Abstract
Objectives:
Our objective was to examine the implementation and associated clinical outcomes of a comprehensive surgical site infection (SSI) reduction bundle in a large statewide surgical quality improvement collaborative leveraging a multifaceted implementation strategy.
Summary Background Data:
Bundled perioperative interventions reduce colorectal SSI rates when enacted at individual hospitals, but the ability to implement comprehensive SSI bundles and to examine the resultant clinical effectiveness within a larger, diverse population of hospitals is unknown.
Methods:
A multifaceted SSI reduction bundle was developed and implemented in a large statewide surgical quality improvement collaborative through a novel implementation program consisting of guided implementation, data feedback, mentorship, process improvement training/coaching, and targeted-implementation toolkits. Bundle adherence and ACS NSQIP outcomes were examined preimplementation versus postimplementation.
Results:
Among 32 hospitals, there was a 2.5-fold relative increase in the proportion of patients completing at least 75% of bundle elements (preimplementation = 19.5% vs. postimplementation = 49.8%, P = 0.001). Largest adherence gains were seen in wound closure re-gowning/re-gloving (24.0% vs. 62.0%, P < 0.001), use of clean closing instruments (32.1% vs. 66.2%, P = 0.003), and preoperative chlorhexidine bathing (46.1% vs. 77.6%, P < 0.001). Multivariable analyses showed a trend toward lower risk of superficial incisional SSI in the postimplementation period compared to baseline (OR 0.70, 95% CI 0.49–10.2, P = 0.06). As the adherence in the number of bundle elements increased, there was a significant decrease in superficial SSI rates (lowest adherence quintile, 4.6% vs. highest, 1.5%, P < 0.001).
Conclusions:
A comprehensive multifaceted SSI reduction bundle can be successfully implemented throughout a large quality improvement learning collaborative when coordinated quality improvement activities are leveraged, resulting in a 30% decline in SSI rates. Lower superficial SSI rates are associated with the number of adherent bundle elements a patient receives, rendering considerable benefits to institutions capable of implementing more components of the bundle.
Keywords: bundle, colectomy, colorectal, proctectomy, quality improvement, surgical site infection
Surgical site infection (SSI) is one of the most common hospital-acquired infections in patients resulting in increased morbidity, mortality, length of stay, costs, and readmissions.1–6 SSI is the mostcommon complication following colorectal resection with an incidence ranging from 5.4% to 23.2%.7 While SSI occurs throughout most of surgery, a disproportionate fraction of SSIs occur in colorectal surgery patients, making colorectal SSIs a common target for quality improvement (QI) initiatives.8–12 Moreover, the impact of SSI has been underscored by inclusion of SSI rates in hospital quality public reporting and pay-for-performance programs.13
SSI are a modifiable complication following colorectal resection. SSI reduction efforts have included the development of best practice bundles which are effective in single institutions and aligned healthcare systems.9,10,14–18 These bundles have included standardization of intravenous (IV) antibiotic prophylaxis, chlorhexidine gluconate (CHG) skin preparations, euglycemia, normothermia, mechanical and oral antibiotic bowel preparation, and various intraoperative interventions intended to minimize wound contamination. Accordingly, SSI prevention bundles are recommended in colon and rectal surgery guidelines.19–23 However, little is known about bundle implementation among a large group of diverse hospitals across a state. A prior study of a statewide collaborative found that 3 elements (preoperative intravenous cefazolin/metronidazole prophylaxis, oral antibiotics after mechanical bowel preparation, and normoglycemia) were associated with reduced SSI rates for a limited set of colon resection operations at participating hospitals; however, a 4-year implementation period was required to implement the 3 relatively simple elements.24
The Illinois Surgical Quality Improvement Collaborative (ISQIC) prospectively evaluated the implementation of a colorectal SSI reduction bundle. ISQIC is a 53-hospital statewide learning and quality improvement collaborative in which hospitals and their surgical quality teams engage in coordinated statewide QI initiatives. A colorectal-specific SSI reduction bundle was developed by ISQIC based on contemporary evidence and best reported practices. Our objectives were to 1) examine the intensive implementation of the ISQIC Colorectal SSI Reduction Bundle in a large statewide surgical quality improvement collaborative, 2) assess changes in clinical outcomes after implementation of the SSI reduction bundle, and 3) examine the association between the extent of bundle adherence and clinical outcomes. We hypothesized coordinated, multifaceted guided implementation strategies would enable successful implementation and decrease risk-adjusted SSI rates and associated complications for colorectal surgeries among collaborative hospitals.
METHODS
In 2016, the ISQIC Colorectal SSI Reduction Bundle (Table 1) was adapted from Keenan et al15 (“the Mantyh bundle”) following a literature review of colorectal SSI reduction efforts in collaboration with ISQIC hospitals. Supplementary measures (CHG skin cleansing, intraoperative skin closing protocol) were added to the Mantyh bundle by ISQIC based on identified updates in contemporary evidence published between 2011 and 2016.9,25 Definitions of the original Mantyh bundle elements were expanded to allow flexibility in local practice inherent to contextual and formulary differences among participating hospitals for IV antibiotic agents and redosing intervals to include several guideline-appropriate agents (Appendix A and B).26 Select Mantyh bundle elements were made congruent with ACS NSQIP definitions where applicable. Four policy-only measures (ie, implemented at the hospital level rather than at individual patient level) were encouraged but not abstracted: 1) operating room (OR) traffic minimization, 2) surgical site hair clipping (no shaving), 3) proper wound classification, and 4) proper hand hygiene for all OR providers.
TABLE 1.
ISQIC Colorectal SSI Reduction Bundle
| Preoperative (outpatient) |
| 1. Oral antibiotics (eg, oral erythromycin, neomycin, metronidazole)* |
| 2. Mechanical bowel preparation (eg, large-volume polyethylene glycol)* |
| 3. Preoperative chlorhexidine skin cleansing day before surgery (eg, shower, wipes) |
| 4. Preoperative chlorhexidine skin cleansing day of surgery (eg, shower, wipes) |
| Preoperative (inpatient) |
| 5. Timely initial administration of appropriate intravenous SSI antibiotic prophylaxis (Appendix A) |
| 6. Same-day, preoperative day-of-surgery blood glucose < 200 mg/dL for ACS-NSQIP-defined diabetics |
| Intraoperative (surgery) |
| 7. Timely intraoperative redosing of appropriate SSI antibiotic prophylaxis (Appendix B) |
| 8. OR traffic limited to essential personnel† |
| 9. Surgical site hair clipping (no shaving)† |
| 10. Proper wound classification† |
| 11. Proper hand hygiene for all OR providers involved in patient caret† |
| 12. First measured temperature on arrival to PACU is ≥ 36.0°C/96.8° F |
| 13. Intraoperative skin preparation with chlorhexidine and alcohol-based solution(s) |
| 14. Impermeable wound protector utilization for all incisions |
| 15. Utilization of a dedicated clean wound closure tray/instruments |
| 16. Gown and glove change for all scrubbed personnel prior to wound closure |
| 17. Redraping prior to wound closure |
| 18. Sterile occlusive incisional wound dressing placed in OR |
| 19. Intraoperative blood glucose at 2h (+/− 30 min) into surgery < 200 mg/dL for AC S-NSQIP-defined diabetics |
| Postoperative (inpatient) |
| 20. Duration of intravenous antibiotic prophylaxis is less than 24 h |
| 21. Removal of the original operating room incisional dressing on postoperative day 2 |
| 22. Daily chlorhexidine incision cleansing after dressing removal until discharge (but not to exceed postoperative day 7) |
ACS-NSQIP defined process measure.
