Abstract
Generation of reactive oxygen species, a critical factor in cisplatin-induced ototoxicity, leads to the formation of peroxynitrite, which in turn results in the nitration of susceptible proteins. Previous studies indicated that LMO4, a transcriptional regulator, is the most abundantly nitrated cochlear protein after cisplatin treatment and that LMO4 nitration facilitates ototoxicity in rodents. However, the role of this mechanism in regulating cisplatin-induced hair cell loss in non-mammalian models is unknown. As the mechanosensory hair cells in the neuromasts of zebrafish share many features with mammalian inner ear and is a good model for studying ototoxicity, we hypothesized that cisplatin treatment induces protein nitration and Lmo4 degradation in zebrafish hair cells, thereby facilitating hair cell loss. Immunostaining with anti-parvalbumin revealed a significant decrease in the number of hair cells in the neuromast of cisplatin treated larvae. In addition, cisplatin treatment induced a significant decrease in the expression of Lmo4 protein and a significant increase in nitrotyrosine levels, in the hair cells. The cisplatin-induced changes in Lmo4 and nitrotyrosine levels strongly correlated with hair cell loss, implying a potential link. Furthermore, a significant increase in the expression of activated Caspase-3 in zebrafish hair cells, post cisplatin treatment, suggested that cisplatin-induced decrease in Lmo4 levels is accompanied by apoptosis. These findings suggest that nitrative stress and Lmo4 degradation are important factors in cisplatin-induced hair cell loss in zebrafish neuromasts and that zebrafish could be used as a model to screen the otoprotective efficacy of compounds that inhibit protein nitration.
Keywords: Ototoxicity, Cisplatin, Neuromast, Lmo4, Nitrative Stress
1. Introduction
Ototoxic insults are usually accompanied by loss of hair cells, which manifest as hearing loss (Abrashkin et al., 2006; Seligmann et al., 1996). Approximately 50% of adults above the age of 75 and 2% of children are affected by hearing loss in America (Lynn L. Chiu et al., 2008). The platinum based chemotherapeutic drug cisplatin, used in the treatment of several adult and pediatric malignancies (Reddel et al., 1982), has a well-known ototoxic side-effect. Nevertheless, the high efficacy of cisplatin in treating solid tumors combined with lack of good alternatives makes it indispensable. Over the past several years, much progress has been made in defining the pathological processes underlying cisplatin-induced ototoxicity and in understanding associated molecular mechanisms (Schacht et al., 2012). Recent studies demonstrated that cisplatin treatment nitrates cochlear proteins in rodents and in UB/OC1 cell lines, which are derived from embryonic mouse inner ear (Jamesdaniel et al., 2012, 2016; Rosati et al., 2019). The most abundantly nitrated cochlear protein was found to be LMO4, a transcriptional factor regulator with a very diverse role. LMO4 belongs to the subclass of LIM domain only proteins and serves as a scaffold on which other proteins can bind (Sang et al., 2014). Additionally, members of this family are known to have roles in cell fate determination, tissue patterning, organ development, hematopoiesis, neural tube closure, altered anterior-posterior patterning among many other functions (Hahm et al., 2004). Though compelling evidences suggest that LMO4 nitration plays a pivotal role in cisplatin-induced ototoxicity in rodents, it is not known if this signaling mechanism is a critical factor in non-mammalian species also.
Zebrafish has emerged as a good model for investigating the ototoxic side-effects of drugs (Buck et al., 2012; d’Alençon et al., 2010; Gompel et al., 2001) and for screening otoprotectants (Kruger et al., 2016; Rocha-Sanchez et al., 2018). Zebrafish has sensory organs called neuromast that run along the length of lateral line, where mechanosensory hair cells are located (Namdaran et al., 2012; Pichler and Lagnado, 2019). The neuromasts help in balancing and are surrounded by supporting cells, which secrete a gelatinous cupula. Furthermore, they are innervated by sensory neurons whose cell bodies lie in a cranial ganglion in apposition to the otic (ear) ganglion (Hernández et al., 2007). The lateral line has evolved into an electrosensory system in some fish species, through specialization of the hair cell receptors (Froehlicher et al., 2009). However, it has disappeared in terrestrial vertebrates except for its derivative, the inner ear. The two systems, lateral line and inner ear, share many features, including the types of cells, their origin, and their central projection in the hindbrain. Studies have shown that cisplatin as well as other ototoxicants can induce hair cell loss in the neuromasts (Chiu et al., 2008; Coffin et al., 2013; Ou et al., 2007; Thomas et al., 2013). The aim of this study is to verify if cisplatin treatment alters the levels of Lmo4 protein and nitrotyrosine in zebrafish hair cells, similar to that observed in the mammalian species.
The Lmo4 in zebrafish is 76% orthologous to that of humans (McCollum et al., 2007). As shown in mammals, the Lmo4 in zebrafish is involved in tissue development and differentiation. Precisely, Lmo4 is known to be a crucial protein in the developmental stages of zebrafish larvae and is found to be expressed in rostral domain, dorsal ectoderm, optic stalk, retinal pigmented epithelium, otic vesicles, branchial arch region, sensory ganglia, cardiovascular expression, olfactory bulb (Duquette et al., 2010; Hao et al., 2013; Lane, 2002; Ochoa and LaBonne, 2009). Moreover, the expression pattern of this protein varies during different stages of larvae development. Lmo4 is also known to have roles in limiting the size of eyes and forebrains in zebrafish by modulating the expression of Six3 and Rx3 (McCollum et al., 2007). Furthermore, the expression pattern of Lmo4 in zebrafish larvae through 24 somite stage shows that it is expressed during gastrulation and segmentation stages, suggesting that it has an essential developmental role (Lane, 2002). As similarities in the expression of zebrafish Lmo4 to that of its murine ortholog has been observed (Sagerström et al., 2001), this study evaluates whether Lmo4 signaling is a factor in cisplatin-induced hair cell loss in the zebrafish.
