The COVID-19 pandemic has exposed longstanding vulnerabilities in our healthcare system, and has laid bare the individuals and communities most threatened by insufficient public health support. In the U.S., persons with HIV (PWH) represent a heterogenous population with complex medical and sociobehavioral needs currently unmet by systemically-flawed care models1,2. Thriving on health disparity, COVID-19 devastates the same communities where HIV prevalence is highest3, illuminating the catastrophic synergy of poverty, policies and structural racism4. This unprecedented time demands reexamination of HIV care delivery, paving the way for a revitalized healthcare infrastructure tailored to the needs of PWH and those at-risk of HIV to realize the goals of the Ending the HIV Epidemic (EHE) initiative5.
The EHE initiative aims to reduce new HIV infections in the U.S. by 90% by 2030 through focusing resources on hardest hit communities. A vital component of this strategy is to treat HIV infection rapidly and effectively so PWH achieve and maintain viral suppression. Prior to COVID-19, HIV care in the U.S. was frequently delivered by facility-based, provider-led visits occurring at 3–6-month intervals, with antiretroviral therapy (ART) dispensed in 30-day increments for the majority of patients. This “one-size-fits-all” model inadequately considers the varied needs of an estimated 1.1 million PWH residing in diverse local contexts across the U.S.6. The consequence is substantial drop-offs at each step in the HIV care continuum; in 2016, only 49% of PWH were retained in care and 53% had viral suppression, the current marker of successful HIV treatment6.
In February 2020, COVID-19 arrived to the Southern U.S., the national epicenter of the HIV/AIDS epidemic and a region crippled by limited healthcare infrastructure and widespread health disparities1,7. As the pandemic rapidly expanded, clinic operations pivoted to minimize in-person visits while ensuring patients had an uninterrupted ART supply. COVID-19 forced health systems to urgently implement mechanisms to reach patients and keep essential medications accessible through telehealth, medication delivery programs, mobile health units and home visits. For implementers of HIV care, the pandemic provides a long-overdue impetus to build innovative, more patient-centered healthcare delivery models.
Differentiated service delivery (DSD) is an HIV care model that combines aspects of facility- and community-based care and healthcare worker- or peer-led care processes8 (Figure). Fundamentally, DSD is an adaptive approach that aims to efficiently use limited resources by tailoring health services to local context, and patients’ clinical status and preferences. This care model originated in Sub-Saharan Africa after global HIV care shifted to a “treat all” strategy in 2016. Existing healthcare infrastructure became severely overwhelmed by the marked expansion of PWH requiring care9. To expand access, healthcare providers, pharmacies, and communities collaborated on remodeling ART delivery, adherence support, and retention tracking by leveraging local resources including personnel and alternative care sites. Employing a more client-centered approach than standard ambulatory-based visits, DSD empowers the primary stakeholder (patient) to find a mode of care conducive to their lifestyle while simultaneously decongesting the traditional healthcare system8,9.
Figure.
