Abstract
Spondyloarthritis Research Consortium Canada (SPARCC) score is an effective magnetic resonance imaging (MRI) evaluation method for inflammation in axial spondyloarthritis. Previously published meta-analyses have shown tumor necrosis factor α inhibitors (TNFi) had great effectiveness on improving disease activity and function in axial spondyloarthritis. However, there still has no one that concentrates on the impact of TNFi on MRI inflammation. We conduct a meta-analysis to summarize the impact of TNFi on MRI inflammation in axial spondyloarthritis using SPARCC score. Comprehensive search was conducted in the databases of OVID Medline, OVID EMBASE, and Cochrane library on November 14, 2020. We investigated the differences in SPARCC score of sacroiliac joint and spine, before and after TNFi treatment in patients with axial spondyloarthritis. SPARCC score was further compared in the subgroup by diagnostic category and TNFi types. In addition, clinical assessment indicators including ankylosing spondylitis disease activity score, bath ankylosing spondylitis disease activity index, bath ankylosing spondylitis functional index, c-reactive protein were also analyzed. Data were pooled by mean differences (MD) with 95% confidence intervals (CI) and publication bias was assessed by Egger’s test. Jadad scale was applied to assess the quality of included trials. Compared with control group, TNFi significantly improved SPARCC score of sacroiliac joints (n = 11, MD = 2.86, 95% CI 2.50, 3.23) and spine (n = 5, MD = 1.87,95%CI 1.27, 2.46). This effect was consistent among subgroups by different diagnostic category (ankylosing spondylitis, non-radiographic axial spondyloarthritis) and TNFi types (adalimumab, certolizumab pegol). Analysis of clinical assessment indicators also confirmed the therapeutic effect on axial spondyloarthritis. Egger’s test suggested no possibility of publication bias. This meta-analysis shows that TNFi are effective to improve MRI inflammation in patients with axial spondyloarthritis and the treatment effectiveness is not affected by diagnostic category and TNFi types.
Introduction
Spondyloarthritis (SpA), a group of chronic inflammatory rheumatic diseases, affect joints, organs, and other tissues. Axial spondyloarthritis (axSpA) is the umbrella term for this group of diseases where the predominant involvement is in the axial skeleton. AxSpA can be further classified to radiographic axSpA, also termed ankylosing spondylitis (AS), and non-radiographic axial spondyloarthritis (nr-axSpA) based on whether definitive structural changes are visible on plain radiographs of the sacroiliac joints (SIJ). Both are characterized by inflammatory pain and functional impairment [1–4].
As the first-line medication for axSpA, non-steroidal anti-inflammatory drugs (NSAIDs) are well recognized for relieving acute symptoms and improving function [5, 6]. But the impact of NSAIDs on radiographic progression was very limited [5] and trial data have arose doubts of NSAIDs on preventing the potential structural progress [7–10]. Local injections of glucocorticoid to the site of musculoskeletal inflammation may be considered an option to treat arthritis and enthesitis [5]. A prior study suggested that short-term glucocorticoid had a mild effect on signs and symptoms [11], but long-term treatment of systemic glucocorticoids was not recommended in patients with axial disease [5]. In spite of the therapeutic effect on rheumatoid arthritis, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as sulfasalazine, methotrexate, and leflunomide have no proven efficacy for axSpA [12–15]. Biological disease-modifying antirheumatic drugs (bDMARDs) are the milestones for the treatment of axSpA [5, 6, 16] Tumor necrosis factor α inhibitors (TNFi) including etanercept, adalimumab, certolizumab pegol, infliximab, and golimumab have shown effect in improving disease activity and physical function [17–19].
To detect and supervise inflammation or structure damage of the joints, among the several available radiology methods, such as X-ray, computed tomography (CT), magnetic resonance imaging (MRI), MRI is considered as the best imaging technique [20], which shows a high sensitivity on detecting the inflammation lesion generally manifested as bone marrow edema (BME) at SIJ and spine [21, 22]. In recent years, MRI has become an important evaluation method for axSpA, especially in patients with inconclusive findings or negative findings on X-rays. The AS working group of the International Association for the Evaluation of Spinal Arthritis (ASAS)/Outcome Measures in Rheumatology (OMERACT) had proposed that MRI should be applied as the first choice to evaluate spinal arthritis [23]. The Spondyloarthritis Research Consortium Canada (SPARCC) score suggested by Landewé et al. [24] had a high sensitivity to reflect inflammation changes and displayed a positive correlation to clinical disease activity. The scoring system was developed by Maksymowych for assessing the inflammatory activity in SIJ and spine relied on the short time inversion recovery (STIR) sequence [25, 26]. For evaluation of inflammation in SIJ reflected by BME, the SPARCC score evaluate the SIJ with highest inflammatory activity, dividing the bilateral joint into 4 quadrants: upper iliac, lower iliac, upper sacral, and lower sacral. Each of these 4 quadrants was scored on a dichotomous way, where 1 = presence of BME and 0 = absent edema signal. Additional score of 1 was given when both sides of joint with a BME signal of depth≥1 cm from the articular surface or a intense signal respectively. The maximal score for a single coronal slice is 12 and the scoring system was repeated in 6 consecutive coronal slices, leading to a total score of 72 [25]. The SPARCC spine score assesses the BME in vertebral bodies of spine based on 6 most severely affected discovertebral unit (DVUs) in 3 consecutive coronal slices and its grading method is similar with the SIJ score, bringing maximum score to 108 [26].
Previously published meta-analysis mainly concerned on the treatment effect of TNFi on disease activity and function in axSpA patients assessed by clinical evaluation methods such as Bath Ankylosing Spondylitis disease activity index (BASDAI), Bath Ankylosing Spondylitis functional index (BASFI) [27, 28], while no one concentrated on the MRI inflammation. To summarize the impact of TNFi on MRI inflammation assessed by SPARCC score, we performed the meta-analysis, accompanied with other assessment indicators including Ankylosing Spondylitis Disease Activity Score (ASDAS), BASDAI, BASFI, and C-reactive protein (CRP).
Materials and methods
Search strategy
Literature retrieval was performed in electronic databases of OVID Medline, OVID EMBASE, and Cochrane library on November 14, 2020. The combination search of key words and MeSH terms were conducted and the search terms were ‘spondyloarthritis’, ‘tumor necrosis factor’ and ‘magnetic resonance imaging’. In addition, references of included studies were also manually checked to identify possibly eligible studies. Detailed search strategy was presented in S1 File. Two authors respectively screened the titles and abstracts, and read the full texts according to predefined study selection criteria. Disagreement was resolved by consensus.
