Abstract
Background
The area under the curve- (AUC-) guided vancomycin dosing is the best strategy for individualized therapy in critical illnesses. Since AUC can be calculated directly using drug clearance (CLvan), any parameter estimating CLvan will be able to achieve the goal of 24-hour AUC (AUC24 h). The present study was aimed to determine CLvan based on 6-hour urine creatinine clearance measurement in critically ill patients with normal renal function.
Method
23 adult critically ill patients with an estimated glomerular filtration rate (eGFR) ≥60 mL/min who received vancomycin infusion were enrolled in this pilot study. Vancomycin pharmacokinetic parameters were determined for each patient using serum concentration data and a one-compartment model provided by MONOLIX software using stochastic approximation expectation-maximization (SAEM) algorithm. Correlation of CLvan with the measured creatinine clearance in 6-hour urine collection (CL6 h) and estimated creatinine clearance by the Cockcroft–Gault formula (CLCG) was investigated.
Results
Data analysis revealed that CL6 h had a stronger correlation with CLvan rather than CLCG (r = 0.823 vs. 0.594; p < 0.001 vs. 0.003). The relationship between CLvan and CL6 h was utilized to develop the following equation for estimating CLvan: CLvan (mL/min) = ─137.4 + CL6 h (mL/min) + 2.5 IBW (kg) (R2 = 0.826, p < 0.001). Regarding the described model, the following equation can be used to calculate the empirical dose of vancomycin for achieving the therapeutic goals in critically ill patients without renal impairment: total daily dose of vancomycin (mg) = (─137.4CL6-h (mL/min) + 2.5 IBW (kg)) × 0.06 AUC24 h (mg.hr/L).
Conclusion
For AUC estimation, CLvan can be obtained by collecting urine in a 6-hour period with good approximation in critically ill patients with normal renal function.
1. Introduction
Vancomycin is still used as the standard treatment for suspected methicillin-resistant Staphylococcus aureus (MRSA) infections in intensive care units (ICUs). This feature has dramatically increased the utilization of vancomycin despite the introduction of some alternative agents [1,2]. Because of the narrow therapeutic range of vancomycin, therapeutic drug monitoring (TDM) is an important issue for achieving optimal levels of this antibiotic particularly among patients with critically ill conditions [3].
According to the vancomycin dosing guidelines, the area under the curve (AUC) is the best predictor for drug dosing [4]. Evidence shows the supratherapeutic threshold of vancomycin in trough levels between 15 and 20 mg/L as recommended before, and dose adjustment based on trough level alone is not an accurate estimation of 24-hour AUC (AUC24 h) [5–7]. AUC-guided dosing is associated with a lower risk of nephrotoxicity due to reducing the irrational overuse of vancomycin [8, 9]. Based on this, converting from the trough level to AUC has occurred for the vancomycin's therapeutic goals, and the AUC24 h target of 400−600 mg·hr/L is recommended regardless of minimum inhibitory concentration (MIC) or treated organism [10, 11].
Since vancomycin AUC is estimated by drug clearance, if there is a way to predict the clearance, the AUC24 h will be easily calculated in the patients. The main route for clearance of vancomycin in the body is almost exclusively through the kidneys. Nearly most of the vancomycin is recovered unchanged in urine through glomerular filtration [12, 13]. If the patient's kidney condition is accurately estimated, then a better decision will be made on the dose of vancomycin; furthermore, accumulation of vancomycin can cause serious side effects, including nephrotoxicity and ototoxicity [14]. The best marker for determining renal function is the glomerular filtration rate (GFR) [15]. There are several methods for measuring GFR, among which creatinine clearance (CrCL) is used based on urine collection [16, 17]. Previous studies reported some positive aspects regarding using creatinine measurement such as easy measurement, as well as not being invasive and expensive [18]. Also, it is endogenous and does not need to be injected. Accordingly, CrCL is used as the clinical surrogate for GFR.
Currently, the guidance on the vancomycin dosing is based on the CrCL obtained from the Cockcroft–Gault (CG) equation. Since the ICU patients have unstable conditions, application of CG equation to adjust drug dosing may lead to ending up with subtherapeutic and supratherapeutic trough concentrations [19]. This equation does not consider increasing the cardiac output and renal flow rate due to hemodynamic changes and medications used in the ICU, thus underestimating CrCL or overestimating the degree of acute kidney injury (AKI) in critically ill patients [20]. As a result, assessment of renal function presents a unique challenge in critical illnesses, and it should be noted that none of the existing methods are capable of truly predicting the clearance of vancomycin (CLvan). Therefore, there is a need to introduce new methods or optimize existing ones. Hence, the current study was aimed to determine CLvan for AUC estimation based on 6-hour urine creatinine clearance (CL6 h) measurement in critically ill patients with normal renal function.