Policy measure, data not abstracted.
The ISQIC Collaborative
ISQIC is a learning collaborative of 53 adult hospitals established in 2014 to improve the quality and safety of surgical care. ISQIC is organized around mutual adoption of the widely recognized American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data platform, an additional best practice measure adherence data platform, and implementation of 21 components designed to facilitate quality improvement (eg, mentors, process improvement curriculum, structured statewide projects).27–29 The objective of the collaborative is to facilitate data-driven QI by arming hospitals with the tools needed to systematically examine their data and develop targeted improvement strategies. ISQIC’s 21 components are intended to facilitate more substantial and rapid improvement compared with hospitals engaging in QI in isolation.4–6 Participating hospitals are supported locally by 1 or more trained surgical clinical reviewers (SCRs) to collect patient characteristics, perioperative processes of care, and 30-day outcome data. Local QI participants from each hospital complete a formal didactic and experiential curriculum in process improvement (Define, Measure, Analyze, Improve, and Control; “DMAIC”). This is then supplemented with project-specific implementation aids as collaborative-wide QI projects are launched. Coaches and the ISQIC Coordinating Center help hospitals tailor the implementation to their local context. Seasoned QI teams have been trained and performing collaborative-wide projects since 2015.
SSI Bundle Implementation
The Colorectal SSI Reduction Bundle was introduced as the annual, statewide Collaborative Quality Improvement Project (CQIP) at the ISQIC Semiannual Conference on January 22, 2016. A detailed SSI bundle abstraction guide containing element definitions and supportive evidence were released to ISQIC teams on May 26, 2016 during a webinar. Participating institutions had approximately 4 months to develop local bundle implementation and data-monitoring strategies. The ISQIC Coordinating Center provided ad hoc telephone and email support for sites. A second edition of the SSI reduction bundle abstraction guide containing minor updates and clarifying examples based on early SCR feedback was released in early September 2016. A final webinar was conducted approximately 2 weeks before data abstraction began on September 8, 2016 to clarify any remaining abstraction issues and to give the hospitals an opportunity to interact with experts. SCRs began abstracting the SSI Reduction Bundle on a target date of September 19, 2016 into the ISQIC data platform, where local teams could review their bundle adherence in near-real time. Due to different NSQIP abstraction cycles among ISQIC hospitals, bundle adherence for operative cases completed up to 100 days before the target bundle abstraction start date was abstracted.
A comprehensive Colorectal SSI Reduction Toolkit was released in October 2016 containing a curated mix of ISQIC-produced and borrowed resources from the literature and other Illinois hospitals containing pragmatic tips to further assist sites with implementation. The toolkit consisted of ready-to-implement solutions, requiring some local adaptation, organized by bundle elements. The Toolkit was bolstered with implementation aids such as frequently asked questions/answers, team building guidance, and ISQIC QI module refreshers specifically targeting bundle implementation barriers. Ordering and prescribing information was provided for material resources (eg, wound protectors, bowel preparation) that were not already available at each site. Electronic slide decks and templated introductory letters announcing the bundle were provided to each institution to assist local efforts in gaining physician and executive support for the bundle. Case reports from early-adopting ISQIC institutions were included highlighting learned lessons, materials, and documents created by local institutions that aided implementation and data abstraction (eg, intraoperative checklists, surgeon operative note dictation cues, situation, background, assessment, recommendation [“SBAR”] forms, and staff communiques). Examples of patient education documents (eg, CHG showering instructions, bowel preparation instructions) were also shared in the toolkit. The toolkit was intended to provide targeted implementation strategies for specific SSI bundle barriers, which were then tailored to the local needs of each hospital.
Data and Patient Population
Adherence with each bundle element was abstracted locally by trained and audited SCRs based on the SSI Reduction Bundle Abstraction Guide using the ISQIC data platform. Shortly after data abstraction began, sites completed a mock case for SSI bundle abstraction that was graded and reviewed during a webinar to guide validity and consistency with abstraction.
Bundle adherence data were merged for each patient with ACS-NSQIP to provide data on patient characteristics, procedure information, and postoperative outcomes. ACS-NSQIP data collection is coordinated by a local SCR, who is specifically trained in NSQIP methods and data definitions, regularly audited, and maintains a degree of separation from individual surgeons. A prominent aspect of the approach is regular assessment of interrater reliability. As a result of multiple reinforcing approaches, data integrity within the program is excellent.30
Elective, nonemergent colectomy and proctectomy surgeries from July 1, 2015 through December 31, 2017 were included (Appendix C). The outcomes of interest included the following 30-day postoperative complications: SSI (any, superficial, deep, and organ-space), overall morbidity, and unplanned readmission. Extended length of stay (LOS), defined as a postsurgical LOS greater than the 75th percentile from the combined 2015 and 2016 ACS-NSQIP PUFs (6 d for colectomy and 8 d for proctectomy), was an additional outcome. To assess changes in implementation and outcomes over time, we created a categorical variable to classify the time period relative to the project implementation (Fig. 1), defined as baseline (pre-toolkit release, covering dates up to October 3, 2016), implementation (6 mo from the date of toolkit release, October 4, 2016 to April 3, 2017), and postimplementation (from April 4, 2017 through December 31, 2017). Congruent with a focused and intensive implementation plan, a 6-month implementation period was chosen based on site feedback estimating the minimum necessary time to operationalize the bundle.
FIGURE 1.
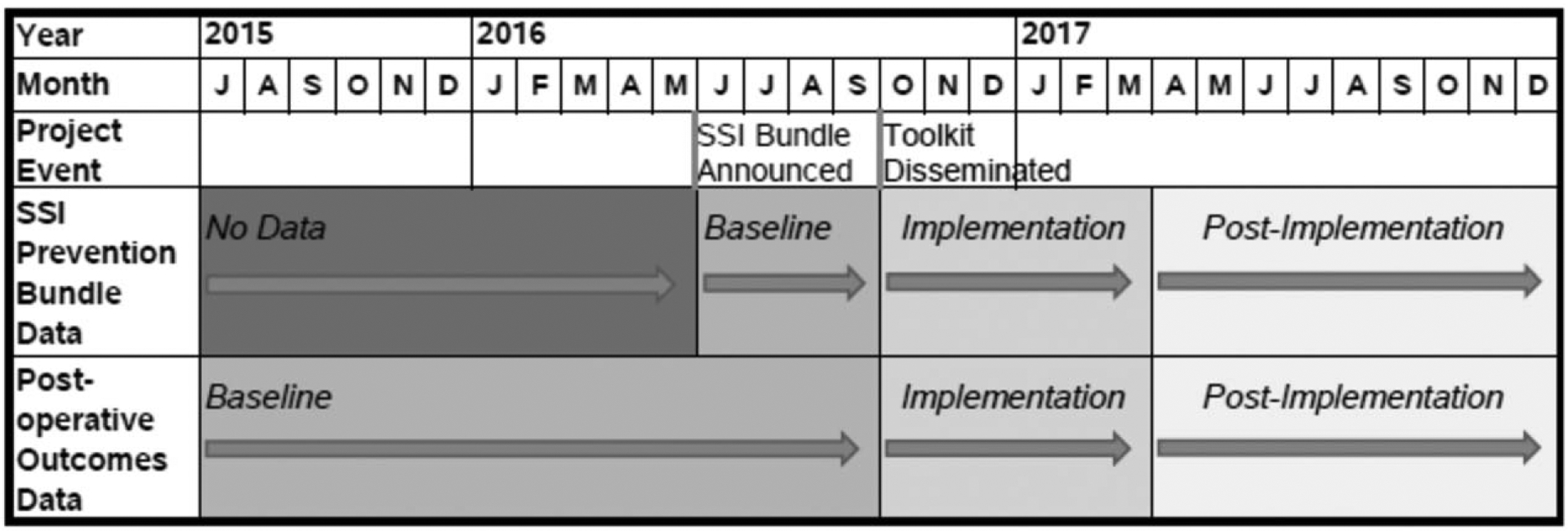
Project and data timeline.
