Abstract
The goal of this study was to evaluate the effects of ovarian hormones on female rat ultrasonic vocalizations (USVs). Twenty (10 control and 10 ovariectomized) 3-month-old female rats were recorded in 3 recording conditions (elicitation, dyad, and isolation) over a full estrous cycle or time-matched duration. There were differences in USV acoustics (frequency and complexity parameters) across recording conditions but no differences in USV acoustics between control and ovariectomized groups. USVs produced in isolation had lower frequency and complexity parameters than elicited USVs for both control and ovariectomized rats. Additionally, for control rats, USV parameters of frequency, complexity, duration, and intensity changed depending on the estrous state. Therefore, although fluctuating hormone levels may influence USV acoustics, this variation can be controlled for by ovariectomizing female rats.
Keywords: Ultrasonic vocalization, ovarian hormones, larynx, rat, menopause, estrous cycle, female
1. Introduction
Rats produce ultrasonic vocalizations (USVs) in a variety of fundamental frequency ranges to communicate affective states in social situations [1]. As such, USVs have been utilized as a behavioral marker to study communicative intent and communicative disorders [2–6]. However, few studies have evaluated sex differences in USV acoustic parameters [7]. In fact, most rat laryngeal mechanistic studies exclude female rats due to the estrous cycle (the hormone cycle) influencing the number of vocalizations produced [8]. Although this exclusion is meant to control for external factors (such as fluctuating ovarian hormones) from influencing results, this rationale fails to acknowledge that female mammals in general experience hormonal fluctuations, limiting the relevance of findings to one sex (male).
Sex hormones may contribute to sexual dimorphism of USV production. Although both male and female rats produce 50-kHz USVs during mating, USV production during sexual encounters may be hormone-dependent for female rats [8]. The communicative intent of USV production during copulation has been hypothesized to serve as a proceptive cue for female rats and to elicit female solicitation behavior by male rats [9]. Because copulation can only take place during the proestrus and estrus portion of the estrous cycle and female USV production during mating indicates proceptivity, ovariectomizing female rats has been reported to eliminate USV production during mating [8]. However, the effect of the estrous cycle on USV production in non-mating environments is unknown.
Sex hormones may also contribute to sexual dimorphism of USV acoustic parameters. Initial investigations have demonstrated that male rats produce all three major USV frequency subtypes (22-kHz, 40-kHz, and 50-kHz) with a lower mean frequency than female rats in a variety of environments suggesting that the acoustic parameters of USVs are sexually dimorphic [10–12]. These initial investigations have primarily focused on number, mean/base frequency, and duration. Nevertheless, USV analysis has evolved since these initial investigations and, therefore, the extent of sex dimorphism of the rat USV acoustics is relatively unknown.
The overall objective of this study was to elucidate how the estrous cycle and surgically induced menopause (via ovariectomy) affected USV acoustics. Using two experiments, we tested the central hypothesis that ovarian hormone deprivation corresponds to changes in USV acoustics.
In Experiment 1, we sought to discover how surgically induced menopause affected USV acoustics by comparing USVs between surgery groups. Because rat vocal folds thicken and swell following menopause (similar to humans) [13–15], we hypothesized menopause would result in increased vocal fold edema and reduce the size of the laryngeal opening necessary for USVs. Therefore, we predicted that menopause would increase USV principal frequency and reduce frequency bandwidth [16]. Additionally, we hypothesized that lack of ovarian hormones should eliminate sexual behaviors associated with estrus, including USV production [8, 9]. Therefore, we predicted that menopause would decrease the number of USVs produced in the elicitation recording.
In Experiment 2, we sought to discover how fluctuating ovarian hormone levels affected USV acoustics in social and nonsocial recording environments by comparing USVs between estrous stages in normally cycling control rats. We hypothesized that the number, duration, and complexity of USVs may be a proceptive cue for mating. Therefore, we predicted that USVs produced during sexually-receptive portions of the estrus cycle (proestrus and estrus) would have greater in number, greater complexity, and longer duration than USVs produced during nonreceptive portions of the estrus cycle (metestrus and diestrus).
2. Materials and methods (Experiment 1 and 2)
A total of 20 3-month-old female Long-Evans rats were obtained from Charles River Laboratories. This strain of rat was chosen due to its high rate of spontaneous vocalizations [5]. Charles River Laboratories surgically induced menopause (ovariectomized) in 10 female rats and did not perform surgery on 10 age-matched rats (controls). Upon arrival at our facility, all rats were placed on a 12-hour reversed light cycle and housed in pairs with a cage mate in the same surgical group for the duration of the experiment. All experimental procedures were approved by New York University Medical Center’s Institutional Animal Care and Use Committee (IACUC).
To fully explore the effects of ovarian hormones on female USV acoustics, all rats were recorded in three conditions during the dark portion of the light cycle: 1) an elicitation condition of a 10-minute recording following a male introduction, 2) a one-hour dyad monitoring condition of female cage mates , and 3) a three-hour social isolation monitoring condition. The three recording conditions allowed us to parse out the different contributions of behavior from the effects of ovarian hormones. We expected the elicitation recording condition to be influenced by copulation behavior during estrous states. The dyad and isolation recording conditions lacked the male interaction and, therefore, would be influenced by ovarian hormone levels alone. Incorporating both the dyad and isolation conditions allowed an evaluation of social influence on USV acoustic parameters and the interaction with different hormone states.
Ten weeks following surgery, both the ovariectomized (OVX) and control rats were recorded in all three conditions on each day of the full estrous cycle (typically four or five days) for the control group or time-matched duration for the OVX group. A ten-week waiting period was chosen based on the timecourse of previous studies investigating neuromuscular adapations to surgerically induced menopause [17, 18].
Estrous state or cessation of ovarian cycle was determined via vaginal lavage [19]. Each estrous stage was tracked during the week of data collection and for one week prior to data collection to acclimate rats to lavage procedure. Estrous stage was determined by the presence and portion of cells within the vaginal lavage that was taken one hour before the start of the dark portion of the light cycle (Figure 1) [20]. Estrus (24-48 hours) had a dominance of cornified cells and/or behavioral estrus signs (darting, spinning, and ear wiggling) during male interaction [20]. Metestrus (6-8 hours) was the non-receptive day following estrus with the presence of cornified cells, nucleated cells, and leukocytes [20]. Diestrus was/were the day(s) (48-72 hours) following metestrus and had a predominance of leukocytes [20]. Proestrus (14 hours) had a predominance of large nucleated cells and behaviorally the female rat would accept the male rat but did not display estrus signs [20]. Sample stained lavages are presented in Figure 1. Using the criteria above recording days for each control rat were categorized into one the following: estrus, metestrus, diestrus 1, diestrus 2, or proestrus. Although most female rats have a 4-5 day estrous cycle, 30% of female rats do not have a typical 4-5 day cycle [19]. Therefore, female rats were recorded for a full estrous cycle containing at least one receptive (estrus) day and one nonreceptive (metestrus) day, regardless of the overall number of days.
Figure 1.
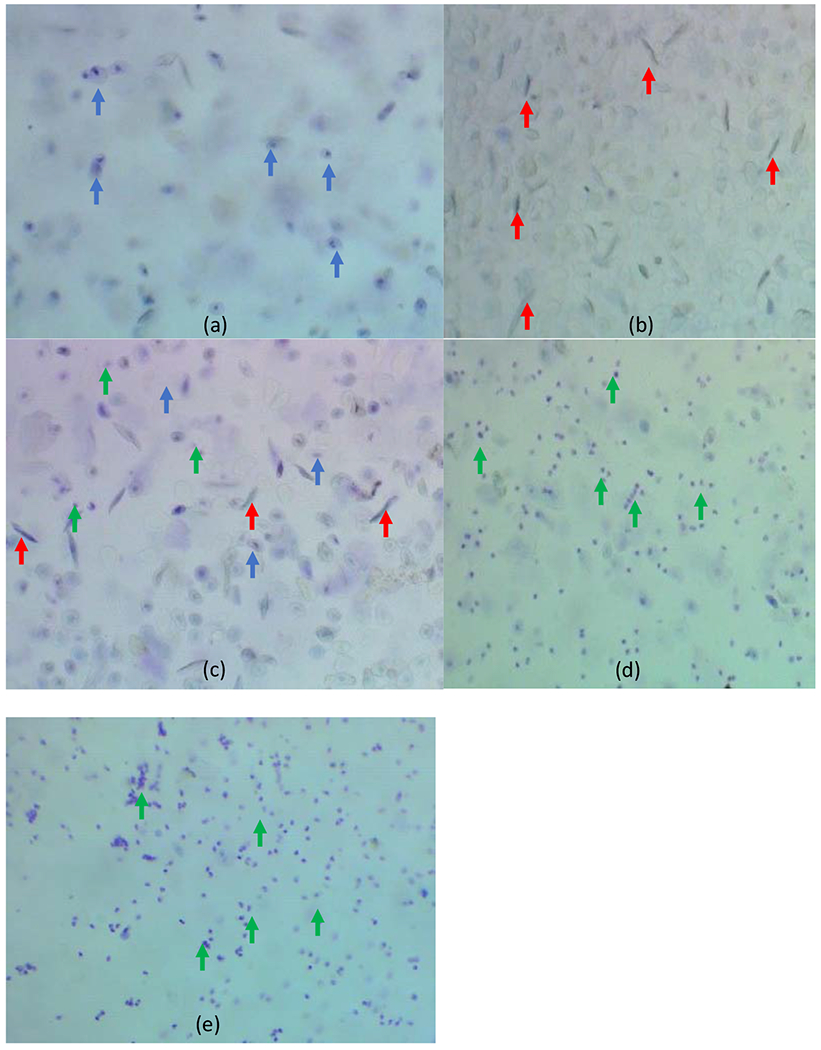
Representative images at 40x magnification of the four stages of the estrous cycle (a-d) and menopause (e) from vaginal lavages stained with Toluidine blue stain. The blue arrows point to nucleated cells predominantly found on the proestrus stage (a). The red arrows point to the cornified cells predominantly found in the estrus stage (b). The green arrow point to the leukocytes predominantly found in the diestrus stage (d). The metestrus stage (c) has all three cell types. The menopause stage (e) is similar to the diestrus stage with predominantly leukocytes.
2.1. Recording
At the beginning of each dark cycle, each rat was recorded for 10 minutes in the elicitation condition, in which the female rat was briefly introduced to a male rat and recorded in the soiled home cage of that male rat [21]. Because female rat odors do not effect sexual behavior of other females, the same soiled male cage was used without cleaning between subjects [21]. If the male rat exposure did not result in elicitation from the female rat, the introduction and removal of the male rat was repeated until each female rat produced a minimum of 30 USVs.
Following the elicitation condition, the dyad monitoring condition took place in the rats’ home cages for 1 hour. Then rats were separated and placed in a clean cage with free access to food and water and monitored for 3 hours in social isolation. USVs were recorded using an ultrasonic microphone (CM16/CMPA, Avisoft Bioacoustics, Germany) and USB recording interface (UltraSoundGate 816H, Avisoft Bioacoustics) connected to a Windows PC running Avisoft-RECORDER (Avisoft Bioacoustics) [22]. In the monitoring conditions (both isolation and dyad), female rats were acoustically monitored using ultrasonic microphones set to bigger a recording when the signal was between 20-125 kHz with additional parameters to reduce broadband frequency cage noise such as locomotion and eating [11].
The rationale for the static (elicitation-dyad-isolation) recording sequence was to avoid interaction between recording environments with the recording condition of primary interest, the elicitation condition. Based on previous investigations, few spontaneous USVs were anticipated from the dyad and isolation conditions, therefore, the elicitation recording condition was prioritized to the other two recording environments to increase the power of statistical analysis [11]. Additionally, recording in this sequential fashion allowed a uniform interpretation of the influence of the estrous cycle on USV parameters by controlling the exact hours of recording. For example, all elicited recordings were taken in the first two hours of the dark cycle. Randomization of recording conditions may have resulted in recording some estrus female rats at the beginning of the estrus phase and some near the end, which would have added an additional confounding variable. Although this recording sequence prevented understanding recording sequence on USV acoustics, controlling the estrous cycle and prioritizing USV collection from the elicitation condition outweighed the benefits of randomizing recording condition.