2. Materials and Methods
2.1. Study Settings
This prospective pilot study was approved by the Institutional Ethics Committee of Tehran University of Medical Sciences (TUMS), Iran.
2.2. Patient Selection
23 critically ill patients aged older than 18 years with a normal renal function (estimated glomerular filtration rate (eGFR) ≥60 mL/min) who received vancomycin infusion for treatment were enrolled in the study after obtaining the informed consent. None of the patients had known hypersensitivity to vancomycin, and all patients had started using this drug by attending the physicians for the treatment of presumed or documented Gram-positive infections. Patients with unstable kidney function during 48 hours before and after the first dose were excluded from the study [21]. All patients initially received a loading dose of 25 mg/kg of vancomycin based on total body weight and with an infusion period of ≥30 minutes for every 500 mg, followed by an intermittent infusion.
2.3. Methods to Collect and Measure Vancomycin Clearance
Serum concentrations of vancomycin were collected from a central venous (CV) line. Peak and trough levels were drawn 1-hour after the end of the infusion and 1-hour before the next dose infusion, respectively. After centrifugation, all plasma samples were analyzed by fluorescence polarization immunoassay through EMIT assays (Siemens Healthcare Diagnosis, United Kingdom, EMIT).
First-dose pharmacokinetics was performed for each individual patient using the one-compartment model. Pharmacokinetic parameters (such as CLvan and AUC24 h) were calculated by MONOLIX software as the mean of their posterior distribution using stochastic approximation expectation-maximization (SAEM) algorithm.
Renal function was monitored for all patients using serum creatinine and urine output. CrCL was estimated using the CG formula.
| (1) |
where CLCG is the Cockcroft–Gault creatinine clearance (mL/min), AGE is the age of the patients (years), IBW is the ideal body weight (kg), and SCr is the serum creatinine (mg/dL).
In addition, a 6-hour urine collection was performed for measuring urinary creatinine concentration and urine volume for all patients. Then, CL6 h can be determined as follows:
| (2) |
where CL6 h is the 6-hour urine creatinine clearance (mL/min), Vu is the urine volume (mL), CuCr is the urine creatinine concentration (mg/dL), T is the duration of urine collection (minutes), equivalent to 3600 minutes for our patients, and CsCr is the creatinine serum concentration (mg/dL).
2.4. Statistical Analysis
Correlation of CLvan with the measured CrCL from the 6-hour urine collection (CL6 h) and the estimated CrCL (CLCG) was investigated by the Pearson correlation test, and the final model was achieved by linear regression analysis within a stepwise protocol. Data are presented as mean (95% confidence interval) or median (1st quarterly and 3rd quarterly), as appropriate. All the collected data were analyzed by SPSS software version 25. For all tests, p value <0.05 was considered as statistically significant.
3. Results
The subjects of the study were the patients admitted to the general and emergency ICU wards of Sina Hospital affiliated to TUMS. A majority of the patients were male (81%) with an average age of 45.1 ± 19.4 years old. The mean Acute Physiology and Chronic Health Evaluation II (APACHE II) score was 12.1 ± 1.5. Regarding the body weight, total body weight (TBW) was 71.1 ± 14.8 kg, and ideal body weight (IBW) was 68.4 ± 9.7 kg. The median (1st quarterly and 3rd quarterly) daily dose of vancomycin therapy was equal to 46.9 [42.9, 48.6] mg/kg in patients. The pharmacokinetic parameters of vancomycin studied in patients are listed in Table 1.
Table 1.
Pharmacokinetic parameters of vancomycin.
| Measures | Mean ± S.D. or median (1st quarterly and 3rd quarterly) |
|---|---|
| t 1/2 (hr) | 5.7 [5.2, 7.0] |
| V d/kg (L/kg) | 0.75 ± 0.23 |
| CLvan (mL/min) | 96.2 ± 29.9 |
| AUC24 h (mg.hr/L) | 518 [447, 641] |
Abbreviations: S.D.: standard deviation, Cp: peak concentration, Ct: trough concentration, t1/2: half-life, Vd/kg: volume of distribution per kilogram, CLvan: clearance of vancomycin, and AUC24 h: 24-hour area under the curve.