SSI bundle adherence was measured at the bundle element level as well as by phase of care (ie, preoperative, intraoperative, and postoperative). Adherence for a phase of care was defined as the case having been compliant on all elements within that phase. Summary adherence across phases was calculated as follows: quintiles of the percentage of elements for which the case was compliant (out of 16 or 18, depending on the patient’s diabetes status since 2 measures apply only to diabetic patients). The percentage of patients adherent to 50%, 75%, 90%, and 100% of bundle elements was determined and used in subsequent analyses. Anticipating difficulty with standardizing dressing-related care among hospitals, patients adherent to all bundle elements minus dressing-related elements (eg, occlusive dressing application, removal, and postoperative wound CHG wipes) were calculated. Only hospitals with continuous abstraction of the SSI bundle (defined as having data during each of the time periods vis-à-vis implementation with ≥40% of eligible cases having available adherence data) were included (n = 32). Fourteen hospitals opted not to participate in the study. Hospitals were excluded due to lacking baseline period adherence data (n = 3), lacking any eligible colorectal cases (n = 2), not meeting the abstraction threshold (n = 1), and discontinuous abstraction (n = 1).
Two analytic cohorts were assembled for the analysis. First, all eligible procedures for which SSI bundle data were abstracted at these 32 hospitals were combined to comprise the “SSI bundle” cohort which allowed temporal examination of bundle adherence. For clinical outcomes analyses, all eligible colorectal procedures at the same hospitals comprised the “clinical outcomes” cohort. Implementation and postimplementation time periods were identical for both cohorts. However, to provide a larger sample of baseline data and better power subsequent analyses, the clinical outcomes cohort contained baseline patients that predated introduction of the SSI bundle by approximately 1 year (Fig. 1).
Statistical Analysis
Descriptive statistics for patient characteristics were calculated separately for the SSI bundle and clinical outcomes cohorts. SSI bundle adherence for individual measures, by phase of care, overall, and by proportion of bundle completed were calculated overall and by time period (eg, baseline, implementation, postimplementation) with design-corrected Rao-Scott chi-square tests clustered by hospital to assess changes in adherence over the course of project implementation.
Outcomes were calculated using the clinical outcomes cohort. The association of patient demographic, comorbidity, and procedure variables used in colorectal SSI risk-adjustment models from recent ACS-NSQIP semiannual reports were examined using design-corrected chi-square tests. Variables with important clinical and statistical significance (P < 0.2) were selected for multivariable analysis.
Logistic regression models with hospital-clustered robust standard errors estimated the association of bundle implementation (in terms of time period) on likelihood of each postoperative complication and extended LOS, adjusting for patient age, American Society of Anesthesiologists (ASA) classification, BMI category (underweight, normal, overweight, or obese), procedure type (proctectomy vs. colectomy), and open procedure (vs. laparoscopic) except for extended LOS (proctectomy indicator not included). The underweight BMI category was excluded from the deep incisional SSI model due to separation of data points with respect to the outcome. Since colectomy and proctectomy groups possessed different procedure-specific 75th percentile LOS thresholds, the extended LOS model excluded the procedure indicator.
Relationships between bundle adherence and postoperative complications were assessed. First, the percentage of bundle elements adhered to for a patient (out of 16 or 18 elements depending on diabetic status) was determined and quintiles across the distribution of adherence were then computed. Design-corrected chi-square tests were used to assess the relationship between adherence quintile and postoperative complications. Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). Statistical significance was set to 0.05 for all tests. The Northwestern University Institutional Review Board office deemed this project to be exempt.
RESULTS
Patient Population
All cases (ie, the clinical outcomes cohort) consisted of 5137 patients (2615 [50.9%] baseline, 1122 [21.8%] implementation, 1400 [27.3%] postimplementation), and the SSI bundle cohort comprised of 2747 cases (459 [16.7%] baseline, 1027 [37.4%] implementation, 1261 [45.9%] postimplementation] from 32 ISQIC hospitals included in the analyses (Table 2). The majority of participating hospitals were nonrural teaching hospitals affiliated with a medical school (Appendix D). Postbaseline period, 235 cases (n = 95 during implementation, n = 140 postimplementation) had outcomes data but no bundle compliance data. Characteristics of bundle and outcomes cohorts were similar.
TABLE 2.
Patient Characteristics
| Frequency (%) | ||
|---|---|---|
| Clinical Outcomes Cohort (N = 5137) | SSI Bundle Cohort (N = 2747) | |
| Age—mean (SD) | 60.4 (14.9) | 59.9 (15.0) |
| Sex | ||
| Female | 2662 (51.8%) | 1392 (50.7%) |
| Male | 2475 (48.2%) | 1355 (49.3%) |
| ASA Classification | ||
| 1–2 | 2501 (48.7%) | 1359 (49.5%) |
| 3 | 2485 (48.4%) | 1320 (48.1%) |
| 4–5 | 151 (2.9%) | 68 (2.5%) |
| Partial or total functional dependence | 99 (1.9%) | 39 (1.4%) |
| Diabetes | ||
| Oral | 526 (10.2%) | 292 (10.6%) |
| Insulin | 254 (4.9%) | 126 (4.6%) |
| Body Mass Index Class | ||
| Underweight (<18.5) | 149 (2.9%) | 82 (3.0%) |
| Normal (18.5–24.9) | 1443 (28.1%) | 754 (27.4%) |
| Overweight (25.0–29.9) | 1667 (32.5%) | 878 (32.0%) |
| Obese (≥30.0) | 1878 (36.6%) | 1033 (37.6%) |
| Current/recent smoker (within last 6 mo) | 850 (16.5%) | 448 (16.3%) |
| History of COPD | 249 (4.8%) | 126 (4.6%) |
| Disseminated cancer | 243 (4.7%) | 125 (4.6%) |
| Wound classification | ||
| Clean | 96 (1.9%) | 63 (2.3%) |
| Clean/contaminated | 4024 (78.3%) | 2082 (75.8%) |
| Contaminated | 730 (14.2%) | 440 (16.0%) |
| Dirty or infected | 287 (5.6%) | 162 (5.9%) |
| Index Procedure | ||
| Colectomy | 4680 (91.1%) | 2549 (92.8%) |
| Proctectomy | 457 (8.9%) | 198 (7.2%) |
| Perioperative blood transfusion* | 386 (7.5%) | 201 (7.3%) |
| Index procedure Work relative value units—mean (SD) | 27.8 (4.5) | 27.9 (4.4) |
| Project time period | ||
| Baseline (Pre-toolkit) | 2615 (50.9%) | 459 (16.7%) |
| Implementation (6-mo period after toolkit release) | 1122 (21.8%) | 1027 (37.4%) |
| Postimplementation | 1400 (27.3%) | 1261 (45.9%) |
Note: Colorectal cases include all eligible operations from July 1, 2015 through December 31, 2017 and comprise the sample for outcomes-only analyses. SSI Prevention Bundle cases include colorectal operations from May 26, 2016 through December 31, 2017 for which the bundle adherence measures were abstracted, and are the sample for all best practice care adherence analyses.