Following data collection, individual USVs were automatically extracted and measured using DeepSqueak software [23] (Figure 2). USVs detected by DeepSqueak software were manually reviewed and categorized as noise or USV. Ten percent of USV files were reviewed by a second trained researcher for interrater reliability (Pearson correlations of USV acoustic parameters between raters ranged from .87 to .98). USV acoustic parameters (frequency, complexity, intensity, and duration) and their operational definitions are summarized in Table 1. Because DeepSqueak calculates USV acoustic parameters based on the USV contour, this program reports different yet robust measurements compared to traditional USV analysis completed using SASLab Pro (Avisoft Bioacoustics) [24]. For example, both DeepSqueak and SASLab Pro analyses report the maximum frequency, minimum frequency, and frequency bandwidth of analyzed USVs; however, SASLab Pro reports mean frequency ( average frequency of the USV) or peak frequency (frequency at the loudest part of the USV) whereas DeepSqueak reports principal frequency (the median frequency of the USV contour). Although these measures differ, both programs report a representative frequency parameter for analysis.
Figure 2.
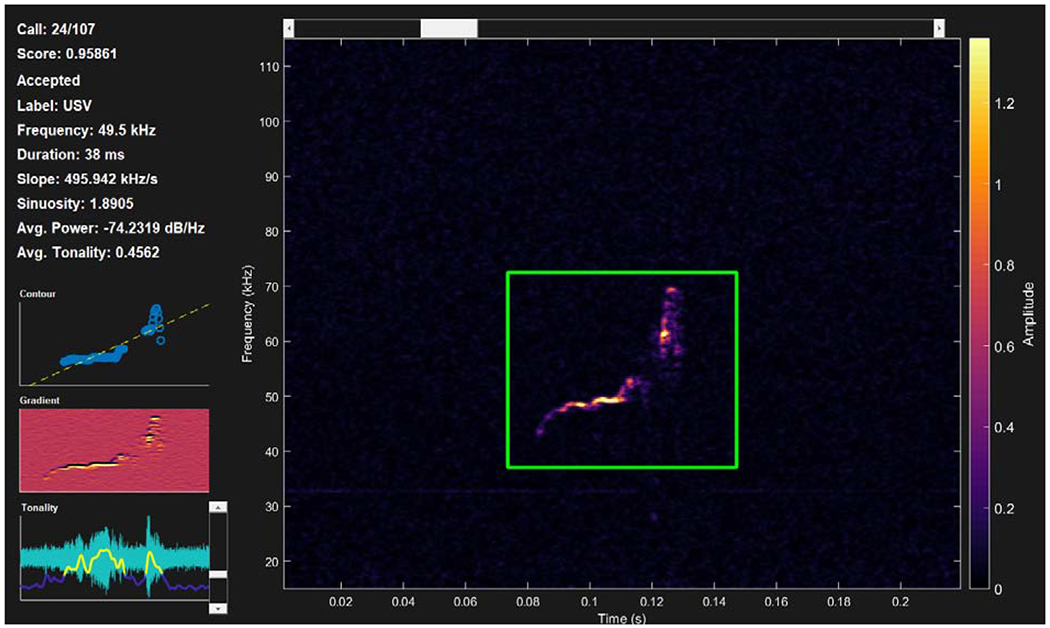
Spectrogram of detected USV in DeepSqueak. The spectrogram contains the detected USV within the detection (green) box with intensity of the signal represented by the heat color gradient bar on the right. The left panel outputs (from top to bottom) display the detected element number out of the total number of detected elements (24/107), confidence score (0.96), status (Accepted), label (USV), principal frequency, duration, slope, sinuosity, average power, and average tonality. The three left panel graphs display (from top to bottom) the contour of the USV, the frequency gradient of the contour, and tonality of the contour.
Table 1.
The definitions and categories of dependent acoustic variables of the ultrasonic vocalizations.
| Acoustic Dependent Variables (unit of measurement) | Definitions/Explanations* | Acoustic Variable Category |
|---|---|---|
| USV production per recording condition (#) | Number of USVs per recording condition per individual rat | Count |
| Principal fundamental frequency (kHz) | Median frequency of the frequencies of the call contour | Frequency parameter |
| Maximum frequency (kHz) | Highest frequency of the call contour | |
| Minimum frequency (kHz) | Lowest frequency of the call contour | |
| Frequency bandwidth (kHz) | Differences between minimum and maximum frequencies of the call contour | |
| Frequency standard deviation (kHz) | Standard Deviation of the frequencies of the call contour | Complexity parameter |
| Slope (kHz/s) | The slope of the least square’s regression line fitted to the detected points in the contour. Therefore, unmodulated USVs will have smaller slopes whereas modulated/complex USVs will have greater slopes. | |
| Sinuosity (#) | Length of the path between the first and last points on the contour, divided by the Euclidean distance between the first and last points. Because the length of the path of the contour (the numerator) is determined by the amount of modulation, unmodulated USVs will have a flat contour and a sinuosity near 1 whereas modulated/complex USVs will have a larger sinuosity | |
| Duration (ms) | Duration of USV | Duration |
| Mean power (dB/Hz) | Average power spectral density of the call contour. By using the call contour this measurement of intensity is not influenced by background noise. | Intensity parameter |
| Tonality (#) | One minus the geometric mean of the spectrogram, divided by the arithmetic mean. Therefore, this intensity measurement is relative to background noise and can be thought of as a signal-to-noise ratio measurement (i.e., the greater the tonality, the louder the signal-to-noise-ratio). |
Definitions from DeepSqueak documentation [23]
2.2. Statistical analysis
The effects of ovarian hormones and recording condition on USV acoustic parameters (Table 1) were analyzed with mixed-effects linear regression models in RStudio [25] using package lme4 [26]. For Experiment 1, to answer how surgery group and recording condition affected USV acoustic parameters, mixed models with surgery group (control and OVX), recording condition (dyad, elicited, and isolation), and their interaction were included as fixed effects and individual rat as the random effect were used to predict USV acoustic variables across the recording period. For the control group, all USVs across the entire estrous cycle were considered together. For the OVX group, USVs across multiple days (time-matched to the control group’s estrous cycle) were combined. After USV acoustic parameter models were fit, Analysis of Variance (ANOVA) was run on each model. Models were reduced if fixed effects were nonsignificant. Reduced and full models were compared using ANOVAs and reduced models were used if there were nonsignificant differences. On each final model, emmeans [27] in RStudio [25]was used to estimate the marginal means and contrasts between fixed effects.
Similarly, for Experiment 2 USVs from the control rats were analyzed to explore how the estrous cycle and recording condition affected USV acoustic parameters. Mixed models with estrous stages (proestrus, estrus, metestrus, and diestrus), recording condition (elicited and isolation), and their interaction were included as fixed effects and individual rat as the random effect were used to predict USV acoustic variables across the recording period (a full estrous cycle). The dyad condition was not used in the second set of models since control female cage mates did not have identical estrous cycles; therefore, the effects of the estrous cycle could not be evaluated in this condition. If fixed predictors were not significant within a given model, models were reduced. ANOVA was used to test for differences between the full and reduced models. Similar to Experiment 1, emmeans was used to estimate means and contrasts between fixed effects.
The number of USVs produced per recording condition were compared using repeated-measures ANOVA with significant main and interaction effects investigated using post hoc pairwise t-tests with Holm-adjusted p-values [25, 27]. For Experiment 1, we tested the effects of surgery group, recording condition, and their interaction on the number of USVs produced. For Experiment 2, we tested the effects of estrous state, recording condition, and their interaction on the number of USVs produced.
3. Results
3.1. Model selection results
For Experiment 1, for all mixed-effects acoustic parameter models that evaluated the effects of surgery group and recording condition, there was no effect of surgery group whereas recording conditions was significant for all models (Appendix A). However, the interaction terms were significant for the following USV parameters: minimum frequency, tonality, and duration (Appendix A). Reduced models were, therefore, chosen based on comparisons between full and reduced models (Appendix B) for the following acoustic parameters: principal frequency, maximum frequency, frequency bandwidth, frequency standard deviation, slope, and mean power. Table 2 summarizes the means and standard deviations of the acoustic parameters for both the ovariectomy and control rats in each recording condition.
Table 2.
The mean and standard deviation of acoustic parameters by group, recording condition, and estrous state.
| Group | Control | Menopause | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recording Condition | Dyad | elicited | isolation | dyad | elicited | isolation | ||||||||
| Estrous State | Diestrus 1 | Diestrus 2 | Estrus | Metestrus | Proestrus | Diestrus 1 | Diestrus 2 | Estrus | Metestrus | Proestrus | menopause | |||
| Number | 25.027 ±19.742 | 152.444 ±106.953 | 265.667 ±195.514 | 346.100 ±199.487 | 177.500 ±95.532 | 254.889 ±173.762 | 12.889 ±17.025 | 23.400 ±23.628 | 78.667 ±172.556 | 18.370 ±22.309 | 15.571 ±16.692 | 29.207 ±20.723 | 119.895 ±126.639 | 17.340 ±12.518 |
| Principal Frequency | 56.068 ±11.402 | 58.189 ±9.281 | 60.617 ±8.69 | 55.234 ±9.891 | 53.944 ±10.671 | 58.308 ±10.605 | 54.506 ±12.779 | 57.105 ±12.435 | 49.638 ±9.285 | 50.18 ±11.33 | 55.287 ±14.2 | 59.629 ±12.148 | 57.731 ±9.154 | 54.515 ±13.721 |
| Minimum Frequency | 50.457 ±10.428 | 52.567 ±8.548 | 53.991 ±8.147 | 49.767 ±9.461 | 49.268 ±9.663 | 52.329 ±9.461 | 50.76 ±11.31 | 52.981 ±11.75 | 45.798 ±9.267 | 46.591 ±10.947 | 50.858 ±12.761 | 52.673 ±10.806 | 51.94 ±8.694 | 50.449 ±12.821 |
| Maximum Frequency | 61.019 ±13.976 | 63.926 ±11.284 | 66.888 ±10.639 | 61.868 ±11.618 | 60.079 ±12.893 | 64.934 ±12.546 | 58.18 ±15.054 | 61.895 ±14.087 | 54.227 ±10.03 | 54.902 ±13.356 | 59.417 ±16.915 | 66.319 ±15.314 | 64.582 ±11.545 | 59.906 ±16.296 |
| Frequency Bandwidth | 10.562 ±9.746 | 11.359 ±9.378 | 12.897 ±9.731 | 12.101 ±9.843 | 10.811 ±9.379 | 12.604 ±10.729 | 7.42 ±8.361 | 8.914 ±7.795 | 8.43 ±8.085 | 8.312 ±7.803 | 8.56 ±10.812 | 13.646 ±11.341 | 12.642 ±10.32 | 9.457 ±10.147 |
| Duration | 37.275 ±29.145 | 30.985 ±17.5 | 34.579 ±19.904 | 32.728 ±18.003 | 29.723 ±17.318 | 38.154 ±27.96 | 50.483 ±69.931 | 55.321 ±75.342 | 47.844 ±30.584 | 37.178 ±35.919 | 44.488 ±45.608 | 37.365 ±36.998 | 33.83 ±20.198 | 52.86 ±76.455 |
| Tonality | 0.447 ±0.126 | 0.414 ±0.13 | 0.377 ±0.118 | 0.431 ±0.135 | 0.428 ±0.132 | 0.376 ±0.121 | 0.446 ±0.128 | 0.468 ±0.132 | 0.475 ±0.131 | 0.441 ±0.117 | 0.452 ±0.136 | 0.422 ±0.115 | 0.408 ±0.135 | 0.471 ±0.14 |
| Mean Power | −75.21 ±5.821 | −75.486 ±6.575 | −79.504 ±5.884 | −74.223 ±6.908 | −74.678 ±6.628 | −76.866 ±6.357 | −73.534 ±6.122 | −72.684 ±6.426 | −70.776 ±6.246 | −73.99 ±5.082 | −72.151 ±5.77 | −74.18 ±5.676 | −75.556 ±7.022 | −73.719 ±6.136 |
| Sinuosity | 1.521 ±0.751 | 1.537 ±0.676 | 1.684 ±0.822 | 1.622 ±0.813 | 1.509 ±0.697 | 1.57 ±0.71 | 1.202 ±0.356 | 1.422 ±1.063 | 1.284 ±0.555 | 1.398 ±0.578 | 1.168 ±0.265 | 1.716 ±0.887 | 1.628 ±0.764 | 1.38 ±0.859 |
| Slope | 228.789 ±360.688 | 276.067 ±286.841 | 272.516 ±321.254 | 258.993 ±300.577 | 275.189 ±349.222 | 251.299 ±288.685 | 197.611 ±267.92 | 177.164 ±188.705 | 139.051 ±231 | 212.749 ±288.364 | 228.31 ±407.183 | 300.736 ±384.354 | 271.827 ±311.69 | 238.393 ±366.931 |
| Frequency Standard Deviation | 3.047 ±2.823 | 3.372 ±2.898 | 3.797 ±3.051 | 3.497 ±2.99 | 3.184 ±2.897 | 3.76 ±3.452 | 2.206 ±2.676 | 2.497 ±2.261 | 2.284 ±2.356 | 2.489 ±2.46 | 2.759 ±3.833 | 3.83 ±3.186 | 3.637 ±3.08 | 2.756 ±3.068 |
For Experiment 2, for all mixed-effects acoustic parameter models that evaluated the effects of estrous stage and recording condition, there was an effect of both estrous cycle and recording condition for all models except slope (Appendix C). Additionally, the interaction terms were significant for all USV parameters except frequency bandwidth, frequency standard deviation, slope, and sinuosity (Appendix C). Reduced models were, therefore, chosen based on comparisons between full and reduced models (Appendix D) for the following acoustic parameters: frequency bandwidth, frequency standard deviation, slope, and sinuosity (Appendix D), the means and standard deviations of the acoustic parameters are summarized by estrous stage in the isolation and elicitation conditions (Table 2).