2. Materials and Methods
2.1. Fish husbandry
Casper (roy −/−; nacre −/−) zebrafish strain was used in this study. Adult zebrafish, used for breeding, were maintained on a recirculating system (Aquaneering Inc., CA, USA) with a 14:10 light/dark cycle. The zebrafish system is supplied with reverse-osmosis (RO) water buffered with salts (Instant Ocean®, Spectrum Brands, VA, USA) with temperature maintained at 27°C– 30°C. Adult fish were fed flakes (Aquatox Fish Diet, Zeigler Bros Inc., PA, USA) twice daily, supplemented with brine shrimp, and were bred in spawning tanks at a sex ratio of 2 females:1 male. Viable embryos were cleaned with concentrated bleach (58 ppm) for 10 minutes, rinsed, and incubated at 28.5°C. Embryos were maintained in media at a density of 100 per petri dish (100 × 100 mm). Embryo media consisted of 600 mg/L Instant Ocean salts, with RO water. Zebrafish use protocols were approved by the Institutional Animal Care and Use Committee at Wayne State University and followed the National Institutes of Health Guide to the Care and Use of Laboratory Animals.
2.2. Drug preparation and treatment
Cisplatin (CAS 68001-283-24, BluePoint Laboratories, India) was diluted to 1000 μM from a 1mg/ml stock solution by dissolving in 5 ml egg water media. Though both 500 μM and 1000 μM doses induced significant changes in the expression of nitrotyrosine in pilot studies, the higher dose was chosen for this study because the cisplatin-induced changes were consistent after 1000 μM treatment and this dose has been used in previous studies investigating cisplatin-induced hair cell loss in zebrafish (Ou et al., 2007). At least 10 larvae, at 5-day post fertilization (dpf) stage, were transferred to each well of a 6-well plate. The larvae in the experimental group were exposed to cisplatin for 4 hours at 37°C in the dark while those in the control group were treated with egg water media. At the end of the treatment the larvae were euthanized with 10 mM tricaine methanesulfonate (ms-222) that was dissolved in sodium bicarbonate.
2.3. Immunohistochemistry
Euthanized larvae were immediately washed three times in 1X phosphate-buffer saline (PBS) for over 30 minutes. The samples were fixed in 4% paraformaldehyde overnight and washed the following day with PBS solution with 1% Triton at room temperature (RT). Samples were blocked in the same solution with 2% goat serum and incubated overnight with primary antibodies at the following dilutions: 1:600 anti-Parvalbumin (Mouse monoclonal, Millipore MAB1572), 1:100 anti-LMO4 (Rabbit polyclonal, Santa cruz sc-22833), 1:500 anti-Nitrotyrosine (Rabbit polyclonal, Millipore 06–284), and 1:100 anti-Caspase-3 (Rabbit polyclonal, Millipore MAB3623). The secondary antibodies, Donkey-anti-mouse AF568 (A100037) and Goat-anti-Rabbit AF647 (A21244) were used at 1:500 dilutions. Phalloidin (A12380, Invitrogen, Oregon US) was used at 1:100 dilution and samples were incubated for 2 hours at RT in dark. Images of the lateral line were captured using a Zeiss confocal microscope (LSM 700) at 63X magnification. The staining intensity of Lmo4, nitrotyrosine, and Caspase-3 was quantified using ImageJ software. Average intensity of three neuromasts in each of the three regions (anterior, middle, and posterior) was calculated by quantifying the staining intensity in three different hair cells selected randomly from each neuromast.
2.4. Hair cell count
The hair cells were counted after immunostaining with anti-parvalbumin. Three neuromasts were chosen from anterior, middle, and posterior regions of the lateral line (Figure 1A) and the hair cells were counted as viable if the entire cell appeared intact without any distortion in its morphology. Total hair cell count from each fish was computed and averaged. A total of 108 neuromasts from 12 larvae were computed and compared between the two groups.
