Introduction:
There is no consensus definition for ventilator-associated tracheitis and limited evidence to guide diagnosis and treatment. To improve acute tracheitis evaluation and management, this quality improvement project aimed to (1) improve the appropriateness of tracheal aspirate cultures while decreasing the number of unnecessary cultures by 20% and (2) decrease antibiotic use for acute tracheitis not consistent with local guidelines by 20% over 12 months among pediatric patients requiring mechanical ventilation.
Methods:
All patients admitted to the Medical Intensive Care Unit requiring mechanical ventilation via an artificial airway were included. Tracheal aspirate sampling criteria, technique, and minimum intervals were standardized. Primary outcome measures were the number of tracheal aspirate cultures obtained per 100 ETT/tracheostomy days and ventilator-associated antibiotic days per 100 ETT/tracheostomy days. Improvement cycles included: Implementation of tracheal aspirate sampling criteria, sampling technique standardization, limiting repeat cultures to >72-hour intervals, and standardizing empiric antibiotic therapy.
Results:
Tracheal aspirate culture rate decreased from 10.70 to 7.10 cultures per 100 ETT/tracheostomy days (P < 0.001). Cultures meeting sampling criteria increased from 28% to 80%. Ventilator-associated antibiotic use decreased from 24.88 to 7.30 ventilator-associated antibiotic days per 100 ETT/tracheostomy days. There were no associated increases in ventilator-associated events or days of mechanical ventilation.
Conclusions:
Implementation of standardized criteria for tracheal aspirate sampling, improved tracheal aspirate sampling technique, limiting repeat tracheal aspirate cultures, and utilizing standardized antibiotic treatment guidelines safely decreased resource utilization and antibiotic use among critically ill children requiring mechanical ventilation.
INTRODUCTION
Healthcare-associated infections contribute to patient morbidity and mortality and increase healthcare resource utilization.1–3 Surveillance definitions for pediatric ventilator-associated events (VAE), including possible ventilator-associated pneumonia (VAP), have been established,4–7 but there is no consensus definition for ventilator-associated tracheitis.8,9 Such infections have previously been called “ventilator-associated tracheobronchitis” (VAT).10 There is limited evidence to guide tracheal aspirate sampling as well as a lack of antibiotic treatment guidelines for acute tracheitis.11–13 Diagnostic uncertainty results in frequent initiation of antibiotics for suspected tracheitis when criteria for pneumonia or lower respiratory tract infections are not met.11
Bacterial colonization may occur in an artificial airway, adding to uncertainty when interpreting tracheal aspirate cultures. Patients with an endotracheal tube (ETT) or tracheostomy tube (trach) can have bacteria mobilize into the lower respiratory tract around the tube cuff or into the lumen with suctioning.14 Also, bacterial biofilm presence may be a contributing factor in infections of both ETT and tracheostomy tubes. In many intubated children, bacterial colony counts will reach >104 cfu/ml within a few days of intubation without clinical infection.13 A Gram stain with rare or few neutrophils usually represents colonization; the presence of moderate or abundant neutrophils (PMN) is more consistent with VAT or VAP.14,15
Best practice for tracheal aspirate specimen collection has not been established.16 Techniques include protective brush tracheal sample, simple tracheal aspirate suction sampling via the artificial airway, deep suctioning, and bronchoalveolar lavage (BAL).17,18 Many studies that report the incidence of and risk factors for VAT do not report their sampling strategy.19–21 Practice variation suggests the need to standardize the collection technique to obtain high-quality, accurate, and undiluted samples.
Early and appropriate antibiotic treatment for VAT improves outcomes by decreasing ventilator days and mortality in adult patients.19,21 However, there is variability in the duration of recommended treatment for VAT. Infectious Diseases Society of America guidelines recommend not administering antibiotics for VAT, although it is a weak recommendation with low-quality evidence.22 One pediatric study concluded that prolonged antibiotics (older than 7 days) for VAT did not protect against hospital-acquired pneumonia or VAP.23 Martin-Loeches et al and Moncayo-Nieto et al found that adult patients with VAT had a resolution of symptoms at 72 hours, supporting a 3-day course of antibiotics.19,20
Preliminary evaluation of our Medical Intensive Care Unit (MICU) practice revealed variation in criteria to obtain a tracheal aspirate culture, with the most common indications being fever (55% of samples), increased ventilator settings (40%), and increased supplemental oxygen requirement (35%). However, criteria with less evidence base also drive decisions to obtain tracheal aspirate cultures, including increased secretions or suctioning needs (25%) and alteration in secretion quality (25%). Clinicians order 30% of all tracheal aspirate cultures within 72 hours of a previous specimen collection. While pediatric VAT is not widely reported, unit evaluation of respiratory cultures and clinical criteria before project initiation detected 10 of 73 patients (13.6%) met the local definition of tracheitis.
