Abstract
Objective:
Investigating intrinsic brain functional connectivity may help identify the neurobiology underlying cognitive patterns and biases contributing to obesity propensity. To address this, the current study used a novel whole-brain, data-driven approach to examine functional connectivity differences in large-scale network interactions between obesity-prone (OP) and obesity-resistant (OR) individuals.
Methods:
OR (N=24) and OP (N=25) adults completed functional magnetic resonance imaging (fMRI) during rest. Large-scale brain networks were identified using independent component analysis (ICA). Voxel-specific between-network connectivity analysis assessed correlations between ICA component time series’ and individual voxel time series, identifying regions strongly connected to many networks, i.e., “hubs”.
Results:
Significant group differences in between-network connectivity (OP vs. OR; FDR-corrected) were observed in bilateral basal ganglia (left: q=0.009; right: q=0.010) and right dorsolateral prefrontal cortex (dlPFC; q=0.026), with OP>OR. Basal ganglia differences were largely driven by a more strongly negative correlation with a lateral sensorimotor network in OP, with dlPFC differences driven by a more strongly negative correlation with an inferior visual network in OP.
Conclusions:
Greater between-network connectivity was observed in the basal ganglia and dlPFC in OP, driven by stronger associations with lateral sensorimotor and inferior visual networks, respectively. This may reflect a disrupted balance between goal-directed and habitual control systems and between internal/external monitoring processes.
Keywords: obesity-prone, obesity-resistant, functional magnetic resonance imaging (fMRI), resting-state, between-network connectivity
1. Introduction
Susceptibility to weight gain varies, with some individuals appearing to be resistant to weight gain, despite being in an obesogenic environment (e.g., easy access to inexpensive, convenient, energy-dense foods; large portion sizes; ubiquitous food and beverage marketing; abundance of screen-based, sedentary leisure activities [1]). Understanding what drives a propensity to gain weight, or why some individuals remain resistant to weight gain, is instrumental in developing novel weight-loss treatments, guiding optimization of current weight management approaches, and creating individualized weight loss and maintenance programs.
Neuronal processes underlying eating behaviors may represent an important factor driving obesity proneness or resistance. A number of studies have identified differences between normal-weight individuals and those with overweight/obesity in neuronal responses to food cues in brain regions involved in energy balance and eating behaviors, such as reward processing, sensory processing, and cognitive control [2, 3]. Studies have also found neuronal response to food cues to be predictive of subsequent food choices [4, 5] and weight gain [4, 6]. A disadvantage of studying individuals who already have obesity, however, is that it is unknown if observed differences are related to the causal factors that contribute to an obese phenotype, or if differences are simply the result of having greater body weight. One possible way to disentangle this issue is to study individuals “at risk” for obesity, but who do not yet have obesity. As described previously by our group, this strategy of studying individuals who are obesity-prone (OP), compared to those who are obesity-resistant (OR), could reveal brain differences that precede weight gain and obesity and thus could reflect a causal mechanism and/or be used to predict obesity risk [7].
Previously, using this OP/OR strategy, we have observed differences in individuals self-identifying as obesity-prone, compared to those identifying as obesity-resistant, in the neuronal response to visual food cues as measured by functional magnetic resonance imaging (fMRI), such that meal-induced reduction in neuronal response (fed compared to fasted states) observed in obesity-resistant individuals was not observed in obesity-prone individuals [7]. This suggests that those prone to obesity may differ in neurobiology underlying food-related processes compared to those resistant to weight gain.
In addition to examining neuronal responses related to food cues, assessing intrinsic brain activity with fMRI during the resting state could also be helpful in identifying the neurobiology underlying overall cognitive patterns and biases that may contribute to obesity propensity. Previous studies have identified alterations in resting-state activity related to overweight/obesity in a number of brain regions, including the hypothalamus [8], insula [9–13], dorsal striatum/putamen [10, 12–17], ventral striatum/caudate [13], somatosensory cortex [13, 17], orbitofrontal cortex [15], medial prefrontal cortex [8, 13, 15, 17], dorsolateral prefrontal cortex [11], and a number of brain networks, including the default mode network [9, 13, 18, 19], salience network [14, 19], and sensorimotor network [19]. Findings have been somewhat inconsistent in terms of location and directionality of effects, however, partially due to the a priori selection of different brain regions and networks in many studies. Nevertheless, obesity has consistently been associated with alterations in intrinsic brain activity and connectivity. Similar to prior observations in the context of food-related tasks, it is unclear, however, whether these alterations are related to propensity or “risk” for obesity or if they reflect a consequence of obesity.
Understanding how intrinsic connectivity patterns between neuronal networks relate to obesity propensity may provide important insight into mechanisms underlying behaviors related to energy balance, such as reward-related processing, goal-directed behaviors and motivation, and how sensory processing relates to inhibitory function [13, 14, 20–22]. The goal of the current study was to use a novel whole-brain, data-driven approach [23] to examine the impact of obesity propensity on intrinsic neuronal connectivity. Participants completing the study identified themselves as being either obesity-prone or obesity-resistant. Those identifying as obesity-prone had at least one first-degree relative with obesity and reported chronically struggling with their weight but did not have obesity themselves. Those identifying as obesity-resistant reported no first-degree relatives with obesity and defined themselves as being “naturally thin,” putting little effort into maintaining their current weight.
The propensity for obesity has, to date, not been associated with any single network or region, and may arise due to interactions between multiple processing systems. For example, maladaptive eating behaviors and weight gain may relate to dysfunctional interactions between inhibitory cognitive control systems and interoceptive somatosensory feedback indicating the body’s current state. However, measuring interactions between multiple networks and processing systems is technically and conceptually challenging. In order to investigate the role of these interacting networks in obesity, new approaches are needed. Toward this end, the current study used a novel between-network connectivity approach [23] to assess whole-brain resting-state functional connectivity in the aforementioned obesity-prone and obesity-resistant groups, with the hypothesis that altered network connectivity may contribute to obesity susceptibility. Based on previous studies focusing on intrinsic network connectivity in obesity [9, 10, 13–15, 17–19, 24], we hypothesized that, if connectivity differences are related to propensity to obesity rather than simply reflecting factors associated with current obesity, differences in between-network connectivity would be observed between obesity-prone and obesity-resistant individuals, specifically in brain regions and networks involved in interoception, sensory processing, salience, reward, and cognitive control.
2. Methods
2.1. Participant characteristics
Fifty-one adults 25–40 years old completed resting-state fMRI scanning for this study. These individuals were drawn from a larger study investigating effects of obesity-proneness on metabolism. Participants were recruited via flyer advertisement in Aurora, CO, between August 2008 and August 2011. All study activities were completed at the University of Colorado Anschutz Medical Campus in Aurora, CO. Half were recruited with a propensity to be resistant to weight-gain and obesity (OR; N=28) and half with a propensity for weight gain and obesity (OP; N=28), as previously described [7, 25, 26]. Briefly, those in the OR group had a body mass index (BMI) of 17–25 kg/m2, responded to advertisements seeking “naturally thin people,” and reported no first-degree relatives with obesity, never being overweight themselves, having weight stability despite few to no attempts to lose weight, and not having high physical activity levels (i.e., not greater than 3 hours planned physical activity per week or more than 12,000 steps per day). Those in the OP group had a BMI of 20–30 kg/m2, responded to advertisements seeking “people who struggle with their weight,” and reported at least one first-degree relative with obesity, a history of weight fluctuations despite efforts to lose or maintain weight, but were not actively attempting to lose weight and were weight-stable (within ± 5 lbs) for at least 3 months prior to study participation. All participants were free of significant medical and psychiatric disease, including eating disorders, as assessed by medical history, physical examination, blood testing (complete metabolic panel, A1c, thyroid stimulating hormone, complete blood count, lipid panel), and behavioral questionnaires (Eating Attitudes Test [27]; Center for Epidemiologic Studies Depression Scale [CES-D] [28]). Participants were right-handed, with no contraindications to MRI scanning. Data from two participants were excluded from analyses due to excessive head movement (>3 mm or 3 degrees in any direction) during fMRI scanning. There were no significant group differences (OP vs. OR) in movement parameters reflecting motion during the scanning session (translational: x [p = 0.95], y [p = 0.32], z [p = 0.09]); rotational: pitch [p = 0.71], roll [p = 0.58], yaw [p = 0.96]). Final analyses included 49 participants, with 24 OR (11 women, 13 men) and 25 OP (13 women, 12 men). Participants provided written informed consent and all procedures were in accordance with and approved by the Colorado Multiple Institutional Review Board.