Figure 1: Cisplatin-induced hair cell loss.
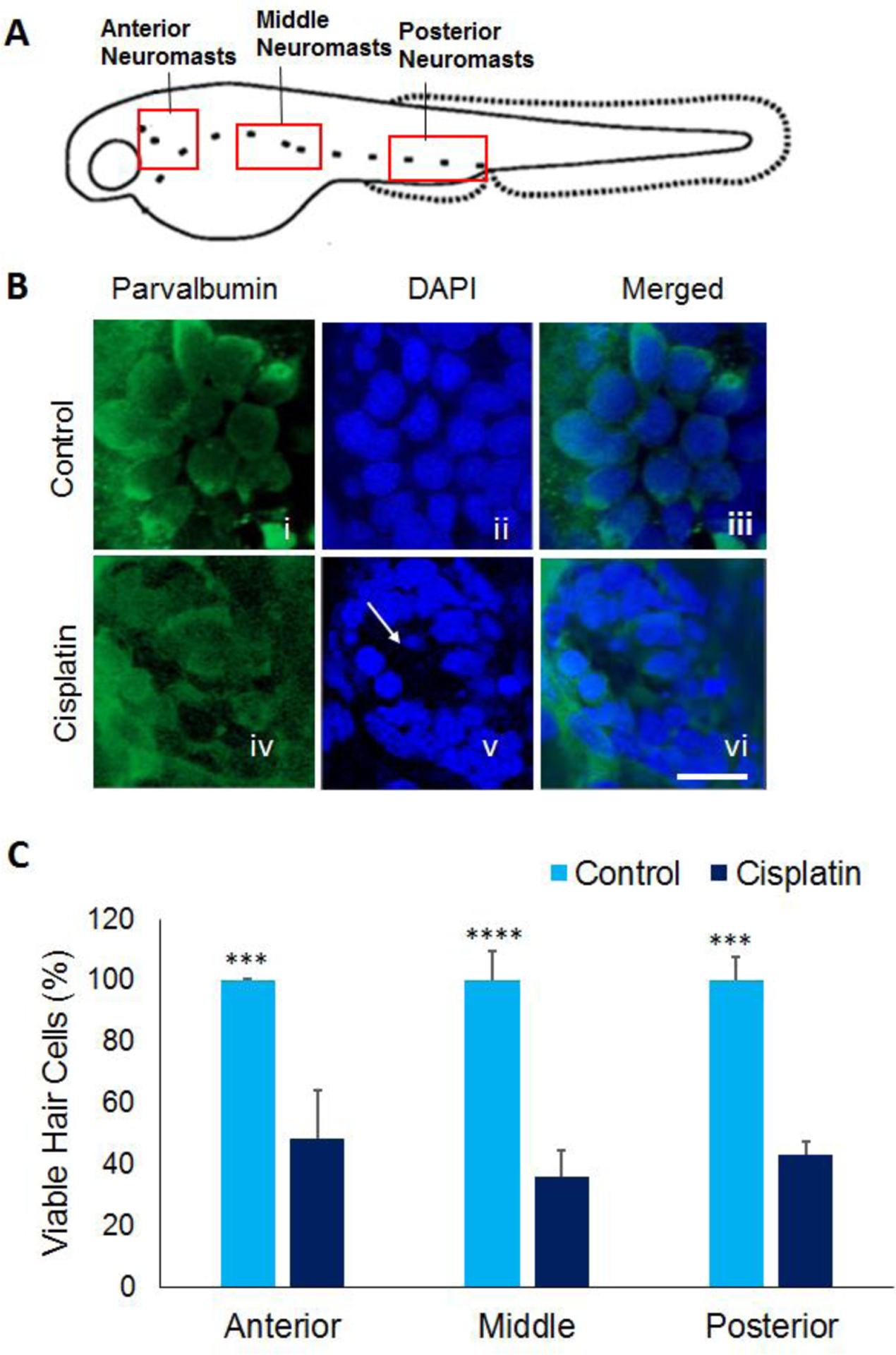
(A) Schematic representation of neuromasts in the anterior, middle, and posterior regions of 5dpf zebrafish larvae. (B) Treatment of 5dpf larvae with 1000 μM cisplatin induced hair cell loss in neuromasts located in the anterior, middle, and posterior regions along the lateral line. The hair cells were labelled with parvalbumin (green), a hair cell marker (i and iv), images were captured at 63X magnification, and the hair cells were counted manually. Cisplatin-induced loss of hair cells is evident from lack of intact hair cells and nuclei (white arrow) in panel iv and v, respectively. Scale bar = 5 μm. (C) Quantification of the hair cells indicated that cisplatin treatment induced a significant decrease in hair cell viability in all three regions along the lateral line suggesting the toxic effect of cisplatin in zebrafish hair cells. The results are expressed as mean ± standard deviation, n = 12, (***p<0.001, ****p<0.0001).
2.5. Neuromast assessment
Cisplatin-induced distortion in the morphology of neuromasts was assessed by using a scoring system employed by Rocha-Sanchez et al. (2018). The scoring protocol assigned a score of 1 for normal rosette-shaped neuromasts with intact hair cells, 2 for normal rosette-shaped neuromasts with fewer hair cells, 3 for normal rosette-shaped neuromasts with several hair cells missing, 4 for rosette-like shaped but distorted neuromasts with several missing hair cells, and 5 for neuromasts that did not have rosette-like shape and had very few dispersed hair cells.
2.6. Data analysis
All experiments were repeated in 6 to 12 biological replicates. All data were statistically analyzed using Microsoft Excel’s Data Analysis Toolkit (Office Professional Plus 2016, Microsoft, Redmond, WA, USA) or GraphPad Prism 6 software (La Jolla, CA). Normality was tested using D’Agostino and Pearson test and homogeneity of variances was tested using Levene’s test. The data was found to be normally distributed and homogenous. Significant differences between the groups were determined by using two-tailed t-tests and p<0.05 was considered significant. The correlation between cisplatin-induced changes in expression levels of Lmo4 and nitrotyrosine and hair cell viability was determined by using the Pearson’s correlation coefficient at 95% confidence interval. Results were expressed as mean ± standard deviation/error.
3. Results
3.1. Cisplatin treatment induced hair cell loss and distorted the neuromasts
Exposure of zebrafish larvae (5dpf) to 1000 μM of cisplatin for 4 hours induced extensive loss of hair cells in the neuromasts in the anterior, middle, and posterior regions (Figure 1). A similar loss of hair cells with a dose of cisplatin ranging from 250–1500 μM cisplatin was reported by Ou et al. (2007). Unlike the rodent cochlea, where the cisplatin-induced damage is focused in the basal region, the hair cell loss in zebrafish lateral line was significant in all three regions (p= 0.00041, p=0.000022 and p=0.00046 respectively). Furthermore, a morphometric analysis of hair cells suggested that cisplatin exposure distorted the neuromasts. More than 77% of neuromasts in the control group had intact and well-defined morphology while 83% of the cisplatin exposed larvae had a score of 5 suggesting that the hair cells were damaged and the neuromasts were distorted by cisplatin treatment (Figure 2).
Figure 2: Morphometric analysis of neuromasts.