Recognizing practice variation, we sought to develop a clinically relevant, standardized approach to diagnosis and treatment of acute tracheitis in patients requiring mechanical ventilation, with the following specific aims: (1) to improve the appropriateness and quality of tracheal aspirate cultures while decreasing the number of cultures not consistent with local guidelines by 20% among patients requiring mechanical ventilation over 12 months and (2) to decrease antibiotic use for acute tracheitis not consistent with local guidelines by 20% over 12 months.
METHODS
Context
We implemented the acute tracheitis quality improvement project in a 22-bed ICU in a pediatric quaternary care hospital. The unit cares for pediatric patients requiring medical ICU care, including the need for variable forms of mechanical ventilation for acute and/or chronic respiratory failure. The MICU staff includes board-certified pediatric intensivists, critical care fellows, pediatric residents, advanced practice nurses, nurses (with a 1:1 or 1:2 nurse-to-patient ratio), and respiratory therapists. The MICU averaged 76 admissions per month in 2018. Stakeholders for this QI project included MICU physicians and nurses, Infection Prevention and Control (nurses, data analyst, and physician), Antimicrobial Stewardship Program (physician and pharmacist), Respiratory Therapy, and the Infectious Disease Diagnostic Laboratory (physician).
All patients admitted to the MICU who required mechanical ventilation via an artificial airway (tracheostomy or ETT) were included.
Intervention
This project included 4 plan-do-study-act cycles. The first 3 cycles sought to standardize tracheal aspirate sampling criteria, technique, and sampling intervals in conjunction with Infection Prevention, Antimicrobial Stewardship, and the Infectious Disease Diagnostic Laboratory. The fourth cycle sought to decrease inappropriate antibiotic use for acute tracheitis by standardizing criteria for the initiation of antibiotics and the duration of treatment. The antibiotic guidelines were developed and implemented in conjunction with the Antimicrobial Stewardship Program.
Cycle 1 intervention consisted of understanding current practice about sampling criteria for tracheal aspirate cultures. The improvement team surveyed key stakeholders and frontline staff, including nurses and respiratory therapists, revealing variable practice. Utilizing literature review and consensus opinion, standardized criteria for obtaining tracheal aspirate culture samples were created and implemented (Fig. 1) (November 2017 to December 2017).
Cycle 2 interventions focused on standardizing the tracheal aspirate sampling technique. Members of the improvement team performed observations of technique by nursing and respiratory therapists. The evaluation revealed variation in technique, including depth of catheter insertion into the airway, the instillation of sterile normal saline during sampling, and the volume of sterile saline instilled during sampling. We standardized recommendations to utilize a new sterile suction catheter, to obtain the specimen on the first pass into the artificial airway, to insert catheter just beyond the end of the artificial airway, not to instill sterile normal saline into the airway during sampling, and to minimize the addition of sterile normal saline to <0.5 ml if necessary to clear the catheter. The improvement team established a recommended minimum sample volume of 1 ml (December 2017 to January 2018).
Cycle 3 intervention included establishing a threshold not to repeat tracheal aspirate culture within the 72 hours following a previous culture. The 72-hour interval was established based upon prior studies and expert consensus opinion.19,20 The intervention included creating a closed-loop communication system between the Infectious Disease Diagnostic Laboratory and the MICU to notify staff of samples requested within the 72-hour interval, which would not be processed. Specifically, the feedback loop consisted of laboratory staff calling to notify the bedside nursing staff of duplicate specimen submission. Prescribers were instructed to call and request a deviation from the sampling recommendation if desired. As a subsequent notification, the lab staff notified the improvement team via email. There were no deviations during the improvement project (January to March 2018).
Cycle 4 interventions consisted of creating consensus criteria to initiate antibiotics with standardized recommendations for initial therapy and duration. Recommendations included the initiation of empiric therapy based upon prior culture results. If prior cultures were not available, empiric therapy included cefepime and vancomycin. A 3-day treatment course was recommended, with guidance to discontinue vancomycin at 48 hours unless there was evidence of MRSA colonization or infection (Fig. 1) (April to July 2018).