From surgery start time through 72 h postprocedure.
ASA indicates American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
SSI Bundle Adherence
Baseline adherence rates for individual bundle elements ranged from 10.1% (daily postoperative chlorhexidine wound cleansing) to 95.0% (intraoperative normothermia; Table 3). Other measures with widespread baseline adherence included timely and appropriate IV antibiotics (94.5%), intraoperative CHG-alcohol skin preparation (90.4%), and discontinuation of prophylactic antibiotics within 24 postoperative hours (93.9%). Bundle element adherence increased from baseline through postimplementation periods for 8 elements, with gains ranging from 8.1% (intraoperative CHG-alcohol skin preparation, P < 0.001) to 37.9% (gown and glove changes; P < 0.001). Adherence decreased 1.9% for the normothermia measure (95.0% to 93.1%%, P = 0.03). Adherence for the overall preoperative phase (baseline 26.0% vs. postimplementation 50.1%, Δ24.1%, P = 0.001) and the overall intraoperative phase (baseline 7% vs. postimplementation 28.9%, Δ 21.9%, P = 0.001) improved between periods, whereas adherence to overall postoperative phase elements did not change between periods. Adherence to the entire bundle (ie, all phases) increased between periods (baseline 0.4% vs. postimplementation 4.0%, Δ3.6%, P = 0.001). When excluding dressing-related bundle elements, (occlusive dressing application, removal, and postoperative CHG cleansing) adherence to the total bundle increased between periods (baseline 5.9% vs. postimplementation 23.1%, Δ17.2%, P < 0.001). The percentage of patients adherent to >75% bundle elements increased from 19.5% to 49.8% between periods (Δ 30.3%, P = 0.001).
TABLE 3.
SSI Prevention Bundle Adherence
| Adherence Rate n (%) | ||||||
|---|---|---|---|---|---|---|
| Total* (N = 2747) | Baseline (N = 459) | Implementation (N = 1027) | Postimplementation (N = 1261) | P Value | Absolute Change, Baseline to Post implementation | |
| Preoperative | ||||||
| Chlorhexidine skin preparation day before surgery | 1920 (67.1) | 211 (46.1) | 660 (62.6) | 1049 (77.6) | <0.001 | 31.6% |
| Chlorhexidine skin preparation day of surgery | 2323 (81.1) | 328 (71.6) | 853 (80.9) | 1142(84.5) | 0.029 | 12.9% |
| Oral antibiotics | 2103 (73.5) | 293 (64.0) | 760 (72.1) | 1050 (77.7) | 0.006 | 13.7% |
| Mechanical bowel preparation | 2088 (72.9) | 303 (66.2) | 751 (71.3) | 1034 (76.5) | 0.070 | 10.4% |
| Appropriate antibiotics within 1 h of incision | 2649 (92.5) | 433 (94.5) | 980 (93.0) | 1236 (91.5) | 0.517 | −3.1% |
| Preoperative day-of-surgery blood sugar <200 mg/dL† | 375 (85.0) | 57 (80.3) | 150 (87.7) | 168 (84.4) | 0.399 | 4.1% |
| Preoperative phase —all elements | 1182 (41.3) | 119 (26.0) | 386 (36.6) | 677 (50.1) | 0.001 | 24.1% |
| Intraoperative | ||||||
| Timely redosing of appropriate antibiotics | 2331 (81.4) | 365 (79.7) | 855 (81.1) | 1111 (82.2) | 0.900 | 2.5% |
| Normothermia (temperature on arrival to PACU ≥96.8°F) | 2666 (93.2) | 435 (95.0) | 973 (92.4) | 1258 (93.1) | 0.034 | −1.9% |
| Intraoperative skin prep with chlorhexidine and alcohol | 2758 (96.4) | 414 (90.4) | 1013 (96.2) | 1331 (98.5) | <0.001 | 8.1% |
| Wound protector utilization | 1925 (67.3) | 262 (57.2) | 652 (61.9) | 1011 (74.8) | 0.010 | 17.6% |
| Dedicated wound closure instruments | 1540 (53.8) | 147 (32.1) | 499 (47.4) | 894 (66.2) | 0.003 | 34.1% |
| Gown/gloves change prior to incision closure | 1381 (48.3) | 110 (24.0) | 434 (41.2) | 837 (62.0) | 0.000 | 37.9% |
| Wound re-draped prior to closure | 1087 (38.0) | 97 (21.2) | 312 (29.6) | 678 (50.2) | 0.001 | 29.0% |
| Wound dressed with occlusive dressing after closure | 1393 (48.7) | 183 (40.0) | 533 (50.6) | 677 (50.1) | 0.258 | 10.2% |
| Intraoperative blood sugar at 2h <200 mg/dL† | 172 (39.0) | 36 (50.7) | 61 (35.7) | 75 (37.7) | 0.083 | −13.0% |
| Intraoperative phase—all elements | 607 (21.2) | 32 (7.0) | 185 (17.6) | 390 (28.9) | 0.001 | 21.9% |
| Postoperative | ||||||
| Prophylactic antibiotics discontinued ≤24 h postop | 2689 (94.0) | 430 (93.9) | 975 (92.6) | 1284 (95.0) | 0.331 | 1.2% |
| Original OR wound dressing removed on postoperative day 2 | 911 (31.8) | 129 (28.2) | 315 (29.9) | 467 (34.6) | 0.187 | 6.4% |
| Daily chlorhexidine cleansing until discharge | 304 (10.6) | 46 (10.1) | 86 (8.2) | 172 (12.7) | 0.314 | 2.6% |
| Postoperative phase—all elements | 204 (7.1) | 29 (6.4) | 54 (5.1) | 121 (9.0) | 0.229 | 2.6% |
| Summary adherence | ||||||
| Quintile of adherence across bundle elements | ||||||
| Bottom quintile: 12.5–44.4% | 527 (18.4) | 132 (28.9) | 221 (21.0) | 174 (12.9) | <0.001 | −16.1% |
| Second quintile: 50.0– 56.3% | 581 (20.3) | 133 (29.2) | 227 (21.6) | 221 (16.4) | −12.8% | |
| Third quintile: 61.1– 68.8% | 598 (20.9) | 94 (20.6) | 251 (23.9) | 253 (18.7) | −1.9% | |
| Fourth quintile: 72.2– 81.3% | 555 (19.4) | 56 (12.3) | 186 (17.7) | 313 (23.2) | 10.9% | |
| Top quintile: 83.3– 100% | 598 (20.9) | 41 (9.0) | 167 (15.9) | 390 (28.9) | 19.9% | |
| Threshold of adherence to the bundle | ||||||
| Adhered to 50% or more of elements | 2332 (81.6) | 324 (71.1) | 831 (79.0) | 1177(87.1) | 0.028 | 16.1% |
| Adhered to 75% or more of elements | 1096 (38.3) | 89 (19.5) | 334 (31.7) | 673 (49.8) | 0.001 | 30.3% |
| Adhered to 90% or more of elements | 265 (9.3) | 19 (4.2) | 73 (6.9) | 173 (12.8) | 0.012 | 8.6% |
| Adhered to entire bundle without dressing-related elements | 406 (14.8) | 27 (5.9) | 89 (8.7) | 290 (23.1) | <0.001 | 17.2% |
| Adhered to entire bundle (all elements) | 73 (2.6) | 2 (0.4) | 17 (1.6) | 54 (4.0) | 0.001 | 3.6% |
Note: Summary adherence items for nondiabetic patients exclude the blood glucose measures, with adherence rate denominators adjusted accordingly. P values from design-corrected chi-square tests with responses clustered by hospital.