2.2. Study design
As previously described [7, 25, 26], body composition was assessed by dual-energy X-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp.), with eating behaviors assessed by the Three Factor Eating Questionnaire (TFEQ [29]). One participant did not complete the TFEQ, resulting in a reduced sample size for the OR group for this measure (N=23). Participants completed a four-day eucaloric run-in diet (50% carbohydrate, 30% fat, 20% protein; estimation of energy needs made using lean body mass plus an activity factor [30]) prior to fMRI scanning, to ensure energy and macronutrient balance. Food was prepared by the Clinical Translational Research Center (CTRC) metabolic kitchen at the University of Colorado Anschutz Medical Campus. Participants reported to the CTRC every morning during the diet period to be weighed, eat breakfast, and be given the remainder of their daily meals to take home. They were asked to maintain usual patterns of physical activity, not to consume alcoholic or calorie-containing beverages, and were regularly questioned regarding activity and compliance. In women, study measures were performed in the follicular phase of their menstrual cycle.
On the study day, participants reported to the CTRC following an overnight fast of at least 10 hours. A visual analog scale (VAS; scale of 0–100) measured hunger (“how hungry are you?” from “not at all hungry” to “extremely hungry”), satiety (“how full do you feel right now?” from “not at all” to “extremely”), and prospective food consumption (“how much do you think you could eat right now?” from “nothing at all” to “a large amount”). Following this, participants were escorted to the Brain Imaging Center at the University of Colorado Anschutz Medical Campus to complete the fMRI sessions (described below). After fasted fMRI measures, participants rated a series of hedonic food images (e.g., pizza, ice cream, cake) for appeal (“how appealing is this food?”), pleasantness (“how pleasant is this picture?”) and desire to eat (“how much do you desire to eat this food?”), on a scale of 0–100. Following this, a liquid breakfast meal was consumed, the caloric content of which equaled 25% of the energy provided during the run-in diet, with the same macronutrient composition. VAS ratings were repeated 30, 90, 120, 150, and 180 minutes following the liquid breakfast meal.
2.3. fMRI data acquisition
As previously described [7, 25, 26], fMRI was performed with a GE 3.0 T MR scanner, using a standard quadrature head coil. A high-resolution, T1-weighted 3D anatomical scan was acquired for each participant using a spoiled gradient echo (SPGR-IR) sequence with the following parameters: TR = 5.5 ms, TE = 1.5 ms, flip angle = 10°, 2562 matrix, 240 mm2 FOV (0.9 × 0.9 mm2 in-plane), 1.2-mm thick slices, 174 slices, coronal plane. Functional images were then acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with the following parameters: TR = 2000 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. An inversion-recovery echo-planar image (IR-EPI; TI=505 ms) volume was acquired to improve coregistration between the echo-planar images and gray matter templates used in preprocessing. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). Functional imaging was performed in the fasted state during 10 minutes of rest (300 image volumes), during which participants were instructed to rest with eyes closed. This study also included fMRI recording while participants viewed food pictures, the results of which are reported elsewhere [7, 25, 26]. In all participants, the resting-state scan followed the food pictures task.
2.4. fMRI preprocessing
fMRI data were preprocessed and analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London, UK). Functional data were corrected for differences in slice timing during acquisition, realigned to the first echo-planar image, normalized to the Montreal Neurological Institute (MNI) EPI template, using the gray-matter-segmented IR-EPI as an intermediate to improve registration, and smoothed with an 8 mm full width at half maximum (FWHM) Gaussian kernel. Global signal was not removed.
2.5. Independent Components Analysis (ICA)
Large-scale networks were identified using group independent components analysis (ICA) in the GIFT toolbox v1.3i (http://icatb.sourceforge.net). The dimensionality of the data from each subject was reduced to 70 components using principle component analysis and concatenated into an aggregate dataset for input into ICA. Forty independent components were estimated using minimum description length (MDL) criteria [31] and extracted using the infomax algorithm [32]. Spatial maps were reconstructed using GICA3 [33]. Ten components, classified as artifacts based on spatial distributions in cerebrospinal fluid (CSF), white matter, or high-frequency oscillations, were excluded from further analysis. To identify common intrinsic connectivity networks (ICNs), group mean ICA spatial maps were correlated with published ICN templates [34]. Templates matching multiple ICA components were classified as subnetworks based on anatomical differences, while ICA components without template matches were classified based on anatomy. Following group ICA, whole-brain networks were back-reconstructed individually for each subject.
Of note, ICA does not parcellate the brain into strictly non-overlapping networks. Instead, it allows partial overlap between and among components. When applied to fMRI data, both spatial overlap and temporal correlations are frequently observed [35] (see [36] for a technical discussion). In essence, ICA fits approximately independent networks, thus allowing a small amount of correlation in order to better fit the observed patterns in the data. Consequently, a voxel may be strongly associated with multiple networks, and correlations between ICA time series are possible. In combination, these observations give rise to analyzing connectivity among ICA networks, termed between-network connectivity analysis.
2.6. Between-network connectivity (BNC) analysis
A novel voxel-specific between-network connectivity (BNC) analysis was used to assess correlations between ICA component time series’ and individual voxel time series. This method has been detailed previously [23]. Similar to other network analysis techniques, such as small-world topology [37], BNC extends social network analysis concepts to fMRI connectivity analyses. Specifically, BNC quantifies two important features of information exchange in networks: (1) how much each voxel, or location within the brain, exchanges information with other voxels in large-scale networks, and (2) how much each voxel can be associated with, or is “a member of” multiple networks. In a social network framework, these would be similar to the concepts that (1) individual social actors interact with large social groups, such as when presenting information to and receiving feedback from a group of peers, and (2) individuals can simultaneously be members of several social groups, thus acting as conduits of information exchange between groups. These network features, although perhaps counterintuitive in brain networks, are consistent with the mathematical model of ICA [38]. For instance, corresponding to the first feature, a coefficient representing an individual voxel’s connectivity to an individual network is derived. Additionally, and consistent with the second feature, the association between every voxel and every network is assessed, as represented by a full set of coefficients for all networks. Furthermore, connectivity between each pair of interacting networks (commonly referred to as either “functional network connectivity” or “between-network connectivity” [35]) likely occurs at voxels that are strongly connected to both networks (hence the term “voxel-specific between-network connectivity”). As such, this technique measures the amount and diversity of connectivity between all large-scale networks occurring at a single voxel. In the current study, this represents connectivity between each voxel and each of the 30 non-artifactual independent components identified. BNC differs from other voxel-specific summary statistics, such as the average value of the connectivity vector [23]. For instance, a voxel with high BNC would indicate that the voxel is strongly connected (i.e., correlated) to many networks, suggesting a high degree of information flow between the networks at that voxel. In contrast, low BNC could indicate either that a voxel’s connections to many intercommunicating resting-state networks are negligible, or, alternatively, that a voxel’s connections to intercommunicating resting-state networks are negligible.
First, voxel time series were processed to remove sources of noise and minimize the influence of movement. Time series were detrended and band-pass filtered between 0.1 and 0.01 Hz. Signals for white matter, cerebral spinal fluid, and six movement parameters were regressed out. Additional movement control was provided by removing volumes with excessive framewise displacement (> 0.5 mm) [39]. Next, multiple regression analysis (Matlab R2012a) was used to assess correlations between each ICA component time series and the time series for each individual voxel. This resulted in a vector of bivariate simple correlations for each voxel representing connectivity between that voxel and all ICA components. As such, a connectivity vector was obtained for each voxel that can be thought of as a “connectional fingerprint” [40]. BNC is a scalar coefficient and was calculated from the connectivity vector, as detailed previously [23]. Briefly, voxel-specific BNC compares the sum of squared elements in the voxel’s connectivity vector to the multiple correlation coefficient. This comparison is possible since, as previously shown [23], “suppressor variables” (defined as a predictor variable in a regression model that, although uncorrelated with the response variable, increases the fit of the model by removing unwanted variance from other predictors [41]) do not appear to contribute to the multiple correlation coefficient in the case of using ICA components as regressors. Notably, both negative and positive correlations between voxels and ICA components are treated equivalently with this method.
Group differences (OP vs. OR) in BNC coefficients were assessed using t-tests in SPM8, after normalizing all voxels by subtracting the whole brain mean and scaling to the whole-brain standard deviation for each subject. Whole-brain results were corrected for multiple comparisons at the cluster level (FDR-corrected, q = 0.05, with a cluster-determining threshold of p < 0.005, resulting in an FDR cluster size threshold of 53 voxels). To explore which networks were driving group differences, correlations between each voxel in the cluster and each individual ICA component were assessed. FDR was then applied to each voxel-to-ICA component correlation coefficient (for all 30 non-artifactual ICA components), for all voxels within all significant clusters (SPM t-tests, FDR-corrected, q = 0.05).