3.2. Effects of menopause and recording condition on USVs (Experiment 1)
Despite our prediction that menopause would increase USV principal frequency and decrease USV frequency bandwidth acoustic parameters, there was no effect of surgery group on any of the mixed-effect models of the acoustic variables but the recording condition was significant in all mixed-effects models.. However, our prediction that menopause would decrease the number of USVs produced in the elicitation condition was confirmed. Overall, both groups of rats produced more USVs in the elicitation condition (f(1,18)=34.19, p<.0001) and the control rats produced a greater number of USVs than OVX rats [f(1,18)=5.19, p = .04] (Figure 3). There was not a significant interaction [f(1,18)= 4.29, p = 0.05] between recording condition and surgery group for USV production, although examination of Figure 4 reveals a trend of the control group producing more USVs in the elicited condition. The lack of a statistical interaction was likely mostly driven by one outlier in the menopause group (Figure 4).
Figure 3.
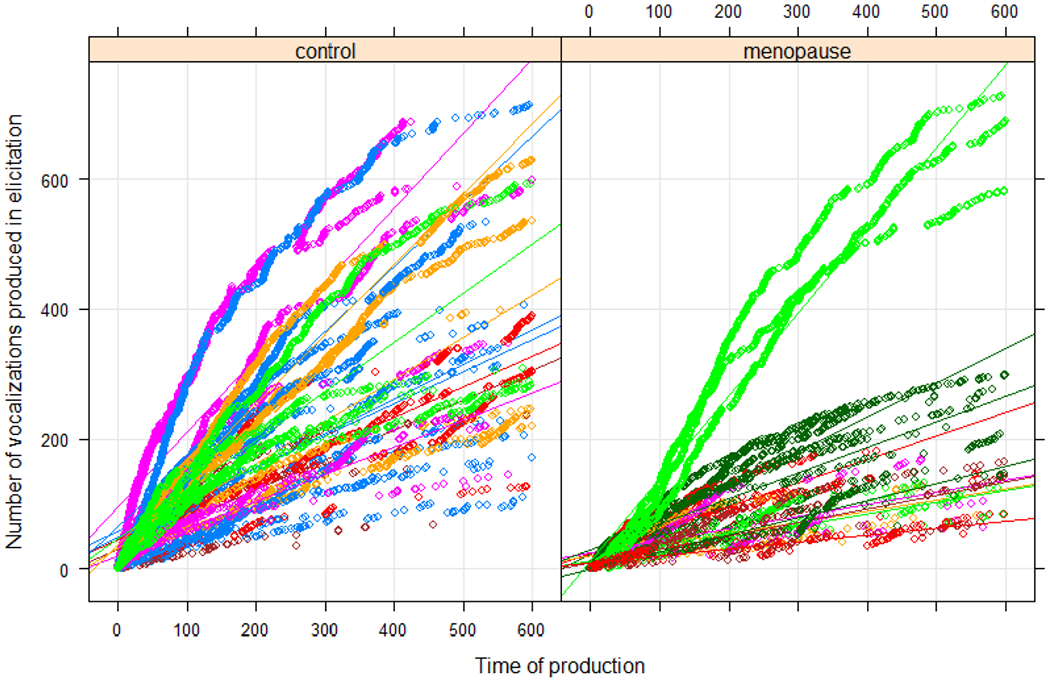
The cumulative number of USVs produced in 10-minute elicitation condition over one estrous cycle or time-matched duration for control rats (left) and menopause rats (right). Note the steeper slopes of the control rats indicating a higher USV production rate following the interaction with the male rat. Colors correspond to individual rats per group.
Figure 4.
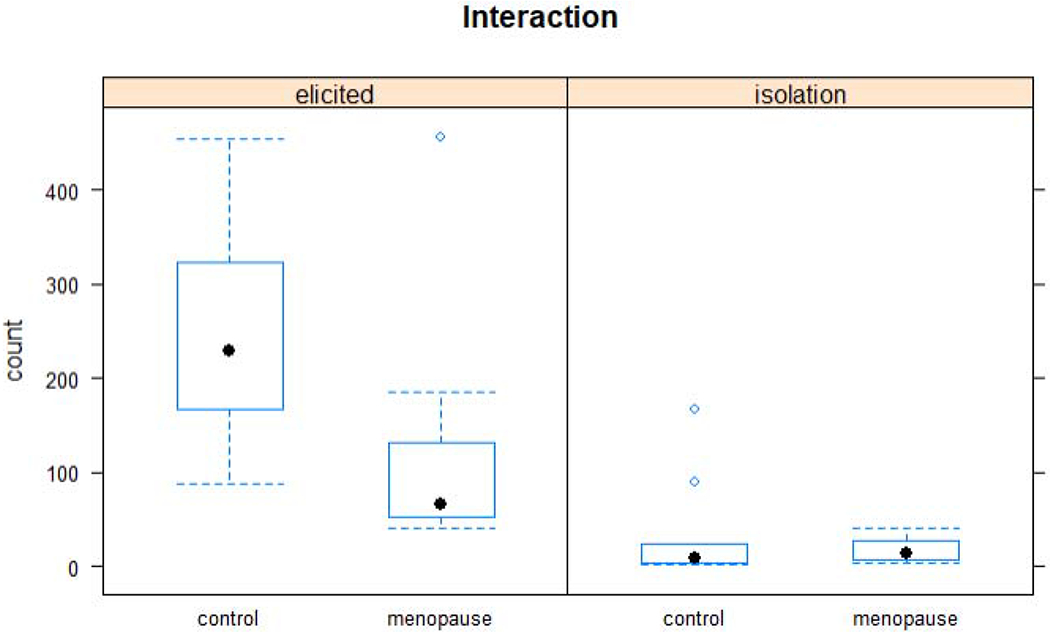
Box and whisker plots of the average number of USVs produced for each recording condition (elicited and isolation) of the control and menopause groups. A greater difference between surgery groups can be observed in the elicited recording condition.
Table 3 reports the pairwise comparisons of recording condition USV acoustics for each surgery group (OVX and control). In summary, in comparison to the elicited recording condition, USVs produced in the isolation condition had lower frequencies parameters, less complexity, greater power, and longer duration for both surgery groups (Table 3).
Table 3.
Pairwise comparisons of the effects of recording condition on predicted estimates of the full or reduced mixed-effects regression models of acoustic variables. Some reduced models do not contain surgery group in the model. P-values are adjusted using Holm’s method. Numbers are rounded to the third decimal place.
| Acoustic parameter | Surgery group | Contrast | Estimate | SE | Z-ratio | P-value |
|---|---|---|---|---|---|---|
| Principal Frequency | control & ovariectomized | elicited - dyad | −0.598 | 1.764 | −0.339 | 0.939 |
| elicited - isolation | 3.180 | 0.243 | 13.084 | <.001** | ||
| dyad - isolation | 3.779 | 1.776 | 2.127 | 0.084 | ||
| Maximum Frequency | control & ovariectomized | elicited - dyad | −0.452 | 1.943 | −0.233 | 0.971 |
| elicited - isolation | 4.088 | 0.297 | 13.781 | <.001** | ||
| dyad - isolation | 4.540 | 1.961 | 2.316 | 0.054 | ||
| Minimum Frequency | control | elicited - dyad | 0.130 | 2.306 | 0.056 | 0.998 |
| elicited - isolation | 2.886 | 0.299 | 9.640 | <.001** | ||
| dyad - isolation | 2.756 | 2.322 | 1.187 | 0.461 | ||
| ovariectomized | elicited - dyad | −0.238 | 2.302 | −0.103 | 0.994 | |
| elicited - isolation | 0.848 | 0.345 | 2.454 | 0.038* | ||
| dyad - isolation | 1.085 | 2.321 | 0.468 | 0.886 | ||
| Frequency Bandwidth | control & ovariectomized | elicited - dyad | −0.422 | 0.820 | −0.515 | 0.864 |
| elicited - isolation | 2.095 | 0.251 | 8.344 | <.001** | ||
| dyad - isolation | 2.518 | 0.849 | 2.967 | 0.008** | ||
| Slope | control | elicited - dyad | −3.876 | 22.805 | −0.170 | 0.984 |
| elicited - isolation | 57.887 | 7.895 | 7.333 | <.001** | ||
| dyad - isolation | 61.763 | 23.817 | 2.593 | 0.026* | ||
| ovariectomized | elicited - dyad | −3.876 | 22.805 | −0.170 | 0.984 | |
| elicited - isolation | 57.887 | 7.895 | 7.333 | <.001** | ||
| dyad - isolation | 61.763 | 23.817 | 2.593 | 0.026* | ||
| Frequency Standard Deviation | control & ovariectomized | elicited - dyad | −0.038 | 0.237 | −0.158 | 0.986 |
| elicited - isolation | 0.658 | 0.077 | 8.594 | <.001** | ||
| dyad - isolation | 0.696 | 0.246 | 2.824 | 0.013* | ||
| Sinuosity | control | elicited - dyad | 0.048 | 0.088 | 0.551 | 0.846 |
| elicited - isolation | 0.156 | 0.025 | 6.176 | <.001** | ||
| dyad - isolation | 0.108 | 0.090 | 1.193 | 0.458 | ||
| ovariectomized | elicited - dyad | −0.058 | 0.087 | −0.665 | 0.784 | |
| elicited - isolation | 0.239 | 0.029 | 8.191 | <.001** | ||
| dyad - isolation | 0.297 | 0.090 | 3.294 | 0.003** | ||
| Tonality | control | elicited - dyad | −0.032 | 0.024 | −1.368 | 0.358 |
| elicited - isolation | −0.024 | 0.004 | −5.591 | <.001** | ||
| dyad - isolation | 0.008 | 0.024 | 0.349 | 0.935 | ||
| ovariectomized | elicited - dyad | −0.035 | 0.023 | −1.501 | 0.290 | |
| elicited - isolation | −0.066 | 0.005 | −13.431 | <.001** | ||
| dyad - isolation | −0.031 | 0.024 | −1.300 | 0.395 | ||
| Mean Power | control & ovariectomized | elicited - dyad | −1.594 | 0.916 | −1.741 | 0.190 |
| elicited - isolation | −1.997 | 0.163 | −12.251 | <.001** | ||
| dyad - isolation | −0.403 | 0.926 | −0.435 | 0.901 | ||
|
Duration |
control | elicited - dyad | −0.005 | 0.004 | −1.344 | 0.371 |
| elicited - isolation | −0.015 | 0.001 | −15.657 | <.001** | ||
| dyad - isolation | −0.010 | 0.004 | −2.559 | 0.028* | ||
| ovariectomized | elicited - dyad | −0.005 | 0.004 | −1.354 | 0.365 | |
| elicited - isolation | −0.021 | 0.001 | −19.730 | <.001** | ||
| dyad - isolation | −0.016 | 0.004 | −4.331 | <.001** |
p-value < .01
p-value < .05
The dyad recording condition USVs did not differ from USVs produced in elicitation for either surgery group. However, both surgery groups produced USVs with greater frequency bandwidth, greater slope, and shorter duration in the dyad recording condition compared to the isolation recording condition. Additionally, OVX rats produced USVs with greater frequency standard deviation and sinuosity in the dyad recording condition compared to the isolation recording condition (Table 3).
