Abstract
Background:
Achalasia subtypes on high-resolution manometry (HRM) prognosticate treatment response and help direct management plan. We aimed to utilize parameters of distension-induced contractility and pressurization on functional luminal imaging probe (FLIP) panometry and machine learning to predict HRM achalasia subtypes.
Methods:
180 adult patients with treatment-naïve achalasia defined by HRM per Chicago Classification (40 type I, 99 type II, 41 type III achalasia) who underwent FLIP-panometry were included: 140 patients were used as the training cohort and 40 patients as the test cohort. FLIP panometry studies performed with 16-cm FLIP assemblies were retrospectively analyzed to assess distensive pressure and distension-induced esophageal contractility. Correlation analysis, single tree, and random forest were adopted to develop classification trees to identify achalasia subtypes.
Key Results:
Intra-balloon pressure at 60ml fill volume, and proportions of patients with absent contractile response, repetitive retrograde contractile pattern, occluding contractions, sustained occluding contractions (SOC), contraction-associated pressure changes >10mmHg all differed between HRM-achalasia subtypes and were used to build the decision-tree-based classification model. The model identified spastic (type III) versus non-spastic (types I and II) achalasia with 90% and 78% accuracy in the train and test cohorts, respectively. Achalasia subtypes I, II, and III were identified with 71% and 55% accuracy in the train and test cohorts, respectively.
Conclusions & Inferences:
Using a supervised machine learning process, a preliminary model was developed that distinguished type III achalasia from non-spastic achalasia with FLIP panometry. Further refinement of the measurements and more experience (data) may improve its ability for clinically relevant application.
Keywords: impedance, manometry, dysphagia, peristalsis, endoscopy
Introduction:
Achalasia is a primary esophageal motor disorder characterized by abnormal deglutitive lower esophageal sphincter (LES) relaxation and absent peristalsis and is traditionally defined by evaluation with esophageal manometry.1 Evaluation with high-resolution manometry (HRM) and esophageal pressure topography (EPT) have allowed further sub-classification of achalasia based on the pressurization and contractility patterns observed in the esophageal body with prognostic implications regarding response to treatment (such as pneumatic dilation and LES myotomy).2–5
Although absent peristalsis is a defining manometric feature of achalasia, we previously demonstrated that esophageal contractility can be observed in patients with achalasia during evaluation with functional luminal imaging probe (FLIP) panometry.6,7 The (FLIP) utilizes high-resolution impedance planimetry to measure luminal dimensions during controlled, volumetric distension of a balloon-positioned within the esophagus. Esophageal contractility can be elicited by FLIP distension and identified when esophageal diameter changes are depicted as a function of time.6–8 FLIP can therefore detect esophageal contractions that both occlude and do not occlude the esophageal lumen (i.e. non-occluding contractions).6 Further, we also observed specific patterns of the contractile response to distension on FLIP panometry. We initially described that both the presence and the patterns of the esophageal contractile response to distension on FLIP panometry differed between the HRM-achalasia subtypes.6,7 An absent contractile response to distension was most commonly observed in patients with type I achalasia and a pattern of repetitive, retrograde contractions (RRCs) was most commonly observed among patients with type III achalasia (neither such pattern was observed among asymptomatic controls).6,7,9 Distension-induced contractility patterns were more varied among patients with type II achalasia on manometry including absent contractile response, RRCs, and some patients that had contractility that was abnormal, but did not occur in an RRC or RAC pattern.6,10 Additionally, we observed unique patterns of esophageal response to distension in sustained occluding contractions (SOCs) and LES-lift events that also appeared to differ between HRM achalasia subtypes.10
Therefore, we aimed to identify and apply parameters of FLIP panometry to patients with achalasia to predict the HRM-achalasia subtype. Supervised machine learning approaches were implemented to help determine important classification variables and cut-offs.
Methods
Subjects
The study protocol was approved by the Northwestern University Institutional Review Board. Adult patients presenting to the Esophageal Center of Northwestern for evaluation of dysphagia between November, 2012 and October, 2018 that completed HRM and FLIP during upper endoscopy were prospectively evaluated. Consecutive patients newly diagnosed with achalasia by HRM were included for this study. Primary analysis and model development was performed on 140 consecutive patients with achalasia (“training cohort”) evaluated between November 2012 and October 2017; this cohort has been previously described.10 A cohort of 40 patients with achalasia evaluated between October 2017 and October 2018 were utilized as a “test cohort”.
Achalasia was diagnosed and sub-classified by HRM (details below).5 None of the patients had previously been treated with pneumatic dilation or LES myotomy. Patients with previous esophageal or gastric surgery (e.g. fundoplication or gastric resection), significant medical co-morbidities that prevented endoscopy with FLIP, eosinophilic esophagitis, severe reflux esophagitis (LA-classification C or D), or hiatal hernia >3cm were also excluded.
FLIP Study Protocol and Analysis
Evaluation was completed after a minimum 6-hour fast. Subjects underwent upper endoscopy in the left lateral decubitus position. Conscious sedation with 2–15 mg midazolam and 50–300 mcg fentanyl was administered during the procedure. Other sedative medications, e.g. propofol, (in addition to midazolam and fentanyl) were used with anesthesiologist assistance at the discretion of the performing endoscopist in some cases.
The 16-cm FLIP (EndoFLIP® EF-322N; Medtronic, Inc, Shoreview, MN) was calibrated to atmospheric pressure prior to trans-oral probe placement. With the endoscope withdrawn, the FLIP was positioned within the esophagus such that 1–3 impedance sensors were observed beyond the EGJ with this positioning maintained throughout the FLIP study. Stepwise 5-ml or 10-ml balloon distensions beginning with 20 ml and increasing to target volume of 60ml or 70 ml were then performed (variations in FLIP study protocol evolved during the course of this study); each stepwise distension volume was maintained for 30–60 seconds.
FLIP data including distension volume, intra-balloon pressure, and 16 channels of luminal diameter were exported to a customized program as previously described.6–8,11 Median EGJ-midline cross-sectional area (CSA) and median intra-balloon pressure over the duration of each the 40-ml, 50-ml, and 60-ml distension volumes were assessed; the EGJ-distensibility index (DI) was calculated as median EGJ CSA divided by median pressure with focus primarily on the 60-ml fill volume as previously described.6,7,12
Esophageal body contractions and contractility patterns were identified similarly to previous studies.6,7,11 Contractility assessment was performed by two independent raters (KPR and DAC); if disagreement between the two raters occurred (which occurred in 8/180 studies), a final classification was determined by group consensus (DAC, KPR, AB, ED, JEP); the group also reviewed all of the FLIP topography plots blinded to clinical data and HRM to provide consensus agreement for classifications among the remainder of the test and training cohorts. Esophageal body contractions were identified by a transient decrease of ≥ 5 mm in the luminal diameter in ≥ 3 adjacent impedance planimetry channels using FLIP topography plots and 16-channel diameter line-tracing output. The axial length of contractions was determined by the number of consecutive impedance planimetry channels (1-cm spacing) with a decrease in luminal diameter. The direction of contractions (antegrade or retrograde) was categorized based on a tangent line placed at the onset of contraction. Specific patterns of the contractile response to distension were further categorized similar to our original descriptions as repetitive if ≥ 3 contractions of similar directionality occurred including repetitive retrograde contractions (RRCs) when these involved retrograde contractions, Figure 1.6,8,10 Contractions were also categorized as occluding if they achieved a minimal esophageal diameter < 6mm or non-occluding contractions if they did not.6 When non-propagating, occluding contractions associated with significant pressure increase (typically >35mmHg) occurred, they were designated as sustained occluding contractions (SOCs), Figure 1. SOCs occurred with and without associated LES-lift. Occurrences of LES-lift events that occurred independently of SOC were also noted if they occurred over the course of the FLIP study and were associated with pressure changes. Pressure-changes associated with contractions were assessed and each FLIP study was dichotomized as occurring with pressure changes ≥ 10 mmHg or pressure changes occurring < 10 mmHg, Figure 1.
Figure 1. Distension-induced contractility patterns in achalasia.