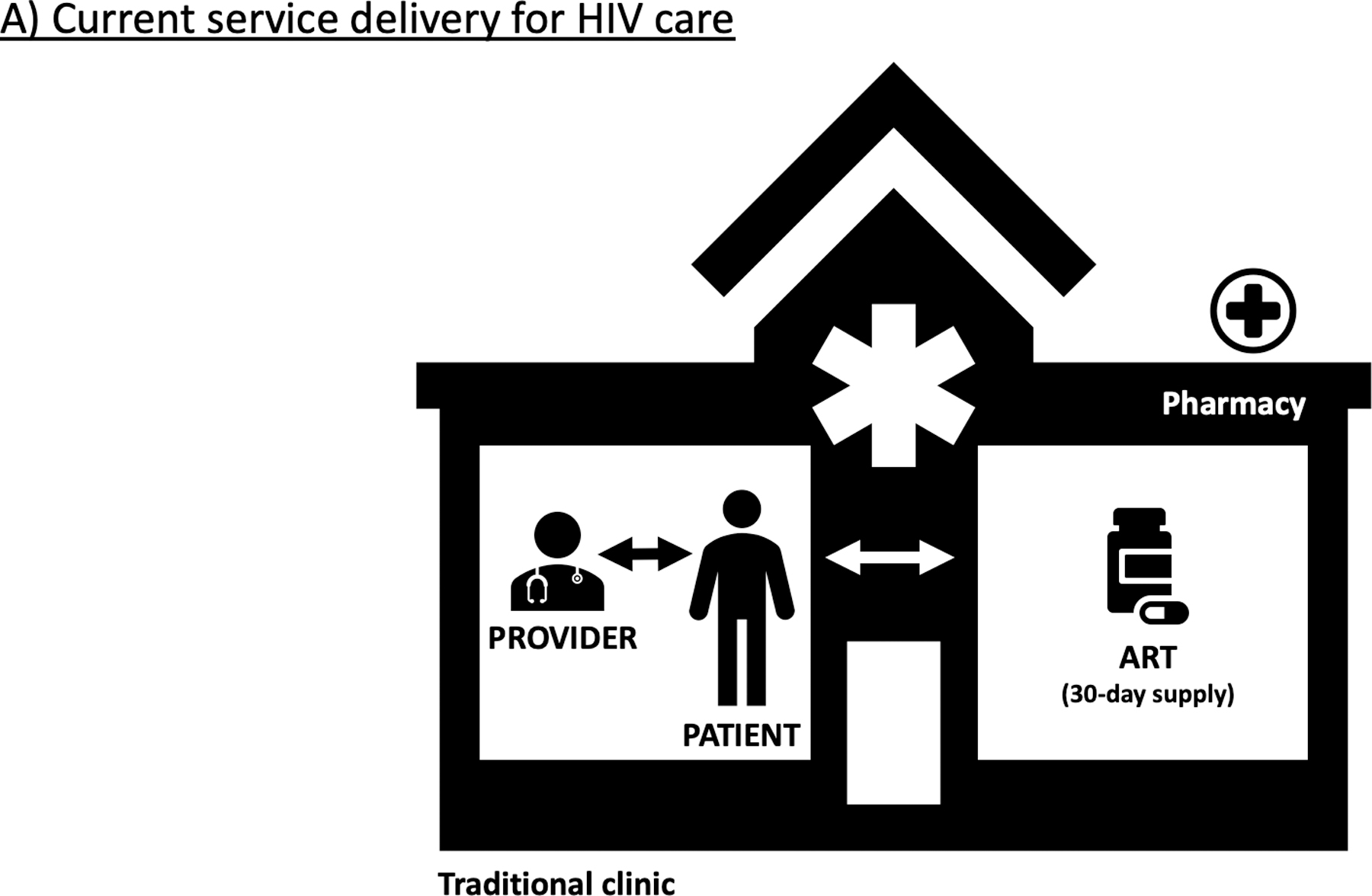

Current care delivery for persons with HIV in the U.S. comprises in-facility, provider-led visits every 3–6 months with antiretroviral therapy (ART) dispensed in 30-day supplies (A). Differentiated service delivery provides a more patient-centered approach adapted to local preference and context, allowing for innovative care platforms via telehealth, mobile units or home visits, and ART delivery by mail order, community distribution points, or in-facility “fast-track” pick-up supported by adherence clubs (B).
DSD has been most widely adopted in high-burden settings, where heterogeneity in HIV micro-epidemics (ranging from stable patients requiring fewer system touches to ill patients requiring intensive medical services)10,11 drives the need for a personalized public health approach12. In the U.S., populations of PWH, barriers to care, and care continuum outcomes differ regionally, with the Deep South falling behind1,13. Implementation of locally-tailored DSD across the country could offer access to care and ART in diverse settings such as clinics, communities and homes, with varying frequency of clinical assessment based on need14. The Ebola crisis in 2014–2015 accelerated use of DSD models in African countries to safeguard continuous ART through 6-month refills and community distribution15. COVID-19 likewise catalyzed adoption of HIV care models in the U.S. that are more accessible, differentiated, and patient-centered, and progress should be sustained beyond the current crisis.