3.3. Estrous cycle results for control rats (Experiment 2)
As in Experiment 1, there was a main effect of recording condition on the number of USVs produced, with more USVs produced in the elicitation condition than the isolation condition [f(1,5)=34.47, p = 0.002], but the interaction was not significant [f(4,24)=1.6, p = 0.21]. Additionally, there was a main effect of the estrous cycle on the number of USVs produced [f(4,26)=3.89, p = 0.01], with post hoc testing revealing the greatest number of USVs was produced in the estrus stage and the fewest in the diestrus I stage [t(26)=3.48,p=.02] of the estrous cycle (Figure 5). Therefore, the prediction that control female rats would produce the greatest number of USVs during receptive stages of the estrous cycle was supported by these findings.
Figure 5.
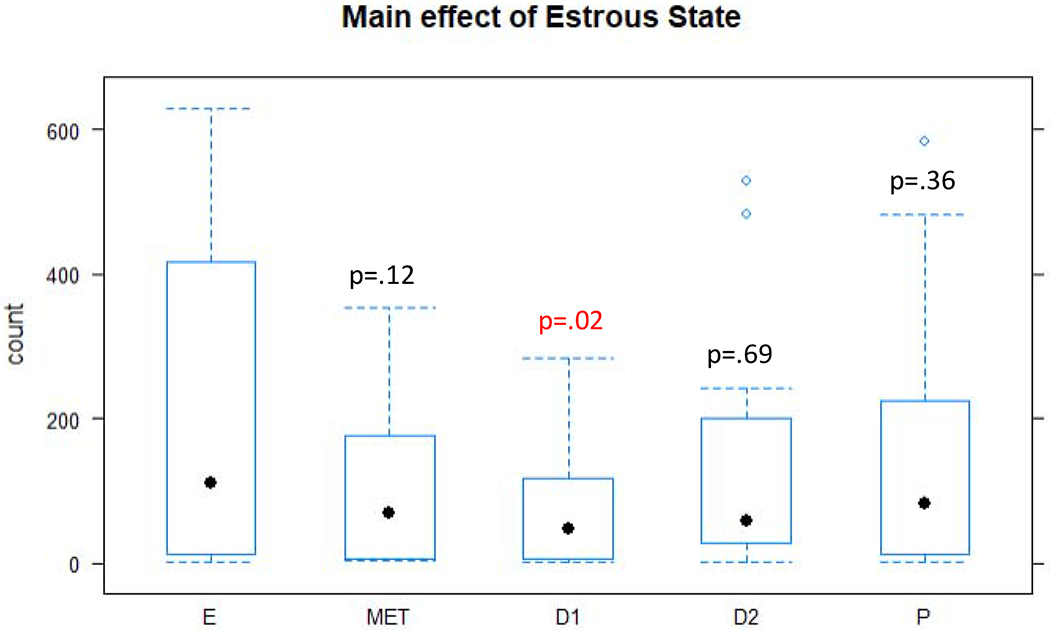
Box and whisker plots of the number of USVs produced in the five estrous states. P-values represent pairwise comparisons between the estrus stage and the other estrous stages. E= estrus, D1=diestrus I, D2=diestrus II, MET=metestrus, and P=proestrus.
All other USV acoustic variables except slope were affected by estrous stages. Appendix E reports the pairwise comparisons of estrous stage by recording condition on USV acoustics. Because of the large number of results (10 pairwise comparisons between the estrous stages for 10 acoustic parameters with 2 recording conditions), we have provided a summary table that indicates the directionality of pairwise comparisons with associated significance values to simplify the interpretation of significant findings (Table 4).
Table 4.
Summary table of pairwise comparisons of the effects of estrous stage on predicted estimates of the mixed-effects regression models for acoustic variables in each recording condition. P-values are adjusted using Holm’s method. Cells indicate directionality (greater or less) of pairwise differences as well as significance levels.
| Recording Condition | Estrous Stage Comparison | Frequency Parameters | Complexity Parameters | Intensity Parameters | Duration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Principal Frequency | Maximum Frequency | Minimum Frequency | Frequency Bandwidth | Slope | Frequency Standard Deviation | Sinuosity | Tonality | Mean Power | Duration | ||
| Elicited | Estrus - Metestrus | > ** | > ** | > | > ** | < | > ** | > ** | > | > ** | > * |
| Estrus - Diestrus 1 | < ** | < * | < ** | > * | < | > | > * | > ** | > ** | > | |
| Estrus - Diestrus 2 | < ** | < ** | < ** | > | < | > | < | > ** | > ** | < | |
| Estrus - Proestrus | < ** | < | < * | > | > | < | > * | > ** | > ** | < ** | |
| Metestrus - Diestrus 1 | < ** | < ** | < ** | < | < | < | < | > | > * | > | |
| Metestrus - Diestrus 2 | < ** | < ** | < ** | < * | > | < | < ** | > ** | > ** | < * | |
| Metestrus - Proestrus | < ** | < ** | < ** | < ** | > | < ** | < * | > ** | > ** | < ** | |
| Diestrus 1 - Diestrus 2 | < | < | > | < | > | < | < * | > | > ** | < * | |
| Diestrus 1 - Proestrus | > * | > | > ** | < * | > | < | < | > ** | > | < ** | |
| Diestrus 2 - Proestrus | > ** | > | > ** | < | > | < | > * | > | < ** | < ** | |
| Isolation | Estrus - Metestrus | < | < | < ** | > ** | < | > ** | > ** | > | > ** | > ** |
| Estrus - Diestrus 1 | < ** | < | < ** | > * | < | > | > * | > | > * | < | |
| Estrus - Diestrus 2 | < ** | < ** | < ** | > | < | > | < | < | < | < ** | |
| Estrus - Proestrus | < ** | < * | < ** | > | > | < | > * | > | > | > | |
| Metestrus - Diestrus 1 | < | < | < | < | < | < | < | < | < | < ** | |
| Metestrus - Diestrus 2 | < | < | < | < * | > | < | < ** | < ** | < ** | < ** | |
| Metestrus - Proestrus | < | < | < | < ** | > | < ** | < * | < | < | < | |
| Diestrus 1 - Diestrus 2 | < | < | < | < | > | < | < * | < * | < ** | < | |
| Diestrus 1 - Proestrus | < | < | > | < * | > | < | < | < | < | > | |
| Diestrus 2 - Proestrus | > | > | > | < | > | < | > * | > | > | > ** | |
p-value < .01
p-value < .05
3.3.1. Effects of estrous stage on USVs produced in elicitation condition (Experiment 2)
All the results described in this section are results from the elicitation recording condition. Because the elicitation involves the presentation of a male rat, differences between estrous stages may be due to hormones and/or copulation behavior. Results are summarized by the following estrous stages respectively: estrus, metestrus, diestrus 1, diestrus 2, and proestrus.
In summary, rats in estrus produced USVs with lower frequencies parameters, greater complexity, and greater intensity than diestrus and proestrus stages. In comparison to metestrus, rats in estrus produce USVs with greater frequencies, greater complexity, greater intensity, and longer duration (Table 4). In comparison to diestrus 1, rats in estrus produce USVs with lower frequencies, greater complexity, and greater intensity (Table 4). Similarly, in comparison to diestrus 2, rats in estrus produce USVs with lower frequencies and greater intensity (Table 4). Finally, in comparison to proestrus, rats in estrus produce USVs with lower frequencies, greater complexity, greater intensity, and shorter duration (Table 4).
In summary, metestrus had the lowest frequency parameters, least complexity, greatest power, and shortest duration when compared to other estrous stages. In comparison to diestrus 1, rats in metestrus produced USVs with lower frequencies and greater intensity (Table 4). In comparison to diestrus 2, rats in metestrus produce USVs with lower frequencies, less complexity, greater intensity, and shorter duration (Table 4). In comparison to proestrus, rats in metestrus produced USVs with lower frequencies, less complexity, greater intensity, and shorter duration (Table 4).
In summary, the diestrus states follow similar directionality in pairwise comparison between other stages. In comparison the diestrus 2, rats in diestrus 1 produce USVs with less complexity, greater intensity, and shorter duration (Table 4). In comparison the proestrus, rats in diestrus 1 produce USVs with higher frequencies, greater intensity, and shorter duration (Table 4). In comparison to proestrus, rats in diestrus 2 produce USVs with higher frequencies, more complexity, greater intensity, and shorter duration (Table 4).
Proestrus differences with other estrous stages have been discussed above. To summarize, USVs in proestrus tend to have frequency parameters higher than estrus and metestrus but lower than diestrus stages. Complexity parameters tend to be greater than metestrus but less than estrus. In general, intensity parameters tend be lowest during proestrus, but duration is longest.
To summarize trends within Table 4, in the elicitation recording condition acoustic parameters of frequency were lowest during low hormonal states (estrus and metestrus), acoustic complexity measures were lowest during the first two days following estrus (metestrus and diestrus 1), intensity parameters were highest during low hormonal states (estrus and metestrus), and duration was longest during proestrus. Therefore, our prediction that rats in sexually receptive states would produce USVs with greater complexity and duration was validated by these findings.
3.3.2. Effects of estrous stage on USVs produced in isolation condition (Experiment 2)
All of the results described in this section are results from the isolation recording condition. Because the isolation condition did not have a social component, differences between estrous stages are assumed to be hormonally driven. Results are summarized by the following estrous stages respectively: estrus, metestrus, diestrus 1, diestrus 2, and proestrus. Fewer overall differences between estrous stages were observed in this isolation recording condition.
In summary, rats in estrus produced USVs with lower frequencies parameters, greater complexity, and greater intensity than other estrous stages. In comparison to metestrus, rats in estrus produced USVs with lower frequencies, greater complexity, greater intensity, and longer duration (Table 4). In comparison to diestrus 1, rats in estrus produced USVs with lower frequencies, greater complexity, and greater intensity (Table 4). In comparison to diestrus 2, rats in estrus produced USVs with lower frequencies and shorter duration (Table 4). Finally, in comparison to proestrus, rats in estrus produced USVs with lower frequencies and greater complexity (Table 4).
In summary, metestrus had the lowest frequency parameters, least complexity, greatest power, and shortest duration when compared to other estrous stages. In comparison to diestrus 1, rats in metestrus produced USVs with shorter duration (Table 4). In comparison to diestrus 2, rats in metestrus produced USVs with less complexity, less intensity, and shorter duration (Table 4). In comparison to proestrus, rats in metestrus produced USVs with less complexity (Table 4).
Few differences between diestrus 1, diestrus 2, and proestrus were noted in the isolation condition. In comparison to diestrus 2, rats in diestrus 1 produced USVs with less sinuosity and less intensity. In comparison to proestrus, rats in diestrus 1 produced USVs with less frequency bandwidth. In comparison to rats in proestrus, rats in diestrus 2 produced USVs with greater sinuosity and longer duration.
To summarize trends within Table 4, acoustic parameters of frequency were lowest during low hormonal states (estrus and metestrus), acoustic complexity measures were greatest during estrus, intensity parameters were lowest during metestrus, and duration did not have a clear directionality in regard to estrous stages.
3.4. Effects of recording condition on USV acoustics in control rats (Experiment 2)
The recording condition also influenced USV acoustics. Table 5 reports the pairwise comparisons of recording condition on USV acoustics for each estrous stage. In summary, Table 5 demonstrates that USVs produced in isolation had lower frequency parameters, less complexity, greater intensity, and longer duration than USVs produced in elicitation. These trends are true for all estrous stages (Table 5).
Table 5.