CL6 h was significantly closer to CLvan than CLCG (r = 0.823 vs. 0.594; p < 0.001 vs. 0.003). The results of comparisons between these parameters are shown in Figures 1 and 2.
Figure 1.
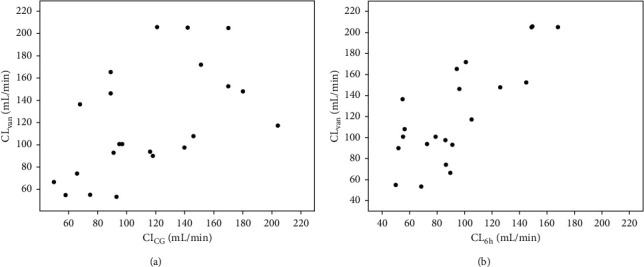
Comparisons between CLvan, CL6 h, and CLCG. (a) Correlation between CLvan and CLCG. (b) Correlation between CLvan and CL6 h. Abbeviations: CLvan: clearance of vancomycin, CL6 h: measured creatinine clearance in 6-hour urine collection, CLCG: estimated creatinine clearance by the Cockcroft–Gault formula.
Figure 2.
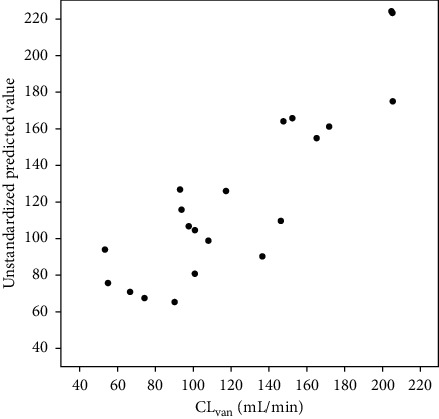
Correlation between predicted values of CLvan and actual values. Abbeviations: CLvan: clearance of vancomycin.
Given that the AUC depends on the clearance, different parameters were included in the regression model to find the most accurate prediction model for CLvan. CLCG, CL6 h, TBW, IBW, and age were tested. CL6 h and IBW were found to be the best predictors for the model. The final equation was identified with respect to the best predictive ability to estimate CLvan in critically ill patients without renal impairment as follows:
| (3) |
Regarding the described model, the practitioners can use the proposed equation to calculate the empirical dose of vancomycin to achieve the AUC24 h goal. For this purpose, the following equation can be used:
| (4) |
Accordingly, AUC24 h may be considered between 400 and 600 mg·hr/L based on the targeted MIC with a goal of AUC24 h/MIC ≥400; as vancomycin MIC for sensitive Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus spp. ranges between 1 and1.5 mg/L,
| (5) |
Table 2 could be simply used to determine the appropriate total daily dose of vancomycin based on measured CL6 h.
Table 2.
Appropriate total daily dose of vancomycin in various ranges of CL6 h to achieve target AUC24 h of 400 or 600 mg·hr/L considering IBW equal to 70 kg.
| CL6 h (mL/min) | A total daily dose of vancomycin (mg) to achieve target AUC24 h | |
|---|---|---|
| Target AUC24 h of 400 (mg·hr/L) | Target AUC24 h of 600 (mg·hr/L) | |
| 60 | 2250 | 3500 |
| 80 | 2750 | 4000 |
| 100 | 3250 | 4750∗ |
| 120 | 3750 | ─ |
∗Target AUC24 h of 400 mg.hr/L may be considered because of the total daily dose >4 g. Abbreviations: CL6 h: measured creatinine clearance in 6-hour urine collection; AUC24 h: 24-hour area under the curve.
4. Discussion
AUC-guided dosing strategy as the critical target value for individualized vancomycin dosing can be estimated using several methods including the Bayesian approach, two-point sampling, and continuous infusion [22]. Bayesian dose-optimizing software used for estimating AUC working by merging a single level of vancomycin with the populations' pharmacokinetics needs to be purchased, and many health systems, particularly in developing countries, have no access to this program [23]. Pharmacokinetic equations based on two or more vancomycin levels provide good accuracy with less bias, but it is associated with the increased laboratory workload and health-care costs [24]. Considering the limitations of current methods for AUC estimation of vancomycin, the present study was conducted to propose a new tool for achieving the therapeutic goals of vancomycin therapy and could be considered as an alternative method for empirical dosing when the lab data are available.