Study selection
The eligible studies met all the following criteria: (1)Study patients fulfilled the spondyloarthritis international society (ASAS) classification criteria for axSpA [29] or the modified New York criteria [30]; (2) Patients in treatment group received one of the five TNFi (etanercept, adalimumab, certolizumab pegol, infliximab, and golimumab) while in control group patients were treated with placebo, NSAIDs, csDMARDs, or any non-TNFi biologics; (3) Using SPARCC score to assess the treatment effect at sites of spine (scale 0–108) and SIJ (scale 0–72), accompanied with other therapeutic effect indicators including ASDAS, BASDAI, BASFI, CRP. (4) Study design was a randomized controlled trial.
A study would be excluded if it met any one of the following statements: (1) a duplicate study; (2) no data related to SPARCC score; (3) TNFi were used in both groups; (4) a letter or a conference abstract without full text.
Data extraction
Two authors independently extracted data and disagreements were addressed by discussion and re-evaluation. We collected information on family name of first author, year of publication, country of first author, subgroup diagnosis of axSpA, treatment information, study duration, and study information in both treatment group and control group (e.g., patient number, mean age, disease duration, interventions, baseline and endpoint or changes of therapeutic effect indicators).
Quality assessment
Jadad scale was applied to assess the methodology quality of included studies [31], containing randomization, double blinding, and drop out and loss of follow-up. For randomization, scores of 0, 1, 2 are given for no mention or inappropriate randomization methods (e.g., based on even or odd number of birth date or admission order), inadequate description like randomization without specific methods, adequate and appropriate randomization (e.g., randomization by random number table or computer). For double blinding, 0 is given to no blinding or inappropriate double binding method, 1 for inadequate description of double blinding, and 2 for adequate and appropriate description of double binding. For drop out and loss of follow-up, 0 is given to no description while 1 is for adequate description. For each study, the highest score is 5, and score less than 2 is considered as low study quality while higher than 3 is deemed as high study quality.
Statistical analysis
Data were analyzed by Review Manager 5.3. The major outcomes were SPARCC score at SIJ and spine. Other outcomes including ASDAS, BASDAI, BASFI, and CRP were also studied when data were available. Data were pooled by the mean difference (MD) with 95% confidence interval (CI). Heterogeneity among the studies was evaluated by I2 statistic and P value at 0.1. Fixed-effect model was adopted when there was no or low heterogeneity (I2≤50%) while random-effect model was applied when there was high heterogeneity (I2>50%).
Subgroup meta-analyses by diagnostic category (AS and nr-axSpA) and TNFi types (etanercept, adalimumab, golimumab, certolizumab pegol, infliximab and golimumab) were also performed (if data were available). Sensitivity analysis was conducted to test the robustness of the outcomes. Egger’s test was applied to assess the publication bias.
Results
Study selection result
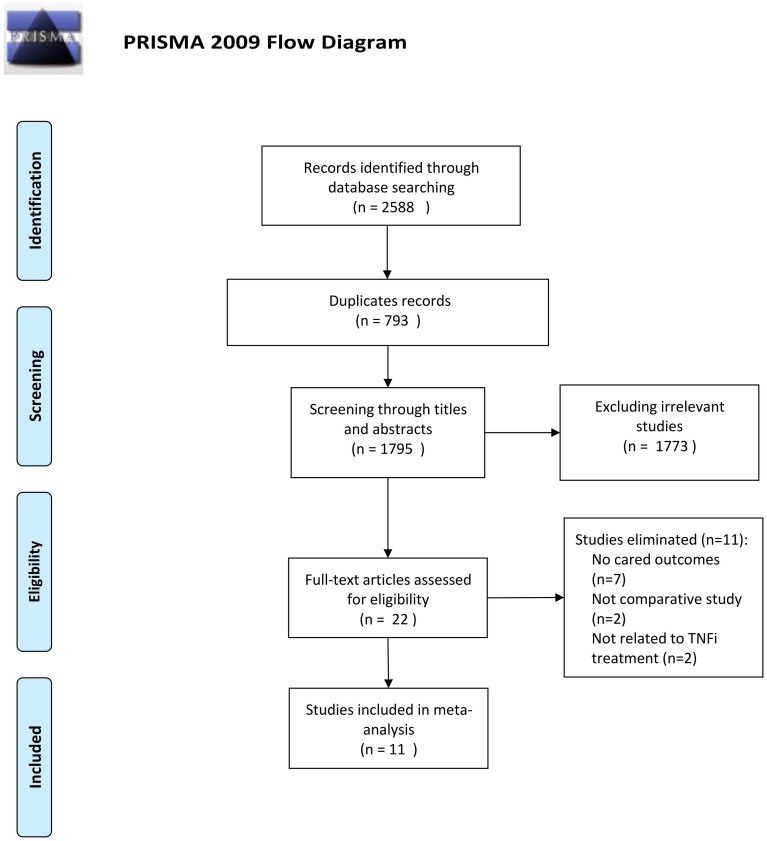
A total of 2588 studies were retrieved from the electronic databases of OVID Medline (n = 410), OVID Embase (n = 1911) and Cochrane library (n = 267). After excluding duplicate studies (n = 793), eliminating irrelevant studies by screening titles and abstracts (n = 1773), and deleting unmet selection criteria studies by reading full texts, 11 studies [32–42] with a total of 685 patients in TNFi group and 638 patients in control group were included (Fig 1). Manually checking the references of included studies did not find extra eligible studies.
Fig 1. Study selection process.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.137l/journal.prned1000097. For more information, visit www.prisma-statement.org.
Study characteristics of included studies
The study characteristics of included studies were summarized in Table 1. Of the 11 included studies, three studies were performed in Germany, two in China, two in Denmark, one in Canada, one in Hong Kong, one in United States and one in France; two studies reported data in AS patients, five studies focused on patients with axSpA, four on nr-axSpA patients; in the treatment group, five trials focused on adalimumab, two on golimumab, two on certolizumab pegol, one on etanercept, and one did not specify individual TNFi; in the control group, nine controls were placebo, one was pamidronate, and one was csDMARDs. Among the included studies, 10 studies clearly pointed out that patients were treated unsuccessfully with ≥ 1 NSAIDs before participation. Other type of biological therapy was not permitted before enrollment. Concomitant treatments, including NSAIDs, csDMARDs, and prednisone, were reported in ten studies. Follow-up duration ranged from 12 weeks to 104 weeks, median 48 weeks. In total, eleven studies reported data on SPARCC score at SIJ concerning the comparison between TNFi group and control group and five studies reported data of SPARCC score at spine. The Jadad scales of all included studies were higher than 3.
