Abstract
Sphingolipids are a unique class of lipids owing to their non-glycerol-containing backbone, ceramide, that is constructed from a long-chain aliphatic amino alcohol, sphinganine, to which a fatty acid is attached via an amide bond. Ceramide plays a star role in the initiation of apoptosis by virtue of its interactions with mitochondria, a control point for a downstream array of signaling cascades culminating in apoptosis. Many pathways converge on mitochondria to elicit mitochondrial outer membrane permeabilization (MOMP), a step that corrupts bioenergetic service. Although much is known regarding ceramides interaction with mitochondria and the ensuing cell signal transduction cascades, how ceramide impacts the elements of mitochondrial bioenergetic function is poorly understood. The objective of this review is to introduce the reader to sphingolipid metabolism, present a snapshot of mitochondrial respiration, elaborate on ceramides convergence on mitochondria and the upstream players that collaborate to elicit MOMP, and introduce a mitochondrial phenotyping platform that can be of utility in dissecting the fine-points of ceramide impact on cellular bioenergetics.
Keywords: Ceramide, Mitochondria, Bioenergetics, Sphingolipids, Cancer
1. Introduction
Mitochondria play a principle role in orchestrating the intrinsic pathway of apoptosis. This cell death spiral can be elicited by cellular stimuli such as radiation, DNA-damaging anticancer drugs, and ER stress. The essential position that the sphingolipid (SL), ceramide exerts in mitochondrial-driven apoptosis has been the subject of extensive studies. It is well known that levels of ceramide increase in cells in response to extracellular insult. Several key SL-metabolizing enzymes are involved in increased ceramide production; this leads to apoptosis via intermediate interaction at the mitochondrial level. When blockers of SL enzymes involved in ceramide synthesis are introduced, apoptotic responses are counteracted, hence the keen connection between ceramide and programmed cell death. Mitochondrial outer membrane permeability (MOMP) is central for the successful apoptotic cascade in which the formation of ceramide channels is believed to participate. Although much is known regarding ceramide interaction with mitochondria and ensuing signaling cascades, how ceramide impacts the elements of mitochondrial bioenergetic function is poorly understood. Establishing the decisive molecular mechanisms by which cellular ceramides directly impinge on mitochondrial bioenergetics is open for exploration. Such studies, aided by an “omics” type of analysis, should be essential in identifying novel, mitochondrial-targeted therapeutics designed to combat diseases, including cancer.
2. Sphingolipids 101, an overview
To most, the mention of lipids recalls our recent blood work with levels of triglycerides, now termed triacylglycerols, and cholesterol, which is not a lipid. And of course, we know that lecithin, also known as phosphatidylcholine, is found in egg yolk and ice cream, for those of us who read labels. Moving on in complexity, many of us connect the term sphingolipids (SL) with sphingomyelin that we understand has something to do with myelin that has a lot to do with nerves and brain. Ceramide, on the other hand, is to many, a wonderous ingredient in hand cream, a skin-restoring moisturizer with considerable fame to the lay public. For the curious, however, how did science come up with the moniker, sphingolipid”?
SL’s were first identified in the 1870’s in extracts of brain, and because of their enigmatic nature they were named after the mythological creature, the Sphinx. Why enigmatic? Many brain SL’s are highly glycosylated and do not dissolve in traditional lipid solvents like chloroform, often used by the chemists of the day. Further adding to the enigma, these lipids frequently contain very long-chain fatty acids coupled as an amide bond that is not labile to mild alkaline treatment, as are the fatty acids in glycerolipids. So, there you have it, “sphinx-o-lipids”, a unique class of lipids with a cornucopia of amazing functions 1.
Sphingolipids comprise an enormous family of lipids found in animals, plants, fungi, and in some prokaryotic organisms and viruses. Appropriate in this 2020 pandemic, FTY720 (Fingolimod), a sphingomimetic drug that promotes SL receptor internalization and degradation, is in clinical trials for management of COVID-19 (NCT04280588 and NCT04276688—ClinicalTrials.gov) 2.
SL’s do not contain the trihydroxy alcohol backbone, glycerol, common to glycerolipids (mono-, di-, and triglycerides, phospholipids) but instead harbor ceramide, a main feature in this review. Ceramide is constructed from sphingosine, an aliphatic amino alcohol to which a fatty acid is attached via an amide bond. Ceramide is the neutral lipid building block of sphingolipids or, if they contain sugars, of glycosphingolipids. These lipids not only serve structural roles in biomembranes, but they also have wide-ranging effects on signal transduction and the regulation of cell function. Chief among these actions is the potentiation of signaling cascades with emphasis on mitochondrial interactions that lead to apoptosis, the “tumor suppressor” property of ceramide.
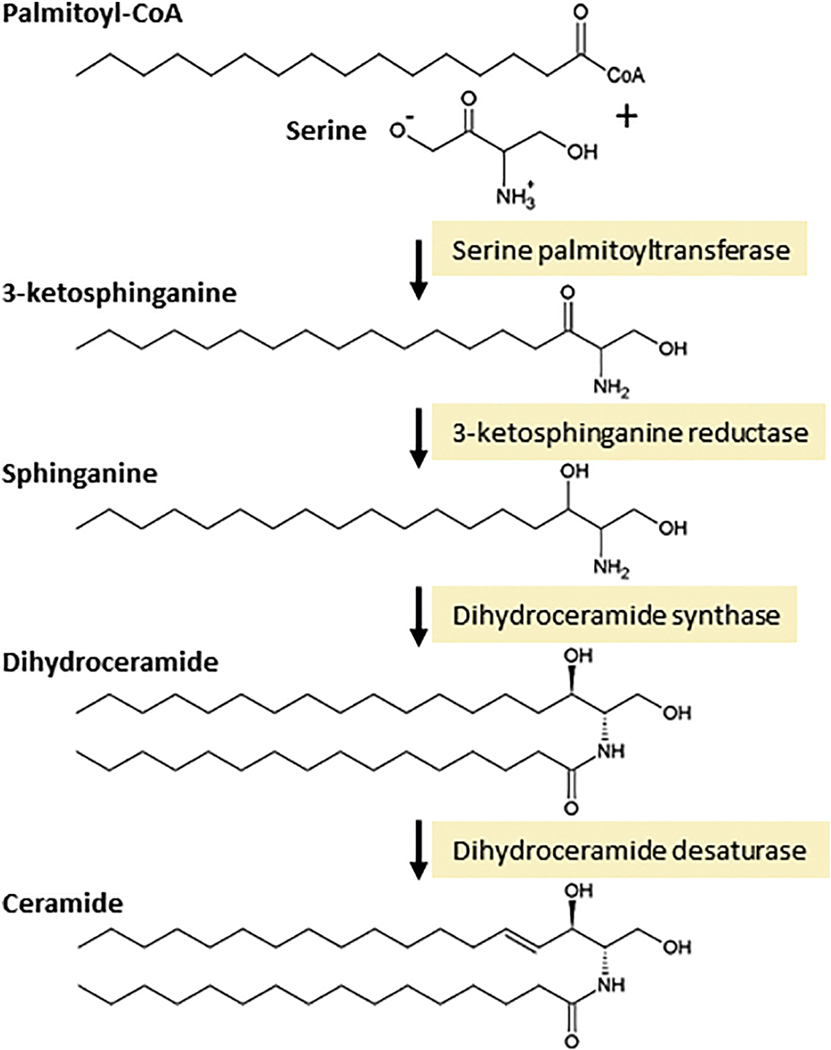
The steps of ceramide biosynthesis, de novo, are illustrated in Fig. 1. In brief, the synthesis of ceramide is initiated by serine palmitoyltransferase, which uses serine and palmitoyl-CoA to generate the ‘sphingoid’ base, sphinganine. On towards the production of ceramide, sphinganine is acylated by dihydroceramide synthases, a sextet of enzymes that produce an astonishing array of chemically diverse dihydroceramides, via dihydroceramide synthase preferences for specific acyl-CoAs. This specificity produces dihydroceramides with carbon chain lengths ranging from C14 to C32, with varying degrees of saturation. Dihydroceramides are not the ceramides we usually read about or buy in hand lotion, until a desaturase step, catalyzed by dihydroceramide desaturase, completes ceramide production via insertion of a 4,5-trans double bond. Ceramide can also be produced by the action of specialized phospholipases, known as sphingomyelinases. These enzymes, which are classified according to their optimum pH and subcellular locations, cleave SM at the phosphodiester bond that is proximal to ceramide, producing ceramide and choline phosphate.