Fig. 1.
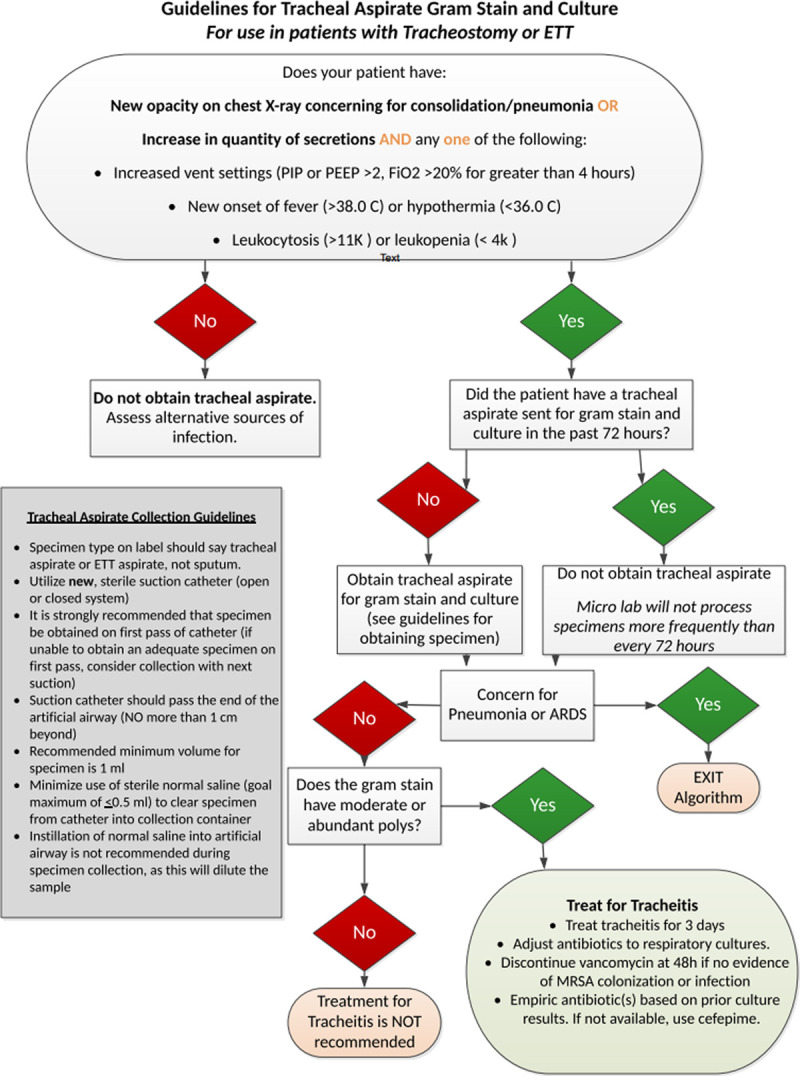
Tracheal aspirate sampling and interpretation guideline.
Study of Intervention
We tracked baseline ventilator use, tracheal aspirate sampling, and antibiotic use for all patients admitted to the MICU during the improvement project. We defined tracheal aspirate cultures as respiratory cultures obtained from an ETT or tracheostomy during the project period. Samples obtained from BAL or cystic fibrosis specific evaluation were excluded. We defined repeat tracheal aspirate cultures as a tracheal aspirate culture obtained when one already had been collected within the prior 72 hours from any patient location (emergency department, inpatient ward, ICU or operating room). Our laboratory reported Gram stain results as having none, rare, few, moderate or abundant PMN, as per standard institutional practice. We defined antibiotic use as the administration of antibiotics among patients with an artificial airway who had a respiratory culture sent. Antiviral, anti-parasitic, and antifungal agents were excluded along with ongoing prophylactic antibiotics or home antibiotics. We also excluded antibiotic days for pneumonia or another non-respiratory infection (eg, urinary tract infection or bacteremia). Antibiotics were counted starting on the day of the culture and included all consecutive antibiotic days until the completion of the treatment of that episode, up to 2 weeks.