Item total N varies due to missing data.
Applies to diabetic patients only
Clinical Outcomes
Unadjusted baseline SSI rates were 7.9% overall (superficial 3.6%, deep 0.8%, organ space 4.2%, Table 4). No statistically significant differences were observed in overall or SSI subtype between periods, although a trend toward lower superficial SSI was observed (3.6% baseline vs. 2.4% postimplementation, Δ −1.2%, P = 0.07). A nonsignificant trend toward decreased overall morbidity was noted between baseline and postimplementation periods (14.1% baseline vs. 11.6% postimplementation, Δ−2.5%, P = 0.102). In multivariable analyses, there was a trend toward a lower likelihood of superficial SSI in the postimplementation period compared with baseline (adjusted odds ratio 0.70, 95% CI 0.49–1.02; Table 4). Model results suggested nonsignificant trends toward decreases in overall morbidity and extended LOS.
TABLE 4.
Thirty-day Postoperative Complications Over Project Implementation
| Outcomes | Event Rate n (%) | Adjusted Odds Ratios (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 2615) | Implementation (n = 1122) | Postimplementation (n = 1400) | |||||
| P Value | Baseline | Implementation | Postimplementation | ||||
| Any SSI | 206 (7.9%) | 74 (6.6%) | 98 (7.1%) | 0.347 | 1.00 (REF) | 0.84 (0.59, 1.20) | 0.92 (0.72, 1.17) |
| Superficial SSI | 95 (3.6%) | 28 (2.5%) | 34 (2.4%) | 0.071 | 1.00 (REF) | 0.69 (0.40, 1.20) | 0.70 (0.49, 1.02)+ |
| Deep SSI | 21 (0.8%) | 7 (0.6%) | 7 (0.5%) | 0.421 | 1.00 (REF) | 0.80 (0.34, 1.86) | 0.66 (0.31, 1.41) |
| Organ space SSI | 109 (4.2%) | 42 (3.8%) | 64 (4.6%) | 0.572 | 1.00 (REF) | 0.92 (0.62, 1.35) | 1.12 (0.82, 1.52) |
| Overall morbidity | 369 (14.1%) | 128 (11.4%) | 162 (11.6%) | 0.102 | 1.00 (REF) | 0.82 (0.59, 1.14) | 0.86 (0.65, 1.14) |
| Extended LOS* | 596 (22.8%) | 241 (21.5%) | 268 (19.1%) | 0.121 | 1.00 (REF) | 0.99 (0.75, 1.33) | 0.90 (0.71, 1.14) |
| Readmission | 235 (9.0%) | 89 (7.9%) | 133 (9.5%) | 0.408 | 1.00 (REF) | 0.89 (0.69, 1.15) | 1.08 (0.87, 1.35) |
P values from design-corrected chi-square tests with responses clustered by hospital.
Statistical significance of odds ratio estimates is denoted as follows:
P = 0.06.
Notes: Based on 4985 to 5315 observations (sample size varies by outcome measure) based on missing data and preexisting complications. Implementation and postimplementation effects are as compared to the Baseline period. SSI subtypes can co-occur, therefore subtype rates do not add up to the any SSI rate.
Models adjusted for patient age, American Society of Anesthesiologists classification, BMI category (underweight, normal, overweight, or obese), procedure type (proctectomy vs. colectomy), and open procedure (vs. laparoscopic) except for extended LOS (proctectomy indicator not included). The underweight BMI category was excluded from the deep incisional SSI model due to separation of data points with respect to the outcome.
Extended LOS defined as surgical length of stay >6 d for colectomy and >8 d for proctectomy based on the 75th percentile of LOS in these elective procedure types in the NSQIP PUF for 2015–2016.
LOS indicates length of stay; REF, referent; SSI, surgical site infection.
Association of Bundle Compliance and Clinical Outcomes
The unadjusted risk of superficial SSI decreased as bundle adherence improved (Fig. 2). Patients in the first quintile of bundle adherence (ie, lowest adherence) experienced a superficial SSI rate of 4.6%, whereas patients in the highest quintile of bundle adherence (ie, best adherence) experienced a 1.5% rate of superficial SSI (P < 0.001 between all quintiles). Similarly, nonsignificant associations were noted as rates of overall SSI, overall morbidity, and extended LOS decreased as bundle adherence increased. Readmission rates varied with percentage bundle adherence, although no linear trend was noted to explain the observed variation: patients in the 4th quintile of bundle adherence experienced the highest risk of readmission (11.5%) while patients in the second quintile of bundle adherence had the lowest risk of readmission (6.5%) (P < 0.05 between all quintiles).
FIGURE 2.
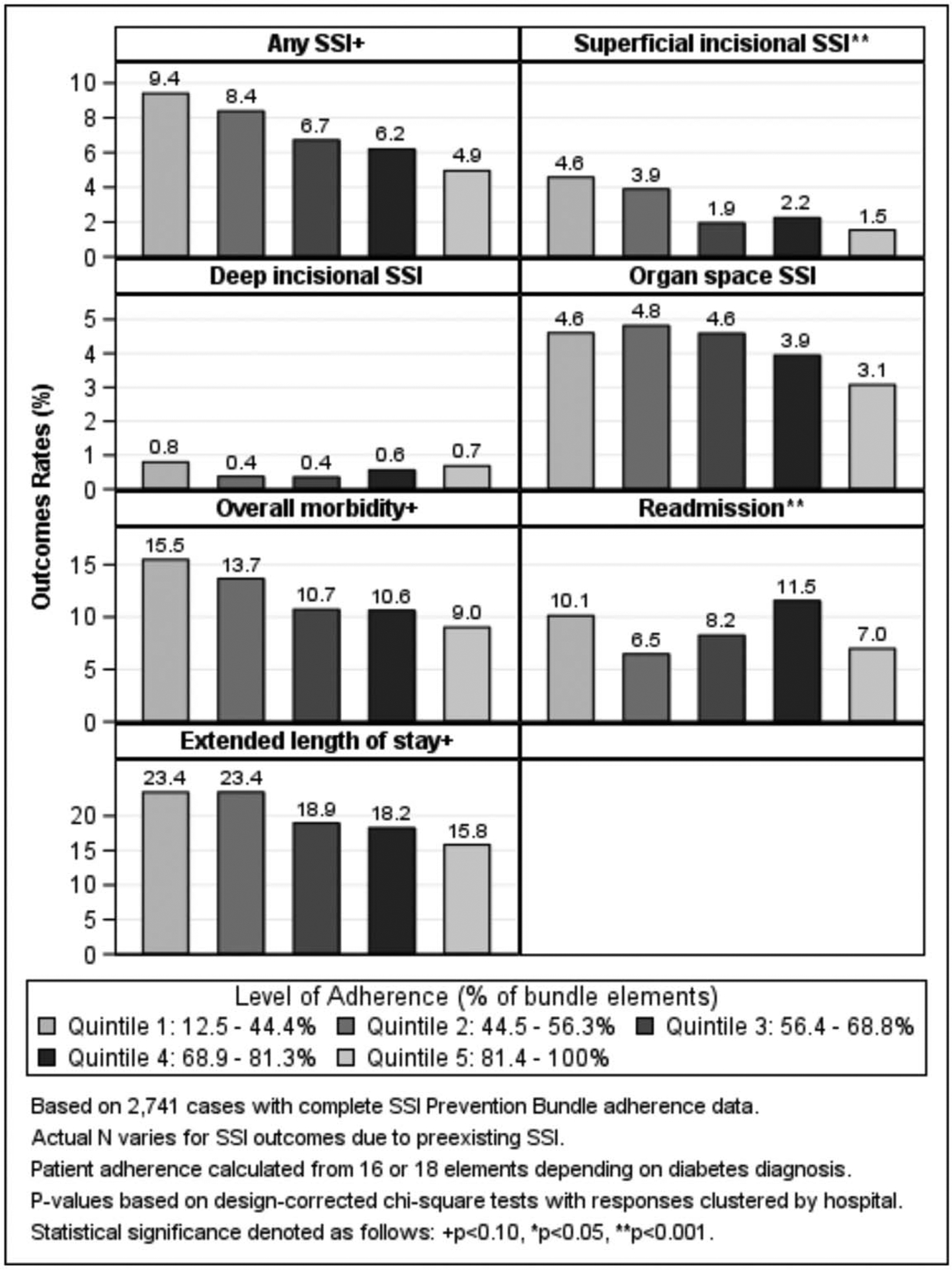
Rates of 30-d postoperative complications by level of adherence to the SSI Prevention Bundle.
