Abstract
Patient: Female, 72-year-old
Final Diagnosis: Invasive aspergillosis
Symptoms: Chest pain • hemoptysis • shortness of breath
Medication: —
Clinical Procedure: Bronchoalveolar lavage
Specialty: Pulmonology
Objective:
Unusual clinical course
Background:
Invasive pulmonary aspergillosis (IPA) is a severe form of the fungal infection with relatively high mortality rates. Risk factors that lead to IPA include immunosuppression through corticosteroid use. IPA complicated by hydropneumothorax is rare and its mechanism of formation is unknown.
Case Report:
A 72-year-old woman recently diagnosed with a right frontal meningioma that was managed with dexamethasone presented with a new 3-day history of nonproductive cough, chest pain, and dyspnea and was managed for pneumonia. The patient failed to improve, prompting a follow-up computed tomography scan, which revealed a right middle lobe cavitary lesion. During the workup of this lesion, the patient’s hospital course was complicated by hemoptysis and development of a large right hydropneumothorax that was successfully managed with a chest tube. Despite initial resolution of hydropneumothorax, the patient developed a right apical pneumothorax that gradually worsened. Bronchoscopy culture revealed Aspergillus fumigatus, leading to the diagnosis of IPA, which was managed with intravenous voriconazole.
Conclusions:
Corticosteroid use with subsequent immunosuppression is a risk factor for developing IPA. Clinicians should include IPA in their differential diagnosis for respiratory infections in patients receiving corticosteroids. Although overall prognosis of IPA is poor, outcomes can be improved with early diagnosis, early empiric initiation of anti-fungals, and withdrawal of immunosuppressive therapy. IPA complicated by hydropneumothorax is a rare phenomenon with a poorly understood mechanism of formation. Based on our case, we propose a mechanism of hydropneumothorax formation from IPA.
MeSH Keywords: Glucocorticoids, Hydropneumothorax, Invasive Pulmonary Aspergillosis
Background
Aspergillus is a hyaline mold that is ubiquitous. It typically dwells in the soil and is usually found in organic debris, dust, compost, foods, and rotted plants. Although over 200 species of Aspergillus exist, only a few are known to cause pathologies in humans, with the most common being Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, and Aspergillus terreus. Exposure to Aspergillus conidia is frequent, but invasive disease is uncommon because of control by host immunity in immunocompetent hosts. Aspergillus can cause a variety of clinical syndromes in the lungs including aspergilloma in patients with preexisting lung cavities, chronic necrotizing aspergillosis in mildly immunocompromised patients or those with chronic lung disease, allergic bronchopulmonary aspergillosis (a type of hypersensitivity reaction in patients with asthma), and invasive pulmonary aspergillosis (IPA), which is typically seen in immunocompromised patients. IPA is a severe form of the fungal infection with relatively high mortality rates. The fungal spores that are easily inhaled and aerosolized can germinate into hyphae in the respiratory mucosa and may invade the mucosa, leading to IPA [1].
Because aspergillosis is not a reportable infection in the United States, its incidence is difficult to determine. The epidemiology of invasive Aspergillus infections has shifted over the years due to the increasing number of solid organ and stem cell transplant recipients and newer immunosuppressive agents. In 2014, the number of aspergillosis-associated hospitalization in the United States was nearly 15 000, after an average increase of 3% per year during 2000–2013 [2].
Immunosuppression is the most important risk factor for IPA. Prolonged neutropenia (for more than 3 weeks) or neutrophil dysfunction (chronic granulomatous disease) impart a significant risk for IPA. Other risk factors for IPA include prolonged, high-dose corticosteroid therapy; lung and bone marrow transplantation; hematologic malignancy (higher risk with leukemia); cytotoxic therapy; and AIDS [3]. Risk factors associated with poor prognosis with aspergilloma include chronic underlying diseases such as sarcoidosis and HIV infection, increasing size and number of lesions on chest radiographs, immunosuppression (including corticosteroid treatment), increasing Aspergillus-specific IgG titers, and repetitive large-volume hemoptysis.
Patients with IPA present with respiratory symptoms similar to bronchopneumonia, such as fever, cough, sputum production, and dyspnea. Other symptoms include pleuritic chest pain and mild to massive hemoptysis. IPA is the most common cause of hemoptysis in neutropenic patients. Other pulmonary symptoms that have been reported include tracheobronchitis and secondary atelectasis [3]. More rare respiratory complications such as a hydropneumothorax can occur, as demonstrated by the current case. Multiple cases of aspergillosis complicated by pneumothorax have previously been reported [4–7]. The purpose of this case report is to present an unusual case of IPA complicated with hydropneumothorax and to propose effective diagnostic methods and management of IPA.