Table 1. Characteristics of included studies.
| Included studies | Country | Diagnosis | Concomitant treatment | Follow up duration (weeks) | TNFi group | Control group | Jadad score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number (male %) | Age (mean ± SD, years) | Disease duration (mean ± SD, years) | TNFi (Usage) | Baseline SPARCC (sacroiliac joint/spine) | Patient number (male %) | Age (mean ± SD, years) | Disease duration (mean ± SD, years) | Controls, (Usage) | Baseline SPARCC (sacroiliac joint/spine) | ||||||
| Lambert 2007 [32] | Canada | AS | SSZ, HCQ, MTX, Pred, NSAIDs | 52 | 38 (81.8%) | 40±10.9 | 12.1±8.7 | Adalimumab (40 mg every other week) | 5.7±9.0/16.0±15.6 | 44 (76.3%) | 41.9±11.1 | 14.5±9.0 | Placebo | 7.5±10.0/19.9±19.8 | 5 |
| Hu 2012 [35] | China | AS | SSZ, MTX, Pred, NSAIDs | 24 | 26 (92.3%) | 28.2±6.9 | 7.4±5.7 | Adalimumab (40 mg every other week) | 10.1±9.5/17.0±12.2 | 20 (100%) | 27.4±7.2 | 7.6±4.6 | Placebo | 9.0±9.1/19.7±12.7 | 5 |
| Sieper 2013 [33] | Germany | nr-axSpA | AZA, SSZ, HCQ, MTX, Pred, NSAIDs | 12 | 91 (48%) | 37.6±11.3 | 10.1±9.0 | Adalimumab (40 mg every other week) | 5.1±6.2/4.1±5.5 | 94 (43%) | 38.4±10.4 | 10.1±8.8 | Placebo | 4.7±5.9/4.6±6.0 | 5 |
| Sieper 2015 [34] | Germany | nr-axSpA | NSAIDs | 16 | 98 (62.2%) | 30.7±7.1 | NR | Golimumab (50mg every 4 weeks) | 9.9±11.82/NR | 100 (52%) | 31.7±7.2 | NR | Placebo | 12.7±15.62/NR | 5 |
| Mok 2015 [38] | Hong Kong | axSpA | NSAIDs | 48 | 20 (80%) | 32±10.7 | 4.2±3.3 | Golimumab (50mg every 4 weeks) | 15.8±17.7/11.4±10.8 | 10 (90%) | 36.3±11.4 | 5.0±3.7 | Pamidronate (60mg every 4 weeks) | 8.25±6.16/12.4±13.6 | 5 |
| Cui 2016 [41] | China | axSpA | NSAIDs | 52 | 18 (16.6%) | 23±NR | 0.67±NR | TNFi(NR) | 27.76±18.38/NR | 17 (17.6%) | 28±NR | 7.5±NR | DMARDs (NR) | 28.67±15.51/NR | 3 |
| Pedersen 2016 [36] | Denmark | axSpA | NSAIDs | 48 | 25 (77.8%) | 39.6±12.4 | 10.9±10.8 | Adalimumab (40 mg every other week) | 6.3±9.1/NR | 27 (76%) | 37.5±9.4 | 8.2±8.1 | Placebo | 10.6±12.2/NR | 5 |
| Braun 2017 [40] | Germany | axSpA | NSAIDs, DMARDs | 96 | 109 (62.3%) | 38.9±NR | NR | Certolizumab pegol (400 mg at weeks 0, 2 and 4 followed by either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks) | 6.9±10.4/NR | 54 (68.5%) | 38.9±NR | NR | Placebo | 12.9±16.6/NR | 4 |
| Dougados 2017 [39] | France | nr-axSpA | NR | 104 | 106 (62%) | 31.9±7.8 | 2.4±1.9 | Etanercept (50mg once weekly) | 8.0±9.7/4.7±7.1 | 109 (53%) | 32.0±7.8 | 2.5±1.8 | Placebo | 7.7±10.1/3.5±5.6 | 5 |
| Hededal 2017 [37] | Denmark | axSpA | NSAIDs | 12 | 27 (75%) | 40.8±12.6 | 11.5±7.2 | Adalimumab (40 mg every other week) | 4.8±10.4/NR | 27 (77.8%) | 38.3±9.5 | 10.2±9.1 | Placebo | 10.1±14.7/NR | 4 |
| Deohar 2019 [42] | United States | nr-axSpA | NSAIDs, DMARDs, corticosteroid | 52 | 159(49.1%) | 37.3±10.5 | 7.8±7.7 | Certolizumab pegol (400 mg at weeks 0, 2, and 4,followed by 200 mg every 2 weeks) | 7.79±10.82/NR | 158(48.1%) | 37.4±10.8 | 8.0±7.5 | Placebo | 8.46±12.31/NR | 5 |
Abbreviations: AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis; NSAIDs, non-steroidal anti-inflammatory drugs; DMARDs, disease-modifying antirheumatic drugs; Pred, prednisone; AZA, azathioprine; SSZ, sulfasalazine; HCQ, hydroxychloroquine; MTX, methotrexate; NR, not report; SD, standard deviation; TNFi,tumor necrosis factor α inhibitor; SPARCC, Spondyloarthritis Research Consortium Canada score.
Treatment outcomes
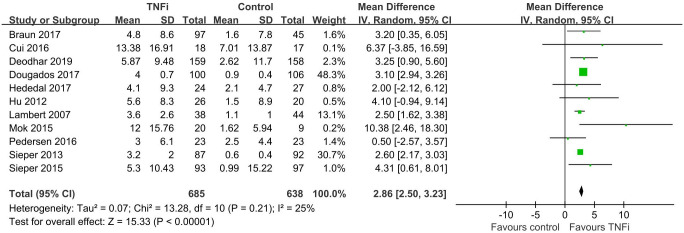
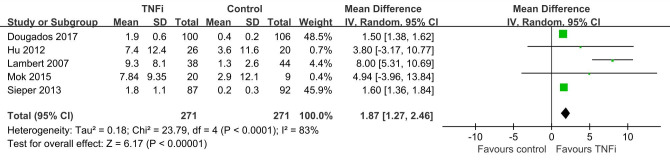
In total, eleven studies [32–42] enrolled 1323 patients investigated SPARCC score of SIJ which reported TNFi were better to reduce MRI inflammation than controls (n = 11, MD = 2.86, 95% CI 2.50, 3.23) (Fig 2). Five studies [32, 33, 35, 38, 39] involving 542 patients suggested that SPARCC spine score achieved a better reduction in TNFi group (n = 5, MD = 1.87,95%CI 1.27, 2.46) (Fig 3). Similar to the SPARCC score of SIJ and spine, treatment effects of TNFi assessed by ASDAS, BASDAI, BASFI and CRP were more effective than controls (n = 7, MD = 0.96, 95% CI 0.71, 1.20; n = 7, MD = 1.05, 95% CI 0.61, 1.49; n = 6, MD = 0.88, 95% CI 0.56, 1.19; n = 7, MD = 3.87, 95% CI 1.09, 6.65) (Table 2).