DISCUSSION
While bundled interventions are increasingly utilized to improve patient outcomes, bundles can be complex, involve multiple providers, and span multiple venues thereby hindering bundle implementation across large and heterogeneous systems. Bundles are increasingly utilized for a variety of healthcare QI programs; however, whether a comprehensive multifaceted bundle could be efficiently implemented across a large collaborative was unknown. Utilizing a structured QI implementation strategy, a complex multifaceted colorectal SSI reduction bundle was successfully implemented in less than 2 years across a large heterogeneous statewide surgical collaborative, resulting in a 30% decrease in the likelihood of a risk-adjusted superficial surgical site infection. Importantly, as adherence to the number of bundle elements increased, superficial SSI rates decreased. Therefore, institutions capable of high bundle adherence conveyed greater benefits to their patients.
SSI Bundle Adherence
Although substantial bundle adherence gains were observed during the study, a wide range of individual bundle element adherence was noted. The wide variation in element adherence likely speaks to the multifaceted nature of the bundle and need for multiple, but different, coordinated implementation strategies. Many intraoperative bundle elements (eg, wound redraping, gown/glove change, clean closing instruments) ultimately experienced the highest adherence gains during the study. It may be that the operating room, which tends to involve 1 preconditioned team in a closed venue that is accustomed to regimented systems, was able to integrate bundle elements more consistently than other domains. Importantly, adherence gains for intraoperative bundle elements demonstrated that surgeons are capable of changing daily practice (eg, wound closure processes), a presumption that was initially met with skepticism from some collaborative members.
Bundle portions that were vestiges of the Surgical Care Improvement Project (SCIP) (eg, timely, appropriate IV antibiotic prophylaxis and normothermia) were already well incorporated at baseline and did not experience adherence gains during the study. Lack of improvement for these measures does not represent bundle implementation failure, but likely demonstrates a ceiling effect for previously well-implemented national SSI reduction endeavors that were once linked to payor reimbursement and are the current focus of public reporting.
Dressing-related bundle elements (occlusive dressing application and removal, postoperative CHG cleansing) were poorly implemented. Poor adherence in this domain may be explained by difficulty implementing or measuring adherence with these elements or physician disbelief in measure efficacy. Since postoperative interventions typically involved a number of providers (eg, physicians, nurses, advanced practice providers, and trainees) over several days, these elements introduced more opportunity for missed treatments compared to bundle elements that occurred at 1 time instance (eg, normothermia). Development of reliable documentation systems at each site likely influenced the accuracy of bundle adherence reporting as informal qualitative feedback from collaborative participants suggested difficulty reliably documenting certain bundle elements—particularly for the postoperative phase (eg, dressing removal and postoperative CHG skin cleansing).
Clinical Outcomes
An approximate 30% decrease in risk-adjusted superficial SSI was noted for participating study hospitals; however, risk-adjusted odds of overall SSI and other complication rates were unaffected by the bundle. The authors hypothesized that a bundle including mechanical bowel preparation and oral antibiotics would decrease organ space infections, commensurate with other large retrospective studies, and a recent prospective study using a similar bundle.12,31–33 The current study findings, however, align with those of Keenan et al,15 which showed reduced rates of superficial SSI but no change in overall, deep, or organ space SSI occurrence with the introduction of a similar bundle at one hospital. This finding suggests that the bundle is most effective in reducing subcutaneous contamination at the wound, but does not affect rates of anastomotic leakage or organ space infections, which are likely driven by myriad factors extending beyond simple contamination.
SSI reduction programs have been promoted for a variety of surgeries dating back to the early 2000s with variable success. SCIP, for example, was designed to reduce surgical site infections by 25% within 5 years.34 While the efficacy of large programs like SCIP was debatable, the current study suggests a robust implementation structure can improve quality on accelerated schedules.35,36 The current study showed a 30% improvement in risk-adjusted odds of superficial SSI in a project that spanned less than 2 years. Various public reporting and pay for performance programs have focused mainly on process measure adherence and resultant clinical outcomes for hospitals; however, the current study suggests that possession of a functional and nimble implementation QI institutional structure may also be a potential quality benchmark to accelerate QI.
Association of Bundle Compliance and Clinical Outcomes
The bundle’s efficacy in decreasing superficial SSI correlated in a somewhat linear fashion with number of successfully completed bundle elements. Therefore, the bundle improved outcomes for institutions capable of higher fidelity implementation. This relationship has been observed with other quality improvement bundles; however, the current study differs with regard to the complexity of the bundle, the focused and intensive implementation timeline, and the diverse multiinstitutional composition of the statewide collaborative.11,18,24 Berian et al37 recently used the ACS-NSQIP PUF database to associate clinical postoperative outcome improvements with an increasing number of completed enhanced recovery bundle elements. Russell et al18 reported empiric associations between degree of compliance with an 8-element SSI reduction bundle with decreasing adjusted SSI rates at a single institution. The current study noted similar, but nonsignificant, trends associating degree of bundle compliance with decreases in overall SSI, overall morbidity, and extended LOS. While these clinical outcomes failed to reach significance, strong putative trends may confirm “dose dependency” of QI bundles.
Implications
The current study provides a unique examination of successful prospective implementation of a complex, comprehensive QI bundle across a large diverse network of hospitals. ISQIC utilized an established QI structure (eg, performance coaches, group learning, focused toolkits, surgical champions, and formal QI training) to implement the bundle. The ability of local hospitals to implement the bundle in a relatively short time speaks to the effectiveness of the collaborative’s QI framework. The highly detailed program attempted to control many variables that are typically uncontrolled in single institution and large retrospective (ie, non-ACS-NSQIP) database studies. Statewide collaboration and organization allowed relatively large samples to detect differences that would be obfuscated in a smaller study. The study provides pragmatic insight to bundle implementation, as well as clinical evidence to further SSI reduction efforts. Lastly, the demonstrated differential effects between high and low bundle adherence stress the importance of high-fidelity bundle implementation.
Limitations
The study has limitations that should be acknowledged. First, a low percentage of patients were able to complete every element of the bundle, likely due to low adherence with postoperative elements and specifically postoperative wound care (dressing removal and postoperative daily CHG cleansing). However, when excluding dressing-related bundle elements from the study, 23% of postimplementation patients were able to complete the entire bundle. Second, the authors do not have insight into why some bundle elements had higher adherence than others. Further qualitative analysis may help to identify barriers to element adherence. Third, this study does not address which bundle elements are most effective in reducing SSI. The authors suspect that some bundle elements are not essential and a simplified bundle may preserve efficacy and future research will explore this issue. Fourth, ISQIC leveraged a seasoned vast network of experienced QI teams who had previously worked on statewide quality initiatives to launch the SSI bundle. Younger or uninitiated QI networks may require alternative implementation strategies or longer implementation timelines. Finally, this study does not permit a comparative control to group to account for temporal trends since ACS NSQIP does not allow identification of hospitals, and we do not have information on what type of SSI bundle, if any, a possible control hospital might have implemented during the study periods.