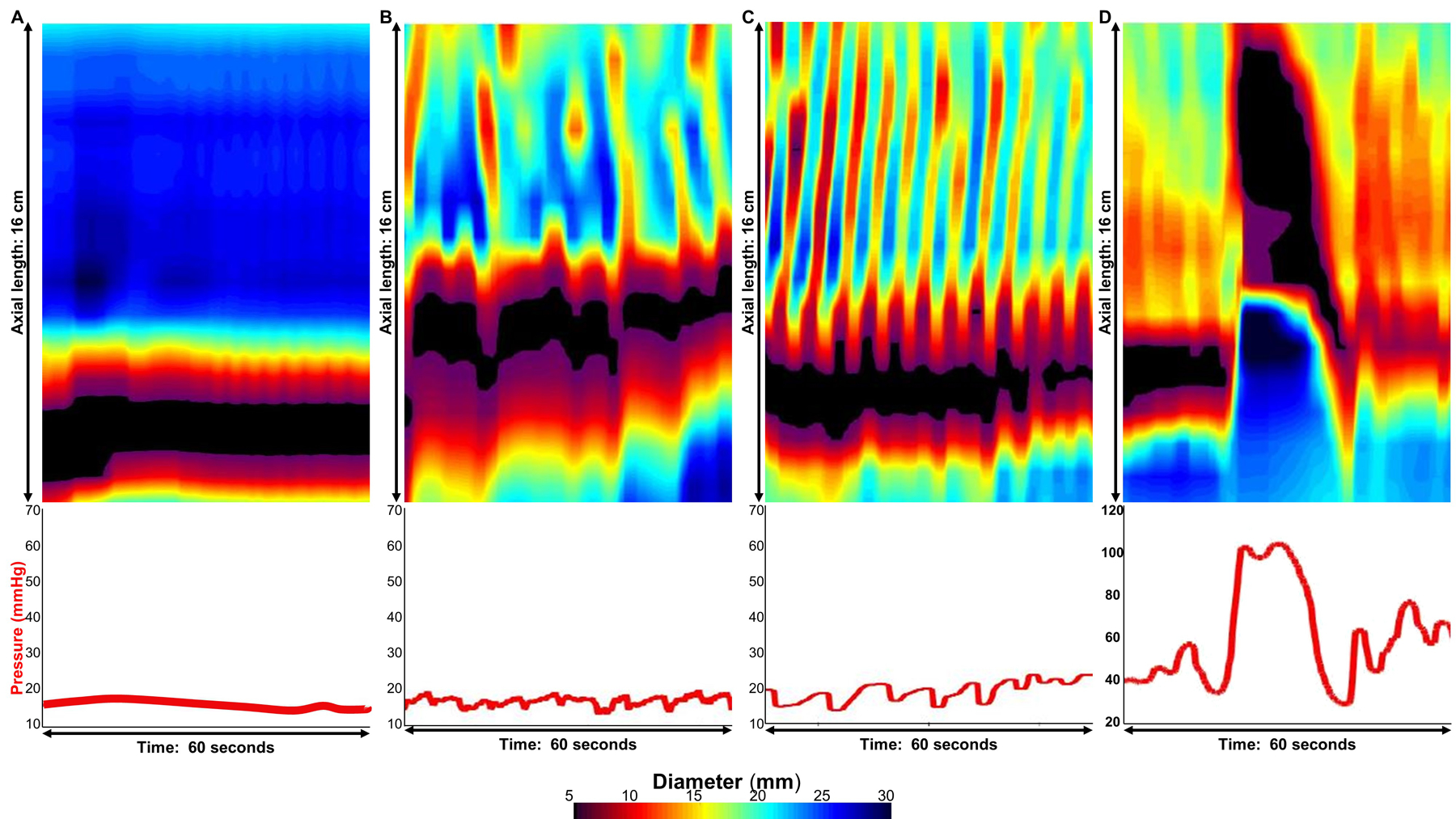
FLIP panometry with 16-cm of interpolated esophageal diameter topography (top panels) and intra-balloon pressure (bottom panel) by time from four patients with achalasia. A.) Absent contractile response, B) Distension-induced contractility without repetitive retrograde contractions (RRCs), C) RRCs, and D) Sustained occluding contraction (SOC). The pressure changes associated with contractions were <10mmHg in B and C and >10mmHg in D. Figure used with permission from the Esophageal Center at Northwestern.
HRM protocol and analysis
After a minimum 6-hour fast, HRM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. After a 2-minute baseline recording, the HRM protocol was performed with ten, 5-ml liquid swallows in a supine position.5
Manometry studies were analyzed using ManoView version 3.0 analysis software (Medtronic) to measure the integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency according to the Chicago Classification.4,5 A median IRP of > 15 mmHg was applied as the upper limit of normal, thus all patients had a median IRP > 15 mmHg as a criterion the achalasia diagnosis. Type I achalasia was defined by absent contractility in 100% of swallows. Type II achalasia was defined by pan-esophageal pressurization at an isobaric contour of 30 mmHg in > 20% of swallows. Type III (spastic) achalasia was defined by ≥20% of premature swallows (i.e. distal latency < 4.5s).
Statistical analysis
Results were expressed as mean (standard deviation, SD) or median and interquartile range (IQR) depending on data distribution. Statistical comparisons were made between HRM achalasia subtypes. Comparison of dichotomous and categorical variables between groups was assessed with X2 test or Exact tests. Continuous variables were compared via t-test, ANOVA or Mann-Whitney U or Kruskal-Wallis tests, depending on data distribution. Statistical significance was considered at a two-tailed p-value < 0.05. Post-hoc comparison testing, as appropriate, was completed using a Bonferroni correction.
Single tree and random forest modeling were utilized to generate models to predict 1) achalasia subtype, i.e. types I vs II vs III and 2) spastic (type III) vs non-spastic (types I and II) achalasia. 140 patients were utilized as a training set and 40 patients were then utilized as a test set. To eliminate redundant features in developing classification model, correlation analysis were first adopted and highly correlated features were identified and removed in subsequent analysis (Supplemental Figure 1). For dimension reduction, a random forest model capable of capturing more complex relationships between features and labels was also studied. The random forest model consisted of 1000 trees with max_depth=5.13 Feature ranking was performed using both correlation analysis illustrating pair-wise linear relationship, and random forest model incorporating nonlinear relationship among multiple features and target labels. The six highest ranked features in importance were then used in subsequent decision tree model. To determine an optimal depth of tree in our model, 10-fold cross-validation was conducted for model selection using the 140 patient training sample using varying branching depths ( referred to as depth) of 2, 3, or 4. Depth = 3 or 4 seemed to achieve high accuracy. A further evaluation with the 40 patient test sample showed that, depth=3 appeared optimal as depth=2 under fitted and depth=4 overfitted. With the choice of depth=3, the model was re-run to build a 3-level tree using 140 patient training data. Additionally, the impact of the imbalanced dataset (i.e. more non-spastic than spastic achalasia patients) was considered. Thus, models with balanced sample weight were also developed but were found to yield comparable performance as the current reported model. All the analysis was written in Python based on the machine-learning library: scikit-learn.
Results
Subjects
Among the total 180 patient cohort, the patients had a mean (SD) age of 53 (78) years; 41% were female. Achalasia sub-classification on HRM included 40 (22%) patients with type I, 99 patients (55%) with type II, and 41 patients (23%) with type III achalasia; proportions of achalasia subtypes were similar (as were other baseline characteristics) between the training and test cohorts, P = 0.353 (Table 1).
Table 1.