Fig 2. Forest plot of SPARCC SIJ score based on TNFi treatment versus controls.
Fig 3. Funnel plot of SPARCC spine score on the basis of TNFi versus controls.
Table 2. Pooled data of clinical indicators.
| Outcomes (TNFi vs. Control) | Study number | Total patient number | Heterogeneity | MD (95%CI) | P value | ||
|---|---|---|---|---|---|---|---|
| TNFi | Control | I2 | P | ||||
| ASDAS | 7 | 367 | 364 | 82% | P<0.00001 | 0.96[0.71, 1.20] | P<0.00001* |
| BASDAI | 7 | 508 | 505 | 77% | 0.0002 | 1.05[0.61, 1.49] | P<0.00001* |
| BASFI | 6 | 485 | 482 | 52% | 0.06 | 0.88[0.56, 1.19] | P<0.00001* |
| CRP | 7 | 503 | 499 | 66% | 0.007 | 3.87[1.09, 6.65] | P = 0.006* |
Abbreviations: TNFi, tumor necrosis factor α inhibitor; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis disease activity index; BASFI, Bath Ankylosing Spondylitis functional index; CRP, C-reactive protein; MD, mean difference; CI, confidence interval;
*p<0.05.
Subgroup analysis by diagnostic category
Subgroup analysis by diagnostic category was performed to test whether the impact of TNFi on MRI inflammation was consistent in nr-axSpA and AS. Data reported by more than two studies were pooled (Table 3). As compared to controls, TNFi were effectively to improve MRI inflammation regardless of disease subgroup assessed by SPARCC SIJ score (nr-axSpA: n = 4, MD = 2.94, 95% CI 2.56, 3.31; AS: n = 2, MD = 2.55, 95% CI 1.68, 3.41), SPARCC spine score (nr-axSpA: n = 2, MD = 1.52, 95% CI 1.41,1.63; AS: n = 2, MD = 7.18, 95% CI 3.92, 10.44). In terms of clinical indicators, TNFi were more effective in nr-axSpA to ameliorate ASDAS (n = 3, MD = 0.87, 95% CI 0.58, 1.16), BASDAI (n = 4, MD = 0.85, 95% CI 0.37, 1.33), BASFI (n = 4, MD = 0.90, 95% CI 0.51, 1.28) and CRP (n = 4, MD = 2.94, 95% CI 0.21, 5.67) in comparisons with controls.
Table 3. Pooled data of subgroup analysis by diagnostic category.
| Outcomes (TNFi vs. Control) | Study number | Total patient number | Heterogeneity | MD (95%CI) | P value | ||
|---|---|---|---|---|---|---|---|
| TNFi | Control | I2 | P | ||||
| SPARCC SIJ score | |||||||
| nr-axSpA | 4 | 439 | 453 | 41% | 0.16 | 2.94[2.56, 3.31] | P<0.00001* |
| AS | 2 | 64 | 64 | 0% | 0.54 | 2.55[1.68, 3.41] | P<0.00001* |
| SPARCC spine score | |||||||
| nr-axSpA | 2 | 187 | 198 | 0% | 0.47 | 1.52[1.41,1.63] | P<0.00001* |
| AS | 2 | 64 | 64 | 18% | 0.27 | 7.18[3.92,10.44] | P<0.0001* |
| ASDAS | |||||||
| nr-axSpA | 3 | 280 | 295 | 91% | P<0.0001 | 0.87[0.58,1.16] | P<0.00001* |
| BASDAI | |||||||
| nr-axSpA | 4 | 439 | 453 | 84% | 0.0003 | 0.85[0.37,1.33] | P = 0.0005* |
| BASFI | |||||||
| nr-axSpA | 4 | 439 | 453 | 71% | 0.01 | 0.90[0.51, 1.28] | P<0.00001* |
| CRP | |||||||
| nr-axSpA | 4 | 439 | 453 | 73% | 0.01 | 2.94[0.21,5.67] | P = 0.03* |
Abbreviations: SPARCC, Spondyloarthritis Research Consortium Canada score; SIJ, sacroiliac joints; TNFi, tumor necrosis factor α inhibitor; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis disease activity index; BASFI, Bath Ankylosing Spondylitis functional index; CRP, C-reactive protein; AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis; MD, mean difference; CI, confidence interval;
*p<0.05.
Subgroup analysis by TNFi types
Subgroup analysis by TNFi types was conducted to assess whether different TNFi affected the treatment outcomes (Table 4). As compared to placebo, adalimumab (ADA) was more potently to reduce SPARCC SIJ score (n = 5, MD = 2.55, 95% CI 2.17, 2.93), ASDAS (n = 3, MD = 1.08, 95% CI 0.63, 1.53), BASFI (n = 2, MD = 0.58, 95% CI 0.09, 1.07), and CRP (n = 2, MD = 3.98, 95% CI 1.04, 6.91), but there were no differences in SPARCC spine score (n = 3, MD = 4.44, 95% CI -0.63, 9.51) and BASDAI (n = 3, MD = 0.85, 95% CI -0.32, 2.02). Similarly, Certolizumab pegol (CZP) was more potently to reduce SPARCC SIJ score (n = 2, MD = 3.23, 95% CI 1.42, 5.04). As compared to control, golimumab (GLM) was more effective to reduce BASFI (n = 2, MD = 1.75, 95% CI 0.93, 2.56) and CRP (n = 2, MD = 8.86, 95% CI 3.19, 14.54).