2.7. Behavioral and body composition measures
Analyses of group differences in behavioral and body composition measures were performed with SPSS 26 (IBM Corp., Armonk, NY). In addition to fasting VAS ratings, total area under the curve for appetite VAS ratings using all post-meal time points was used. Independent samples t-tests were used to assess group differences (OP vs. OR) in age, body composition measures (BMI, lean body mass, fat mass, body fat), and eating-related behavioral measures (TFEQ, VAS ratings, food image ratings), with an alpha of 0.05.
Correlations between BNC and body composition/behavioral measures were assessed in R version 3.6.0 [43]. For clusters identified as being significantly different between groups in the BNC analysis, the average across all voxels in that cluster was taken as the mean BNC value for that cluster for correlation analyses. Notably, since BNC is a scalar statistic calculated from the voxels’ connectivity vector, the averaging was over the voxel-specific BNC scores and not over the elements of the connectivity vector itself. Pearson’s correlation was then used to determine the relationship between the mean normalized BNC for that cluster and each of the body composition/behavioral measures.
3. Results
3.1. Behavioral and body composition measures
Significant group differences were observed in BMI, fat mass, and percent body fat, with OP > OR (Table 1). No significant group differences were observed for age or lean body mass. For behavioral measures, groups were significantly different on all three of the TFEQ metrics (Restraint, Disinhibition, and Hunger) and ratings of “desire to eat” the food images, with OP > OR (Table 2). No significant group differences were observed for VAS measures (fasting or AUC) or for ratings of appeal or pleasantness of the food images.
Table 1.
Participant characteristics.
| Group | Group Differences | ||
|---|---|---|---|
| Characteristic |
OR (N=24) |
OP (N=25) |
p1 |
| Age (years)2 | 31.1 ± 2.9 | 30.2 ± 3.8 | 0.386 |
| BMI (kg/m2)2 | 20.8 ± 2.1 | 26.2 ± 3.1 | <0.001 |
| Lean body mass (kg)2 | 49.1 ± 10.6 | 54.2 ± 10.8 | 0.100 |
| Fat mass (kg)2 | 15.0 ± 13.9 | 24.2 ± 11.6 | 0.016 |
| Body fat (%)2 | 18.9 ± 4.5 | 28.9 ± 7.9 | <0.001 |
Significant p-values in bold;
Mean ± SD
OP: obesity-prone; OR: obesity-resistant.
Table 2.
Appetite and food-related behaviors.
| Group | Group Differences | ||
|---|---|---|---|
| Measure |
OR (N=24) |
OP (N=25) |
p1 |
| TFEQ: Restraint2,3 | 4.3 ± 2.7 | 9.0 ± 4.5 | <0.001 |
| TFEQ: Disinhibition2,3 | 3.1 ± 2.3 | 7.8 ± 3.4 | <0.001 |
| TFEQ: Hunger2,3 | 4.3 ± 2.5 | 6.2 ± 3.0 | 0.021 |
| Fasting Hunger VAS2 | 65.0 ± 20.2 | 68.4 ± 23.6 | 0.592 |
| Hunger AUC4 | 7196.0 ± 3264.1 | 8367.5 ± 3241.0 | 0.218 |
| Fasting Satiety VAS2 | 17.2 ± 19.0 | 22.5 ± 25.0 | 0.413 |
| Satiety AUC4 | 8231.2 ± 2945.4 | 8774.4 ± 3377.1 | 0.556 |
| Fasting PFC VAS2 | 66.4 ± 20.3 | 66.4 ± 19.9 | 1.000 |
| PFC AUC4 | 10315.63 ± 3704.7 | 9179.4 ± 3125.1 | 0.257 |
| Food Image Appeal2 | 65.8 ± 2.7 | 71.4 ± 2.2 | 0.106 |
| Food Image Desire To Eat2 | 60.2 ± 2.8 | 68.3 ± 2.4 | 0.032 |
| Food Image Pleasantness2 | 65.7 ± 2.5 | 70.1 ± 2.7 | 0.235 |
Significant p-values in bold;
Mean ± SEM;
Sample size for OR group reduced to N=23 for TFEQ measures;
Mean total area under the curve (mm × 180 min)
AUC: area under the curve; OP: obesity-prone; OR: obesity-resistant; PFC: prospective food consumption; TFEQ: Three Factor Eating Questionnaire; VAS: visual analog scale.
3.2. fMRI
Significant group differences (OP vs. OR; Table 3) were observed in bilateral basal ganglia BNC, primarily in the putamen (left basal ganglia: FDR-corrected q = 0.009; right basal ganglia: FDR-corrected q = 0.010), and right dorsolateral prefrontal cortex (dlPFC, FDR-corrected q = 0.026), with greater BNC in the OP compared to OR group (Figure 1).
Table 3.
Coordinates and brain regions showing differential BNC between OP and OR.
| Brain region | MNI coordinates | T value | qFDR | Cluster size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Basal ganglia (L) | −21 | −13 | 13 | 3.96 | 0.009 | 104 |
| Basal ganglia (R) | 21 | −13 | 10 | 3.87 | 0.010 | 89 |
| dlPFC (R) | 42 | 29 | 43 | 3.41 | 0.026 | 66 |
All values in table significant at a voxel-level threshold of p < 0.005 and a cluster-corrected FDR threshold of q < 0.05.
T values reported for local maxima within clusters.
Figure 1.
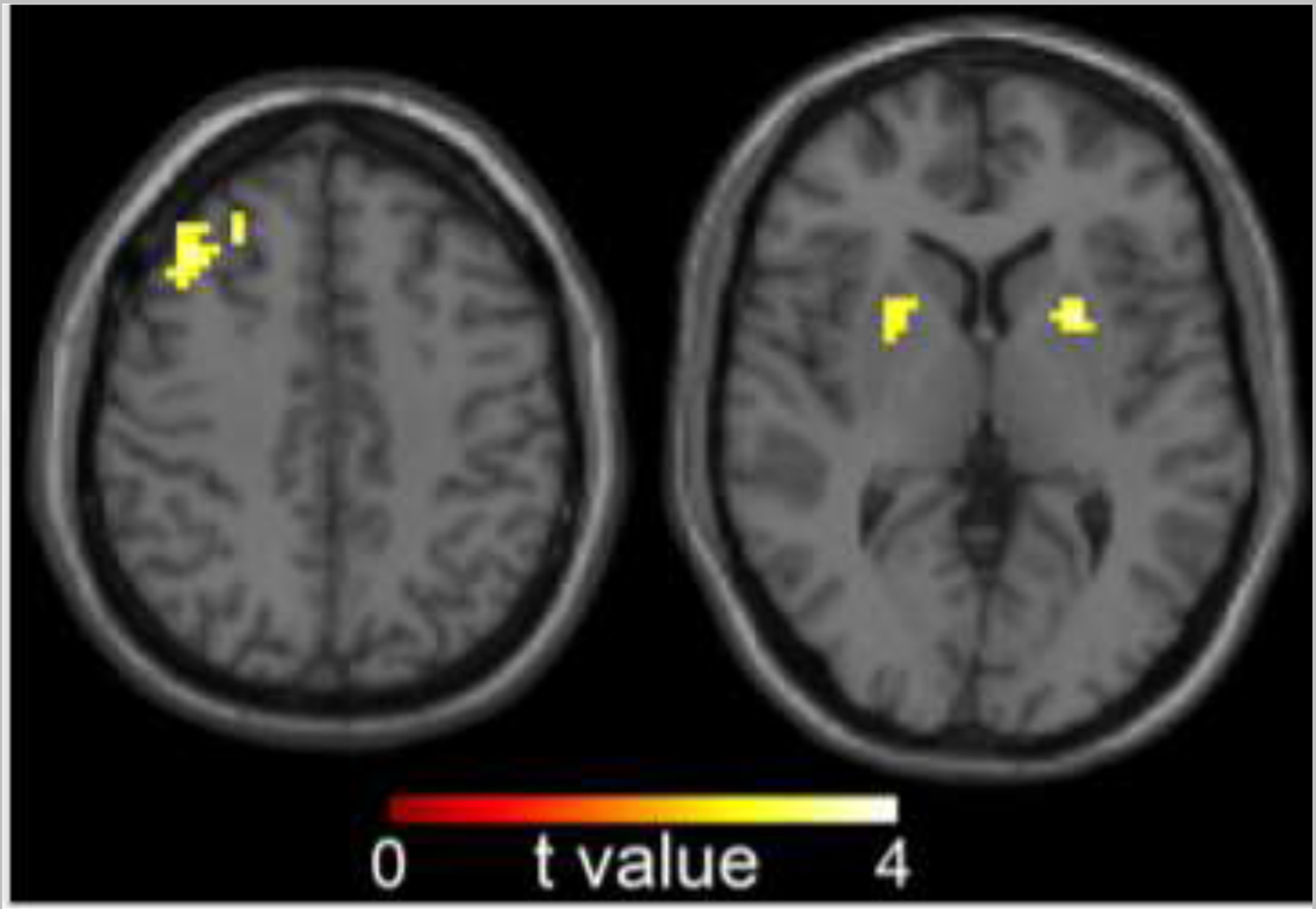
Group differences (OP vs. OR) in between-network connectivity (BNC). Significant group difference observed in basal ganglia, with OP > OR (voxel-level threshold of p < 0.005 and a cluster-corrected FDR threshold of q < 0.05). Data are shown in the radiologic convention (i.e., right hemisphere on the left).