Trained data abstractors collected data from the enterprise data warehouse for all patients with an artificial airway requiring mechanical ventilation which had a tracheal aspirate culture obtained. Data included temperature (>38.0 or <36.0 in the previous 24 hours) and a white blood cell count measured in the previous 24 hours. The abstractors then performed medical record review, evaluating for documentation of increased quantity of secretions and/or increased ventilator settings (increase in PIP or PEEP >2 cm H2O, FiO2 >20% for greater than 4 hours). We defined pneumonia through documentation of radiographic pneumonia per an attending radiologist report or documentation of treatment for pneumonia in the attending critical care physician note. All cases underwent an independent review by 2 team members, resulting in the exclusion of 4 cases from the cohort.
Measures
The pre-intervention period was from January 2016 to October 2017, and the intervention period was from November 2017 to October 2018. The tracheal aspirate process measure is the proportion of tracheal aspirate cultures obtained from pediatric patients requiring mechanical ventilation via an artificial airway in the MICU who met criteria for sampling (number of respiratory cultures that met criteria divided by the total number of respiratory cultures). The process measure for treatment compliance with the guideline is the proportion of tracheal aspirate cultures with moderate or abundant PMN for which antibiotics were administered for younger than 4 days. Outcome measures included: tracheal aspirate culture rate, repeat culture rate, and tracheal aspirate culture associated with antibiotic use. Balancing measures included mortality, ICU length of stay (LOS), VAE and ventilator days.5,6
Data Analysis
We used Shewhart control charts to evaluate process variability, specifically tracheal aspirate culture and ventilator-associated antibiotic use rates, during baseline and intervention periods. U-charts were created using SQCpack Version 7 software (PQ Systems, Dayton, OH). Centerlines were re-computed based on special cause when 7 consecutive data points were above or below the mean. The control limits varied based on sample size for each period, set at 3 SDs above and below the mean. The QI team met at 2-week intervals to review data, assess the compliance with sampling criteria and antibiotic initiation/duration through chart review, and explore special cause variation. At each meeting, the team made decisions regarding subsequent plan-do-study-act cycle interventions based on group discussion, special cause analysis, and quantitative methods to draw inferences from data. Cumulative data on tracheal aspirate culture sampling and antibiotic use were analyzed using Stata (StataCorp. 2015. Stata Statistical Software: Release 14: StataCorp LP, College Station, TX).
Ethical Considerations
This project met our institutional standards for quality improvement and therefore did not require institutional review board review. All data were de-identified and stored in a password-protected secure location behind the hospital firewall.
RESULTS
During the intervention period, 251 tracheal aspirate cultures were sent from 101 unique patients. All tracheal aspirate samples were obtained at any time point during admission to the MICU except 1 specimen collected in the emergency department before admission to the MICU. The patients’ median age was 7 years old, and 50% were male (Table 1). The mean ventilator days per month within the MICU during the intervention period was 295 days. There was no statistically significant change in the population age, sex, and mortality between the pre- and postintervention periods.
Table 1.
Population Characteristics and Balancing Measures
| Baseline (January 2016 to October 2017) (N = 301) | Intervention (November 2017 to October 2018) (N = 101) | P | |
|---|---|---|---|
| Age (y), median (IQR) | 9 (2–26) | 7 (2–16) | 0.697 |
| Male, N (%) | 160 (53) | 50 (50) | 0.525 |
| Balancing measures | |||
| Mortality events, N (%) | 16 (5.3) | 8 (7.9) | 0.339 |
| Ventilator-associated events (VAE), N | 3 | 3 | 0.157 |
| Ventilator days per month, mean (SD) | 230 (68.5) | 295 (64.6) | 0.011 |
| ICU length of stay (d), median (IQR) | 5 (2–11) | 16 (6–28) | <0.001 |
| Hospital length of stay (d), median (IQR) | 9 (3–25) | 24 (9–52) | <0.001 |
The mean tracheal aspirate culture rate decreased from 10.70 (SD = 2.27) cultures per 100 ETT/tracheostomy days during the pre-intervention period to 7.10 (SD = 1.91) over the 12-month improvement project period (t(32) = 4.76, P < 0.001) (Fig. 2). Additionally, in the last 6 months of the intervention period, 5 out of the 6 points on the U-chart were below the new mean, consistent with a trend to process improvement. The proportion of tracheal aspirate cultures meeting local guideline criteria increased from 7% to 73% during the intervention period. Implementation of standardized criteria to obtain tracheal aspirate culture samples in Cycle 1 from November to December 2017 resulted in an initial decrease in culture rates to 8.63 cultures per 100 ETT/tracheostomy days. During cycle 2, upon standardization of the tracheal aspirate sampling technique, we observed an initial increase in tracheal aspirate culture rates. In response to the increase, technique recommendations were incorporated in sampling criteria signage to reinforce compliance, which leads to a subsequent decrease in culture rates to 7.50 cultures per 100 ETT/tracheostomy days (December 2017 to January 2018). Standardization of sampling technique included a minimum specimen volume of 1 ml. Specimens that did not meet the minimum 1 ml requirement were not to be processed. The Infectious Disease Diagnostic Laboratory staff notified the clinical team of the lack of sufficient quantity with a request to re-send an appropriate sample. There were no rejected samples due to sample volume <1 ml during the improvement project. The staff did not instill additional saline to specimens exclusive of volume used to clear suction catheters.