Case Report
A 72-year-old woman with a medical history of urinary incontinence initially presented for a ground-level fall and was diagnosed with a right frontal meningioma. Neurosurgery deferred surgical intervention at that admission given that the tumor was benign and indolent but scheduled outpatient surgery for brain mass resection. The meningioma was managed with dexamethasone (4 mg orally every 6 h for 20 days) and outpatient surveillance. The patient returned to the hospital following a second ground-level fall and with a new 3-day history of dyspnea, nonproductive cough, chest pain, and generalized weakness. The initial physical examination revealed crackles in the right lung base, and a chest X-ray demonstrated consolidation in the right lung base suggestive of pneumonia. Laboratory test results displayed elevated white blood cell counts, elevated neutrophils, thrombocytopenia, and normal renal function. The patient was admitted for suspected community-acquired pneumonia and further management of meningioma. Pneumonia was managed with intravenous (IV) ceftriaxone and azithromycin. The patient was also noted to have abruptly discontinued a month-long course of 4 mg dexamethasone 3 days before admission. She was restarted on dexamethasone 4 mg IV every 6 h and tapered up to 6 mg IV every 6 h in the next 20 days for concerns about acquired adrenal insufficiency.
A week after admission, the patient reported continued chest pain and developed a productive cough with hemoptysis. Repeat chest X-ray demonstrated a possible cavitary lesion, and a subsequent computer tomography scan confirmed the presence of a cavitary mass in the right middle lobe measuring 6×3.5 cm (Figure 1). The cavitary lesion prompted collection of 2 sputum samples for acid fast bacilli (AFB) stain and culture, and the results were negative. An initial serum QuantiFERON gold test (interferon gamma release assay) was indeterminate, and a repeat test was negative. The serum Aspergillus antigen enzyme immunoassay that is used for detecting galactomannan antigen in the serum was negative. Antibiotic treatment was broadened to vancomycin and piperacillin-tazobactam.
Figure 1.
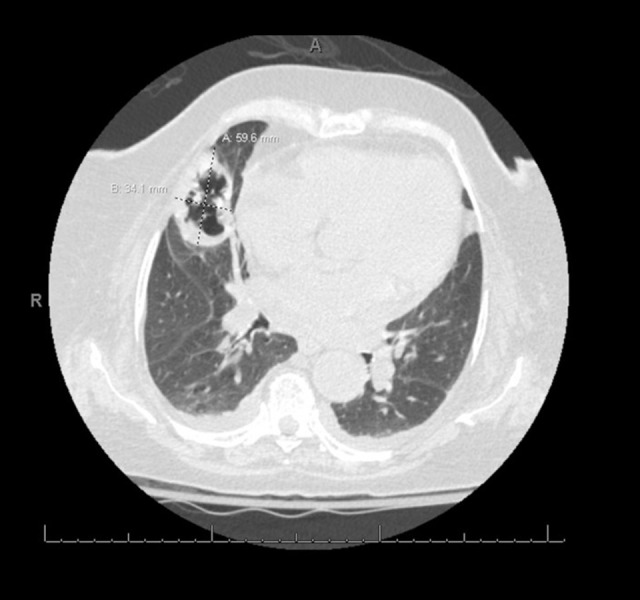
High-resolution computed tomography scan of a new cavitary mass in the right middle lobe measuring 6×3.5 cm, indicating formation of invasive pulmonary aspergillosis.
Despite the antibiotics, the patient developed a large right-sided hydropneumothorax, requiring a chest tube placement, which resolved the pneumothorax (Figure 2). Owing to the worsening of the patient’s condition, a pulmonary consult was requested for diagnostic bronchoscopy for tissue sampling. The patient underwent bronchoscopy with bronchoalveolar lavage. The AFB stain and culture were again negative, but the BAL culture grew Streptococcus viridians and A. fumigatus. Cytology revealed the presence of acute-angle hyphae septate consistent with A. fumigatus (Figure 3). Given the results indicating IPA, the patient was started on voriconazole 490 mg IV every 12 h. She was then switched to isavuconazole 372 mg IV every 24 h after developing thrombocytopenia and a rash. Despite the initial resolution of hydropneumothorax after the chest tube placement, the patient had a continued pleural leak and persistent and progressive pneumothorax.
Figure 2.
