Abstract
While post-operative bacterial infections can trigger rejection of pulmonary allografts, the impact of bacterial colonization of donor grafts on alloimmune responses to transplanted lungs remains unknown. Here, we tested the hypothesis that bacterial products present within donor grafts at the time of implantation promote lung allograft rejection. Administration of the TLR2 agonist Pam3Cys4 to Balb/c wildtype grafts triggered acute cellular rejection after transplantation into B6 wildtype recipients that received peri-operative costimulatory blockade. Pam3Cys4-triggered rejection was associated with an expansion of CD8+ T lymphocytes and CD11c+CD11bhiMHC class II+ antigen presenting cells within the transplanted lungs. Rejection was prevented when lungs were transplanted into TLR2-deficient recipients, but not when MyD88-deficient donors were used. Adoptive transfer of B6 wildtype monocytes, but not T cells, following transplantation into B6 TLR2-deficient recipients restored the ability of Pam3Cys4 to trigger acute cellular rejection. Thus, we have demonstrated that activation of TLR2 by a bacterial lipopeptide within the donor airways prevents the induction of lung allograft tolerance through a process mediated by recipient-derived monocytes. Our work suggests that donor lungs harboring bacteria may precipitate an inflammatory response that can facilitate allograft rejection.
1. Introduction
Lung transplantation is the only established lifesaving option for many patients suffering from end-stage pulmonary disease. The selection of appropriate lung donors is a critical element of the transplant process. Infiltrates on chest imaging studies and the presence of bacteria in the donor airways have long been considered contraindications to the use of lungs for transplantation (1). Due to a scarcity in suitable lungs for transplantation and a rising waitlist mortality rate, strategies have been increasingly employed to expand the donor pool. To this end, one such approach is the use of extended criteria lung donors, which may harbor infections. The use of donor lungs with bacterial colonization or pneumonia, however, is controversial. While some studies have shown that positive donor airway gram stains do not predict poor outcomes, others have challenged this notion (2). To this end, Avlonitis reported that positive bacterial cultures in donor bronchoalveolar fluid were associated with worse lung function in the immediate post-operative period, longer median times of mechanical ventilation and inferior survival at 6 months, 1 year, 2 years and 4 years after transplantation (3). A more recent study also found that the presence of potentially pathogenic bacteria in donor airway cultures was associated with prolonged mechanical ventilation following lung transplantation (4).
It is well recognized that innate immune sensing of pathogens or endogenous ligands by Toll like receptors can regulate alloimmune responses. A seminal study in 2003 showed that rejection of HY-incompatible skin allografts was dependent on expression of MyD88, a downstream adaptor protein for most Toll like receptors (5). It was subsequently reported that Staphylococcus aureus infection prevents skin allograft acceptance in wildtype, but not MyD88-deficient hosts (6). Similarly, signaling through MyD88 in recipient mice triggers rejection of tolerant heart grafts after infection with Listeria monocytogenes (7). Our laboratory has previously shown that treatment of lung transplant recipients with low molecular weight hyaluronic acid abrogates tolerance, where TLR2/4 / MyD88-dependent signaling in recipient mice results in an expansion of alloreactive T cells (8). While we have not specifically examined innate immune signaling, we have also reported that post-operative infection of lung allograft recipients with Pseudomonas aeruginosa results in acute rejection (9). Infection with Pseudomonas aeruginosa activates graft-infiltrating neutrophils, which interact with CD4+ T cells and CD11c+ dendritic cells and promote inflammation. Whether and how the presence of bacteria or their products in donor lungs at the time of transplantation influences alloimmunity is poorly understood.
The aim of the current study was to examine whether the presence of bacterial products within donor lungs at the time of transplantation influence alloimmune responses. We have taken advantage of our previously established mouse model whereby peri-operative costimulatory blockade induces tolerance after lung transplantation; this process depends on local immunoregulation through Foxp3+ T cells that accumulate within induced bronchus-associated lymphoid tissue in the grafts (10). In this model, mouse lung allografts remain free of rejection without the need for maintenance immunosuppression and recipients accept donor-matched heart grafts. Successful tolerance induction in the clinics, for example through mixed chimerism or adoptive transfer of regulatory T cells, would be desirable to avoid adverse effects of immunosuppressive drugs, such as pharmacological toxicity as well as an increased risk for opportunistic infections and malignancies (11). We now show that administration of Pam3Cys4, a bacterial lipopeptide, into the donor airway prior to implantation abrogates tolerance induction. Pam3Cys4-triggered rejection is associated with an expansion of CD8+ T cells in the grafts and depends on TLR2 signaling in recipient bone marrow-derived monocytes. Our findings suggest new therapeutic avenues aimed at preventing rejection after lung transplantation and are of relevance in the changing landscape of lung donor selection criteria.
2. Materials and methods
2.1. Mice
C57BL/6 (wild-type) (B6 CD45.2 WT), B6 CD45.1, Balb/c (wild-type) (BALB/c WT), and B6.129-Tlr2tm1Kir (B6 TLR2KO) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). MyD88-deficient Balb/c mice (Balb/c MyD88KO) were purchased from Oriental Bio Service (Kyoto, Japan). 6–8 week old male and female mice were used for all experiments. Transplants were matched for gender. All procedures were approved by the Institutional Animal Studies Committee in Washington University in St. Louis.