Figure 2 displays a summary of individual t-tests, highlighting which anatomical voxels were influenced by which ICNs. Each line/edge represents a t-test that was significant after correcting for multiple comparisons (FDR, q = 0.05). The circle size for ICN nodes and voxel nodes both represent unweighted degree, i.e., the number of edges (i.e., significant t-tests) for each node. Greater circle size indicates a greater number of significant t-tests. For example, a voxelnode with an unweighted degree of 3 would indicate that the voxel is connected to 3 ICNs. An ICN node with an unweighted degree of 50 would indicate that 50 voxels are significantly connected to that ICN. Larger circles indicate a greater number of significant connections in both cases. For basal ganglia BNC, group differences were most apparent in connectivity between basal ganglia voxels (largely putamen) and a lateral sensorimotor network (bilateral postcentral gyri), with a more strongly negative relationship between basal ganglia activity and the lateral sensorimotor network in the OP group than the OR group. Post-hoc analyses identified a negative correlation between basal ganglia and the lateral sensorimotor network in the OP group that was not observed in the OR group. Group differences in dlPFC BNC were largely driven by group differences in connectivity between dlPFC and an inferior visual network (fusiform gyri), with a negative correlation between dlPFC and the inferior visual network observed in the OP group, but not in the OR group.
Figure 2.
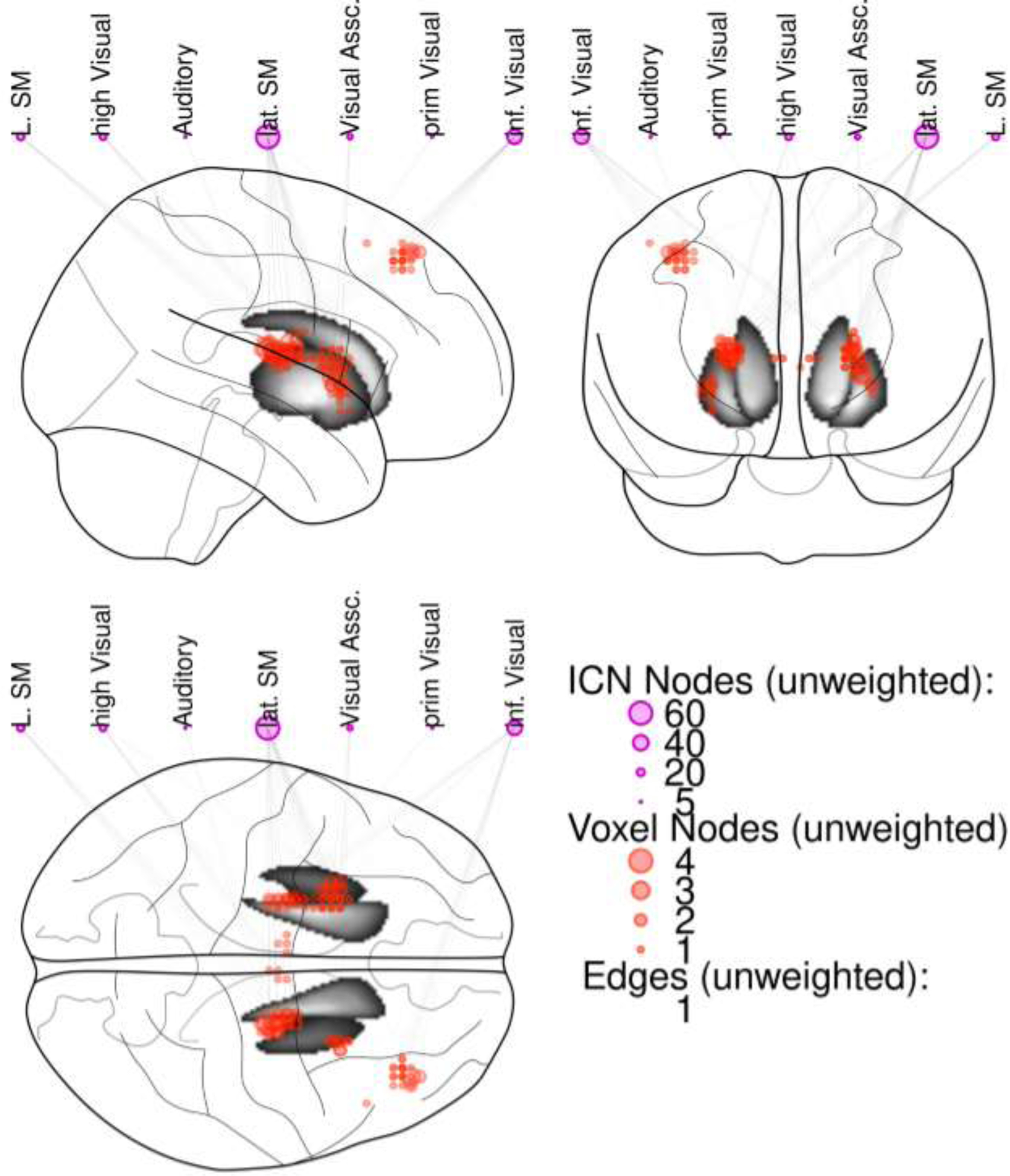
Summary of t-tests assessing group differences (OP vs. OR) in BNC between each voxel in the significant clusters identified in Figure 1 (bilateral basal ganglia; right dorsolateral prefrontal cortex [dlPFC]) and each ICN. Each line represents a significant t-test, with larger circle size indicating greater number of significant t-tests for that ICN or voxel (e.g., voxel node 3 = significant t-tests [OP vs. OR] between that voxel and 3 ICNs). Group differences were most apparent in connectivity between basal ganglia voxels and a lateral sensorimotor network, and between dlPFC voxels and an inferior visual network. Abbreviations: ICN= Intrinsic connectivity network; lat. SM = lateral sensorimotor network; L. SM = left sensorimotor network; high Visual = high visual network; inf. Visual = inferior visual network; Visual Assc. = visual association network.
To evaluate potential effects of sex, analyses were repeated in a two-way ANOVA, with sex and group (OP vs. OR) as predictor variables. No main effect of sex was observed (largest cluster of 35 voxels, non-significant at p = 0.20, corrected) and the inclusion of sex did not alter the main group effect from the initial analysis.
3.3. fMRI and body composition/behavioral measure associations
Correlations between mean BNC and body composition/behavioral measures were assessed for the bilateral basal ganglia and right dlPFC. Across all subjects, BNC in the basal ganglia was significantly correlated with hunger AUC (r = 0.38, t (46) = 2.75, p = 0.008; Figure 3) and satiety AUC (r = −0.55, t (46) = −4.47, p < 0.001; Figure 4). To determine if this relationship differed by group, analyses were repeated including group in the model. Associations between BNC and hunger AUC/satiety AUC remained significant (hunger AUC: F(3, 44) = 3.11, p = 0.036; satiety AUC: F(3, 44) = 6.82, p < 0.001), but with no significant group interactions (hunger AUC: t(48) = −0.67, p = 0.25; satiety AUC: t(48) = 0.17, p = 0.57), suggesting the relationship between BNC and hunger/satiety was consistent across groups. No other significant correlations were observed.
Figure 3.