Fig. 1. Biosynthesis of ceramide by the de novo pathway.
Ceramide is composed of a sphingolipid base, sphinganine, joined by an amide bond to a fatty acid. Ceramide is synthesized in the endoplasmic reticulum. The de novo steps begin with the condensation of serine and palmitoyl-CoA, catalyzed by serine palmitoyltransferase. The product, 3-ketosphinganine, contains 18 carbons and is reduced to sphinganine by 3-ketosphinganine reductase. The next step generates ceramides saturated precursor, dihydroceramide, via the action of dihydroceramide synthase, of which there are several isoforms that ultimately give rise to a multitude of molecular species of ceramide with distinct roles. Finally, although dihydroceramide is nearly identical in structure to ceramide, it lacks the 4, 5-trans double bond, which is inserted by dihydroceramide desaturase to form ceramide.
As nature would have it, many of the actions of ceramide are molecular species specific in biology ranging from cancer to diabetes to coronary artery disease to cystic fibrosis 3–11. For example, work by Siddique et al, 12 illustrated that ceramide molecular species nuances focusing on the 4,5-trans double bond were required for cancer cell sensitivity to etoposide, a semisynthetic derivative of podophyllotoxin from the rhizome of the wild mandrake that is used in the treatment of Kaposi’s and Ewing’s sarcoma, lung and testicular cancer, and glioblastoma. Rudd and Devaraj 13 showed that the apoptotic impact of several ceramide species was dependent on acyl-chain saturation, and in aging where loss of skeletal muscle mass is widespread and unwelcome, CerS1 and 5 were identified as potential regulators of fiber size and strength 14.
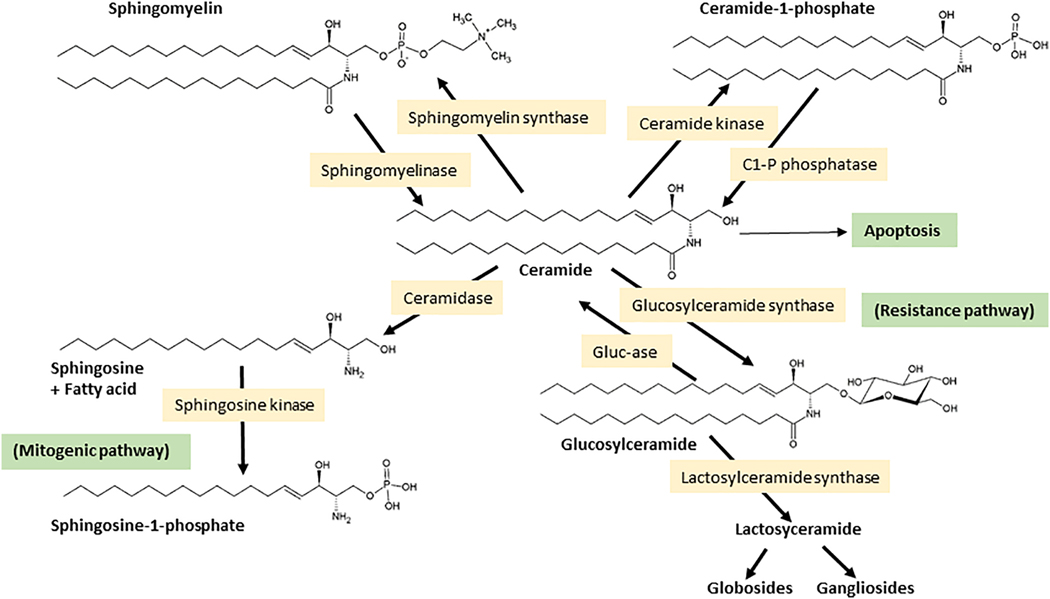
Two additional points of ceramide generation merit mention. These are essentially the back reactions to ceramide kinase, catalyzed by ceramide 1-phosphate phosphatase, and glucosylceramide synthase, catalyzed by glucosylcerebrosidase (Fig. 2). So, what becomes of ceramide once generated? By and large, tumor cells can be protected from ceramide’s apoptosis-inducing effects by two major constructive metabolic steps that produce a wide variety of SL’s such as sphingomyelin (SM), glucosylceramide (GC), the precursor of higher cerebrosides, globosides, and gangliosides, and galactosylceramides, the precursors of sulfatides (Fig. 2). Of particular interest here is ceramide glycosylation, a voracious metabolic pathway utilized in multidrug resistant cancer cells to facilitate ceramide clearance 15–17. Whereas ceramide is a powerful tumor suppressor, the glycosylated product, GC, formed by the action of glucosylceramide synthase (GCS) (Ceramide: UDP-Glc Glucosyltransferase), is ineffectual in this realm. Upregulated ceramide glycosylation is an avenue through which cancer cells skirt the deleterious effects of ceramide 18–22. Ceramide can also be converted to ceramide1-phosphate (C1-P) by ceramide kinase. C1-P plays various, novel roles in cell proliferation, wound healing, inflammation, and tumor cell metastasis 23–25; signaling aspects of C1-P have recently been reviewed 26. Also relevant is ceramide hydrolysis, specifically by acid ceramidase (AC), another sentinel enzyme regulator of cancer cell growth 27–32. Similar with GCS, AC thwarts the tumor-killing properties of ceramide via hydrolysis, producing fatty acids and sphingosine, the latter a substrate for sphingosine kinase (SPHK), the enzyme catalyzing production of sphingosine 1-phosphate (S1-P), a cancer cell mitogen 33–35. In summary, ceramide can be generated de novo or via sphingomyelin hydrolysis by a host of agents, anticancer drugs included 36, and ceramide’s elimination can be hastened by hydrolysis or conversion to higher SL’s. What little ceramide manages to hang around has, as we shall read, a tremendous impact on mitochondrial function.
Fig. 2. Cellular fate of ceramide.
Ceramide can be produced by the action of specialized phospholipases, known as sphingomyelinases. Sphingomyelinases, which are characterized according to their optimum pH and subcellular locations, cleave sphingomyelin at the phosphodiester bond that is proximal to ceramide, producing ceramide and choline phosphate. Ceramide can also be generated by the action of ceramide 1-phosphate phosphatase (C1-P phosphatase) and via glucosylcerebrosidase (Gluc-ase). Once produced, ceramide can be hydrolyzed by ceramidase, glycosylated by glucosylceramide synthase (GCS), or phosphorylated by ceramide kinase producing ceramide 1-phosphate (C1-P). Strategic points in de novo synthesis and in subsequent ceramide metabolism can be activated or inhibited, providing useful avenues for studying ceramide-regulated events and for controlling cell fate. Enzyme inhibitors and P-glycoprotein (P-gp) antagonists are often used to amplify the induction of cell death by ceramide. Enzymes that “remove” ceramide either by destructive, ceramidase, or constructive metabolism, glucosylceramide synthase (GCS), can contribute to cancer cell growth.