2.2. Lung transplantation
Left orthotopic vascularized lung transplants were performed as previously described (10). Mice were treated with co-stimulatory blockade consisting of MR1 (250 μg intraperitoneally (i.p.)) and CTLA4-Ig (200 μg i.p.), on days 0 and 2, respectively (Bio X Cell, West Lebanon, NH). Pam3Cys4 was dissolved in distilled water at a concentration of 1 μg/μl and 50 μL were administered into the donor airway just prior to completing the bronchial anastomosis (Invivo Gen, San Diego, CA). In select experiments, LPS (20 μg) was dissolved in 50 μl of phosphate buffered saline and injected into the donor airway before connecting donor and recipient bronchi (Sigma, St. Louis, MO). The concentrations and administration route were based on previous studies examining the impact of Pam3Cys4 and LPS on pulmonary immune responses (12) (13) (14). For select experiments, 10x106 CD8+ T cells (Ly-2 microbeads, Miltenyi Biotec, Bergisch Gladbach, Germany) (15) or 3x106 monocytes (Monocyte Isolation Kit, Miltenyi Biotec) (16), isolated from the spleen or bone marrow, respectively, of naïve B6 WT mice were injected intravenously into B6 TLR2KO recipients at the time of transplantation.
2.3. Histology
Portions of transplanted lungs were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (H&E). Lung histology was assessed for rejection in a blinded manner by a pathologist (JHR) using standard criteria from The International Society of Heart and Lung Transplantation (17).
2.4. Flow cytometry
Single cell suspensions were prepared from transplanted lungs as previously described (10). Cells were stained with fluorochrome-labeled antibodies against CD90.2 (clone 30-H12, Biolegend, San Diego, CA), CD8 (clone 53–6.7, Thermo Fisher Scientific, Waltham MA), CD4 (clone RM4–5, Thermo Fisher Scientific), CD45 (clone 30-F11, Thermo Fisher Scientific), Ki67 (clone SolA15, Thermo Fisher Scientific), CD11b (clone M1/70, Thermo Fisher Scientific), CD11c (clone N418, Thermo Fisher Scientific), CD45.2 (clone 104, BD Bioscience, San Jose, CA), CD45.1 (clone A20, Biolegend) and I-Ab (clone AF6–120.1, Biolegend).
2.5. Gene expression analysis
Homogenized lung tissue was lysed with TRIzol (Thermo Fisher Scientific). Total RNA was isolated using QIAGEN RNeasy Mini Kit (QIAGEN), and quantitative PCR was performed as previously described (16). Primer sequences were as follows: IL-1beta, 5′- GCAACTGTTCCTGAACTCAACT −3′ and 5′- ATCTTTTGGGGTCCGTCAACT−3′; TNFalpha, 5’ - CCCTCACACTCAGATCATCTTCT−3’ and 5’- GCTACGACGTGGGCTACAG−3’; IL-6, 5’- TAGTCCTTCCTACCCCAATTTCC-3’ and 5’-TTGGTCCTTAGCCACTCCTTC −3’.
2.6. Statistics
Data are reported as mean ± standard error of the mean (SEM). The Mann–Whitney U test was performed using GraphPad Prism version 7.0 (GraphPad Software, La Jolla, CA). p < 0.05 was considered to be statistically significant.
3. Results
3.1. Pam3Cys4 within the donor airway prevents the induction of tolerance after lung transplantation
We have previously shown that peri-operative costimulatory blockade results in tolerance after transplantation of Balb/c lungs into allogeneic B6 recipients (10). To examine the impact of bacterial lipopeptide within donor lungs at the time of engraftment on alloimmune responses, we transplanted Balb/c wildtype lungs into costimulatory blockade-treated B6 wildtype mice and administered Pam3Cys4 into the donor airways immediately prior to anastomosing the left bronchus. At 3 days after transplantation, expression levels of IL-1beta and TNF-alpha, but not IL-6 were significantly elevated in Pam3Cys4- compared to vehicle-treated pulmonary allografts or naïve lungs (Supplemental Fig. 1). We found that at 7 days after transplantation Pam3Cys4-treated lung grafts were not ventilated and had histological evidence of acute cellular rejection (Grade A≥2) (Figure 1A). Conversely, control vehicle (distilled, sterile water)-treated transplants were ventilated and developed no rejection or had only mild inflammation (Grades A0–1) (Fig. 1B).