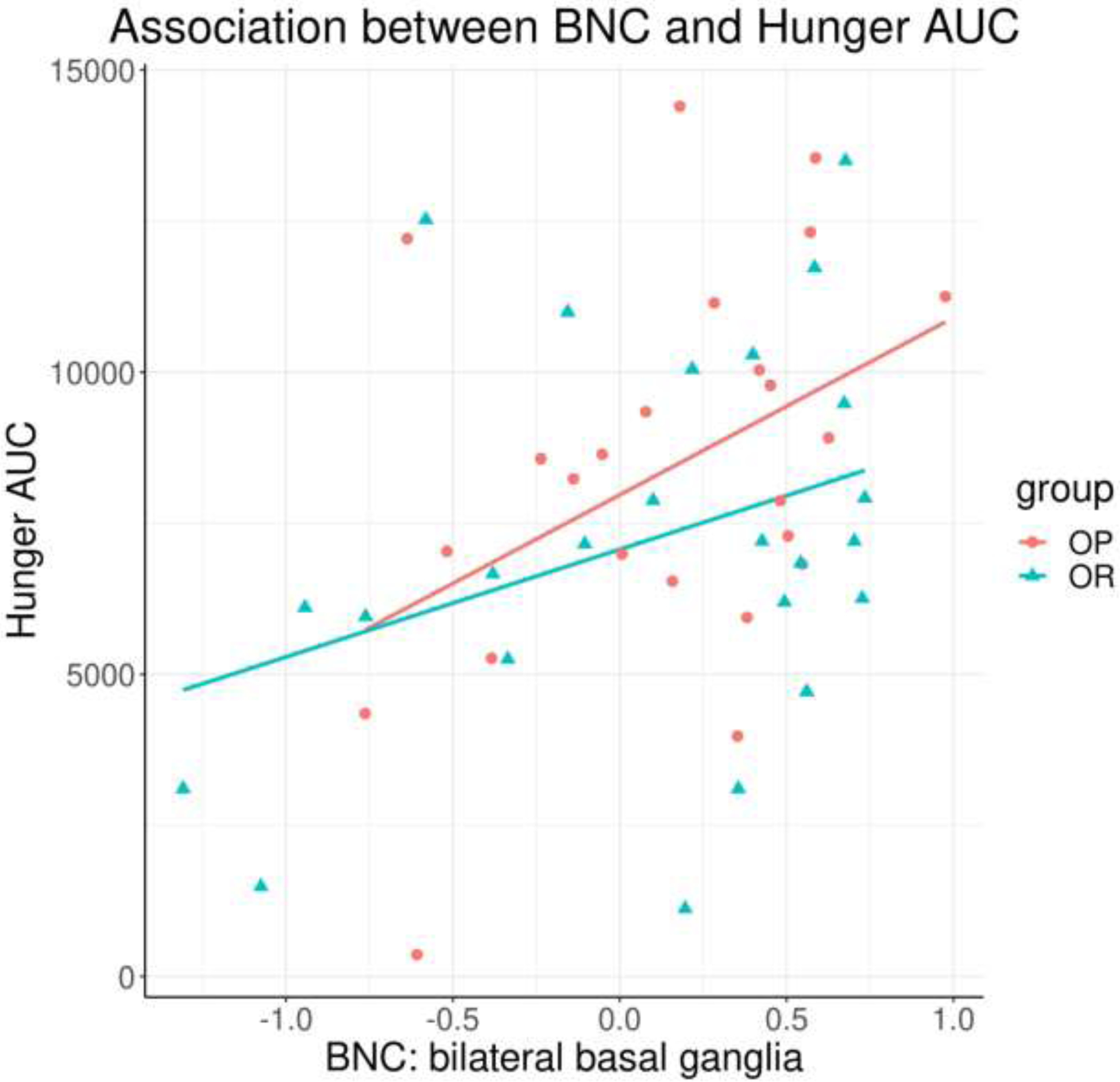
Significant positive correlation between basal ganglia BNC and hunger AUC (p = 0.008), across all participants. BNC = between-network connectivity; AUC = area under the curve.
Figure 4.
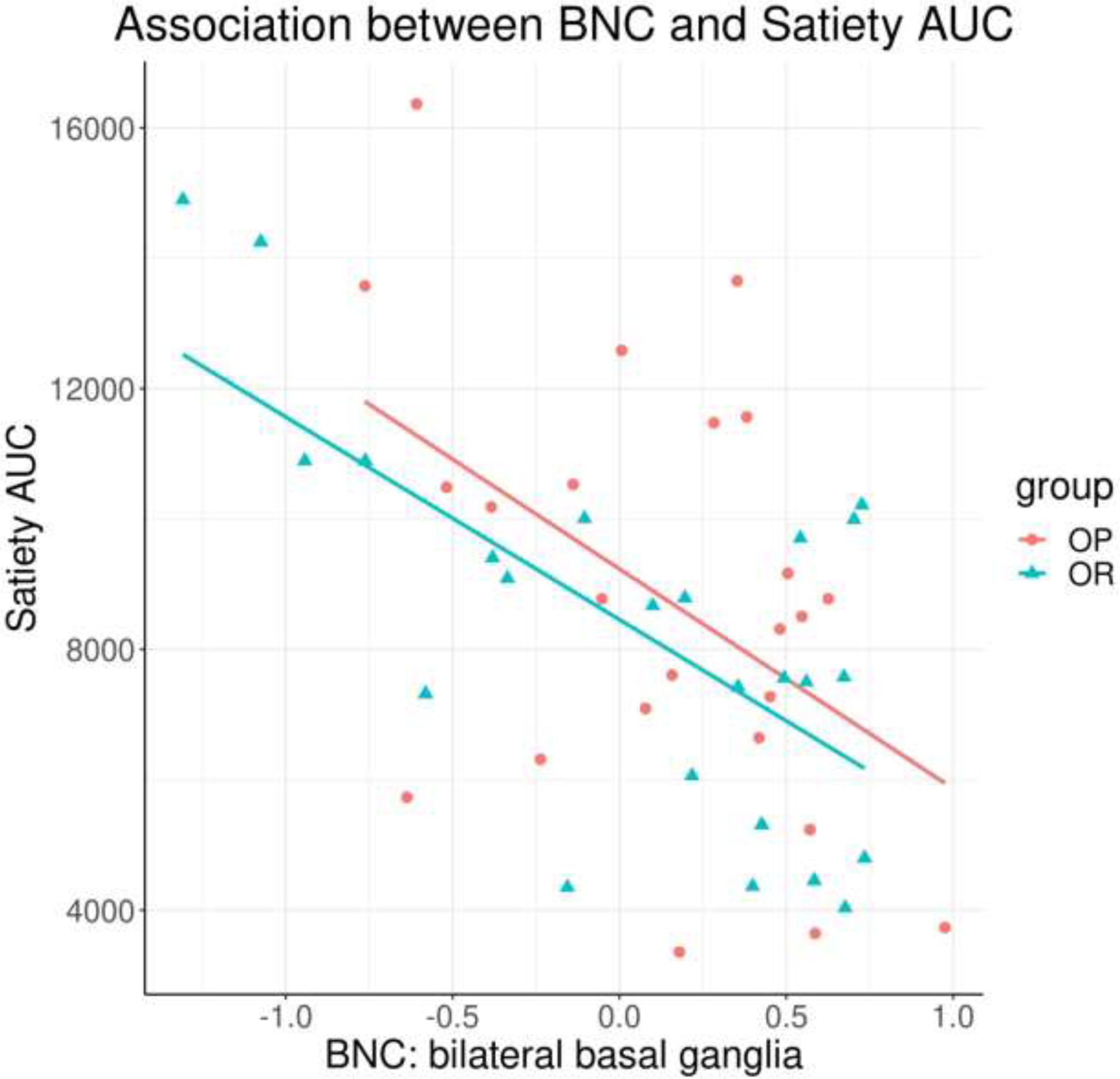
Significant negative correlation between basal ganglia BNC and satiety AUC (p < 0.001), across all participants. BNC = between-network connectivity; AUC = area under the curve.
4. Discussion
The current study used a novel whole-brain, data-driven approach to investigate intrinsic between-network connectivity (BNC) in individuals identifying as obesity-prone (OP) or obesity-resistant (OR). In OP compared to OR individuals, greater BNC was observed in bilateral basal ganglia and right dorsolateral prefrontal cortex (dlPFC). For the basal ganglia, this effect was driven by a more strongly negative association with a lateral sensorimotor network (lSMN; postcentral gyri) in the OP group; i.e., stronger negative connectivity between the lSMN and basal ganglia voxels in OP. For the dlPFC, this group difference was driven by a more strongly negative association with an inferior visual network (fusiform gyri) in the OP group; i.e., stronger negative connectivity between the dlPFC and inferior visual network in OP.