3. Mitochondrial respiration in a nutshell
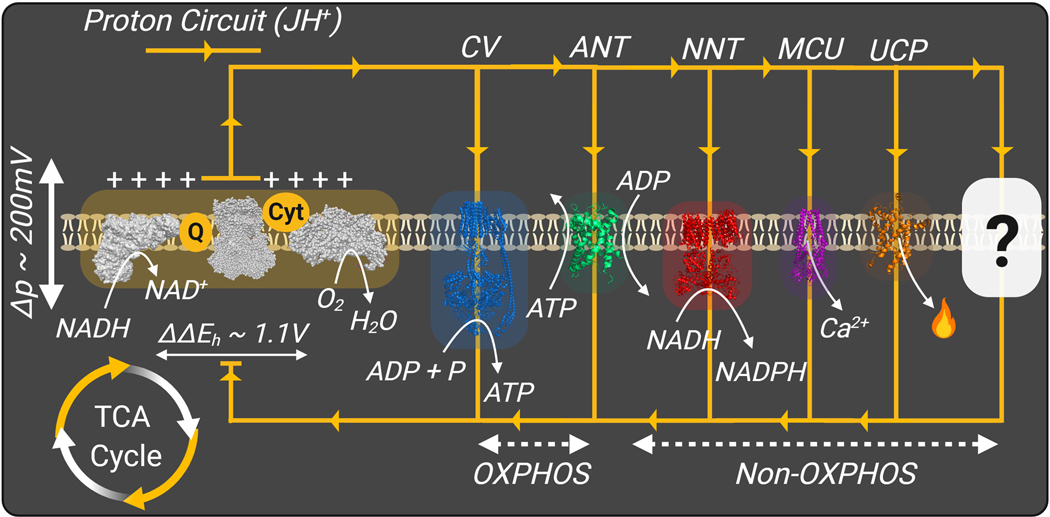
Mitochondria are double-membrane organelles ubiquitous to all mammalian cells, sans red blood cells. Across eukaryotic life, mitochondria are critically involved in generating and maintaining cellular energy charge. They accomplish this task through a series of energy transduction reactions that ultimately transduce the chemical potential energy of electron-rich carbon intermediates to ATP free energy (ΔGATP). Oxidation of carbon intermediates is carried out in the matrix space by a network of dehydrogenase enzymes that deliver electrons to the electron transport system (ETS) that upon electron transport through a series of multi-subunit protein complexes leads to O2 reduction to generate H2O. Driven by the redox potential energy span across the ETS (NADH/NAD+ ~ H2O/O2), three of the respiratory complexes pump protons across the inner-mitochondrial membrane to generate an electrochemical proton gradient known as the proton motive force (Δp). The mitochondrial proton motive force is in turn coupled to a variety of cellular processes that carry out essential cellular functions, including ATP synthesis (ATP synthase) and exchange (phosphorylation system; ATP synthase ‘Complex V’, adenine nucleotide translocase ‘ANT’), as well as, NADPH generation (nicotinamide nucleotide transhydrogenase ‘NNT’), calcium cycling, and metabolite exchange (Fig. 3).
Fig. 3. Mitochondrial Energy Transduction.
Mitochondrial energy transfer mediated by the dehydrogenase network, ETS, and the phosphorylation system. Mitochondrial flux is depicted as a H+ circuit conducted via various ‘OXPHOS’ and ‘Non-OXPHOS’ resistors; ‘?’–predicted ‘Non-OXPHOS’ resistors. Proton motive force (Δp), Complex V (CV), Adenine nucleotide translocase (ANT), Nicotinamide nucleotide transhydrogenase (NNT), mitochondrial calcium uniporter (MCU), Uncoupling protein (UCP).
Although best known for their role in cellular ATP generation through the process of oxidative phosphorylation (OXPHOS), it is now understood that mitochondria are central to a wide variety of cellular functions (e.g., reactive oxygen species production, calcium buffering, macromolecular synthesis). In addition, mitochondria are critically involved in cellular survival through regulation of the intrinsic apoptotic pathway (discussed below). Given that mitochondria are present in all cell types (RBCs excluded), the assumption has been that all mitochondria are alike, and that function declines due to disease or aging. However, new evidence is emerging that all mitochondria are not alike but, in fact, are unique in composition and function within each cell type 37,38, including cancer cells 39,40. This raises the exciting possibility that identifying the unique bioenergetic signature(s) of cancer cells may hold the key to designing mitochondrial-targeted chemotherapeutics that specifically target only those cancer cells. Related to this, mounting evidence indicates that in addition to alterations in ceramide handling, multi-drug resistance in cancer involves alterations in mitochondrial form and function that facilitates cancer cell survival 41,42.
4. Ceramide convergence on mitochondria— the end game
Mitochondria instigate the intrinsic apoptotic pathway, obviously initiated from within the cell; the consequences are executed downstream. It is important to understand that through ceramide, whether by intracellular generation 43 or exogenous administration 44,45, there exists the means to promote tumor cell death via a myriad of signal transduction pathways that converge on mitochondria to elicit release of proapoptotic proteins; a process which results in mitochondrial damage. Although the present work is focused on ceramide, note that Obeid and colleagues 46 presented an excellent recap dealing with the broader subject of SL’s and mitochondria.
Regarding cancer and the intrinsic apoptotic pathway, resistance to mitochondrial apoptosis is a prominent feature of cancer cells; however, in some situations, ceramide can override this. As ceramide is highly insoluble, an energy barrier hinders its intracellular movement from the plasma membrane or other intracellular membranes in which it is produced. Therefore, how ceramide reaches mitochondria to modulate function is relevant and will be briefly discussed.
Upon generation in the plasma membrane, ceramide forms platforms that subsequently invaginate and fuse with mitochondria 47,48. This event has been termed ‘the kiss of death’ in other words, the end game. Via this commute, ceramide can be transferred directly from the plasma membrane to the mitochondria, a function promoting mitochondrial ceramide accumulation and the induction of apoptosis 47,48. Some studies indicate that endoplasmic reticulum (ER) membranes, called mitochondria-associated membranes, are physically and functionally allied with mitochondria to integrate several aspects of ER and mitochondrial function 49,50. Experiments with isolated mitochondria have revealed that these mitochondria-associated membranes (known as ER-like membranes) can produce enough ceramide through ceramide synthases or the salvage pathway to transiently permeabilize the outer mitochondrial membrane 49. Ceramide also reaches mitochondria through the localization of CerS (ceramide synthases), neutral SMase, and neutral ceramidase in (or associated with) mitochondria to orchestrate in situ ceramide production 51–54.
5. Ceramide and mitochondrial outer membrane permeabilization (MOMP)—same old, same old
The following summarizes the effects of ceramide on mitochondrial-governed apoptosis. These ideas have been the mainstay in the field; however, there is more to ceramide’s impact than meets the eye. For example, what are the bioenergetic consequences of a “ceramide storm”, a storm elicited either by ceramide-generating drugs or by administration of ceramide itself 55. This question will be visited in a latter section entitled, Mitochondrial Diagnostics and deciphering the fine points of ceramide’s impact —what we can learn.
Cancer cells typically display dysfunctional apoptotic pathways that in most cases arise through a defect in intrinsic pathway sensitivity. Therefore, the idea that mitochondria might serve as a target in cancer therapy 56–59 makes sense. A common cause of tumorigenesis lies in the imbalance between the proapoptotic (BAX and BAK) and the antiapoptotic (Bcl-2) members of the Bcl-2 family. Bcl-2 expression is linked to cell survival and resistance to chemotherapy 60,61, whereas Bcl-2 mutations suppress the normal functions of the proapoptotic proteins BAX and BAK 62. Elevated ceramide levels can leverage MOMP, which is crucial for apoptotic signaling 63,64. MOMP is closely regulated via interactions between the for- and against- apoptosis members of the Bcl-2 family 65, a delicate minuet that can elicit the “point of no return”. Stress caused by elevated ceramide coax a dialogue between Bcl-2 family proteins that control activation of effector relatives, BAX and BAK. Some have shown that ceramide alone is not enough to induce MOMP 66,67, but instead ceramide and BAX are thought to synergistically permeabilize the outer mitochondrial membrane and induce apoptosis 66,67. Ceramide-rich macrodomains and/or channels in the outer mitochondrial membrane are essential for BAX insertion, oligomerization, and pore formation in order to induce MOMP 68,69. Short-chain ceramides can also induce MOMP 70. MOMP promotes leakage of apoptotic proteins, like cytochrome c and SMAC (second mitochondria-derived activator of caspases), and the release of intermembrane space proteins with a molecular mass of less than ~60,000 Da 71–73. SMAC-DIABLO triggers caspase 3 activation. Of note and on point, MOMP directly correlates with the levels of ceramide in the outer mitochondrial membrane 72. Together, we see that ceramide upregulates mitochondrial proapoptotic proteins, which is a good thing, and at the same time directly targets mitochondria. This relationship between ceramide and mitochondria and the consequences of this interaction highlight the appeal of ceramide as a therapeutic agent 55,74,75 that can stymie cancer cell resistance to the intrinsic apoptotic pathway.