While DSD has thus far been most commonly implemented for PWH with stable viral suppression, similar strategies may be useful for populations with retention and adherence challenges, especially if difficulty attending in-person provider or pharmacy appointments leads to ART disruptions. Rather than dispensing 1-month ART refills by pharmacy pick-up, DSD models encourage 3-month or 6-month ART supplies obtained via “fast-track” intra-facility retrieval or community distribution points16. Models are individualized based on community context and patient status (i.e., newly initiating ART, unstable or stable on treatment)17,18. Task-shifting has been widely used in South Africa since 2012 when the STRETCH trial demonstrated nurse-initiated and monitored ART improved mortality for PWH with CD4 counts >200 cells/µL and overall quality of care19. Another South African study found that when compared to usual care, participation in client-led “adherence clubs” improved retention in care (81.6% vs. 89.5%) and had comparable viral suppression (79.6% vs. 80.0%)20. While “fast-track” models were cost-saving in Malawi (10% reduction in annual unit cost of providing care to stable patients)18, it will be important to evaluate cost-effectiveness in U.S. settings and among patients of varying clinical stability.
Reduced frequency of in-person visits and ramping-up telehealth has potential to further decongest clinics, reduce patient inconvenience and improve outcomes. In 2015, the Veterans Affairs healthcare system offered telehealth in HIV specialty clinics given the large proportion (21%) of PWH who must travel ≥1 hour for care. A cluster-randomized trial of 1,670 veterans found telehealth uptake increased as the time saved from travel increased; viral suppression was higher among telehealth users than controls (91.5% vs. 80.0%)21. Telehealth is generally acceptable and multibeneficial22, however, the availability of and proficiency with technology varies among patients, presenting challenges to effective patient-provider communication. Ideally programs should offer flexibility in format (video, telephonic, face-to-face, co-provider) to accommodate patient-provider preferences and also guide implementation science to determine which approaches are most effective.
COVID-19 has starkly exposed gaps in our healthcare infrastructure beyond service delivery, namely social determinants of health that are exacerbated by the current crisis. Without addressing the intersectionality of structural racism, food insecurity, housing instability, stigma, mental health and substance use disorders – synergized by the COVID-19 and HIV epidemics – we will not be successful at EHE in the U.S1. DSD offers a strategic platform to confront social determinants of health that obstruct HIV treatment outcomes (i.e., retention and viral suppression) and deliver on the other EHE pillars: diagnosis, prevention, outbreak response5. Critical to this mission is building a robust public health workforce trained in testing, counseling and triaging community referrals23. Community health worker and patient navigator interventions among PWH in the U.S. and high-burden settings have been shown to promote psychosocial outcomes and housing security, respectively, and ultimately improve success across the care continuum24,25. Investing in community-based public health personnel equipped with multifaceted skills and resources allows for bundling of health promotion activities26. For example, performing culturally-competent COVID-19 contact tracing could double as an opportunity to conduct HIV testing and provide pre-exposure prophylaxis (PrEP) for HIV prevention27. Such integrated service delivery has the potential to destigmatize and scale-up testing (for COVID-19, HIV, other STIs and non-communicable diseases, such as hypertension and diabetes), build trust among communities, and restore public health infrastructure for long-lasting impacts on individual and community health23,26,28.
The current era prompts reevaluation of the standard metrics used to evaluate the quality of HIV care and to inform programming. Current measures are imperfect; they require several visits per year, involve longitudinal measurement, are not immediately actionable, and do not consider the patient’s psychosocial health and overall wellbeing. Among the six core HIV/AIDS Bureau clinical care performance measures, three require a visit in the last six months (HIV medical visit frequency, gap in HIV medical visits, annual retention in care)29. Using current definitions, if a patient stably suppressed was seen annually, this would result in misclassification as “not retained.” Moving forward, patients with stable viral suppression should be allowed annual HIV-1 RNA measurements – instead of biannual – without forfeiting ART coverage or retention status. Acceptable clinical encounters should include telehealth, in addition to face-to-face visits. Lastly, the pandemic has accentuated the psychologic toll of health, economic, and social stressors on overall wellbeing30. Validated tools exist to measure quality of life (QOL) among PWH31 and should be the final step in the care continuum, beyond viral suppression. For example, the WHOQOL-HIV BREF is a cross-culturally validated scale assessing six QOL domains, can be self-administered in ≤10 minutes, and has been used in Ethiopia and Nigeria among PWH to guide targeted interventions32,33. This scale could be integrated into U.S. DSD models once adapted to local context.