Fig. 2.
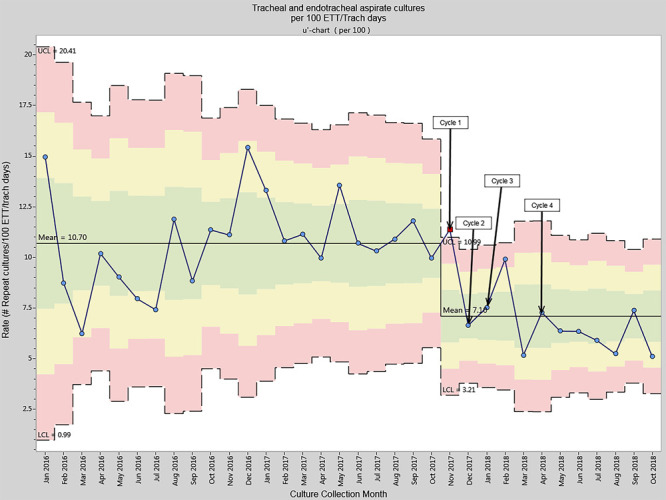
Tracheal aspirate culture rate per 100 ETT/tracheostomy days.
Cycle 3 implemented a standardized protocol with the Infectious Disease Diagnostic Laboratory not to process repeat tracheal aspirate specimens within 72 hours of prior samples, resulting in a sustained decrease in the overall culture rate to 5.20 cultures per 100 ETT/tracheostomy days by March 2018. The mean repeat culture rate within 72 hours decreased from 2.10 (SD = 1.1) to 1.19 (SD = 0.8) when comparing the pre-intervention to the intervention period (t (32) = 2.17, P = 0.038) (Fig. 3).
Fig. 3.
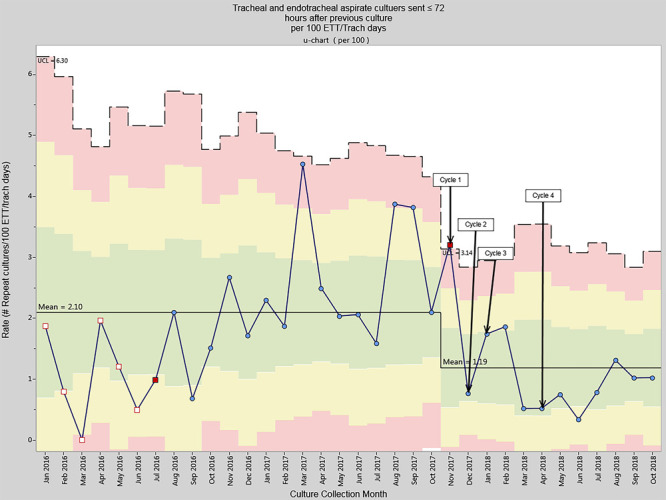
Repeat tracheal aspirate cultures rate per 100 ETT/tracheostomy days.
While there was a consistent decrease in culture rates, compliance with meeting sampling criteria remained variable over the first 2 improvement cycles. Cycles 3 and 4 incorporated feedback to nursing and physicians when samples did not meet established criteria and compliance improved from 28% to 80%.
Ventilator-associated antibiotic use decreased from 24.88 to 7.30 antibiotic days per 100 ETT/tracheostomy days (Fig. 4). Cycle 4 implemented standardized criteria for antibiotic therapy initiation with recommendations for empiric choices and a duration of 3 days. The hospital antibiogram guided empiric antibiotic choice in conjunction with the Antimicrobial Stewardship Program, including Infectious Diseases physicians, pharmacists, and Laboratory Medicine representatives. While this cycle focused specifically on antibiotic initiation and duration, the ventilator-associated antibiotic use rate had already decreased by the conclusion of cycle 3.
Fig. 4.