CONCLUSION
The study provides evidence that complex multifaceted QI initiatives involving a large number of diverse hospitals can be implemented when a QI network is built through training and collaboration. As a result, implementation of an SSI reduction bundle decreased superficial SSI by 30% for participating Illinois hospitals. SSI bundle effectiveness is associated individual patient bundle adherence, rendering considerable benefits to institutions capable of implementing more elements of the bundle. Analysis is needed to determine which bundle elements most effectively reduce SSI, and which hospital and patient factors best predict bundle adherence and optimal clinical outcomes. Simplifying the SSI bundle to eliminate unnecessary elements will allow institutions to focus on maximizing adherence with a smaller set of highly effective bundle elements.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank surgeons, nurses, and quality improvement professionals for all 53 ISQIC hospitals.
This work is supported by the Agency for Healthcare Research and Quality (R01HS024516 [PI: K.Y.B.]), National Institutes of Health (K08HL145139 [PI: A.Y.]), and the Health Care Services Corporation/Blue Cross Blue Shield of Illinois (PI: K.Y.B.).
DISCUSSANTS
Dr Justin B. Dimick (Ann Arbor, MI):
Thank you, Dr McGee, for a really great body of work. Obviously, this is really impressive and a standard. Thank you for not just measuring care across your collaborative but working to improvement. As a famous quality improve guru once again, weighing a pig doesn’t make it fatter, and so measurement isn’t enough. Thank you for feeding the pig and actively working to improve quality.
I have a couple of questions.
First, can you give us a little more details on implementation? Was this the generic implementation structure of ISQIC, or was there tailored implementation study, both tailored for this specific program, meaning the SSI bundle, and did you tailor it for individual hospitals? Did you look at where they were falling short and tailor your strategy according to where they needed improvement?
My second question, probably doesn’t have an answer, but I’m going to ask it anyway. One of the most interesting papers on this topic was by Anthony in 2011 in Archives of Surgery. That was a randomized trial, so it looked at all these observational studies of bundle and implementation similar to yours but on a smaller scale and showed that cross-sectionally bundles were associated with better outcomes. If you implement the bundle, you do better. This is a randomized trial by Anthony that showed a doubling of the SSI rate after bundle implementation. So they implemented a bundle and doubled the SSI rate, which is really interesting and lends us to think a lot about, well, maybe there is this idea that when we multitask and we get distracted by focusing on this whole bundle thing, we forget to do something that we were doing otherwise that might be the right thing to do.
How do you reconcile your results with the only randomized trial that I know that’s SSI bundle and implementation. Is your bundle different? If you were to do just a randomized trial, would you expect this result or that result? Thank you.
Dr Michael F. McGee
Thank you very much, Dr Dimick, for the cogent and very insightful thoughts. I’d like to tackle your questions in order.
Regarding implementation, there is a baseline curriculum that is quick, employs for all of the projects, and that is the mentoring the DMAIC model, and frequent in-person meetings as well as teleconferences when a project is launched. Particularly to the SSI bundle, however, we released a tool kit. We didn’t have a ton of time to go into what the tool kit entailed, but this is the specific component of the implementation plan that specifically addresses the project itself, so for the SSI bundle, we had a tool kit that included case reports from early adopters, so we had what hospitals were already up and running, what did they learn worked? We had ordering information particular to the things that you needed to do, for instance, getting a wound protector was challenging for some hospitals. So we provided them what they needed. And we also shared a lot of lessons learned, frequently asked questions, things like that. So it’s a mix of a baseline implementation structure that we have within the collaborative plus tailored adjuncts needed ad hoc to help push a particular project through.
The second question, the discrepancy in findings with the 2011 study that you had mentioned, the study unfortunately is not the freshest in my mind. I can tell you a couple of different things, though. A lot of people aim to implement a bundle, but the interesting thing about this is not only do we have the clinical outcomes but we had extremely high level process measure compliance. So it’s interesting to see—and I would have to go back and look at the paper to more properly address your questions—but I would be curious to see, well, their SSI rate went up, but did they just say they implemented a bundle or were they actually tracking the process measures to show that they did successfully implement the bundle? We’ll have to go back and look at that. Thank you.
Dr Liane Feldman (Montreal, QC):
Congratulations. It is a huge amount of work and really nicely presented and well done. But at the end of the day, it’s kind of disappointing—I thought the punch line was going to be that SSIs went down. But the SSI rate is already very low in laparoscopic colectomy, as was mentioned. The issues tend to be more with proctectomy where SSI rates can be quite high. I’m interested in that particular subset.
The other question is, for this kind of huge intervention, you have a lot of centers that already have extremely low SSI rates. Were you able to look at targeting or analyzing those centers that started off as having a problem and then look specifically at their results rather than in addition to the entire 40-some hospitals?
Dr Michael F. McGee
Very insightful points. So regarding the disappointment, I would agree. We were expecting to see a more dramatic decrease in overall SSI. Anastomotic leaks have been published in some studies with utilization of the bowel prep and oral antibiotics. When we got the data, although we were happy to see that we did make a difference with reducing the superficial component of this, we were somewhat disappointed that we thought this would have a more dramatic impact.
All I can say add to, Dr Feldman, is that the data is what the data is. We have very high-level compliance. We can pull out the people who were able to complete the bundle and show that when you completed the bundle, we could drive that level down.
The data presented here, by and large, is the entire collaborative. These are people who didn’t complete a lot of the bundle versus people who did, so we did see that differential. I think future work will help identify high performers versus low performers, which I think ties into your last question, low-performing hospitals, what can we do to focus and feedback that data?
More work is going on currently with our research group to look at hospital and patient level characteristics to better answer that question about both compliance and result in SSIs.
Dr Rebecca Minter (Madison, WI):
Thank you for the opportunity to comment. Really impressive work, and the Illinois collaborative has really been leading the way in many ways, many initiatives.
My question really relates to back to kind of your tool kit and your implementation scalability strategy. I think one of the real limitations here, as you noted, is that you’re a very mature statewide collaborative. It’s based on NSQIP hospitals, so that’s already a very labor intensive and resource intensive sort of approach, nurse abstraction for all of the data. In what ways have you considered rolling out your bundle by leveraging informatic strategies, in particular, the EMR, tools like Procedure Pass in EPIC or other similar perioperative EMR tools. Obviously people are on different EMRs, but there are largely 2 dominant products, and even if you could reach a number of hospitals by protocolizing at least a piece of this that’s related to orders and things of that nature it would serve 2 purposes. One, it would make this more scalable and attainable, and then you can focus your efforts around the things that can’t be protocolized in that way. Also, you would be able to facilitate data extraction without having nurses dive into the record to try to figure this out at every hospital.
Dr Michael F. McGee
I think that’s so important to what we’re trying to do. I think the biggest thing we can do to hospitals to aid implementation if we’re looking going beyond NSQIP or to non-NSQIP hospitals in our state is to make a shorter, more succinct bundle. This is an onerous thing to implement. Clearly, there’s some dead weight in it. I think, first, we’re beholden to make this as succinct and potent as possible and eliminate perhaps some of the dead weight that might be in the bundle. We fully acknowledge that.