Pairwise comparisons (emmeans) of recording conditions (elicitation vs isolation) for each estrous stage effects on predicted estimates of the mixed-effects regression models for acoustic variables. P-values are adjusted using Holm’s. Numbers are rounded to the third decimal place.
| Acoustic parameter | Estrous State | Estimate | SE | Z-ratio | P-value |
|---|---|---|---|---|---|
| Principal Frequency | Estrus | 4.848 | 0.421 | 11.525 | <.001** |
| Metestrus | 1.568 | 0.800 | 1.959 | 0.050* | |
| Diestrus 1 | 3.177 | 0.901 | 3.526 | <.001** | |
| Diestrus 2 | 2.476 | 0.899 | 2.754 | 0.006** | |
| Proestrus | 1.858 | 0.917 | 2.027 | 0.043* | |
| Maximum Frequency | Estrus | 5.739 | 0.508 | 11.296 | <.001** |
| Metestrus | 2.975 | 0.967 | 3.078 | 0.002** | |
| Diestrus 1 | 4.440 | 1.088 | 4.079 | <.001** | |
| Diestrus 2 | 3.220 | 1.086 | 2.965 | 0.003** | |
| Proestrus | 3.309 | 1.107 | 2.987 | 0.003** | |
| Minimum Frequency | Estrus | 4.538 | 0.390 | 11.624 | <.001** |
| Metestrus | 0.956 | 0.743 | 1.288 | 0.198 | |
| Diestrus 1 | 2.099 | 0.836 | 2.511 | 0.012* | |
| Diestrus 2 | 0.350 | 0.834 | 0.419 | 0.675 | |
| Proestrus | 1.074 | 0.851 | 1.262 | 0.207 | |
| Frequency Bandwidth | Estrus | 1.762 | 0.325 | 5.427 | <.001** |
| Metestrus | 1.762 | 0.325 | 5.427 | <.001** | |
| Diestrus 1 | 1.762 | 0.325 | 5.427 | <.001** | |
| Diestrus 2 | 1.762 | 0.325 | 5.427 | <.001** | |
| Proestrus | 1.762 | 0.325 | 5.427 | <.001** | |
| Slope | Estrus | 66.921 | 10.167 | 6.582 | <.001** |
| Metestrus | 66.921 | 10.167 | 6.582 | <.001** | |
| Diestrus 1 | 66.921 | 10.167 | 6.582 | <.001** | |
| Diestrus 2 | 66.921 | 10.167 | 6.582 | <.001** | |
| Proestrus | 66.921 | 10.167 | 6.582 | <.001** | |
| Frequency Standard Deviation | Estrus | 0.574 | 0.101 | 5.706 | <.001** |
| Metestrus | 0.574 | 0.101 | 5.706 | <.001** | |
| Diestrus 1 | 0.574 | 0.101 | 5.706 | <.001** | |
| Diestrus 2 | 0.574 | 0.101 | 5.706 | <.001** | |
| Proestrus | 0.574 | 0.101 | 5.706 | <.001** | |
| Sinuosity | Estrus | 0.172 | 0.025 | 6.949 | <.001** |
| Metestrus | 0.172 | 0.025 | 6.949 | <.001** | |
| Diestrus 1 | 0.172 | 0.025 | 6.949 | <.001** | |
| Diestrus 2 | 0.172 | 0.025 | 6.949 | <.001** | |
| Proestrus | 0.172 | 0.025 | 6.949 | <.001** | |
| Tonality | Estrus | −0.017 | 0.006 | −3.000 | 0.003** |
| Metestrus | 0.004 | 0.011 | 0.404 | 0.686 | |
| Diestrus 1 | −0.015 | 0.012 | −1.248 | 0.212 | |
| Diestrus 2 | −0.070 | 0.012 | −5.831 | <.001** | |
| Proestrus | −0.040 | 0.012 | −3.257 | 0.001** | |
| Mean Power | Estrus | −1.398 | 0.284 | −4.925 | <.001** |
| Metestrus | 0.236 | 0.540 | 0.438 | 0.662 | |
| Diestrus 1 | −0.821 | 0.608 | −1.351 | 0.177 | |
| Diestrus 2 | −5.940 | 0.607 | −9.790 | <.001** | |
| Proestrus | −2.609 | 0.619 | −4.217 | <.001** | |
| Duration | Estrus | −0.015 | 0.001 | −13.637 | <.001** |
| Metestrus | −0.009 | 0.002 | −4.267 | <.001** | |
| Diestrus 1 | −0.019 | 0.002 | −8.204 | <.001** | |
| Diestrus 2 | −0.023 | 0.002 | −9.987 | <.001** | |
| Proestrus | −0.007 | 0.002 | −2.980 | 0.003** |
p-value < .01
p-value < .05
4. Discussion
4.1. Effects of ovariectomy on USVs (Experiment 1)
We hypothesized that menopause would increase vocal fold edema and reduce the size of the laryngeal opening necessary for USVs and subsequently predicted that menopause would increase USV principal frequency and reduce frequency bandwidth. However, when USVs were compared between ovariectomized and normally-cycling groups across a full estrous cycle or time-matched duration, the only difference was that control female rats produced a greater number of USVs on average in the elicitation condition than the age-matched ovariectomized rats. The difference in number of USVs produced was predicted since the elicited recording condition used a male rat introduction to elicit the USVs. Female rat USVs have been hypothesized to be a proceptive cue to male rats; therefore, control female rats were expected to vocalize the most during estrus when receptive to copulation, which was confirmed in this study. In the elicited recording condition, control female rats produced the most USVs during their receptive estrus state, which is also the lowest hormonal state; thus, the higher number of USVs produced by the control females in the elicited recording condition was likely behaviorally driven (a proceptive cue to male rats to indicate receptivity) rather than hormonally-driven.
The ovariectomy did not have a robust effect on USV acoustic parameters relative to the effect of the estrous cycle on USV acoustic parameters. Therefore, in future investigations rather than excluding the female rat from laryngeal mechanism studies, the cycle can be eliminated by ovariectomizing the female rats without changing the overall acoustic properties of the USVs. A few considerations to this statement are pertinent. First, female rats in this study were young (3-months old) and, therefore, the interaction between age and ovarian hormones is unknown. Second, the effects of the estrous cycle were confounded by day (1 day per estrous stage), therefore, it is unclear if effects of the estrous cycle are consistent. Third, because estrous state was confounded by day, it is unclear what daily variation of acoustic parameters is normal. In future studies, male rats should be recorded along with female counterparts to serve as a control and to compare if recording condition affects USVs similarly between sexes. Nevertheless, the effects of recording condition were robust whereas surgery group was not, which indicates that ovarian hormone deprivation did not have a functional impact on the USVs of young female rats.
4.1. Effects of the estrous cycle on USVs (Experiment 2)
We hypothesized that the number, duration, and complexity of USVs may be a proceptive cue for mating and subsequently predicted that USVs produced during sexually-receptive portions of the estrus cycle (proestrus and estrus) would have greater in number, greater complexity, and longer duration than USVs produced during nonreceptive portions of the estrus cycle (metestrus and diestrus). Estrous stage clearly affected USV acoustic parameters. Vocalizations produced during low hormone levels (estrus and metestrus estrous stages) had lower frequency ranges than USVs produced during higher hormone levels (diestrus and proestrus estrous stages). Vocalizations produced during receptive estrous states (estrus and proestrus) were greater in complexity and duration measures than USVs produced in nonreceptive estrous states (metestrus and diestrus), thus, confirming our hypotheses and predictions that duration and complexity may server a proceptive cue to male rats. Furthermore, USV production was greatest in the estrus state and least in diestrus I stage.
A caveat to the lack of differences observed in the USV production per recording condition between all the estrous stages is that in the elicitation model, male rats were reintroduced to female rats until at least 30 USVs were obtained. This method allowed for a robust statistical analysis of USV acoustics; however, non-receptive stages of the estrous cycle required repeated male rat exposures whereas proestrus and estrus stages always resulted in more than 30 USVs following a male introduction. Therefore, although the only statistically significant difference was between estrus and diestrus I states, in general USV production was greatest for estrus and proestrus and lowest in metestrus and diestrus (Figure 5).
Frequency ranges of USVs may be regulated by hormones, as evidenced by the two low hormonal states having the lowest principal, minimum, and maximum frequencies. Because estradiol levels were not taken during recording days, this hypothesis currently cannot be directly substantiated by correlational analyses between hormone levels and USV frequency. It is possible the differences were driven by behavior. However, estrous copulation behavior alone cannot entirely explain this difference, as evidenced by the low frequency parameters of USVs in the isolation recording condition in both low hormonal stages (metestrus and estrus). This finding supports the hypothesis that USV frequency parameters are influenced by hormonal levels in the absence of sexual motivation.
The intensity and complexity of USVs may be regulated by estrous copulation behavior. USV parameters that measure the complexity of the vocalizations were highest during proestrus and estrous (receptive) stages of the cycle and lowest during the first two days following estrus (nonreceptive stages). Thus, female rats produced USVs with less complexity during nonreceptive stages of the cycle. In elicitation, both tonality and mean power were greatest during estrus compared to other estrous stages. Therefore, both intensity and complexity of the USV may serve as a proceptive cue for the male rats.
4.3. Limitations
One limitation to this study is that recordings were collected over one estrous cycle, which prevented analysis of cycle-to-cycle variation. Future investigations should validate these findings and include at least two consecutive estrous cycles to ensure that acoustic differences between estrous stages are consistent and not merely daily variation.
A primary difference between the acoustic analysis of this study in comparison to previous reports is the lack of USV categorization. Although the analysis of this study only included 50-kHz USV types and excluded any “alarm calls” with frequencies below 30 kHz. Several studies have indicated that rats produce subtypes of USVs depending on age [28], social context [1, 4, 21, 29–31], and sex [32]. However, no current consensus has been consistently utilized to categorize the 50-kHz USVs [23]. In fact, a goal of DeepSqueak software was to create a more time-efficient method of USV analysis so that future investigators can combined USV databases and identify a validated USV classification system using statistical clustering models based on USV acoustic features [23]. Thus, this study declined using USV classification and interpretation of USV subtypes until a widely-adopted USV classification has been validated.
5. Conclusion
The effects of the estrous cycle on rat USVs have been understudied in part due to earlier studies that found the number of USVs were influenced by estrous states [8, 33]. Nevertheless, several key findings from this study are pertinent to debunking myths and demystifying the rat estrous cycle as it relates to USV production and acoustics.
First, ovariectomized rats produce vocalizations with similar acoustic parameters as age-matched regular cycling female rats in a variety of social conditions: in response to a male rat, with a cage mate, and during social isolation. This finding conflicts with the previous study that claimed elimination of USVs following ovariectomy [8]. This difference may be due to several differences between studies. First, we waited 10 weeks following the ovariectomy procedure to record rats whereas it is unclear how soon after ovariectomy Thomas and Barfield’s rats were recorded. Also, we recorded OVX female rats for 10 minutes following the introduction of the male rat while Thomas and Barfield recorded OVX females for 5 minutes while the devocalized male was still present. Therefore, it is unclear if Thomas and Barfield’s OVX female rats did not vocalize due to a recent surgery, insufficient recording time, or the physical presence of the male; nevertheless, OVX female rats from this study vocalized in all three recording conditions.
Second, USV acoustic parameters across a full estrous cycle are similar to an ovariectomized (non-cycling) rat USVs. Therefore, the influence of the estrous cycle on USV acoustic parameters can be mediated by ovariectomizing female rats.
This study helped elucidate some of the effects of the estrous cycle and ovariectomy on female rat USVs. Although many hypothesized differences were not confirmed in this study, the null effects further substantiate the argument that female rats no longer should be excluded from mechanistic studies on the sole basis of estrous cycle effects.
Highlights:
USVs produced in isolation have lower frequency and complexity parameters than elicited USVs.
Menopause does not change USV acoustics in young female rats.
The frequency, complexity, duration, and intensity of USVs depend on estrous states.
Acknowledgements:
This research was supported by the 2018 American Laryngological Association Research Grant and grants K23DC014517 (PI: Johnson), F31DC017053 (PI: Lenell), and 5T32DC009401 (PI: Thibeault) from the National Institute on Deafness and other Communication Disorders of the National Institutes of Health.