6. From plasma membrane to mitochondria—a concerto of ceramide-involved signaling
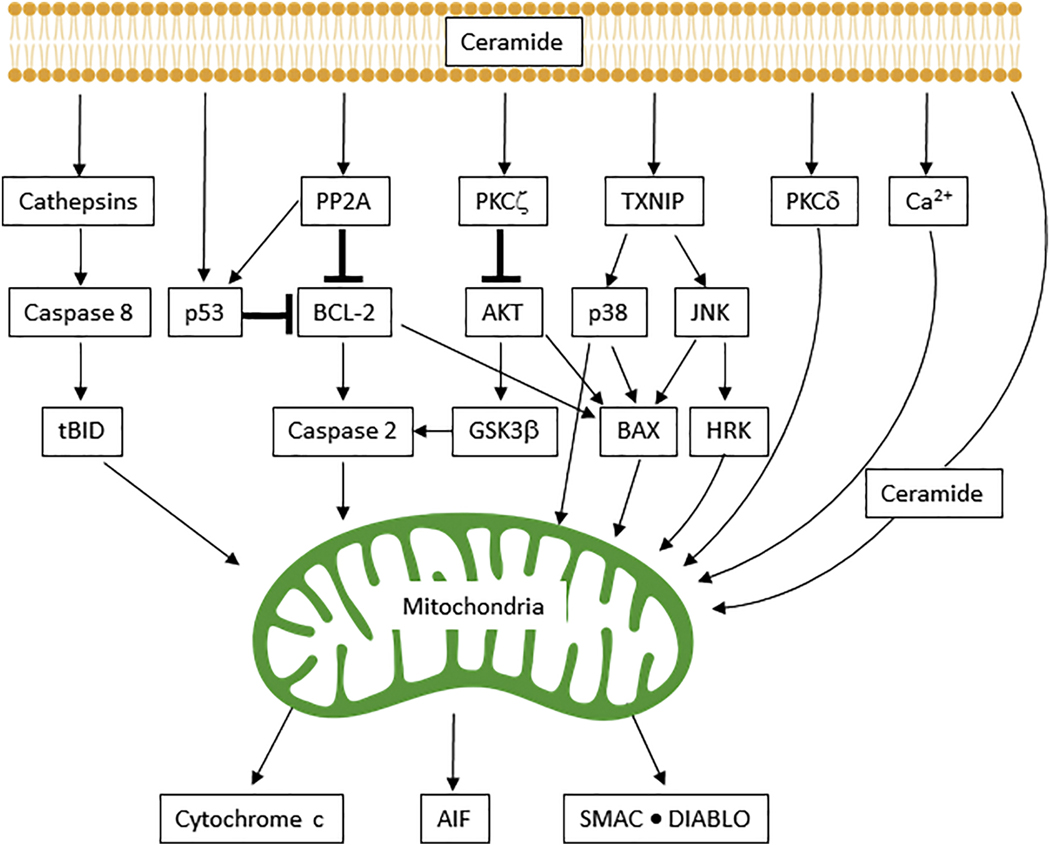
The translocation of BAX from 14-3-3 proteins, a family of regulatory molecules that bind a multitude of signaling elements 76,77 in the cytoplasm, to mitochondria requires JUN N-terminal kinase (JNK) activation 78,79. Ceramide-mediated activation of p38 MAPK 80,81 or downregulation of AKT can elicit BAX translocation to mitochondria 81. Ceramide can also contribute to MOMP via glycogen synthase kinase 3β (GSK3β) 82, by activation of protein phosphatase 2A (PP2A), via inactivation of AKT, and via activation of the endolysosomal protease cathepsin D 83–86 (Fig. 4). This leads to caspase 2 and caspase 8 activation, and the cleavage of BID to form tBID, which translocates to mitochondria 83,87–89. Ceramide can also impact mitochondrial function by inducing translocation and activation of protein kinase Cδ (PKCδ) to mitochondria. This promotes cytochrome c release and caspase 9 activation, as demonstrated in a model of prostate cancer 90. Bcl-2 89,91, calcium overload 87,91, and cell death receptors 92 are also involved in inducing MOMP. Revisiting the omni-potent Bcl-2 family, ceramide can activate caspase 2 through the downregulation of Bcl-2 via activation of PP2A, which also leads to MOMP (Fig. 4). On the other hand, overexpression of Bcl-2 or low calcium can prevent ceramide-induced caspase 2 activation and mitochondrial apoptosis 89,91,93. In summary, ceramide-mitochondria interactions are a complex aspect of cancer biology that put mitochondria in the position of “hired gun”.
Fig. 4. Musicians in the intrinsic pathway of apoptosis—a large ensemble.
The intrinsic mitochondrial pathway of ceramide-assisted apoptosis is largely regulated by caspases and Bcl-2 family members. Crosstalk between signaling members is designated by arrows. Loss of mitochondrial outer membrane permeability (MOMP) subsequently leads to the release of proapoptotic proteins: cytochrome c, apoptosis-inducing factor (AIF), and second mitochondria-derived activator of caspase (SMAC)–direct inhibitor of apoptosis protein (IAP)-binding protein with low PI (DIABLO). GSK3β, glycogen synthase kinase 3β; HRK, harakiri; JNK, JUN N-terminal kinase; PKC, protein kinase C; PP2A, protein phosphatase 2A; TXNIP, thioredoxin interacting protein.
Neutral and acid sphingomyelinases, ceramide synthases, and ceramidases manufacture ceramide for the initiation of mitochondrial-directed apoptosis. These enzymes are often cell type-specific in cancer; some phenotypes might harbor suppressed ceramide synthase activity, whereas others, as with ceramide overproduction, might overexpress GCS to decrease ceramide levels. Overexpression of GCS is particularly relevant to multidrug-resistant cancer 36,94–96. Moreover, the ablation of ceramide synthase 2, which catalyzes synthesis of very long acyl chain ceramides (C22-C24), was shown to produce chronic oxidative stress via disruption of Complex IV, thought by the authors to result from compensatory increases in C16 ceramide 97.
7. Mitochondrial Diagnostics and deciphering the fine points of ceramide’s impact —what we can learn.
Ceramide plays a premier role in the initiation of cell death by virtue of its interactions with mitochondria, a control point for a downstream array of signaling cascades culminating in apoptosis. Many pathways converge on mitochondria to elicit MOMP, a step that corrupts bioenergetic service. Although much is known regarding ceramides interaction with mitochondria and the ensuing cell signal transduction cascades, how ceramide impacts the elements of mitochondrial bioenergetic function is poorly understood. To begin to fill in this gap in knowledge, we leveraged a recently described mitochondrial diagnostics workflow 37. The key technological advancement of this assay workflow is the utilization of the creatine kinase (CK) clamp that allows bioenergetic assays to be performed across physiological ATP free energies (ΔGATP). Such conditions mimic live cell thermodynamic energy constraints and in so doing provide a quantitative snapshot of mitochondrial OXPHOS kinetics, relative to total respiratory capacity. Exposure of energized mitochondria to more negative ΔGATP values (i.e, increasing ATP free energies) inversely impacts respiratory flux, as the build-up of ATP/ADP is transmitted throughout the entire energy transduction system to slow flux. By evaluating OXPHOS kinetics with a variety of distinct carbon substrate combinations, in combination with parallel assays designed to quantify total respiratory capacity, it becomes possible to assign a given change in bioenergetic flux to a specific energy transduction control node: 1. Matrix Dehydrogenases, 2. Electron transport system (ETS), 3. ATP synthesis.