Table 4. Pooled data of subgroup analysis by TNFi types.
| Outcomes (ADA/GLM/CZP vs. Control) | Study number | Total patient number | Heterogeneity | MD (95%CI) | P value | ||
|---|---|---|---|---|---|---|---|
| TNFi | Control | I2 | P | ||||
| SPARCC SIJ score | |||||||
| ADA vs. Placebo | 5 | 198 | 206 | 0% | 0.7 | 2.55[2.17, 2.93] | P<0.00001* |
| CZP vs. Placebo | 2 | 256 | 203 | 0% | 0.98 | 3.23[1.42,5.04] | P = 0.0005* |
| SPARCC spine score | |||||||
| ADA vs. Placebo | 3 | 151 | 156 | 91% | P<0.0001 | 4.44[-0.63, 9.51] | P = 0.09 |
| ASDAS | |||||||
| ADA vs. Placebo | 3 | 136 | 135 | 54% | 0.11 | 1.08[0.63,1.53] | P<0.00001* |
| BASDAI | |||||||
| ADA vs. Placebo | 3 | 136 | 135 | 74% | 0.02 | 0.85[-0.32,2.02] | P = 0.16 |
| BASFI | |||||||
| ADA vs. Placebo | 2 | 113 | 112 | 0% | 0.53 | 0.58[0.09,1.07] | P = 0.02* |
| GLM vs. Control | 2 | 113 | 106 | 11% | 0.29 | 1.75[0.93, 2.56] | P<0.0001* |
| CRP | |||||||
| ADA vs. Placebo | 2 | 113 | 112 | 0% | 0.95 | 3.98[1.04,6.91] | P = 0.008** |
| GLM vs. Control | 2 | 113 | 106 | 0% | 0.87 | 8.86[3.19,14.54] | P = 0.002* |
Abbreviations: SPARCC, Spondyloarthritis Research Consortium Canada score; SIJ, sacroiliac joints; TNFi, tumor necrosis factor α inhibitor; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis disease activity index; BASFI, Bath Ankylosing Spondylitis functional index; CRP, C-reactive protein; AS, ankylosing spondylitis; axSpA, axial spondyloarthritis; nr-axSpA, non-radiographic axial spondyloarthritis; ADA, Adalimumab; GLM, Golimumab; Certolizumab pegol (CZP); MD, mean difference; CI, confidence interval;
*p<0.05.
Sensitivity analysis
Sensitivity analysis of treatment outcomes was performed by deleting those studies that controls were not placebo, resulting in comparing TNFi versus placebo and results showed the treatment effect of TNFi versus placebo were consistent with TNFi versus controls, suggesting the results of the study were robust (S1 Table).
Publication bias
We assessed the public bias using Egger’s test, suggesting there had no possibility for potential publication bias in the analysis of SPARCC SIJ score(P = 0.320) and spine score(P = 0.249).
Discussion
This meta-analysis is the first study to evaluate the impact of TNFi on MRI inflammation in axSpA patients and shows TNFi are capable of relieving MRI inflammation in SIJ and spine, as well as ASDAS, BASDAI, BASFI, and CRP. Inflammatory changes are objective signs of axSpA. Inflammation in the SIJ (sacroiliitis) and in spine (spondylitis) can be visualized as BME [20]. If the inflammation is not controlled, structural changes such as fat metaplasia, erosions, sclerosis, or ankylosis in SIJ and vertebral edge would appear in successive years, which is so called radiographic progression. Radiographic progression takes place in 20–45% of AS patients and 7% of nr-axSpA within 2 years [43, 44]. The role of TNFi in slowing radiographic progression is still unclear. A meta-analysis reported no significant effect of TNFi on delaying spinal radiographic progression in AS [45]. More recently, another meta-analysis showed that TNFi might slow radiographic progression at spine in AS patients with treatment time for more than 4 years but not for shorter time like 2 years [46]. Nevertheless, both the studies were concentrated on the change of modified Stoke AS Spine Score (mSASSS) for axial damage detected by conventional radiography.
Controlling symptoms and inflammation, preventing progressive structural damage, preserving joint function are the treatment goals of axSpA [5] and early detection of inflammatory is the key link of diagnosis and treatment. MRI, not only directly shows the edema signal of inflammation site, but also displays the bone erosion and osteosclerosis of joint surface, can sensitively find the edema of the bone marrow and the surrounding soft tissue of SIJ and spine [20, 47], and is capable of making a diagnosis of inflammation before the obvious morphological change in X-ray and CT [48]. Moreover, previous studies indicated that lesions identified by MRI had a predictive capacity for the development of sacroiliitis [49, 50]. In addition, MRI inflammation score could be a predictor for disease remission. A study by Sieper et al. revealed that higher SPARCC SIJ score and lower SPARCC spine score at baseline would predict ASDAS inactive disease at week 12 in nr-axSpA [51].
Application of TNFi in axSpA is recommended in the patients who failed to reach disease relief after NSAIDs treatment or had high CRP level [5, 6]. In our study, adalimumab is the most used drugs in the reviewed researches, followed by golimumab and Certolizumab pegol in axSpA patients. It is reported male are more common than female in patients with established AS while female are dominant in nr-axSpA and patients with established AS are tended to have increased CRP level, MRI inflammation, and more structure changes than patients with nr-axSpA [52]. Our results suggest TNFi are effective to treat axSpA regardless of the disease subgroups assessed by SPARCC score. Regrettably, treatment effect of TNFi in disease subgroup could not relate to gender owing to the limited data report. The novel biologic agents targeting interleukin (IL)-17 such as secukinumab and ixekizumab were superior to placebo with respect to improving objective inflammation assessed by MRI [53] and long-term IL-17 inhibitor exposure might slow down radiographic progression in axSpA patients [54]. Another treatment option is tofacitinib, an oral Janus kinase (JAK) inhibitor, achieving meaningful reductions in SIJ and spinal MRI inflammation in AS patients [55, 56]. But the effectiveness of tofacitinib on delaying radiographic progression is limited.
Minimally important change (MIC) of imaging score can be helpful to reflect treatment responses and understand the number of patients showing significant changes. Maksymowych et al. defined the MIC of SPARCC score as ≥2.5-point change for SIJ score, and≥5-point change for spine score [57]. The MIC of spine score indicates a change of 5 quadrants in DVUs with BME. Similarly, the MIC of SIJ score indicates a change of at least 2–3 quadrants with BME in sacroiliac joint. Among the enrolled 10 studies, changes of SPARCC SIJ score in TNFi group all exceeded the MIC, and three of five studies reported that the changes of SPARCC spine score were greater than MIC. In trials of nr-axSpA, the most serious inflammation located in the SIJ but almost no inflammation in the spine. Conversely, in trials of AS, inflammation mostly located in the spine [3]. As shown in our study, after TNFi treatment, the improvement of SPARCC SIJ score in nr-axSpA was greater than in AS. In contrast, the changes of SPARCC spine score had a better reduction in AS patients.