One interpretation of the observed greater basal ganglia BNC in OP, which was focused in the putamen, is that this could reflect an altered balance between goal-directed and habitual control systems. Stronger putaminal BNC could reflect a shift towards a habitual control bias, as the putamen, particularly within the context of sensorimotor cortico-basal ganglia network connectivity, is implicated in habitual rather than goal-directed action control [44, 45]. This interpretation would be consistent with previous work suggesting a link between overweight/obesity and a shift from goal-directed to habitual control dominance [16, 17, 46]. A shift toward a habit-driven bias could reflect a more reflexive, less adaptive system [45, 47], which could have important implications for weight-loss approaches (i.e., if behaviors are more resistant to change). The current findings correspond with previous imaging studies observing greater resting-state putamen functional connectivity, in the context of global brain connectivity (GBC; a metric encompassing both within- and between-network connectivity) [13], temporal synchronicity of putamen activity [15], and salience network connectivity strength [14], in individuals with obesity, compared to lean/healthy-weight individuals. Additionally, Contreras-Rodriguez et al. observed increased resting-state functional connectivity between basal ganglia (dorsal striatum) and somatosensory cortex in participants with overweight/obesity compared to normal-weight participants, using a seed-based approach [17], which was also associated with subsequent BMI gains after 12 weeks. It is difficult, however, to determine if the directionality of effects in the current study corresponds with previous studies. The current study observed increased basal ganglia BNC, focused in the putamen, in the OP group. That this was largely driven by a negative correlation between basal ganglia and the lSMN may suggest a stronger inhibitory influence of the basal ganglia on that network. Thus, although the relationship between the basal ganglia and lSMN connectivity is in a negative direction, results may still corroborate the increased resting-state putaminal connectivity observed in previous studies [13–15]. Directionality in the current study does differ from that in Contreras-Rodriguez et al. [17], however, in which a greater positive correlation between dorsal striatum and somatosensory cortex was observed in participants with “excess weight” compared to “normal weight.” Differences in study methodology may contribute to this discrepancy. The average BMI of the “excess weight” group in the Contreras-Rodriguez et al. study was 30.5 kg/m2, whereas the OP group in the current study had an average BMI of 26.2 kg/m2. As such, group differences observed by Contreras-Rodriguez et al. may reflect effects of current obesity in addition to those related to obesity propensity. Given differences in BMI between the groups, it is also possible that the participants in the Contreras-Rodriguez et al. study regularly maintained an obesogenic diet to a greater extent than those in the current study. Continued consumption of obesogenic foods, coupled with reinforcement of conditioned responses to those foods, may result in altered connectivity patterns between regions relevant to reward and attentional bias toward food cues [22, 48]. Furthermore, differences in satiety state between the two studies may affect results, as previous studies have suggested effects of satiation (i.e., fasted compared to fed states) on resting-state brain activity [10, 15, 49, 50]. Results presented here were with participants in the fasted state (following an overnight fast of at least 10 hours), while those in the Contreras-Rodriguez et al. study completed MRI scanning ~2–4 hours postprandially. Accordingly, VAS measures of hunger (scale of 0–100) prior to MRI scanning were greater in the current study (OR: 65.0, OP: 68.4) compared to those in Contreras-Rodriguez et al. (normal weight: 15.0, excess weight: 16.3). Future studies can further investigate how satiety state may influence group differences in BNC. The observed greater basal ganglia BNC in OP in the current study, driven by increased anticorrelation to the lSMN, could also reflect a greater attention to internal state. In other words, the opposing directionality of response in the two regions could indicate increased internal monitoring, at the expense of external monitoring, which may result in reduced reliance on external sensorimotor stimuli. This would also be congruent with a shift in cognitive bias towards a habit-driven system. An increase in internal monitoring would also be consistent with previous reports of increased intrinsic default mode network activity in individuals with obesity, compared to normal-weight individuals [9, 18, 19].
Although basal ganglia BNC was correlated with appetite (greater BNC was associated with greater hunger and reduced satiety ratings following a meal), an association between basal ganglia BNC and body composition metrics (BMI, fat mass, percent body fat) was not observed, suggesting that the greater BNC observed in the OP group may not be driven by the BMI differences between the groups. As such, results may reflect a predisposition towards obesity in the OP group, or perhaps be related to potential differences in eating behaviors between the two groups. Although food intake was not directly measured in the current study, the OP group rated high-calorie food images as more desirable to eat than did the OR group, which could suggest a greater inclination to consume more high-calorie foods. Supporting a link between eating behaviors and basal ganglia function, binge-like consumption of sweetened condensed milk in rats has been found to alter dorsolateral striatum (analogous to human putamen) responsivity and to promote a more rapid shift towards habitual action control [51]. A recent meta-analysis failed to observe a relationship between BMI and neuronal response to food cues [52], further highlighting the importance of taking other factors relevant to obesity propensity into consideration, such as impulsivity and inhibitory control, stress responsivity, comorbid health conditions, genetic contributions, and learned eating patterns [22, 53–56]. Multiple theories surrounding neural vulnerability factors for obesity risk focus on such factors, including the incentive sensitization theory of obesity, which suggests that repeated high-calorie food intake may lead to altered striatal responsivity, contributing to further overconsumption [22, 57].
A potential mechanism underlying the altered basal ganglia connectivity observed in the current study is altered dopaminergic signaling. Volkow et al. have demonstrated an association between striatal dopamine D2 receptors and somatosensory cortex metabolism in humans, hypothesizing that dopaminergic modulation of somatosensory cortex may influence the reinforcing value of foods and conditioned associations between environmental cues and food intake [58]. Both obesity and exposure to an obesogenic diet have been consistently associated with alterations in striatal dopaminergic signaling, in human and rodent studies [59–63], but the directionality of this relationship (i.e., contributing to vs. a consequence of obesity) remains unclear. In mice, overexpression of dopamine D2 receptors during development was associated with having a predisposition to later obesity, suggesting that early alterations in dopamine signaling may relate to obesity propensity [62]. Interestingly, evidence suggested this was due to reduced energy expenditure, through brown adipose tissue thermogenesis, rather than increased food intake. Other rodent studies also support a contribution of altered striatal dopaminergic signaling to reductions in energy expenditure [59, 60]. As such, the altered intrinsic basal ganglia BNC in the current study could reflect a propensity towards reduced energy expenditure, rather than a propensity towards increased intake. Previous observations of reduced dopamine D2 receptors in animal and human studies of obesity contributed to the reward deficit theory of obesity, positing that food overconsumption reflects heightened reward pursuit in an attempt to compensate for reduced reward signaling [64, 65]. Mounting evidence suggests that this theory may be overly simplistic, though, including human and animal studies that have observed reductions in striatal dopamine to be associated with reduced consumption and/or appetite [22, 57, 66]. A recent study by Mourra et al. [67] found dopamine D2 receptor blockade in mice to reduce willingness to expend physical effort for food, without affecting body weight or the amount of food consumed, further suggesting dopaminergic effects on energy expenditure may also be a key consideration in the development of obesity. Although evaluation of dopaminergic signaling and energy expenditure was beyond the scope of the current study, investigating the contribution of these factors to obesity propensity may be a promising direction for future research.
Increased BNC was also observed in the dlPFC, a region with a key role in cognitive control and inhibitory function. Previous studies have observed reduced dlPFC responsivity to food cues and following feeding in individuals with obesity compared to healthy-weight individuals, with increased dlPFC responsivity observed following weight reduction [3]. Few studies, however, have investigated how intrinsic dlPFC connectivity relates to obesity. The current results were primarily driven by increased connectivity between the right dlPFC and an inferior visual network in the OP group. Specifically, this result was driven by a stronger negative correlation between the right dlPFC and a network of inferior visual regions, including the fusiform gyri. In addition to playing an important role in cognitive inhibition, the dlPFC has been implicated in set shifting and attention [68]. Given the anticorrelation, it is possible that this reflects dlPFC-driven attenuation of sensory input responsivity, relating to reduced attention to the external environment, similar to the interpretation suggested for altered basal ganglia connectivity. The current results are consistent with findings from a previous study in adolescents, in which increased negative connectivity between dlPFC and primary visual cortex was observed in adolescents with overweight/obesity compared to normal-weight adolescents [11]. Differences in intrinsic dlPFC BNC in the OP group may also relate to cognitive biases that contribute to obesogenic behaviors, such as enhanced attentional bias to food cues or discounting negative consequences in favor of immediate reward [56, 69]. This would correspond with both the incentive sensitization theory of obesity, which proposes that elevated food cue responsivity contributes to excess intake, and with the inhibitory control deficit theory of obesity, suggesting that deficits in inhibitory control lead to overconsumption [22, 57]. These theories are not mutually exclusive, in that reduced inhibitory control could increase the likelihood of overeating in the presence of appealing food cues [53, 70]. Subsequent studies can assess BNC in the context of tasks targeting behaviors relevant to these theories (e.g., food cues, delay discounting) to determine how intrinsic connectivity relates to task-related connectivity, and how this is impacted by obesity propensity.