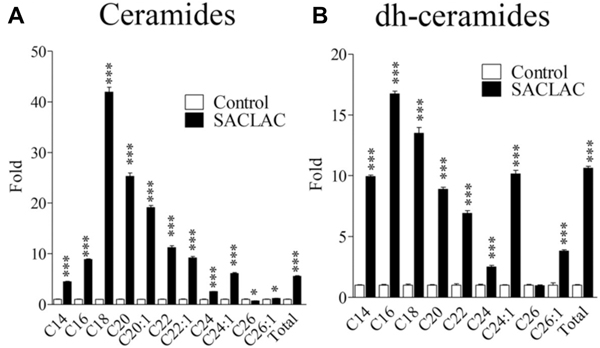
To demonstrate the utility of our novel, mitochondrial phenotyping system, we tested the effect of SACLAC, an AC inhibitor 96,98 on mitochondrial function using a chemotherapy resistant leukemia cell model, HL-60/dnr (daunorubicin resistant) 96. SACLAC blocks the hydrolysis of ceramide into its components, sphingosine, and fatty acid (see Fig. 2), and thus introduction should result in the buildup of intracellular ceramides. We anticipated that ceramide measurement data (mass spectroscopy) in response to inhibition of AC would allow understanding of how ceramide changes support response. As shown in Fig. 5, SACLAC exposure promoted phenomenal increases in nearly all ceramide and dihydroceramide molecular species in HL-60/dnr cells, a striking outcome. This model is thus well suited for studying the impact of ceramide molecular species on mitochondrial function 3,99–102 and opens avenues for exploring novel dihydroceramides, players that deserve at least some attention 103–108.
Fig. 5. Effect of SACLAC exposure on ceramide levels in drug-resistant leukemia cells.
HL-60/dnr cells (resistant to daunorubicin) (800,000/ml dnr-free, RPMI-1640 medium, 10% FBS) were exposed to 10 μM SACLAC (DMSO vehicle) or DMSO (control) for 24 hr. Cells were then harvested by centrifugation, washed three times in PBS, and subjected to lipidomic evaluation by LC/ESI/MS/MS. A. Ceramide molecular species. B. Dihydroceramide molecular species. Viability in SACLAC-treated cells was 80% at harvest. Data are expressed as fold change compared with untreated controls. Data from Kao L-P, et al., J. Lipid Res., 60: 1590–1602, 2019, and reproduced with permission from the publisher.
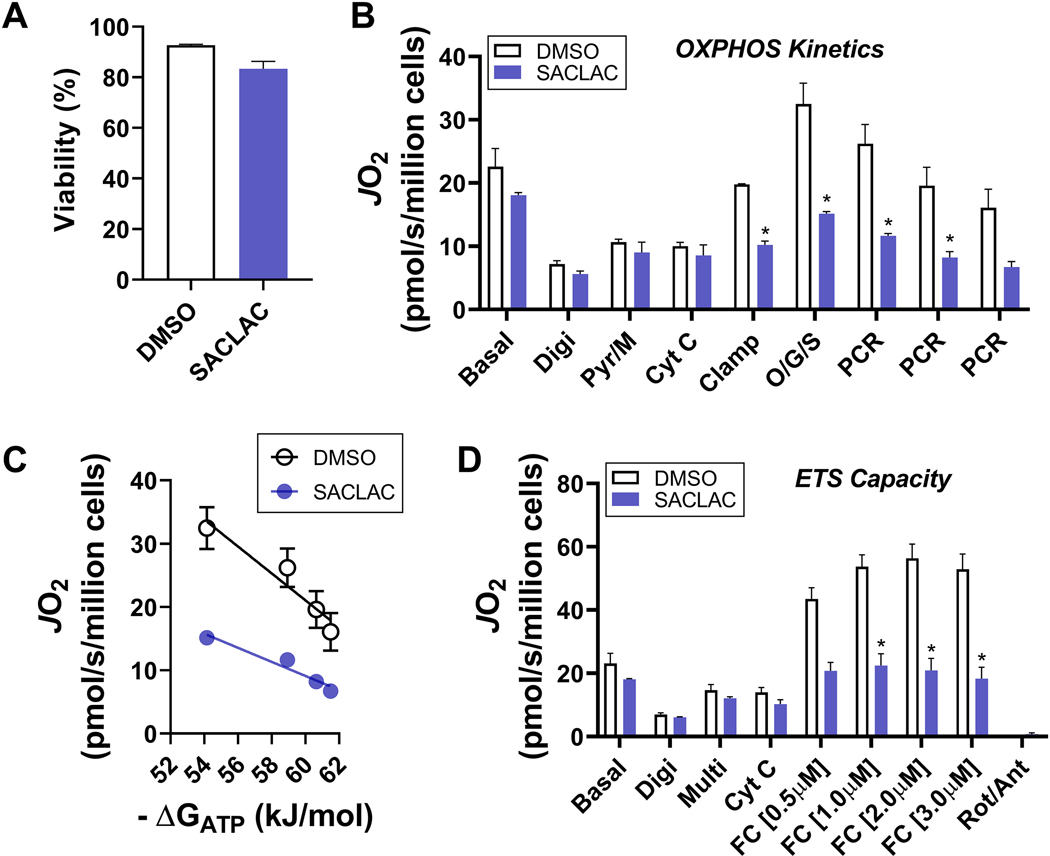
The influence of the “dihydroceramide/ceramide storm” on cellular bioenergetic function is summarized in Fig. 6. Importantly, these 24 hr changes were observed before overt cytotoxicity ensued after a 72 hr exposure to SACLAC 96. At the 24 hr timepoint, despite minimal changes in cell viability (Fig. 6A) and basal respiration (Fig. 6B & 6D, ‘Basal’), respiration stimulated by physiological ΔGATP was dramatically impaired in SACLAC exposed cells (Fig. 6B). Importantly, these changes were apparent using distinct carbon substrate combinations (pyruvate/malate and pyruvate/malate/octanoyl-carnitine/glutamate/succinate) and occurred despite no evidence of mitochondrial outer membrane permeabilization (i.e., no change in respiration induced in response to exogenous cytochrome C, ‘Cyt C’) (Fig. 6B). Quantification of OXPHOS impairment induced by SACLAC was done by plotting the relationship between mitochondrial respiration and ΔGATP, the slope of which represents global OXPHOS conductance (Fig. 6C). In this way, relative to vehicle control, SACLAC reduced OXPHOS conductance by ~ 2 -fold. In a parallel assay, SACLAC reduced maximal uncoupled respiration in substrate replete permeabilized cells, indicating overt disruptions in ETS capacity (Fig. 6D). The combination of impaired OXPHOS conductance and lowered respiratory capacity suggest a model whereby elevated dihydroceramide/ceramide, induced by SACLAC, impinges on the protein subunits that comprise the ETS. Taken together, these findings highlight the unique ability of comprehensive mitochondrial phenotyping to reveal nuanced crosstalk between cellular sphingolipids and mitochondrial bioenergetics and suggest that direct ETS disruption likely precedes MOMP in the context of elevated dihydroceramide/ceramide.
Fig. 6. Effect of SACLAC exposure on mitochondrial bioenergetics in drug-resistant leukemia.