Figure 1. Pam3Cys4 in the donor airway prevents the induction of lung allograft tolerance through TLR2 signaling in the recipient.
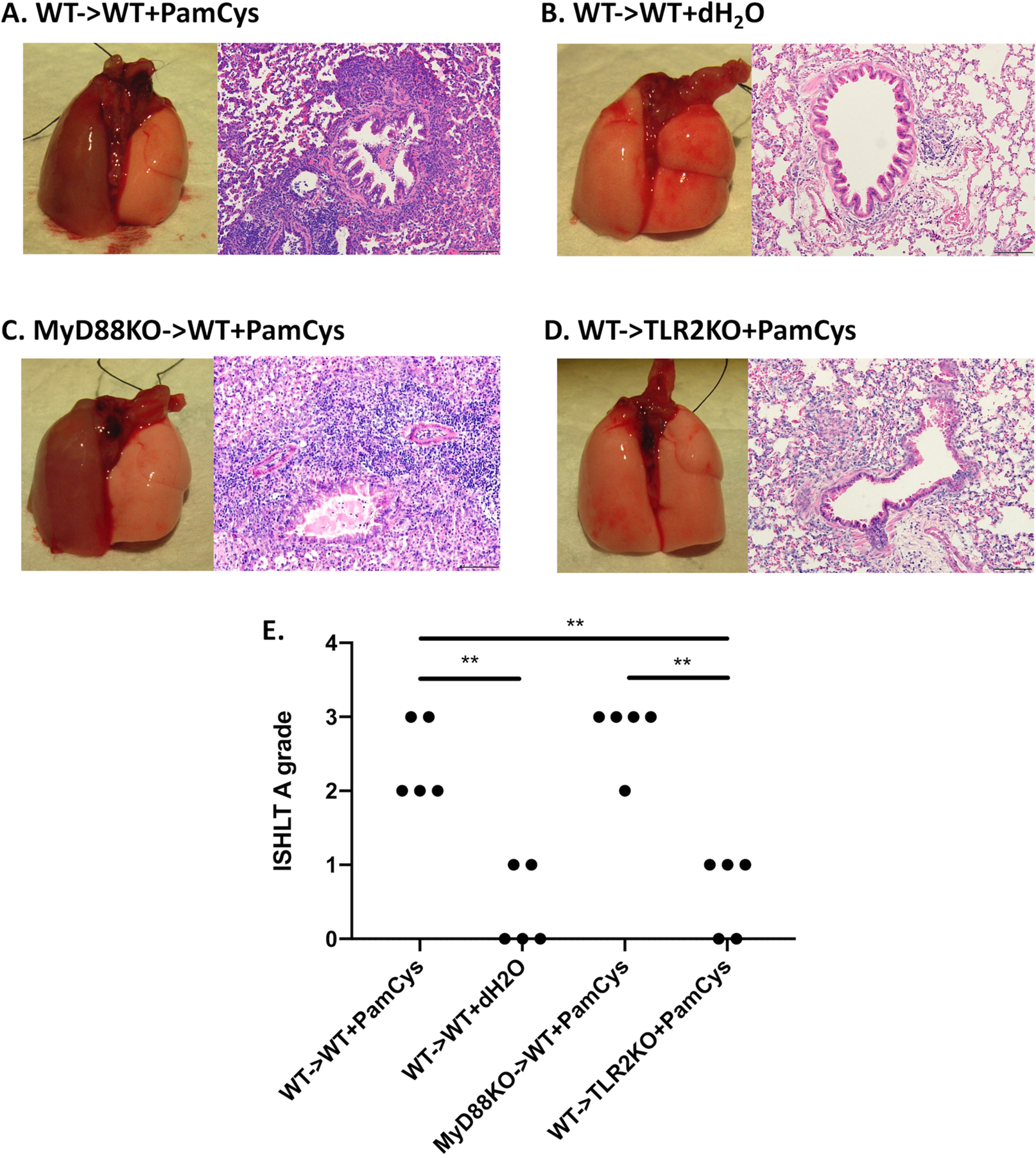
Gross findings and histology (hematoxylin and eosin (H&E) staining) for Balb/c WT lung grafts that were treated with (A) Pam3Cys4 (PamCys) or (B) vehicle (dH2O) prior to transplantation into B6 WT recipients, as well as (C) Balb/c MyD88KO or (D) Balb/c WT lung grafts treated with Pam3Cys4 prior to transplantation into B6 WT or B6 TLR2KO recipients, respectively. For all experiments, Pam3Cys4 or vehicle was instilled into the donor bronchus just before anastomosis, co-stimulatory blockade was given to recipient mice, and grafts were harvested at day 7. (A) – (D) represent one experiment out of 5 for the respective condition. (E) ISHLT A grade rejection scores are shown for each condition. n=5 for all conditions. Original magnification: 200x. Scale bars: 100μm. ** denotes p < 0.01.
3.2. Pam3Cys4-mediated rejection depends on TLR2 signaling in the recipient
We next set out to investigate whether Pam3Cys4 triggered acute rejection by signaling through donor or recipient cells. Pam3Cys4 is a known TLR2 agonist, which stimulates innate immune responses in a MyD88-dependent manner (19). To examine the effect of Pam3Cys4 on donor tissues, we transplanted Pam3Cys4-treated Balb/c MyD88-deficient lungs into costimulatory blockade-treated B6 wildtype mice. Seven days later these grafts were not ventilated and had histological evidence of severe acute rejection (Grade A≥2) (Fig. 1C), similar to our observations after transplantation of Pam3Cys4-treated Balb/c wildtype lungs into costimulatory blockade-treated B6 wildtype hosts (Fig. 1A). In stark contrast, however, Balb/c wildtype lung grafts transplanted into B6 TLR2-deficient mice after treatment with Pam3Cys4 were well ventilated and had only mild inflammatory changes (Grades A0–1) (Fig. 1D). Thus, TLR2 signaling in recipient, but not donor cells, is responsible for Pam3Cys4-triggered acute graft rejection (Fig. 1E).
3.3. Pam3Cys4-mediated rejection is associated with an accumulation of CD8+ T cells within the lung grafts
We have previously shown that acute lung rejection is associated with a predominance of CD8+ over CD4+ T cells within the grafts and can occur independent of CD4+ T cells (20). Compared to control conditions, we found that Pam3Cys4-treatment was associated with a significant increase in the ratio of CD8+ to CD4+ T lymphocytes within Balb/c lungs after transplantation into B6 wildtype, but not B6 TLR2-deficient recipients (Figs. 2A–C). Notably, CD8+ T cells infiltrating acutely rejecting grafts demonstrated increased proliferative responses, as evidenced by their Ki-67 expression (Figs. 2D–E). Of note, a higher percentage of CD8+ T cells expressed Ki67 in MyD88-deficient compared to wildtype grafts (Fig. 2E). TLR2 ligation can directly activate human and mouse T lymphocytes, where it has been shown to function as a costimulatory molecule (21, 22). To examine whether TLR2 signaling via recipient T cells is sufficient to trigger rejection, we transplanted Balb/c lungs into Pam3Cys4-treated B6 TLR2-deficient mice that received CD8+ T cells derived from the spleens of wildtype B6 mice at the time of engraftment. These grafts did not show any evidence of rejection indicating that TLR2 signaling via recipient CD8+ T cells is not sufficient to trigger rejection (Fig. 2F–G).
Figure 2. Pam3Cys4-mediated rejection results in increased intragraft CD8+ T cells.