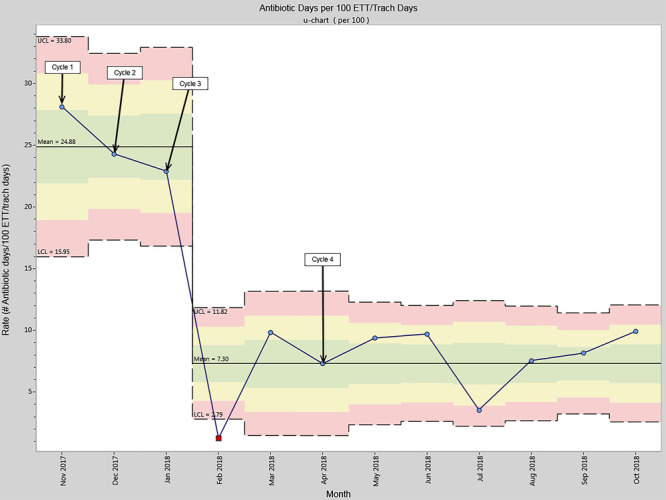
Ventilator-associated antibiotic days per 100 ETT/tracheostomy days.
Balancing measures revealed no increase in VAE or mortality during the improvement project. Ventilator days, ICU LOS and hospital LOS increased in the intervention period; however, review of patient-specific data revealed that 10% of patients in the intervention period had ICU/hospital LOS greater than 100 days due to chronic complex medical needs including continuous mechanical ventilation which precluded disposition to home or a long-term care facility. None of the patients with a LOS older than 100 days in the cohort met tracheitis treatment criteria during the last 2 weeks of hospitalization, and acute tracheitis was not the etiology of prolonged admission among these patients. Ongoing surveillance of improvement efforts over the subsequent months through July 2019 revealed a sustained tracheal aspirate culture rate of 7.00 cultures per 100 ETT/tracheostomy days and a decreased mean repeat culture rate of 0.63 per 100 ETT/tracheostomy days.
DISCUSSION
This quality improvement initiative successfully decreased the rate of tracheal aspirate cultures by 35% from 10.70 to 7.10 cultures per 100 ETT/tracheostomy days among patients requiring mechanical ventilation via an artificial airway. It decreased the rate of repeat cultures within 72 hours by 36%. Besides, ventilator-associated antibiotic use decreased by 71% from 24.88 to 7.30 antibiotic days per 100 ETT/tracheostomy days without an increase in VAE. Together these efforts have improved resource utilization for diagnosing acute tracheitis and improved treatment by decreasing potentially unnecessary antibiotic exposure. The establishment of an interdisciplinary team with active engagement from MICU, Infection Prevention, Pharmacy, Antimicrobial Stewardship, Respiratory Therapy, and Infectious Disease Diagnostic Laboratory staff facilitated these improvements. The project provides a standardized approach to tracheal aspirate culture sampling and treatment of acute tracheitis in the face of a limited evidence base in the literature.
Respiratory cultures are often used to evaluate ventilator-associated infections, but positive culture results cannot distinguish bacteria colonizing artificial airways from those causing infection. In this context, excess testing may promote unnecessary antibiotic use and increase antibiotic resistance among patients requiring mechanical ventilation. Our project demonstrates how diagnostic stewardship efforts can play a pivotal role in antibiotic stewardship overall. Expansion of this improvement effort to other pediatric ICU environments (eg, cardiac or neonatal ICUs) could further refine the population.
While our local improvement effort achieved the initial aims, there are significant limitations to consider that may inform future multicenter quality improvement efforts. Our MICU exists in a quaternary pediatric hospital with other ICUs and, as a result, does not provide care for the full range of patients who may receive care in an interdisciplinary pediatric ICU, specifically patients requiring perioperative care, patients with a primary oncologic diagnosis, those receiving a hematopoietic stem cell transplant, and patients requiring extracorporeal membrane oxygenation. A retrospective review of the patient cohort utilizing CPT codes determined that there was 1 patient with a primary surgical diagnosis in the baseline cohort and none in the intervention group. Patients were not excluded a priori during the improvement cycles based upon diagnosis; all patients admitted to the MICU requiring mechanical ventilation via an artificial airway were eligible for inclusion. Tailored approaches to diagnosis and management of acute tracheitis may be needed among these specialized patient populations, although the basic principles of our intervention remain relevant.