HL-60/dnr cells (resistant to daunorubicin) (800,000/ml dnr-free, RPMI-1640 medium, 10% FBS) were exposed to 10 μM SACLAC (DMSO vehicle) or DMSO (control) for 24 hr. Cells were then harvested by centrifugation, washed three times in PBS, and subjected to bioenergetic characterization. For respiration experiments, cells were suspended in a potassium-based respiration buffer, permeabilized with digitonin (10μg/mL), and energized with various carbon substrates and respiratory stimuli/inhibitors. All data were normalized to live cell count. A. Cell viability. B. Respiration under basal conditions, as well as in response to digitonin (Digi, 10μg/mL), pyruvate/malate (Pyr/M, 5mM/1mM), cytochrome c (Cyt C, 10μM), CK clamp (CK 20U/mL; phosphocreatine, PCR, 1mM; ATP, 5mM), octanoyl-carnitine/glutamate/succinate (O/G/S, 0.2mM/5mM/5mM); and multiple PCR additions to titrate ATP free energy (ΔGATP) across a physiological span. C. Relationship between oxygen consumption (JO2) and ATP free energy (ΔGATP). Calculation of ΔGATP done using the online resource https://dmpio.github.io/bioenergetic-calculators/ck_clamp/. D. Respiration under basal conditions, as well as in response to digitonin (Digi, 10μg/mL), Pyr/M/O/G/S (Multi), cytochrome c (Cyt C, 10μM), and FCCP titration (0.5–3.0μM). Rotenone (0.5μM) and antimycin A (0.5μM) were added at the end to control from any non-mitochondrial respiration. Data are mean ± SEM, N=3/group, *P<0.05.
Interestingly, in earlier work Siskind et al 73 showed that C16-ceramide, but not dihydroceramide, formed large, stable channels in membranes, albeit the system employed planar membranes formed by the monolayer method. The same study also revealed that apoptotic and channel-forming activities were greatly reduced with dihydroceramide (C-18). Moving forward, elucidating the precise molecular mechanisms by which cellular ceramide species directly impinge on mitochondrial bioenergetics is a fruitful area of research, ripe for identifying novel, mitochondrial-targeted therapeutics designed to combat cancer.
8. Coda
Defining the actions of dihydroceramides, ceramides, and their prominent, biologically potent molecular species is paramount in unravelling the fine-points of the ceramide-mitochondrial union, as it is this bond that can be all-important in directing lucrative therapeutic direction. MOMP is fine; however, comprehensive mitochondrial phenotyping as described herein can pave the way to new discovery. Our findings highlight the unique ability of mitochondrial phenotyping to unmask exacting crosstalk between cellular sphingolipids and mitochondrial bioenergetics and suggest that direct ETS disruption likely precedes MOMP in the context of elevated ceramides. We curiously await work on specific ceramide molecular species, but if you are in the lipid field, there is always the question: yeah, but what about “solubility”?!
Research highlights.
-
►
Introductory review on sphingolipid metabolism in the context of cancer.
-
►
Brief discussion of the interaction between cellular sphingolipids and mitochondrial bioenergetics.
-
►
Introduction to a mitochondrial phenotyping platform that can be of utility in dissecting the fine-points of ceramide impact on cellular bioenergetics.
Acknowledgments
The work was supported by DOD-W81XWH-19-1-0213 (K.H.F.-W.) and NIH P01 CA171983 (M.C.C).
Footnotes
Abbreviations: Listed throughout, in order of appearance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zheng W. et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochimica et biophysica acta 1758, 1864–1884, doi: 10.1016/j.bbamem.2006.08.009 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Abu-Farha M. et al. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int J Mol Sci 21, doi: 10.3390/ijms21103544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dany M. et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 128, 1944–1958, doi: 10.1182/blood-2016-04-708750 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grösch S, Schiffmann S. & Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res 51, 50–62, doi: 10.1016/j.plipres.2011.11.001 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Helke K. et al. Ceramide Synthase 6 Deficiency Enhances Inflammation in the DSS model of Colitis. Sci Rep 8, 1627, doi: 10.1038/s41598-018-20102-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch A. et al. Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients. Int J Mol Sci 18, 1532, doi: 10.3390/ijms18071532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullen TD, Hannun YA & Obeid LM Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochemical Journal 441, 789–802, doi: 10.1042/BJ20111626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleem M. et al. A Lipidomics Approach to Assess the Association between Plasma Sphingolipids and Verbal Memory Performance in Coronary Artery Disease Patients Undertaking Cardiac Rehabilitation: A C18:0 Signature for Cognitive Response to Exercise. J Alzheimers Dis 60, 829–841, doi: 10.3233/JAD-161292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tidhar R. et al. Acyl chain specificity of ceramide synthases is determined within a region of 150 residues in the tram-lag-CLN8 (TLC) domain. The Journal of biological chemistry 287, 3197–3206, doi: 10.1074/jbc.M111.280271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z. et al. Overexpression of ceramide synthase 1 increases C18-ceramide and leads to lethal autophagy in human glioma. Oncotarget 8, 104022–104036, doi: 10.18632/oncotarget.21955 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galea S. An Unhealthy Mismatch. The Milbank quarterly 95, 486–489, doi: 10.1111/1468-0009.12275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddique MM et al. Ablation of dihydroceramide desaturase confers resistance to etoposide-induced apoptosis in vitro. PLoS One 7, e44042-e44042, doi: 10.1371/journal.pone.0044042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd AK & Devaraj NK Traceless synthesis of ceramides in living cells reveals saturation-dependent apoptotic effects. Proceedings of the National Academy of Sciences of the United States of America 115, 7485–7490, doi: 10.1073/pnas.1804266115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosetti B. et al. A tissue-specific screen of ceramide expression in aged mice identifies ceramide synthase-1 and ceramide synthase-5 as potential regulators of fiber size and strength in skeletal muscle. Aging Cell 19, e13049, doi: 10.1111/acel.13049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavie Y, Cao H, Bursten SL, Giuliano AE & Cabot MC Accumulation of glucosylceramides in multidrug-resistant cancer cells. The Journal of biological chemistry 271, 19530–19536, doi: 10.1074/jbc.271.32.19530 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Morjani H. et al. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. International journal of cancer 94, 157–165, doi: 10.1002/ijc.1449 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Gutierrez-Iglesias G, Hurtado Y, Palma-Lara I. & Lopez-Marure R. Resistance to the antiproliferative effect induced by a short-chain ceramide is associated with an increase of glucosylceramide synthase, P-glycoprotein, and multidrug-resistance gene-1 in cervical cancer cells. Cancer chemotherapy and pharmacology 74, 809–817, doi: 10.1007/s00280-014-2552-3 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Xie P. et al. Overexpression of glucosylceramide synthase in associated with multidrug resistance of leukemia cells. Leukemia research 32, 475–480, doi: 10.1016/j.leukres.2007.07.006 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Liu YY et al. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. International journal of oncology 39, 425–431, doi: 10.3892/ijo.2011.1052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouaze-Andersson V. et al. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochimica et biophysica acta 1771, 1407–1417, doi: 10.1016/j.bbalip.2007.09.005 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YY, Han TY, Giuliano AE & Cabot MC Ceramide glycosylation potentiates cellular multidrug resistance. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 15, 719–730, doi: 10.1096/fj.00-0223com (2001). [DOI] [PubMed] [Google Scholar]
- 22.Baran Y, Bielawski J, Gunduz U. & Ogretmen B. Targeting glucosylceramide synthase sensitizes imatinib-resistant chronic myeloid leukemia cells via endogenous ceramide accumulation. Journal of cancer research and clinical oncology 137, 1535–1544, doi: 10.1007/s00432-011-1016-y (2011). [DOI] [PubMed] [Google Scholar]