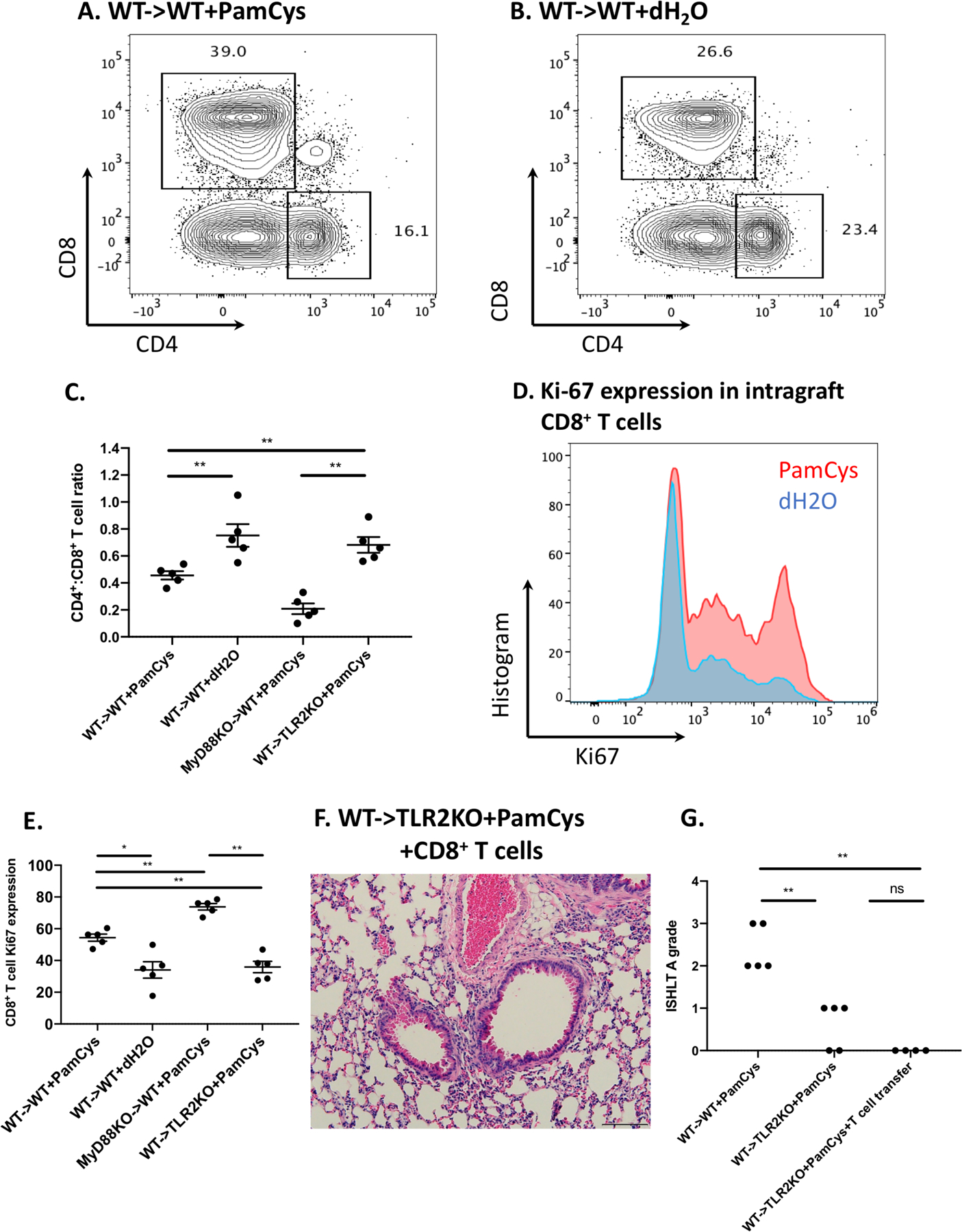
(A) Representative contour plot depicting the percentage of CD4+ and CD8+ T cells, gated on live, single CD45+CD90.2+ cells within Balb/c WT lung grafts treated with (A) Pam3Cys4 (PamCys) or (B) vehicle (dH2O) prior to transplantation into B6 WT recipients. (C) Ratio of intragraft CD4+ T cells to CD8+ T cells among various experimental groups. n=5 for all conditions. (D) Histogram of Ki-67 expression for intragraft CD8+ T cells from Balb/c WT lung grafts treated with Pam3Cys4 (red) or vehicle (blue) prior to transplantation into B6 WT recipients. (E) Comparison of Ki-67 expression for intragraft CD8+ T cells among various experimental groups. Plots and quantifications shown are gated on live, single, CD45+CD90.2+CD8+CD4− cells. n=5 for all conditions. (F) Histological analysis (H&E) and (G) ISHLT A grade rejection scores of Pam3Cys4-treated Balb/c lung grafts after transplantation into B6 TL2KO recipients that received spleen-derived B6 WT CD8+ T cells on the day of transplantation (n=4). Original magnification 200x. Scale bar: 100μm. For all experiments, Pam3Cys4 or vehicle was instilled into the donor bronchus just before anastomosis, co-stimulatory blockade was given to recipient mice, and grafts were harvested at day 7. Data represent mean ± SEM. * and ** denote p < 0.05 and p < 0.01, respectively; ns=not significant.