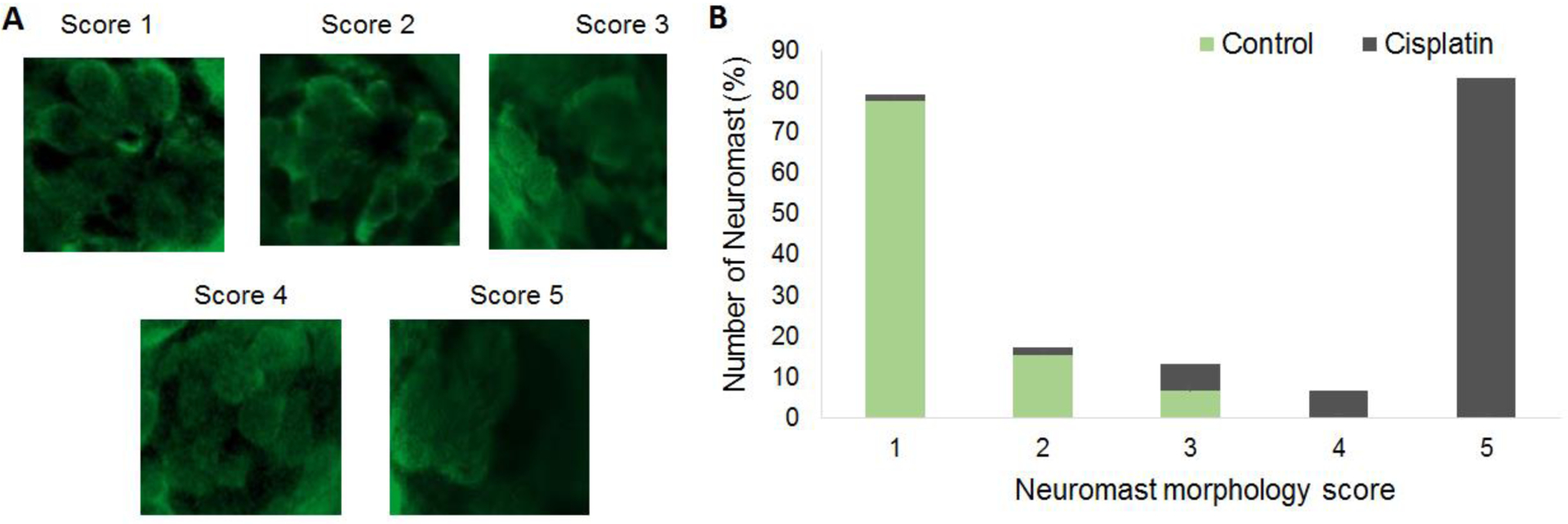
(A) Representative images indicating the degree of distortion in the morphology of hair cells and neuromasts and their corresponding scores are illustrated. The hair cells were labelled with parvalbumin (green) and the images were captured at 63X magnification. The morphometric analysis indicated that the morphology of hair cells and the neuromasts were distorted after cisplatin treatment. (B) Morphometric scores indicated that more than 77% of the neuromasts in the controls had intact and well-defined morphology. However, 83% of the neuromasts in cisplatin treated larvae received a score of 5 suggesting that the hair cells were dispersed and the neuromasts were distorted, n = 6. Evaluation of the difference in the morphology by Mann-Whitney’s U test indicated that the differences were significant between the control and cisplatin-treated groups (mean scores of control and cisplatin-treated groups were 1 and 5, respectively; U=155; p<0.001).
3.2. Cisplatin-induced decrease in Lmo4 levels positively correlated with hair cell viability
Immunohistochemistry analysis with anti-Lmo4 indicated that cisplatin induced a decrease in Lmo4 levels in the hair cells (red staining) compared to the controls (Figure 3A). Quantification of the staining intensity revealed a significant decrease in the Lmo4 expression (p= 0.004591, p=0.000031 and p=0.00014, n=6) in the anterior, middle, and posterior regions (Figure 3B). In addition, a correlation analysis indicated that hair cell viability positively correlated with the protein levels of Lmo4 (R2= 0.761858, Figure 3C). This suggested a potential link between Lmo4 levels and survival of hair cells in cisplatin treated zebrafish larvae.
Figure 3: Lmo4 protein expression in zebrafish hair cells.
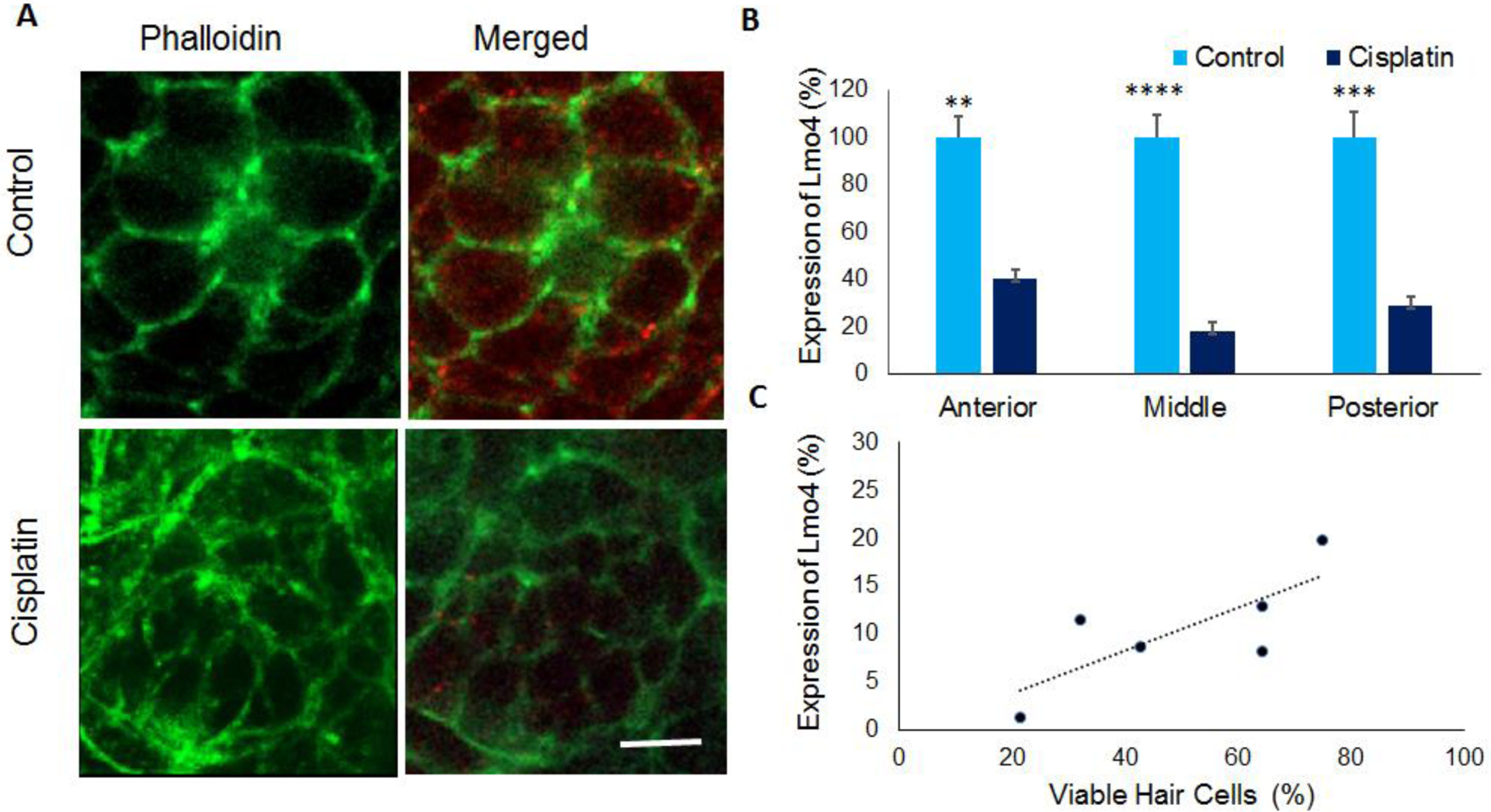
(A) Immunohistochemistry analysis indicated that cisplatin treatment decreased the expression of Lmo4 protein (red) in the hair cells of 5dpf stage larvae. Phalloidin (green) was used to stain actin in the hair cells. Images are representative of six replicates. Scale bar= 5 μm. (B) Quantification of the staining intensity in neuromasts located in the anterior, middle, and posterior regions indicated a significant decrease in the Lmo4 protein levels in cisplatin treated zebrafish. The results are expressed as mean ± standard error, n = 6, (**p<0.01, ***p<0.001, ****p<0.0001). (C) Analysis of the correlation between cisplatin-induced changes in hair cell viability and Lmo4 protein expression indicated a positive correlation (R2=0.76), n=6.