Differentiated care models have been needed for decades, but the COVID-19 pandemic has served as a catalyst to harness tragedy for change16,34. In refocusing our health systems to become patient-centered and community-invested, we have an unparalleled opportunity to use this crisis to restructure HIV care delivery permanently. Not only will these changes lead to progress toward EHE in the U.S., but may improve health disparities and clinical outcomes for PWH and all people in vulnerable populations. The HIV/COVID-19 syndemic makes it apparent that we must prioritize robust and sustained investment in addressing social determinants of health and in rebuilding our public health infrastructure to provide destigmatized and accessible care for all.
Acknowledgments
Financial support: This work was supported by the Emory Center for AIDS Research (award number P30-AI-050409). L. F. C. is also supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) (award numbers UL1TR002378 and TL1TR002382). C.A.M. is also supported by the National Heart, Lung, and Blood Institute of the NIH (award number K23-HL152903). C.D.L. is also supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number K23-AI124913).
References
- 1.Colasanti JA & Armstrong WS Challenges of reaching 90-90-90 in the Southern United States. Curr Opin HIV AIDS 14, 471–480 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Collins LF & Armstrong WS What It Means to Age With HIV Infection: Years Gained Are Not Comorbidity Free. JAMA Netw Open 3, e208023 (2020). [DOI] [PubMed] [Google Scholar]
- 3.COVID-19 and HIV Prevalence by U.S. County. Presented at the 54th meeting of the National Institutes of Health Office of AIDS Research (OAR) Director briefing of OAR Advisory Council on June 25, 2020. [Google Scholar]
- 4.Yancy CW COVID-19 and African Americans. JAMA (2020) 10.1001/jama.2020.6548. [DOI] [PubMed]
- 5.Giroir BP The Time Is Now to End the HIV Epidemic. Am J Public Health 110, 22–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2017. HIV Surveillance Supplemental Report 2019;24(No. 3). http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published June 2019. Accessed [June 20, 2020]. [Google Scholar]
- 7.Georgia Department of Public Health COVID-19 Daily Status Report. Available at: https://dph.georgia.gov/covid-19-daily-status-report. Accessed 6/20/2020.
- 8.International AIDS Society (IAS). It is time to deliver differently. Available at: https://www.differentiatedcare.org. Accessed June 29, 2020.
- 9.Grimsrud A et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc 19, 21484 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavhu W et al. Effect of a differentiated service delivery model on virological failure in adolescents with HIV in Zimbabwe (Zvandiri): a cluster-randomised controlled trial. Lancet Glob Health 8, e264–e275 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Ford N et al. Emerging priorities for HIV service delivery. PLoS Med. 17, e1003028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng EH, Holmes CB, Moshabela M, Sikazwe I & Petersen ML Personalized public health: An implementation research agenda for the HIV response and beyond. PLoS Med. 16, e1003020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summers NA et al. Predictors for Poor Linkage to Care Among Hospitalized Persons Living with HIV and Co-Occurring Substance Use Disorder. AIDS Res. Hum. Retroviruses (2020) 10.1089/AID.2019.0153. [DOI] [PMC free article] [PubMed]
- 14.Jiang H, Zhou Y & Tang W Maintaining HIV care during the COVID-19 pandemic. Lancet HIV 7, e308–e309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson L & Grimsrud A The time is now: expedited HIV differentiated service delivery during the COVID-19 pandemic. J Int AIDS Soc 23, e25503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AIDS Vaccine Advocacy Coalition (AVAC). We Need DSD Now More Than Ever: The frontier of human rights-centered services for HIV treatment & prevention. (2020).