In case of critical illnesses, equations used for estimating renal function including CG formula are not able to provide correct judgment because this formula is based on the kidney status of the healthy men, and factors such as muscle mass, excess fat, or fluid in obese subjects and secretion of creatinine from renal tubules are not considered in this formula [6, 25]. Moreover, the CG equation is insensitive and is not capable of showing abrupt and acute changes in kidney condition since changes in the serum creatinine status do not occur quickly [26, 27]. Besides, this formula is no longer recommended for estimating vancomycin clearance and consequently for maintenance dosing's [28, 29]. According to the results of a current systematic review [30], using urinary CrCL is the best diagnostic method for estimating drug dosing in augmented renal clearance (ARC). 24-hour urine collection is often difficult, and some limitations are reducing the accuracy of collecting urine within 24 hours [31, 32]. In case of the critical condition of the ICU, requiring quick decision-making urine collection can be implemented by decreasing the time of urine collection. Several studies have been conducted to compare the results of 24-hour urine collection with those collected in less duration, and it has been concluded that 24-hour urine collection can be replaced with urine collection in less time [32–34]. Nevertheless, few of them have compared the urine CrCL with drug clearance such as vancomycin, which is almost completely removed from the kidneys. Rodvold et al. included a larger sample of 37 patients into three groups based on measured 24-hour CrCL including patients with renal impairment. The resulting equation (CLvan (mL/min/1.73 m2) = 15.7 + 0.79 CL24 h (mL/min/1.73 m2)) produces similar results to the equation derived in this study [35]. Zokufa et al. have suggested that renal biomarkers measurement (e.g., cystatin C) may be used to estimate CrCL and to determine the vancomycin dosing in ICU patients [36].
To our knowledge, this is the first research using 6-hour urine collection in ICU and comparing it with CLvan and CG equation in patients with normal renal function and including ARC. The 6-hour urine collection was selected as urine collection in our ICU is performed every 3 hours under the supervision of nurses, and the 6-hour duration only involves the participation of one nurse in this process and may reduce the errors. In this study, a strong relationship was found between CLvan and CL6 h. Therefore, a new modeling trend was determined for vancomycin dosing based on urine collection as an alternative method for empirical initiation while waiting for serum level measurements.
4.1. Study Limitations
Since this research was a single-center pilot study, the described model should be confirmed by further studies with large multicenter data to evaluate vancomycin AUC24 h based on 6-hour urine CrCL measurement in ICU patients. The described model only fits the patients with an eGFR ≥60, and this could be considered as another important limitation of this study. On the other hand, as shown in the plots, this model may fit accurately in patients with ARC. Since the sample size of this study was small, we could not perform multiple adjustments for any other independent variables. However, we included one variable for every ten patients (1 : 10) into the multiple linear regression model to have enough power.
5. Conclusions
Considering the cost and labor intensity related to applying the TDM process, the results of the present study revealed that, for AUC estimation, CLvan can be obtained by collecting urine in a 6-hour period with good approximation in critically ill patients with normal renal function.
Acknowledgments
The authors would like to thank the support of Sina Hospital in Tehran, Iran.