Patient characteristics.
| Training cohort - Total | Type I achalasia | Type II achalasia | Type III achalasia | |
|---|---|---|---|---|
| Sample size (n; % cohort) | 140 | 29 (21) | 81 (58) | 30 (21) |
| *Age, years, mean (SD) | 52 (18) | 51 (14) | 49 (19) | 62 (14) |
| *n (% )Female | 55 (39) | 15 (52) | 32 (40) | 8 (27) |
| *On chronic opioids, n (%) | 12 (9) | 0 | 4 (5) | 8 (27) |
| Median IRP, mmHg, mean (SD) | 33 (13) | 35 (17) | 34 (13) | 30 (11) |
| *EGJ-distensibility index, mm2/mmHg, median (IQR) | 0.8 (0.5 – 1.2) | 1.3 (0.9 – 1.9) | 0.8 (0.6 – 1.2) | 0.6 (0.4 – 0.9) |
| Test cohort - Total | Type I achalasia | Type II achalasia | Type III achalasia | |
| Sample size (n; % cohort) | 40 | 11 (28) | 18 (45) | 11 (28) |
| Age, years, mean (SD) | 55 (15) | 57 (15) | 53 (13) | 58 (19) |
| n (% )Female | 20 (50) | 5 (45) | 7 (39) | 8 (73) |
| *On chronic opioids, n (%) | 5 (13) | 0 | 0 | 5 (46) |
| Median IRP, mmHg, mean (SD) | 32 (13) | 26 (9) | 35 (13) | 35 (15) |
| EGJ-distensibility index, mm2/mmHg, median (IQR) | 0.9 (0.7 – 1.2) | 0.9 (0.8 – 1.4) | 0.9 (0.7 – 1.5) | 0.6 (0.4 – 1.0) |
P < 0.05 with comparing across achalasia subtypes within cohort. Included parameters were similar between training and test cohorts. IRP – integrated relaxation pressure. EGJ – esophagogastric junction
Endoscopic sedation associated with FLIP testing was similar between training and test cohorts: midazolam dosage median (IQR) 6.0 (4.0 – 8.8) mg vs 6.0 (2.2 – 8.0) mg (P = 0.340); fentanyl dosage median (IQR) 150 (100 – 194) mcg vs 125 (81 – 175) mcg (P = 0.267); propofol proportion of cases 19% vs 23% (P = 0.651); ketamine proportion of case 11% vs 8% (P = 0.573).
Distension-induced contractility and pressurization parameters among achalasia patients.
Among the 140 patient training cohort, an absent contractile response, i.e. no distension-induced contractility observed during a FLIP panometry study, was observed in 48 (34%) achalasia patients. Conversely, distension-induced contractility was observed in 92 (66%) achalasia patients. A RRC pattern was observed in 41/140 (29%) patients. Occluding contractions (occlusion of the esophageal lumen occurring at any point during the FLIP study) were observed in 48 (34%) of patients. SOCs were observed in 18 (13%) patients and isolated LES-lift events were observed in 11 (8%).
The median (IQR) intra-balloon pressure from the cohort was 17 (11 – 27) mmHg at 40ml fill volume, 23 (15 – 37) mmHg at 50ml fill volume, and 32 (24 – 47) at the 60ml fill volume. 72 patients (51%) had a contraction that was associated with a pressure change > 10 mmHg.
No differences in presence or pattern of distension-induced contractility were detected with relationship to endoscopic sedation, but chronic opioid use was more common in patients with vs without contractility (p=0.02) and with vs without RRCs (p=0.02), Supplementary Table 1.
Comparison of distension-induced contractility and pressurization parameters between achalasia subtypes
In the training cohort, differences in distension-induced contractility patterns were observed between HRM subtypes such that an absent contractile response was most commonly observed in type I achalasia and an RRC pattern was most commonly observed in type III achalasia, Table 2. Contractility without RRCs was observed in a similar proportion of type II and type III achalasia patients, but more commonly than in type I achalasia. Neither the duration (in number of consecutive contractions) of RRCs, mean (SD) 8 (5) vs 9 (6) retrograde contractions, nor the rate of RRCs, mean (SD) 11(2) vs 12 (2) contractions per minute, differed between non-spastic (types I and II) vs spastic (type III) achalasia; P 0.351 and 0,533, respectively.
Table 2.
Comparison of distension-induced contractility and pressurization parameters between high resolution manometry (HRM) achalasia subtypes.
| Training cohort; n = 140 | HRM subtype | |||
|---|---|---|---|---|
| Type I achalasia | Type II achalasia | Type III achalasia | P-value | |
| FLIP contractile response pattern, n (%) | ||||
| Absent contractile response | 22 (76) | 26 (32) | 0 | <0.001 |
| Contractility without RRCs | 5 (17) | 34 (42) | 12 (40) | 0.054 |
| RRCs | 2 (7) | 21 (26) | 18 (60) | <0.001 |
| Occluding contractions, n (%) | 4 (14) | 22 (27) | 22 (73) | <0.001 |
| SOCs present, n (%) | 0 | 5 (6) | 13 (43) | <0.001 |
| LES-lift present, n (%) | 1 | 7 (9) | 4 (13) | <0.001 |
| Intra-balloon Pressure - 40ml, mmHg, Median (IQR) | 11 (7 – 20) | 16 (12 – 25) | 29 (22 – 37) | <0.001 |
| Intra-balloon Pressure - 50ml, mmHg, Median (IQR) | 16 (11 – 22) | 21 (15 – 31) | 41 (33 – 52) | <0.001 |
| Intra-balloon Pressure - 60ml, mmHg, Median (IQR) | 20 (18 – 30) | 31 (24 – 43) | 56 ( 42 – 66) | <0.001 |
| Pressure changes > 10mmHg, n (%) | 6 (21) | 41 (51) | 27 (90) | <0.001 |
| Test cohort; n = 40 | HRM subtype | |||
| Type I achalasia | Type II achalasia | Type III achalasia | P-value | |
| FLIP contractile response pattern, n (%) | ||||
| Absent contractile response | 9 (82) | 11 (55) | 1 (5) | 0.002 |
| Contractility without RRCs | 2 (18) | 7 (35) | 3 (27) | 0.485 |
| RRCs | 0 | 0 | 6 (55) | <0.001 |
| Occluding contractions, n (%) | 1 (13) | 1 (6) | 6 (55) | 0.003 |
| SOCs present, n (%) | 1 (9) | 0 | 3 (27) | 0.040 |
| LES-lift present, n (%) | 1 (9) | 2 (11) | 6 (55) | 0.011 |
| Intra-balloon Pressure - 40ml, mmHg, Median (IQR) | 15 (12 – 22) | 15 (12 – 20) | 26 (22 – 33) | <0.001 |
| Intra-balloon Pressure - 50ml, mmHg, Median (IQR) | 19 (15 – 24) | 17 (13 – 22) | 37 (28 – 49) | 0.002 |
| Intra-balloon Pressure - 60ml, mmHg, Median (IQR) | 25 (23 – 30) | 23 (18 – 31) | 42 (36 – 58) | <0.001 |
| Pressure changes > 10mmHg, n (%) | 2 (18) | 4 (22) | 8 (73) | 0.008 |
Percentages indicate percent within HRM subtype. Comparisons are between achalasia subtypes within each cohort. RRC – repetitive retrograde contractions. SOC – sustained occluding contraction. LES – lower esophageal sphincter.
The presence of occluding contractions differed by achalasia subtype and most commonly occurred in type III achalasia patients, Table 2. SOCs also occurred most commonly in type III achalasia patients. LES-lift events were observed in a similar proportion of type II and type III achalasia patients, but less frequently in type I achalasia.
The intra-balloon pressures differed between achalasia subtypes at the 40ml, 50ml, and 60ml fill volumes; Table 2. Contraction-associated pressure changes greater than 10mmHg were observed more commonly in type III than in type II than in type I achalasia patients.
Differences between the achalasia subtypes were also observed in the test cohort in a similar manner as with the training cohort (Table 2).
Predication of HRM achalasia-subtype with FLIP panometry
Parameters that differed between the achalasia subtypes were then evaluated by correlation analysis (Supplemental Figure 1). As the three pressure-related variables were highly correlated, the intra-balloon pressure at 40ml and at 50ml were removed from subsequent analysis. Feature ranking based on correlational analysis and random forest model revealed several shared important features (Supplemental Figure 1). The six highest ranking features were then included in development of a decision tree model: intra-balloon pressure at 60ml fill volume, presence of SOCs, an absent contractile response, presence of occluding contractions, presence of RRC pattern, and contraction associated pressure changes > 10mmHg.
Hence, decision trees with depth of three levels were then developed to predict HRM achalasia subtype (I vs II vs III), Figure 2A and B, and non-spastic (HRM subytpes I and II) vs spastic (subtype III) achalasia, Figure 2C and D. The decision tree to predict three achalasia subtypes yielded an accuracy of 71% (99/140) in the training cohort and 55% (22/40 patients) in the test cohort. The model’s sensitivity/specificity for HRM subtype prediction in the training cohort were 45%/95% for type I, 72%/73% for type II, and 93%/82% for type III achalasia, respectively. The model’s sensitivity/specificity for HRM subtype prediction in the test cohort were 9%/86% for type I, 72%/23% for type II, and 64%/97% for type III achalasia, respectively.