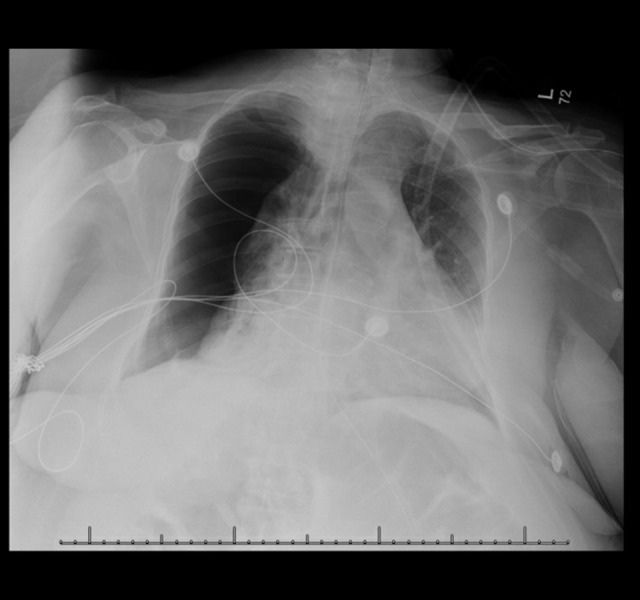
Chest X-ray with large right-sided pneumothorax with a minimal right to left mediastinal shift that initially resolved with a chest tube but then reformed.
Figure 3.
Three microphotographs of Aspergillus organisms and a background photo of heavy mixed, neutrophilic, and lymphocytic infiltrate with numerous macrophages. The 3 microphotographs show dichotomous hyphae of 2.5–4.5 μm in diameter with frequent septations and branching at 45°. Counterclockwise from the upper left corner are different histological stains with internal scales: hematoxylin eosin (HE) ×40, Grocott-Gömöri methenamine silver (GMS) ×40, periodic acid Schiff (PAS) ×40. The background photo used a Romanowsky-type stain called Diff-Quik (DQ) with an internal scale of ×10.
Her hospital course was complicated by prolonged ileus and worsening renal function. Cardiothoracic Surgery was consulted for evaluation for lobectomy, but given the patient’s poor surgical candidacy and risk of open thoracotomy, the family declined surgical intervention. While the Aspergillus cavitary lesion continued to be treated, the ongoing right pneumothorax became concerning for either a bronchopulmonary fistula or complications from the aspergilloma. Despite continuing antifungal therapy and supportive care, the patient’s condition continued to decline. She developed severe septic shock and multiorgan failure and eventually died.
Discussion
Corticosteroids are a recognized risk factor for developing IPA. Patients receiving steroids constitute a far broader population than those considered classically or most severely immunocompromised, such as HIV/AIDS patients and hematopoietic stem cell transplant patients. The current case involved a patient taking high-dose dexamethasone, with no underlying lung disease, malignancy, or other immunosuppression, who developed severe IPA with drastic complications. In patients taking high-dose steroids, IPA should be included in the differential diagnosis for pulmonary infections; failure to respond appropriately to empiric pneumonia therapy should provoke further diagnostic workup and initiation of early antifungal therapy when warranted.
The overall prognosis of patients with IPA is poor. Data on mortality are the most robust for hematopoietic stem cell transplant patients. One older retrospective case study of 89 hematopoietic stem cell transplant patients with IPA showed a 51% overall 2-year mortality attributed to IPA [8]. A recent retrospective database review of 412 patients with IPA in the Intensive Care Unit, excluding transplant, cancer, AIDS, and neutropenic patients, found an overall hospital mortality rate of 46% (although this is not IPA-attributable mortality) [9]. Out of the 412 patients that were studied, 315 (76.5%) received acute high-dose corticosteroid therapy, which included corticosteroids such as dexamethasone, methylprednisolone, prednisolone, and prednisone during their hospital stay [9].
The cornerstone of IPA management consists of early initiation of antifungals. Typically, voriconazole or isavuconazole can be used as first-line agents, requiring an extended duration (6–12 weeks) of therapy, and tapering and removal of immunosuppressive therapy to allow for immune system reconstitution [10,11]. Our patient was initially managed for community-acquired pneumonia, and was she was diagnosed with IPA and initiated on antifungals only after 24 days had passed. Her risk factor for acquiring IPA was her treatment with high-dose corticosteroids. Her symptoms of pleuritic chest pain and hemoptysis, while nonspecific, could have raised the clinical suspicion for IPA. While the prognosis for IPA remains poor, early initiation of antifungals has been shown to improve outcomes. One study indicated that a delay in antifungal treatment increased the length of stay and hospital costs; for example, a 1-day delay resulted in an extra 1.28-day stay and a 4% increase in cost [9]. Among patients that fail to respond to initial therapy, some may respond to different antifungal agents, while select cases may require surgery [10].