Further limitations include conducting the practice improvement within the context of current practice rather than controlling all factors that may have influenced tracheal aspirate sampling. Specifically, tracheostomy tubes are not routinely changed upon MICU admission but are changed on a 30-day schedule. As the timing of tracheostomy change was not standardized as part of the improvement effort, variable timing of tube changes may have influenced biofilm development and tracheal aspirate sampling results.
Several patients in the intervention and baseline cohorts had prolonged ICU and hospital LOS. The baseline cohort contained 9 patients with ICU LOS older than 60 days (mean 143 days, range 62–646), representing 3% of the cohort, and 30 patients with hospital LOS older than 60 days (mean 138, range 61–697). In comparison, the intervention cohort contained ten patients with ICU LOS older than 60 days (mean 165 days, range 63–645 days), representing 10% of the cohort, and 24 patients with hospital LOS older than 60 days (mean 140 days, 62–645). Among patients with ICU and hospital LOS older than 60 days, 60% of the baseline cohort and 95% of the intervention cohort required baseline mechanical ventilation, which precluded transfer from the ICU when the critical illness was resolved. Still, the patient was not yet eligible for hospital discharge. Among the patients with ICU LOS older than 60 days, there were higher proportions in the intervention group who remained in the ICU for management of tracheobronchomalacia requiring a high PEEP strategy (30% versus 10%, intervention versus baseline), those awaiting solid organ transplantation ineligible for discharge due to illness severity (20% versus 10%), and those awaiting care coordination/approval for transfer to a skilled nursing facility or home (60% versus 20%). We acknowledge that there is a nearly 3-fold increase in ICU LOS in the intervention group that may have been influenced by the quality improvement interventions alone. Given the number of confounders in interpreting this increase in LOS, we cannot state with certainty that the increased LOS is not a result of the intervention alone and look forward to other’s efforts in improving tracheitis care to understand the influence of these interventions better.
Despite the increase in ICU and hospital LOS in the intervention period, evaluation of the Pediatric Index of Mortality-3 Standardized Mortality Ratios (SMR)24 revealed higher acuity scores in the baseline period, PIM3 SMR 0.48 versus 0.22, respectively. Lower SMR scores in the intervention group and a higher proportion of patients ineligible for transfer from the ICU suggest the increased LOS was not due to illness severity but rather to care coordination needs. During the improvement period, this phenomenon suggests that the interventions alone were not responsible for the increase in LOS during the improvement period. Furthermore, among patients with LOS older than 30 days, none fit criteria for or were treated for acute tracheitis within 2 weeks of the unit or hospital discharge. We did not exclude patients with anticipated prolonged LOS a priori when screening for tracheitis as it remained difficult to accurately predict LOS at the time of initial entry into the cohort.
The efforts to change practice amongst many disciplines within a complex hospital system were significant and required active engagement of key stakeholders and frontline staff to achieve the initial aims. The team identified key barriers important for other teams to consider when adopting this improvement work within their local units. The need for sustained engagement of multiple teams, including quality improvement leadership, nursing, respiratory therapy, laboratory staff, and physicians, is vital when accounting for staff turnover and implementing serial practice changes. Regular communication of improvement cycle data and request for input into future cycles served to sustain engagement. The lack of pediatric-specific data to guide practice improvement and engage stakeholders is an important consideration. Extending local improvement to other centers with diverse patient populations will expedite closing this knowledge gap.
Despite the effort required and the limitations to the project, we were able to successfully decrease our tracheal aspirate culture rate and ventilator-associated antibiotic use among critically ill children requiring mechanical ventilation without an associated increase in VAE. Through the establishment of standardized criteria, active interdisciplinary engagement, and antimicrobial stewardship principles, improvement in the care of acute tracheitis is feasible. This initial single-center effort will hopefully inform multicenter improvement efforts to decrease resource utilization, potentially unnecessary antibiotic use, and risk of antibiotic resistance in this vulnerable population.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Footnotes
Published online December 28, 2020
To cite: Ormsby J, Conrad P, Blumenthal J, Carpenter J, Jones S, Sandora TJ, Vaughan A, Vincuilla J, McAdam AJ, Fogg LF, Flett K, Kelly DP. Practice Improvement for Standardized Evaluation and Management of Acute Tracheitis in Mechanically Ventilated Children. Pediatr Qual Saf 2021;6:e368.
