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 25.Kohler S, Wojcik M, Xu K, Dernburg AF. Superresolution microscopy reveals the three-dimensional organization of meiotic chromosome axes in intact Caenorhabditis elegans tissue. Proc Natl Acad Sci U S A. 2017;114(24):E4734–E43. 10.1073/pnas.1702312114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frokjaer-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nature Methods. 2012;9(2):117–8. 10.1038/nmeth.1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen SP, et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40(11):1375–83. 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32(4):728–30. 10.2144/02324bm01 [DOI] [PubMed] [Google Scholar]
- 29.Machaca K, DeFelice LJ, L'Hernault SW. A novel chloride channel localizes to Caenorhabditis elegans spermatids and chloride channel blockers induce spermatid differentiation. Dev Biol. 1996;176(1):1–16. 10.1006/dbio.1996.9999 [DOI] [PubMed] [Google Scholar]
- 30.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 31.Sigurdson DC, Spanier GJ, Herman RK. Caenorhabditis elegans deficiency mapping. Genetics. 1984;108(2):331–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minniti AN, Sadler C, Ward S. Genetic and molecular analysis of spe-27, a gene required for spermiogenesis in Caenorhabditis elegans hermaphrodites. Genetics. 1996;143:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstein P. The synaptonemal complexes of Caenorhabditis elegans: pachytene karyotype analysis of hermaphrodites from the recessive him-5 and him-7 mutants. J Cell Sci. 1986;82:119–27. [DOI] [PubMed] [Google Scholar]
- 34.Phillips CM, Wong C, Bhalla N, Carlton PM, Weiser P, Meneely PM, et al. HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell. 2005;123(6):1051–63. Epub 2005/12/20. 10.1016/j.cell.2005.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.L'Hernault SW, Roberts TM. Cell biology of nematode sperm In: Epstein HF, Shakes D, editors. Caenorhabditis elegans Modern Biological Analysis of an Organism. 48 San Diego: Academic Press, Inc; 1995. p. 273–99. [DOI] [PubMed] [Google Scholar]
- 36.Stevens L, Félix M-A, Beltran T, Braendle C, Caurcel C, Fausett S, et al. Comparative genomics of 10 new Caenorhabditis species. Evolution Letters. 2019;3(2):217–36. 10.1002/evl3.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soria PS, McGary KL, Rokas A. Functional divergence for every paralog. Mol Biol Evol. 2014;31(4):984–92. 10.1093/molbev/msu050 [DOI] [PubMed] [Google Scholar]
- 38.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction Mutants of the Nematode Caenorhabditis elegans. Genetics. 1979;91(1):67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips CM, Meng X, Zhang L, Chretien JH, Urnov FD, Dernburg AF. Identification of chromosome sequence motifs that mediate meiotic pairing and synapsis in C. elegans. Nat Cell Biol. 2009;11(8):934–42. 10.1038/ncb1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabeshima K, Mlynarczyk-Evans S, Villeneuve AM. Chromosome painting reveals asynaptic full alignment of homologs and HIM-8-dependent remodeling of X chromosome territories during Caenorhabditis elegans meiosis. PLoS Genet. 2011;7(8):e1002231 10.1371/journal.pgen.1002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun H, Nelms BL, Sleiman SF, Chamberlin HM, Hanna-Rose W. Modulation of Caenorhabditis elegans transcription factor activity by HIM-8 and the related Zinc-Finger ZIM proteins. Genetics. 2007;177(2):1221–6. 10.1534/genetics.107.070847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The image used in Fig 2 is on the right. The set of bands to the left had a larger volume of RT-PCR products loaded into the wells than the set of bands that were included in Fig 2.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.