Appendix A
Statistical results used to determine model selection for mixed-effects models. Full models included surgery group (control and ovariectomized), recording condition (dyad, elicited, and isolation), and their interaction as fixed effects and individual rat as the random effect. If the fixed terms were not significant within a given model, models were reduced. Numbers were rounded to the third decimal place.
| ANOVAs of mixed-effect models for each USV acoustic parameter. | ||||||
|---|---|---|---|---|---|---|
| USV acoustic parameter | Sum Sq | Mean Sq | NumDF | DenDF | F value | Pr(>F) |
| Principal Frequency | ||||||
| Surgery group | 217.842 | 217.842 | 1.000 | 26.446 | 2.408 | 0.133 |
| Recording condition | 14712.640 | 7356.320 | 2.000 | 52.199 | 81.330 | <.001** |
| Interaction | 215.016 | 107.508 | 2.000 | 52.199 | 1.189 | 0.313 |
| Maximum Frequency | ||||||
| Surgery group | 393.061 | 393.061 | 1.000 | 26.324 | 2.918 | 0.099 |
| Recording condition | 24263.776 | 12131.888 | 2.000 | 52.278 | 90.065 | <.001** |
| Interaction | 413.338 | 206.669 | 2.000 | 52.278 | 1.534 | 0.225 |
| Minimum Frequency | ||||||
| Surgery group | 185.435 | 185.435 | 1.000 | 26.485 | 2.367 | 0.136 |
| Recording condition | 5231.458 | 2615.729 | 2.000 | 52.294 | 33.385 | <.001** |
| Interaction | 1559.169 | 779.584 | 2.000 | 52.294 | 9.950 | <.001** |
| Frequency Bandwidth | ||||||
| Surgery group | 112.657 | 112.657 | 1.000 | 29.300 | 1.164 | 0.289 |
| Recording condition | 7145.211 | 3572.606 | 2.000 | 63.450 | 36.912 | <.001** |
| Interaction | 525.766 | 262.883 | 2.000 | 63.450 | 2.716 | 0.074 |
| Slope | ||||||
| Surgery group | 598720.280 | 598720.280 | 1.000 | 29.019 | 6.253 | 0.018* |
| Recording condition | 4782199.521 | 2391099.760 | 2.000 | 64.267 | 24.974 | <.001** |
| Interaction | 251884.069 | 125942.034 | 2.000 | 64.267 | 1.315 | 0.275 |
| Frequency Standard Deviation | ||||||
| Surgery group | 8.618 | 8.618 | 1.000 | 31.066 | 0.957 | 0.336 |
| Recording condition | 687.495 | 343.748 | 2.000 | 67.956 | 38.165 | <.001** |
| Interaction | 31.805 | 15.903 | 2.000 | 67.956 | 1.766 | 0.179 |
| Sinuosity | ||||||
| Surgery group | 1.500 | 1.500 | 1.000 | 27.940 | 2.677 | 0.113 |
| Recording condition | 58.842 | 29.421 | 2.000 | 60.365 | 52.492 | <.001** |
| Interaction | 3.086 | 1.543 | 2.000 | 60.365 | 2.753 | 0.072 |
| Tonality | ||||||
| Surgery group | 0.001** | 0.001** | 1.000 | 27.793 | 0.081 | 0.778 |
| Recording condition | 3.098 | 1.549 | 2.000 | 56.281 | 96.800 | <.001** |
| Interaction | 0.673 | 0.337 | 2.000 | 56.281 | 21.038 | <.001** |
| Mean Power | ||||||
| Surgery group | 3.069 | 3.069 | 1.000 | 27.862 | 0.075 | 0.786 |
| Recording condition | 6367.284 | 3183.642 | 2.000 | 55.943 | 78.262 | <.001** |
| Interaction | 215.272 | 107.636 | 2.000 | 55.943 | 2.646 | 0.080 |
| Duration | ||||||
| Surgery group | <.001** | <.001** | 1.000 | 27.833 | 0.003 | 0.957 |
| Recording condition | 0.487 | 0.244 | 2.000 | 58.751 | 317.163 | <.001** |
| Interaction | 0.017 | 0.008 | 2.000 | 58.751 | 10.863 | <.001** |
p < .01
p <.05
Appendix B
Statistical results used to determine model selection for mixed-effects models. Full models included surgery group (control and ovariectomized), recording condition (dyad, elicited, and isolation), and their interaction as fixed effects and individual rat as the random effect. Reduced models for principal frequency, maximum frequency, frequency bandwidth, frequency standard deviation, sinuosity, and mean only included recording condition as the fixed effect and individual rat as the random effect. Reduced model for slope included both recording condition and surgery group as fixed effects and individual rat as the random effect. ANOVAs were used to test full versus reduced models. No significance resulted in selection of reduced models. Numbers were rounded to the third decimal place.
| ANOVAs of full vs reduced models for acoustic variables with nonsignificant interaction terms. | ||||||||
|---|---|---|---|---|---|---|---|---|
| USV Acoustic parameter | npar | AIC | BIC | logLik | deviance | Chisq | Df | Pr(>Chisq) |
| Principal Frequency | ||||||||
| 5 | 135623.862 | 135662.977 | −67806.931 | 135613.862 | ||||
| 8 | 135625.190 | 135687.773 | −67804.595 | 135609.190 | 4.672 | 3 | 0.197 | |
| Maximum Frequency | ||||||||
| 5 | 142966.900 | 143006.015 | −71478.450 | 142956.900 | ||||
| 8 | 142967.147 | 143029.730 | −71475.573 | 142951.147 | 5.753 | 3 | 0.124 | |
| Bandwidth Frequency | ||||||||
| 5 | 136824.889 | 136864.004 | −68407.445 | 136814.889 | ||||
| 8 | 136823.509 | 136886.093 | −68403.755 | 136807.509 | 7.380 | 3 | 0.061 | |
| Slope | ||||||||
| 6 | 264076.941 | 264123.879 | −132032.471 | 264064.941 | ||||
| 8 | 264078.185 | 264140.768 | −132031.093 | 264062.185 | 2.756 | 2 | 0.252 | |
| Frequency Standard Deviation | ||||||||
| 5 | 93005.222 | 93044.337 | −46497.611 | 92995.222 | ||||
| 8 | 93006.229 | 93068.812 | −46495.114 | 92990.229 | 4.993 | 3 | 0.172 | |
| Sinuosity | ||||||||
| 5 | 41772.678 | 41811.793 | −20881.339 | 41762.678 | ||||
| 8 | 41769.172 | 41831.755 | −20876.586 | 41753.172 | 9.507 | 3 | 0.023* | |
| Mean Power | ||||||||
| 5 | 120865.701 | 120904.816 | −60427.851 | 120855.701 | ||||
| 8 | 120866.006 | 120928.589 | −60425.003 | 120850.006 | 5.696 | 3 | 0.127 | |
p <.05
Appendix C
Statistical results used to determine model selection for mixed-effects models. Full models included estrous stage (proestrus, estrus, Metestrus, diestrus 1, and diestrus 2), recording condition (dyad, elicited, and isolation), and their interaction as fixed effects and individual rat as the random effect. If the interaction term was not significant within a given model, models were reduced. Numbers were rounded to the third decimal place.
| ANOVAs of mixed effect models for each USV acoustic parameter. | ||||||
|---|---|---|---|---|---|---|
| USV acoustic parameter | Sum Sq | Mean Sq | NumDF | DenDF | F value | Pr(>F) |
| Principal Frequency | ||||||
| Estrous stage | 7014.743 | 1753.686 | 4 | 11677.341 | 20.526 | <.001** |
| Recording condition | 4786.693 | 4786.693 | 1 | 11678.790 | 56.025 | <.001** |
| Interaction | 1839.763 | 459.941 | 4 | 11675.524 | 5.383 | <.001** |
| Maximum Frequency | ||||||
| Estrous stage | 4397.198 | 1099.300 | 4 | 11678.540 | 8.819 | <.001** |
| Recording condition | 9561.051 | 9561.051 | 1 | 11680.464 | 76.705 | <.001** |
| Interaction | 1398.448 | 349.612 | 4 | 11676.188 | 2.805 | 0.024* |
| Minimum Frequency | ||||||
| Estrous stage | 8096.334 | 2024.084 | 4 | 11677.212 | 27.512 | <.001** |
| Recording condition | 2006.671 | 2006.671 | 1 | 11678.609 | 27.276 | <.001** |
| Interaction | 2997.126 | 749.282 | 4 | 11675.458 | 10.185 | <.001** |
| Frequency Bandwidth | ||||||
| Estrous stage | 1787.047 | 446.762 | 4 | 11676.385 | 4.883 | 0.001** |
| Recording condition | 2847.772 | 2847.772 | 1 | 11675.261 | 31.123 | <.001** |
| Interaction | 352.016 | 88.004 | 4 | 11679.715 | 0.962 | 0.427 |
| Slope | ||||||
| Estrous stage | 566018.447 | 141504.612 | 4 | 11662.284 | 1.576 | 0.178 |
| Recording condition | 1745764.810 | 1745764.810 | 1 | 11651.616 | 19.444 | <.001** |
| Interaction | 751174.903 | 187793.726 | 4 | 11679.328 | 2.092 | 0.079 |
| Freq Standard Deviation | ||||||
| Estrous stage | 95.209 | 23.802 | 4 | 11678.130 | 2.709 | 0.029* |
| Recording condition | 239.924 | 239.924 | 1 | 11678.256 | 27.303 | <.001** |
| Interaction | 20.931 | 5.233 | 4 | 11679.538 | 0.596 | 0.666 |
| Sinuosity | ||||||
| Estrous stage | 9.749 | 2.437 | 4 | 11673.049 | 4.590 | 0.001** |
| Recording condition | 21.180 | 21.180 | 1 | 11669.585 | 39.889 | <.001** |
| Interaction | 1.424 | 0.356 | 4 | 11679.839 | 0.670 | 0.613 |
| Tonality | ||||||
| Estrous stage | 0.318 | 0.079 | 4 | 11679.599 | 5.184 | <.001** |
| Recording condition | 0.469 | 0.469 | 1 | 11681.911 | 30.608 | <.001** |
| Interaction | 0.389 | 0.097 | 4 | 11676.876 | 6.340 | <.001** |
| Mean Power | ||||||
| Estrous stage | 1828.423 | 457.106 | 4 | 11678.953 | 11.752 | <.001** |
| Recording condition | 2737.150 | 2737.150 | 1 | 11681.046 | 70.370 | <.001** |
| Interaction | 2604.143 | 651.036 | 4 | 11676.425 | 16.738 | <.001** |
| Duration | ||||||
| Estrous Stage | 0.022 | 0.005 | 4 | 11661.795 | 9.577 | <.001** |
| Recording condition | 0.132 | 0.132 | 1 | 11650.861 | 230.763 | <.001** |
| Interaction | 0.021 | 0.005 | 4 | 11679.251 | 8.998 | <.001** |
p < .01
p <.05
Appendix D
Statistical results used to determine model selection for mixed-effects models. Full models included estrous stage (proestrus, estrus, Metestrus, diestrus 1, and diestrus 2), recording condition (dyad, elicited, and isolation), and their interaction as fixed effects and individual rat as the random effect. Reduced models did not include the interaction term but all other fixed and random effects. ANOVAs were used to test full versus reduced models. No significance resulted in selection of reduced models. Numbers were rounded to the third decimal place.
| ANOVAs of full vs reduced models for acoustic variables with nonsignificant interaction terms. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Acoustic Parameter | npar | AIC | BIC | logLik | deviance | Chisq | Df | Pr(>Chisq) |
| Frequency Bandwidth | 8 | 86042.014 | 86100.948 | −43013.007 | 86026.014 | |||
| 12 | 86046.176 | 86134.577 | −43011.088 | 86022.176 | 3.837 | 4 | 0.428 | |
| Slope | 8 | 166593.567 | 166652.501 | −83288.783 | 166577.567 | |||
| 12 | 166593.189 | 166681.590 | −83284.595 | 166569.189 | 8.378 | 4 | 0.079 | |
| Frequency Standard Deviation | 8 | 58644.592 | 58703.526 | −29314.296 | 58628.592 | |||
| 12 | 58650.211 | 58738.612 | −29313.106 | 58626.211 | 2.381 | 4 | 0.666 | |
| Sinuosity | 8 | 25827.782 | 25886.716 | −12905.891 | 25811.782 | |||
| 12 | 25833.094 | 25921.495 | −12904.547 | 25809.094 | 2.688 | 4 | 0.611 | |
Appendix E
Pairwise comparisons (emmeans) of the effects of estrous stages on predicted estimates of the mixed-effects (full or reduced) regression models for acoustic variables for each recording condition. P-values are adjusted using Elolm’s method. Numbers are rounded to the third decimal place.