With regard to specific EMR implements, we struggle with this like everyone else does. There are 2 dominant EMR players in our state. A lot of information in the tool kit was work-arounds for hospitals who figured out how to reliably document these things to make life easier for their abstracters. We do not have a universal solution yet to the EMR issue. I think it is a good for us to focus on going forward, though.
Dr Jeffrey Drebin (New York, NY):
I have a mirror image question to Justin’s question. What if you implement a bundle and it doesn’t work.
My question relates—and you sort of mentioned it in your conclusions—when you implement a very complex bundle, and it works, how you break it down from there can be problematic. Peter Allen, when he was with us, put in a Whipple bundle, and it dramatically reduced postsurgical infections, but it had a lot of aspects that were annoying like doing regloving, regowning, redraping, separate instruments. At the end of a long case, it’s sort of not what anyone necessarily wants to do. The occlusive dressing with the vacuum pump actually whistles all night long and the patients hated it and got no sleep the first night.
How do you decide when you put 20 things into a bundle, and it’s work, what’s your next step? A randomized trial of 10 versus 10, take out 5 and just see what happens? You already educated your hospitals to start doing these things, and now you are going to tell them, well, maybe not. How do you handle that?
Dr Michael F. McGee
Because of the large number of hospitals and the large number of patients, we’re fairly well suited to perform a factor analysis to determine what are the big drivers for SSI. That, I think, is the ongoing focus of much of the work that’s coming out of our group in the next few months.
So that’s the first thing we could do with the data that we have, what things can be eliminated. When you have a bundle that’s this big, and even though we have over 5100 patients, we’re pushing the limits of what we can do with a factor analysis to help determine that.
So more to come, and I think we owe it to everyone to figure out what are the main 5 or 6 things that we can do rather than the 22 things. Yes, the complaints that I’m hearing, by the way, are the same complaints I heard for the last 2 years. I don’t want to change my gowns and glove. This is the way that slows me down. But we know that when you put these things together, we do see some benefit.
Footnotes
This work was presented at the American Surgical Association Annual Meeting in Dallas, TX on April 13, 2019.
The authors report no conflicts of interest.
REFERENCES
- 1.Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. [DOI] [PubMed] [Google Scholar]
- 2.Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96:1–15. [DOI] [PubMed] [Google Scholar]
- 3.Astagneau P, Rioux C, Golliot F, et al. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect. 2001;48:267–274. [DOI] [PubMed] [Google Scholar]
- 4.Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg. 2019;85:142–149. [PubMed] [Google Scholar]
- 5.Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313:483–495. [DOI] [PubMed] [Google Scholar]
- 6.Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young H, Knepper B, Moore EE, et al. Surgical site infection after colon surgery: National Healthcare Safety Network risk factors and modeled rates compared with published risk factors and rates. J Am Coll Surg. 2012;214:852–859. [DOI] [PubMed] [Google Scholar]
- 8.de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–397. [DOI] [PubMed] [Google Scholar]
- 9.Cima R, Dankbar E, Lovely J, et al. Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program-driven multidisciplinary single-institution experience. J Am Coll Surg. 2013;216:23–33. [DOI] [PubMed] [Google Scholar]
- 10.Anthony T, Murray BW, Sum-Ping JT, et al. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Arch Surg. 2011;146:263–269. [DOI] [PubMed] [Google Scholar]
- 11.Waits SA, Fritze D, Banerjee M, et al. Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surgery. 2014;155:602–606. [DOI] [PubMed] [Google Scholar]
- 12.Gorgun E, Rencuzogullari A, Ozben V, et al. An effective bundled approach reduces surgical site infections in a high-outlier colorectal unit. Dis Colon Rectum. 2018;61:89–98. [DOI] [PubMed] [Google Scholar]
- 13.Sajankila N, Como JJ, Claridge JA. Upcoming rules and benchmarks concerning the monitoring of and the payment for surgical infections. Surg Clin North Am. 2014;94:1219–1231. [DOI] [PubMed] [Google Scholar]
- 14.Thompson KM, Oldenburg WA, Deschamps C, et al. Chasing zero: the drive to eliminate surgical site infections. Ann Surg. 2011;254:430–436. [DOI] [PubMed] [Google Scholar]
- 15.Keenan JE, Speicher PJ, Thacker JK, et al. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014;149:1045–1052. [DOI] [PubMed] [Google Scholar]
- 16.Gorgun E, Benlice C, Abbas MA, et al. Experience in colon sparing surgery in North America: advanced endoscopic approaches for complex colorectal lesions. Surg Endosc. 2018;32:3114–3121. [DOI] [PubMed] [Google Scholar]
- 17.Bull A, Wilson J, Worth LJ, et al. A bundle of care to reduce colorectal surgical infections: an Australian experience. J Hosp Infect. 2011;78:297–301. [DOI] [PubMed] [Google Scholar]
- 18.Russell TA, Chung H, Riad C, et al. Sustaining improvement: implementation and spread of a surgical site infection bundle. Am Surg. 2018;84:1665–1669. [PubMed] [Google Scholar]
- 19.Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guideline for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons (ASCRS) and Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Surg Endosc. 2017;31:3412–3436. [DOI] [PubMed] [Google Scholar]
- 20.Allegranzi B, Bischoff P, de Jonge S, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e276–e287. [DOI] [PubMed] [Google Scholar]
- 21.Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288–e303. [DOI] [PubMed] [Google Scholar]
- 22.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 23.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 update. J Am Coll Surg. 2017;224:59–74. [DOI] [PubMed] [Google Scholar]
- 24.Vu JV, Collins SD, Seese E, et al. Evidence that a regional surgical collaborative can transform care: surgical site infection prevention practices for colectomy in Michigan. J Am Coll Surg. 2018;226:91–99. [DOI] [PubMed] [Google Scholar]
- 25.Edmiston CE Jr, Lee CJ, Krepel CJ, et al. Evidence for a standardized preadmission showering regimen to achieve maximal antiseptic skin surface concentrations of chlorhexidine gluconate, 4%, in surgical patients. JAMA Surg. 2015;150:1027–1033. [DOI] [PubMed] [Google Scholar]
- 26.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. [DOI] [PubMed] [Google Scholar]
- 27.Berian JR, Thomas JM, Minami CA, et al. Evaluation of a novel mentor program to improve surgical care for US hospitals. Int J Qual Health Care. 2017;29:234–242. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan HC, Provost LP, Froehle CM, et al. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21:13–20. [DOI] [PubMed] [Google Scholar]
- 29.Wandling MW, Minami CA, Johnson JK, et al. Development of a conceptual model for surgical quality improvement collaboratives: facilitating the implementation and evaluation of collaborative quality improvement. JAMA Surg. 2016;151:1181–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210:6–16. [DOI] [PubMed] [Google Scholar]
- 31.Kiran RP, Murray AC, Chiuzan C, et al. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg. 2015;262:416–425. [DOI] [PubMed] [Google Scholar]
- 32.Morris MS, Graham LA, Chu DI, et al. Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg. 2015;261:1034–1040. [DOI] [PubMed] [Google Scholar]
- 33.Scarborough JE, Mantyh CR, Sun Z, et al. Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg. 2015;262:331–337. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt). 2011;12:163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stulberg JJ, Delaney CP, Neuhauser DV, et al. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. [DOI] [PubMed] [Google Scholar]
- 36.Hawn MT, Itani KM, Gray SH, et al. Association of timely administration of prophylactic antibiotics for major surgical procedures and surgical site infection. J Am Coll Surg. 2008;206:814–819. [DOI] [PubMed] [Google Scholar]
- 37.Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019; 269:486–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