- 17.Eshun-Wilson I et al. Differentiated Care Preferences of Stable Patients on Antiretroviral Therapy in Zambia: A Discrete Choice Experiment. J. Acquir. Immune Defic. Syndr 81, 540–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prust ML et al. Multi-month prescriptions, fast-track refills, and community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. J Int AIDS Soc 20, 21650 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairall L et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 380, 889–898 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MP et al. Adherence clubs and decentralized medication delivery to support patient retention and sustained viral suppression in care: Results from a cluster-randomized evaluation of differentiated ART delivery models in South Africa. PLoS Med 16, e1002874 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohl ME et al. Impact of Availability of Telehealth Programs on Documented HIV Viral Suppression: A Cluster-Randomized Program Evaluation in the Veterans Health Administration. Open Forum Infect Dis 6, ofz206 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandachi D, Dang BN, Lucari B, Teti M & Giordano TP Exploring the Attitude of Patients with HIV About Using Telehealth for HIV Care. AIDS Patient Care STDS 34, 166–172 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Armstrong WS The HIV Workforce in Crisis: An Urgent Need to Build the Foundation Required to End the Epidemic. Clin. Infect. Dis (2020) 10.1093/cid/ciaa302. [DOI] [PubMed]
- 24.Han H-R et al. Community health worker interventions to promote psychosocial outcomes among people living with HIV-A systematic review. PLoS ONE 13, e0194928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajabiun S et al. The Influence of Housing Status on the HIV Continuum of Care: Results From a Multisite Study of Patient Navigation Models to Build a Medical Home for People Living With HIV Experiencing Homelessness. Am J Public Health 108, S539–S545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peretz PJ, Islam N & Matiz LA Community Health Workers and Covid-19 - Addressing Social Determinants of Health in Times of Crisis and Beyond. The New England Journal of Medicine (2020) 10.1056/NEJMp2022641. [DOI] [PubMed]
- 27.Nosyk B, Armstrong WS & Del Rio C Contact tracing for COVID-19: An opportunity to reduce health disparities and End the HIV/AIDS Epidemic in the US. Clin. Infect. Dis (2020) 10.1093/cid/ciaa501. [DOI] [PMC free article] [PubMed]
- 28.Armstrong WS. HIV, COVID-19 and the importance of public health. Available at: https://thehill.com/opinion/healthcare/505676-hiv-covid-19-and-the-importance-of-public-health. Accessed July 5, 2020.
- 29.HRSA Ryan White & Global HIV/AIDS Programs. HIV/AIDS Bureau Performance Measures. Available at https://hab.hrsa.gov/sites/default/files/hab/clinical-quality-management/coremeasures.pdf. Accessed on July 5, 2020.
- 30.Shiau S, Krause KD, Valera P, Swaminathan S & Halkitis PN The Burden of COVID-19 in People Living with HIV: A Syndemic Perspective. AIDS Behav (2020) 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed]
- 31.Cooper V, Clatworthy J, Harding R, Whetham J & Emerge Consortium. Measuring quality of life among people living with HIV: a systematic review of reviews. Health Qual Life Outcomes 15, 220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legesse Tesemma A, Girma Abate M, Hailemariam Abebo Z & Estifanos Madebo W Determinants of Poor Quality of Life Among Adults Living with HIV and Enrolled in Highly Active Anti-Retroviral Therapy at Public Health Facilities of Arba Minch Town Administration in Southern Ethiopia. HIV AIDS (Auckl) 11, 387–394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suleiman BA, Yahaya M, Olaniyan FA, Sule AG & Sufiyan MB Determinants of health-related quality of life among human immunodeficiency virus positive (HIV-positive) patients at Ahmadu Bello University teaching hospital, Zaria, Nigeria-2015. BMC Public Health 20, 531 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoptaw S, Goodman-Meza D & Landovitz RJ Collective Call to Action for HIV/AIDS Community-Based Collaborative Science in the Era of COVID-19. AIDS Behav (2020) 10.1007/s10461-020-02860-y. [DOI] [PMC free article] [PubMed]