3.3. Cisplatin-induced increase in nitrotyrosine levels negatively correlated with hair cell viability
Immunohistochemistry analysis with anti-nitrotyrosine indicated that nitrotyrosine levels (purple staining) were markedly increased in the hair cells after cisplatin treatment (Figure 4A). Quantification of the staining intensity indicated that the cisplatin-induced increase was significant (p=0.0003, p=0.0001, and p=0.000035) in the anterior, middle, and posterior regions (Figure 4B). In addition, a negative correlation was observed between hair cell viability and nitrotyrosine levels (R2=−0.74), suggesting that nitrative stress is a factor in cisplatin-induced hair cell loss (Figure 4C).
Figure 4: Nitrotyrosine in zebrafish hair cells.
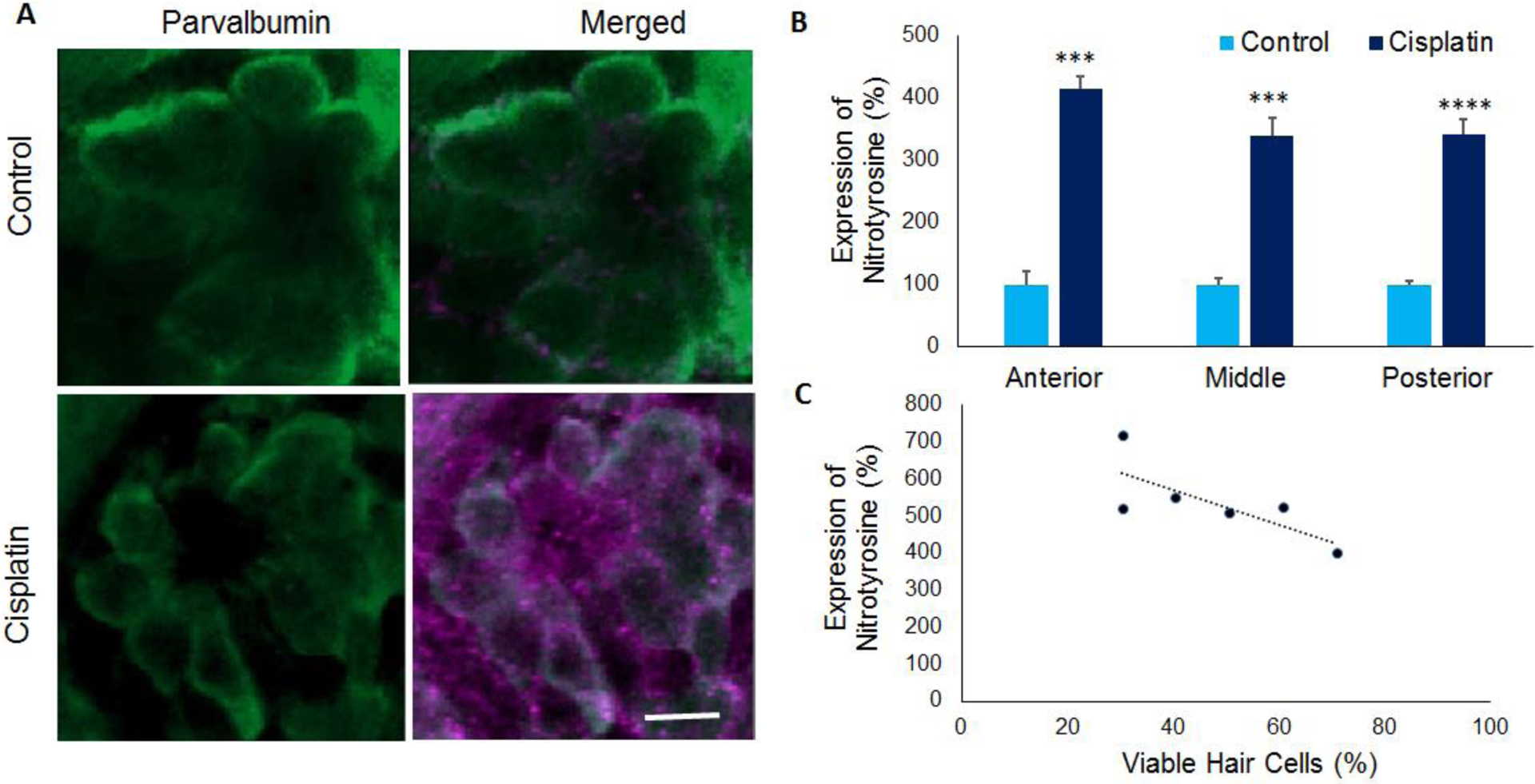
(A) Immunolocalization with anti-nitrotyrosine indicated that cisplatin treatment increased the nitrotyrosine levels (purple) in the hair cells of 5dpf stage larvae. Parvalbumin (green) was used to stain the hair cells. Images are representative of six replicates. Scale bar= 5 μm. (B) Quantification of the staining intensity in neuromasts located in the anterior, middle, and posterior regions indicated a significant increase in nitrotyrosine levels in cisplatin treated zebrafish. The results are expressed as mean ± standard error, n = 6, (***p<0.001, ****p<0.0001). (C) Analysis of the correlation between cisplatin-induced changes in hair cell viability and nitrotyrosine levels indicated a negative correlation (R2=0.74), n=6.