- 23.Berwick ML, Dudley BA, Maus K. & Chalfant CE The Role of Ceramide 1-Phosphate in Inflammation, Cellular Proliferation, and Wound Healing. Advances in experimental medicine and biology 1159, 65–77, doi: 10.1007/978-3-030-21162-2_5 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Presa N. et al. Regulation of cell migration and inflammation by ceramide 1-phosphate. Biochimica et biophysica acta 1861, 402–409, doi: 10.1016/j.bbalip.2016.02.007 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Muñoz A. The Role of Ceramide 1-Phosphate in Tumor Cell Survival and Dissemination. Advances in cancer research 140, 217–234, doi: 10.1016/bs.acr.2018.04.012 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Presa N, Gomez-Larrauri A, Dominguez-Herrera A, Trueba M. & Gomez-Muñoz A. Novel signaling aspects of ceramide 1-phosphate. Biochimica et biophysica acta. Molecular and cell biology of lipids 1865, 158630, doi: 10.1016/j.bbalip.2020.158630 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Zeidan YH et al. Molecular targeting of acid ceramidase: implications to cancer therapy. Current drug targets 9, 653–661, doi: 10.2174/138945008785132358 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camacho L. et al. Acid ceramidase as a therapeutic target in metastatic prostate cancer. Journal of lipid research 54, 1207–1220, doi: 10.1194/jlr.M032375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X. et al. Acid ceramidase upregulation in prostate cancer: role in tumor development and implications for therapy. Expert opinion on therapeutic targets 13, 1449–1458, doi: 10.1517/14728220903357512 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan SF et al. Acid ceramidase promotes drug resistance in acute myeloid leukemia through NF-κB-dependent P-glycoprotein upregulation. Journal of lipid research 60, 1078–1086, doi: 10.1194/jlr.M091876 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan SF, Pearson JM, Feith DJ & Loughran TP Jr. The emergence of acid ceramidase as a therapeutic target for acute myeloid leukemia. Expert opinion on therapeutic targets 21, 583–590, doi: 10.1080/14728222.2017.1322065 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morad SA et al. Novel off-target effect of tamoxifen--inhibition of acid ceramidase activity in cancer cells. Biochimica et biophysica acta 1831, 1657–1664, doi: 10.1016/j.bbalip.2013.07.016 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Orr Gandy KA & Obeid LM Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochimica et biophysica acta 1831, 157–166, doi: 10.1016/j.bbalip.2012.07.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hait NC, Oskeritzian CA, Paugh SW, Milstien S. & Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochimica et biophysica acta 1758, 2016–2026, doi: 10.1016/j.bbamem.2006.08.007 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Maceyka M, Rohrbach T, Milstien S. & Spiegel S. Role of Sphingosine Kinase 1 and Sphingosine-1-Phosphate Axis in Hepatocellular Carcinoma. Handbook of experimental pharmacology 259, 3–17, doi: 10.1007/164_2019_217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senchenkov A, Litvak DA & Cabot MC Targeting ceramide metabolism--a strategy for overcoming drug resistance. Journal of the National Cancer Institute 93, 347–357, doi: 10.1093/jnci/93.5.347 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Fisher-Wellman KH et al. Mitochondrial Diagnostics: A Multiplexed Assay Platform for Comprehensive Assessment of Mitochondrial Energy Fluxes. Cell reports 24, 3593–3606.e3510, doi: 10.1016/j.celrep.2018.08.091 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benador IY et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell metabolism 27, 869–885.e866, doi: 10.1016/j.cmet.2018.03.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sriskanthadevan S. et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood 125, 2120–2130, doi: 10.1182/blood-2014-08-594408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurmi K. et al. Tyrosine Phosphorylation of Mitochondrial Creatine Kinase 1 Enhances a Druggable Tumor Energy Shuttle Pathway. Cell metabolism 28, 833–847.e838, doi: 10.1016/j.cmet.2018.08.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farge T. et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer discovery 7, 716–735, doi: 10.1158/2159-8290.cd-16-0441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones CL et al. Nicotinamide Metabolism Mediates Resistance to Venetoclax in Relapsed Acute Myeloid Leukemia Stem Cells. Cell stem cell, doi: 10.1016/j.stem.2020.07.021 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles AG et al. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer chemotherapy and pharmacology 47, 444–450, doi: 10.1007/s002800000265 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Flowers M. et al. C6-ceramide and targeted inhibition of acid ceramidase induce synergistic decreases in breast cancer cell growth. Breast cancer research and treatment 133, 447–458, doi: 10.1007/s10549-011-1768-8 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Chapman JV et al. Metabolism of short-chain ceramide by human cancer cells--implications for therapeutic approaches. Biochemical pharmacology 80, 308–315, doi: 10.1016/j.bcp.2010.04.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández-Corbacho MJ, Salama MF, Canals D, Senkal CE & Obeid LM Sphingolipids in mitochondria. Biochimica et biophysica acta. Molecular and cell biology of lipids 1862, 56–68, doi: 10.1016/j.bbalip.2016.09.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babiychuk EB et al. The targeting of plasmalemmal ceramide to mitochondria during apoptosis. PLoS One 6, e23706, doi: 10.1371/journal.pone.0023706 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babiychuk EB, Monastyrskaya K. & Draeger A. Fluorescent annexin A1 reveals dynamics of ceramide platforms in living cells. Traffic (Copenhagen, Denmark) 9, 1757–1775, doi: 10.1111/j.1600-0854.2008.00800.x (2008). [DOI] [PubMed] [Google Scholar]
- 49.Stiban J, Caputo L. & Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. Journal of lipid research 49, 625–634, doi: 10.1194/jlr.M700480-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Mignard V. et al. Sphingolipid distribution at mitochondria-associated membranes (MAMs) upon induction of apoptosis. Journal of lipid research 61, 1025–1037, doi: 10.1194/jlr.RA120000628 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C. & Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? The Biochemical journal 382, 527–533, doi: 10.1042/bj20031819 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Novgorodov SA et al. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. The Journal of biological chemistry 286, 25352–25362, doi: 10.1074/jbc.M110.214866 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu BX, Rajagopalan V, Roddy PL, Clarke CJ & Hannun YA Identification and characterization of murine mitochondria-associated neutral sphingomyelinase (MA-nSMase), the mammalian sphingomyelin phosphodiesterase 5. The Journal of biological chemistry 285, 17993–18002, doi: 10.1074/jbc.M110.102988 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birbes H, El Bawab S, Hannun YA & Obeid LM Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 15, 2669–2679, doi: 10.1096/fj.01-0539com (2001). [DOI] [PubMed] [Google Scholar]
- 55.Barth BM et al. Sphingolipid metabolism determines the therapeutic efficacy of nanoliposomal ceramide in acute myeloid leukemia. Blood advances 3, 2598–2603, doi: 10.1182/bloodadvances.2018021295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hockenbery DM Targeting mitochondria for cancer therapy. Environmental and molecular mutagenesis 51, 476–489, doi: 10.1002/em.20552 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Panina SB, Pei J, Baran N, Konopleva M. & Kirienko NV Utilizing Synergistic Potential of Mitochondria-Targeting Drugs for Leukemia Therapy. Frontiers in oncology 10, 435, doi: 10.3389/fonc.2020.00435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guièze R. et al. Mitochondrial Reprogramming Underlies Resistance to BCL-2 Inhibition in Lymphoid Malignancies. Cancer cell 36, 369–384.e313, doi: 10.1016/j.ccell.2019.08.005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee EA et al. Targeting Mitochondria with Avocatin B Induces Selective Leukemia Cell Death. Cancer research 75, 2478–2488, doi: 10.1158/0008-5472.can-14-2676 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Zhang L. & Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer gene therapy 12, 228–237, doi: 10.1038/sj.cgt.7700792 (2005). [DOI] [PubMed] [Google Scholar]
- 61.Kang MH & Reynolds CP Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 1126–1132, doi: 10.1158/1078-0432.ccr-08-0144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnstone RW, Ruefli AA & Lowe SW Apoptosis: a link between cancer genetics and chemotherapy. Cell 108, 153–164, doi: 10.1016/s0092-8674(02)00625-6 (2002). [DOI] [PubMed] [Google Scholar]
- 63.Bock FJ & Tait SWG Mitochondria as multifaceted regulators of cell death. Nature reviews. Molecular cell biology 21, 85–100, doi: 10.1038/s41580-019-0173-8 (2020). [DOI] [PubMed] [Google Scholar]
- 64.Tait SW & Green DR Mitochondrial regulation of cell death. Cold Spring Harbor perspectives in biology 5, doi: 10.1101/cshperspect.a008706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youle RJ & Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular cell biology 9, 47–59, doi: 10.1038/nrm2308 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Ganesan V. et al. Ceramide and activated Bax act synergistically to permeabilize the mitochondrial outer membrane. Apoptosis : an international journal on programmed cell death 15, 553–562, doi: 10.1007/s10495-009-0449-0 (2010). [DOI] [PubMed] [Google Scholar]