REFERENCES
- 1.Richards MJ, Edwards JR, Culver DH, et al. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000; 21:510–515 [DOI] [PubMed] [Google Scholar]
- 2.Haque M, Sartelli M, McKimm J, et al. Health care-associated infections—an overview. Infect Drug Resist. 2018; 11:2321–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorden AL, Jiang L, Radcliff TA, et al. Potentially preventable hospitalizations and the burden of healthcare-associated infections. Health Serv Res Manag Epidemiol. 2017; 4:2333392817721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cocoros NM, Kleinman K, Priebe GP, et al. ; Pediatric Ventilator-Associated Conditions Study Team. Ventilator-associated events in neonates and children–a new paradigm. Crit Care Med. 2016; 44:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocoros NM, Priebe GP, Logan LK, et al. A pediatric approach to ventilator-associated events surveillance. Infect Control Hosp Epidemiol. 2017; 38:327–333 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Pediatric ventilator-associated event (PedVAE). 2019. Available at https://www.cdc.gov/nhsn/pdfs/pscmanual/pedvae-current-508.pdf. Accessed December 6, 2019
- 7.Lilly CM, Landry KE, Sood RN, et al. ; UMass Memorial Critical Care Operations Group; UMass Memorial Critical Care Operations Group. Prevalence and test characteristics of national health safety network ventilator-associated events. Crit Care Med. 2014; 42:2019–2028 [DOI] [PubMed] [Google Scholar]
- 8.Klompas M. Complications of mechanical ventilation—the CDC’s new surveillance paradigm. N Engl J Med. 2013; 368:1472–1475 [DOI] [PubMed] [Google Scholar]
- 9.Magill SS, Klompas M, Balk R, et al. Developing a new, national approach to surveillance for ventilator-associated events*. Crit Care Med. 2013; 41:2467–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36:309–332 [DOI] [PubMed] [Google Scholar]
- 11.Beardsley AL, Nitu ME, Cox EG, et al. An evaluation of various ventilator-associated infection criteria in a PICU. Pediatr Crit Care Med. 2016; 17:73–80 [DOI] [PubMed] [Google Scholar]
- 12.Nseir S, Povoa P, Salluh J, et al. Is there a continuum between ventilator-associated tracheobronchitis and ventilator-associated pneumonia? Intensive Care Med. 2016; 42:1190–1192 [DOI] [PubMed] [Google Scholar]
- 13.Willson DF, Conaway M, Kelly R, et al. The lack of specificity of tracheal aspirates in the diagnosis of pulmonary infection in intubated children. Pediatr Crit Care Med. 2014; 15:299–305 [DOI] [PubMed] [Google Scholar]
- 14.Craven DE, Hudcova J, Lei Y. Diagnosis of ventilator-associated respiratory infections (VARI): microbiologic clues for tracheobronchitis (VAT) and pneumonia (VAP). Clin Chest Med. 2011; 32:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willson DF, Kirby A, Kicker JS. Respiratory secretion analyses in the evaluation of ventilator-associated pneumonia: a survey of current practice in pediatric critical care. Pediatr Crit Care Med. 2014; 15:715–719 [DOI] [PubMed] [Google Scholar]
- 16.Robinson J. Colonization and infection of the respiratory tract: what do we know? Paediatr Child Health. 2004; 9:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muszynski JA, Sartori J, Steele L, et al. Multidisciplinary quality improvement initiative to reduce ventilator-associated tracheobronchitis in the PICU. Pediatr Crit Care Med. 2013; 14:533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson VS, Bailey A, Higgerson RA, et al. Ventilator-associated tracheobronchitis in a mixed medical/surgical pediatric ICU. Chest. 2013; 144:32–38 [DOI] [PubMed] [Google Scholar]
- 19.Martin-Loeches I, Povova P, Rodriguez A. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicenter, prospective, observational study. Lancet Respir Med. 2015; 3:859–868 [DOI] [PubMed] [Google Scholar]
- 20.Moncayo-Nieto OL, Reid P, Laurenson IF, et al. Improving the use of sputum cultures in lower respiratory tract infection. J R Coll Physicians Edinb. 2013; 43:108–113 [DOI] [PubMed] [Google Scholar]
- 21.Nseir S, Favory R, Jozefowicz E, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008; 12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamma PD, Turnbull AE, Milstone AM, et al. Ventilator-associated tracheitis in children: does antibiotic duration matter? Clin Infect Dis. 2011; 52:1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straney L, Clements A, Parslow RC, et al. ; ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med. 2013; 14:673–681 [DOI] [PubMed] [Google Scholar]