Data Availability
The figure and table data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Hanberger H., Walther S., Leone M., et al. Increased mortality associated with meticillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. International Journal of Antimicrobial Agents. 2011;38(4):331–335. doi: 10.1016/j.ijantimicag.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Barie K. A., Cannon J. P., Grim S. A. Alternative agents to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. American Journal of Therapeutics. 2013;20(2):200–212. doi: 10.1097/mjt.0b013e31821109ec. [DOI] [PubMed] [Google Scholar]
- 3.Felton T. W., Hope W. W., Roberts J. A. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagnostic Microbiology and Infectious Disease. 2014;79(4):441–447. doi: 10.1016/j.diagmicrobio.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Rybak M. J., Le J., Lodise T. P., et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of America, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. American Journal of Health-System Pharmacy. 2020;77(11):835–864. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 5.Shahrami B., Najmeddin F., Mousavi S., Ahmadi A., Mojtahedzadeh M. Therapeutic drug monitoring of vancomycin following critical care illnesses: peak concentration determination maybe critical. Journal of Pharmaceutical Care. 2014;2(3):137–139. [Google Scholar]
- 6.Haeseker M., Croes S., Neef C., Bruggeman C., Stolk L., Verbon A. Evaluation of vancomycin prediction methods based on estimated creatinine clearance or trough levels. Therapeutic Drug Monitoring. 2016;38(1):120–126. doi: 10.1097/ftd.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 7.Turner R. B., Kojiro K., Won R., Chang E., Chan D., Elbarbry F. Prospective evaluation of vancomycin pharmacokinetics in a heterogeneous critically ill population. Diagnostic Microbiology and Infectious Disease. 2018;92(4):346–351. doi: 10.1016/j.diagmicrobio.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Finch N. A., Zasowski E. J., Murray K. P., et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrobial Agents and Chemotherapy. 2017;61(12):e01293–e01217. doi: 10.1128/aac.01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahrami B., Najmeddin F., Mousavi S., et al. Achievement of vancomycin therapeutic goals in critically ill patients: early individualization may be beneficial. Critical care research and practice. 2016;2016:7. doi: 10.1155/2016/1245815.1245815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto K., Takesue Y., Ohmagari N., et al. Practice guidelines for therapeutic drug monitoring of vancomycin: a consensus review of the japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. Journal of Infection and Chemotherapy. 2013;19(3):365–380. doi: 10.1007/s10156-013-0599-4. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki E. L., Claeys K. C., Mynatt R. P., et al. Making the change to area under the curve-based vancomycin dosing. American Journal of Health-System Pharmacy. 2018;75(24):1986–1995. doi: 10.2146/ajhp180034. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins L., Krcmova L. K., Solich P., Kaska M. Simple and rapid quantification of vancomycin in serum, urine and peritoneal/pleural effusion via UHPLC-MS/MS applicable to personalized antibiotic dosing research. Journal of Pharmaceutical and Biomedical Analysis. 2017;142:59–65. doi: 10.1016/j.jpba.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Spadaro S., Berselli A., Fogagnolo A., et al. Evaluation of a protocol for vancomycin administration in critically patients with and without kidney dysfunction. BMC Anesthesiology. 2015;15(1):p. 95. doi: 10.1186/s12871-015-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruniera F., Ferreira F., Saviolli L., et al. The use of vancomycin with its therapeutic and adverse effects: a review. European Review for Medical and Pharmacological Sciences. 2015;19(4):694–700. [PubMed] [Google Scholar]
- 15.Eppenga W. L., Kramers C., Derijks H. J., Wensing M., Wetzels J. F. M., De Smet P. A. G. M. Drug therapy management in patients with renal impairment: how to use creatinine-based formulas in clinical practice. European Journal of Clinical Pharmacology. 2016;72(12):1433–1439. doi: 10.1007/s00228-016-2113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartet-Farnier E., Goutelle-Audibert L., Maire P., De la Gastine B., Goutelle S. Implications of using the MDRD or CKD-EPI equation instead of the cockcroft-Gault equation for estimating renal function and drug dosage adjustment in elderly patients. Fundamental & Clinical Pharmacology. 2017;31(1):110–119. doi: 10.1111/fcp.12241. [DOI] [PubMed] [Google Scholar]
- 17.Baptista J. P. Augmented renal clearance. In: Udy A. A., Roberts J. A., Lipman J., editors. Antibiotic Pharmacokinetic/Pharmacodynamic Considerations in the Critically Ill. Singapore, Singapore: Springer Singapore; 2018. pp. 125–150. [Google Scholar]