| Acoustic parameter | Recording condition | Contrast | Estimate | SE | Z-ratio | P-value |
|---|---|---|---|---|---|---|
| Principal Frequency | elicited | Estrus - Metestrus | 1.046 | 0.278 | 3.770 | 0.002** |
| Estrus - Diestrus 1 | −1.945 | 0.306 | −6.352 | <.001** | ||
| Estrus - Diestrus 2 | −2.472 | 0.297 | −8.333 | <.001** | ||
| Estrus - Proestrus | −1.005 | 0.260 | −3.870 | 0.001** | ||
| Metestrus - Diestrus 1 | −2.992 | 0.341 | −8.767 | <.001** | ||
| Metestrus - Diestrus 2 | −3.519 | 0.348 | 10.124 | <.001** | ||
| Metestrus - Proestrus | −2.052 | 0.310 | −6.614 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.527 | 0.359 | −1.468 | 0.584 | ||
| Diestrus 1 - Proestrus | 0.940 | 0.328 | 2.866 | 0.034* | ||
| Diestrus 2 - Proestrus | 1.467 | 0.306 | 4.796 | <.001** | ||
| isolation | Estrus - Metestrus | −2.234 | 0.854 | −2.616 | 0.068 | |
| Estrus - Diestrus 1 | −3.617 | 0.932 | −3.881 | 0.001** | ||
| Estrus - Diestrus 2 | −4.844 | 0.961 | −5.040 | <.001** | ||
| Estrus - Proestrus | −3.996 | 0.959 | −4.167 | <.001** | ||
| Metestrus - Diestrus 1 | −1.382 | 1.158 | −1.194 | 0.755 | ||
| Metestrus - Diestrus 2 | −2.610 | 1.165 | −2.240 | 0.165 | ||
| Metestrus - Proestrus | −1.761 | 1.179 | −1.494 | 0.566 | ||
| Diestrus 1 - Diestrus 2 | −1.228 | 1.227 | −1.000 | 0.855 | ||
| Diestrus 1 - Proestrus | −0.379 | 1.234 | −0.307 | 0.998 | ||
| Diestrus 2 - Proestrus | 0.849 | 1.241 | 0.684 | 0.960 | ||
| Maximum Frequency | elicited | Estrus - Metestrus | 1.619 | 0.335 | 4.829 | <.001** |
| Estrus - Diestrus 1 | −1.112 | 0.370 | −3.008 | 0.022* | ||
| Estrus - Diestrus 2 | −1.639 | 0.358 | −4.574 | <.001** | ||
| Estrus - Proestrus | −0.775 | 0.314 | −2.471 | 0.097 | ||
| Metestrus - Diestrus 1 | −2.731 | 0.412 | −6.627 | <.001** | ||
| Metestrus - Diestrus 2 | −3.258 | 0.420 | −7.762 | <.001** | ||
| Metestrus - Proestrus | −2.394 | 0.375 | −6.391 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.527 | 0.434 | −1.214 | 0.743 | ||
| Diestrus 1 - Proestrus | 0.337 | 0.396 | 0.851 | 0.914 | ||
| Diestrus 2 - Proestrus | 0.864 | 0.369 | 2.339 | 0.133 | ||
| isolation | Estrus - Metestrus | −1.144 | 1.032 | −1.109 | 0.802 | |
| Estrus - Diestrus 1 | −2.412 | 1.125 | −2.143 | 0.202 | ||
| Estrus - Diestrus 2 | −4.158 | 1.161 | −3.582 | 0.003** | ||
| Estrus - Proestrus | −3.206 | 1.158 | −2.768 | 0.045* | ||
| Metestrus - Diestrus 1 | −1.267 | 1.399 | −0.906 | 0.895 | ||
| Metestrus - Diestrus 2 | −3.013 | 1.407 | −2.141 | 0.203 | ||
| Metestrus - Proestrus | −2.061 | 1.424 | −1.448 | 0.597 | ||
| Diestrus 1 - Diestrus 2 | −1.746 | 1.482 | −1.178 | 0.764 | ||
| Diestrus 1 - Proestrus | −0.794 | 1.491 | −0.533 | 0.984 | ||
| Diestrus 2 - Proestrus | 0.952 | 1.499 | 0.635 | 0.969 | ||
| Minimum Frequency | elicited | Estrus - Metestrus | 0.281 | 0.258 | 1.089 | 0.812 |
| Estrus - Diestrus 1 | −1.954 | 0.284 | −6.877 | <.001** | ||
| Estrus - Diestrus 2 | −1.852 | 0.275 | −6.727 | <.001** | ||
| Estrus - Proestrus | −0.678 | 0.241 | −2.813 | 0.039* | ||
| Metestrus - Diestrus 1 | −2.235 | 0.317 | −7.057 | <.001** | ||
| Metestrus - Diestrus 2 | −2.133 | 0.323 | −6.612 | <.001** | ||
| Metestrus - Proestrus | −0.959 | 0.288 | −3.331 | 0.008 | ||
| Diestrus 1 - Diestrus 2 | 0.102 | 0.333 | 0.306 | 0.998 | ||
| Diestrus 1 - Proestrus | 1.276 | 0.304 | 4.193 | <.001** | ||
| Diestrus 2 - Proestrus | 1.174 | 0.284 | 4.136 | <.001** | ||
| isolation | Estrus - Metestrus | −3.301 | 0.793 | −4.165 | <.001** | |
| Estrus - Diestrus 1 | −4.392 | 0.865 | −5.080 | <.001** | ||
| Estrus - Diestrus 2 | −6.040 | 0.892 | −6.772 | <.001** | ||
| Estrus - Proestrus | −4.142 | 0.890 | −4.655 | <.001** | ||
| Metestrus - Diestrus 1 | −1.092 | 1.074 | −1.016 | 0.848 | ||
| Metestrus - Diestrus 2 | −2.739 | 1.081 | −2.534 | 0.083 | ||
| Metestrus - Proestrus | −0.841 | 1.094 | −0.769 | 0.940 | ||
| Diestrus 1 - Diestrus 2 | −1.647 | 1.139 | −1.447 | 0.597 | ||
| Diestrus 1 - Proestrus | 0.251 | 1.145 | 0.219 | 0.999 | ||
| Diestrus 2 - Proestrus | 1.898 | 1.152 | 1.648 | 0.467 | ||
| Frequnecy Bandwidth | elicited | Estrus - Metestrus | 1.439 | 0.273 | 5.266 | <.001** |
| Estrus - Diestrus 1 | 0.974 | 0.302 | 3.229 | 0.011* | ||
| Estrus - Diestrus 2 | 0.354 | 0.296 | 1.194 | 0.755 | ||
| Estrus - Proestrus | 0.007 | 0.259 | 0.028 | 1.000 | ||
| Metestrus - Diestrus 1 | −0.465 | 0.340 | −1.367 | 0.649 | ||
| Metestrus - Diestrus 2 | −1.085 | 0.348 | −3.121 | 0.016* | ||
| Metestrus - Proestrus | −1.432 | 0.311 | −4.598 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.620 | 0.358 | −1.731 | 0.415 | ||
| Diestrus 1 - Proestrus | −0.967 | 0.328 | −2.951 | 0.026* | ||
| Diestrus 2 - Proestrus | −0.347 | 0.308 | −1.126 | 0.793 | ||
| isolation | Estrus - Metestrus | 1.439 | 0.273 | 5.266 | <.001** | |
| Estrus - Diestrus 1 | 0.974 | 0.302 | 3.229 | 0.011* | ||
| Estrus - Diestrus 2 | 0.354 | 0.296 | 1.194 | 0.755 | ||
| Estrus - Proestrus | 0.007 | 0.259 | 0.028 | 1.000 | ||
| Metestrus - Diestrus 1 | −0.465 | 0.340 | −1.367 | 0.649 | ||
| Metestrus - Diestrus 2 | −1.085 | 0.348 | −3.121 | 0.016* | ||
| Metestrus - Proestrus | −1.432 | 0.311 | −4.598 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.620 | 0.358 | −1.731 | 0.415 | ||
| Diestrus 1 - Proestrus | −0.967 | 0.328 | −2.951 | 0.026* | ||
| Diestrus 2 - Proestrus | −0.347 | 0.308 | −1.126 | 0.793 | ||
| Slope | elicited | Estrus - Metestrus | −7.422 | 8.557 | −0.867 | 0.909 |
| Estrus - Diestrus 1 | −16.838 | 9.445 | −1.783 | 0.384 | ||
| Estrus - Diestrus 2 | −1.873 | 9.274 | −0.202 | 1.000 | ||
| Estrus - Proestrus | 9.271 | 8.121 | 1.142 | 0.784 | ||
| Metestrus - Diestrus 1 | −9.416 | 10.663 | −0.883 | 0.903 | ||
| Metestrus - Diestrus 2 | 5.549 | 10.883 | 0.510 | 0.986 | ||
| Metestrus - Proestrus | 16.694 | 9.750 | 1.712 | 0.426 | ||
| Diestrus 1 - Diestrus 2 | 14.965 | 11.217 | 1.334 | 0.670 | ||
| Diestrus 1 - Proestrus | 26.109 | 10.257 | 2.546 | 0.081 | ||
| Diestrus 2 - Proestrus | 11.144 | 9.642 | 1.156 | 0.777 | ||
| isolation | Estrus - Metestrus | −7.422 | 8.557 | −0.867 | 0.909 | |
| Estrus - Diestrus 1 | −16.838 | 9.445 | −1.783 | 0.384 | ||
| Estrus - Diestrus 2 | −1.873 | 9.274 | −0.202 | 1.000 | ||
| Estrus - Proestrus | 9.271 | 8.121 | 1.142 | 0.784 | ||
| Metestrus - Diestrus 1 | −9.416 | 10.663 | −0.883 | 0.903 | ||
| Metestrus - Diestrus 2 | 5.549 | 10.883 | 0.510 | 0.986 | ||
| Metestrus - Proestrus | 16.694 | 9.750 | 1.712 | 0.426 | ||
| Diestrus 1 - Diestrus 2 | 14.965 | 11.217 | 1.334 | 0.670 | ||
| Diestrus 1 - Proestrus | 26.109 | 10.257 | 2.546 | 0.081 | ||
| Diestrus 2 - Proestrus | 11.144 | 9.642 | 1.156 | 0.777 | ||
| Frequency Standard Deviation | elicited | Estrus - Metestrus | 0.342 | 0.085 | 4.040 | 0.001** |
| Estrus - Diestrus 1 | 0.196 | 0.093 | 2.098 | 0.221 | ||
| Estrus - Diestrus 2 | 0.091 | 0.092 | 0.997 | 0.857 | ||
| Estrus - Proestrus | −0.074 | 0.080 | −0.920 | 0.889 | ||
| Metestrus - Diestrus 1 | −0.146 | 0.106 | −1.385 | 0.638 | ||
| Metestrus - Diestrus 2 | −0.251 | 0.108 | −2.326 | 0.137 | ||
| Metestrus - Proestrus | −0.416 | 0.097 | −4.311 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.105 | 0.111 | −0.942 | 0.881 | ||
| Diestrus 1 - Proestrus | −0.270 | 0.101 | −2.660 | 0.060 | ||
| Diestrus 2 - Proestrus | −0.165 | 0.095 | −1.735 | 0.413 | ||
| isolation | Estrus - Metestrus | 0.342 | 0.085 | 4.040 | 0.001** | |
| Estrus - Diestrus 1 | 0.196 | 0.093 | 2.098 | 0.221 | ||
| Estrus - Diestrus 2 | 0.091 | 0.092 | 0.997 | 0.857 | ||
| Estrus - Proestrus | −0.074 | 0.080 | −0.920 | 0.889 | ||
| Metestrus - Diestrus 1 | −0.146 | 0.106 | −1.385 | 0.638 | ||
| Metestrus - Diestrus 2 | −0.251 | 0.108 | −2.326 | 0.137 | ||
| Metestrus - Proestrus | −0.416 | 0.097 | −4.311 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.105 | 0.111 | −0.942 | 0.881 | ||
| Diestrus 1 - Proestrus | −0.270 | 0.101 | −2.660 | 0.060* | ||
| Diestrus 2 - Proestrus | −0.165 | 0.095 | −1.735 | 0.413 | ||
| Sinuosity | elicited | Estrus - Metestrus | 0.127 | 0.021 | 6.116 | <.001** |
| Estrus - Diestrus 1 | 0.067 | 0.023 | 2.927 | 0.028* | ||
| Estrus - Diestrus 2 | −0.021 | 0.023 | −0.917 | 0.890 | ||
| Estrus - Proestrus | 0.054 | 0.020 | 2.735 | 0.049* | ||
| Metestrus - Diestrus 1 | −0.060 | 0.026 | −2.316 | 0.140 | ||
| Metestrus - Diestrus 2 | −0.148 | 0.026 | −5.588 | <.001** | ||
| Metestrus - Proestrus | −0.073 | 0.024 | −3.089 | 0.017* | ||
| Diestrus 1 - Diestrus 2 | −0.088 | 0.027 | −3.222 | 0.011* | ||
| Diestrus 1 - Proestrus | −0.013 | 0.025 | −0.529 | 0.984 | ||
| Diestrus 2 - Proestrus | 0.075 | 0.023 | 3.187 | 0.013* | ||
| isolation | Estrus - Metestrus | 0.127 | 0.021 | 6.116 | <.001** | |
| Estrus - Diestrus 1 | 0.067 | 0.023 | 2.927 | 0.028* | ||
| Estrus - Diestrus 2 | −0.021 | 0.023 | −0.917 | 0.890 | ||
| Estrus - Proestrus | 0.054 | 0.020 | 2.735 | 0.049* | ||
| Metestrus - Diestrus 1 | −0.060 | 0.026 | −2.316 | 0.140 | ||
| Metestrus - Diestrus 2 | −0.148 | 0.026 | −5.588 | <.001** | ||
| Metestrus - Proestrus | −0.073 | 0.024 | −3.089 | 0.017 | ||
| Diestrus 1 - Diestrus 2 | −0.088 | 0.027 | −3.222 | 0.011* | ||
| Diestrus 1 - Proestrus | −0.013 | 0.025 | −0.529 | 0.984 | ||
| Diestrus 2 - Proestrus | 0.075 | 0.023 | 3.187 | 0.013* | ||
| Tonality | elicited | Estrus - Metestrus | 0.006 | 0.004 | 1.741 | 0.409 |
| Estrus - Diestrus 1 | 0.017 | 0.004 | 4.074 | <.001** | ||
| Estrus - Diestrus 2 | 0.023 | 0.004 | 5.814 | <.001** | ||
| Estrus - Proestrus | 0.031 | 0.003 | 8.929 | <.001** | ||
| Metestrus - Diestrus 1 | 0.010 | 0.005 | 2.240 | 0.165 | ||