3.4. Pam3Cys4-mediated rejection depends on TLR2 signaling in recipient bone marrow-derived monocytes
We have previously reported that recipient monocyte-derived CD11c+ antigen presenting cells infiltrate lung allografts where they encounter and activate T cells (23). Three days after transplantation, we observed a significantly increased abundance of recipient-derived CD11c+CD11bhi cells within Pam3Cys4-treated compared to vehicle control-treated lung allografts. We also observed an increase in CD11c+CD11blow cells in Pam3Cys4-treated lungs, which approached statistical significance (p=0.05). CD11c+CD11bhi cells have been described as dendritic cells and CD11c+CD11blow cells as macrophages, both of which can differentiate from monocytes in the lung under inflammatory conditions (24). Of note, stimulation of donor lungs with LPS, a component of the cell wall of gram-negative bacteria resulted in an increased graft infiltration of recipient CD11c+CD11bhi and CD11c+CD11blow cells compared to control conditions (Supplemental Fig. 2). Seven days after transplantation, Pam3Cys4-triggered rejection was associated with an increased abundance of MHC class II-expressing CD11c+CD11bhi cells within the allograft (Fig 3A–C). Of note, the abundance of MHC class II-expressing CD11c+CD11bhi cells was higher in MyD88-deficient when compared to wildtype grafts (Fig. 3C). Next, we next set out to examine whether Pam3Cys4 could mediate lung rejection by TLR2 signaling through recipient monocytes. To this end, we adoptively transferred bone marrow-derived B6 wildtype monocytes into B6 TLR2-deficient hosts that received Pam3Cys4-treated Balb/c lungs. These grafts developed perivascular cuffing, consistent with acute rejection (Grades A2–3) (Fig. 3D–E), similar in appearance to B6 wildtype recipients of Pam3Cys4-treated Balb/c lungs (Fig. 1A). We have recently shown that monocytes that originate from the spleen infiltrate lung grafts early after reperfusion and play a critical role in mediating ischemia reperfusion injury (16). To evaluate whether recipient monocytes that respond to Pam3Cys4 are derived from the spleen or require the spleen for their maturation, we transplanted Balb/c lungs that were treated with Pam3Cys4 into B6 wildtype mice that underwent a splenectomy at the time of engraftment; under these conditions, lung grafts developed acute rejection (Grade A3) (Supplemental Fig. 3A–B). Taken together, these results demonstrate that Pam3Cys4-mediated rejection can be mediated through TLR2 signaling in recipient bone marrow-derived monocytes.
Figure 3. Pam3Cys4-mediated rejection is associated with an increased in CD11c+CD11bhiMHCII+ cells and depends on TLR2 signaling in recipient-derived monocytes.
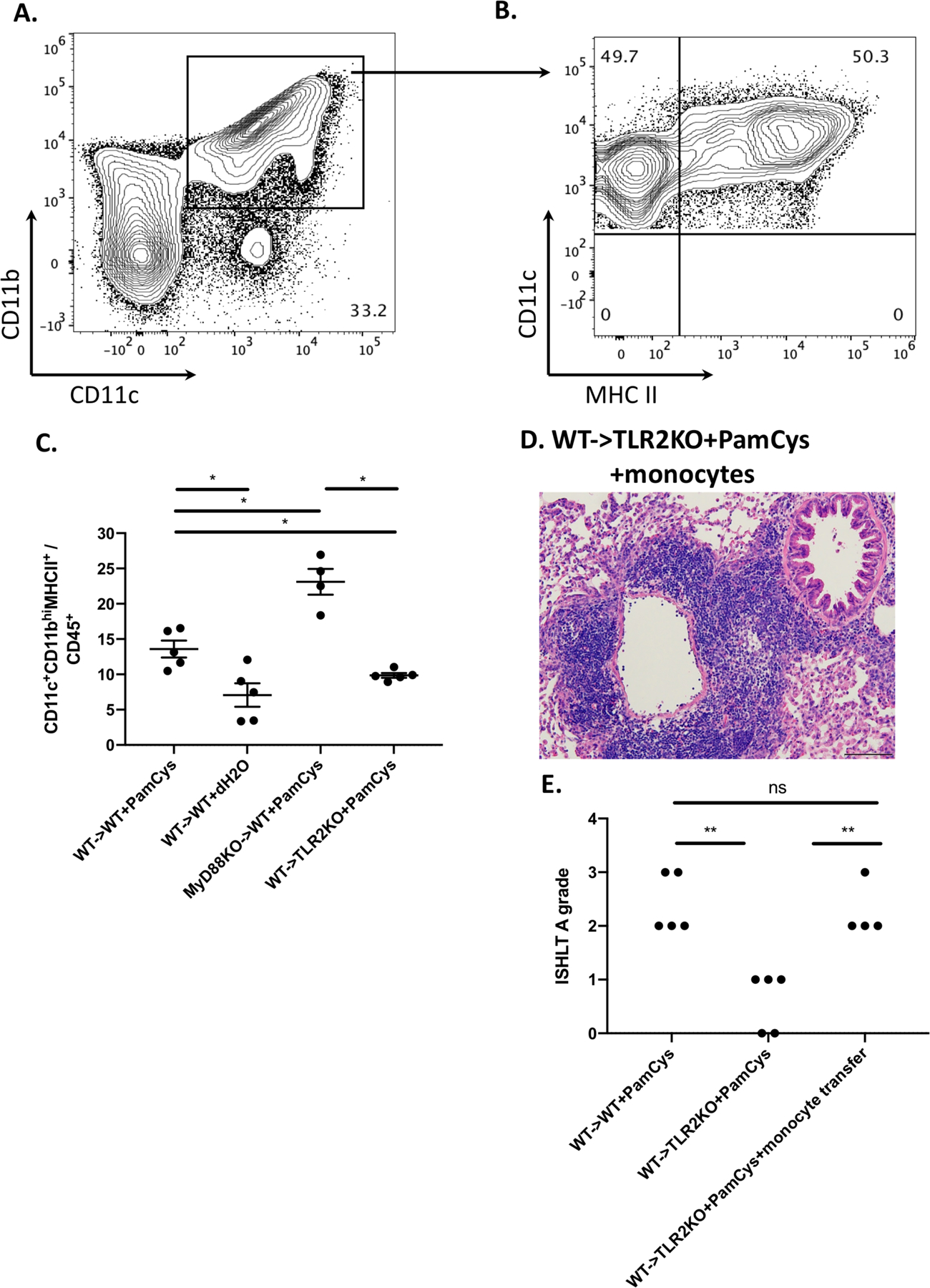
Representative contour plots depicting the percentage of (A) CD11c+CD11bhi cells and (B) CD11c+CD11bhiMHCII+ cells within Balb/c WT lung grafts treated with Pam3Cys4 prior to transplantation into B6 WT recipients. (C) Comparison of CD11c+CD11bhiMHCII+ cell abundance in various experimental groups. Plots and quantifications shown are pre-gated on live, single, CD45+ cells (n=4 or 5 per group as depicted). (D) Histological analysis (H&E) and (E) ISHLT A grade rejection scores of Pam3Cys4-treated Balb/c lung grafts after transplantation into B6 TL2KO recipients that received bone marrow-derived B6 WT monocytes at the time of transplantation (n=4). Original magnification 200x. Scale bar: 100μm. For all experiments, Pam3Cys4 (PamCys) or vehicle (dH2O) was instilled into the donor bronchus just before anastomosis, co-stimulatory blockade was given to recipient mice, and grafts were harvested at day 7. Data represent mean ± SEM. * and ** denote p < 0.05 and p < 0.01, respectively; ns=not significant.