There are a number of potential study limitations to consider. As resting-state fMRI scans were performed after a food cues task, it is possible that task-related response could influence subsequent resting-state measures (e.g., [71, 72]). Since both groups completed the scans in this order, this is unlikely to have affected observed group differences. It is possible, though, that those prone to obesity may experience lasting effects from a food cues task to a different degree than those who are obesity-resistant, an intriguing possibility to be explored in future studies. Itis also possible that the increased negative correlation between the basal ganglia and lSMN in the OP group could reflect greater discomfort during scanning compared to the OR group, i.e., perhaps indicating a stronger basal ganglia-driven inhibitory response to sensorimotor regions. That we did not observe significant group differences in movement during scanning suggests that OP and OR groups experienced similar comfort levels in the scanner. Additionally, the average BMI in both groups was below the obese range, which also increases the likelihood of similar comfort levels during scanning. It is possible, however, that some group differences in discomfort existed that were not captured by assessing movement during scanning. Another possible limitation is that because the OP group studied did not have BMIs in the obese range, despite having multiple obesity risk factors, this group could itself be resistant to weight gain. Given their self-reported difficulties maintaining their weight, however, we believe the low BMIs in this group more likely reflect the fact that the group is relatively young (average age 30.2 yrs), resulting in a lower cumulative exposure to obesity risk factors. To explore this idea, we investigated the association between age and BMI in both OP and OR groups separately. We did not observe a relationship between age and BMI in the OR group (r = .12, p = .568), but did observe a trend toward a significant correlation between age and BMI in the OP group (r = .80, p = .053), with increased age associated with increased BMI. This supports the idea that the relative youth of the participants in the study may have contributed to a lower average BMI and that these individuals are likely on a trajectory towards more significant overweight/obesity. This effect in fact underscores the potential value of studying individuals in the OP state, as identifying neuronal mechanisms that precede weight gain and obesity may point to an opportunity to therapeutically target those mechanisms (e.g., transcranial magnetic stimulation [TMS], transcranial direct current stimulation [tDCS] [73]) and prevent obesity onset.
Additional limitations relate to the participant groups studied. While the OP group potentially allows for the identification of factors that precede or predict weight gain, the lack of a group with BMIs in the obese range does limit our ability parse these effects from direct effects of obesity itself, and reduces comparability of the present study results with other studies of individuals with obesity. Also, because the OR group had a relatively low average BMI (20.8 kg/m2), it is possible that this group is not fully representative of the general population. It is possible that this low BMI relates to the study being conducted in Colorado, where the overweight/obesity prevalence is consistently lower than in all other states [74].
In conclusion, the current study found differences in between-network connectivity in obesity-prone compared to obesity-resistant individuals, using a data-driven, whole-brain approach. Increased BNC was observed in bilateral basal ganglia and right dlPFC, largely driven by more strongly negative associations with lateral sensorimotor and inferior visual networks, respectively. These alterations in functional connectivity may reflect a disrupted balance between goal-directed and habitual control systems and between internal and external monitoring processes. These connectivity differences may contribute to a predisposition towards obesity, potentially related to obesogenic eating behaviors or perhaps reflecting a propensity towards reduced energy expenditure. Additional research is needed, however, to further advance our understanding of factors that underlie a propensity or resistance to weight-gain.
Highlights.
Between-network connectivity (BNC) assessed in adults prone or resistant to obesity
Increased basal ganglia and dorsolateral prefrontal cortex BNC in obesity-prone group
May reflect disrupted balance between goal-directed and habitual control systems
Acknowledgments
We thank the research participants, and Debra Singel and Yiping Du for their assistance with fMRI data collection. This work was supported by NIH Colorado CTSI grant UL1TR00154, NIH Nutrition Obesity Research Center grant P30DK48520, and NIH grants R01MH102224 (JRT), R01DK103691 (JRT), R01DK089095 (MAC and JRT), R01DK072174 (MAC), R21DK102052 (JRT), and K01DK100445 (KTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Corsica JA, Hood MM Eating disorders in an obesogenic environment. J Am Diet Assoc. 2011,111:996–1000. doi: 10.1016/j.jada.2011.04.011 [DOI] [PubMed] [Google Scholar]
- [2].Stoeckel LE, Birch LL, Heatherton T, Mann T, Hunter C, Czajkowski S, et al. Psychological and neural contributions to appetite self-regulation. Obesity (Silver Spring). 2017,25 Suppl 1:S17–S25. doi: 10.1002/oby.21789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A Neuroimaging and obesity: Current knowledge and future directions. Obes Rev. 2012,13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012,63:415–22. doi: 10.1016/j.neuroimage.2012.06.070 [DOI] [PubMed] [Google Scholar]
- [5].Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012,96:989–99. doi: 10.3945/ajcn.112.042341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yokum S, Stice E Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: A repeated-measures fMRI study. Am J Clin Nutr. 2019,110:1275–86. doi: 10.1093/ajcn/nqz204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Eichman LS, Bessesen DH, et al. Differences in the neuronal response to food in obesity-resistant as compared to obesity-prone individuals. Physiol Behav. 2013. doi: 10.1016/j.physbeh.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lips MA, Wijngaarden MA, van der Grond J, van Buchem MA, de Groot GH, Rombouts SA, et al. Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. Am J Clin Nutr. 2014,100:524–31. doi: 10.3945/ajcn.113.080671 [DOI] [PubMed] [Google Scholar]
- [9].Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, et al. The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp. 2012,33:1052–61. doi: 10.1002/hbm.21268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, et al. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int J Obes (Lond). 2016,40:1687–92. doi: 10.1038/ijo.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C, Stamatakis EA, Verdejo-Garcia A Disrupted functional connectivity in adolescent obesity. NeuroImage. Clinical 2016,12:262–8. doi: 10.1016/j.nicl.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Avery JA, Powell JN, Breslin FJ, Lepping RJ, Martin LE, Patrician TM, et al. Obesity is associated with altered mid-insula functional connectivity to limbic regions underlying appetitive responses to foods. J Psychopharmacol. 2017,31:1475–84. doi: 10.1177/0269881117728429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Geha P, Cecchi G, Todd Constable R, Abdallah C, Small DM Reorganization of brain connectivity in obesity. Hum Brain Mapp. 2017,38:1403–20. doi: 10.1002/hbm.23462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Garcia-Garcia I, Jurado MA, Garolera M, Segura B, Sala-Llonch R, Marques-Iturria I, et al. Alterations of the salience network in obesity: A resting-state fMRI study. Hum Brain Mapp. 2013,34:2786–97. doi: 10.1002/hbm.22104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang B, Tian D, Yu C, Zhang J, Tian X, von Deneen KM, et al. Altered baseline brain activities before food intake in obese men: A resting state fMRI study. Neurosci Lett. 2015,584:156–61. doi: 10.1016/j.neulet.2014.10.020 [DOI] [PubMed] [Google Scholar]
- [16].Baek K, Morris LS, Kundu P, Voon V Disrupted resting-state brain network properties in obesity: Decreased global and putaminal cortico-striatal network efficiency. Psychol Med. 2017,47:585–96. doi: 10.1017/S0033291716002646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A Ventral and dorsal striatum networks in obesity: Link to food craving and weight gain. Biol Psychiatry. 2017,81:789–96. doi: 10.1016/j.biopsych.2015.11.020 [DOI] [PubMed] [Google Scholar]
- [18].Tregellas JR, Wylie KP, Rojas DC, Tanabe J, Martin J, Kronberg E, et al. Altered default network activity in obesity. Obesity (Silver Spring). 2011,19:2316–21. doi: 10.1038/oby.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S Elevated body mass index is associated with increased integration and reduced cohesion of sensory-driven and internally guided resting-state functional brain networks. Cereb Cortex. 2018,28:988–97. doi: 10.1093/cercor/bhx008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dong D, Lei X, Jackson T, Wang Y, Su Y, Chen H Altered regional homogeneity and efficient response inhibition in restrained eaters. Neuroscience. 2014,266:116–26. doi: 10.1016/j.neuroscience.2014.01.062 [DOI] [PubMed] [Google Scholar]
- [21].Dong D, Jackson T, Wang Y, Chen H Spontaneous regional brain activity links restrained eating to later weight gain among young women. Biol Psychol. 2015,109:176–83. doi: 10.1016/j.biopsycho.2015.05.003 [DOI] [PubMed] [Google Scholar]