Figure 2. Tree models for prediction of HRM achalasia subtypes by FLIP panometry.
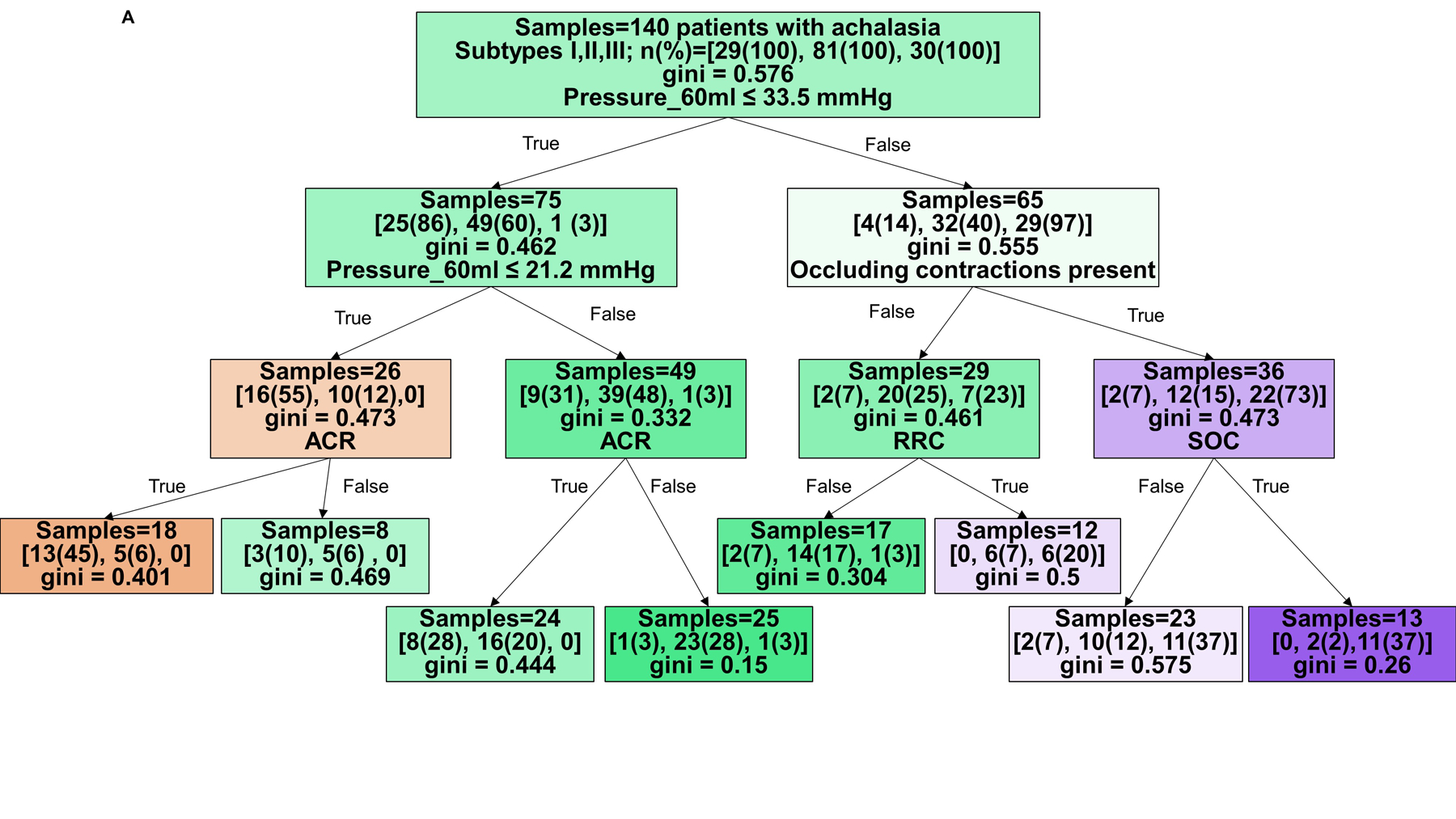
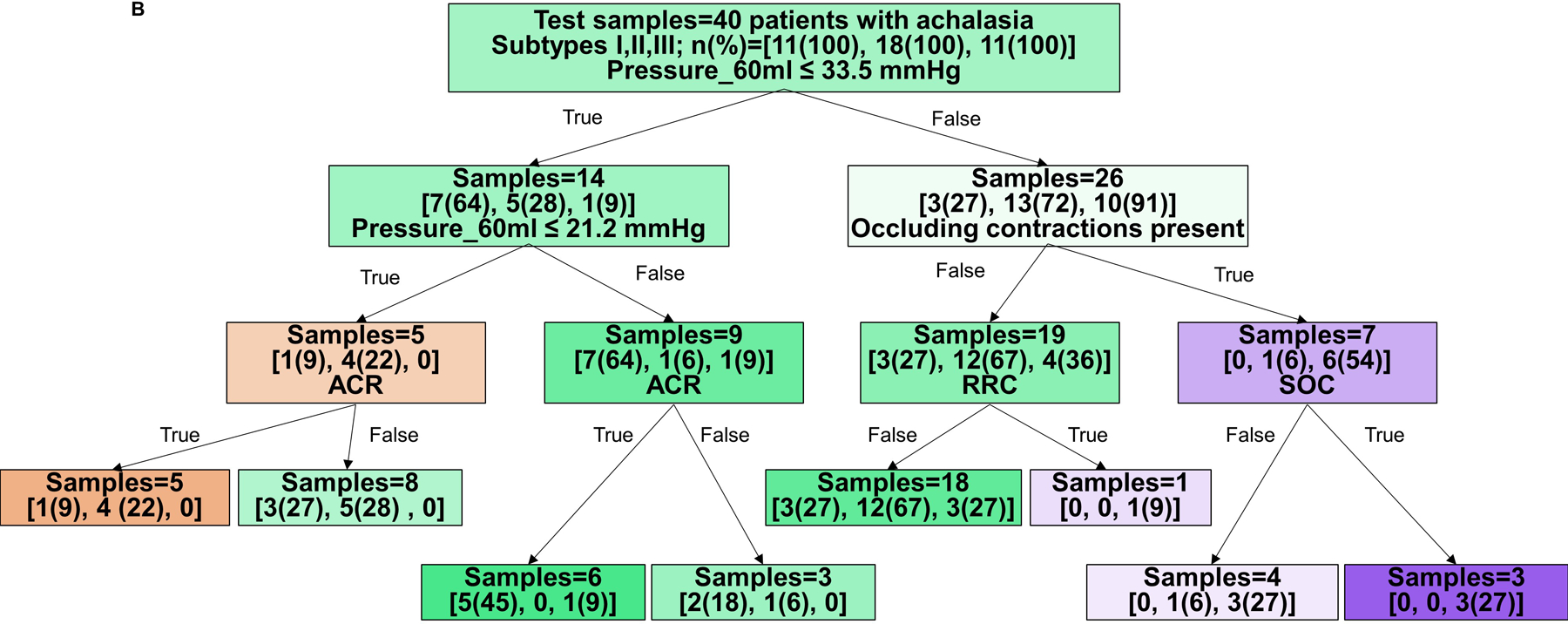
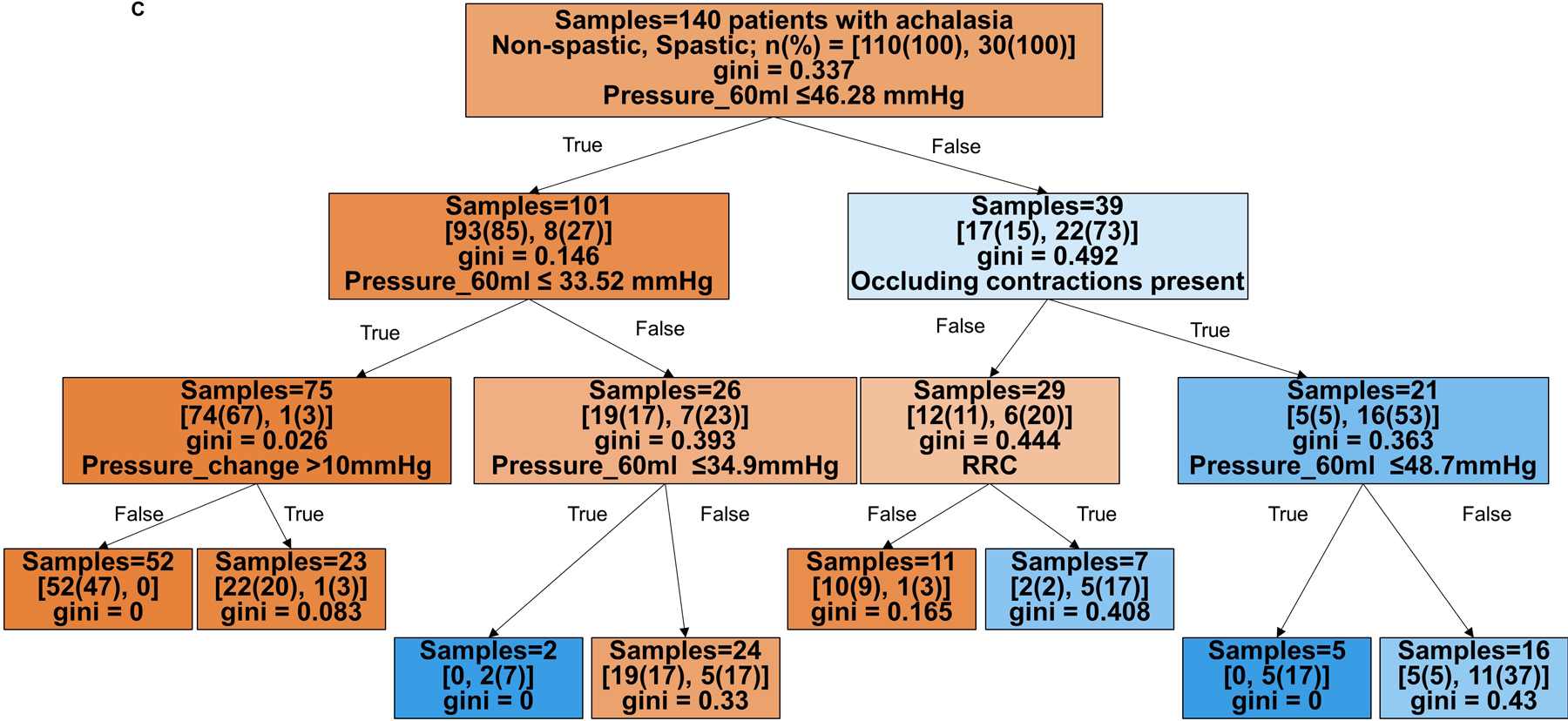
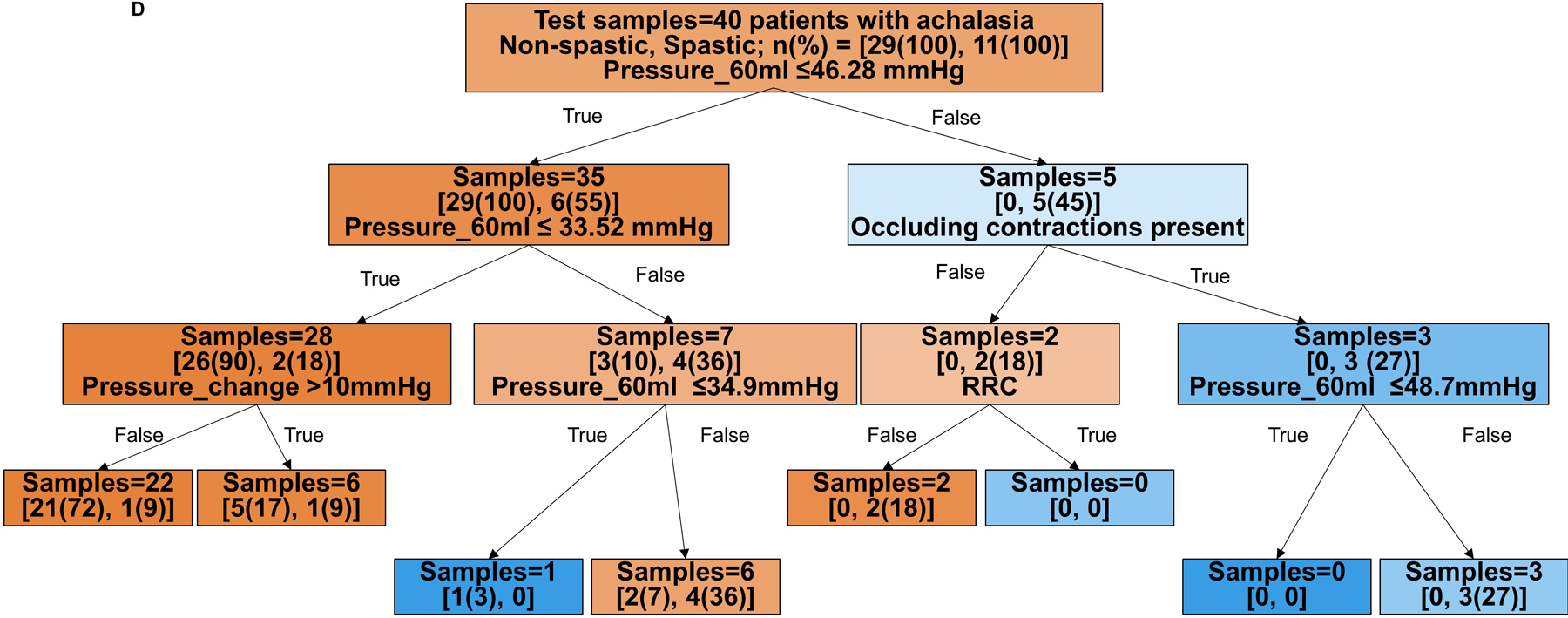
Branch points are color coded by HRM classification. The intensity of shading indicates the ‘impurity’ within that branch point and gini indicates the decrease in node impurity related to the variable derived from the training cohort. A and B) Prediction for HRM subtypes: I vs II vs III. The training cohort is reflected in A and the test cohort in B. Branch points are color coded by predicted HRM subtype with orange for type I, green for type II, and purple for type III. The number of patients per achalasia classification are indicated by [n(%) type I; n(%) type II; n(%) type III]. C and D) Prediction of non-spastic vs spastic achalasia subtypes. Branch points are color coded by predicted HRM subtype with orange for non-spastic achalasia (subtypes I and II) and blue for spastic (type III) achalasia. The number of patients per classification are indicated by [n(%) non-spastic, n(%) = spastic]. ACR – absent contractile response. RRC – repetitive retrograde contractions. SOC – sustained occluding contractions. Figure used with permission from the Esophageal Center at Northwestern.
The decision tree to predict non-spastic vs spastic achalasia yielded an accuracy of 90% (126/140 patients) with HRM subtype accurately identified in the training cohort and 78% (31/40 patients) in the test cohort. The model’s sensitivity/specificity to identify non-spastic vs spastic prediction in the training cohort were 94%/77% and 97%/27% in the test cohort, respectively.
Based on the decision trees, FLIP panometry with 60-ml intra-balloon pressure < 21mmHg and absent contractile response was predictive of type I achalasia. Type II achalasia was predicted by 60-ml intra-balloon pressure 21–34mmHg and absent contractile response, or intra-balloon pressure ≥34mmHg without occluding contractions, RRC pattern, or SOCs. Type III achalasia was predicted by 60-ml intra-balloon pressure > 46mmHg, and presence of occluding contractions, presence of RRC pattern, and presence of SOC. Examples of FLIP panometry prediction of achalasia subtype along with the corresponding HRM findings are depicted in Figure 3.
Figure 3. Examples of achalasia subtypes predicted by FLIP panometry.