4. Discussion
Our study demonstrates that a bacterial lipoprotein, Pam3Cys4, within the donor airway prior to lung transplantation can prevent the induction of tolerance and lead to acute cellular rejection. In this setting, the immune response to Pam3Cys4 is dependent on TLR2 signaling in recipient, but not donor cells. While CD8+ T cell abundance is increased in rejecting grafts, TLR2 signaling in this cell population is not sufficient to induce rejection in lungs treated with Pam3Cys4 prior to implantation. Conversely, TLR2 signaling in recipient monocytes is required for Pam3Cys4-triggered rejection and results in an increase in CD11c+CD11bhiMHCII+ cells within rejecting lungs.
TLRs are transmembrane glycoproteins that recognize antigens from an array of pathogens, including bacteria, viruses, fungi, and parasites, as well as endogenous ligands that are released during non-apoptotic cell death (25, 26). TLR signaling generates innate immune responses, such as the production of inflammatory cytokines and activation of antigen presenting cells, that protect hosts from such pathogens. We and others have demonstrated that lung allograft rejection can be induced in previously tolerant lung grafts after innate immune responses are activated (7–9). In human lung transplant recipients certain TLR4 polymorphisms that are hyporesponsive to bacterial endotoxin have been associated with significantly fewer acute rejection episodes and increased chronic lung allograft dysfunction-free survival; importantly, these improved outcomes were associated with TLR4 polymorphisms in recipients, but not donors (27, 28). Similarly, in our study TLR2 signaling in recipients, but not donors, was required for Pam3Cys4-triggered rejection. TLR2 signaling is activated by lipoproteins from both Gram-positive and Gram-negative bacteria. Triacylated liporoteins such as Pam3Cys4 are known to bind to a heterodimer complex formed by TLR2 and TLR1 (29); the significance of this TLR2/TLR1 complex in mediating inflammatory responses to infected lung grafts needs to be determined, as some reports have suggested that triacylated lipoproteins can be recognized through TLR2 independent of TLR1 (30). At the time of lung transplantation Gram-positive bacteria, such as Staphylococcus aureus and Streptococcus pneumoniae, are the most common pathogens detected in donor airways (3, 31). Extending previous experimental and clinical studies showing that post-operative bacterial infections or graft airway colonizations are associated with pulmonary allograft rejection, our results suggest that the presence of such organisms in the donor graft prior to transplantation may enhance alloimmune responses against the transplanted lung (9, 32). Nakajima has shown that treatment of infected human donor grafts with antibiotics during ex vivo lung perfusion results in a significant reduction in endotoxin levels in the perfusate (33). Interestingly, a correlation existed between endotoxin concentrations and levels of IL-1β and TNF-α, proinflammatory cytokines that are also increased in Pam3Cys4-treated lungs in our model.
Consistent with our previous studies, we have observed that CD8+ T cells outnumber CD4+ T cells in acutely rejected grafts (20). Despite published reports, however, demonstrating that TLR2 signaling can result in the direct activation of T cells, we found that grafts were not rejected when - among recipient cells – only CD8+ T lymphoyctes were able to repond to Pam3Cys4 (22). Our findings rather suggest a model whereby rejection depends on recipient bone-marrow derived monocytes responding to the TLR2 ligand. In previous work we have shown that monocytes are mobilized from the bone marrow in CCR2-dependent fashion after lung transplantation (23). We have reported that CCR2-deficient recipients have a relative reduction in graft accumulation of CD11c+CD11bhi cells, which is associated with lower local levels of IL12p40. Using two-photon microscopy we have shown that CD11c+ antigen presenting cells interact with T lymphocytes within lung grafts, where they can activate them via both direct and indirect allorecognition (23). We have also previously reported that, following lung transplantation, recipient monocytes preferentially differentiate into CD11c+CD11blow rather than CD11c+CD11bhi cells when recipients lack expression of MyD88 (34). Similarly, others have shown that the activation of inflammatory cytokine production by monocyte-derived antigen presenting cells depends on MyD88 signaling (35). TLR2 signaling leads to monocyte differentiation into macrophage and dendritic cell subsets that upregulate cytokine receptors, release proinflammatory cytokines, and activate T cells (36). Thus, our findings raise the possibility that bacterial products within graft airways trigger the activation of monocytes that infiltrate the transplanted lung, which in turn promotes the local expansion of T cells. Interestingly, lack of graft inflammation when transplanting Pam3Cys4-treated wildtype grafts into TLR2-deficient recipients indicates that the local activation of donor antigen presenting cells is not sufficient to mediate rejection. It is noteworthy that CD8+ T cells and antigen presenting cells appear more activated in lung grafts that lack expression of MyD88. While the precise mechanism underlying this observation deserves further study, this finding is consistent with the notion that TLR signaling plays an important role in tissue repair and recovery from acute lung injury (37).
While we have shown that TLR2 expression on recipient CD8+ T cells is not sufficient to trigger acute rejection after treatment of donor lungs with Pam3Cys4, it is important to point out that other cell populations in the recipient can also express TLR2. For example, NK cells, which can contribute to both rejection and tolerance after organ transplantation, are known to express TLR2 (38). While we have previously shown that T cells are essential to mediate rejection of mouse lung allografts it is possible that activation of NK cells through TLR2 stimulation contributes to the activation of alloreactive T cells in our model (15). Furthermore, evidence exists that recipient NK cells may contribute to the downregulation of alloimmune responses after mouse lung transplantation, possibly through killing of donor antigen presenting cells (39). Therefore, possible augmentation of a tolerogenic role of NK cells through TLR2 stimulation cannot overcome detrimental effects that result from TLR2 activation of recipient monocytes.