- [22].Stice E, Burger K Neural vulnerability factors for obesity. Clin Psychol Rev. 2019,68:38–53. doi: 10.1016/j.cpr.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wylie KP, Kronberg E, Maharajh K, Smucny J, Cornier MA, Tregellas JR Between-network connectivity occurs in brain regions lacking layer IV input. Neuroimage. 2015,116:50–8. doi: 10.1016/j.neuroimage.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Donofry SD, Stillman CM, Erickson KI A review of the relationship between eating behavior, obesity, and functional brain network organization. Soc Cogn Affect Neurosci. 2019. doi: 10.1093/scan/nsz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cornier MA, McFadden KL, Thomas EA, Bechtell JL, Bessesen DH, Tregellas JR Propensity to obesity impacts the neuronal response to energy imbalance. Front Behav Neurosci. 2015,9:52. doi: 10.3389/fnbeh.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Legget KT, Cornier MA, Bessesen DH, Mohl B, Thomas EA, Tregellas JR Greater reward-related neuronal response to hedonic foods in women compared with men. Obesity (Silver Spring). 2018,26:362–7. doi: 10.1002/oby.22082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Garner DM, Olmsted MP, Bohr Y, Garfinkel PE The Eating Attitudes Test: Psychometric features and clinical correlates. Psychol Med. 1982,12:871–8. [DOI] [PubMed] [Google Scholar]
- [28].Radloff LS The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977,1:385–401. [Google Scholar]
- [29].Stunkard AJ, Messick S The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985,29:71–83. [DOI] [PubMed] [Google Scholar]
- [30].Grunwald GK, Melanson EL, Forster JE, Seagle HM, Sharp TA, Hill JO Comparison of methods for achieving 24-hour energy balance in a whole-room indirect calorimeter. Obes Res. 2003,11:752–9. doi: 10.1038/oby.2003.105 [DOI] [PubMed] [Google Scholar]
- [31].Li YO, Adal T, Calhoun VD Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping. 2007,28:1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bell AJ, Sejnowski TJ An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995,7:1129–59. [DOI] [PubMed] [Google Scholar]
- [33].Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011,32:2075–95. doi: 10.1002/hbm.21170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012,22:158–65. doi: 10.1093/cercor/bhr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Calhoun VD, de Lacy N Ten key observations on the analysis of resting-state functional mr imaging data using independent component analysis. Neuroimaging Clin N Am. 2017,27:561–79. doi: 10.1016/j.nic.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hyvarinen A Independent component analysis: Recent advances. Philos Trans A Math Phys Eng Sci. 2013,371:20110534. doi: 10.1098/rsta.2011.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watts DJ, Strogatz SH Collective dynamics of ‘small-world’ networks. Nature. 1998,393:440–2. doi: 10.1038/30918 [DOI] [PubMed] [Google Scholar]
- [38].Hyvarinen A, Karhunen J, Oja E Independent Component Analysis. New York: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- [39].Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012,59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Passingham RE, Stephan KE, Kotter R The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002,3:606–16. doi: 10.1038/nrn893 [DOI] [PubMed] [Google Scholar]
- [41].Brady RO Jr., McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar disorders. 2013. doi: 10.1111/bdi.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994,1:210–20. doi: 10.1002/hbm.460010306 [DOI] [PubMed] [Google Scholar]
- [43].Team, R. C. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- [44].Yin HH, Knowlton BJ The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006,7:464–76. doi: 10.1038/nrn1919 [DOI] [PubMed] [Google Scholar]
- [45].Balleine BW, O’Doherty JP Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010,35:48–69. doi: 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Horstmann A, Dietrich A, Mathar D, Possel M, Villringer A, Neumann J Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite. 2015,87:175–83. doi: 10.1016/j.appet.2014.12.212 [DOI] [PubMed] [Google Scholar]
- [47].de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012,32:12066–75. doi: 10.1523/JNEUROSCI.1088-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Burger KS, Berner LA A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Physiol Behav. 2014,136:121–7. doi: 10.1016/j.physbeh.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wiemerslage L, Zhou W, Olivo G, Stark J, Hogenkamp PS, Larsson EM, et al. A resting-state fMRI study of obese females between pre- and postprandial states before and after bariatric surgery. Eur J Neurosci. 2017,45:333–41. doi: 10.1111/ejn.13428 [DOI] [PubMed] [Google Scholar]
- [50].Al-Zubaidi A, Heldmann M, Mertins A, Jauch-Chara K, Munte TF Influences of hunger, satiety and oral glucose on functional brain connectivity: A multimethod resting-state fmri study. Neuroscience. 2018,382:80–92. doi: 10.1016/j.neuroscience.2018.04.029 [DOI] [PubMed] [Google Scholar]
- [51].Furlong TM, Jayaweera HK, Balleine BW, Corbit LH Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum. J Neurosci. 2014,34:5012–22. doi: 10.1523/JNEUROSCI.3707-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Morys F, Garcia-Garcia I, Dagher A Is obesity related to enhanced neural reactivity to visual food cues? A review and meta-analysis. Soc Cogn Affect Neurosci. 2020. doi: 10.1093/scan/nsaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].van den akker K, Stewart K, Antoniou EE, Palmberg A, Jansen A Food cue reactivity, obesity, and impulsivity: Are they associated? Current Addiction Reports. 2014,1:301–8. [Google Scholar]
- [54].Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015,518:197–206. doi: 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sinha R Role of addiction and stress neurobiology on food intake and obesity. Biol Psychol. 2018,131:5–13. doi: 10.1016/j.biopsycho.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Janssen LK, Herzog N, Waltmann M, Breuer N, Wiencke K, Rausch F, et al. Lost in translation? On the need for convergence in animal and human studies on the role of dopamine in diet-induced obesity. Current Addiction Reports. 2019,6:229–57. [Google Scholar]
- [57].Stice E, Yokum S Neural vulnerability factors that increase risk for future weight gain. Psychol Bull. 2016,142:447–71. doi: 10.1037/bul0000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage. 2008,42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kravitz AV, O’Neal TJ, Friend DM Do dopaminergic impairments underlie physical inactivity in people with obesity? Frontiers in human neuroscience. 2016,10:514. doi: 10.3389/fnhum.2016.00514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR, et al. Basal ganglia dysfunction contributes to physical inactivity in obesity. Cell Metab. 2017,25:312–21. doi: 10.1016/j.cmet.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Li G, Ji G, Hu Y, Xu M, Jin Q, Liu L, et al. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018,39:4755–65. doi: 10.1002/hbm.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Labouesse MA, Sartori AM, Weinmann O, Simpson EH, Kellendonk C, Weber-Stadlbauer U Striatal dopamine 2 receptor upregulation during development predisposes to diet-induced obesity by reducing energy output in mice. Proc Natl Acad Sci U S A. 2018,115:10493–8. doi: 10.1073/pnas.1800171115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Leite F, Ribeiro L Dopaminergic pathways in obesity-associated inflammation. J Neuroimmune Pharmacol. 2020,15:93–113. doi: 10.1007/s11481-019-09863-0 [DOI] [PubMed] [Google Scholar]
- [64].Wang JD, Kuo TB, Yang CC An alternative method to enhance vagal activities and suppress sympathetic activities in humans. Auton Neurosci. 2002,100:90–5. doi:S1566–0702(02)00150–9 [pii] [DOI] [PubMed] [Google Scholar]
- [65].Blum K, Thanos PK, Gold MS Dopamine and glucose, obesity, and reward deficiency syndrome. Front Psychol. 2014,5:919. doi: 10.3389/fpsyg.2014.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wise RA Dopamine, learning and motivation. Nat Rev Neurosci. 2004,5:483–94. doi: 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]
- [67].Mourra D, Gnazzo F, Cobos S, Beeler JA Striatal dopamine D2 receptors regulate cost sensitivity and behavioral thrift. Neuroscience. 2020,425:134–45. doi: 10.1016/j.neuroscience.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Feil J, Sheppard D, Fitzgerald PB, Yucel M, Lubman DI, Bradshaw JL Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Rev. 2010,35:248–75. doi: 10.1016/j.neubiorev.2010.03.001 [DOI] [PubMed] [Google Scholar]
- [69].Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015,109:318–27. doi: 10.1016/j.neuroimage.2014.12.073 [DOI] [PubMed] [Google Scholar]
- [70].Vainik U, Garcia-Garcia I, Dagher A Uncontrolled eating: A unifying heritable trait linked with obesity, overeating, personality and the brain. Eur J Neurosci. 2019,50:2430–45. doi: 10.1111/ejn.14352 [DOI] [PubMed] [Google Scholar]
- [71].Hasson U, Nusbaum HC, Small SL Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009,106:10841–6. doi: 10.1073/pnas.0903253106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pyka M, Beckmann CF, Schoning S, Hauke S, Heider D, Kugel H, et al. Impact of working memory load on fMRI resting state pattern in subsequent resting phases. PLoS One. 2009,4:e7198. doi: 10.1371/journal.pone.0007198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gobel CH, Tronnier VM, Munte TF Brain stimulation in obesity. Int J Obes (Lond). 2017,41:1721–7. doi: 10.1038/ijo.2017.150 [DOI] [PubMed] [Google Scholar]
- [74].Centers for Disease Control. Prevalence of self-reported obesity among U.S. adults by state and territory, 2018. https://www.cdc.gov/obesity/downloads/2018-overall-obesity-prevalence-map-508.pdf (accessed 4 August 2020).