Several inflammation scoring methods based on MRI including Berlin, Aarhus, Leeds, and SPARCC have been reported. The Berlin method grades inflammation lesions according to the percent involvement of the bone marrow: 0: no high signal on quadrant area, 1: <33% of quadrant area, 2: high signal is ≥33% and <66% of the quadrant area, and 3: ≥66% of the quadrant area [58]. Similarly, both Aarhus and Leeds are based on semi-qualitative grading methods [59, 60]. SPARCC score is a quantitative method on sacroiliitis and spondylitis with excellent intraobserver and good interobserver reproducibility [25, 36]. Besides, SPARCC scoring system is reliable for measurement of enthesitis [61]. Afterwards the same research team developed and validated the SPARCC structure score for the assessment of structural lesions on MRI in the SIJ of patients with SpA [62], also with feasibility and reliability for pediatric SIJ MRI evaluation [63]. Newer measures, such as quantitative low-dose CT may provide higher sensitivity to find small changes in syndesmophyte size [64], and has a good correlation with Schober test in AS [65].
Five of 11 included studies had open-label period and data from open-label phase were not been analyzed in our study. It is often unclear what type of data is used for these trials (e.g. observed case or imputed data). Moreover, there is often considerable missing data at the point when the follow up MRI is typically performed. In addition, the control group will have received active treatment with TNFi in the open label period, the absolute changes in inflammation became evident in control group. So, this should be confused to compare the treatment effect in both groups in open-label phase. Thus, it is sufficient to show the data from analyses of the double-blind phase.
Heterogeneity was found among the studies in several indicators, which might originate from the following aspects. It is unclear whether the SPARCC method was correctly used in the different studies. As reported by the studies, Hu et al. [35] only calculated the SPARCC spine score from the lower T12 to upper S1 level instead of standardized method in which scoring the most serious involved 6 DVUs in the entire spine [26]. Additionally, we only searched studies in English, and some relevant studies in non-English might not be included. Small numbers of studies included in our analysis would limit the statistic power. Furthermore, the included studies had different inclusion and exclusion criteria, causing the heterogeneity in the efficacy of TNFi between studies. At last, our study was unable to distinguish whether the dose in TNFi group were responsible for treatment effects.
Conclusion
TNFi are effective to improve MRI inflammation of SIJ and spine in axSpA patients and treatment effectiveness is consistent among different diagnostic category and TNFi types. SPARCC score is a reliable way to reflect the treatment impact of TNFi on MRI-detected inflammation. Nevertheless, more studies are needed to warrant the results owing to small study number.
Supporting information
(DOC)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009; 68(6):777–783. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 2.Sieper J. New treatment targets for axial spondyloarthritis. Rheumatology (Oxford). 2016; 55 (suppl 2):ii38–ii42. 10.1093/rheumatology/kew349 [DOI] [PubMed] [Google Scholar]
- 3.Braun J. Axial spondyloarthritis including ankylosing spondylitis. Rheumatology (Oxford). 2018; 57(suppl 6):vi1–vi3. 10.1093/rheumatology/key079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011; 377(9783): 2127–2137. 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- 5.van der Heijde D, Ramiro S, Landewé R, Baraliakos X, van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017; 76(6):978–991. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 6.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology / Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (Hoboken). 2019; 71(10):1285–1299. 10.1002/acr.24025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanders A, van der Heijde D, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Non-steroidal anti-inflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005; 52(6):1756–1765. 10.1002/art.21054 [DOI] [PubMed] [Google Scholar]
- 8.Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012; 71(10):1623–1629. 10.1136/annrheumdis-2012-201370 [DOI] [PubMed] [Google Scholar]
- 9.Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012; 71(10):1616–1622. 10.1136/annrheumdis-2011-201252 [DOI] [PubMed] [Google Scholar]
- 10.Sieper J, Listing J, Poddubnyy D, Song IH, Hermann KG, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016; 75(8):1438–1443. 10.1136/annrheumdis-2015-207897 [DOI] [PubMed] [Google Scholar]
- 11.Haibel H, Fendler C, Listing J, Callhoff J, Braun J, Sieper J. Effificacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomised, placebo-controlled short-term trial. Ann Rheum Dis. 2014; 73(1):243–246. 10.1136/annrheumdis-2012-203055 [DOI] [PubMed] [Google Scholar]
- 12.Dougados M. Treatment of spondyloarthropathies. Recent advances and prospects in 2001. Joint Bone Spine. 2001; 68(6):557–563. 10.1016/s1297-319x(01)00328-1 [DOI] [PubMed] [Google Scholar]
- 13.Braun J, Zochling J, Baraliakos X, Alten R, Burmester G, Grasedyck K, et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicenter randomized controlled trial. Ann Rheum Dis. 2006; 65(9):1147–1153. 10.1136/ard.2006.052878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun J, Sieper J. Treatment of rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009; 27 (4 suppl 55): S146–147. [PubMed] [Google Scholar]
- 15.Haibel H, Specker C. Disease-modifying anti-rheumatic drugs in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009; 27 (4 suppl 55): S159–163. [PubMed] [Google Scholar]
- 16.Sepriano A, Regel A, van der Heijde D, Braun J, Baraliakos X, Landewé R, et al. Efficacy and safety of biological and targeted-synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open. 2017; 3(1): e000396 10.1136/rmdopen-2016-000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Heijde D, Sieper J, Maksymowych W, Dougados M, Burgos-Vargas R, Landewé R, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011; 70(6):905–908. 10.1136/ard.2011.151563 [DOI] [PubMed] [Google Scholar]