Unfortunately, during this patient’s diagnostic workup prior to her diagnosis of IPA, she experienced the complication of hydropneumothorax, which eventually led to respiratory failure. Hydropneumothorax is formed by free fluid and air entering the pleural space. Hydropneumothorax is commonly associated with malignancy, chest trauma (including chest tube placement or after thoracentesis), rheumatologic diseases that affect lung parenchyma, and pulmonary infections [12]. In this case, hydropneumothorax developed due to IPA, an infectious process. There is little to no literature around the mechanism of hydropneumothorax formation from IPA. However, there has been some research on hydropneumothorax formation from other infections such as Mycobacterium tuberculosis. Tuberculosis has been found to be the most common etiology of hydropneumothorax, followed by bacterial infections and pleural or pulmonary malignancies [13]. A proposed mechanism for hydropneumothorax formation from tuberculosis is through the formation of a bronchopleural fistula. The bronchopleural fistula is formed through liquefaction of subpleural caseous nodules that then undergo pleural necrosis and rupture [14]. The resulting inflammatory response initially seals off the fistula, but it also causes serosanguineous fluid to accumulate and allows intermittent release of air into the pleura [14].
In the case of IPA, the fungal hyphae can proliferate in the pulmonary parenchyma and invade the pulmonary bronchi and blood vessels, resulting in thrombosis, hemorrhagic infarction, and a necrotizing pneumonitis. However, 1 study showed that nonneutropenic patients, similar to the patient in the current case, tend to not show angioinvasion [15]. Little is known about the way in which this fungal disease influences pulmonary function and general physiology. We propose a mechanism of hydropneumothorax formation from IPA that is similar to what occurs with tuberculosis. Specifically, we suggest that the fungus breaks down the lung parenchyma, creating an inflammatory reaction that results in edema and eventual air flowing from the lungs into the pleural space. Further investigation in the development of hydropneumothorax in IPA patients is needed.
Conclusions
Development of IPA in patients on high-dose corticosteroid should be considered. If clinicians have a high suspicion of IPA, then initiation of antifungal treatment and withdrawal of immunosuppressive agents, such as corticosteroids, should occur as early as possible. Additionally, the current case had a unique presentation of IPA complicated with hydropneumothorax, and further studies are needed to understand the underlying pathophysiology.
Acknowledgments
We thank Grady Memorial Hospital and Austin Chan, MD, for their support of this case report.
Footnotes
Conflicts of interest
None.
References:
- 1.Naaraayan A, Kavian R, Lederman J, et al. Invasive pulmonary aspergillosis – case report and review of literature. J Community Hosp Intern Med Perspect. 2015;5(1):26322. doi: 10.3402/jchimp.v5.26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Aspergillosis statistics. 2019. https://www.cdc.gov/fungal/diseases/aspergillosis/statistics.html.
- 3.Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. 2002;121(6):1988–99. doi: 10.1378/chest.121.6.1988. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Hu Y, Chen L, et al. Pleural aspergillosis complicated by recurrent pneumothorax: A case report. J Med Case Rep. 2010;4:180. doi: 10.1186/1752-1947-4-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suri T, Makkar N, Ray A, Sood R. A unique case of hydropneumothorax in allergic bronchopulmonary aspergillosis. Med Mycol Case Rep. 2019;25:29–31. doi: 10.1016/j.mmcr.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricketti AJ, Greenberger PA, Glassroth J. Spontaneous pneumothorax in allergic bronchopulmonary aspergillosis. Arch Intern Med. 1984;144:151–52. [PubMed] [Google Scholar]
- 7.Kant S, Saheer S, Singh A, Hassan G. Pyopneumothorax secondary to Aspergillus infection: A case report. Oman Med J. 2012;27(6):494–96. doi: 10.5001/omj.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeghen T, Kibbler CC, Prentice HG, et al. Management of invasive pulmonary aspergillosis in hematology patients: A review of 87 consecutive cases at a single institution. Clin Infect Dis. 2000;31(4):859–68. doi: 10.1086/318133. [DOI] [PubMed] [Google Scholar]
- 9.Baddley JW, Stephens JM, Ji X, et al. Aspergillosis in Intensive Care Unit (ICU) patients: Epidemiology and economic outcomes. BMC Infect Dis. 2013;13:29. doi: 10.1186/1471-2334-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63(4):e1–60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMMERS guideline. Clin Microbiol Infect. 2018;24:e1–38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Miller JC, Boyce TG. Hydropneumothorax as a complication of necrotizing pneumonia in a young girl. Clin Case Rep. 2019;7(8):1559–61. doi: 10.1002/ccr3.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suri T, Makkar N, Ray A, Sood R. A unique case of hydropneumothorax in allergic bronchopulmonary aspergillosis. Med Mycol Case Rep. 2019;25:29–31. doi: 10.1016/j.mmcr.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kates DE, Pollack CV., Jr Hydropneumothorax due to tuberculosis. J Emerg Med. 1995;13(1):27–30. doi: 10.1016/0736-4679(94)00108-1. [DOI] [PubMed] [Google Scholar]
- 15.Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–77. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]