We have recently shown that during ischemia reperfusion injury following lung transplantation classical monocytes are mobilized from the recipient spleen to the pulmonary graft, where they mediate neutrophil extravasation (16). In fact, ischemia reperfusion injury is significantly attenuated if the recipient’s spleen is removed prior to transplantation. Mechanistically, we have shown that graft-infiltrating monocytes produce IL-1β in MyD88-dependent fashion, which downregulates junctional proteins in pulmonary endothelial cells thereby facilitating neutrophil entry into the graft tissue and airways. We suggested that graft-infiltrating monocytes are activated through endogenous ligands that are known to be released from injured grafts. While splenectomy did not prevent Pam3Cys4-mediated graft rejection, future studies will need to determine whether bacterial colonization of donor airways exacerbates ischemia reperfusion injury after lung transplantation. Interestingly, ischemia reperfusion injury has been shown to cause inflammation through the TLR2 pathway in other solid organ transplants such as kidneys and hearts (40, 41).
In conclusion, we have shown that a bacterial lipopeptide within the donor airways is capable of preventing tolerance after lung transplantation through a signaling cascade that is dependent on TLR2 in recipient bone marrow-derived monocytes. These findings extend our understanding of the role of monocytes after lung transplantation as key orchestrators of ischemia reperfusion injury as well as graft rejection (16, 20). While most clinical protocols include a bolus of steroids before reperfusion, our tolerance model relied on peri-operative costimulatory blockade. As TLR signaling can be modulated by steroids, future studies will need to determine whether steroids interfere with the monocyte-dependent enhancement of alloreactivity in our current model (42). Similar considerations apply to the use of peri-operative antibiotics in the clinics. Furthermore, we have used a single concentration of Pam3Cys4 is the current study. Future experiments will need to examine whether activation of alloimmune responses is dependent on dose or type of pathogen. To this end, evidence is emerging that the pulmonary allograft microbiome may play an important role in shaping the fate to the transplanted lung (43, 44). For example, a recent study has suggested that the microbiota composition of lung allografts may impact airway remodeling that could have implications for the development of chronic rejection (45). Since lungs are not sterile and the donor microbiome is likely to impact the composition of the microbiome of the allograft after transplantation, future studies need to address how the composition of the donor microbiome impacts the host immune response to the pulmonary graft. Such studies are especially relevant in light of our recent findings that regulatory immune pathways are established locally within the lung graft (10). Therefore, it is not surprising that respiratory infections can disrupt an immunoquiescent state after lung transplantation and trigger the development of chronic lung allograft dysfunction (46). Given the push in recent years to expand donor criteria in hopes of increasing the pool of transplantable lungs, our findings draw attention to possible drawbacks that could be associated with the use of organs that may be colonized with bacteria or harbor pneumonia. A more widespread use of computed tomography in the evaluation of donor lungs will likely result in more frequent detection of radiographic findings that are concerning for infectious processes (47). Ex vivo perfusion with high dose antibiotics may be warranted for infected lungs as two studies have demonstrated that bacterial loads in graft airways can be significantly reduced using this novel approach (33, 48). Also, as human anti-TLR2 antibodies are being evaluated in clinical trials, our findings highlight a novel target for such therapy following lung transplantation (49).
Supplementary Material
Acknowledgments
DK is supported by NIH grants 1P01AI116501, R01HL094601, R01HL151078, Veterans Administration Merit Review grant 1I01BX002730 and The Foundation for Barnes-Jewish Hospital. JMG is supported by 1F32HL143950.
Footnotes
This work was presented at the 2019 American Transplant Congress
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Van Raemdonck D, Neyrinck A, Verleden GM, Dupont L, Coosemans W, Decaluwe H, et al. Lung donor selection and management. Proc Am Thorac Soc 2009;6(1):28–38. [DOI] [PubMed] [Google Scholar]
- 2.Weill D, Dey GC, Hicks RA, Young KR Jr., Zorn GL Jr., Kirklin JK, et al. A positive donor gram stain does not predict outcome following lung transplantation. J Heart Lung Transplant 2002;21(5):555–8. [DOI] [PubMed] [Google Scholar]
- 3.Avlonitis VS, Krause A, Luzzi L, Powell H, Phillips JA, Corris PA, et al. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiothorac Surg 2003;24(4):601–7. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad O, Shafii AE, Mannino DM, Choate R, and Baz MA. Impact of donor lung pathogenic bacteria on patient outcomes in the immediate post-transplant period. Transpl Infect Dis 2018;20(6):e12986. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein DR, Tesar BM, Akira S, and Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest 2003;111(10):1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed EB, Wang T, Daniels M, Alegre ML, and Chong AS. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant 2011;11(5):936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Ahmed EB, Chen L, Xu J, Tao J, Wang CR, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant 2010;10(7):1524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med 2014;189(5):556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Nava RG, Zhu J, Huang HJ, Ibrahim M, Mohanakumar T, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol 2012;189(9):4221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Gauthier JM, Higashikubo R, Hsiao HM, Tanaka S, Vuong L, et al. Bronchus-associated lymphoid tissue-resident Foxp3+ T lymphocytes prevent antibody-mediated lung rejection. J Clin Invest 2019;129(2):556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finger EB, Strom TB, and Matas AJ. Tolerance--is it worth it? Cold Spring Harb Perspect Med 2014;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakes JL, O’Connor BP, Warg LA, Burton R, Hock A, Loader J, et al. Ozone enhances pulmonary innate immune response to a Toll-like receptor-2 agonist. Am J Respir Cell Mol Biol 2013;48(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HS, Xu F, Bagchi A, Herrup E, Prakash A, Valentine C, et al. Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J Immunol 2011;186(2):1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni K, Gill A, Cao D, Koike K, Schweitzer KS, Garantziotis S, et al. Intravascular heavy chain-modification of hyaluronan during endotoxic shock. Biochem Biophys Rep 2019;17:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest 2014;124(3):1130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao HM, Fernandez R, Tanaka S, Li W, Spahn JH, Chiu S, et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1beta. J Clin Invest 2018;128(7):2833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26(12):1229–42. [DOI] [PubMed] [Google Scholar]