- 18.Baraliakos X, Listing J, Fritz C, Haibel H, Alten R, Burmester GR, et al. Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years–early clinical response predicts long-term outcome. Rheumatology (Oxford). 2011; 50(9):1690–1699. 10.1093/rheumatology/ker194 [DOI] [PubMed] [Google Scholar]
- 19.Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010; 11:180–210. 10.1159/000289205 [DOI] [PubMed] [Google Scholar]
- 20.Baraliakos X, Braun J. Imaging scoring Methods in axial spondyloarthritis. Rheum Dis Clin North Am. 2016; 42(4):663–678. 10.1016/j.rdc.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 21.Bollow M, Biedermann T, Kannenberg J, Paris S, Schauer-Petrowski C, Minden K, et al. Use of dynamic magnetic resonance imaging to detect sacroiliitis in HLA-B27 positive and negative children with juvenile arthritides. J Rheumatol. 1998; 25(3):556–564. [PubMed] [Google Scholar]
- 22.Weber U, Kissling RO, Hodler J. Advances in musculoskeletal imaging and their clinical utility in the early diagnosis of spondyloarthritis. Curr Rheum Rep. 2007; 9(5):353–360. 10.1007/s11926-007-0057-3 [DOI] [PubMed] [Google Scholar]
- 23.van der Heijde D, Landewé R. Selection of a method for scoring radiographs for ankylosing spondylitis clinical trials, by the Assessment in Ankylosing Spondylitis Working Group and OMERACT. J Rheumatol. 2005;32(10):2048–2049. [PubMed] [Google Scholar]
- 24.Landewé R, Hermann KG, van der Heijde D, Baraliakos X, Jurik AG, Lambert R, et al. Scoring sacroiliac joints by magnetic resonance imaging. A multiple-reader reliability experiment. J Rheumatol. 2005; 32(10):2050–2055. [PubMed] [Google Scholar]
- 25.Maksymowych W, Inman R, Salonen D, Dhillon S, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005; 53(5):703–709. 10.1002/art.21445 [DOI] [PubMed] [Google Scholar]
- 26.Maksymowych W, Inman R, Salonen D, Dhillon S, Krishnananthan R, Stone M, et al. Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2005; 53(4):502–509. 10.1002/art.21337 [DOI] [PubMed] [Google Scholar]
- 27.Callhoff J, Sieper J, Weiß A, Zink A, Listing J. Efficacy of TNF α blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 2015; 74(6):1241–1248. 10.1136/annrheumdis-2014-205322 [DOI] [PubMed] [Google Scholar]
- 28.Machado M, Barbosa M, Almeida A, de Araújo V, Kakehasi A, Andrade E, et al. Treatment of ankylosing spondylitis with TNF blockers: a meta-analysis. Rheumatol Int. 2013; 33(9):2199–2213. 10.1007/s00296-013-2772-6 [DOI] [PubMed] [Google Scholar]
- 29.Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of assessment of spondyloArthritis international society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. 2009; 68(6):777–783. [DOI] [PubMed] [Google Scholar]
- 30.van der Linden S, Valkenburg H, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27(4):361–368. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 31.Jadad A, Moore R, Carroll D, Jenkinson C, Reynolds D, Gavaghan D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17(1):1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 32.Lambert R, Salonen D, Rahman P, Inman R, Wong R, Einstein S, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis:a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2007; 56(12):4005–4014. 10.1002/art.23044 [DOI] [PubMed] [Google Scholar]
- 33.Sieper J, van der Heijde D, Dougados M, Mease P, Maksymowych W, Brown M, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomized placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013; 72(6):815–822. 10.1136/annrheumdis-2012-201766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieper J, van der Heijde D, Dougados M, Maksymowych W, Scott B, Boice J, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheum. 2015; 67(10):2702–2712. 10.1002/art.39257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu ZY, Xu ML, Li QX, Lin ZM, Liao ZT, Gao SY, et al. Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int J Rheum Dis. 2012; 15(4):358–365. 10.1111/j.1756-185X.2012.01734.x [DOI] [PubMed] [Google Scholar]
- 36.Pedersen S, Poddubnyy D, Sørensen I, Loft A, Hindrup J, Thamsborg G, et al. Course of magnetic resonance imaging-detected inflammation and structural lesions in the sacroiliac joints of patients in the randomized, double-blind, placebo-controlled danish multicenter study of adalimumab in spondyloarthritis, as Assessed by the Berlin and Spondyloarthritis Research Consortium of Canada Methods. Arthritis Rheumatol. 2016; 68(2):418–429. 10.1002/art.39434 [DOI] [PubMed] [Google Scholar]
- 37.Hededal P, Østergaard M, Sørensen I, Loft A, Hindrup J, Thamsborg G, et al. Development and validation of MRI sacroiliac joint scoring methods for the semiaxial scan plane corresponding to the Berlin and SPARCC MRI scoring methods, and of a new global MRI sacroiliac joint method. J Rheumatol. 2017; 45(1):70–77. 10.3899/jrheum.161583 [DOI] [PubMed] [Google Scholar]
- 38.Mok CC, Li OC, Chan KL, Ho LY, Hui PK. Effect of golimumab and pamidronate on clinical efficacy and MRI inflammation in axial spondyloarthritis: a 48-week open randomized trial. Scand J Rheumatol. 2015; 44(6):480–486. 10.3109/03009742.2015.1038300 [DOI] [PubMed] [Google Scholar]
- 39.Dougados M, van der Heijde D, Sieper J, Braun J, Citera G, Lenaerts J, et al. Effects of long-term etanercept treatment on clinical outcomes and objective signs of inflammation in early non-Radiographic axial spondyloarthritis: 104-week results from the EMBARK study. Arthritis Care Res (Hoboken). 2017; 69(10):1590–1598. 10.1002/acr.23276 [DOI] [PubMed] [Google Scholar]
- 40.Braun J, Baraliakos X, Hermann KG, Landewé R, Machado P, Maksymowych W, et al. Effect of certolizumab pegol over 96 weeks of treatment on inflammation of the spine and sacroiliac joints, as measured by MRI, and the association between clinical and MRI outcomes in patients with axial spondyloarthritis. RMD Open. 2017; 3(1): e000430 10.1136/rmdopen-2017-000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Y, Zheng JP, Zhang X, Zeng H, Luo RQ. Evaluation of treatments for sacroiliitis in spondyloarthropathy using the Spondyloarthritis Research Consortium Canada scoring system. Arthritis Res Ther. 2016; 18:38 10.1186/s13075-016-0916-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deodhar A, Gensler L, Kay J, et al. A Fifty-Two-Week, Randomized, Placebo-Controlled Trial of Certolizumab Pegol in Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol, 2019,71(7):1101–1111. 10.1002/art.40866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baraliakos X, Listing J, von der Recke A, Braun J. The natural course of radiographic progression in ankylosing spondylitis—evidence for major individual variations in a large proportion of patients. J Rheumatol. 2009; 36(5):997–1002. 10.3899/jrheum.080871 [DOI] [PubMed] [Google Scholar]