3.4. Cisplatin induced apoptosis in zebrafish hair cells
Immunohistochemistry analysis with anti-Caspase-3 suggested that cisplatin treatment induced an increase in activated Caspase-3 (violet staining), which is an indicator of apoptosis (Figure 5A). Quantification of the staining intensity of Caspase-3 in the anterior, middle, and posterior regions of the neuromast indicated a significant increase in all three regions (p=0.00187) in cisplatin treated larvae (Figure 5B). This suggested that cisplatin-induced hair cell loss in the neuromasts probably occurrs by apoptosis.
Figure 5: Activated Caspase-3 expression in zebrafish hair cells.
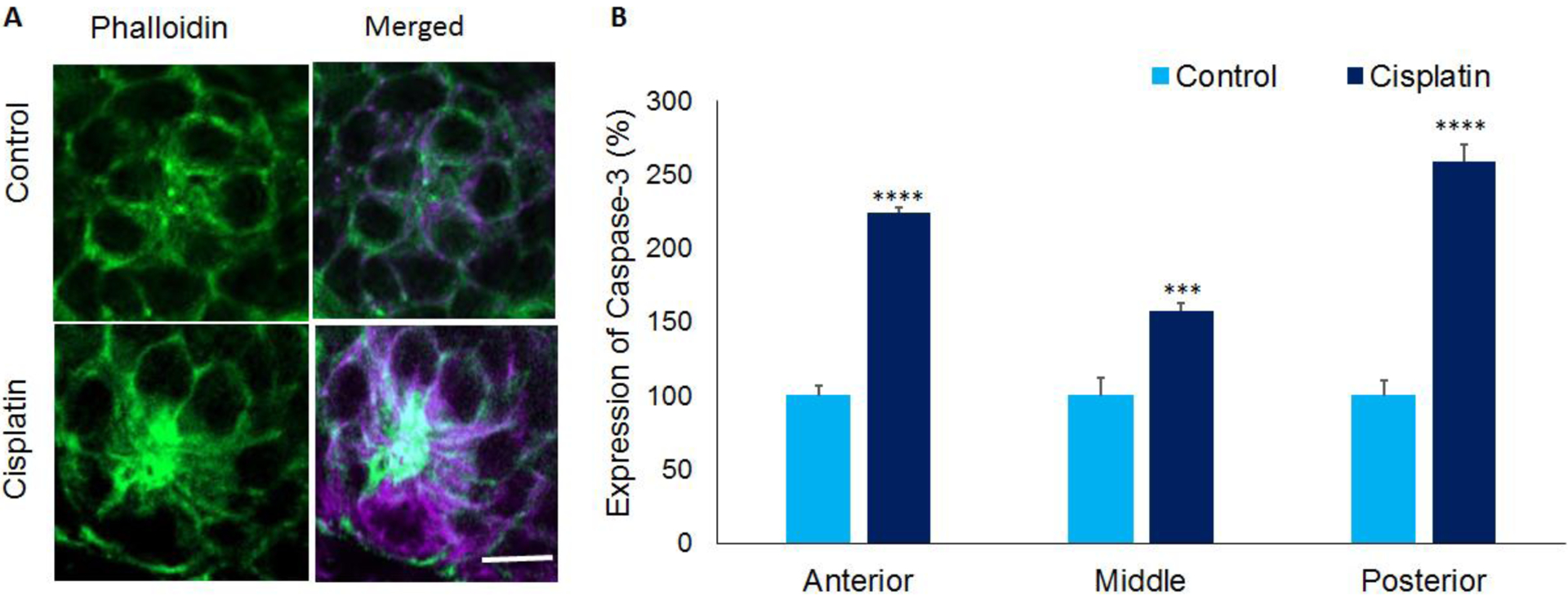
(A) Immunolocalization with anti- Caspase-3 indicated that cisplatin treatment increased the expression of activated Caspase-3 (purple) in the hair cells of 5dpf stage larvae. This suggested cisplatin-induced apoptosis in zebrafish hair cells. Phalloidin (green) was used to stain actin in the hair cells. Images are representative of six replicates. Scale bar= 5 μm. (B) Quantification of the staining intensity in neuromasts located in the anterior, middle, and posterior regions indicated a significant increase in activated Caspase-3 levels in cisplatin treated zebrafish. The results are expressed as mean ± standard error, n = 6, (***p<0.001, ****p<0.0001).
4. Discussion
Cisplatin treatment is known to induce hair cell loss in zebrafish neuromasts (Chiu et al., 2008; Kruger et al., 2016). However, it is not clear if the underlying molecular mechanisms and pathological processes in non-mammalian models are similar to that observed in cisplatin-induced ototoxicity in mammals. This study provides evidence that cisplatin-induced hair cell loss in zebrafish is accompanied by protein nitration and Lmo4 degradation, which are emerging as critical factors in cisplatin-induced ototoxicity in rodents (Jamesdaniel et al., 2012). Unlike the mammalian cochlea, where cisplatin-induced damage is focused predominantly in the basal region (Slattery and Warcho, 2010), the hair cell loss and structural damage to neuromasts were similar in the anterior, middle and posterior regions of zebrafish lateral line. Cisplatin treatment significantly increased the levels of nitrotyrosine, decreased the levels of Lmo4, and increased the levels of activated Caspase-3 in the hair cells, in all three regions. These observations suggest that, similar to previous findings indicating cisplatin-induced LMO4 degradation and hearing loss in mammalian models (Jamesdaniel et al., 2012), cisplatin treatment alters Lmo4 signaling in zebrafish hair cells and induces apoptosis resulting in hair cell loss.