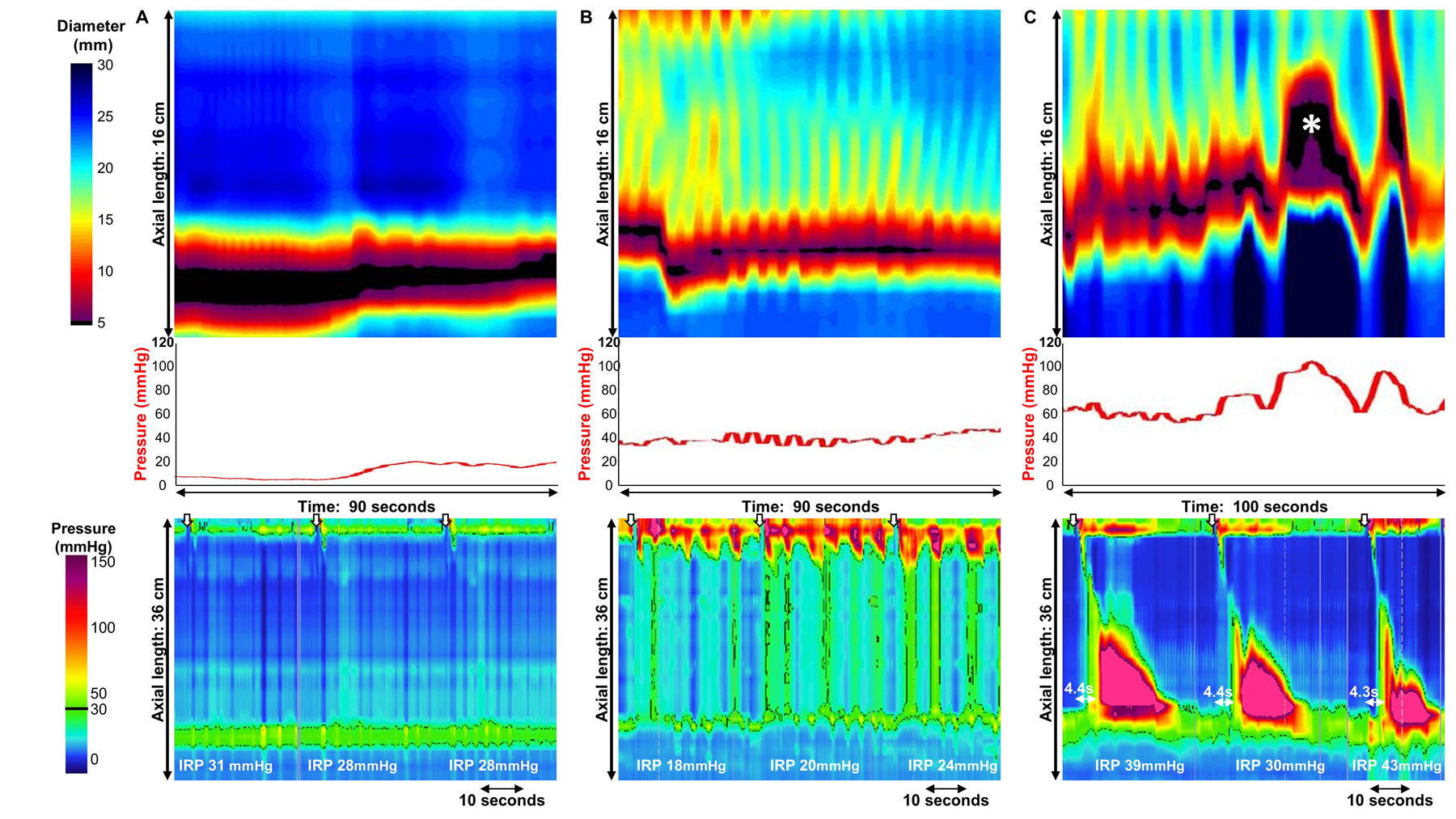
FLIP panometry (top) and high-resolution manometry (HRM; bottom); supine water swallows on HRM indicated by white arrows. A) FLIP panometry demonstrated an intra-balloon pressure < 34 mmHg and an absent contractile response predictive of type I achalasia as seen on HRM B) FLIP panometry demonstrated an intra-balloon pressure > 34mmHg (median pressure value of 40mmHg) and contractility with both antegrade and retrograde contractions. But without occluding contractions, repetitive retrograde contractions, nor sustained occluding contraction (SOC). Type II achalasia was thus predicted based on FLIP panometry and observed on HRM. C) Contractility was observed on FLIP panometry that included a SOC (white asterisk); intra-balloon pressure was also >48 mmHg. Type III achalasia was thus predicted based on FLIP panometry and observed on HRM. Figure used with permission from the Esophageal Center at Northwestern.
Discussion
In this study, a prospectively maintained achalasia natural history cohort was utilized to assess the esophageal response to distension using FLIP panometry in 180 patients with achalasia and a decision tree analysis derived though machine learning was developed to predict HRM subtype based on FLIP panometry features. We found that distension-induced contractility and pressurization parameters frequently differed by HRM achalasia sub-type. Further, by identifying key differentiating FLIP panometry features and applying these parameters to decision tree modeling we were able to accurately differentiate HRM-classified non-spastic from spastic achalasia (i.e. the key achalasia subtype differentiation with management implications) with 90% and 78% accuracy in the training and test cohorts, respectively. Application of these features of the esophageal response to distension may provide a method to sub-classify achalasia with FLIP panometry, either independently, or in a complementary nature to the HRM subytpes.
Classification of achalasia patients into the HRM subtypes provides clinical utility by providing prognostic information in predicting response to treatment.2,3,14,15 Type II achalasia is the most likely to have a positive treatment outcome, followed closely by type I, while type III may predict the poorest response rate to treatment. Additionally, post-hoc analysis of the prospective randomized European achalasia trial that compared pneumatic dilation and Heller myotomy, demonstrated that patients with type III achalasia may yield greater benefit from Heller myotomy.3,16,17 Thus, myotomy that is extended or tailored to be inclusive of the entire spastic segment is considered the preferred treatment approach in type III (spastic) achalasia and identification of this subtype is an important component of the Chicago Classification.18,19
We previously reported studies describing the detection of contractility with FLIP panometry, even among type I and type II achalasia patients that did not have contractility observed on HRM.6,7 We postulated that FLIP was able to detect non-occluding esophageal contractions (as opposed to the lumen occluding contractions created to create contact pressure for detection with manometry); subsequently, non-occluding esophageal contractions were also observed in patients with achalasia using high-frequency, intra-luminal ultrasound, and demonstrated to be a contributing mechanism to the pan-esophageal pressurization of the type II achalasia.2,20 In this expansion of our achalasia cohort, we demonstrate a consistency in our observations that distension-induced contractility was frequently observed in achalasia with FLIP panometry such that the contractile response to distension was often absent in type I achalasia, heterogeneous in type II achalasia, and commonly with RRCs in type III achalasia.6,7
However, while our initial descriptions of distension-induced contractility in achalasia demonstrated differences across HRM-achalasia subtypes, there was overlap in distension-induced contractility patterns, particularly between type II and type III.6,7 We subsequently recognized that additional differences among FLIP panometry findings existed between the HRM achalasia subtypes. SOCs, for example, appeared to be an abnormal contractile response to distension as they have not been observed among our asymptomatic controls.10 Further, we came to appreciate the differences in pressure generation within the FLIP balloon, particularly with often very high pressures generated during the FLIP study in type III patients. Application of the machine learning approaches further demonstrated the importance of the intraballoon pressure with regards to differentiation between the HRM achalasia subtypes. Application of these additional parameters facilitated approaches to identify the HRM subtypes using FLIP panometry that we describe here through machine-learning derived decision trees.
A decision tree model was chosen for this study due to its relative simplicity and transparency for application in clinical practice. Other models based on boosting/bagging techniques or gradient-based trees could also be used as a classifier and they may be more capable of achieving higher accuracy with reduced variances. However, those model techniques would be more obscure for interpretation as they utilize ‘black-box’ processes when accuracy is the main goal. Incorporation of these decision trees into real-time software programs could potentially facilitate predicting achalasia subtype via FLIP panometry at the time of sedated endoscopy. Additionally, application of the machine learning approach to diagnostic classification demonstrated that different thresholds for the same metric (e.g. intra-balloon pressure) are needed based on mediation by other factors (e.g. contractile properties) as opposed to single threshold decision points that are commonly applied, notably with the IRP in the Chicago Classification.5,21
The patients with apparent discordant FLIP identification of HRM subtype draw particular attention. On closer examination of these patients, we observed that patients with type III-like features (e.g. SOCs) on FLIP panometry often had spastic features on HRM as well that were not captured via the type II designation from CCv3.0 (Figure 4). Additionally, patients with type III achalasia on HRM based on rigid Chicago Classification criteria but without spastic features on FLIP panometry had some similar-appearing features on manometry that could suggest more of a hybrid type II achalasia (Figure 5). Differentiation between type I and type II achalasia than identifying type III because it has less impact on management decisions;17 the convention of using the 30mmHg isobaric contour threshold of pan-esophageal pressurization to differentiate between type I and II is also somewhat arbitrary. Ultimately, FLIP panometry may provide a mechanism to both identify the HRM subtype as well as provide adjunctive characterization to further sub-classify phenotypes of achalasia. For example, the overlap between distension-induced contractility pattern and characteristics between type II and type III achalasia, may aid identification of specific sub-subtypes of disease that could help further refine therapeutic approaches, e.g. pursuit of POEM in type II achalasia patients with SOCs. However, future study remains needed to test this hypothesis and this future study will be facilitated by the paradigms described here.