- 44.Poddubnyy D, Haibel H, Listing J, Märker-Hermann E, Zeidler H, Braun J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 2012; 64(5):1388–1398. 10.1002/art.33465 [DOI] [PubMed] [Google Scholar]
- 45.Zong HX, Xu SQ, Tong H, Wang XR, Pan MJ, Teng YZ, et al. Effect of anti-tumor necrosis factor α treatment on radiographic progression in patient with ankylosing spondylitis: a systematic review and meta-analysis. Mod Rheumatol. 2019; 29(3):503–509. 10.1080/14397595.2018.1525017 [DOI] [PubMed] [Google Scholar]
- 46.Karmacharya P, Duarte-Garcia A, Dubreuil M, Murad MH, Shahukhal R, Shrestha P, et al. The effect of therapy on radiographic progression in axial spondyloarthritis: a systematic review and meta-analysis. Arthritis Rheumatol. 2020; 75(5):733–749. 10.1002/art.41206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puhakka K, Jurik AG, Schiottz-Christensen B, Hansen G, Egund N, Christiansen J, et al. Magnetic resonance imaging of sacroiliitis in early seronegative spondylarthropathy. Abnormalities correlated to clinical and laboratory findings. Rheumatology (Oxford). 2004; 43(2):234–237. 10.1093/rheumatology/keh008 [DOI] [PubMed] [Google Scholar]
- 48.Grigoryan M, Roemer F, Mohr A, Genant H. Imaging in spondyloarthropathies. Curr Rheumatol Rep. 2004; 6(2):102–109. 10.1007/s11926-004-0054-8 [DOI] [PubMed] [Google Scholar]
- 49.Blum U, Buitrago-Tellez C, Mundinger A, Krause T, Laubenberger J, Vaith P, et al. Magnetic resonance imaging (MRI) for detection of active sacroiliitis: a prospective study comparing conventional radiography, scintigraphy, and contrast enhanced MRI. J Rheumatol. 1996; 23(12):2107–2115. [PubMed] [Google Scholar]
- 50.Oostveen J, Prevo R, den Boer J, van de Laar M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography: a prospective, longitudinal study. J Rheumatol. 1999; 26(9):1953–1958. [PubMed] [Google Scholar]
- 51.Sieper J, Landewé R, Magrey M, Anderson JK, Zhong S, Wang X, et al. Predictors of remission in patients with non-radiographic axial spondyloarthritis receiving open-label adalimumab in the ABILITY-3 study. RMD Open. 2019; 5(1): e000917 10.1136/rmdopen-2019-000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiltz U, Heldmann F, Baraliakos X, Braun J. Treatment of ankylosing spondylitis in patients refractory to TNF-inhibition: are there alternatives? Curr Opin Rheumatol. 2012; 24(3):252–260. 10.1097/BOR.0b013e3283524b82 [DOI] [PubMed] [Google Scholar]
- 53.Dougados M, Wei JC, Landewé R, Sieper J, Baraliakos X, Van den Bosch F, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis. 2020; 79(2):176–185. 10.1136/annrheumdis-2019-216118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashany D, Stein E, Goto R, Goodman S. The effect of TNF inhibition on bone density and fracture risk and of IL17 inhibition on radiographic progression and bone density in patients with axial spondyloarthritis: a systematic literature review. Curr Rheumatol Rep. 2019; 21(5): 20 10.1007/s11926-019-0818-9 [DOI] [PubMed] [Google Scholar]
- 55.van der Heijde D, Deodhar A, Wei JC, Drescher E, Fleishaker D, Hendrikx T, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis, 2017;76(8):1340–1347. 10.1136/annrheumdis-2016-210322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maksymowych W, van der Heijde D, Baraliakos X, Deodhar A, Sherlock S, Li D, et al. Tofacitinib is associated with attainment of the minimally important reduction in axial magnetic resonance imaging inflammation in ankylosing spondylitis patients. Rheumatology. 2018; 57(8):1390–1399. 10.1093/rheumatology/key104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maksymowych W, Lambert R, Brown L, Pangan A. Defining the minimally important change for the SpondyloArthritis Research Consortium of Canada spine and sacroiliac joint magnetic resonance imaging indices for ankylosing spondylitis. J Rheumatol. 2012; 39(8): 1666–1674. 10.3899/jrheum.120131 [DOI] [PubMed] [Google Scholar]
- 58.Song IH, Hermann KG, Haibel H, Althoff C, Poddubnyy D, Listing J, et al. Relationship between active inflammatory lesions in the spine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week 48. Ann Rheum Dis. 2011; 70(7):1257–1263. 10.1136/ard.2010.147033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madsen K, Jurik AG. Magnetic resonance imaging grading system for active and chronic spondylarthritis changes in the sacroiliac joint. Arthritis Care Res (Hoboken). 2010; 62(1):11–18. 10.1002/acr.20008 [DOI] [PubMed] [Google Scholar]
- 60.van der Heijde D, Landewé R, Hermann KG, Jurik AG, Maksymowych W, Rudwaleit M, et al. Application of the OMERACT filter to scoring methods for magnetic resonance imaging of the sacroiliac joints and the spine. Recommendations for a research agenda at OMERACT. J Rheumatol. 2005; 32(10):2042–2047. [PubMed] [Google Scholar]
- 61.Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski W, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index. Ann Rheum Dis. 2009; 68(6):948–953. 10.1136/ard.2007.084244 [DOI] [PubMed] [Google Scholar]
- 62.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert R, Pedersen S, et al. Development and preliminary validation of the Spondyloarthritis Research Consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol. 2015; 42(1):79–86. 10.3899/jrheum.140519 [DOI] [PubMed] [Google Scholar]
- 63.Weiss P, Maksymowych W, Lambert R, Jaremko J, Biko D, Paschke D, et al. Feasibility and reliability of the Spondyloarthritis Research Consortium of Canada sacroiliac joint structural score in children. J Rheumatol. 2018; 45(10):1411–1417. 10.3899/jrheum.171329 [DOI] [PubMed] [Google Scholar]
- 64.de Koning A, de Bruin F, van den Berg R, Ramiro S, Baraliakos X, Braun J, et al. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis. 2018; 77(2):293–299. 10.1136/annrheumdis-2017-211989 [DOI] [PubMed] [Google Scholar]
- 65.Tan S, Yao JH, Flynn J, Yao L, Ward M. Quantitative measurement of syndesmophyte volume and height in ankylosing spondylitis using CT. Ann Rheum Dis. 2014;73(3):544–550. 10.1136/annrheumdis-2012-202661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.