Nitrative stress is an important factor in cisplatin-induced ototoxicity in rodents (Li et al., 2006). Although cisplatin binds to DNA to form DNA adducts in mitotically active cells (Dasari and Bernard Tchounwou, 2014), in mitotically quiescent cells, such as hair cells, cisplatin treatment leads to the generation of reactive oxygen species (ROS), which can lead to the death of the hair cells (Rybak et al., 1995). In addition, cisplatin treatment depletes antioxidant systems and increases the production of nitric oxide by upregulating iNOS (Callejo et al., 2015; Chirino and Pedraza-Chaverri, 2009; Ramesh and Reeves, 2005). All of this can eventually lead to an increase in peroxynitrite production, which can nitrate susceptible proteins. In this study, an increase in nitrotyrosine levels in zebrafish hair cells was observed after cisplatin treatment. This is consistent with previous reports indicating that cisplatin induced an increase in cochlear nitrotyrosine levels in rodents (Jamesdaniel et al., 2012). Moreover, the increase in nitrotyrosine levels in zebrafish hair cells negatively correlated with cisplatin-induced decrease in hair cell viability, suggesting a critical role of nitrative stress in cisplatin-induced hair cell loss in non-mammalian models. To further understand the role of the nitrative stress mechanism in cisplatin-induced hair cell loss in zebrafish model, it is essential to evaluate potential molecular targets of cisplatin-induced nitration.
Previous studies indicated that cisplatin treatment nitrates LMO4 and decreases its levels in mammalian models (Jamesdaniel et al., 2012). As nitrated proteins are susceptible to degradation (Radi, 2012; Souza et al., 2008), the decrease in Lmo4 levels in zebrafish hair cells, observed in this study, along with an increase in nitrotyrosine levels suggests that Lmo4 could be an important target of cisplatin in zebrafish models. In addition, the hair cell viability positively correlated with Lmo4 protein levels and negatively correlated with nitrotyrosine levels. This suggests that Lmo4 signaling plays a role in cisplatin-induced hair cell loss in zebrafish. This is consistent with the findings of earlier studies, which supported an otoprotective role of Lmo4 in cisplatin-induced ototoxicity because overexpression of Lmo4 promoted cell survival (Jamesdaniel et al., 2016, 2012; Rathinam et al., 2015), while knockout of Lmo4 increased susceptibility to cisplatin-induced cell death. In addition, co-treatment with a peroxynitrite decomposition catalyst attenuated cisplatin-induced decrease in cochlear LMO4 levels and hearing loss (Jamesdaniel et al., 2016; Rosati et al., 2019).
The cisplatin-induced increase in the expression of activated Caspase-3 in zebrafish hair cells suggests cisplatin-induced apoptosis. This observation is consistent with previous findings on cisplatin-induced apoptosis of hair cells in both mammalian and non-mammalian models (Chiu et al., 2008; Jamesdaniel, 2014; Jamesdaniel et al., 2016; Ou et al., 2007; Rathinam et al., 2015) and is in agreement with reports that indicate ototoxic drugs induce mitochondrial dysfunction and apoptosis in hair cells (Baker et al., 2015; Owens et al., 2008; Schacht et al., 2012). Overall, the results of this study suggest that cisplatin-induced hair cell loss in zebrafish involve similar mechanistic pathways that have been reported in rodents. Although additional studies are needed to demonstrate a causal role of Lmo4 nitration and delineate downstream signaling patterns through which cisplatin treatment leads to hair cell loss in non-mammalian models, the present findings suggests that zebrafish model could be useful for understanding emerging targets through which cisplatin mediate its ototoxic side-effects and for high throughput screening of potential otoprotectant compounds that modify these novel targets.
Highlights.
Cisplatin treatment increases nitrotyrosine levels in zebrafish hair cells.
Cisplatin-induced degradation of Lmo4 positively correlates with hair cell loss.
Lmo4 signaling has a role in cisplatin-induced ototoxicity in non-mammalian models.
Funding
This work was supported by faculty startup grant to SJ from Wayne State University and P30 Grant (P30 ES020957) to Center for Urban Responses to Environmental Stressors (CURES).