| Metestrus - Diestrus 2 | 0.017 | 0.005 | 3.573 | 0.003** | ||
| Metestrus - Proestrus | 0.025 | 0.004 | 5.921 | <.001** | ||
| Diestrus 1 - Diestrus 2 | 0.006 | 0.005 | 1.329 | 0.673 | ||
| Diestrus 1 - Proestrus | 0.014 | 0.004 | 3.270 | 0.009** | ||
| Diestrus 2 - Proestrus | 0.008 | 0.004 | 1.946 | 0.293 | ||
| isolation | Estrus - Metestrus | 0.028 | 0.011 | 2.421 | 0.110 | |
| Estrus - Diestrus 1 | 0.019 | 0.012 | 1.486 | 0.571 | ||
| Estrus - Diestrus 2 | −0.030 | 0.013 | −2.349 | 0.130 | ||
| Estrus - Proestrus | 0.008 | 0.013 | 0.621 | 0.972 | ||
| Metestrus - Diestrus 1 | −0.009 | 0.016 | −0.590 | 0.977 | ||
| Metestrus - Diestrus 2 | −0.058 | 0.016 | −3.713 | 0.002** | ||
| Metestrus - Proestrus | −0.020 | 0.016 | −1.249 | 0.722 | ||
| Diestrus 1 - Diestrus 2 | −0.049 | 0.016 | −2.969 | 0.025* | ||
| Diestrus 1 - Proestrus | −0.011 | 0.017 | −0.640 | 0.969 | ||
| Diestrus 2 - Proestrus | 0.038 | 0.017 | 2.299 | 0.145 | ||
| Mean Power | elicited | Estrus - Metestrus | 0.633 | 0.187 | 3.378 | 0.007** |
| Estrus - Diestrus 1 | 1.282 | 0.207 | 6.208 | <.001** | ||
| Estrus - Diestrus 2 | 3.698 | 0.200 | 18.477 | <.001** | ||
| Estrus - Proestrus | 1.507 | 0.175 | 8.596 | <.001** | ||
| Metestrus - Diestrus 1 | 0.650 | 0.230 | 2.823 | 0.038* | ||
| Metestrus - Diestrus 2 | 3.066 | 0.234 | 13.077 | <.001** | ||
| Metestrus - Proestrus | 0.874 | 0.209 | 4.178 | <.001** | ||
| Diestrus 1 - Diestrus 2 | 2.416 | 0.242 | 9.972 | <.001** | ||
| Diestrus 1 - Proestrus | 0.224 | 0.221 | 1.014 | 0.849 | ||
| Diestrus 2 - Proestrus | −2.192 | 0.206 | 10.622 | <.001** | ||
| isolation | Estrus - Metestrus | 2.266 | 0.576 | 3.933 | 0.001** | |
| Estrus - Diestrus 1 | 1.859 | 0.629 | 2.957 | 0.026 | ||
| Estrus - Diestrus 2 | −0.844 | 0.648 | −1.301 | 0.691 | ||
| Estrus - Proestrus | 0.296 | 0.647 | 0.457 | 0.991 | ||
| Metestrus - Diestrus 1 | −0.407 | 0.781 | −0.521 | 0.985 | ||
| Metestrus - Diestrus 2 | −3.110 | 0.786 | −3.956 | 0.001** | ||
| Metestrus - Proestrus | −1.971 | 0.795 | −2.478 | 0.096 | ||
| Diestrus 1 - Diestrus 2 | −2.703 | 0.828 | −3.265 | 0.010* | ||
| Diestrus 1 - Proestrus | −1.563 | 0.833 | −1.877 | 0.330 | ||
| Diestrus 2 - Proestrus | 1.139 | 0.837 | 1.361 | 0.653 | ||
| Duration | elicited | Estrus - Metestrus | 0.002 | 0.001** | 2.791 | 0.042* |
| Estrus - Diestrus 1 | 0.002 | 0.001** | 2.573 | 0.075 | ||
| Estrus - Diestrus 2 | −0.001** | 0.001** | −0.680 | 0.961 | ||
| Estrus - Proestrus | −0.004 | 0.001** | −6.393 | <.001** | ||
| Metestrus - Diestrus 1 | <.001** | 0.001** | 0.039 | 1.000 | ||
| Metestrus - Diestrus 2 | −0.003 | 0.001** | −2.813 | 0.039* | ||
| Metestrus - Proestrus | −0.006 | 0.001** | −7.858 | <.001** | ||
| Diestrus 1 - Diestrus 2 | −0.003 | 0.001** | −2.757 | 0.046* | ||
| Diestrus 1 - Proestrus | −0.006 | 0.001** | −7.469 | <.001** | ||
| Diestrus 2 - Proestrus | −0.004 | 0.001** | −4.769 | <.001** | ||
| isolation | Estrus - Metestrus | 0.008 | 0.002 | 3.613 | 0.003** | |
| Estrus - Diestrus 1 | −0.002 | 0.002 | −0.944 | 0.880 | ||
| Estrus - Diestrus 2 | −0.009 | 0.002 | −3.598 | 0.003** | ||
| Estrus - Proestrus | 0.003 | 0.002 | 1.392 | 0.633 | ||
| Metestrus - Diestrus 1 | −0.010 | 0.003 | −3.424 | 0.006** | ||
| Metestrus - Diestrus 2 | −0.017 | 0.003 | −5.615 | <.001** | ||
| Metestrus - Proestrus | −0.005 | 0.003 | −1.485 | 0.572 | ||
| Diestrus 1 - Diestrus 2 | −0.007 | 0.003 | −2.099 | 0.220 | ||
| Diestrus 1 - Proestrus | 0.006 | 0.003 | 1.793 | 0.377 | ||
| Diestrus 2 - Proestrus | 0.012 | 0.003 | 3.859 | 0.001** |
p-value < .01
p-value < .05
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wohr M, Schwarting RK Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013,354:81–97. [DOI] [PubMed] [Google Scholar]
- [2].Burgdorf J, Panksepp J, Moskal JR Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2011,35:1831–6. [DOI] [PubMed] [Google Scholar]
- [3].Willey AR, Spear LP The effects of pre-test social deprivation on a natural reward incentive test and concomitant 50 kHz ultrasonic vocalization production in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2013,245:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright JM, Gourdon JC, Clarke PB Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl.). 2010,211:1–13. [DOI] [PubMed] [Google Scholar]
- [5].Ciucci MR, Vinney L, Wahoske EJ, Connor NP A translational approach to vocalization deficits and neural recovery after behavioral treatment in Parkinson disease. J. Commun. Disord 2010,43:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson AM, Grant LM, Schallert T, Ciucci MR Changes in Rat 50-kHz Ultrasonic Vocalizations During Dopamine Denervation and Aging: Relevance to Neurodegeneration. Curr. Neuropharmacol. 2015,13:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lenell C, Sandage MJ, Johnson AM A Tutorial of the Effects of Sex Hormones on Laryngeal Senescence and Neuromuscular Response to Exercise. J. Speech. Lang. Hear. Res 2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thomas DA, Barfield RJ Ultrasonic vocalization of the female rat (Rattus norvegicus) during mating. Anim. Behav 1985,33:720–5. [Google Scholar]
- [9].White NR, Gonzales RN, Barfield RJ Do vocalizations of the male rat elicit calling from the female? Behav. Neural Biol 1993,59:76–8. [DOI] [PubMed] [Google Scholar]
- [10].Bowers JM, Perez-Pouchoulen M, Edwards NS, McCarthy MM Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J. Neurosci 2013,33:3276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lenell C, Johnson AM Sexual dimorphism in laryngeal muscle fibers and ultrasonic vocalizations in the adult rat. Laryngoscope. 2017,127:E270–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus). J. Comp. Psychol 1992,106. [DOI] [PubMed] [Google Scholar]
- [13].Tatlipinar A, Gunes P, Ozbeyli D, Cimen B, Gokceer T Effects of ovariectomy and estrogen replacement therapy on laryngeal tissue: a histopathological experimental animal study. Otolaryngol. Head Neck Surg 2011,145:987–91. [DOI] [PubMed] [Google Scholar]
- [14].Oyarzún P, Sepúlveda A, Valdivia M, Roa I, Cantín M, Trujillo G, et al. Variations of the Vocal Fold Epithelium in a Menopause Induced Model. Int J Morphol. 2011,29:377–81. [Google Scholar]
- [15].Kim JM, Shin SC, Park GC, Lee JC, Jeon YK, Ahn SJ, et al. Effect of sex hormones on extracellular matrix of lamina propria in rat vocal fold. The Laryngoscope. 2019. [DOI] [PubMed] [Google Scholar]
- [16].Riede T, Borgard HL, Pasch B Laryngeal airway reconstruction indicates that rodent ultrasonic vocalizations are produced by an edge-tone mechanism. Royal Society Open Science. 2017,4:170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wirakiat W, Udomuksorn W, Vongvatcharanon S, Vongvatcharanon U Effects of estrogen via estrogen receptors on parvalbumin levels in cardiac myocytes of ovariectomized rats. Acta Histochem. 2012,114:46–54. [DOI] [PubMed] [Google Scholar]
- [18].Bunratsami S, Udomuksorn W, Kumarnsit E, Vongvatcharanon S, Vongvatcharanon U Estrogen replacement improves skeletal muscle performance by increasing parvalbumin levels in ovariectomized rats. Acta Histochem. 2015,117:163–75. [DOI] [PubMed] [Google Scholar]
- [19].Marcondes FK, Bianchi AP, Tanno. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol 2002,62:609–14. [DOI] [PubMed] [Google Scholar]
- [20].Cora MC, Kooistra L, Travlos G Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol. Pathol 2015,43:776–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Snoeren EM, Agmo A The incentive value of males’ 50-kHz ultrasonic vocalizations for female rats (Rattus norvegicus). J Comp Psychol. 2014,128:40–55. [DOI] [PubMed] [Google Scholar]
- [22].Johnson AM, Ciucci MR, Connor NP Vocal training mitigates age-related changes within the vocal mechanism in old rats. J. Gerontol. A Biol. Sci. Med. Sci 2013,68:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coffey KR, Marx RG, Neumaier JF DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology. 2019,44:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J Vis Exp. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].RStudio Team: Integrated Development Environment for R. Boston, MA2020.
- [26].Bates D, Mächler M, Bolker B, Walker S Fitting linear mixed-effects models using lme4. arXiv preprint arXiv: 1406.5823. 2014. [Google Scholar]
- [27].Lenth R emmeans: Estimated marginal means, aka least-squares means. R package version 1.4. 3.01. 2019.
- [28].Portfors CV Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science. 2007,46:28–34. [PubMed] [Google Scholar]
- [29].Kosten TA, Lee HJ, Kim JJ Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006,1087:142–50. [DOI] [PubMed] [Google Scholar]
- [30].Blanchard RJ, Agullana R, McGee L, Weiss S, Blanchard DC Sex differences in the incidence and sonographic characteristics of antipredator ultrasonic cries in the laboratory rat (Rattus norvegicus). J. Comp. Psychol 1992,106. [DOI] [PubMed] [Google Scholar]
- [31].Mallo T, Matrov D, Herm L, Koiv K, Eller M, Rinken A, et al. Tickling-induced 50-kHz ultrasonic vocalization is individually stable and predicts behaviour in tests of anxiety and depression in rats. Behav Brain Res. 2007,184:57–71. [DOI] [PubMed] [Google Scholar]
- [32].Brown RE The 22-kHz pre-ejaculatory vocalizations of the male rat. Physiol. Behav 1979,22:483–9. [DOI] [PubMed] [Google Scholar]
- [33].Matochik JA, White NR, Barfield RJ Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol. Behav 1992,51:783–6. [DOI] [PubMed] [Google Scholar]