Figure 4. Examples of discordance in FLIP panometry prediction: potential spastic features on FLIP panometry among patients with type II achalasia on high-resolution manometry (HRM).
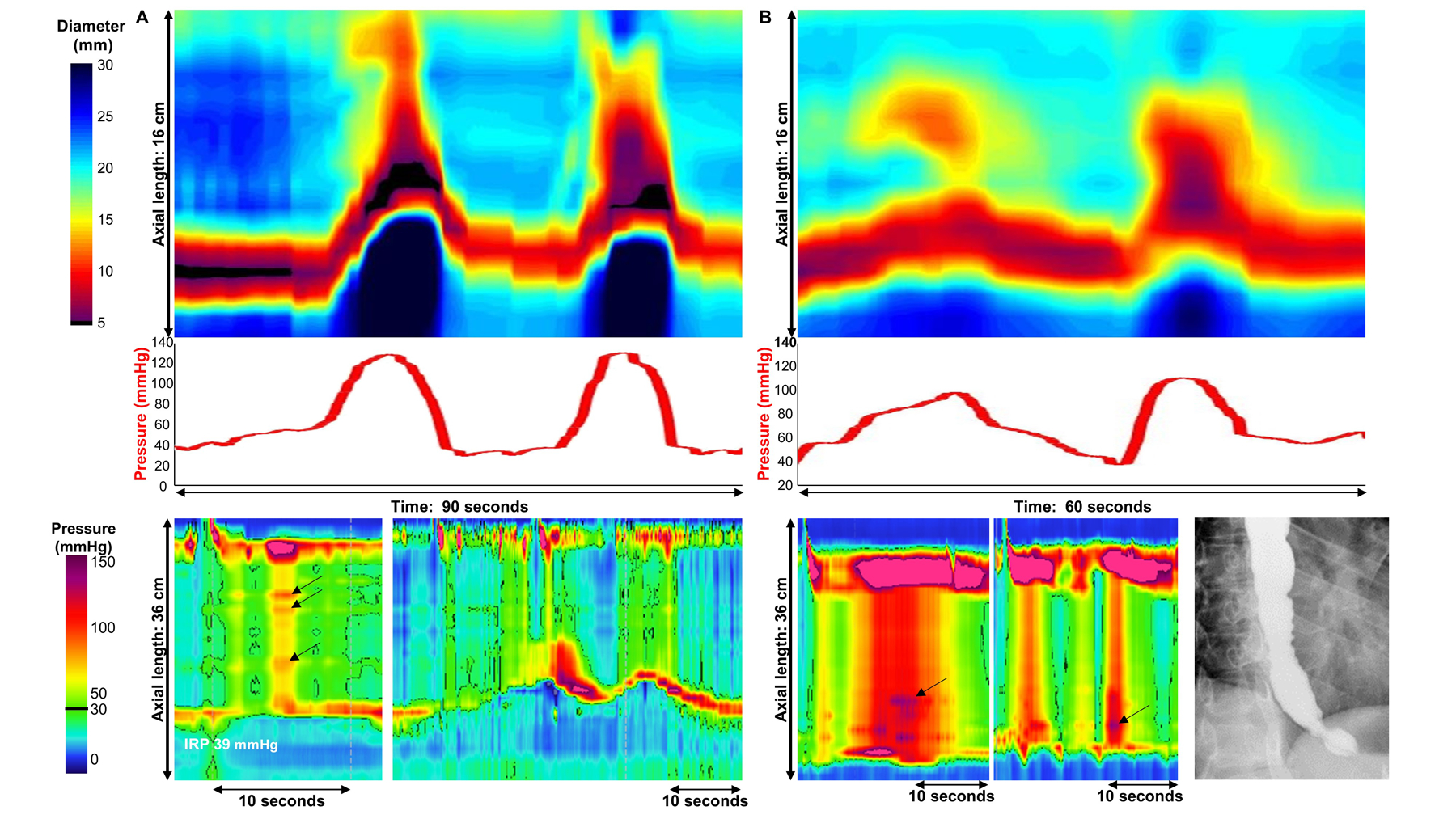
A) FLIP panometry (top) demonstrated sustained occluding contractions (SOC) and associated intra-balloon pressure exceeding 100 mmHg, thus predictive of type III achalasia on HRM. The actual HRM (left-bottom) derived a classification of type II achalasia based on absent peristalsis with pan-esophageal pressurization on supine test swallows. However, subtle focal contractions can be visualized within the esophageal pressurization (arrows). Esophageal shortening events observed following a 200ml rapid-drink challenge (right-bottom) were also observed, similar in appearance to the FLIP panometry. This patient was treated with pneumatic dilation and had sustained symptom improvement at 1-year follow-up. B) FLIP panometry (top) demonstrated sustained occluding contractions (SOC) and associated intra-balloon pressure exceeding 100 mmHg, thus predictive of type III achalasia on HRM. The actual HRM (bottom left and center) derived a classification of type II achalasia based on absent peristalsis with pan-esophageal pressurization on supine test swallows. However, the degree of pan-esophageal pressure was high (>100 mmHg) and subtle focal contractions are evident within the esophageal pressurization (arrows). On barium esophagram (bottom-right) tertiary contractions were observed. The patient did not improve after 30 and 35mm pneumatic dilation but did improve with subsequent treatment with per-oral endoscopic myotomy; standard myotomy length extending 6-cm proximal and 3-cm distal to the squamocolumnar junction was performed. Figure used with permission from the Esophageal Center at Northwestern.
Figure 5. Examples of discordance in FLIP panometry prediction: type III achalasia on high-resolution manometry (HRM) without spastic features on FLIP panometry.