- 67.von Haefen C. et al. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene 21, 4009–4019, doi: 10.1038/sj.onc.1205497 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Lee H. et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One 6, e19783, doi: 10.1371/journal.pone.0019783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martínez-Abundis E, Correa F, Pavón N. & Zazueta C. Bax distribution into mitochondrial detergent-resistant microdomains is related to ceramide and cholesterol content in postischemic hearts. The FEBS journal 276, 5579–5588, doi: 10.1111/j.1742-4658.2009.07239.x (2009). [DOI] [PubMed] [Google Scholar]
- 70.Morad SA et al. Ceramide--antiestrogen nanoliposomal combinations--novel impact of hormonal therapy in hormone-insensitive breast cancer. Molecular cancer therapeutics 11, 2352–2361, doi: 10.1158/1535-7163.mct-12-0594 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siskind LJ, Kolesnick RN & Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. The Journal of biological chemistry 277, 26796–26803, doi: 10.1074/jbc.M200754200 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siskind LJ, Kolesnick RN & Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6, 118–125, doi: 10.1016/j.mito.2006.03.002 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siskind LJ & Colombini M. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. The Journal of biological chemistry 275, 38640–38644, doi: 10.1074/jbc.C000587200 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kester M. et al. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biological chemistry 396, 737–747, doi: 10.1515/hsz-2015-0129 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Li G. et al. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 154, 1024–1036.e1029, doi: 10.1053/j.gastro.2017.10.050 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenquist M. 14-3-3 proteins in apoptosis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 36, 403–408, doi: 10.1590/s0100-879x2003000400001 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Diallo K, Oppong AK & Lim GE Can 14-3-3 proteins serve as therapeutic targets for the treatment of metabolic diseases? Pharmacological research 139, 199–206, doi: 10.1016/j.phrs.2018.11.021 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Tsuruta F. et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. The EMBO journal 23, 1889–1899, doi: 10.1038/sj.emboj.7600194 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshida K, Yamaguchi T, Natsume T, Kufe D. & Miki Y. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nature cell biology 7, 278–285, doi: 10.1038/ncb1228 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Kong JY, Klassen SS & Rabkin SW Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Molecular and cellular biochemistry 278, 39–51, doi: 10.1007/s11010-005-1979-6 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Kim HJ, Oh JE, Kim SW, Chun YJ & Kim MY Ceramide induces p38 MAPK-dependent apoptosis and Bax translocation via inhibition of Akt in HL-60 cells. Cancer letters 260, 88–95, doi: 10.1016/j.canlet.2007.10.030 (2008). [DOI] [PubMed] [Google Scholar]
- 82.Lin CF et al. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. Journal of cell science 120, 2935–2943, doi: 10.1242/jcs.03473 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Sanvicens N. & Cotter TG Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. Journal of neurochemistry 98, 1432–1444, doi: 10.1111/j.1471-4159.2006.03977.x (2006). [DOI] [PubMed] [Google Scholar]
- 84.De Stefanis D. et al. Increase in ceramide level alters the lysosomal targeting of cathepsin D prior to onset of apoptosis in HT-29 colon cancer cells. Biological chemistry 383, 989–999, doi: 10.1515/bc.2002.106 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Heinrich M. et al. Ceramide as an activator lipid of cathepsin D. Advances in experimental medicine and biology 477, 305–315, doi: 10.1007/0-306-46826-3_33 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Heinrich M. et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. The EMBO journal 18, 5252–5263, doi: 10.1093/emboj/18.19.5252 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Darios F, Lambeng N, Troadec JD, Michel PP & Ruberg M. Ceramide increases mitochondrial free calcium levels via caspase 8 and Bid: role in initiation of cell death. Journal of neurochemistry 84, 643–654, doi: 10.1046/j.1471-4159.2003.01590.x (2003). [DOI] [PubMed] [Google Scholar]
- 88.Yuan H, Williams SD, Adachi S, Oltersdorf T. & Gottlieb RA Cytochrome c dissociation and release from mitochondria by truncated Bid and ceramide. Mitochondrion 2, 237–244, doi: 10.1016/s1567-7249(02)00106-x (2003). [DOI] [PubMed] [Google Scholar]
- 89.Lin CF et al. Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage of caspase-2 activation. The Journal of biological chemistry 280, 23758–23765, doi: 10.1074/jbc.M412292200 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Sumitomo M. et al. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. The Journal of clinical investigation 109, 827–836, doi: 10.1172/jci14146 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pinton P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. The EMBO journal 20, 2690–2701, doi: 10.1093/emboj/20.11.2690 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raisova M. et al. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS letters 473, 27–32, doi: 10.1016/s0014-5793(00)01491-5 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Morales MC et al. 4-HPR-mediated leukemia cell cytotoxicity is triggered by ceramide-induced mitochondrial oxidative stress and is regulated downstream by Bcl-2. Free radical research 41, 591–601, doi: 10.1080/10715760701218558 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Liu YY et al. Glycosylation of ceramide potentiates cellular resistance to tumor necrosis factor-alpha-induced apoptosis. Experimental cell research 252, 464–470, doi: 10.1006/excr.1999.4649 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Bleicher RJ & Cabot MC Glucosylceramide synthase and apoptosis. Biochimica et biophysica acta 1585, 172–178, doi: 10.1016/s1388-1981(02)00338-4 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Kao LP et al. Chemotherapy selection pressure alters sphingolipid composition and mitochondrial bioenergetics in resistant HL-60 cells. Journal of lipid research 60, 1590–1602, doi: 10.1194/jlr.RA119000251 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zigdon H. et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. The Journal of biological chemistry 288, 4947–4956, doi: 10.1074/jbc.M112.402719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pearson JM et al. Ceramide Analogue SACLAC Modulates Sphingolipid Levels and MCL-1 Splicing to Induce Apoptosis in Acute Myeloid Leukemia. Molecular cancer research : MCR 18, 352–363, doi: 10.1158/1541-7786.mcr-19-0619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Osawa Y. et al. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. The Journal of biological chemistry 280, 27879–27887, doi: 10.1074/jbc.M503002200 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Seumois G. et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. Journal of leukocyte biology 81, 1477–1486, doi: 10.1189/jlb.0806529 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Frahm R, Fritz H. & Drescher E. [Angular measurements of the hindfoot in CT]. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 151, 77–81, doi: 10.1055/s-2008-1047133 (1989). [DOI] [PubMed] [Google Scholar]
- 102.Fugio LB, Coeli-Lacchini FB & Leopoldino AM Sphingolipids and Mitochondrial Dynamic. Cells 9, doi: 10.3390/cells9030581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siddique MM, Li Y, Chaurasia B, Kaddai VA & Summers SA Dihydroceramides: From Bit Players to Lead Actors. The Journal of biological chemistry 290, 15371–15379, doi: 10.1074/jbc.R115.653204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muñoz-Guardiola P. et al. The anti-cancer drug ABTL0812 induces ER stress-mediated cytotoxic autophagy by increasing dihydroceramide levels in cancer cells. Autophagy, 1–18, doi: 10.1080/15548627.2020.1761651 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fabrias G. et al. Dihydroceramide desaturase and dihydrosphingolipids: debutant players in the sphingolipid arena. Prog Lipid Res 51, 82–94, doi: 10.1016/j.plipres.2011.12.002 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Gagliostro V. et al. Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. The international journal of biochemistry & cell biology 44, 2135–2143, doi: 10.1016/j.biocel.2012.08.025 (2012). [DOI] [PubMed] [Google Scholar]
- 107.Hernández-Tiedra S. et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 12, 2213–2229, doi: 10.1080/15548627.2016.1213927 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H. et al. N-(4-Hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Molecular cancer therapeutics 7, 2967–2976, doi: 10.1158/1535-7163.mct-08-0549 (2008). [DOI] [PubMed] [Google Scholar]