Abbreviations
- Dpf
Day post fertilization
- Lmo4
LIM-domain only 4
- iNOS
Inducible nitric oxide synthase
- Nacre
Non-crest-derived retinal pigment epithelium
- PBS
Phosphate-buffer saline
- RO
Reverse-osmosis
- ROS
Reactive oxygen species
- Roy
Mitochondrial inner membrane protein
- RT
Room Temperature
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors declare no competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang C-H, Crumling MA, Swiderski DL, Beyer LA, Gong T-WL, Raphael Y, 2006. The fate of outer hair cells after acoustic or ototoxic insults. Hear. Res 218(1–2):20–9. [DOI] [PubMed] [Google Scholar]
- Baker TG, Roy S, Brandon CS, Kramarenko IK, Francis SP, Taleb M, Marshall KM, Schwendener R, Lee F-S, Cunningham LL, 2015. Heat shock protein-mediated protection against Cisplatin-induced hair cell death. J. Assoc. Res. Otolaryngol. JARO 16(1):67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck LMJ, Winter MJ, Redfern WS, Whitfield TT, 2012. Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear. Res 284(1–2):67–81. [DOI] [PubMed] [Google Scholar]
- Callejo A, Sedó-Cabezón L, Domènech Juan I, Llorens J, 2015. Cisplatin-Induced Ototoxicity: effects, mechanisms and protection strategies. Toxics 3(3): 268–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirino YI, Pedraza-Chaverri J, 2009. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol 61(3):223–42. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC, 2008. Using the zebrafish lateral line to screen for ototoxicity. JARO - J. Assoc. Res. Otolaryngol 9(2):178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin AB, Williamson KL, Mamiya A, Raible DW, Rubel EW, 2013. Profiling drug-induced cell death pathways in the zebrafish lateral line. Apoptosis 18(4):393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Alençon CA, Peña OA, Wittmann C, Gallardo VE, Jones RA, Loosli F, Liebel U, Grabher C, Allende ML, 2010. A high-throughput chemically induced inflammation assay in zebrafish. BMC Biol. 8:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Bernard Tchounwou P, 2014. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette PM, Zhou X, Yap NL, MacLaren EJ, Lu JJ, Wallace VA, Chen HH, 2010. Loss of LMO4 in the retina leads to reduction of GABAergic amacrine cells and functional deficits. PLoS One 5(10): e13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlicher M, Liedtke A, Groh KJ, Neuhauss SCF, Segner H, Eggen RIL, 2009. Zebrafish (Danio rerio) neuromast: promising biological endpoint linking developmental and toxicological studies. Aquat. Toxicol 95(4):307–19. [DOI] [PubMed] [Google Scholar]
- Gompel N, Cubedo N, Thisse C, Thisse B, Dambly-Chaudière C, Ghysen A, 2001. Pattern formation in the lateral line of zebrafish. Mech. Dev 105(1–2):69–77. [DOI] [PubMed] [Google Scholar]
- Hahm K, Sum EYM, Fujiwara Y, Lindeman GJ, Visvader JE, Orkin SH, 2004. Defective neural tube closure and anteroposterior patterning in mice lacking the LIM Protein LMO4 or its interacting partner Deaf-1. Mol. Cell. Biol 24(5): 2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao R, Bondesson M, Singh AV, Riu A, McCollum CW, Knudsen TB, Gorelick DA, Gustafsson JÅ, 2013. Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS One 8(11): e79020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML, 2007. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker Sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev. Neurobiol 7(5):637–54. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, 2014. Downstream targets of Lmo4 are modulated by cisplatin in the inner ear of wistar rats. PLoS One 9(12): e115263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Coling D, Hinduja S, Ding D, Li J, Cassidy L, Seigel GM, Qu J, Salvi R, 2012. Cisplatin-induced ototoxicity is mediated by nitroxidative modification of cochlear proteins characterized by nitration of Lmo4. J. Biol. Chem 287(22): 18674–18686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Rathinam R, Neumann WL, 2016. Targeting nitrative stress for attenuating cisplatin-induced downregulation of cochlear LIM domain only 4 and ototoxicity. Redox Biol. 10, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Boney R, Ordoobadi AJ, Sommers TF, Trapani JG, Coffin AB, 2016. Natural bizbenzoquinoline derivatives protect zebrafish lateral line sensory hair cells from aminoglycoside toxicity. Front. Cell. Neurosci 10: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Liu W, Frenz D, 2006. Cisplatin ototoxicity to the rat inner ear: A role for HMG1 and iNOS. Neurotoxicology 27(1):22–30 [DOI] [PubMed] [Google Scholar]
- McCollum CW, Amin SR, Pauerstein P, Lane ME, 2007. A zebrafish LMO4 ortholog limits the size of the forebrain and eyes through negative regulation of six3b and rx3. Dev. Biol 309(2): 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW, 2012. Identification of modulators of hair cell regeneration in the zebrafish lateral line. J. Neurosci 32(10): 3516–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa SD, LaBonne C, 2009. The role of LMO4 in neural crest development. Dev. Biol 361(2): 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW, 2007. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear. Res 233(1–2): 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW, 2008. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 4(2): e1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler P, Lagnado L, 2019. The transfer characteristics of hair cells encoding mechanical stimuli in the lateral line of zebrafish. J. Neurosci 39(1):112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, 2012. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc Chem Res. 46(2):550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB, 2005. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Physiol 289(1): F166–74. [DOI] [PubMed] [Google Scholar]
- Rathinam R, Ghosh S, Neumann WL, Jamesdaniel S, 2015. Cisplatin-induced apoptosis in auditory, renal, and neuronal cells is associated with nitration and downregulation of LMO4. Cell Death Discov. 1:15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH, 1982. Ototoxicity in patients receiving cisplatin: Importance of dose and method of drug administration. Cancer Treat. Rep 66, 19–23. [PubMed] [Google Scholar]
- Rocha-Sanchez SM, Fuson O, Tarang S, Goodman L, Pyakurel U, Liu H, He DZ, Zallocchi M, 2018. Quinoxaline protects zebrafish lateral line hair cells from cisplatin and aminoglycosides damage. Sci. Rep 8(1):15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati R, Shahab M, Neumann WL, Jamesdaniel S, 2019. Inhibition of protein nitration prevents cisplatin-induced inactivation of STAT3 and promotes anti-apoptotic signaling in organ of Corti cells. Exp. Cell Res 381(1):105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Ravi R, Somani SM, 1995. Mechanism of protection by diethyldithiocarbamate against cisplatin ototoxicity: Antioxidant System. Fundam. Appl. Toxicol 26(2):293–300. [DOI] [PubMed] [Google Scholar]
- Sagerström CG, Kao BA, Lane ME, Sive H, 2001. Isolation and characterization of posteriorly restricted genes in the zebrafish gastrula. Dev. Dyn 220(4):402–8. [DOI] [PubMed] [Google Scholar]
- Sang Meixiang, Ma L, Sang Meijie, Zhou X, Gao W, Geng C, 2014. LIM-domain-only proteins: Multifunctional nuclear transcription coregulators that interacts with diverse proteins. Mol. Biol. Rep 4(12): 1132–1137. [DOI] [PubMed] [Google Scholar]
- Schacht J, Talaska AE, Rybak LP, 2012. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. (Hoboken) 295(11): 1837–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann H, Podoshin L, Ben-David J, Fradis M, Goldsher M, 1996. Drug-induced tinnitus and other hearing disorders. Drug Saf. 14(3):198–212. [DOI] [PubMed] [Google Scholar]
- Slattery EL, Warcho ME, 2010. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J. Neurosci 30(9):3473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Peluffo G, Radi R, 2008. Protein tyrosine nitration-functional alteration or just a biomarker? Free Radic. Biol. Med 45(4):357–66. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC, 2013. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J. Neurosci 33(10): 4405–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]