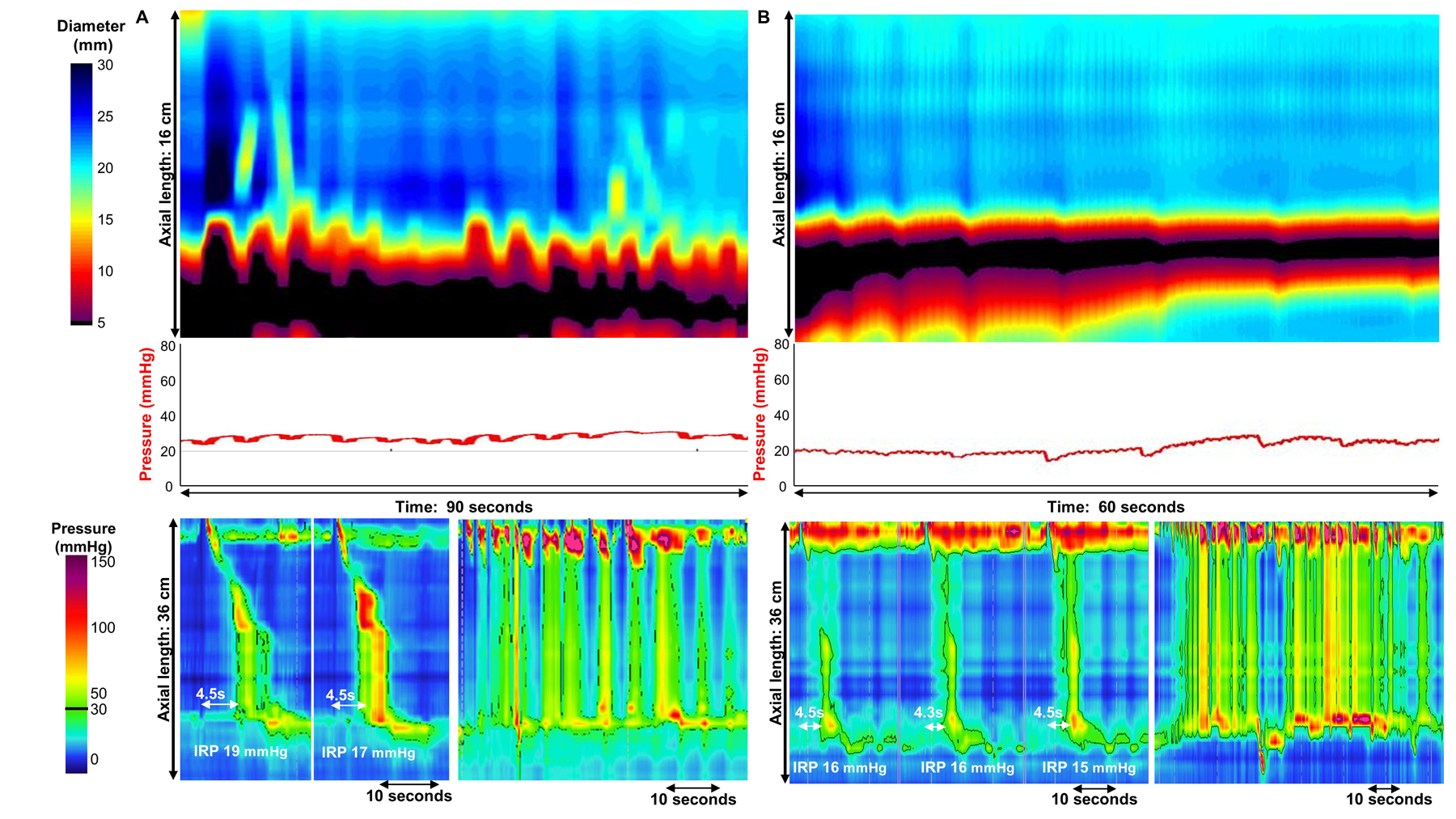
A) FLIP panometry (top) demonstrated contractility without repetitive contractions with intra-balloon pressure of 25mmHg, thus predictive of type II achalasia on HRM. The actual HRM (left-bottom) yielded a classification of type III achalasia based on elevated integrated relaxation pressure (IRP) and premature swallows; however, the distal latency measure was obscured by compartmentalized pressurization from the proximal contraction. Pan-esophageal pressurization was observed during the rapid drink challenge (bottom-right). The patient was treated with per-oral endoscopy myotomy with extended myotomy due to the type III HRM pattern. Symptomatic improvement was achieved, but ongoing dysphagia, chest pain, and daily regurgitation were reported at 1 year follow-up (Eckardt score was 4). B) FLIP panometry (top) demonstrated an absent contractile response and intra-balloon pressure <34 mmHg (median pressure at 60ml was of 23 mmHg) predictive of non-spastic achalasia on HRM. The actual HRM (left-bottom) yielded a classification of type III achalasia based on elevated integrated relaxation pressure (IRP) and premature contractions within pan-esophageal pressurization. Pan-esophageal pressurization was also observed during the rapid drink challenge (bottom-right). Given the shared type II achalasia features, pneumatic dilation was offered, chosen by the patient as primary treatment modality, and performed. The patient for unfortunately lost to follow-up. Figure used with permission from the Esophageal Center at Northwestern.
While the strengths of this study include a novel and detailed analysis paradigm applied to a large cohort of patients with achalasia, the study does have limitations. The study evaluated achalasia patients that were identified for purpose of this study based on their HRM diagnoses of achalasia. However, similar patterns on FLIP panometry have been observed among patients without conclusive achalasia on HRM;7 thus there is anticipated overlap of these FLIP panometry patterns among patients that have less distinct motility diagnosis, such as those meeting the EGJ outflow obstruction diagnosis on HRM via the Chicago Classification. Additionally, the human scoring of the variables by experienced and well-trained raters that were input into the machine learning model could limit generalizability to broader applications. Future study to formally assess learning curves and inter-rater agreement of FLIP panometry interpretation, as well as development of artificial intelligence models with automated feature extraction and evaluation, can address these concerns. Future study may also evaluate the significance of these FLIP panometry findings among these other cohorts of patients. We hope that the detailed characterization of this achalasia cohort will provide a basis for comparison with other esophageal motility patterns in future studies. Additionally, the aim of this study was not to assess direct clinical management implications or predication of treatment outcomes; evaluation of these outcomes is also planned for future study.
In conclusion, we evaluated patients with achalasia identified by HRM and utilized supervised machine learning methods to differentiate HRM based achalasia subtypes using FLIP panometry parameters. While our findings can be considered preliminary, the FLIP panometry parameters and approaches we describe here appear to have value in identifying specific clinical phenotypes among patients with achalasia – either as a complement to HRM or as an independent assessment. We theorize that FLIP panometry supplemented with machine learning models may enhance clinical characterization and may aid prognostication of treatment response in achalasia. However, future study is needed to assess the clinical implications of this FLIP panometry approach to achalasia and extend this approach to a larger and more heterogeneous clinical population.
Supplementary Material
Grant support:
This work was supported by P01 DK117824 (JEP) from the Public Health service and American College of Gastroenterology Junior Faculty Development Award (DAC).
Footnotes
Conflicts of interest:
Dustin A. Carlson, Peter J. Kahrilas, and John E. Pandolfino hold shared intellectual property rights and ownership surrounding FLIP panometry systems, methods, and apparatus with Medtronic Inc.
Dustin A. Carlson:Medtronic (Speaking. Consulting)
Wenjun Kou: Crospon, Inc (Consulting)
Ezra N. Teitelbaum: Boston Scientific (Consulting), Cook Medical (Speaking)
Peter J. Kahrilas: Ironwood (Consulting)
Eric Hungness: Boston Scientific (Consulting), Baxter (Consulting), Cook Medical (Consulting)
John E. Pandolfino: Crospon, Inc (stock options), Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking. Consulting), Torax (Speaking, Consulting), Ironwood (Consulting), Impleo (Grant).
None: Katharine P. Rooney, Joseph R. Triggs, Alexandra J. Baumann, Erica Donnan, Amy Holmstrom, Sajiv Sethi
References
- 1.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. The American journal of gastroenterology. 2013;108(8):1238–1249; quiz 1250. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135(5):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology. 2013;144(4):718–725; quiz e713–714. [DOI] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. The American journal of gastroenterology. 2008;103(1):27–37. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27(2):160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149(7):1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol. 2016;111(12):1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson DA, Lin Z, Rogers MC, Lin CY, Kahrilas PJ, Pandolfino JE. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015;27(7):981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson DA, Kahrilas PJ, Ritter K, Lin Z, Pandolfino JE. Mechanisms of repetitive retrograde contractions in response to sustained esophageal distension: a study evaluating patients with postfundoplication dysphagia. American journal of physiology Gastrointestinal and liver physiology. 2018;314(3):G334–G340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson DA, Kou W, Pandolfino JE. The rhythm and rate of distension-induced esophageal contractility: A physiomarker of esophageal function. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2020:e13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019;17(4):674–681 e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, de Ruigh A, Nicodeme F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2013;25(6):496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. Springer Science & Business Media; 2009. [Google Scholar]
- 14.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. Journal of neurogastroenterology and motility. 2011;17(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvador R, Costantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14(11):1635–1645. [DOI] [PubMed] [Google Scholar]
- 16.Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. The New England journal of medicine. 2011;364(19):1807–1816. [DOI] [PubMed] [Google Scholar]
- 17.Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut. 2016;65(5):732–739. [DOI] [PubMed] [Google Scholar]
- 18.Hungness ES, Sternbach JM, Teitelbaum EN, Kahrilas PJ, Pandolfino JE, Soper NJ. Per-oral Endoscopic Myotomy (POEM) After the Learning Curve: Durable Long-term Results With a Low Complication Rate. Ann Surg. 2016;264(3):508–517. [DOI] [PubMed] [Google Scholar]
- 19.Werner YB, Hakanson B, Martinek J, et al. Endoscopic or Surgical Myotomy in Patients with Idiopathic Achalasia. N Engl J Med. 2019;381(23):2219–2229. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Zifan A, Kumar D, Mittal RK. Genesis of Esophageal Pressurization and Bolus Flow Patterns in Patients with Achalasia Esophagus. Gastroenterology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Kahrilas PJ, Roman S, Boris L, Carlson D, Pandolfino JE. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil. 2012;24(8):e356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