- 18.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant 2007;7(6):1672–9. [DOI] [PubMed] [Google Scholar]
- 19.Zahringer U, Lindner B, Inamura S, Heine H, and Alexander C. TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 2008;213(3–4):205–24. [DOI] [PubMed] [Google Scholar]
- 20.Gelman AE, Okazaki M, Lai J, Kornfeld CG, Kreisel FH, Richardson SB, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol 2008;180(7):4754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komai-Koma M, Jones L, Ogg GS, Xu D, and Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A 2004;101(9):3029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercier BC, Cottalorda A, Coupet CA, Marvel J, and Bonnefoy-Berard N. TLR2 engagement on CD8 T cells enables generation of functional memory cells in response to a suboptimal TCR signal. J Immunol 2009;182(4):1860–7. [DOI] [PubMed] [Google Scholar]
- 23.Gelman AE, Okazaki M, Sugimoto S, Li W, Kornfeld CG, Lai J, et al. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant 2010;10(5):1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landsman L, Varol C, and Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 2007;178(4):2000–7. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, and Chadban SJ. Roles of Toll-like receptors in transplantation. Curr Opin Organ Transplant 2014;19(1):1–7. [DOI] [PubMed] [Google Scholar]
- 26.Opitz B, van Laak V, Eitel J, and Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med 2010;181(12):1294–309. [DOI] [PubMed] [Google Scholar]
- 27.Palmer SM, Burch LH, Trindade AJ, Davis RD, Herczyk WF, Reinsmoen NL, et al. Innate immunity influences long-term outcomes after human lung transplant. Am J Respir Crit Care Med 2005;171(7):780–5. [DOI] [PubMed] [Google Scholar]
- 28.Palmer SM, Burch LH, Davis RD, Herczyk WF, Howell DN, Reinsmoen NL, et al. The role of innate immunity in acute allograft rejection after lung transplantation. Am J Respir Crit Care Med 2003;168(6):628–32. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 2002;169(1):10–4. [DOI] [PubMed] [Google Scholar]
- 30.Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, Akira S, et al. TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 2006;281(14):9049–57. [DOI] [PubMed] [Google Scholar]
- 31.Bonde PN, Patel ND, Borja MC, Allan SH, Barreiro CJ, Williams JA, et al. Impact of donor lung organisms on post-lung transplant pneumonia. J Heart Lung Transplant 2006;25(1):99–105. [DOI] [PubMed] [Google Scholar]
- 32.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85(5):771–4. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima D, Cypel M, Bonato R, Machuca TN, Iskender I, Hashimoto K, et al. Ex Vivo Perfusion Treatment of Infection in Human Donor Lungs. Am J Transplant 2016;16(4):1229–37. [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto S, Lin X, Okazaki M, Lai J, Tietjens JR, Huang H, et al. Monocyte differentiation is controlled by MyD88 after mouse orthotopic lung transplantation. Transplant Proc 2009;41(1):388–90. [DOI] [PubMed] [Google Scholar]
- 35.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, and Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 2003;19(6):891–901. [DOI] [PubMed] [Google Scholar]
- 36.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med 2005;11(6):653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11(11):1173–9. [DOI] [PubMed] [Google Scholar]
- 38.Martinez J, Huang X, and Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog 2010;6(3):e1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jungraithmayr W, Codarri L, Bouchaud G, Krieg C, Boyman O, Gyulveszi G, et al. Cytokine complex-expanded natural killer cells improve allogeneic lung transplant function via depletion of donor dendritic cells. Am J Respir Crit Care Med 2013;187(12):1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WB, Tobias PS, et al. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 2007;178(10):6252–8. [DOI] [PubMed] [Google Scholar]
- 41.Arslan F, Smeets MB, O’Neill LA, Keogh B, McGuirk P, Timmers L, et al. Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation 2010;121(1):80–90. [DOI] [PubMed] [Google Scholar]
- 42.Broering R, Montag M, Jiang M, Lu M, Sowa JP, Kleinehr K, et al. Corticosteroids shift the Toll-like receptor response pattern of primary-isolated murine liver cells from an inflammatory to an anti-inflammatory state. Int Immunol 2011;23(9):537–44. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AB. The lung microbiome and transplantation. Curr Opin Organ Transplant 2019;24(3):305–10. [DOI] [PubMed] [Google Scholar]
- 44.Becker J, Poroyko V, and Bhorade S. The lung microbiome after lung transplantation. Expert Rev Respir Med 2014;8(2):221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouraux S, Bernasconi E, Pattaroni C, Koutsokera A, Aubert JD, Claustre J, et al. Airway microbiota signals anabolic and catabolic remodeling in the transplanted lung. J Allergy Clin Immunol 2018;141(2):718–29 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentine VG, Gupta MR, Walker JE Jr., Seoane L, Bonvillain RW, Lombard GA, et al. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2009;28(2):163–9. [DOI] [PubMed] [Google Scholar]
- 47.Gauthier JM, Bierhals AJ, Liu J, Balsara KR, Frederiksen C, Gremminger E, et al. Chest computed tomography imaging improves potential lung donor assessment. J Thorac Cardiovasc Surg 2019;157(4):1711–8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreasson A, Karamanou DM, Perry JD, Perry A, zalp F, Butt T, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant 2014;33(9):910–6. [DOI] [PubMed] [Google Scholar]
- 49.Anwar MA, Shah M, Kim J, and Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev 2019;39(3):1053–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