- 18.Jelliffe R. Chapter 5 - evaluation of renal function. In: Jelliffe R. W., Neely M., editors. Individualized Drug Therapy for Patients. Boston, MA, USA: Academic Press; 2017. pp. 47–56. [Google Scholar]
- 19.Truong J., Smith S. R., Veillette J. J., Forland S. C. Individualized pharmacokinetic dosing of vancomycin reduces time to therapeutic trough concentrations in critically ill patients. The Journal of Clinical Pharmacology. 2018;58(9):1123–1130. doi: 10.1002/jcph.1273. [DOI] [PubMed] [Google Scholar]
- 20.Lin Wu F.-L., Liu S.-S., Yang T.-Y., et al. A larger dose of vancomycin is required in adult neurosurgical intensive care unit patients due to augmented clearance. Therapeutic Drug Monitoring. 2015;37(5):609–618. doi: 10.1097/ftd.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 21.Win R. L., Kellum J. A., Shah S. V., et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molitoris N. E. Using AUC/MIC to guide vancomycin dosing: ready for prime time? Clinical Microbiology and Infection. 2020;26(4):406–408. doi: 10.1016/j.cmi.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Turner R. B., Kojiro K., Shephard E. A., et al. Review and validation of bayesian dose‐optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2018;38(12):1174–1183. doi: 10.1002/phar.2191. [DOI] [PubMed] [Google Scholar]
- 24.Won L., Wong T., Huang S., et al. Conversion from vancomycin trough concentration–guided dosing to area under the curve–guided dosing using two sample measurements in adults: implementation at an academic medical center. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2019;39(4):433–442. doi: 10.1002/phar.2234. [DOI] [PubMed] [Google Scholar]
- 25.Den Bakker E., Gemke R., van Wijk J. A. E., Hubeek I., Stoffel-Wagner B., Bökenkamp A. Combining GFR estimates from cystatin C and creatinine-what is the optimal mix? Pediatric Nephrology. 2018;33(9):1553–1563. doi: 10.1007/s00467-018-3973-8. [DOI] [PubMed] [Google Scholar]
- 26.Udy A. A., Roberts J. A., Lipman J. Implications of augmented renal clearance in critically ill patients. Nature Reviews Nephrology. 2011;7(9):539–543. doi: 10.1038/nrneph.2011.92. [DOI] [PubMed] [Google Scholar]
- 27.Shahrami B., Najmeddin F., Rouini M. R, et al. Evaluation of amikacin pharmacokinetics in critically ill patients with intra-abdominal sepsis. Advanced Pharmaceutical Bulletin. 2020;10(1):114–118. doi: 10.15171/apb.2020.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MojtahedzadehNajafi M., Hartinger J., Netíková I. Š, Slanar O. Creatinine clearance estimations for vancomycin maintenance dose adjustments. American Journal of Therapeutics. 2018;25(5):e602–e604. doi: 10.1097/MJT.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 29.Šíma M., Hartinger J., Grus T., Slanař O. Initial dosing of intermittent vancomycin in adults: estimation of dosing interval in relation to dose and renal function. European Journal of Hospital Pharmacy. 2019;54(6):702–708. doi: 10.1136/ejhpharm-2019-002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilbao-Meseguer I., Rodríguez-Gascón A., Barrasa H., Isla A., Solinís M. Á. Augmented renal clearance in critically ill patients: a systematic review. Clinical Pharmacokinetics. 2018;57(9):1107–1121. doi: 10.1007/s40262-018-0636-7. [DOI] [PubMed] [Google Scholar]
- 31.Young T., Daniel M., Baumhover S., Eidson D., Green J. Methodological study of vancomycin dosing in elderly patients using actual serum creatinine versus rounded serum creatinine. Drugs in R&D. 2017;17(3):435–440. doi: 10.1007/s40268-017-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera-Gutiérrez M. E., Seller-Pérez G., Banderas-Bravo E., Muñoz-Bono J., Lebrón-Gallardo M., Fernandez-Ortega J. F. Replacement of 24 h creatinine clearance by 2 h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Medicine. 2007;33(11):1900–1906. doi: 10.1007/s00134-007-0745-5. [DOI] [PubMed] [Google Scholar]
- 33.Baptista J. P., Roberts J. A., Sousa E., Freitas R., Deveza N., Pimentel J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Critical Care. 2014;18(6):p. 654. doi: 10.1186/s13054-014-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pong S., Seto W., Abdolell M., et al. 12-hour versus 24-hour creatinine clearance in critically ill pediatric patients. Pediatric Research. 2005;58(1):83–88. doi: 10.1203/01.pdr.0000156225.93691.4f. [DOI] [PubMed] [Google Scholar]
- 35.Trope K. A., Blum R. A., Fischer J. H., et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrobial Agents and Chemotherapy. 1988;32(6):848–852. doi: 10.1128/aac.32.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zokufa M. G., Hilpert J. W., Gnewuch C., Kees F., Voegeler S. Clearance of vancomycin during continuous infusion in intensive care unit patients: correlation with measured and estimated creatinine clearance and serum cystatin C. International Journal of Antimicrobial Agents. 2010;36(6):545–548. doi: 10.1016/j.ijantimicag.2010.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The figure and table data used to support the findings of this study are included within the article.


